Abstract
Introduction
The maximum standardized uptake value (SUVmax) in 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) may be of prognostic significance for patients with malignant pleural mesothelioma (MPM). This retrospective study aimed to investigate the prognostic value of the SUVmax in patients with MPM.
Materials and methods
Medical records were retrospectively reviewed for the patients who were diagnosed with histopathologically proven MPM between 2009 and 2018 at Samsung Medical Center. For each patient, SUVmax was calculated for the primary lesion on PET/CT. To determine optimal cutoff values for predicting mortality, receiver operating characteristic curves were used.
Results
Among the 54 study patients, 34 (63.0%) had epithelioid subtype, 13 (24.1%) had sarcomatoid or biphasic subtype, and 7 (13.0%) had mesothelioma, not otherwise specified (NOS). The median overall survival (OS) was 8.7 months, and the median SUVmax was 9.9. The median values of SUVmax were 5.5 in patients with epithelioid subtype, 11.7 in those with sarcomatoid/biphasic subtype, and 13.3 in those with NOS subtype (P = 0.003). The optimal cutoff values of SUVmax to predict mortality were 10.1 in all patients, and 8.5 in patients with epithelioid subtype. In multivariate analysis, SUVmax was significantly associated with overall survival in all patients (P = 0.003) and in patients with epithelioid subtype (P = 0.012), but not in those with non-epithelioid subtype.
Conclusions
SUVmax in PET/CT is an independent prognostic factor in patients with MPM, especially those with epithelioid subtype. The histologic subtype of MPM should be considered when evaluating the prognostic significance of SUVmax.
Introduction
Malignant pleural mesothelioma (MPM) is a rare but aggressive tumor that arises from pleural mesothelial cells. The prognosis of patients with MPM is poor, with a median survival of 20–29 months despite tri-modality treatment including surgery, chemotherapy, and radiotherapy [1, 2]. Surgical methods (e.g., extra-pleural pneumonectomy [EPP] or pleurectomy/decortication) should be selected in accordance with the patient's condition [3, 4]. Among chemotherapeutic agents, a pemetrexed and platinum-based regimen has been recommended as a first-line treatment because of its proven ability to improve the survival rate [5, 6]. Immune checkpoint inhibitors, vinorelbine and gemcitabine are recommended as subsequent systemic therapy in the most recent guideline [6]. Pembrolizumab or nivolumab with (or without) ipilimumab showed promising results in recent clinical trials [7–9].
Predicting the prognosis of patients with MPM is important for determining treatment options. There are multiple prognostic prediction models for MPM, such as the model developed by the European Organization for the Research and Treatment of Cancer (EORTC) and that developed by Cancer and Leukemia Group B (CALGB) [10, 11]. Several studies have reported that 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) parameters, including maximum standardized uptake value (SUVmax), are associated with the prognosis of MPM [12–19]. Few studies have considered clinical factors such as stage, histology, or chemotherapeutic agents as confounding factors in determining the prognosis of patients with MPM. Because most previous studies are based on PET rather than integrated PET/computed tomography (PET/CT), the applications of the results of these studies in the medical field are limited.
The purpose of this study was to investigate the prognostic value of SUVmax of 18F-FDG PET/CT in patients with MPM and to define its impact on survival prognosis in those patients. The prognostic value of SUVmax was evaluated for each subgroup based on clinical characteristics.
Materials and methods
Patients
We conducted a retrospective review of the medical records of 123 patients who were diagnosed with histopathologically proven MPM during the period between January 2009 and June 2018 at Samsung Medical Center in Seoul, South Korea. In all patients, surgical biopsy was performed for diagnosis of MPM. Patients who were lost to follow-up (n = 4), who did not undergo 18F-FDG PET/CT (n = 49), or who had no available data for SUV (n = 16) were excluded. Ultimately, 54 patients were enrolled in this retrospective study (Fig 1).
Fig 1. Flow chart of patients in the study.
We reviewed clinical records for age, gender, smoking history, exposure to asbestos, location of tumor, presence of bilateral pleural plaque, histologic subtype, stage, SUVmax, type of surgery, and chemotherapy. All patients underwent diagnostic contrast-enhanced CT of the chest and abdomen and 18F-FDG PET/CT. Disease stage was classified in accordance with the eighth edition of the tumor-node-metastasis (TNM) classification for MPM by the Union for International Cancer Control (UICC) and the American Joint Commission on Cancer (AJCC) [20].
EPP, pleurectomy/decortication, or partial pleurectomy was performed in patients with resectable MPM who could tolerate aggressive surgery. Neoadjuvant or adjuvant chemotherapy with four to six cycles of pemetrexed and cisplatin or carboplatin was administered in combination with surgery. In patients who were not candidates for surgery, palliative chemotherapy was administered with pemetrexed and cisplatin or carboplatin. Cycles of chemotherapy were repeated at 21-day intervals.
This review was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2018-07-081), which waived the requirement for informed consent by individual patients because of the retrospective nature of the study.
FDG PET/CT
18F-FDG PET/CT was performed prior to surgery or chemotherapy for baseline analysis in all patients. All patients fasted for at least 6 h and had a blood glucose level <150 mg at the time of PET/CT. Imaging was performed 60 min after injection of 5 MBq/kg 18F-FDG (without intravenous or oral contrast) on a Discovery LS (GE Healthcare, Waukesha, WI, USA) or a Discovery STe PET/CT scanner (GE Healthcare Waukesha, WI, USA). Continuous spiral CT was performed using an 8-slice helical CT (140 keV; 40–120 mA; Discovery LS) or with 16-slice helical CT (140 keV; 30–170 mA; Discovery STe). Further details were described in our previous published study [21].
The 18F-FDG PET/CT data were evaluated using the SUVmax by one experienced nuclear medicine physician (J.Y.C) who was blinded to patient outcome. Region of interest analysis tools included with the scanner were used to calculate the SUVmax over the primary tumor after correction for the injected dose of 18F-FDG and patient weight.
Statistical analysis
The data are presented as number (%) or median (interquartile range) unless otherwise stated. To compare SUVmax according to clinical characteristics, we performed independent sample t-tests or Mann–Whitney U tests. Receiver operating characteristic (ROC) curves were plotted to determine the optimal cutoff values of SUVmax that yielded the maximal sensitivity plus specificity of predicting the overall survival. The patient population was subdivided using the cutoff values of SUVmax from the ROC curves, and the duration of overall survival was compared between groups. Overall survival (OS) was calculated as the time (months) from diagnosis until death from any cause. Patients who were alive on the date of the most recent follow-up were censored on that date. Median OS was calculated using the Kaplan–Meier method and compared using a log-rank test. To assess the potential independent effects of SUVmax on OS, we performed univariate and multivariate analyses using Cox proportional hazards models with variables that had P-values <0.05. Statistical analyses were performed using a statistical software package (SPSS version 19.0, SPSS, Chicago, IL, USA).
Results
Patients
The characteristics of the 54 study patients are summarized in Table 1. The median age was 64 years and 75.9% of patients were men. Thirty-four patients (63.0%) had epithelioid subtype, 13 patients (24.1%) had sarcomatoid (n = 10) or biphasic (n = 3) subtype, and 7 patients (13.0%) had mesothelioma, not otherwise specified (NOS). Nineteen patients (35.2%) underwent surgical resection (EPP [n = 10], pleurectomy/decortication [n = 4] or pleurectomy alone without decortication [n = 5]). Thirty-six patients (66.7% underwent chemotherapy with pemetrexed plus cisplatin or carboplatin (neoadjuvant or adjuvant [n = 11] or palliative chemotherapy [n = 25]). During a median follow-up of 8.7 months (3.8–21.9 months), 30 of 54 (55.6%) MPM patients died. The median OS of patients was 12.6 months.
Table 1. Baseline characteristics of study subjects.
| Characteristics | N (%) or Median (IQR) |
|---|---|
| Age (years) | 64 (53–71) |
| Male/female | 41 (75.9)/13 (24.1) |
| Smoker/nonsmoker | 30 (55.6)/24 (44.4) |
| Asbestos exposure | |
| Yes | 15 (27.8) |
| No | 20 (37.0) |
| Unknown | 19 (35.2) |
| Location of tumor | |
| Right | 31 (57.4) |
| Left | 23 (42.6) |
| Bilateral pleural plaque | |
| Yes | 10 (18.5) |
| No | 44 (81.5) |
| Histologic subtype | |
| Epithelioid | 34 (63.0) |
| Sarcomatoid | 10 (18.5) |
| Biphasic | 3 (5.5) |
| NOS | 7 (13.0) |
| T stage | |
| T1 | 15 (27.8) |
| T2 | 6 (11.1) |
| T3 | 14 (25.9) |
| T4 | 19 (35.2) |
| N stage | |
| N0 | 27 (50.0) |
| N1 | 16 (29.6) |
| N2 | 11 (20.4) |
| M stage | |
| M0 | 42 (77.8) |
| M1 | 12 (22.2) |
| Stage | |
| IA | 3 (5.6) |
| IB | 15 (27.8) |
| II | 2 (3.7) |
| IIIA | 4 (7.4) |
| IIIB | 20 (37.0) |
| IV | 10 (18.5) |
| SUVmax | 9.9 (4.4–13.5) |
| Type of surgery | |
| Extrapleural pneumonectomy | 10 (18.5) |
| Pleurectomy/decortication | 4 (7.4) |
| Partial pleurectomy | 5 (9.3) |
| None | 35 (64.8) |
| Chemotherapy | |
| Pemetrexed/platinum | 36 (66.7) |
| None | 18 (33.3) |
IQR, interquartile range; NOS, not otherwise specified; SUVmax, maximum standardized uptake value
SUVmax according to clinical characteristics
The median value of SUVmax was significantly lower in patients with epithelioid subtype (5.5) than in those with sarcomatoid/biphasic subtype (11.7) or mesothelioma, NOS (13.3) (Table 2). The SUVmax was also significantly associated with stage and surgery. The ROC curve showed that the optimal cutoff value of SUVmax for predicting death was 10.1 (area under the curve [AUC] = 0.681) in all patients. Because there was a significant difference in the median SUVmax in relation to tumor subtype, we calculated the optimal cutoff values of SUVmax in relation to tumor subtype. In patients with epithelioid subtype (n = 34), the optimal cutoff value of SUVmax for predicting death was 8.5 (AUC = 0.611). In patients with non-epithelioid subtype (n = 20) including sarcomatoid/biphasic subtype and mesothelioma, NOS, the optimal cutoff value of SUVmax was 10.3 (AUC = 0.453).
Table 2. Comparison of SUVmax according to clinical characteristics.
| SUVmax | P | |
|---|---|---|
| Gender | 0.424 | |
| Male (n = 41) | 9.7 (3.5–13.5) | |
| Female (n = 13) | 10.1 (7.0–13.5) | |
| Histologic subtype | 0.003 | |
| Epithelioid (n = 34) | 5.5 (3.2–10.8) | |
| Sarcomatoid/biphasic (n = 13) | 11.7 (9.9–14.7) | |
| NOS (n = 7) | 13.3 (9.5–15.8) | |
| Stage | 0.031 | |
| Stage I–II (n = 20) | 5.5 (3.4–10.8) | |
| Stage III–IV (n = 34) | 10.4 (7.3–13.7) | |
| Surgery | 0.037 | |
| Yes (n = 19) | 5.1 (3.0–10.4) | |
| No (n = 35) | 10.3 (5.8–13.7) | |
| Chemotherapy | 0.565 | |
| Pemetrexed/platinum (n = 36) | 9.1 (4.3–13.5) | |
| None (n = 18) | 10.3 (4.2–13.3) |
Data are presented as median (interquartile range).
NOS, not otherwise specified; SUVmax, maximum standardized uptake value
Univariate survival analysis in relation to clinical characteristics
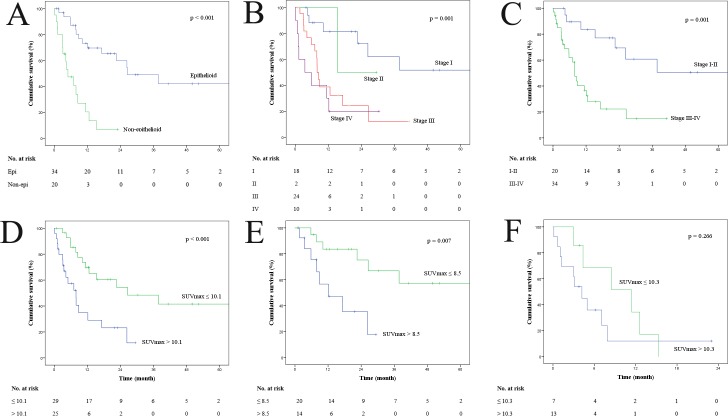
Univariate analysis of OS included age, gender, smoking history, exposure to asbestos, tumor location, histologic subtype, stage, SUVmax, EPP, and chemotherapy (Table 3). Among all patients, histologic subtype (P < 0.001) (Fig 2A), stage (P = 0.001) (Fig 2B and 2C), SUVmax (P < 0.001) (Fig 2D), and chemotherapy (P = 0.031) were significantly associated with OS. In patients with epithelioid subtype, stage (P = 0.013) and SUVmax (P = 0.007) (Fig 2E) were associated with OS. However, in patients with non-epithelioid subtype, chemotherapy was associated with OS (P = 0.005) but SUVmax was not associated with OS (P = 0.266) (Fig 2F).
Table 3. Univariate analysis for overall survival.
| Total (n = 54) | Epithelioid (n = 34) | Non-epithelioid (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median Survival (months) |
1-year Survival (%) |
Log-rank P | Median Survival (months) |
1-year Survival (%) |
Log-rank P |
Median Survival (months) |
1-year Survival (%) |
Log-rank P |
|
| Age | 0.269 | 0.367 | 0.307 | ||||||
| >64 | 11.4 | 30.8 | 22.5 | 41.2 | 4.2 | 11.1 | |||
| ≤64 | 17.2 | 53.6 | NR | 76.5 | 7.1 | 18.2 | |||
| Gender | 0.558 | 0.923 | 0.750 | ||||||
| Male | 15.3 | 41.5 | 37.8 | 55.6 | 5.0 | 14.3 | |||
| Female | 12.2 | 46.2 | 26.6 | 71.4 | 3.0 | 16.7 | |||
| Smoking history | 0.480 | 0.568 | 0.870 | ||||||
| Nonsmoker | 12.6 | 40.0 | 37.8 | 66.7 | 11.4 | 11.1 | |||
| Smoker | 8.9 | 45.8 | 26.3 | 52.6 | 5.0 | 18.2 | |||
| Asbestos exposure | 0.124 | 0.272 | 0.007 | ||||||
| Yes | 15.3 | 60.0 | NR | 60.0 | 12.6 | 60.0 | |||
| No/unknown | 12.2 | 35.9 | 26.3 | 58.3 | 4.2 | 0.0 | |||
| Location of tumor | 0.792 | 0.625 | 0.298 | ||||||
| Right | 12.6 | 45.2 | 26.6 | 63.2 | 2.9 | 16.7 | |||
| Left | 15.3 | 39.1 | NR | 53.3 | 1.4 | 12.5 | |||
| Bilateral pleural plaque | 0.744 | 0.479 | 0.746 | ||||||
| Yes | 12.6 | 60.0 | NR | 80.0 | 7.1 | 40.0 | |||
| No | 17.2 | 38.6 | 26.6 | 55.2 | 5.0 | 6.7 | |||
| Histologic subtype | <0.001 | ||||||||
| Epithelioid | 26.6 | 58.8 | |||||||
| Non-epithelioid | 5.0 | 15.0 | |||||||
| Stage | 0.001 | 0.013 | 0.028 | ||||||
| I–II | NR | 70.0 | NR | 85.7 | 15.3 | 33.3 | |||
| III–IV | 7.9 | 26.5 | 17.2 | 40.0 | 3.1 | 7.1 | |||
| SUVmax* | 0.002 | 0.007 | 0.266 | ||||||
| > cutoff | 7.9 | 24.0 | 12.2 | 42.9 | 4.2 | 7.7 | |||
| ≤ cutoff | 26.6 | 58.6 | NR | 70.0 | 11.4 | 28.6 | |||
| EPP | 0.816 | 0.437 | 0.646 | ||||||
| Yes | 8.5 | 40.0 | NR | 66.7 | 4.4 | 0.0 | |||
| No | 12.6 | 43.2 | 26.3 | 57.1 | 5.0 | 18.8 | |||
| Chemotherapy | 0.031 | 0.931 | 0.005 | ||||||
| Pemetrexed/platinum | 22.5 | 47.2 | 26.6 | 60.0 | 8.5 | 18.2 | |||
| None | 4.4 | 33.3 | 37.8 | 55.6 | 3.0 | 11.1 | |||
*SUVmax cutoff; Total = 10.1, Epithelioid = 8.5, Non-epithelioid = 10.3
NR, not reached; SUVmax, maximum standardized uptake value; EPP, extra-pleural pneumonectomy
Fig 2.
(A) Kaplan–Meier overall survival (OS) curve of all patients according to histologic subtype. (B, C) Kaplan–Meier OS curve of all patients according to stage. (D) Kaplan–Meier OS curve of all patients according to cutoff value of SUVmax. (E) Kaplan–Meier OS curve of patients with epithelioid subtype according to cutoff value of SUVmax. (F) Kaplan–Meier OS curve of patients with non-epithelioid subtype according to cutoff value of SUVmax.
Multivariate survival analysis
SUVmax, subtype, stage, and chemotherapy were included in multivariate analysis (Table 4). SUVmax (P = 0.003), histologic subtype (P = 0.003), stage (P = 0.001), and chemotherapy (P = 0.015) remained significant in all patients. Furthermore, SUVmax (P = 0.012) and stage (P = 0.014) remained significant in patients with epithelioid subtype. In patients with non-epithelioid subtype, chemotherapy (P = 0.044) showed significance in multivariate analysis.
Table 4. Multivariate analysis for overall survival.
| Total (n = 54) | Epithelioid (n = 34) | Non-epithelioid (n = 20) | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| SUVmax* (> cutoff vs. ≤ cutoff#) | 3.77 (1.58–9.01) | 0.003 | 5.65 (1.45–21.98) | 0.012 | 2.83 (0.79–10.1) | 0.111 |
| Histologic subtype (Epithelioid vs. non-epithelioid#) | 0.25 (0.10–0.64) | 0.003 | ||||
| Stage (I–II vs. III–IV#) | 0.20 (0.08–0.52) | 0.001 | 0.15 (0.03–0.68) | 0.014 | 0.31 (0.06–1.61) | 0.163 |
| Chemotherapy (Pemetrexed/platinum vs. None#) | 0.34 (0.14–0.81) | 0.015 | 0.29 (0.06–1.47) | 0.134 | 0.28 (0.08–0.97) | 0.044 |
*SUVmax cutoff; Total = 10.1, Epithelioid = 8.5, Non-epithelioid = 10.3
#Reference
Discussion
In the present study, we confirmed that SUVmax in PET/CT was an independent prognostic factor for OS in multivariate analysis. Furthermore, subgroup analysis revealed that the SUVmax was a prognostic factor in patients with epithelioid subtype, but not in those with non-epithelioid subtype. Previous studies suggested a relationship between SUVmax and OS in MPM patients [12–19]. However, this was the first study to suggest that the prognostic role of SUVmax could be limited to the epithelioid subtype only. Histologic subtype, stage, and platinum-based chemotherapy were prognostic factors in the univariate analysis in this study, which were consistent with previous studies [5, 10, 11], and were evaluated in the multivariate analysis.
Previous studies compared SUVmax between MPM patients with epithelioid and non-epithelioid subtypes. Kadota et al. showed that pleomorphic subtype of epithelioid histology showed higher SUVmax than epithelioid non-pleomorphic subtype and was similar to non-epithelioid histology [14]. However, two studies reported no statistically significant differences in SUVmax between epithelioid and non-epithelioid subtypes in patients with MPM [16, 19]. And these studies have a limited number of patients with sarcomatoid subtype. In the present study, OS and SUVmax were significantly different between MPM patients with epithelioid subtype and those with non-epithelioid subtype. Furthermore, SUVmax was significantly higher in stage III–IV than in stage I–II.
In the present study, the cutoff value of SUVmax for death was 10.1 for all patients. However, the cutoff value of SUVmax was lower in patients with epithelioid subtype than in those with non-epithelioid subtype. In patients with non-epithelioid subtype, SUVmax was not associated with prognosis. Therefore, the cutoff value of SUVmax should be carefully interpreted with respect to tumor subtype. In previous studies, the cutoff values for SUVmax to discriminate prognosis varied from 6 to 10 [12, 14, 16–18]. To serve as a prognostic factor in the clinical setting, a standardized method to determine the optimal cutoff value of SUVmax should be established.
There have been few biological explanations with respect to the relationship between SUVmax and survival in MPM patients. A previous study suggested that 18F-FDG uptake in MPM is influenced by glucose metabolism, phosphorylation of glucose, hypoxia, angiogenesis, cell proliferation (Ki-67), cell cycle regulators, and the mTOR pathway [22]. In addition, a positive correlation between mitotic count and SUVmax was reported in another study [14]. Further studies are needed to provide a biological explanation for the impact of SUVmax as a prognostic factor in MPM.
There are several staging systems available to demonstrate the prognostic significance of tumor stage on the survival of MPM patients. The eighth edition of the UICC/AJCC staging system for MPM has recently been developed [20]. Previous studies have reported that advanced AJCC clinical stage is associated with poor prognosis in MPM [23, 24], and the present study showed similar results. In addition, subgroup analysis showed that advanced stage was associated with poor prognosis in epithelioid subtype, but not in non-epithelioid subtype. The underlying cause of these results is unclear, but the non-epithelioid type may be associated with poor prognosis; moreover, the survival period is very short, even in early stages. Therefore, it is necessary to consider the histologic subtype when using clinical stage to predict prognosis in MPM patients; this should be confirmed by a prospective study in the future. Chemotherapy based on pemetrexed/platinum has been shown to improve survival in MPM patients [5, 24, 25]. In the present study, pemetrexed/platinum was administered to all patients receiving chemotherapy, and the survival rate was significantly improved, as in previous studies.
The present study had several limitations. First, relatively small sample size and limited number of events may invalidate the stability of the multivariable regression model in this study. The generalization of our results might potentially be limited by its retrospective nature and single-institution population. We also performed propensity score adjustment for histology subtype, stage and chemotherapy to validate the prognostic significance of SUVmax instead of the multivariable regression analysis. The hazard ratios (95% confidence interval) for total, epithelioid, and non-epithelioid histology were 1.83 (0.86–3.87; P = 0.114), 2.53 (0.83–7.67; P = 0.101), and 1.84 (0.59–5.75; P = 0.295), respectively. The hazard ratios of SUVmax after propensity score adjustment showed similar trends with the multivariable regression model but all the results from the propensity score adjustment were not statistically significant. Therefore, the results of current study from the multivariable model should be interpreted conservatively. Although there was no association between SUVmax and overall survival in non-epithelioid histology, a further prospective study using the multivariable model or propensity score adjustment is needed for the larger population in the future to elucidate the association between SUVmax and prognosis in epithelioid and non-epithelioid histology. Second, the histologic subtypes of the study subjects were not specifically defined in seven subjects who also underwent surgery. Finally, there were insufficient data on exposure to asbestos in 19 patients (35.2%).
In conclusion, the SUVmax on PET/CT is an independent prognostic factor in patients with MPM, especially in those with epithelioid subtype. The histologic subtype of MPM should be considered in evaluating the prognostic significance of SUVmax.
Data Availability
All relevant data are within the paper.
Funding Statement
S-W.U. was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (HI14C3418). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Krug LM, Pass HI, Rusch VW, Kindler HL, Sugarbaker DJ, Rosenzweig KE, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. Journal of Clinical Oncology. 2009;27(18):3007 10.1200/JCO.2008.20.3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thieke C, Nicolay NH, Sterzing F, Hoffmann H, Roeder F, Safi S, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiation Oncology. 2015;10(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. The lancet oncology. 2011;12(8):763–72. 10.1016/S1470-2045(11)70149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf AS, Flores RM. Current treatment of mesothelioma: extrapleural pneumonectomy versus pleurectomy/decortication. Thoracic surgery clinics. 2016;26(3):359–75. 10.1016/j.thorsurg.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. Journal of clinical oncology. 2003;21(14):2636–44. 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 6.Network NCC. NCCN malignant pleural mesothelioma guidelines, version 1.2020 Nov 27, 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf.
- 7.Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. The Lancet Oncology. 2019;20(2):239–53. 10.1016/S1470-2045(18)30765-4 [DOI] [PubMed] [Google Scholar]
- 8.Disselhorst MJ, Quispel-Janssen J, Lalezari F, Monkhorst K, de Vries JF, van der Noort V, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. The Lancet Respiratory Medicine. 2019;7(3):260–70. 10.1016/S2213-2600(18)30420-X [DOI] [PubMed] [Google Scholar]
- 9.Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. The Lancet Oncology. 2017;18(5):623–30. 10.1016/S1470-2045(17)30169-9 [DOI] [PubMed] [Google Scholar]
- 10.Herndon JE II, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113(3):723–31. 10.1378/chest.113.3.723 [DOI] [PubMed] [Google Scholar]
- 11.Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. Journal of Clinical Oncology. 1998;16(1):145–52. 10.1200/JCO.1998.16.1.145 [DOI] [PubMed] [Google Scholar]
- 12.Flores RM, Akhurst T, Gonen M, Zakowski M, Dycoco J, Larson SM, et al. Positron emission tomography predicts survival in malignant pleural mesothelioma. The Journal of thoracic and cardiovascular surgery. 2006;132(4):763–8. 10.1016/j.jtcvs.2006.03.068 [DOI] [PubMed] [Google Scholar]
- 13.Ceresoli GL, Chiti A, Zucali PA, Rodari M, Lutman RF, Salamina S, et al. Early response evaluation in malignant pleural mesothelioma by positron emission tomography with [18F] fluorodeoxyglucose. Journal of Clinical Oncology. 2006;24(28):4587–93. 10.1200/JCO.2006.06.8999 [DOI] [PubMed] [Google Scholar]
- 14.Kadota K, Kachala SS, Nitadori J-i, Suzuki K, Dunphy MP, Sima CS, et al. High SUVmax on FDG-PET indicates pleomorphic subtype in epithelioid malignant pleural mesothelioma: supportive evidence to reclassify pleomorphic as nonepithelioid histology. Journal of Thoracic Oncology. 2012;7(7):1192–7. 10.1097/JTO.0b013e3182519d96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutani Y, Takuwa T, Miyata Y, Fukuoka K, Hasegawa S, Nakano T, et al. Prognostic significance of metabolic response by positron emission tomography after neoadjuvant chemotherapy for resectable malignant pleural mesothelioma. Annals of oncology. 2012;24(4):1005–10. 10.1093/annonc/mds537 [DOI] [PubMed] [Google Scholar]
- 16.Klabatsa A, Chicklore S, Barrington SF, Goh V, Lang-Lazdunski L, Cook GJ. The association of 18 F-FDG PET/CT parameters with survival in malignant pleural mesothelioma. European journal of nuclear medicine and molecular imaging. 2014;41(2):276–82. 10.1007/s00259-013-2561-1 [DOI] [PubMed] [Google Scholar]
- 17.Lopci E, Zucali PA, Ceresoli GL, Perrino M, Giordano L, Gianoncelli L, et al. Quantitative analyses at baseline and interim PET evaluation for response assessment and outcome definition in patients with malignant pleural mesothelioma. European journal of nuclear medicine and molecular imaging. 2015;42(5):667–75. 10.1007/s00259-014-2960-y [DOI] [PubMed] [Google Scholar]
- 18.Billé A, Krug LM, Woo KM, Rusch VW, Zauderer MG. Contemporary analysis of prognostic factors in patients with unresectable malignant pleural mesothelioma. Journal of Thoracic Oncology. 2016;11(2):249–55. 10.1016/j.jtho.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Ghanem M, Herbertson R, Berlangieri SU, Byrne AJ, Tabone K, et al. Prognostic value of 18 F-FDG PET/CT in patients with malignant pleural mesothelioma. Molecular Imaging and Biology. 2009;11(6):473 10.1007/s11307-009-0203-6 [DOI] [PubMed] [Google Scholar]
- 20.Nowak AK, Chansky K, Rice DC, Pass HI, Kindler HL, Shemanski L, et al. The IASLC Mesothelioma Staging Project: proposals for revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for pleural mesothelioma. Journal of Thoracic Oncology. 2016;11(12):2089–99. 10.1016/j.jtho.2016.08.147 [DOI] [PubMed] [Google Scholar]
- 21.Lim C, Seok H, Hyun S, Moon S, Cho Y, Lee K-H, et al. Evaluation of a diagnostic 18F-FDG PET/CT strategy for differentiating benign from malignant retroperitoneal soft-tissue masses. Clinical radiology. 2019;74(3):207–15. 10.1016/j.crad.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 22.Kaira K, Serizawa M, Koh Y, Takahashi T, Hanaoka H, Oriuchi N, et al. Relationship between 18F-FDG uptake on positron emission tomography and molecular biology in malignant pleural mesothelioma. European Journal of Cancer. 2012;48(8):1244–54. 10.1016/j.ejca.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 23.Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. Journal of Thoracic Oncology. 2007;2(10):957–65. 10.1097/JTO.0b013e31815608d9 [DOI] [PubMed] [Google Scholar]
- 24.Kao SC, Vardy J, Chatfield M, Corte P, Pavlakis N, Clarke C, et al. Validation of prognostic factors in malignant pleural mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales Dust Diseases Board. Clinical lung cancer. 2013;14(1):70–7. 10.1016/j.cllc.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 25.Ceresoli G, Grosso F, Zucali P, Mencoboni M, Pasello G, Ripa C, et al. Prognostic factors in elderly patients with malignant pleural mesothelioma: results of a multicenter survey. British journal of cancer. 2014;111(2):220 10.1038/bjc.2014.312 [DOI] [PMC free article] [PubMed] [Google Scholar]