Abstract
Background
Lymphomatoid papulosis (LyP) can be associated with other haematological malignancies (HM), but reported percentages vary from 20% to over 50%.
Objective
To evaluate the frequency and prognostic significance of associated HM and non‐HM in LyP patients.
Methods
In this multicentre cohort study, the complete Dutch LyP population was included from the Dutch Cutaneous Lymphoma Registry between 1985 and 2018. Clinical and histopathological information was retrieved from every individual patient.
Results
After a median follow‐up of 120 months (range, 6–585), an associated HM was observed in 78/504 (15.5%) patients. Most common associated HM were mycosis fungoides (MF; n = 31) and anaplastic large‐cell lymphoma (ALCL; n = 29), while 19 patients had another HM of B‐cell (n = 14) or myeloid origin (n = 5). Even after a 25‐year follow‐up period, percentages of associated HM did not exceed 20%. Thirty‐nine of 465 patients (8.4%) without a prior or concurrent associated HM developed an associated HM during follow‐up, after a median of 68 months (range of 3–286 months). Nine of 78 patients died of associated HM, including 6/22 patients developing extracutaneous ALCL, while all patients with associated MF or skin‐limited ALCL had an excellent prognosis. Compared with the general population, LyP patients showed an increased risk (relative risk, 2.8; 95% confidence intervals, 2.4–3.3) for non‐HM, in particular cutaneous squamous cell carcinoma, melanoma and intestinal/lung/bladder cancer.
Conclusions
An associated HM was reported in 15.5% of the LyP patients, particularly MF and ALCL. Although the frequency of associated HM is lower than suggested and the prognosis of most patients with associated HM is excellent, a small subgroup will develop aggressive disease, in particular extracutaneous ALCL. Furthermore, LyP patients have a higher risk of developing other malignancies. Clinicians should be aware of these risks, and LyP patients require close monitoring.
Short abstract
Linked article: F. Rongioletti. J Eur Acad Dermatol Venereol 2020; 34: 216–217. https://doi.org/10.1111/jdv.16157.
Introduction
Lymphomatoid papulosis (LyP) is a chronic, recurrent skin disease, clinically characterized by the presence of self‐healing papular, papulonecrotic and/or nodular skin lesions with histologic features of a CD30+ cutaneous T‐cell lymphoma (CTCL). LyP has overlapping clinical and histopathological features with primary cutaneous anaplastic large‐cell lymphoma (C‐ALCL), and both conditions form a spectrum of primary cutaneous CD30+ lymphoproliferative diseases.1, 2, 3, 4 LyP has an excellent prognosis with a 5‐year disease‐specific survival near 100%.1, 2, 4 However, in a subset of patients, LyP may be associated with another type of haematological malignancy (HM), most commonly mycosis fungoides (MF), ALCL or Hodgkin lymphoma, and these patients may have an unfavourable prognosis.5, 6 The incidence of these LyP‐associated HM reported in the literature shows wide variation, while most studies described associated HM in approximately 15–20% of LyP patients, and more recent studies report percentages up to 50%.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 These differences may be due to referral bias, differences in duration of follow‐up or differences in criteria used for the diagnosis of an associated HM. However, more precise information about the risk to develop an associated HM or non‐HM is important, since it may have consequences for the management and treatment of patients with LyP.18 In the present study, we therefore evaluated the frequency and prognostic significance of associated HM as well as non‐HM in the largest, most comprehensive and unbiased cohort to date of 504 LyP patients with long‐term follow‐up included in the Dutch registry of cutaneous lymphomas.
Methods
Patient selection
Between October 1985 and February 2018, 515 patients with LyP were included in the Dutch Registry of Cutaneous Lymphomas (DRCL). In all cases, the diagnosis was based on the clinicopathologic criteria of the WHO‐EORTC classification and confirmed by an expert panel of dermatologists and pathologists of the Dutch Cutaneous Lymphoma Group.4 Yearly collected follow‐up data were retrieved from the DRCL. Missing information was collected from medical records and referring clinicians.
In addition, the presence of associated HM and non‐HM was investigated by retrieving the results of all histologic examinations of every patient using the nationwide network and registry of histo‐ and cytopathology in the Netherlands (PALGA Foundation).19 Living patients with a follow‐up duration <12 months and/or incomplete follow‐up data were excluded (n = 11). The final study group consisted of 504 LyP patients, including 118 patients evaluated in 2000 by Bekkenk et al.5 This multicentre study was evaluated by the institutional review board and provided with a waiver of consent.
Definition of associated haematological malignancies
In all cases, the diagnosis of an associated HM was confirmed by review of the histologic sections. Many LyP patients have eczematous skin lesions with variable clinical resemblance to early‐stage MF, but do not meet the histopathologic criteria of MF. Therefore, a diagnosis of associated MF was only made if confirmed histologically using well‐established criteria.4, 20
Patients with LyP may be preceded by or develop lesions with clinicopathologic features consistent with an ALCL in skin and/or extracutaneous sites. It is, however, arbitrary if and when tumours developing in patients with LyP should be considered as C‐ALCL or still as LyP. In the present study, the occurrence of tumours was recorded in every patient. Diagnosis of C‐ALCL was only made in case of a skin lesion that did not disappear spontaneously and was treated with radiotherapy or excision. Tumours with a diameter of 1–4 cm coexisting with papular lesions and disappearing spontaneously, recorded in three patients, were not considered to represent progression to ALCL. Each associated lymphoma was counted only once; relapsed lymphoma or disease progression was not counted as a new malignancy.
Statistical analysis
All statistical analyses were performed using SPSS version 23 statistical software (IBM Corp, Armonk, NY). All analyses of time to lymphoma and incidence rates were based on patients free of associated malignancies at time to diagnosis. The Kaplan–Meier technique was used to compute time to associated malignancy, measured from diagnosis of LyP. The incidence rates, relative risk (RR) and 95% confidence intervals (CIs) of associated malignancies in LyP cases in our cohort were calculated and were compared with 1‐year incidence rates, corrected for age and gender, of (non‐)HM in persons from the Dutch cancer registration (IKNL/NKR), which includes the complete Dutch population and is based on the guidelines of the World Health Organization.21 A P‐value of <0.05 was considered significant.
Results
The final study group included 286 males and 218 females (male‐to‐female ratio: 1.3 : 1). Median age at time of diagnosis was 47 years (range, 2–92 years). The study group contained 52 patients younger than 20 years of age at the time of diagnosis (median, 11 years; range: 2–19 years). After a median follow‐up of 120 months (range, 6–585 months), an associated HM was found in 78 of 504 patients (15.5%; Table 1). Thirty‐nine patients developed an associated HM before (n = 25) or concurrent with (n = 14) diagnosis of LyP and 39 patients 3–286 months (median 68 months) after the diagnosis of LyP. One patient developed two different associated lymphomas (MF and C‐ALCL).
Table 1.
Associated haematological malignancies in lymphomatoid papulosis (LyP)
| Before | Concurrent | After | Total | D+‡ | |
|---|---|---|---|---|---|
| Mycosis fungoides | 7 | 13 | 11 | 31 | 0 |
| Anaplastic large‐cell lymphoma | 10 | 0 | 19 | 29 | 6 |
| Skin | 3 | 0 | 5 | 8 | 1 |
| Extracutaneous ± skin | 7 | 0 | 14 | 21 | 5 |
| Other† | 8 | 1 | 10 | 19 | 3 |
†Includes Hodgkin lymphoma, B‐cell lymphoma/leukaemia and myeloid leukaemia. ‡Died of (associated) lymphoma or leukaemia.
Three groups of associated HM were distinguished: patients with MF (n = 31), patients with ALCL in skin and/or extracutaneous sites (n = 29) and a miscellaneous group of malignancies of B‐cell and myeloid origin (n = 19).
Since previous studies suggested that increased percentages of associated HM are due to prolonged follow‐up periods, we performed a subgroup analysis of patients included in the database before 2005. After a median of 217 months, 42/227 (18.5%) of the patients developed an associated HM, which is not different from the percentage observed in the whole study group. This is further supported by a Kaplan–Meier curve illustrating the development of an associated HM plotted against time, which seems to stabilize around 20% (Fig. 1).
Figure 1.
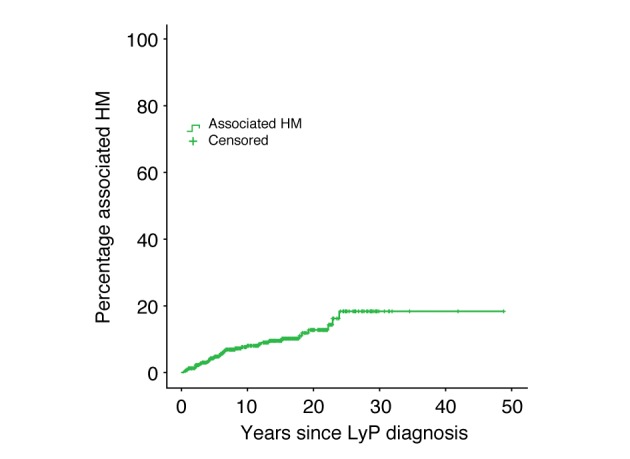
Time to associated haematological malignancy, measured from diagnosis of lymphomatoid papulosis.
Compared with the general population, LyP patients without prior or concurrent associated HM at time of diagnosis have a significantly increased risk of developing an associated HM (RR, 11.9; 95% CI, 8.3–15.5). When evaluating the risk of developing MF or C‐ALCL apart, the RR was increased dramatically (RR, 460.7; 95% CI, 298.5–622.8). An increased, but not significant, risk was found when evaluating the risk of developing a B‐cell lymphoma separately (RR, 2.95; 95% CI, 0.6–5.3).
LyP associated with mycosis fungoides
This subgroup of 31 patients contained 21 males and 10 females. The median age at diagnosis of LyP was 56 years (range, 30–87 years) and 54 years (range, 32–88 years) for MF. MF was diagnosed in seven patients before, in 13 patients concurrent with, and in 11 patients after the diagnosis of LyP (Table 2). Twenty‐two patients had MF stage IA and nine patients MF stage IB. None of the 31 patients with the combination of LyP and MF progressed to tumour stage MF or developed extracutaneous localizations. Treatment of MF was expectative (n = 18), or with PUVA/UVB (n = 12), and only one patient received radiotherapy for extensive plaques. After a median follow‐up period of 67 months after the diagnosis of MF (range, 10–409 months), six patients were alive without MF, 17 patients were alive with MF, and 8 patients died of unrelated disease.
Table 2.
Individual patient characteristics of LyP patients with an associated MF
| No | Sex | Age | Time interval (months)† | Stage‡ | Treatment MF | Current status (follow‐up in months) | |
|---|---|---|---|---|---|---|---|
| MF | LyP | ||||||
| 1. | M | 58 | A (52) | IA | PUVA | Do (232) | Do (284) |
| 2. | F | 71 | B (3) | IB | PUVA | A+ (326) | A+ (323) |
| 3. | F | 56 | B (72) | IB | PUVA | Do (409) | Do (337) |
| 4. | F | 59 | A (274) | IA | Expectative | Do (57) | Do (331) |
| 5. | M | 44 | C | IA | Expectative | Do (177) | Do (177) |
| 6. | F | 87 | C | IB | PUVA | Do (23) | Do (23) |
| 7. | M | 80 | A (97) | IB | UVB | Do (13) | Do (110) |
| 8. | M | 58 | B (148) | IB | RT | Ao (225) | A+ (77) |
| 9. | M | 64 | B (91) | IB | UVB | Do (163) | Do (72) |
| 10. | M | 66 | A (24) | IA | UVB | Ao (178) | Ao (202) |
| 11. | F | 55 | B (208) | IA | Expectative | Ao (236) | A+ (28) |
| 12. | M | 51 | C | IA | PUVA | A+ (42) | A+ (42) |
| 13. | M | 70 | B (143) | IB | PUVA | A+ (261) | A+ (118) |
| 14. | M | 56 | A (144) | IB | UVB | A+ (92) | A+ (236) |
| 15. | M | 62 | A (50) | IA | Expectative | A+ (107) | Ao (157) |
| 16. | F | 64 | C | IA | Expectative | Do (87) | Do (87) |
| 17. | M | 42 | A (108) | IA | Expectative | Ao (15) | A+ (123) |
| 18. | M | 37 | C | IA | Expectative | A+ (124) | A+ (124) |
| 19. | M | 43 | C | IA | UVB | A+ (129) | Ao (129) |
| 20. | M | 54 | A (71) | IA | Expectative | A+ (31) | A+ (102) |
| 21. | M | 51 | A (36) | IA | Expectative | A+ (49) | A+ (85) |
| 22. | M | 69 | C | IA | Expectative | A+ (76) | A+ (76) |
| 23. | M | 48 | B (18) | IA | Expectative | Ao (67) | Ao (49) |
| 24. | F | 30 | A (34) | IA | Expectative | A+ (24) | A+ (58) |
| 25. | M | 35 | A (48) | IA | Expectative | A+ (10) | A+ (58) |
| 26. | F | 49 | C | IB | UVB | A+ (41) | A+ (41) |
| 27. | M | 50 | C | IA | Expectative | A+ (41) | Ao (41) |
| 28. | M | 36 | C | IA | Expectative | A+ (24) | A+ (24) |
| 29. | F | 77 | C | IA | Expectative | A+ (12) | A+ (12) |
| 30. | M | 65 | C | IA | Expectative | Ao (16) | A+ (16) |
| 31. | M | 53 | C | IA | Expectative | A+ (14) | A+ (14) |
†Associated lymphoma before (B) concomitant with (C) or after (A) diagnosis of lymphomatoid papulosis. ‡IA, patches/plaques <10% body surface. IB, patches/plaques >10% body surface.
A+, alive with disease; Ao, alive no evidence of disease; Do, died of unrelated disease; LyP, lymphomatoid papulosis; MF, mycosis fungoides; RT, radiotherapy.
LyP with associated (cutaneous) ALCL
This subgroup contained 29 patients including 19 males and 10 females (male‐to‐female ratio 1.9 : 1). The median age at diagnosis of LyP was 47 years (range, 20–77 years) and 49 years (range, 30–83 years) for ALCL. Diagnosis of ALCL was made in 10 patients before and in 19 patients after LyP (Table 3). Eight of 29 patients (nos. 1–8) presented with only a persistent skin tumour before (n = 3) or after (n = 5) the diagnosis of LyP had been made. Treatment with local radiotherapy in six cases, excision in one case and CHOP courses in one case resulted in complete remission in all of them. After a median follow‐up of 175 months (range, 82–365 months), only one of these patients developed systemic disease and died of ALCL (no. 8).
Table 3.
Individual patient characteristics of LyP patients with an associated CD30+ anaplastic large‐cell lymphoma
| Sex/Age (y) | Time interval (months)† | Localization | Treatment (response) | Current status (follow‐up in months) | |||
|---|---|---|---|---|---|---|---|
| Skin | EC | Lymphoma | LyP | ||||
| 1. | F/71 | A (48) | + | − | RT (CR) | Ao (275) | A+ (323) |
| 2. | M/57 | B (16) | + | − | CHOP (CR) | Do (365) | Do (349) |
| 3. | F/45 | A (3) | + | − | RT (CR) | Do (277) | Do (280) |
| 4. | M/20 | A (182) | + | − | RT (CR) | Ao (147) | A+ (329) |
| 5. | M/52 | A (118) | + | − | RT (CR) | Ao (114) | A+ (232) |
| 6. | M/62 | A (75) | + | − | RT (CR) | Do (82) | Do (157) |
| 7. | M/31 | B (7) | + | − | RT (CR) | Ao (203) | Ao (196) |
| 8. | M/59 | B (47) | + | − | Excision (CR) | D+ (94) | D+ (47) |
| A (25) | − | + | CHOP (PR) | ||||
| 9. | M/27 | A (51) | + | + | CHOP (CR) | Do (348) | Do (399) |
| 10. | F/33 | B (33) | + | +‡ | CHOP (CR) | Ao (309) | Ao (276) |
| 11. | M/31 | A (68) | + | +‡ | CHOP (CR) | Ao (255) | A+ (323) |
| 12. | F/55 | B (136) | + | + | CHOP (CR) | Ao (241) | A+ (105) |
| 13. | M/71 | A (47) | + | + | Died before treatment | D+ (4) | D+ (51) |
| 14. | M/55 | B (5) | + | +‡ | CHOP (CR) | Ao (136) | A+ (131) |
| 15. | M/42 | A (70) | + | + | CHOP (PR) | Ao (64) | Ao (134) |
| A (81) | − | + | BV + alloSCT (CR) | ||||
| 16. | F/42 | B (24) | + | +‡ | RT + CHOP (CR) | Ao (114) | A+ (90) |
| 17. | F/52 | A (60) | + | +‡ | Spontaneous regression | Ao (300) | Ao (360) |
| 18. | F/45 | B (24) | − | + | RT (CR) | Do (113) | Do (89) |
| 19. | M/43 | A (78) | − | + | CHOP (CR) | Ao (177) | Ao (255) |
| 20. | M/37 | A (230) | − | + | CHOP (CR) | Ao (71) | A+ (301) |
| 21. | M/49 | B (157, 49) | − | + | RT (CR), MOPP (CR) | Ao (395) | A+ (238) |
| 22. | M/46 | A (140) | − | + | CHOP (NR) | D+ (7) | D+ (147) |
| 23. | F/47 | A (10) | − | + | Refused treatment | D+ (4) | D+ (14) |
| 24. | M/43 | A (25) | − | + | Alemtuzumab + CHOP + alloSCT (CR) | Ao (122) | Ao (147) |
| 25. | M/77 | A (78) | − | + | CHOP (PD) | D+ (6) | D+ (84) |
| 26. | M/43 | A (4) | − | + | CHOP (NR) | D+ (7) | D+ (11) |
| 27. | M/65 | A (12) | − | + | CHOP + RT (CR) | Ao (94) | Ao (106) |
| 28. | F/64 | B (16) | − | + | CHOP (CR) | Ao (34) | A+ (18) |
| 29. | F/71 | A (25) | − | + | RT (CR) | Ao (16) | Ao (41) |
†Associated lymphoma before (B) or after (A) the diagnosis of lymphomatoid papulosis. ‡Patients with a solitary tumour with extracutaneous disease restricted to one regional draining lymph node.
A+, alive with disease; AlloSCT, allogeneic stem cell transplantation; Ao, alive no evidence of disease; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CR, complete remission (100%); D+, died of lymphoma; Do, died of unrelated disease; EC, extracutaneous localizations of CD30+ lymphoproliferative disease (CTCL); LyP, lymphomatoid papulosis; NR, no remission (0–50%); PD, progressive disease; PR, partial remission (>50%); RT, radiotherapy.
Altogether, 22 patients developed ALCL at extracutaneous sites with or without persistent skin lesions. Systemic localizations preceded LyP in seven cases and developed after the diagnosis of LyP in the other 15 patients. Notably, none of the seven LyP patients with preceding systemic localizations died of lymphoma. Sixteen patients were treated with CHOP or CHOP‐like courses, in two of them followed by an allogeneic stem cell transplantation, two patients were treated with local radiotherapy, while two patients received no treatment and in one patient both skin and nodal lesions disappeared spontaneously. Six patients, who developed ALCL 4–140 months (median 36 months) after the diagnosis of LyP had been made, died 4–94 months (median, 6.5 months) after the diagnosis.
Interestingly, five patients (cases 10, 11, 14, 16 and 17) who developed a single persistent tumour and extracutaneous involvement restricted to one draining lymph node had an excellent prognosis. Treatment with CHOP courses in four patients and spontaneous regression of both skin and nodal lesions in one patient resulted in sustained complete remissions with no new ALCL lesions after a follow‐up period of 9.5–25 years.
Other haematological malignancies
In total, 19 other associated HM were reported, mainly of B‐cell (n = 14) or myeloid origin (n = 5; Table 4). Four cases were initially diagnosed as Hodgkin lymphoma. However, after reviewing the histologic sections two cases were reclassified as ALCL, because of expression of T‐cell and/or cytotoxic markers, without expression of B‐cell markers (PAX‐5, CD79a, CD20). Nine patients developed a lymphoma/leukaemia before or concurrent with diagnosis of LyP, and 10 patients after. Three patients died of associated disease, one of Hodgkin lymphoma and two of myeloid leukaemias.
Table 4.
Frequencies of associated miscellaneous lymphomas/leukaemias
| Before | Concurrent | After | Total | |
|---|---|---|---|---|
| Hodgkin lymphoma | 1 | 0 | 1 | 2 |
| B‐cell lymphoma/leukaemia | 6 | 0 | 6 | 12 |
|
0 | 0 | 1 | 1 |
|
1 | 0 | 1 | 2 |
|
3 | 0 | 1 | 4 |
|
0 | 0 | 2 | 2 |
|
1 | 0 | 0 | 1 |
|
0 | 0 | 1 | 1 |
|
1 | 0 | 0 | 1 |
| Myeloid proliferation | 1 | 1 | 3 | 5 |
|
1 | 0 | 1 | 2 |
|
0 | 0 | 1 | 1 |
|
0 | 1 | 0 | 1 |
|
0 | 0 | 1 | 1 |
| Total | 8 | 1 | 10 | 19 |
Bold value indicates total number of patients within this group.
Non‐haematological malignancies and atopic dermatitis
After retrieving the results of all histologic examinations of 456 patients (PALGA Foundation), a total number of 195 non‐HM were observed in 146 (32%) patients (Table 5). Comparative statistical analysis was performed, in which basal cell carcinomas (n = 52) and Bowen's (n = 10) disease were excluded as associated non‐HM, since these are not included in the national cancer registry. Compared with the general population, LyP patients have a significantly increased risk of developing a non‐HM (RR, 2.8; 95% CI, 2.4–3.3). Subsequent risk evaluation of separate types of malignancies resulted in a significantly increased risk of developing cutaneous squamous cell carcinoma (RR, 4.3; CI, 2.5–6.1), melanoma (RR, 4.2; CI, 1.8–6.6), lung cancer (RR, 3.7; CI, 2.1–5.2), intestinal cancer (RR, 2.4; CI, 1.2–3.7) and bladder cancer (RR, 8.1; CI, 3.1–13.1; Table 5). Also, an atopic dermatitis prevalence of 83 of 288 (29%) evaluable patients was found.
Table 5.
Risk of non‐haematological malignancies in lymphomatoid papulosis (LyP) patients
| Malignancy | Number (%†) | RR | 95% CI |
|---|---|---|---|
| Cutaneous squamous cell carcinoma | 26 (6) | 4.3 | 2.5 to 6.1 |
| Pulmonary | 21 (5) | 3.7 | 2.1 to 5.2 |
| Melanoma | 15 (3) | 4.2 | 1.8 to 6.6 |
| Breast cancer | 13 (3) | 1.3 | 0.3 to 2.3 |
| Intestinal (+rectal) | 13 (3) | 2.4 | 1.2 to 3.7 |
| Prostate | 13 (3) | 1.7 | 0.7 to 2.7 |
| Bladder | 11 (2) | 8.1 | 3.1 to 13.1 |
| Kidney | 4 (1) | 0.8 | −0.8 to 2.5 |
| Ovary | 4 (1) | 10 | 0.2 to 19.7 |
| Stomach | 4 (1) | 6.9 | 0.1 to 13.7 |
| Uterus | 3 (1) | 3.6 | −0.5 to 7.7 |
| Oesophagus | 2 (<1) | 1.7 | −0.7 to 4.1 |
| Liver | 1 (<1) | 2.8 | −2.7 to 8.4 |
| Gall bladder | 1 (<1) | 3.5 | −3.3 to 10.2 |
| Thyroid | 1 (<1) | 2.7 | −2.6 to 8 |
| Cerebral | 1 (<1) | 2.3 | −2.2 to 6.8 |
†Percentage of LyP cohort.
CI, confidence interval; RR, relative risk.
Bold value indicates statistically significant values.
Discussion
In the present comprehensive study of 504 patients with LyP, an associated HM was found in 78 patients (15.5%). MF (31 cases; 6.2%) and ALCL (29 cases; 5.8%) were the most common associated HM, while there were 19 patients (3.8%) with another type of HM including Hodgkin lymphoma, different B‐cell neoplasms and myeloid leukaemias. Whereas detection of shared clonality in skin lesions of LyP and associated MF as well as in lesions of LyP and associated ALCL suggest a biologic relationship between LyP and MF/ALCL, the relationship between LyP and these other types of HM is probably coincidental.
LyP patients with an associated HM do not necessarily have a poor outcome. The prognosis of patients with the combination of LyP and MF is usually excellent, and MF‐related deaths have rarely been reported.12 In the present study, all 31 patients with associated MF had early‐stage disease and none of them progressed to tumour stage disease or developed extracutaneous disease. In the group of patients with associated ALCL, patients with skin‐limited tumours generally had a favourable prognosis. Only one of eight patients developed systemic disease and died of ALCL. LyP patients developing nodal or visceral localizations are more at risk. In the present study, six of 22 patients developing extracutaneous disease died of associated ALCL. Interestingly, five patients with a single persistent tumour and involvement of only one draining lymph node had an excellent prognosis. Altogether, 9 of 78 patients with an associated HM died of lymphoma or leukaemia, including six of ALCL, one of Hodgkin lymphoma and two of myeloid leukaemia.
Several risk factors for an associated HM have been suggested, such as age, male sex, histologic subtype of LyP and the presence of a T‐cell clone.7, 8, 9, 10, 11, 12, 22 However, the results of these studies are conflicting, and risk factors for those rare patients developing systemic and potentially fatal disease are unknown.
The result of the present study shows an associated HM in 15.5% of LyP patients, but deviates significantly from the results of recent studies, which reported percentages up to 50%.5, 7, 8, 9, 10, 12, 16 There are several explanations for the discrepancy in the frequency of associated HM in different studies, and referral bias has been suggested as the major cause.12 All dermatologists working in peripheral institutes in the Netherlands refer patients with a diagnosis of (suspected) cutaneous lymphoma, including patients with LyP, to dermatologists in university hospitals. Since 1985, all patients with a CTCL, seen in one of the eight Dutch university hospitals, are discussed periodically by a multidisciplinary panel of dermatologists and pathologists and all cases are included in the DRCL. In this way, it is highly unlikely that referral bias plays a significant part in our study.
A second explanation might be selection bias resulting from differences in the criteria used for diagnosis of these associated HM. Notably, in none of the previous studies definitions for associated HM were provided. In the present study, diagnosis of MF was only made if confirmed by histologic examination. This is important since a considerable proportion of LyP patients may show, in addition to the typical LyP lesions, skin lesions clinically suspect of early‐stage MF. The association between LyP or other cutaneous lymphomas and atopy/atopic dermatitis has been described before and was found in 83 of 288 (29%) evaluable patients in the present study.23, 24, 25 This might even be an underestimation, since the presence of atopic dermatitis was not recorded adequately in the past. It is more difficult how C‐ALCL should be defined in this setting. Diagnosis of C‐ALCL was made in case of persistent tumours, treated with radiotherapy or excision, while tumours showing complete spontaneous resolution were not considered to represent progression to ALCL.
Our results argue against the suggestion that differences in the percentages of associated HM could be explained by differences in the duration of follow‐up between studies.8 Evaluation of a subgroup of 227 LyP patients diagnosed between 1985 and 2004 with a median follow‐up of 217 months showed an associated HM in 42 patients (18.5%), which is similar to the whole cohort. Because patients are monitored yearly, and associated HM were also identified by evaluating pathology reports using the national histopathology (PALGA) database, it is unlikely that associated HM were missed.
The present study also revealed a significant higher incidence of associated non‐HM compared with the general Dutch population (RR, 2.8), in particular for cutaneous squamous cell carcinoma, malignant melanoma and a number of internal cancers. An increased risk for cutaneous squamous cell carcinoma and malignant melanoma might be explained by common external factors, such as UV exposure and frequent physical examinations of the skin, or shared (epi)genetic alterations.18, 26, 27 Thus, clinicians should be aware of this increased risk, during skin examination of an LyP patient. An explanation for the increased risk for internal non‐HM is currently lacking.
In conclusion, the results of the present study of 504 patients with LyP show an associated HM in 15.5% of patients and do not support the recently reported high percentages up to 50%. It should also be noted that the risk of a LyP patient without prior or concurrent MF or ALCL at the time of diagnosis to develop one of these biologically related CTCLs during follow‐up is only 7% and the risk to develop extracutaneous localizations of these conditions only 3%. The observation that LyP patients have also an increased risk to develop cutaneous squamous cell carcinoma, melanoma and some internal cancers has not been reported before. Clinicians should be aware of these risks, and LyP patients require close monitoring and regular follow‐up.
Conflict of interest
None.
Funding source
None.
References
- 1. Kempf W, Pfaltz K, Vermeer MH et al EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30‐positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large‐cell lymphoma. Blood 2011; 118: 4024–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki‐1)‐positive lymphoproliferative disorders. A proposal for classification and guidelines for management and treatment. J Am Acad Dermatol 1993; 28: 973–980. [DOI] [PubMed] [Google Scholar]
- 3. Macaulay WL. Lymphomatoid papulosis. A continuing self‐healing eruption, clinically benign–histologically malignant. Arch Dermatol 1968; 97: 23–30. [DOI] [PubMed] [Google Scholar]
- 4. Willemze R, Jaffe ES, Burg G et al WHO‐EORTC classification for cutaneous lymphomas. Blood 2005; 105: 3768–3785. [DOI] [PubMed] [Google Scholar]
- 5. Bekkenk MW, Geelen FA, van Voorst Vader PC et al Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long‐term follow‐up data of 219 patients and guidelines for diagnosis and treatment. Blood 2000; 95: 3653–3661. [PubMed] [Google Scholar]
- 6. Beljaards RC, Willemze R. The prognosis of patients with lymphomatoid papulosis associated with malignant lymphomas. Br J Dermatol 1992; 126: 596–602. [DOI] [PubMed] [Google Scholar]
- 7. AbuHilal M, Walsh S, Shear N. Associated hematolymphoid malignancies in patients with lymphomatoid papulosis: a Canadian retrospective study. J Cutan Med Surg 2017; 21: 507–512. [DOI] [PubMed] [Google Scholar]
- 8. Cordel N, Tressieres B, D'Incan M et al Frequency and risk factors for associated lymphomas in patients with lymphomatoid papulosis. Oncologist 2016; 21: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Souza A, el‐Azhary RA, Camilleri MJ, Wada DA, Appert DL, Gibson LE. In search of prognostic indicators for lymphomatoid papulosis: a retrospective study of 123 patients. J Am Acad Dermatol 2012; 66: 928–937. [DOI] [PubMed] [Google Scholar]
- 10. Kunishige JH, McDonald H, Alvarez G, Johnson M, Prieto V, Duvic M. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol 2009; 34: 576–581. [DOI] [PubMed] [Google Scholar]
- 11. Nikolaou V, Papadavid E, Ekonomidi A et al Association of clinicopathological characteristics with secondary neoplastic lymphoproliferative disorders in patients with lymphomatoid papulosis. Leuk Lymphoma 2015; 56: 1303–1307. [DOI] [PubMed] [Google Scholar]
- 12. Wieser I, Oh CW, Talpur R, Duvic M. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol 2016; 74: 59–67. [DOI] [PubMed] [Google Scholar]
- 13. Gan EY, Tang MB, Tan SH. Lymphomatoid papulosis: is a second lymphoma commoner among East Asians? Clin Exp Dermatol 2012; 37: 118–121. [DOI] [PubMed] [Google Scholar]
- 14. Sina B, Burnett JW. Lymphomatoid papulosis. Case reports and literature review. Arch Dermatol 1983; 119: 189–197. [DOI] [PubMed] [Google Scholar]
- 15. Wang HH, Myers T, Lach LJ, Hsieh CC, Kadin ME. Increased risk of lymphoid and nonlymphoid malignancies in patients with lymphomatoid papulosis. Cancer 1999; 86: 1240–1245. [PubMed] [Google Scholar]
- 16. El Shabrawi‐Caelen L, Kerl H, Cerroni L. Lymphomatoid papulosis: reappraisal of clinicopathologic presentation and classification into subtypes A, B, and C. Arch Dermatol 2004; 140: 441–447. [DOI] [PubMed] [Google Scholar]
- 17. Kempf W. Cutaneous CD30‐positive lymphoproliferative disorders. Surg Pathol Clin 2014; 7: 203–228. [DOI] [PubMed] [Google Scholar]
- 18. Olsen EA, Delzell E, Jegasothy BV. Second malignancies in cutaneous T cell lymphoma. J Am Acad Dermatol 1984; 10(2 Pt 1): 197–204. [DOI] [PubMed] [Google Scholar]
- 19. Casparie M, Tiebosch AT, Burger G et al Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007; 29: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pimpinelli N, Olsen EA, Santucci M et al Defining early mycosis fungoides. J Am Acad Dermatol 2005; 53: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 21. https://www.iknl.nl/home.
- 22. Gruber R, Sepp NT, Fritsch PO, Schmuth M. Prognosis of lymphomatoid papulosis. Oncologist 2006; 11: 955–957; author reply 7. [DOI] [PubMed] [Google Scholar]
- 23. Nijsten T, Curiel‐Lewandrowski C, Kadin ME. Lymphomatoid papulosis in children: a retrospective cohort study of 35 cases. Arch Dermatol 2004; 140: 306–312. [DOI] [PubMed] [Google Scholar]
- 24. Legendre L, Barnetche T, Mazereeuw‐Hautier J, Meyer N, Murrell D, Paul C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta‐analysis. J Am Acad Dermatol 2015; 72: 992–1002. [DOI] [PubMed] [Google Scholar]
- 25. Zychowska M, Wozniak Z, Maj J. Primary cutaneous CD30+ lymphoproliferative disorders in a patient with severe atopic dermatitis: is there a causative link? Acta Derm Venereol 2018; 98: 123–125. [DOI] [PubMed] [Google Scholar]
- 26. Woollard WJ, Pullabhatla V, Lorenc A et al Candidate driver genes involved in genome maintenance and DNA repair in Sezary syndrome. Blood 2016; 127: 3387–3397. [DOI] [PubMed] [Google Scholar]
- 27. DeWane ME, Kelsey A, Oliviero M, Rabinovitz H, Grant‐Kels JM. Melanoma on chronically sun damaged skin: lentigo maligna and desmoplastic melanoma. J Am Acad Dermatol 2019; 81: 823–833. [DOI] [PubMed] [Google Scholar]


