Abstract
Background:
Myocardial injury after non-cardiac surgery (MINS) is the most common peri-operative cardiovascular complication independently associated with 30-day mortality but its relationship with pre-operative glucose is unknown.
Methods:
Prospective evaluation of patients aged ≥45 years in the Vascular events In non-cardiac Surgery patients cOhort evaluatioN (VISION) Study assessed relationships between pre-operative casual or fasting glucose and post-operative MINS using logistic regression, and 30-day mortality using Cox regression in people with and without diabetes.
Findings:
Among 11,954 patients (23.5% with diabetes), 813 experienced MINS (6.8%), and 249 30-day mortality (2.1%). More patients with diabetes experienced MINS (OR 1.98, 95%CI [1.70-2.30]; p<0.0001), and death (OR 1.41, [1.08-1.86]; p=0.016). Casual glucose levels were associated with MINS in all patients (adjusted OR 1.06 per 1 mmol/L increment in glucose, [1.04-1.09]; p<0.001), and with death only in patients without diabetes (aHR 1.13 per mmol/L, [1.05-1.23]; p=0.002). There was a progressive relationship between unadjusted fasting glucose levels and both MINS (OR 1.14 per mmol/L, [1.08-1.20]; p<0.001) - which was due to the effect in the subgroup without prior diabetes (interaction-p=0.025) - and 30 day death (OR 1.10 per mmol/L, [1.02-1.19]; p=0.013). For patients without diabetes, casual glucose of more than 6.86 mmol/L and fasting glucose of more than 6.41 mmol/L predicted MINS (OR 1.71 [1.36-2.15]; p<0.0001, and OR 2.71 [1.85-3.98]; p<0.0001, respectively). For patients with diabetes, only casual glucose concentration more than 7.92 mmol/L predicted MINS (OR 1.47 [1.10-1.96]; p=0.0096).
Interpretation:
Pre-operative glucose, particularly casual glucose predicts risk for post-operative cardiovascular outcomes, especially among patients without diabetes.
Funding:
Multiple non-profit and industry (see footnote).
Introduction
Diabetes is a well-established risk factor for cardiovascular disease and cardiovascular mortality (1). Though the mechanisms remain unclear, glucose levels themselves show a continuous relationship with future cardiovascular disease in patients with or without diabetes, and in the general population, beginning at levels well below those used to define diabetes (1). Whereas these relationships have been observed in ambulatory settings (1), and in specialized hospital settings (2-16), the relationship between pre-operative glucose levels and morbidity in patients undergoing a broad range of non-cardiac surgeries has not been systematically studied in large numbers of patients.
Hundreds of millions of non-cardiac surgeries are performed worldwide every year (17), and post-operative cardiac complications are not uncommon (18). The relationship between glucose and myocardial injury after non-cardiac surgery (MINS) – a recently discovered complication that affects 8-18% of patients, is under-recognized due to frequent absence of typical ischemic symptoms or signs in the post-operative period, yet strongly predicts the risk of death (19, 20) – has not been studied.
The Vascular events In non-cardiac Surgery patients cOhort evaluatioN (VISION) Study is a multicentre, international prospective cohort study evaluating the incidence of, and clinical risk factors for post-operative cardiovascular events in a representative sample of patients undergoing a broad range of elective, urgent and emergent non-cardiac surgeries. Pre-operative glucose levels were documented in almost 12,000 VISION participants, providing an opportunity to determine whether they provide additional value in assessing the risk of subsequent post-operative myocardial ischemia and death in patients with and without diabetes.
Methods
The VISION study was conducted at 12 centres in 8 countries in accordance with the Declaration of Helsinki and was approved by local ethics committees at all sites. A detailed description of the methods has been published (21). Briefly, all patients on the operating lists at each participating centre during the recruitment periods were screened. Consecutive consenting patients aged 45 years or older who required at least one overnight hospital admission for non-cardiac surgery of any type or acuity, occurring at all times of the day and all days of the week were recruited from 2007 to 2013. Informed consent was obtained before surgery or, if consent could not be obtained due to emergency surgery, within 24 hours after surgery. The current analysis was planned in 2011 before viewing any blood glucose data.
The clinical data collected at the time of enrolment by interview or from the medical chart included demographics, pre-existing comorbidities (including history of diabetes documented in the medical record or reported by the patient if not documented), medication use, pre-operative glucose level and whether it was a venous laboratory measure or point of care finger-stick measure, and its timing in relation to last caloric intake (enteral or parenteral), if available. Type (vascular, major orthopedic, major general surgery, and other surgery including neurosurgery, urologic/gynecologic, thoracic, head and neck, ophthalmic, plastic, and hernia surgery) and urgency (elective, urgent, emergent) of surgery were noted. Blood was systematically collected in all participants after surgery at 6-12 hours, and on the mornings of the 1st, 2nd and 3rd post-operative days (as long as patients were still admitted to hospital) to measure troponin T (TnT) levels using the Roche 4th-generation Elecsys TnT assay (coefficient of variation < 10% at 0.035 ng/mL (22)). Death and date of death were ascertained from medical records until the end of the hospital stay and, after discharge, by phone 30 days after surgery.
VISION patients were included in the current analysis if pre-operative glucose values were documented in the medical chart, and at least 1 post-operative TnT was measured. Glucose values were classified as fasting if there was confirmation that at least 8 hours had elapsed since the last caloric intake (enteral or parenteral). All other glucose values were considered casual. The primary outcome was myocardial injury after non-cardiac surgery (MINS) within 3 days after surgery. MINS, defined as any TnT value of 0.03 ng/mL or more, judged by trained adjudicators as resulting from myocardial ischemia (i.e., no evidence of a nonischemic etiology causing the TnT elevation such as sepsis, pulmonary embolism, cardioversion), is a strong independent predictor of death after non-cardiac surgery, even in the absence of ischemic symptoms (20). The secondary outcome was time to death from any cause.
Statistical methods
Baseline characteristics of all participants together and separately for those with and without known diabetes were presented as n (%) and mean ± SD. The distribution of both casual and fasting pre-operative glucose values in 0.5mmol/L increments were plotted in frequency histograms for patients with and without known diabetes. Odds ratios (OR) and 95% confidence intervals (95%CI) per 1 mmol/L increment in glucose levels for MINS were calculated by logistic regression, and hazard ratios (HR) and 95%CIs per 1 mmol/L increment in glucose levels for post-operative death were calculated by Cox proportional regression. Both unadjusted models and models adjusted for age, sex, history of diabetes, pre-operative use of insulin or other anti-diabetic agents, lab versus finger-stick glucose, previously reported clinical predictors of MINS in VISION (20) (i.e., type and urgency of surgery, current atrial fibrillation, history of congestive heart failure, coronary artery disease, stroke or transient ischemic attack, hypertension, deep vein thrombosis or pulmonary embolus, chronic obstructive pulmonary disease) and pre-operative use of antiplatelet agents, ACEi/ARBs, beta blockers, and statins were run. Separate analyses were run for fasting and casual glucose values in all participants and in those with and without known diabetes. For those with diabetes, the interaction between glucose values and glucose-lowering medication (i.e. insulin, oral agents alone or no drug therapy) on the outcomes was also assessed. Pre-specified subgroup analyses included those with and without diabetes and glucose lowering medications as described above. Post-hoc subgroup analyses were completed after peer-review for research centre, year of enrolment, sex, type of surgery and urgency of surgery. The effect of differences across subgroups was assessed by including an interaction term in the models. Youden's Index (which maximises the sum of sensitivity and specificity) in receiver operating characteristic analysis (23) was used to identify clinically useful cut-points for casual and fasting pre-operative glucose levels to predict MINS, for all participants and separately according to diabetes status. The assumptions of Cox regression were assessed and no violations were identified. All analyses were completed with IBM-SPSS (V22) software. P-values <0.05 were considered nominally significant for the pre-specified main and subgroup analyses. Bonferroni p-values of 0.01 were considered significant for the 5 post-hoc subgroup analyses.
Role of funding sources
Funding sources had no input into study design, data collection, analysis, interpretation, reporting or the decision to report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
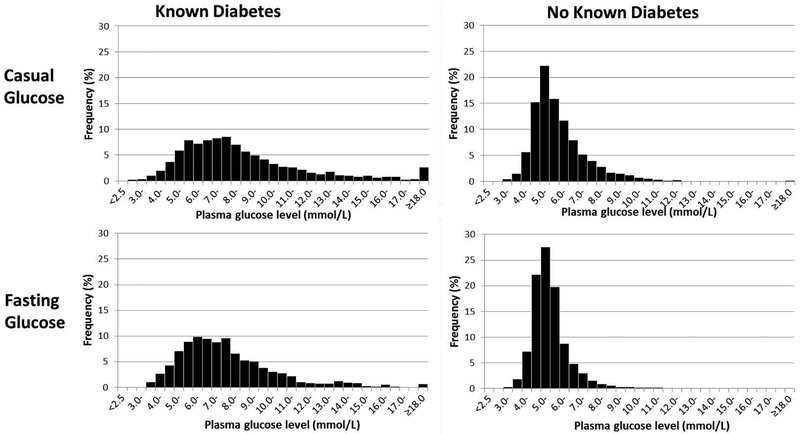
In the VISION study, of 23,603 eligible patients, 22,609 were screened in time to measure a post-operative troponin, and 16,087 consented and were enrolled (21). There were 15,296 participants who had at least one post-operative TnT measurement, of whom, 11,954 participants, including 2809 (23.5%) with diabetes, had either a casual or fasting glucose value documented pre-operatively in their medical records. The proportion of VISION patients with documented glucose values varied between research centres from 42% to 98% (median 80%). Vital status was known for 100% of patients at 27-30 days, and data on covariates were complete in all but 4 patients. Compared to VISION patients not included in this analysis, GlucoVISION participants were older and more likely to have diabetes and other comorbidities and more likely to be undergoing vascular or orthopedic, and elective surgery (see Appendix Table A1). As noted in Table 1, patients with diabetes were more likely to have cardiovascular disease and hypertension, were more likely to be undergoing vascular surgery, and had higher casual and fasting glucose levels than those without diabetes (Figure 1).
Table 1.
Characteristics of patients undergoing non-cardiac surgery according to diabetes status
| All | Known Diabetes | No Known Diabetes | |
|---|---|---|---|
| n= 11954 | n= 2809 | n= 9145 | |
| Age (years) | 66.2 ± 11.7 | 67.9 ± 10.8 | 65.7 ± 12.0 |
| Female | 6107 (51.1) | 1316 (46.8) | 4791 (52.4) |
| Coronary artery disease | 1584 (13.3) | 623 (22.2) | 961 (10.5) |
| Congestive heart failure | 646 (5.4) | 240 (8.5) | 406 (4.4) |
| Stroke or transient ischemic attack | 928 (7.8) | 320 (11.4) | 608 (6.6) |
| Peripheral vascular disease | 722 (6.0) | 341 (12.1) | 381 (4.2) |
| Current atrial fibrillation | 451 (3.8) | 130 (4.6) | 321 (3.5) |
| Hypertension | 6450 (54.0) | 2207 (78.6) | 4242 (46.4) |
| Chronic obstructive pulmonary disease | 1095 (9.2) | 281 (10.0) | 814 (8.9) |
| Type of surgery | |||
| Vascular (including amputation) | 422 (3.5) | 142 (5.1) | 280 (3.1) |
| Orthopedic | 2768 (23.2) | 642 (22.9) | 2126 (23.2) |
| General | 2220 (18.6) | 503 (17.9) | 1717 (18.8) |
| Other | 6544 (54.7) | 1522 (54.2) | 5022 (54.9) |
| Urgency of surgery | |||
| Urgent/Emergent | 1630 (13.6) | 398 (14.2) | 1232 (13.5) |
| Elective | 1034 (86.4) | 2411 (85.8) | 7913 (86.5) |
| Taking no diabetes drugs pre-op | 9507 (79.5) | 448 (15.9) | 9059 (99.1) |
| Taking only oral diabetic drugs | 1022 (8.5) | 1000 (35.6) | 22 (0.2) |
| Taking insulin pre-op | 1425 (11.9) | 1361 (48.5) | 64 (0.7) |
| Casual glucose was measured | 7690 (64.3) | 1828 (65.1) | 5862 (64.1) |
| Casual glucose level (mmol/L) | 6.6 ± 2.5 | 8.6 ± 3.6 | 6.0 ± 1.7 |
| Fasting glucose was measured | 4264 (35.7) | 981 (34.9) | 3283 (35.9) |
| Fasting glucose level (mmol/L) | 6.1 ± 1.9 | 7.9 ± 2.7 | 5.5 ± 1.1 |
Values are n (%) or mean ± SD.
Figure 1.
Distribution of casual and fasting pre-operative glucose values among patients with and without diabetes in 0.5 mmol/L increments
Within the first 3 post-operative days, MINs occurred in 813 (6.8%) patients comprising 297/2809 (10.5%) with diabetes and 516/9145 (5.6%) without diabetes (OR 1.98, 95%CI 1.70 to 2.30; p<0.0001). Within 30 days of surgery, 249 (2.1%) patients died, comprising 75/2809 (2.7%) with diabetes and 174/9145 (1.9%) without diabetes (OR 1.41, 95%CI 1.08 to 1.86; p=0.016).
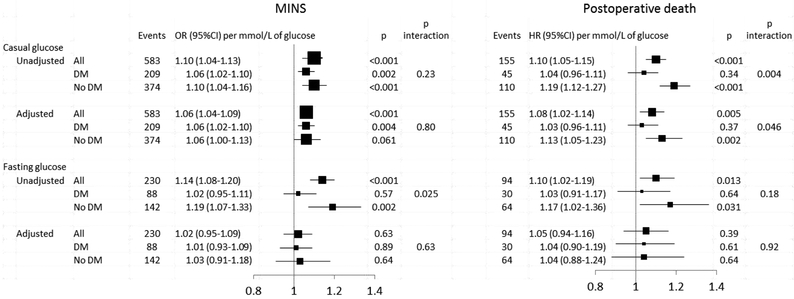
Linearity of the relationship between pre-operative glucose and MINS or death is demonstrated in Appendix Figure A1. Both unadjusted and adjusted casual glucose levels were progressively related to a higher incidence of MINS such that the adjusted odds ratio increased by 6% for every 1 mmol/L higher glucose level (adjusted OR 1.06, 95%CI 1.04 to 1.09; p<0.001)(Figure 2). This relationship was present regardless of diabetes status (interaction p=0.23). Both unadjusted and adjusted casual glucose levels were also progressively related to 30 day post-operative mortality such that the adjusted hazard ratio increased by 8% for every 1 mmol/L higher glucose level (adjusted HR 1.08, 95%CI 1.02 to 1.14; p=0.005). This relationship was due to the effect of glucose in the subgroup without known diabetes but not in those with diabetes (interaction p=0.046)
Figure 2. Relationship between pre-operative glucose levels and risk of MINS or death in those with and without diabetes.
Adjusted models are adjusted for age, sex, history of diabetes, type and urgency of surgery, current atrial fibrillation, history of congestive heart failure, coronary artery disease, stroke or transient ischemic attack, hypertension, deep vein thrombosis or pulmonary embolism, chronic obstructive pulmonary disease, pre-operative use of insulin or other anti-diabetic agent, antiplatelet, ACEi/ARB, beta blocker, and statin, and lab vs. fingerstick glucose.
Unadjusted but not adjusted fasting glucose levels were progressively related to a higher incidence of MINS such that the unadjusted odds ratio increased by 14% for every 1 mmol/L higher glucose level (OR 1.14, 95%CI 1.08 to 1.20; p<0.001) (Figure 2). This relationship was due to the effect of glucose in the subgroup without known diabetes but not in those with diabetes (interaction p=0.025). Unadjusted but not adjusted fasting glucose levels were also progressively related to 30 day post-operative mortality such that the unadjusted hazard ratio increased by 10% for every 1 mmol/L higher glucose level (HR 1.10, 95% CI 1.02 to 1.19; p=0.013) with no differences according to diabetes status (interaction p=0.18).
The relationships between fasting or casual glucose and MINS or death did not differ by research centre, year of enrolment, sex, type or urgency of surgery (all interaction p-values >0.01).
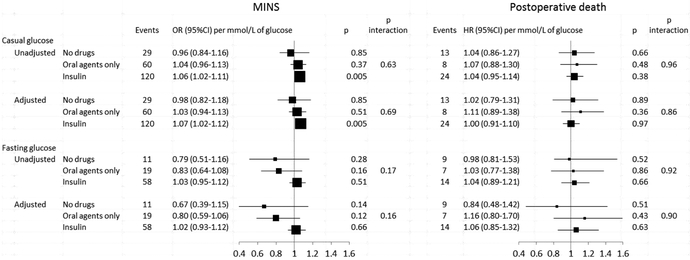
As noted in Figure 3, there was no evidence that the relationships between pre-operative glucose and either MINS or death were affected by the type of diabetes therapy (all interaction p>0.15).
Figure 3. Relationship between pre-operative glucose levels and risk of MINS or death by treatment type for patients with diabetes.
Adjusted models are adjusted for age, sex, history of diabetes, type and urgency of surgery, current atrial fibrillation, history of congestive heart failure, coronary artery disease, stroke or transient ischemic attack, hypertension, deep vein thrombosis or pulmonary embolism, chronic obstructive pulmonary disease, pre-operative use of insulin or other anti-diabetic agent, antiplatelet, ACEi/ARB, beta blocker, and statin, and lab vs. fingerstick glucose
Casual and fasting glucose cut-points that best predicted MINS are shown in Table 2. For casual glucose levels, patients with diabetes and a level above 7.92 mmol/L were most likely to develop MINS (OR 1.47 [95% CI 1.10-1.96]; p=0.0096). The optimal cut-point for patients without diabetes was 6.86 mmol/L (OR 1.71 [1.36-2.15]; p<0.001). For fasting glucose levels, no such cut-point was identified in patients with known diabetes. However, patients without diabetes who had fasting glucose level above 6.41 mmol/L were most likely to develop MINS (OR 2.71 [1.85-3.98]; p<0.0001).
Table 2.
Pre-operative blood glucose cut-points most predictive of MINS
| Glucose cut-point (mmol/L) |
Odds ratio (95% CI) |
p-value | |
|---|---|---|---|
| Casual glucose | |||
| All patients | 6.97 | 1.76 (1.49-2.09) | <0.0001 |
| Known diabetes | 7.92 | 1.47 (1.10-1.96) | 0.0096 |
| No known diabetes | 6.86 | 1.71 (1.36-2.15) | <0.0001 |
| Fasting glucose | |||
| All patients | 6.32 | 2.19 (1.67-2.87) | <0.0001 |
| Known diabetes | N/A* | 0.571 | |
| No known diabetes | 6.41 | 2.71 (1.85-3.98) | <0.0001 |
We were not able to find a cut-point in this group
Discussion
This large prospective cohort study representative of patients undergoing a wide range of non-cardiac surgeries demonstrates that pre-operative glucose levels are predictive of post-operative myocardial injury in people undergoing non-cardiac surgery (MINS) as well as post-operative mortality. These relationships are particularly evident for casual glucose levels and for people without known diabetes before surgery. Moreover for casual glucose levels these relationships cannot be explained by other common cardiac risk factors. Casual and fasting glucose thresholds of 6.9 and 6.4 mmol/L, respectively are most predictive of MINS in people without diabetes, and a casual glucose threshold of 7.9 mmol/L is most predictive of MINS in people with diabetes.
Main findings in context
The observation that the degree of dysglycemia is an independent risk factor for ischemic heart disease and mortality in people with and without a history of diabetes has been made in a variety of acute and ambulatory settings (1-16). In particular, similar or slightly larger effect sizes have been noted in smaller single centre studies of non-cardiac elective surgeries (OR for a composite CV outcome of 1.19 per mmol/L)(4) and cardiac surgeries (aOR for death of 1.39 per mmol/L [95% CI 1.15-1.67])(7). The higher risk among people with higher glucose levels may reflect underlying myocardial damage or dysfunction due to oxidative stress, acute inflammatory responses, reduced nitric oxide-mediated vasodilation and ischemia-reperfusion injury, and/or coronary artery disease due to associations with more chronic inflammation and advanced glycation endproduct (AGE) effects, and other cardiovascular risk factors (24) or may simply reflect the degree of catecholamine and cortisol response in the peri-operative setting. Therefore, it is not surprising that the stresses of the peri-operative period will precipitate a serious outcome in these susceptible individuals. Regardless of the mechanism, glucose levels clearly identify patients with higher post-operative risk who may benefit from closer monitoring.
Furthermore, the mechanism underlying myocardial ischemia causing troponin elevation is heterogeneous in the post-operative setting and may have been thrombotic, or related to hypoperfusion or hypoxia. Non-ischemic causes (25) including sepsis, pulmonary embolism, cardioversion, end stage renal disease, intracranial hemorrhage, chest wall trauma, myocarditis and non-ischemic cardiomyopathies including Takotsubo were excluded by adjudication to the extent possible with the available information.
It is notable that there was a more robust relationship between glucose levels and post-operative outcomes in patients without known diabetes than in those with diabetes, despite diabetes itself increasing the odds of MINS and death 2- and 1.4-fold, respectively. Similar differences have been noted in non-surgical (26, 27) and surgical literature (8, 10, 15), though not universally (6, 12, 14). This observation may be due to the fact that in patients with diabetes, glucose levels are monitored and intervened upon, and are therefore a less reliable reflection of the patient’s underlying metabolic state (27). An alternative explanation may be that a diagnosis of diabetes already reflects the underlying mechanisms described above that increase risk of post-operative ischemia and death, which in patients without diabetes manifest as higher glucose levels. There is also evidence that acute rises or fluctuations in blood glucose of people without diabetes who usually have normal levels may cause more endothelial dysfunction and oxidative stress than chronic hyperglycemia (28) which is seen in diabetes. Finally, high glucose levels in those without known diabetes may reflect undiagnosed, unmonitored and untreated diabetes.
It is also notable that casual glucose levels were more strongly related to both MINS and death than fasting glucose. This may be due to the higher variability in casual glucose levels that could have made an underlying relationship more apparent in the statistical models. Alternatively, glucose levels in the non-fasting state may be a better ‘test’ of neurohormonal homeostatic mechanisms that both aim to normalize blood glucose and affect function of the vasculature and the heart in the acute setting. Others have shown a rise in oxidative stress, inflammation and prothrombotic markers after ingestion of glucose (i.e. the non-fasting state)(29). Another marker of glycemic status not collected in this study is glycated hemoglobin (HbA1c) which reflects glycemic exposure over the previous several months with less day-to-day random variability. However, it is also subject to error in those with severe renal and liver disease, anemia and recent blood transfusions, and would not reflect the short term neurohormonal stress of the pre-operative period. Pre-operative HbA1c has been shown to predict outcomes (9, 15, 16), with one small elective vascular surgery study showing similar effect sizes for 1% increment in HbA1c (OR 1.3 [95%CI 1.0-1.7] for post-op TnT elevation and HR 1.5 [1.0-2.1] for death) as for 1mmol/L increment in pre-operative glucose (OR 1.4 [1.2-1.5] for TnT elevation and HR 1.2 [1.1-1.3] for death) (16). However, it is not known if HbA1c would be a better indicator of short-term peri-operative outcomes than glucose levels measured near the time of surgery.
Whether attempts to modify peri-operative glucose level have an impact on outcomes in non-cardiac surgery remains to be studied. However, randomized trials of predominantly cardiac surgery patients indicate that tight peri-operative glycemic control does not reduce cardiovascular events or mortality, and increases the risk of hypoglycemia in patients with diabetes (30).
Strengths and limitations
Strengths of this study include its large sample size and statistical power. Reliability and generalizability of results were enhanced by the prospective international evaluation in a representative group of non-cardiac surgery patients, including large numbers of patients with and without diabetes, and accounting for a large number of systematically gathered covariates. Nevertheless, other unmeasured confounders such as body mass index, smoking and insulin resistance were not included. Furthermore, relevant glucose cut-points were derived empirically.
These findings are limited by the absence of information describing the circumstances under which casual glucose levels were drawn, and the fact that diabetes status before surgery was based on patient self-report and was not verified independently, which may have led to underestimation of effects and underestimation of differences between those with and without diabetes. In addition, variability in casual glucose may have been due to a variety of factors including recent meal size or timing, in addition to intrinsic glucometabolic differences, which would have also led to underestimation of effects. Glucose measurements were recorded from the clinical chart, which may have led to non-random selection of individuals with fasting or casual or no available pre-operative glucose values; however, the evaluation of glucose values obtained clinically from patient charts enhances generalizability and translation into clinical practice. Finally, several pre-specified analyses were completed for which adjustment for multiple testing was not done.
Implications for practice and research
For patients undergoing non-cardiac surgery, glucose measurement is an additional tool that can be used to inform risk assessment, and identify patients needing closer monitoring postoperatively. Furthermore, glucose levels measured at any time irrespective of the last meal are easier to obtain and more predictive than fasting levels, thus facilitating their use for this purpose.
There is potential clinical utility in considering pre-operative glucose levels. Surgeons and cardiologists, internists and anesthetists who consult on surgical patients can easily obtain glucose measurements in a matter of minutes and can now evaluate the incremental risk using empirically determined glucose thresholds, even after considering all the other known preo-perative prognostic factors. These results have the potential to enhance conversations about prognosis and decision making before going to surgery.
Identifying a better risk marker in people with diabetes could be the subject of future studies. Furthermore, it is not known if delaying surgery until glucose levels can be normalized pre-operatively would positively or negatively impact outcomes, a question which may need to be answered separately for elective and urgent surgical patients.
Supplementary Material
Research in Context.
Evidence before this study
We searched Medline using the terms “glucose” or “glycemia”, and “peri-operative” or “preoperative” or “surgery”, and “post-operative cardiovascular events” or “post-operative mortality”. Glucose levels measured in the pre-operative period have been associated with post-operative cardiovascular outcomes or death in small studies or in specialized groups of surgical patients, particularly cardiac surgery patients, but not in a broad range of non-cardiac surgery patients. Furthermore, cutpoints used to identify high risk patients have been arbitrary or taken from diabetes diagnostic definitions which have uncertain relevance in the acute care setting.
Added value of this study
This study prospectively evaluated a large number of patients with and without diabetes internationally undergoing a broad range of non-cardiac surgeries of varying acuity. We found that pre-operative glucose levels, particularly casual glucose levels predict myocardial ischemia and death post-operatively, especially among patients without diabetes. In addition, we empirically determined that the risk of myocardial injury after non-cardiac surgery significantly increases for people without diabetes if the pre-operative casual glucose is above 6.9 mmol/L or the fasting glucose is above 6.4 mmol/L, and for people with diabetes if the pre-operative casual glucose is above 7.9 mmol/L.
Implications of all the available evidence
Pre-operative glucose levels predict risk of post-operative myocardial injury and death in a broad range of surgical patients even after accounting for other risk factors. Clinically useful pre-operative glucose cut-points for increased risk have now been identified. Surgeons and cardiologists, internists and anesthetists who consult on surgical patients can easily obtain glucose measurements in a matter of minutes and can now evaluate the incremental risk using empirically determined glucose thresholds, even after considering all the other known pre-operative prognostic factors. These results have the potential to enhance conversations about prognosis and decision making before going to surgery. Future studies should assess whether other measures of glycemia such as HbA1c may be better markers of risk, and whether pre-operative glycemic control impacts outcomes in non-cardiac surgery.
Acknowledgement:
The authors would like to thank Diane Heels-Ansdell for statistical advice.
Funding: Funders had no role in the study design, collection, analysis or interpretation of data, writing of the report, or the decision to submit the article for publication. Funding for VISION and its substudies was provided by the following institutions from Canada: Canadian Institutes of Health Research (6 grants, including one specifically for GlucoVISION) (Ottawa, Ontario, Canada); Heart and Stroke Foundation of Ontario (2 grants) (Toronto, Ontario, Canada); Academic Health Science Centres Alternative Funding Plan Innovation Fund Grant (Toronto, Ontario, Canada); Population Health Research Institute Grant (Hamilton, Ontario, Canada); CLARITY Research Group Grant; McMaster University, Department of Surgery, Surgical Associates Research Grant (Hamilton, Ontario, Canada); Hamilton Health Science New Investigator Fund Grant (Hamilton, Ontario, Canada); Hamilton Health Sciences Grant (Hamilton, Ontario, Canada); Ontario Ministry of Resource and Innovation Grant (Toronto, Ontario, Canada); Stryker Canada (Waterdown, Ontario, Canada); McMaster University, Department of Anesthesiology (2 grants) (Hamilton, Ontario, Canada); Saint Joseph’s Healthcare, Department of Medicine (2 grants) (Hamilton, Ontario, Canada); Father Sean O’Sullivan Research Centre (2 grants) (Hamilton, Ontario, Canada); McMaster University, Department of Medicine (2 grants) (Hamilton, Ontario, Canada); Roche Diagnostics Global Office (3 grants) (Basel, Switzerland); Hamilton Health Sciences Summer Studentships (6 grants) (Hamilton, Ontario, Canada); McMaster University, Department of Clinical Epidemiology and Biostatistics Grant (Hamilton, Ontario, Canada); McMaster University, Division of Cardiology Grant (Hamilton, Ontario, Canada); Canadian Network and Centre for Trials Internationally Grant (Hamilton, Ontario, Canada); Winnipeg Health Sciences Foundation Operating Grant (Winnipeg, Manitoba, Canada); University of Manitoba, Department of Surgery Research Grant (2 grants) (Winnipeg, Manitoba, Canada); Diagnostic Services of Manitoba Research Grant (2 grants) (Winnipeg, Manitoba, Canada); Manitoba Medical Services Foundation Grant (Winnipeg, Manitoba, Canada); Manitoba Health Research Council Grant (Winnipeg, Manitoba, Canada); University of Manitoba, Faculty of Dentistry Operational Fund (Winnipeg, Manitoba, Canada); University of Manitoba, Department of Anesthesia Grant (Winnipeg, Manitoba, Canada); University Medical Group, Department of Surgery, University of Manitoba, start-up Fund (Winnipeg, Manitoba, Canada). Funding from Australia: National Health and Medical Research Council Program Grant (Canberra, Australia). Funding from Brazil: Projeto Hospitais de Excelência a Serviço do SUS (PROADI-SUS) grant from the Brazilian Ministry of Health in Partnership with Hcor (Cardiac Hospital Sao Paulo-SP) (Sao Paulo, Brazil). Funding from China: Public Policy Research Fund, Research Grant Council, Hong Kong SAR (Hong Kong); General Research Fund, Research Grant Council, Hong Kong SAR (Hong Kong); Australian and New Zealand College of Anesthesiologists Grant (Sydney, Australia). Funding from Colombia: School of Nursing, Universidad Industrial de Santander (Bucaramanga, Colombia); Grupo de Cardiología Preventiva, Universidad Autónoma de Bucaramanga (Bucaramanga, Colombia); Fundación Cardioinfantil – Instituto de Cardiología (Bogota, Colombia); Alianza Diagnóstica S.A. (Bucaramanga, Colombia). Funding from India: St. John’s Medical College and Research Institute Grant, Division of Clinical Research and Training Grant (Bangalore, India). Funding from Malaysia: University of Malaya Research Grant (UMRG) (Kuala Lumpur, Malaysia); University of Malaya, Penyelidikan Jangka Pendek Grant (PJP) (Kuala Lumpur, Malaysia). Funding from Spain: Instituto de Salud Carlos III (Madrid, Spain); Fundació La Marató de TV3 (Esplugues de Llobregat, Spain). Funding from United States: American Heart Association Grant (Dallas, Texas). Funding from United Kingdom: National Institute for Health Research (NIHR) (London, United Kingdom). Dr. Nagele was funded by a grant from the National Institute for General Medical Sciences (K23 GM087534), National Institutes of Health (Bethesda, Maryland), and a grant to Washington University Institute of Clinical and Translational Sciences (UL1RR024992).
Footnotes
Declarations of interests: ZP reports receipt of grants from Amgen, Astra Zeneca/Bristol Myers Squibb, Lexicon, Merck, NovoNordisk, and Sanofi, and personal fees for speaking and advising from Abbott, Astra Zeneca/Bristol Myers Squibb, Boehringer Ingelheim/Eli Lilly, Janssen, Merck, NovoNordisk, Pfizer, and Sanofi,. HCG reports research grant support from Sanofi, Lilly, AstraZeneca, Boehringer Ingelheim, Merck, Abbott, and Novo Nordisk; honoraria for speaking from Sanofi, Novo Nordisk, Merck, Boehringer Ingelheim and AstraZeneca, and consulting fees from Sanofi, Lilly, AstraZeneca, Merck, Novo Nordisk, Abbot, Janssen, and Boehringer Ingelheim. PJD reports receipt of grants from Abbott Diagnostics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Octapharma, Philips Healthcare, Roche Diagnostics, and Stryker, and honoraria for advising from AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline. PPI, PAC, IG, II, GM, RDJ declare no financial relationships with any organizations that might have an interest in the submitted work in the previous two years.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med 2012; 40:3180–8. [DOI] [PubMed] [Google Scholar]
- 3.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355:773–8. [DOI] [PubMed] [Google Scholar]
- 4.Biteker M, Dayan A, Can MM, et al. Impaired fasting glucose is associated with increased perioperative cardiovascular event rates in patients undergoing major non-cardiothoracic surgery. Cardiovasc Diabetol 2011; 10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 2013; 257:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson RS, Amdur RL, White JC, Macsata RA. Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg 2012; 214:68–80. [DOI] [PubMed] [Google Scholar]
- 7.Zindrou D, Taylor KM, Bagger JP. Admission plasma glucose: an independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care 2001; 24:1634–9. [DOI] [PubMed] [Google Scholar]
- 8.Imran SA, Ransom TP, Buth KJ, et al. Impact of admission serum glucose level on in-hospital outcomes following coronary artery bypass grafting surgery. Can J Cardiol 2010; 26:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodenough CJ, Liang MK, Nguyen MT, et al. Preoperative Glycosylated Hemoglobin and Postoperative Glucose Together Predict Major Complications after Abdominal Surgery. J Am Coll Surg 2015; 221:854–61. [DOI] [PubMed] [Google Scholar]
- 10.Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015; 261:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc 2005; 80:862–6. [DOI] [PubMed] [Google Scholar]
- 12.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2005; 130:1144. [DOI] [PubMed] [Google Scholar]
- 13.Mansur A, Popov AF, Hanna AA, et al. Perioperative Blood Glucose Levels <150 mg/dL are Associated With Improved 5-Year Survival in Patients Undergoing On-Pump Cardiac Surgery: A Prospective, Observational Cohort Study. Medicine (Baltimore) 2015; 94:e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology 2010; 112:860–71. [DOI] [PubMed] [Google Scholar]
- 15.van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1C and Glucose on Postoperative Mortality in Noncardiac and Cardiac Surgeries. Diabetes Care 2018; 41:782–8. [DOI] [PubMed] [Google Scholar]
- 16.Feringa HH, Vidakovic R, Karagiannis SE, et al. Impaired glucose regulation, elevated glycated haemoglobin and cardiac ischaemic events in vascular surgery patients. Diabet Med 2008; 25:314–9. [DOI] [PubMed] [Google Scholar]
- 17.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–44. [DOI] [PubMed] [Google Scholar]
- 18.Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–47. [DOI] [PubMed] [Google Scholar]
- 19.Devereaux PJ, Biccard BM, Sigamani A, et al. Association of Postoperative High-Sensitivity Troponin Levels With Myocardial Injury and 30-Day Mortality Among Patients Undergoing Noncardiac Surgery. JAMA 2017; 317:1642–51. [DOI] [PubMed] [Google Scholar]
- 20.Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–78. [DOI] [PubMed] [Google Scholar]
- 21.Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–304. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux PJ, Bradley D, Chan MT, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med 2011; 5:e193–e200. [PMC free article] [PubMed] [Google Scholar]
- 23.Mazumdar M, Smith A, Bacik J. Methods for categorizing a prognostic variable in a multivariable setting. Stat Med 2003; 22:559–71. [DOI] [PubMed] [Google Scholar]
- 24.Mapanga RF, Essop MF. Damaging effects of hyperglycemia on cardiovascular function: spotlight on glucose metabolic pathways. Am J Physiol Heart Circ Physiol 2016; 310:H153–H173. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Singh A, Collins B, et al. Causes of Troponin Elevation and Associated Mortality in Young Patients. Am J Med 2018; 131:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstein HC, Swedberg K, Carlsson J, et al. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med 2008; 168:1699–704. [DOI] [PubMed] [Google Scholar]
- 27.Gerstein HC. Dysglycemia and cardiovascular risk in the general population. Circulation 2009; 119:773–5. [DOI] [PubMed] [Google Scholar]
- 28.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57:1349–54. [DOI] [PubMed] [Google Scholar]
- 29.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Anti-inflammatory effects of insulin and the pro-inflammatory effects of glucose. Semin Thorac Cardiovasc Surg 2006; 18:293–301. [DOI] [PubMed] [Google Scholar]
- 30.Buchleitner AM, Martinez-Alonso M, Hernandez M, Sola I, Mauricio D. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst Rev 2012;CD007315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.