Abstract
N-Butylbenzenesulfonamide (NBBS) is a plasticizer detected in the environment suggesting potential human exposure. These studies investigated the in vitro hepatic clearance and disposition of [14C]NBBS in rodents following a single gavage (2, 20 or 200 mg/kg) or intravenous (IV) administration (20 mg/kg). NBBS was cleared slower in hepatocytes from humans compared to rodents. [14C]NBBS was well-absorbed in male rats following gavage administration and excreted extensively in urine (70–76%) and feces (11–15%) 72 h following administration. Following a 20 mg/kg gavage dose in male rats, 25% of the dose was excreted in bile by 24 h suggesting that observed fecal excretion was due to biliary excretion. The radioactivity was distributed to tissues with 14% and 8% of the administered dose remaining in tissues at 24 and 72 h, respectively. There was no apparent dose-dependent effect in disposition in male rats. Disposition patterns were similar in female rats (urine, 83%; feces, 14%) and male (urine, 69%; feces, 11%) and female (urine, 72%; feces, 9%) mice following gavage administration of 20 mg/kg. The disposition following IV administration was similar to that of gavage. Urinary radiochemical profiles were similar between doses, routes, species, and sexes. Among numerous metabolites identified, oxidative metabolites of NBBS predominated.
Keywords: N-butylbenzenesulfonamide, absorption, distribution, metabolism, excretion, plasticizer
Introduction
N-Butylbenzenesulfonamide (NBBS) is a common plasticizer used primarily in polyamides (e.g., Nylon 6, 11, and 12), with reported use in medical devices, films, and the automotive industry (Wypych 2017). NBBS is not listed as a priority pollutant by the Environmental Protection Agency and, therefore, is not routinely monitored. However, when NBBS is detected in environmental monitoring studies, it is often among the most frequently detected and highest concentration contaminant with reported levels typically in the ng/L - μg/L range (Di Carro et al. 2018; Dsikowitzky et al. 2004a; Dsikowitzky et al. 2004b; Grigoriadou et al. 2008; Huppert et al. 1998; Oros et al. 2003; Pedersen et al. 2005; Plumlee et al. 2012). Examples of studies that measured relatively high concentrations of NBBS in environmental samples include one reporting up to 16 μg/L NBBS in secondary effluent from a water reclamation plant in California (Soliman et al. 2007) and another reporting a concentration of 4000 μg/L NBBS in a groundwater sample from an agricultural land-use area in the United Kingdom (Manamsa et al. 2016). NBBS has also been identified as a leachate from polyamide cooking utensils (Skjevrak et al. 2005) and as a contaminant in wine with measured concentrations up to 2 μg/L (Duffield et al. 1994). NBBS isolated from the bark of Pygeum africanum has been used in traditional medicine to treat prostate cancer (Komakech et al. 2017). Taken together, there is potential for human exposure to NBBS via an oral route.
There are limited toxicity data available for NBBS. The majority of data available in the public domain followed the discovery that NBBS, which had leached from a plastic dosing vial into the saline vehicle, elicited neurotoxic effects in rabbits when administered via intracisternal injection (Strong et al. 1990). Subsequent studies in rabbits further characterized the original finding and demonstrated that intraperitoneal injection also elicited neurotoxicity (Strong et al. 1991). Another study found that intraperitoneal doses of 300 mg/kg resulted in neurotoxicity in Wistar rats (Lee et al. 1995). However, no signs of neurotoxicity were observed in a 27-day oral gavage study in Sprague-Dawley rats with doses up to 300 mg/kg (Rider et al. 2012). Marrocco et al. (2015) evaluated the immunotoxic potential of NBBS following a 28-day dermal exposure in mice to 0 (control) or 100% NBBS; while they did not observe meaningful effects on the immune parameters measured, they did note significant increases in liver and kidney weights. NBBS, isolated from the bark of Pygeum africanum was found to be an androgen receptor antagonist in vitro (Schleich et al. 2006; Papaioannou et al. 2010). Therefore, the potential for endocrine disruption is a concern for this compound, as with many other plasticizers.
Due to limited data on NBBS, the National Toxicology Program (NTP) is investigating the toxicity of NBBS in mice and rats (https://ntp.niehs.nih.gov/testing/status/agents/ts-10057.html). Absorption, distribution, metabolism, and excretion (ADME) and toxicokinetics (TK) data are essential for the design and interpretation of toxicology study data; however, there are limited ADME and TK data in the literature, especially following routes of exposure relevant to humans. Following intravenous (IV) administration of 1 mg/kg [13C6]NBBS in male Sprague Dawley rats, the elimination of NBBS in plasma was triphasic with respective half-lives of 0.78, 11, and 1036 min (Kumar et al. 2007). In this study, NBBS was detected in the liver, kidney, muscle, fat, and brain with the tissue:plasma ratio for liver being the highest. NBBS preferentially partitioned into red blood cells (RBC) with a RBC:plasma ratio of 3. In female Wistar rats, following IV administration of 1 mg/kg, the maximum NBBS concentration was reached within 2 min in liver and skeletal muscle and within 5 min in kidney and fat (Kumar et al., 2007). In female rats, the oral bioavailability following administration of 1 mg/kg was reported as 52% to 79%; the plasma elimination was triphasic with half-lives of 0.32, 27, and 500 min (summarized in NTP 2000). In vitro metabolism studies in liver S9 fraction from rat, rabbit, and human showed that NBBS was metabolized to 2-hydroxy-NBBS but conjugation of the metabolite was not observed (summarized in NTP 2010).
To aid in design and provide data for interpretation of toxicology studies, we conducted ADME studies in Hsd:Sprague Dawley® SD® (HSD) rats and B6C3F1/N mice, the two rodent models used in NTP studies. The study design is given in Table 1. A dose-response study was conducted in male rats following a single gavage administration of 2, 20, and 200 mg/kg, representing 0.001X, 0.01X and 0.1X of the reported oral LD50 of NBBS in rats (1725–2050 mg/kg; summarized in NTP 2010). Sex and species differences were investigated in limited studies following a single gavage administration of a 20 mg/kg dose in female rats and male and female mice. To aid in interpretation of oral data, IV studies were conducted in male and female rats and mice following administration of a 20 mg/kg dose. In addition, comparative clearance and metabolism of NBBS were investigated in vitro in hepatocytes from rat, mouse and human to compare in vivo and in vitro data as well as to predict potential ADME behavior of NBBS in humans.
Table 1.
Study design of [14C]NBBS in Harlan Sprague Dawley rats and B6C3F1/N mice
| Species (Sex) | Dose (mg/kg) | Route | Study Duration (h) | Endpoint |
|---|---|---|---|---|
| Rat (M) | 2, 20, 200 | Gavage | 72 | Dose response |
| Rat (M) | 20 | Gavage | 24 | Tissue distribution |
| Rat (F) | 20 | Gavage | 72 | Sex difference |
| Mouse (M, F) | 20 | Gavage | 72 | Species difference |
| Rat (M, F) | 20 | Intravenous | 72 | Route difference |
| Mouse (M, F) | 100 | Intravenous | 72 | Route difference |
Materials and methods
Chemicals and reagents:
NBBS was obtained from Ivy Fine Chemicals (CAS RN 3622–84-2; Lot IF10505; Cherry Hill, NJ). Identity of NBBS was confirmed by 1H and 13C NMR and mass spectrometry (MS) and the purity (>99.9%) was determined by gas chromatography-with flame ionization detection. [14C]-labeled NBBS ([14C]NBBS) (uniformly ring labelled, 12.1 mCi, 0.19 mCi/mg in ethanol) was obtained from MRIGlobal (Kansas City, MO) and was stored at −20 °C. The radiochemical purity was determined to be >96% by high performance liquid chromatography (HPLC) methods 1 or 2 given below. A radiochemical purity of >94% was maintained throughout the studies by purifying the material using a 3-cc Oasis® HLB solid phase extraction cartridge (Waters, Milford, MA).
Ultima Gold™ liquid scintillation cocktail and Soluene®−350 were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Potential NBBS metabolites, benzenesulfonamide and 4-hydroxybenzenesulfonamide, β-Glucuronidase (from E. coli), sulfatase (from Aerobacter aerogenes), acylase (Type I, porcine kidney) were from Sigma-Aldrich (St. Louis, MO). Rapid Equilibrium Dialysis (RED) devices were from Thermo Scientific (Waltham, MA). All other reagents were obtained from commercial sources.
HPLC Method 1:
The HPLC instrumentation included two Waters (Milford, MA) 515 HPLC pumps, an Applied BioSystems (Foster City, CA) 759A absorbance detector, and a β-RAM Model 3 radioactivity detector with a 50-μL lithium glass solid scintillant cell (IN/US, Tampa, FL). A Zorbax Eclipse XDB-C18 column (4.6 X 150 mm; Agilent, Santa Clara) was used with a flow rate of 1 mL/min. An isocratic mobile phase of 60:40 water:acetonitrile was used with the eluent monitored for both UV absorbance at 220 nm and radioactivity.
HPLC Method 2:
The system used was the same as in HPLC Method 1. Mobile phases A: water and B: acetonitrile were used with an initial composition of 60:40 A:B held for 10 min followed by a linear gradient to 10:90 A:B over 15 min, and held for 5 min. The eluent was monitored for both UV absorbance at 220 nm and radioactivity.
HPLC Method 3:
The system used was the same as in HPLC Method 1. Mobile phases A: water and B: acetonitrile were used with an initial composition of 98:2 A:B held for 10 min followed by a linear gradient to 10:90 A:B over 30 min, and held for 5 min. The eluent was monitored for both UV absorbance at 220 nm and radioactivity.
HPLC Method 4:
The system used was the same as in HPLC Method 1. Mobile phases A: 0.1% formic acid and B: 0.1% formic acid in acetonitrile were used with an initial composition of 98:2 A:B held for 2 min followed by a linear gradient to 65:35 A:B over 33 min, and then to 10:90 A:B over 1 min and held for 9 min. The eluent was monitored for both UV absorbance at 220 nm and radioactivity.
LC-MS Method 1:
The LC-MS instrumentation included an API 5000 Triple Quadrupole Mass Spectrometer (Applied Biosystems, Foster City, CA) with Turboion Spray source coupled with an Agilent 1200 Liquid Chromatograph (Santa Clara, CA). A Phenomenex Luna C18 column (2 X 50 mm) was used (Torrance, CA) with a flow rate of 1 mL/min. Mobile phases A: 0.1% formic in water and B: 0.1% formic acid in methanol were used with an initial composition of 95:5 A:B held for 1 min followed by a linear gradient to 10:90 A:B over 4 min, and held for 3 min. The ion source was operated in positive ion mode with a source temperature of 650 °C and voltage spray of 4500 V. The transition monitored for quantitation of NBBS was m/z 214.1 → 157.8.
LC-MS Method 2:
The LC-MS instrumentation consisted of an API 4000 Triple Quadrupole Mass Spectrometer (Applied Biosystems, Foster City, CA) with Turboion Spray source coupled with an Agilent 1100 Liquid chromatograph (Santa Clara, CA). A Zorbax Eclipse XDB-C18 column (4.6 X 250 mm) was used (Agilent, Santa Clara) with a flow rate of 1 mL/min. Mobile phases A: 0.1% formic acid in water and B: 0.1% formic acid in acetonitrile were used with an initial composition of 98:2 A:B held for 10 min followed by a linear gradient to 10:90 A:B over 30 min, and held for 5 min. The ion source was operated in positive ion mode with a source temperature of 650°C and voltage spray of 4500 V. The mass spectrometer was operated in full scan (150–600 amu), neutral loss of m/z 176, or product ion scan (m/z 230 and 246) modes.
LC-MS Method 3:
The LC-MS instrumentation consisted of a Thermo LTQ Orbitrap Velos mass spectrometer equipped with a FTMS analyzer (Thermo Finnegan, Waltham, MA) coupled with a Waters Acquity Ultra Performance Liquid Chromatography system (Milford, MA). A Zorbax Eclipse XDB-C18 column (4.6 X 250 mm) (Agilent, Santa Clara) was used with a flow rate of 1 mL/min. Mobile phases A: 0.1% formic acid in water and B: 0.1% formic acid in acetonitrile was used with an initial composition of 98:2 A:B held for 2 min followed by a linear gradient to 65:35 A:B over 33 min, and to 10:90 A:B over 9 min. The mass spectrometer was operated in positive ion mode or negative ion mode in a data-dependent fashion. The instrument resolution and the mass range were 30,000 and 100–700 amu, respectively. MS/MS spectra were acquired by collection of the top 6 spectra in a data dependent mode. Data analysis was conducted with Compound Discoverer software (Thermo Scientific, San Jose, CA). Metabolites were identified based on the exact mass, isotope ratio, and if available, MS/MS spectra.
In vitro studies.
The clearance and metabolism (males only) were investigated in human, B6C3F1 mouse and Sprague Dawley rat cryopreserved hepatocytes. Hepatocyte suspensions are commonly used to investigate hepatic clearance and metabolism (Wetmore et al 2012). Human (Caucasian, 20–47 years of age, pooled hepatocytes from n = 6; male lot HC3–5; female lot 789), rat (Harlan Sprague Dawley (HSD), 8–10 weeks old; male lot 1110181; female lot, 1110182) and mouse (B6C3F1/N, 8–10 weeks, male lot 1110183, female lot 1110184) hepatocytes were obtained from Xenotech LLC (Lenexa, KS). Cells were thawed and resuspended according to the supplier’s protocol. Viability was assessed by trypan blue exclusion. The cell concentrations were 0.5 to 0.8 × 106 cells/mL and viabilities were between 79–92%.
NBBS stock solution was prepared at 0.1 mM in acetonitrile. Incubations to determine clearance were performed at a final NBBS concentration of 1 μM. Incubations were performed in triplicate with 1 mL cell suspension in 24-well polystyrene cell culture plates in a 37°C incubator with 5% CO2 atmosphere. Incubations of media (no hepatocytes) were carried out to assess analyte losses over the duration of the experiment; no losses of analytes were observed. Following the addition of NBBS, aliquots (50 μL) were removed from the incubation at 0, 15, 30, 60, 120, 180, and 300 min for mouse and human and at 0, 1, 10, 15, 30, 60, 120, 180, and 300 min for rat and added to 50 μL acetonitrile. Each sample was vortexed and centrifuged (11,000 g for 1 min), and supernatants were collected and stored at −20 °C until analysis.
Standard curves were prepared and analyzed to determine linearity, accuracy, and precision of the method. Two stock solutions of NBBS (5 and 10 mM) were prepared in acetonitrile. Working solutions were prepared in water using alternate stock solutions as appropriate. Standard curves were prepared in triplicate in hepatocyte incubation medium using alternate stock solutions to final concentrations from 0.05 to 2.5μM, except at the limit of quantitation (LOQ) of 0.05 μM, where 6 replicates were prepared and processed as described above for samples.
All standards and samples were analyzed using LC-MS Method 1. A linear regression was used to relate peak area response of analyte to analyte concentration. The correlation coefficient was > 0.99, the precision (estimated as percent relative standard deviation, RSD) was ≤ 11% except at 0.05 μM where it was 27%, and accuracy (estimated as percent relative error) was ≤ ± 25% demonstrating the suitability of the analytical method.
The concentration (ng/mL) of NBBS in the supernatant from hepatocyte incubations was calculated using peak area of analyte and the regression equation. Concentration versus time data were evaluated by noncompartmental analysis using Phoenix WinNonlin 6.3 (Pharsight, Cary, NC) and half-lives of elimination (t½) were estimated. Intrinsic clearance (Clint, mL/(min/kg body wt.)) was calculated using the equation: Clint = 0.693/t½ * mL incubation/cells * 120 million cells/g liver * g liver/kg body wt. using the physiological parameters for liver weight of 25.7 g/kg body wt. for human, and 34 g/kg body wt. for rat and mouse (Brown et al. 1997).
To assess metabolite formation, [14C]NBBS (0.4μCi, 10 μM) was incubated in duplicate with rat, mouse and human hepatocytes from males as described above. At 300 min following incubation, 1mL acetonitrile was added to each sample, centrifuged, and the supernatant was analyzed by HPLC Method 2. Aliquots of supernatants were also assayed for radiochemical content by liquid scintillation spectroscopy (LSS) using a Packard 1900CA Tri-Carb Liquid Scintillation Analyzer (Perkin Elmer) to verify that the majority of the radioactivity was recovered in the supernatants.
To assess plasma protein binding, NBBS was added to HSD rat, B6C3F1/N mouse, and human plasma to a final concentration of 10 μM. Following addition of NBBS, 200 μL of plasma was placed into the sample chamber of RED device and 350 μL PBS buffer (containing 100 mM sodium phosphate and 150 mM sodium chloride) was added to the buffer chamber and incubated for 4h at 37°C. Fifty microliter aliquots from each chamber were removed; to each sample from the buffer chamber 5 μL of control plasma was added. Following addition of 0.3mL aqueous 90% aqueous acetonitrile/0.1% formic acid to all samples and mixing, they were centrifuged (13000 g, 10 min) and 0.1 mL supernatant was combined with 0.1 mL of 50% aqueous methanol for analysis by LC-MS Method 1. Percent free (unbound) was determined using peak area of NBBS in the buffer chamber divided by the peak area of NBBS in the plasma sample chamber multiplied by 100. Percent bound was determined by subtracting the percent free from 100.
Animals.
Studies were conducted at RTI International (RTP, NC) and were approved by Institutional Animal Care and Use Committee. Animals were housed in facilities that are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (Council 2011). Male and female Hsd:Sprague Dawley® SD® (HSD) rats, including the animals with closed loop bile catheters, were obtained from Harlan Laboratories (Dublin, VA). B6C3F1/N mice were obtained from Taconic Farms (Germantown, NY). The animals were quarantined for at least one week before they were randomized into dosing groups for the study. All animals had ad libitum access to certified, irradiated NTP 2000 feed (Ziegler Bros, Inc., Gardners, PA) and city (Durham, NC) tap water. Environmental conditions included: room temperature 72 ± 3°F (22 ± 2 °C), relative humidity 35 to 65% and a 12-h light/dark cycle. Animals were acclimated overnight in individual metabolism cages prior to dosing and were returned to cages following dosing to allow for separate collection of excreta. Animals were 8–10 weeks old at the time of dosing.
Formulation and administration of NBBS.
All dose formulations contained [14C]NBBS and unlabeled NBBS to achieve the final desired NBBS concentration and specific activity. The target radioactivity per animal was ~ 50 μCi/rat and 10 μCi/mouse. Oral dose formulations were prepared in 0.5% methylcellulose; a single dose was administered in a volume of 5 mL/kg for rat and 10 mL/kg for mouse by intragastric gavage. IV formulations were prepared in Cremophor®:ethanol:water (1:1:8, v/v/v); a single dose was administered in a volume of 2 mL/kg for rats and 4 mL/kg for mice into a lateral tail vein. The concentration of [14C]NBBS in the dose formulations was determined by liquid scintillation spectroscopy (LSS) analysis of weighed aliquots of formulations collected before, during, and after dosing for a total of five aliquots sampled on the day of dosing. Radiochemical purity of each formulation was assessed using HPLC Method 1 on the day of dosing and was ≥ 94%.
Collection and analysis of biological samples.
Urine and feces were collected into round-bottom flasks cooled over dry ice at 8 (urine only) and 24 h, and at 24-h intervals through study termination. Any urine present in the bladder at the time of necropsy was added to the final urine collection vessels. At the end of each 24 h excreta collection, the cages were rinsed with water and ethanol and the rinsate was stored separately from urine. Bile was collected from bile duct cannulated animals over the intervals 0–1, 1–3, 3–8, 8–12, and 12–24 h. At the termination of each study group, the animals were euthanized by asphyxiation with CO2 and blood was collected via cardiac puncture and added to tubes containing K2EDTA. Plasma was prepared by centrifugation at 2000 g for 10 min at 4 °C. The following organs and tissues were collected and weighed: liver, kidney, heart, lung, spleen, brain, bladder, thyroid, pancreas, testes or uterus and ovaries, small intestine, large intestine, cecum and stomach (all gastrointestinal (GI) tract tissues were collected without contents) and samples of muscle (hind leg), abdominal skin and adipose (perirenal). The contents from the GI tract were combined by animal. The GI tract tissues were rinsed with deionized water and the rinsate was collected separately. All samples were stored at −20 °C until analysis.
Feces were homogenized with an approximately equal amount of water. Triplicate feces and tissue aliquots were digested in 2 mL Soluene®−350; after digestion samples requiring bleaching were decolorized with 125 μL of 70% perchloric acid and 300 μL of hydrogen peroxide. GI tract tissues, GI contents, skin, and carcass were digested in 2N ethanolic NaOH. Triplicate aliquots of all samples (except urine, cage rinse, bile, and plasma where duplicate aliquots were used) were added to vials containing Ultima Gold™ scintillation cocktail. All samples were analyzed for radioactivity content by LSS with external standards quench correction.
A Debra™ data collection and reporting system (LabLogic Systems, Ltd., Sheffield, England) was used for calculation of all radiolabel disposition data. For determination of total [14C]NBBS in dispersed tissues, the total rat weight was assumed to be comprised of 7.4% blood, 4.3% plasma, 7.0% adipose, 40.4% muscle, and 19% skin and the total mouse weight was assumed to be comprised of 4.9% blood, 2.8% plasma, 7.0% adipose, 38.4% muscle, and 16.5% skin (Brown et al. 1997).
Metabolite profiling and identification.
Urine pools were created by combining a set percentage (5%) of each animal’s urine collection per time period and group. Pools were created for the time intervals 0–24, 24–48 or 48–72 h as appropriate, when greater than 5% of the radioactivity was recovered in urine. Urine pools were analyzed before and after deconjugation with β-glucuronidase, sulfatase, and acylase. In deconjugation experiments, equal volumes (200 μL) of urine and 0.1 M phosphate buffer (pH 7.4) (for glucuronidase and acylase) or 0.1 M Trizma buffer (pH 7.4) (for sulfatase) in amber glass vials were incubated at 37 °C for 6 h with one of the following: 4 μL of β-glucuronidase (19600 U/mL in water to a final enzyme content of 180 U), 40 μL of sulfatase solution (10 U sulfatase/mL in glycerol to a final enzyme content of 1 U) or 40 μL of acylase solution (18000 U/mL in 0.1 M phosphate buffer to a final enzyme content of 1637 U). Incubation controls were prepared as above except no enzyme was added. Each sample was filtered using an Ultrafree® filter 0.45 μm (Millipore, Billerica, MA) and profiled using HPLC Method 4. Pooled urine samples were also analyzed using LC-MS Methods 2 and 3 for metabolite identification.
Bile collected following administration of 200 mg/kg [14C]NBBS in male rats was filtered by Ultrafree®-MC 0.45 μm PVDF centrifugal filter and 25-μL aliquots were analyzed by HPLC Method 3. NBBS, benzenesulfonamide, and 4-hydroxybenzenesulfonamide standards were added to control bile and analyzed using the same method. Plasma collected 6 h following administration of 200 mg/kg [14C]NBBS was also analyzed by HPLC Method 3.
Results
In vitro studies.
Clearance of NBBS was investigated in male and female rat, mouse, and human hepatocytes. Blank matrix incubations conducted alongside the rat hepatocyte incubations and analyzed using the same methods contained about 2.6 ng/mL (0.012 μM) NBBS at all time points, which is likely due to background levels in the incubation system (plate, solvents, pipettes etc.). Half-life and clearance data are presented in Table S1. In rats, the clearance of NBBS in males (38 mL/(min*kg)) was slightly higher than in females (11 mL/(min*kg)); corresponding half-lives were 152 (male) and 252 min (female). In the mice, females (34 mL/(min*kg)) cleared NBBS faster than males (12 mL/(min*kg)) with half-lives of 110 (female) to 319 min (male). However, the clearance of NBBS in humans (~ 3 to 4 mL/(min*kg)) was much slower than in rodents with no apparent sex difference; corresponding half-lives were 909 to 947 min. For male human hepatocytes, data for a single replicate was shown due to no discernable clearance of NBBS in two replicates.
Metabolism was investigated in limited studies following incubation of [14C]NBBS in male mouse, rat, and human hepatocytes and representative radiochemical profiles are shown in Figure S1. NBBS (~ 30 min) and potential metabolite standards, benzenesulfonamide (~ 20 min) and 4-hydroxybenzenesulfonamide (~ 23 min), were chromatographed under the same condition to tentatively identify metabolites of NBBS in these incubations. In mouse hepatocytes, one metabolite (~ 24 min) containing 12% of the radioactivity in the chromatogram was observed and tentatively identified as a hydroxybenzenesulfonamide; parent NBBS comprises about 85% of the radioactivity in the chromatogram. In rat hepatocyte incubations, two metabolites were observed, one was similar to that observed in mouse hepatocytes (~ 24 min, 26% radioactivity) and the other one was eluting at the same retention time as benzenesulfonamide (~ 20 min, 16%). Human hepatocytes had little capacity to metabolize NBBS, with 95% of the radioactivity associated with NBBS (~ 30 min) after the 5 h incubation.
Plasma protein binding was investigated in rat, mouse, and human plasma. NBBS was highly bound to plasma protein with 80, 79, and 75% bound in rat, mouse, and human plasma, respectively.
Disposition of [14C]NBBS in rats.
Disposition of radioactivity 72 h following a single gavage administration in male (2, 20, and 200 mg/kg) and female (20 mg/kg) rats are shown in Table 2. In male rats, [14C]NBBS was well-absorbed following gavage administration and excreted mainly via urine with 70–76% of the administered dose recovered in urine (+ cage rinse) by 72 h (Table 2). The excretion was rapid with the majority of the dose recovered in urine within the first 24 h. Excretion in feces was low with 11–15% of the administered dose recovered within 72 h in male rats. There was no apparent sex difference in excretion following administration of 20 mg/kg [14C]NBBS in rats (males: urine, 75%; feces, 15%; females: urine, 83%; feces, 14%) (Table 2). The secretion of radioactivity in bile was investigated in male rats following a single gavage dose of 20 mg/kg; most of the radioactivity secreted in bile occurred in the first 12 h following dose administration with 25% of the administered dose secreted within 24 h.
Table 2.
Disposition of radioactivity following a single gavage administration of [14C]NBBS in male and female Hsd:Sprague Dawley® SD® ratsa
| Sample | Collection Interval (h) | Percent of dose | ||||
|---|---|---|---|---|---|---|
| 72h | 24h | |||||
| Male | Male | Male | Female | Male | ||
| 2 mg/kg | 20 mg/kg | 200 mg/kg | 20 mg/kg | 20 mg/kg | ||
| Urine | 0 – 8 | 22.0 ± 4.2 | 20.0 ± 4.5 | 15.4 ± 4.6 | 32.5 ± 10.5 | 18.3 ± 6.0 |
| 8 – 24 | 30.0 ± 4.4 | 27.8 ± 3.3 | 32.8 ± 4.8 | 25.6 ± 8.1 | 25.4 ± 6.9 | |
| 24 – 48 | 10.5 ± 1.7 | 8.56 ± 1.3 | 14.1 ± 1.6 | 3.9 ± 1.3 | - | |
| 48 – 72b | 3.2 ± 0.4 | 2.5 ± 0.2 | 5.9 ± 1.6 | 1.3 ± 0.2 | - | |
| Cage rinse | 0 – 8 | - | 9.9 ± 1.7 | - | 14.6 ± 2.8 | 9.4 ± 2.7 |
| 8 – 24 | - | 3.9 ± 0.4 | - | 3.3 ± 1.1 | 3.5 ± 1.2 | |
| 24 – 48 | - | 1.1 ± 0.4 | - | 0.8 ± 0.1 | - | |
| 48 – 72 | 4.6 ± 2.0 | 1.1 ± 0.4 | 7.9 ± 4.9c | 0.8 ± 0.5 | - | |
| Urine and cage rinse | Sub totald | 70.2 ± 1.6 | 74.8 ± 1.0 | 76.1 ± 2.6 | 82.8 ± 2.7 | 56.7 ± 3.3 |
| Feces | 0 – 24 | 7.7 ± 2.5 | 10.7 ± 1.7 | 4.5 ± 2.7 | 9.2 ± 4.8 | 14.3 ± 2.2 |
| 24 – 48 | 4.1 ± 1.1 | 3.3 ± 1.0 | 5.1 ± 0.4 | 3.8 ± 2.9 | - | |
| 48 – 72 | 0.9 ± 0.3 | 1.1 ± 0.1 | 1.6 ± 0.3 | 0.5 ± 0.2 | - | |
| Subtotal | 12.6 ± 1.3 | 15.0 ± 1.1 | 11.2 ± 2.8 | 13.5 ± 2.2 | 14.3 ± 2.2 | |
| GI contentse | 0.7 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.4 ± 0.1 | 5.4 ± 0.7 | |
| Tissuese | 7.6 ± 0.7 | 4.9 ± 0.4 | 5.2 ± 0.8 | 2.8 ± 0.1 | 13.8 ± 1.6 | |
| Total recovered | 91.1 ± 1.7 | 95.3 ± 0.7 | 93.1 ± 4.3 | 99.5 ± 0.7 | 90.2 ± 2.0 | |
Mean (±SD) for N= 4 animals.
Includes urine present in bladder at time of sacrifice.
0–72 h data is shown.
Total of urine plus cage rinse is shown.
Collected at study termination which is either 24 or 72 h.
Radioactivity was distributed to all tissues examined in male rats with the concentration increasing with the dose administered (Table 3); the total radioactivity in tissues at 72 h was 5–8% (Table 2). In male rats following gavage administration of 20 mg/kg, the concentration of radioactivity in tissues (Table 3) at 24 h was were much higher than at 72 h with 14 and 5% of the administered dose recovered, respectively (Table 2). At 24 h, the highest concentrations of [14C]NBBS-derived radioactivity were found in muscle, blood, skin, and liver (Table 3) with 4.4%, 3.1%, 1.9%, and 1.1%, respectively, of the total administered dose recovered at 24 h (data not shown).
Table 3.
Tissue concentration (ng-equivalents/g tissue) following a single gavage administration of [14C]NBBS to male and female Hsd:Sprague Dawley® SD® ratsa
| Tissue | Male, 2 mg/kg | Male, 20 mg/kg | Male, 200 mg/kg | Female, 20 mg/kg | Male, 20 mg/kg |
|---|---|---|---|---|---|
| 72 h | 72 h | 72 h | 72 h | 24 h | |
| Adipose Perirenal | 85.6 ± 21.6 | 445 ± 32.6 | 7220 ± 5540 | 212 ± 75.1 | 1290 ± 355 |
| Bladder | 285 ± 64.8 | 1160 ± 94 | 28800 ± 8550 | 411 ± 131 | 7560 ± 1760 |
| Muscle (hind leg) | 88.6 ± 21.1 | 575 ± 117 | 10100 ± 2700 | 281 ± 43.6 | 2160 ± 313 |
| Skin (abdominal) | 84.1 ± 28.8 | 631 ± 119 | 11600 ± 2930 | 297 ± 24.1 | 1940 ± 393 |
| Blood | 1230 ± 92.9 | 5030 ± 508 | 20100 ± 2350 | 3740 ± 173 | 8210 ± 681 |
| Plasma | 45 ± 1.91 | 302 ± 59 | 10500 ± 1950 | 143 ± 25.6 | 1900 ± 267 |
| Spleen | 360 ± 55.4 | 1840 ± 113 | 12200 ± 1600 | 992 ± 76.8 | 4540 ± 309 |
| Liver | 276 ± 47.5 | 1670 ± 241 | 18900 ± 3660 | 884 ± 74.5 | 4820 ± 698 |
| Kidney | 547 ± 30.9 | 3310 ± 546 | 36000 ± 5280 | 1830 ± 169 | 8260 ± 1070 |
| Brain | 208 ± 12.8 | 1210 ± 182 | 11900 ± 1850 | 658 ± 80 | 3170 ± 245 |
| Heart | 320 ± 29.7 | 1700 ± 255 | 13300 ± 2140 | 880 ± 102 | 3880 ± 420 |
| Lung | 475 ± 77.9 | 2000 ± 181 | 14200 ± 2980 | 1160 ± 143 | 4030 ± 485 |
| Stomach | 266 ± 17.6 | 1330 ± 135 | 13600 ± 2780 | 585 ± 342 | 4140 ± 550 |
| Cecum | 567 ± 114 | 3200 ± 942 | 19400 ± 5110 | 1120 ± 681 | 14200 ± 6000 |
| Large Intestine | 438 ± 134 | 1810 ± 395 | 15300 ± 2840 | 882 ± 529 | 7630 ± 1810 |
| Small Intestine | 193 ± 28.1 | 1130 ± 237 | 20200 ± 3140 | 484 ± 227 | 9980 ± 1420 |
| Thyroid | 225 ± 55.7 | 1110 ± 95.7 | 18800 ± 3760 | 790 ± 249 | 3100 ± 234 |
| Pancreas | 187 ± 46.7 | 1050 ± 184 | 13000 ± 2530 | 537 ± 60.8 | 3040 ± 672 |
| Testes | 75 ± 13.9 | 503 ± 84.2 | 10800 ± 1990 | NAb | 2020 ± 336 |
| Ovaries | NA | NA | NA | 526 ± 83.6 | NA |
| Uterus (with Fallopian tubes) | NA | NA | NA | 576 ± 138 | NA |
Mean (±SD) for N= 4 animals.
NA = not applicable
The pattern of disposition following IV administration of 20 mg/kg [14C]NBBS in male and female rats was similar to that following gavage administration of a similar dose; in males 80%, 15% and 5% and in females 77%, 11% and 3% of the administered dose was recovered in urine, feces and tissues, respectively (Tables S2 and S3). There was no apparent sex difference in disposition of [14C]NBBS-derived radioactivity following IV administration. The total administered dose recovered following gavage and IV administration of [14C]NBBS in male and female rats ranged from 90–101% (Tables 2 and S2).
Disposition of [14C]NBBS in mice.
Disposition of [14C]NBBS in mice was investigated using a limited design. Following gavage administration of 20 mg/kg, [14C]NBBS was well-absorbed in male and female mice and excreted mainly via urine (69–72%) and feces (9–11%) (Table 4). Excretion was rapid with 62–65% of the dose recovered in urine (+ cage rinse) by 24 h. Radioactivity was distributed to all tissues examined in male and female mice (Table 5); however, the total radioactivity in tissues at 72 h was low (0.1–0.2%) (Table 4). The pattern of disposition in mice following IV administration of 20 mg/kg [14C]NBBS was similar to that following gavage administration of a similar dose. In males 66%, 20%, and 0.1% and in females 69%, 19% and 0.1% were recovered in urine, feces, and tissues, respectively (Tables S2 and S3). There was no apparent sex difference in the disposition of [14C]NBBS-derived radioactivity following IV or gavage administration. The total administered dose recovered following gavage and IV administration of [14C]NBBS in male and female mice ranged from 80–89%.
Table 4.
Disposition of radioactivity 72 h following gavage administration of 20 mg/kg [14C]NBBS to male and female B6C3F1/N micea
| Sample | Collection Interval (h) | Percent of dose | |
|---|---|---|---|
| Male | Female | ||
| Urine | 0 – 8 | NDb | ND |
| 8 – 24 | 45.9 ± 24.4 | 25.6 ± 19.0 | |
| 24 – 48 | 2.7 ± 1.6 | 3.9 ± 2.4 | |
| 48 – 72c | 0.3 ± 0.1 | 1.2 ± 0.9 | |
| Cage rinse | 0 – 8 | 6.5 ± 12.9 | 24.8 ± 31.1 |
| 8 – 24 | 12.2 ± 8.8 | 11.8 ± 8.0 | |
| 24 – 48 | 1.3 ± 1.2 | 2.9 ± 1.8 | |
| 48 – 72 | 0.5 ± 0.6 | 1.3 ± 1.1 | |
| Urine and cage rinse | Sub totald | 69.2 ± 10.7 | 71.5 ± 1.6 |
| Feces | 0 – 24 | 9.8 ± 8.7 | 7.3 ± 5.4 |
| 24 – 48 | 0.6 ± 0.1 | 1.0 ± 0.3 | |
| 48 – 72 | 0.4 ± 0.7 | 0.5 ± 0.6 | |
| Subtotal | 10.7 ± 8.9 | 8.8 ± 5.2 | |
| GI contentse | 0.01 ± 0.0 | 0.0 ± 0.0 | |
| Tissuese | 0.1 ± 0.0 | 0.2 ± 0.0 | |
| Total recovered | 80.0 ± 5.2 | 80.5 ± 5.3 | |
Mean (±SD) for N= 4 animals.
ND, not detected.
Includes urine present in bladder at time of sacrifice.
Total of urine plus cage rinse is shown.
Collected at study termination which is either 24 or 72 h.
Table 5.
Tissue concentration (ng-equivalents/g tissue) 72 h following gavage administration of 20 mg/kg [14C]NBBS to male and female B6C3F1/N micea
| Tissue | Male | Female |
|---|---|---|
| Adipose Perirenal | NSb | 28.2 ± 16.4 |
| Bladder | 39.4 ± 37.1 | 26.0 ± 3.88 |
| Muscle (hind leg) | 14.3c | 12.8 ± 2.94 |
| Skin (abdominal) | 25.4 ± 4.85 | 43.6 ± 21.8 |
| Blood | 119 ± 25.7 | 156 ± 30.1 |
| Plasma | 10.0 ± 1.31 | 14.3 ± 3.55 |
| Spleen | 26.8 ± 4.53 | 44.5 ± 9.68 |
| Liver | 84.4 ± 13.7 | 113 ± 10.1 |
| Kidney | 320 ± 20.0 | 244 ± 11.6 |
| Brain | 15.0 ± 3.70 | 19.5 ± 4.15 |
| Heart | 26.2 ± 10.1 | 27.3 ± 5.10 |
| Lung | 56.0 ± 14.8 | 68.5 ± 11.7 |
| Stomach | 55.5 ± 31.7 | 28.9 ± 1.21 |
| Cecum | 402 ± 237 | 282 ± 148 |
| Large Intestine | 104 ± 59.0 | 133 ± 81.1 |
| Small Intestine | 23.9 ± 11.8 | 29.2 ± 15.6 |
| Thyroid | 326c | 157c |
| Pancreas | 27.3 ± 10.7 | 31.7 ± 6.24 |
| Testes | 8.88 ± 2.56 | NA |
| Ovaries | NA | 49.7 ± NA |
| Uterus (with Fallopian tubes) | NA | 36.4 ± 18.5 |
Mean (±SD) for N= 4.
NS, no sample, NA= not applicable.
Data from N=1
Profiling and identification of NBBS metabolites in rats and mice.
Radiochemical profiles of urine showed numerous peaks and were generally similar across dose groups, species, sexes, and routes (data not shown); a representative chromatogram of male rat urine following administration of a 200 mg/kg gavage dose is shown in Figure 2A. Under these chromatographic conditions, the retention times of NBBS, and two potential metabolites, benzenesulfonamide, and 4-hydroxybenzenesulfonamide, were ~ 35, ~21 and ~9 min, respectively. Upon treatment of urine with β-glucuronidase, peaks eluting at retention times ~ 12, ~ 21, and ~ 25 min were reduced with concomitant increase in peaks at the retention times ~ 28 and 29 min suggesting the presence of glucuronide conjugates (Figure 2B). The treatment with acylase slightly increased the peak at ~ 21 min with concomitant reduction in peaks at ~ 28 and 29 min suggesting the presence of a glycine conjugate (Figure 2C). Incubation of urine with sulfatase had little or no effect on the metabolite profile (Figure 2D). Profiling of bile following administration of 200 mg/kg [14C]NBBS to male rats showed one major peak, eluting near the retention time of benzenesulfonamide, and up to three less prominent peaks eluting between 20 and 25 min (chromatogram not shown).
Figure 2.
HPLC radiochromatograms of urine (0–24 h) following gavage administration of 200 mg/kg NBBS in male rats A) undigested urine B) following β-glucuronidase digestion C) following acylase digestion D) following sulfatase digestion
Identification of metabolites in urine was achieved using high resolution mass spectrometry in both positive and negative ion modes. Potential metabolites, retention times, molecular formulae, expected and found masses, as well as mass error are given in Table S4. Based on these analyses, the proposed metabolic pathway of NBBS in rodent is shown in Figure 3.
Figure 3.
Proposed metabolism of NBBS in rodents
In the positive ion mode, four peaks with molecular ions at m/z 406.1154 (M+H+) and retention times ~23 to 27 min were identified as glucuronides of hydroxylated NBBS (Table S4). In extracted ion chromatograms, peaks corresponding to hydroxylated NBBS (m/z 230.08, Figure 4B) were observed at the same retention times and with the same peak shape as the glucuronides of hydroxylated NBBS (m/z 406.12, Figure 4A) and are presumed to arise from in-source fragmentation of the glucuronides. The mass spectrum (Figures 4C) for the peak at 24 min is shown as an example, with detection of the M+Na+ adduct ion at 428.0966, M+H+ at 406.1553, and fragment ions at 230.0837, consistent with the loss of the glucuronide, and 212.0733 consistent with loss of the OH group. MS/MS spectrum of the ion at m/z 230 showed ions at m/z 212.0733–212.0737 indicating loss of OH group (Figure 4D). Additional fragments at m/z 170.0265–170.0267 indicated the presence of an unmodified ring, a sulfonamide and an intact alpha carbon. Furthermore, the presence of an ion at m/z 141.000 indicated that ring hydroxylation did not occur (Figure 4D). N-hydroxylation would also yield a metabolite with m/z 230 with fragments at m/z 141 and 172; no ions matching the expected pattern for N-hydroxylation were observed in the hydroxylated metabolites. Based on these data, phase 1 metabolism likely leads to the formation of four hydroxylated NBBS metabolites with the hydroxylation on the butyl side chain (Table S4, Figure 3) which subsequently underwent phase 2 metabolism to form corresponding glucuronides representing peaks ~ 23 to 27 min. An additional peak ~ 26.7 min was observed suggesting the presence of the fifth hydroxylated metabolite and subsequently its glucuronide. These hydroxylated metabolites were assigned metabolites 1–5 and corresponding glucuronides are assigned metabolite 8 (Figure 3).
Figure 4.
Extracted positive ion chromatograms and mass spectra of glucuronides of hydroxylated NBBS in urine from male rats administered 200 mg/kg NBBS by gavage: A) Extracted ion chromatogram of hydroxylated glucuronide of NBBS ([M+H]+, m/z 406.1–406.2); B) Extracted ion chromatogram of [M+H minus glucuronide]+ (m/z 230.0 – 230.1); C) Mass spectrum of peak at ~24 min; D) MS/MS spectrum of m/z 230 ion at ~ 24 min
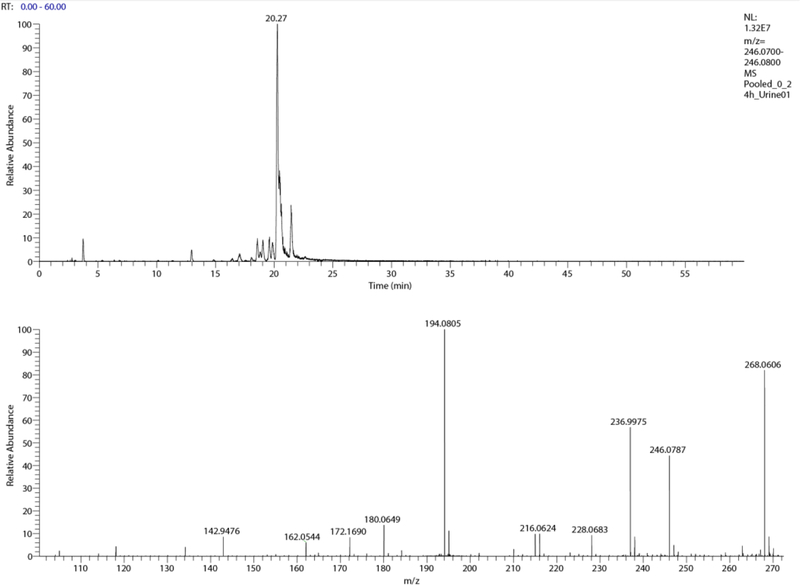
A peak with ions of m/z 244.06 ([M+H]+) and m/z 266.04 ([M+Na]+) consistent with a doubly oxidized and reduced metabolite of NBBS (m/z 213) was observed at ~ 27.4 min; it is probable that this metabolite is the carboxylic acid, 4-[(phenylsulfonyl)amino]butanoic acid, formed by oxidation of the terminal carbon on the butyl group (metabolite 6, Figure 3); the corresponding extracted ion chromatogram and spectrum are shown in Figure 5, with a fragment ion at 226.05 consistent with loss of water. The peak at ~ 29 min with a m/z 228.0684 ([M+H]+) gave a mass spectrum (not shown) consistent with an oxidization and desaturation of NBBS, and hence is potentially an oxobutyl metabolite, 3-oxobutylbenzenesulfonamide (metabolite 7, Table S4, Figure 3). The peak at ~ 20 min gave ions consistent with dihydroxylation of NBBS; the mass spectrum contained ions at m/z 246.0787 ([M+H+]) and 268.0606 ([M+Na+]), loss of OH (228.0683), (Figure 6). This metabolite is depicted as 4-(phenylsulfonyl)-1,2-butanediol (metabolite 9, Table S4, Figure 3).
Figure 5.
Extracted positive ion chromatogram (m/z 266.04 – 266.05, top) and mass spectrum of 4-[(phenylsulfonyl)amino]butanoic acid (bottom) in urine from male rats administered 200 mg/kg NBBS by gavage
Figure 6.
Extracted positive ion chromatogram (m/z 246.07 – 246.08, top) and mass spectrum of (phenylsulfonyl)-1,2-butanediol (bottom) in urine from male rats administered 200 mg/kg NBBS by gavage
In negative ion mode, NBBS was detected with peak at ~ 31 min, and an ion at m/z 212.0761 (([M-H]-) (Table S4). A peak at ~ 26 min with a molecular ion of m/z 156.0138 (Figure 7) was confirmed as benzenesulfonamide using the retention time and mass spectrum of an authentic standard (metabolite 5, Table S4, Figure 3). A metabolite consistent with the oxidation and β-oxidation of the butane side chain was also detected (spectrum not shown); the spectrum and the retention time matched with a standard of phenylsulfonylglycine (metabolite 10, Table S4, Figure 3).
Figure 7.
Extracted ion chromatogram (m/z 156.01 – 156.02) and mass spectrum of benzene sulfonamide in urine from male rats administered 200 mg/kg NBBS by gavage
Discussion
Here we report the ADME data for NBBS in male and female rats and mice following gavage and IV administration. To the best of our knowledge, this is the first comprehensive study investigating the disposition, metabolism, and mass balance of [14C]NBBS in rodents. [14C]NBBS was well-absorbed following gavage administration in rats and mice and excreted rapidly and extensively via urine. Following gavage administration, 70–76% and 83% of the dose was excreted in urine of male and female rats, respectively (Table 2), with no dose- or sex-related difference in urinary excretion. Approximately 11–15% of the administered dose was excreted in feces which could be from either the absorbed or unabsorbed dose. Distinguishing between the unabsorbed dose and the dose potentially secreted to the intestine via bile and subsequently excreted in feces is an important consideration. In bile duct cannulated rats, 25% of an orally administered 200 mg/kg dose was excreted via bile in 24 h providing evidence that the observed fecal excretion following gavage administration was due to biliary excretion and not the unabsorbed dose. The lower percent of dose excreted in 72 h feces (11%) compared to that excreted in 24 h bile (25%) following administration of a similar dose in male rats suggests that the radioactivity secreted in bile may undergo enterohepatic recirculation and some of it is subsequently excreted via feces. These observations, together with high fecal excretion in rats following IV administration (11–15%), support the conclusion that NBBS was completely absorbed following gavage administration. As in rats, NBBS was well absorbed in male and female mice following gavage administration and excreted mainly via urine (69–72%) and feces (9–11%) with no apparent sex or species difference (Table 4).
[14C]NBBS-derived radioactivity was distributed to all tissues examined in both rats and mice of both sexes following gavage administration. The total radioactivity remaining in tissues following a single 20 mg/kg gavage dose in male rats at 24 h was 14% demonstrating significant tissue distribution (Table 2). The total administered dose recovered in tissues at 72 h was 5% following administration of a similar dose demonstrating elimination of radioactivity from tissues over time. There was no dose-related difference in total tissue radioactivity. recovered. The percent total radioactivity remaining in tissues at 72 h (5%) following a single gavage administration of [14C]NBBS in rats was higher than those observed previously in mass balance studies with other xenobiotics such as hydroquinone and sulfolane (1–2%) (Black et al. 2018; Waidyanatha et al. 2018). Unlike in rats, the percent radioactivity remaining in mice at 72 h following a gavage dose of 20 mg/kg [14C]NBBS was <0.1% (Table 4) demonstrating a significant species difference in tissue retention.
In all dose groups, the radioactivity in blood was highly associated with RBCs (Tables 3 and 5) and could be either non-covalently or covalently bound with proteins. For example, in male rats following a gavage dose of 2 and 20 mg/kg, >95% of blood radioactivity was associated with the RBCs at 72 h and represented about 2% of the administered dose (data not shown). However, at 200 mg/kg, 58% of the radioactivity in blood was associated with RBCs at 72 h and represented about 0.7% of the dose administered, indicating that retention of radioactivity in RBCs is likely capacity-limited. Radioactivity in RBCs from male rats administered a 200 mg/kg gavage dose was extractable, indicating that the NBBS-derived products are not covalently bound (data not shown). Treatment of blood from mice administered a 20 mg/kg gavage dose with the carbonic anhydrase inhibitor, acetazolamide, displaced radioactivity from RBCs (data not shown). This suggests that NBBS-derived products in RBCs are likely associated with carbonic anhydrases and therefore likely contain an -SO2NH2 group per the well-established structure activity relationships known for sulfonamides that bind strongly to that enzyme (Supuran et al. 2003). In vitro plasma protein binding showed that NBBS was highly bound to plasma protein (75–80%) which is in reasonable agreement to the 70% bound to rat serum as reported by Kumar et al. (2007).
Urinary radiochemical profiles of NBBS were complex with up to 14 peaks detected. In general, profiles were similar across doses, species, sexes, and routes. Enzyme deconjugation experiments suggested the presence of glucuronide and glycine conjugates. Using a combination of mass spectrometric analyses, numerous metabolites were identified representing hydroxylation and glucuronidation and subsequent oxidation to a ketone and several carboxylic acids (Figure 3). The presence of 4 glucuronides of hydroxylated metabolites was confirmed with a fifth one possible; whether these are isomers or a mixture of isomers and diastereoisomers, resulting from an introduction of a chiral center upon hydroxylation, is not confirmed. A doubly oxidized and desaturated metabolite, consistent with the formation of a carboxylic acid, was detected, identified as 4-[(phenylsulfonyl)amino]butanoic acid; β-oxidation of this compound would give rise to the observed phenylsulfonyl glycine. This is consistent with the presence of a glycine metabolite confirmed during deconjugation experiments. The detection of benzenesulfonamide as a metabolite in hepatocytes, in extracted red blood cells in vivo (data not shown), and in urine, suggests the hydroxylation of the butyl group on the carbon α to the NH group occurs, followed by dealkylation.
In order to gauge the disposition of NBBS in humans, metabolism and clearance of NBBS by hepatocytes was investigated in male mouse, rat, and human cryopreserved cells. Of the three species, rat showed the most extensive clearance and metabolism. Human hepatocytes showed the lowest clearance and capacity to metabolize NBBS, with 95% of parent remaining at the end of the incubation suggesting that hepatic disposition of NBBS may be different from that of rodents. As mentioned previously, studies are ongoing at NTP to evaluate the potential toxicity of NBBS following perinatal (rat) or adult (mouse) exposure (https://ntp.niehs.nih.gov/testing/status/agents/ts-10057.html). In order to determine the applicability of the rodent toxicology study data for human safety assessment, we have also investigated the TK and systemic exposure of NBBS in rodents following oral (gavage and feed) administration and will be published elsewhere (Waidyanatha et al., 2019).
Consistent with the background levels of NBBS reported in the literature, background levels of NBBS were observed during incubation of NBBS with hepatocytes as indicated above demonstrating potential ubiquitous presence of NBBS in the environment. Experiments are underway to determine the sources and levels of NBBS background in humans and will be published in a subsequent publication.
Conclusion
Our data demonstrated that NBBS was completely absorbed in male and female rats and mice following a single gavage administration and excreted extensively via urine. It was well-distributed to tissues with some tissue retention. NBBS was highly metabolized in rodents with numerous phase 1 and phase 2 metabolites. There was no apparent dose, species or sex difference in excretion and metabolism of NBBS, although retention in tissues was higher in rats compared with mice. Although the clearance of NBBS in rodent hepatocytes was moderate, NBBS was cleared slowly in human hepatocytes.
Supplementary Material
Figure 1.
HPLC radiochemical profiles following incubation of 10 μM [14C]NBBS with mouse (top), rat (middle) and human (bottom) hepatocytes
Acknowledgements
The authors are grateful to Drs Madelyn Huang and Jason Stanko for their review of this manuscript. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316–04, and performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract HHSN29120077563 (RTI International, RTP, NC).
Footnotes
Declaration of Interest
The authors report no declarations of interest.
References
- Black SR, Fennell TR, Mathews JM, Snyder RW, Patel PR, Watson SL, Sutherland V, Waidyanatha S. (2018). Disposition of [C-14]hydroquinone in Harlan Sprague-Dawley rats and B6C3F1/N mice: species and route comparison. Xenobiotica, 48, 1128–41. [DOI] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. (1997). Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health, 13, 407–84. [DOI] [PubMed] [Google Scholar]
- Council NR, (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. [Google Scholar]
- Di Carro M, Magi E, Massa F, Castellano M, Mirasole C, Tanwar S, Olivari E, Povero P. (2018). Untargeted approach for the evaluation of anthropic impact on the sheltered marine area of Portofino (Italy). Mar Pollut Bull, 131, 87–94. [DOI] [PubMed] [Google Scholar]
- Dsikowitzky L, Schwarzbauer J, Kronimus A, Littke R. (2004a). The anthropogenic contribution to the organic load of the Lippe River (Germany). Part 1: qualitative characterisation of low-molecular weight organic compounds. Chemosphere, 57, 1275–88. [DOI] [PubMed] [Google Scholar]
- Dsikowitzky L, Schwarzbauer J, Littke R. (2004b). The anthropogenic contribution to the organic load of the Lippe River (Germany). Part II: quantification of specific organic contaminants. Chemosphere, 57, 1289–300. [DOI] [PubMed] [Google Scholar]
- Duffield P, Bourne D, Tan K, Garruto RM, Duncan MW. (1994). Analysis of the neurotoxic plasticizer n-butylbenzenesulfonamide by gas chromatography combined with accurate mass selected ion monitoring. J Anal Toxicol, 18, 361–8. [DOI] [PubMed] [Google Scholar]
- Grigoriadou A, Schwarzbauer J, Georgakopoulos A. (2008). Molecular indicators for pollution source identification in marine and terrestrial water of the industrial area of Kavala city, North Greece. Environ Pollut, 151, 231–42. [DOI] [PubMed] [Google Scholar]
- Huppert N, Wurtele M, Hahn HH. (1998). Determination of the plasticizer N-butylbenzenesulfonamide and the pharmaceutical Ibuprofen in wastewater using solid phase microextraction (SPME). Fresen J Anal Chem, 362, 529–36. [Google Scholar]
- Komakech R, Kang YM, Lee JH, Omujal F. (2017). A Review of the Potential of Phytochemicals from Prunus africana (Hook f.) Kalkman Stem Bark for Chemoprevention and Chemotherapy of Prostate Cancer. Evid-Based Compl Alt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Smith QR, Hokari M, Parepally J, Duncan MW. (2007). Brain uptake, pharmacokinetics, and tissue distribution in the rat of neurotoxic N-butylbenzenesulfonamide. Toxicol Sci, 97, 253–64. [DOI] [PubMed] [Google Scholar]
- Lee WY, Hwang SJ, Cho DH, Kim JS. (1995). Behavioral changes with alterations of choline acetyltransferase immunoreactivities induced by N-butyl benzenesulfonamide. Vet Hum Toxicol, 37, 537–42. [PubMed] [Google Scholar]
- Manamsa K, Crane E, Stuart M, Talbot J, Lapworth D, Hart A. (2016). A national-scale assessment of micro-organic contaminants in groundwater of England and Wales. Sci Total Environ, 568, 712–26. [DOI] [PubMed] [Google Scholar]
- Marrocco A, Meade BJ, Long CM, Lukomska E, Marshall NB, Anderson SE. (2015). Investigations into the Immunotoxicity and Allergic Potential Induced by Topical Application of N-Butylbenzenesulfonamide (Nbbs) in a Murine Model. J Toxicol Env Heal A, 78, 1122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP, 2010. Chemical information review document for N-butylbenznesulfonamide [CAS No. 3622–84-2]. Research Triangle Park: National Toxicology Program; Available at: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/n_butylbenzensulfonamide_508.pdf. [Google Scholar]
- Oros DR, Jarman WM, Lowe T, David N, Lowe S, Davis JA. (2003). Surveillance for previously unmonitored organic contaminants in the San Francisco Estuary. Mar Pollut Bull, 46, 1102–10. [DOI] [PubMed] [Google Scholar]
- Papaioannou M, Schleich S, Roell D, Schubert U, Tanner T, Claessens F, Matusch R (2010). NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth. Investigational New Drugs, 28, 729–743. [DOI] [PubMed] [Google Scholar]
- Pedersen JA, Soliman M, Suffet IH. (2005). Human Pharmaceuticals, Hormones, and Personal Care Product Ingredients in Runoff from Agricultural Fields Irrigated with Treated Wastewater. J Agric Food Chem, 53, 1625–32. [DOI] [PubMed] [Google Scholar]
- Plumlee MH, Gurr CJ, Reinhard M. (2012). Recycled water for stream flow augmentation: Benefits, challenges, and the presence of wastewater-derived organic compounds. Sci Total Environ, 438, 541–8. [DOI] [PubMed] [Google Scholar]
- Rider CV, Janardhan KS, Rao D, Morrison JP, McPherson CA, Harry GJ. (2012). Evaluation of N-butylbenzenesulfonamide (NBBS) neurotoxicity in Sprague-Dawley male rats following 27-day oral exposure. Neurotoxicology, 33, 1528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjevrak I, Brede C, Steffensen I-L, Mikalsen A, Alexander J, Fjeldal P, Herikstad H. (2005). Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment. Food Addit Contam, 22, 1012–22. [DOI] [PubMed] [Google Scholar]
- Soliman MA, Pedersen JA, Park H, Castaneda-Jimenez A, Stenstrom MK, Suffet IH. (2007). Human pharmaceuticals, antioxidants, and plasticizers in wastewater treatment plant and water reclamation plant effluents. Water Environ Res, 79, 156–67. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Garruto RM, Wolff AV, Chou SM, Fox SD, Yanagihara R. (1991). N-butyl benzenesulfonamide: a neurotoxic plasticizer inducing a spastic myelopathy in rabbits. Acta Neuropathol, 81, 235–41. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Garruto RM, Wolff AV, Yanagihara R, Chou SM, Fox SD. (1990). N-butylbenzenesulphonamide, a novel neurotoxic plasticising agent. Lancet, 336, 640. [DOI] [PubMed] [Google Scholar]
- Supuran CT, Scozzafava A, Casini A. (2003). Carbonic anhydrase inhibitors. Med Res Rev, 23, 146–89. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, Black SR, Snyder RW, Yueh YL, Sutherland V, Patel PR, Watson SL, Fennell TR. (2018). Disposition and metabolism of the bisphenol analogue, bisphenol S, in Harlan Sprague Dawley rats and B6C3F1/N mice and in vitro in hepatocytes from rats, mice, and humans. Toxicol Appl Pharm, 351, 32–45. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, South N, Gibbs S , Smith JP, Mutlu E, Burback B, Rider CV (2019). Toxicokinetics of N-butylbenzenesulfonamide, in plasma and brain following exposure in rodents; route, species, and sex comparison Toxicol Appl Pharm, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ III, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS (2012) Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicological Sciences, 125, 157–174. [DOI] [PubMed] [Google Scholar]
- Wypych G, (2017). Plasticizer Types. In: Wypych G, ed. Handbook of Plasticizers, 3rd Edition Toronto: ChemTec Publishing; 7–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.