Abstract
BACKGROUND:
The optimal approach for reducing iron depletion (ID) in blood donors may vary depending on biologic or behavioral differences across donors.
STUDY DESIGN AND METHODS:
More than 12,600 successful whole blood donors were enrolled from four US blood centers for ferritin testing. The study population was enriched for racial/ethnic minorities (1605 African American, 1616 Asian, 1023 Hispanic). Subjects completed questionnaires on ID risk factors. Logistic regression identified predictors of absent iron stores (AIS; ferritin <12 ng/mL) and low ferritin (LF; ferritin <26 ng/mL).
RESULTS:
Across all subjects, 19% had AIS and 42% had LF, with a sharp increase in risk observed with increasing donation intensity and among women a large decrease in risk in those more than 50 years old. When other factors were controlled for, African American and Asian donors showed 20% to 25% decreased risk for AIS compared to non-Hispanic Caucasian donors, while Hispanic donors had 25% higher risk. Daily iron supplementation reduced risk for LF and AIS by 30% to 40%, respectively, while the benefit from less frequent use was lower (7%-19% protection). Regular antacid use was associated with at least 20% increment to risk. Use of oral contraceptives or estrogen in females reduced risk by 16% to 22%, while males who reported supplemental testosterone use had a 50% to 125% greater risk for LF and AIS.
CONCLUSIONS:
This study confirms high prevalence of LF and AIS in US donors and the principal risk factors of age, sex, and donation frequency. Additional demographic and behavioral risk factors of secondary importance might allow for refinement of ID mitigation strategies.
Iron depletion (ID) is common in blood donors, with estimated prevalence of intermediate ID (ferritin <26 ng/mL) of 33% to 40% in United States and Canadian donors.1,2 Other jurisdictions have also found high rates of ID in their donor populations.3–5 No US blood center uniformly tests the iron status of all donors and little systematic testing is performed elsewhere,6,7 but donor age, sex, and donation frequency are routinely documented risk factors for ID.2–5,8–11 Iron deficiency, particularly with concurrent anemia, has been associated with a number of adverse outcomes, including cognitive dysfunction, fatigue, decreased exercise endurance, pica, and pregnancy-related complications.12 This study identifies similarities and differences in risk for ID in a largely unselected, racially diverse US blood donor population.
MATERIALS AND METHODS
Subject recruitment and enrollment
Subjects were a subset of donors enrolled in the REDS-III RBC-Omics study designed to examine the genetic and metabolic basis of blood donor susceptibility to ID, iron-related symptoms, and red blood cell (RBC) hemolysis at end of storage.13 The parent RBC-Omics study was enriched for racial/ethnic minorities and for high-intensity donors who gave at least 9 RBC units in 2 years without a hemoglobin (Hb) deferral. Target enrollment was 2000 each African American, Asian, Hispanic, and high-intensity donors and 6000 non-Hispanic white (NHW) donors.
Each of four REDS-III blood centers tailored recruitment strategies to maximize efficiency, subject to the same eligibility criteria: signed informed consent, successful whole blood donation (minimum Hb level of 12.5 g/dL for male and female subjects), age at least 18 years old, and willingness to consider being recalled for another blood draw. No additional blood volume was drawn, nor was additional phlebotomy or fingerstick required for enrollment. At enrollment, participants self-reported race/ethnicity and completed a questionnaire on factors that could affect iron status (iron supplementation, menstrual status, antacid use, exogenous hormone use, and smoking) and on symptoms potentially associated with ID (pica, restless leg syndrome). Donation history was sourced from blood center operational databases for statistical modeling.
Ferritin testing
Samples for ferritin testing came from EDTA retention tubes, which were centrifuged and plasma separated and frozen within 5 days of collection.14 Ferritin testing was performed on a chemistry analyzer (Model AU680, Beckman-Coulter; linear range, 8-450 ng/mL). “Low ferritin” (LF) was defined as a ferritin level of less than 26 ng/mL, and “absent iron stores” (AIS) as ferritin levels of less than 12 ng/mL, with LF inclusive of AIS (e.g., they are overlapping categories).8
Statistical analysis
The primary objective of this study was to characterize the iron status of a racially/ethnically diverse blood donor population. Qualitative comparisons of iron status were made by summarizing the average ferritin value in first-time and “reactivated” (no donations in prior 2 years) donors, whose ferritin levels would not be strongly influenced by previous blood donation.8 Median and interquartile range (IQR) ferritin values were stratified by race/ethnicity, sex, and age, and the proportion with LF was determined. No statistical tests were performed for these initial assessments.
The prevalence of LF and AIS was summarized for all donors in each race/ethnicity group, and logistic regression was used to develop unadjusted odds ratios (ORs) for demographic (age, sex, weight), donation (donations in past 2 years and interval since last donation), and behavioral characteristics (iron supplementation, smoking, use of antacids, and use of hormones). Adjusted ORs were derived with multivariable logistic regression. Models stratified by race/ ethnicity allowed for comparison of demographic factors and donation intensity across race/ethnicity. Models for NHW donors were run both with and without the white high-intensity donors; after it was determined that the model results were unchanged with high-intensity donors included, the remaining models retained them. Model parameters from the stratified analyses allowed for estimates of the prevalence of LF and AIS by sex and age group within each race/ethnicity group as well as the amount of unexplained variation (“residuals”) remaining by age and sex after accounting for donation frequency, body weight, use of supplemental iron, and blood center. A model including all subjects allowed for formal statistical assessments of differences by race/ethnicity as well as inclusion of behavioral factors not routinely collected from blood donors. Data analysis took place with computer software (SAS/STAT software, Version 9.3, of the SAS System for Windows, SAS Institute).
RESULTS
Study population
Of 13,323 subjects enrolled in RBC-Omics with ferritin measured, 640 reported American Indian, Native Hawaiian, or multiple race and were excluded from this analysis. Less than 1% of participants were excluded from the analysis for lack of ferritin value.13 The remaining 12,683 participants in the analysis included 1605 African Americans, 1616 Asians, 1023 Hispanic donors of Caucasian race, and 8439 NHW (1959 high-intensity and 6480 unselected donors). Table S1 (available as supporting information in the online version of this paper) shows the distributions of demographic and behavioral factors by race/ethnicity groups. As expected, the NHW group, which included high-intensity donors, were more likely to be older and to donate more frequently than the other groups. Female donors were more likely to take iron supplementation than male donors whereas African Americans and Hispanics were more likely to report smoking than Asians and NHW donors (Table S1).
Among subjects with no recent donations, females and the youngest donors have lower iron stores
In first-time donors and reactivated donors with no donations in 2 years, LF and AIS were examined to assess ferritin levels uninfluenced by blood donation. The proportion of first-time and reactivated donors was greater in the three minority race/ethnicity groups (37%-55%) compared to NHW donors (17%-22% overall, 25% excluding high-intensity donors). The median and IQR of ferritin values by age, sex, and race/ethnicity (Table 1) mirrored patterns observed in previous population studies:2–5,8–10,15,16 males with higher values than females, younger subjects with lower values than older subjects in both sexes, and higher values for females older than age 50 across all race/ethnicities. In contrast, peak median values for male subjects varied by race/ethnicity, occurring in age group 20 to 29 in Asian males and at ages 50 to 59 in African American males. A decline in median ferritin values was observed in all racial/ethnic groups in older males. The proportion of female donors with LF was most pronounced in the youngest age group, varying from 45% to 65% in Asian and African American females less than 20 years old, respectively. The prevalence of LF declined after age 50 across all race/ethnicities in females. In male donors, prevalence of LF was less than 10% in most age strata, with an increased prevalence after 50 observed for all race/ethnicity groups (Table 2).
TABLE 1.
Ferritin levels by age, sex, and race/ethnicity of first-time and “reactivated” donors*
| Donation history and age | African American (n = 1605) |
Asian (n = 1616) |
Hispanic (n = 1023) |
NHW (n = 8439) |
||||
|---|---|---|---|---|---|---|---|---|
| Female (n = 862) | Male (n = 743) | Female (n = 735) | Male (n = 881) | Female (n = 614) | Male (n = 409) | Female (n = 4141) | Male (n = 4298) | |
| FTD† | ||||||||
| Number | 397 | 278 | 404 | 453 | 297 | 179 | 919 | 747 |
| Percent | 46 | 37 | 55 | 51 | 48 | 44 | 22 | 17 |
| Age group (years) | ||||||||
| 18-19 | 23 (10-38) | 64 (51-87) | 27 (17-53) | 99 (64-143) | 15 (6-32) | 75 (55-107) | 24 (11-38) | 69 (38.5-104) |
| 20-29 | 24 (13-54) | 87.5 (56-147) | 34 (16-66) | 128 (74-191) | 26.5 (15-50) | 87 (63-143) | 30 (17-49) | 99 (63-146) |
| 30-39 | 44 (25-81) | 123 (70-178) | 48.5 (25-73) | 93 (57-194) | 23.7 (13-43) | 144 (81-206) | 36 (21-62) | 103.5 (58-159) |
| 40-49 | 42.5 (19-83) | 117 (69-198) | 35 (20-64) | 127.5 (58-239) | 37 (14-58) | 141 (70-212) | 36.5 (19-62) | 121 (67-171) |
| 50-59 | 73 (48-103) | 149.5 (65-235) | 56 (43-82) | 93 (44-178) | 41.5 (29-82) | 54 (40-141) | 45 (26-91) | 95 (45-172) |
| 60+ | 66 (51-116) | 113 (52-177) | 63.5 (46-132) | 61 (39-145) | 39 (35-99) | 30.5 (15-60) | 56 (37-99) | 75 (39146) |
Data are reported as median (IQR).
FTD = first-time donors and includes “reactivated” donors who are repeat donors with no donations in the prior 2 years.
TABLE 2.
Prevalence of ferritin levels of less than 26 ng/mL by age, sex, and race/ethnicity of first-time and “reactivated” donors (%)*
| Age group (years) | African American |
Asian |
Hispanic |
NHW |
||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| 18-19 | 54.6 | 12.1 | 45.3 | 3.2 | 64.7 | 9.5 | 55.9 | 2.9 |
| 20-29 | 51.2 | 5.2 | 38.4 | 2.7 | 49.1 | 2.7 | 40.2 | 6.1 |
| 30-39 | 26.0 | 0.0 | 25.5 | 5.5 | 52.9 | 5.4 | 30.9 | 4.6 |
| 40-49 | 32.4 | 2.6 | 33.9 | 2.6 | 38.9 | 3.3 | 37.3 | 7.1 |
| 50-59 | 10.9 | 4.8 | 8.7 | 9.4 | 15.0 | 7.7† | 24.3 | 11.7 |
| 60+ | 9.7% | 9.1% | 21.4%† | 13.3%† | 14.3%† | 50.0%† | 17.5% | 14.5% |
Ferritin level of less than 12 ng/mL not shown due to its scarcity in male donors with no recent donation history.
Fewer than 20 subjects for the reported combination of race/ethnicity, sex, and age.
Prevalence of ID varies by race/ethnicity and is higher in Hispanic donors
Across all participants, the prevalence of AIS ranged from 20% in African American females to 30% in Hispanic females (Table S2), and from 4.4% in African American males to 16% in NHW males (10% excluding the high-intensity donors, whose high donation frequency was associated with high frequency of AIS and LF). The prevalence of LF ranged from 43% in African American and Asian females to 59% in Hispanic females and from 16% in Asian males to 38% in all NHW males (27% excluding high-intensity males). The aggregate prevalences of AIS and LF were 19 and 42%, respectively (17 and 38%, respectively, excluding high-intensity donors).
Unadjusted estimates indicate higher ID risk for females, younger donors, those with higher donation count and with shorter donation intervals
Unadjusted ORs for LF and AIS, stratified by race/ethnicity, identified much higher risk for females than males, higher risk in 18- to 19-year-olds, a strong increase in risk with higher donation frequency, and shorter interval since one’s last donation (Table S2, available as supporting information in the online version of this paper). Supplemental iron use showed little association with LF and AIS, whether for daily or less frequent consumption. In contrast, subjects who reported having smoked in the past 30 days, altogether representing less than 10% of subjects, had unadjusted ORs for AIS and LF between 0.4 and 0.7. Regular or occasional use of any of 10 listed prescription antacids, reported by 14% of subjects, showed no association with iron status.
Multivariable analysis confirms similar patterns of risk across race/ethnicities, indicates that race/ethnicity is an independent predictor of iron status, and allows for evaluation of other risk factors
Multivariable logistic regression, stratified by race/ethnicity, provides a qualitative comparison of the association between demographic and behavioral variables and risk for ID. In Table 4 and Table S3, the modeled risk for ferritin levels of less than 12 and less than 26 ng/mL, respectively, generally reinforces risk factors identified in prior studies and in univariable analyses here: female sex, higher donation count, and lower body weight were associated with higher risk for AIS and LF, while postmenopausal status in females was associated with a lower risk. Using a reference age range of 40 to 49 years old, females have odds for ferritin levels of less than 12 ng/mL that are approximately threefold (Hispanic and NHW) to 16-fold (African American) greater than males of their respective race/ethnicity stratum, and approximately fourfold (NHW) to sixfold (African American) greater for ferritin levels of less than 26 ng/mL. After age 50 years, the odds for LF in females decrease by at least one-third compared to females 40 to 49 years old. Compared to donors with no donations in 2 years, the first one to two previous donations during the preceding 2 years roughly double the odds for ferritin levels of less than 12 or less than 26 ng/mL, and the estimated risk progressively increases commensurate with additional units donated. Donating 7+ units in the prior 2-year period increases the odds for a ferritin level of 12 ng/mL approximately 10-fold, and for ferritin levels of less than 26 ng/mL by roughly 15-fold compared to those with no donations. Across all groups, each additional pound of body weight is associated with approximately 0.5- to 1-percentage-point decline in odds for LF and AIS, with most comparisons statistically significant.
TABLE 4.
Adjusted ORs for ferritin levels of less than 12 ng/mL in reduced models, stratified by race/ethnicity*
| Ferritin <12 ng/mL |
||||
|---|---|---|---|---|
| Risk factor | African American | Asian | Hispanic | NHW |
| % <12 ng/mL in reference group* | 0.9 | 3.5 | 3.0 | 6.0 |
| Sex | p < 0.0001 | p = 0.0001 | p < 0.05 | p < 0.0001 |
| Female | 16.3 (4.7-57.2) | 6.6 (2.5-17.0) | 3.2 (1.0-10.4) | 2.7 (1.9-3.6) |
| Male | Ref | Ref | Ref | Ref |
| Age (years), female/male | p = 0.0220/p = 0.0102 | p = 0.0003/p = 0.02 | p = 0.4029/p = 0.7374 | p < 0.0001/p = 0.0773 |
| 18-19 | 1.9 (0.99-3.7)/8.8 (2.1-36.4) | NA†/NA† | 5.6 (2.8-11.3)/2.0 (0.5-7.9) | 2.7 (1.8-4.1)/1.5 (0.72-3.15) |
| 20-29 | 1.6 (0.93-2.8)/2.1 (0.49-8.8) | 1.5† (0.81-2.6)/0.71† (0.24-2.0) | 2.6 (1.4-4.9)/1.8 (0.53-5.9) | 1.7 (1.3-2.3)/1.1 (0.77-1.64) |
| 30-39 | 1.1 (0.6-1.9)/1.5 (0.29-7.8) | 1.4 (0.72-2.6)/2.6 (1.1-6.5) | 2.4 (1.3-4.7)/1.2 (0.37-4.9) | 0.98 (0.73-1.3)/1.1 (0.79-1.6) |
| 40-49 | Ref | Ref | Ref | Ref |
| 50-59 | 0.27 (0.13-0.55)/2.0 (0.53-7.9) | 0.33 (0.14-0.83)/1.3 (0.41-4.0) | 0.6 (0.26-1.4)/1.12 (0.28-4.5) | 0.64 (0.51-0.82)/0.87 (0.66-1.2) |
| 60+ | 0.27 (0.13-0.56)/1.1 (0.23-5.2) | 0.07 (0.01-0.53)/2.6 (0.79-8.7) | 0.66 (0.19-2.3)/0.47 (0.05-4.7) | 0.44 (0.35-0.57)/0.79 (0.61-1.04) |
| Weight | p = 0.002 | p = 0.1046 | p < 0.0001 | p < 0.0001 |
| Per pound | 0.99 (0.98-1.0) | 0.99 (0.98-1.0) | 0.99 (0.985-0.995) | 0.994 (0.992-0.996) |
| Donation frequency | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| 0 donations past 2 years | Ref | Ref | Ref | Ref |
| 1-2 | 1.8 (1.2-2.7) | 2.7 (1.8-4.1) | 2.1 (1.4-3.2) | 1.7 (1.3-2.1) |
| 3-4 | 4.7 (2.8-8.1) | 3.4 (1.0-5.6) | 5.3 (3.1-9.1) | 3.5 (2.7-4.4) |
| 5-6 | 9.9 (5.1-19.2) | 3.5 (1.6-7.5) | 11.3 (5.2-24.7) | 6.4 (4.9-8.2) |
| 7+ | 13.1 (6.3-27.5) | 7.8 (3.9-15.6) | 10.4 (4.6-23.5) | 9.5 (7.5-11.9) |
| Iron Supplement | p = 0.34 | p = 0.053 | p = 0.64 | p < 0.0001 |
| Daily | 0.84 (0.55-1.3) | 0.54 (0.32-0.92) | 0.8 (0.48-1.34) | 0.57 (0.50-0.66) |
| Less than daily | 0.69 (0.41-1.2) | 0.74 (0.46-1.2) | 1.1 (0.65-1.8) | 0.84 (0.71-0.99) |
| None | Ref | Ref | Ref | Ref |
| Blood center | p = 0.90 | p < 0.0001 | p = 0.54 | p = 0.001 |
| ARC | Ref | Ref | Ref | Ref |
| BCW | 0.89 (0.57-1.4) | 1.3 (0.64-2.76) | 1.2 (0.58-2.7) | 0.94 (0.80-1.09) |
| BSRI | 1.0 (0.6-1.9) | 0.44 (0.3-0.65) | 0.84 (0.58-1.2) | 0.66 (0.54-0.82) |
| ITxM | 0.9 (0.6-1.3) | 0.93 (0.51-1.7) | 1.4 (0.62-3.1) | 0.87 (0.74-1.02) |
Data are reported as OR (95% CI). Reference group is 40-year-old male with 0 donations in last 24 months, not taking supplemental iron, weight 180 pounds, at ARC blood center.
For the Asian population the age group of “18–19” was combined with “20–29”.
Some differences emerge in comparing results across racial/ethnic groups. In the reference group—male donor age 40 to 49, with no donations in the prior 2 years, not taking iron, and weighing 180 pounds (Table 4)—there is a six-fold difference in prevalence for ferritin levels of less than 12 ng/mL, from less than 1% in African Americans to 6% in NHW. A similar finding occurs in the model for ferritin levels of less than 26 ng/mL, with a range from 4.1% in African Americans to 11.3% in NHW donors (Table S3). In both ferritin models, the youngest African American males stand out for having estimated risk that was statistically distinguishable from 40- to 49-year-olds of their same race, and for ferritin levels of less than 26 ng/mL the African Americans who are 60+ years of age also have a higher modeled risk (OR, 2.8; 95% CI, 1.3-6.0). Otherwise, with the sole exception of Asian male donors age 30 to 39 in the ferritin <12 ng/mL model, the CIs for male donors of all age groups include 1, indicating that observed differences in prevalence of ID in male donors are largely accounted for by factors distinct from age. In contrast, as noted, the risk in females uniformly declines in age groups 50 to 59 and 60+ years old for all groups in both ferritin models, with CIs excluding 1 for nearly all comparisons. While females less than 20 years old appear at higher risk for African Americans, Hispanic, and NHW donors, the OR is highest for Hispanic donors less than 20 years old (OR, 5.6; 95% CI, 2.8-11.3) in the ferritin <12 ng/mL model, and the elevated risk compared to 40- to 49-year-olds extends to Hispanic females 20 to 29 and 30 to 39 years old. For ferritin <26 ng/mL model, a similar phenomenon is observed.
Another difference across race/ethnicity is the association between self-reported iron supplementation and risk for LF. Only for Asian and NHW donors was the expected protective effect of iron supplementation observed, with a gradient in ORs indicating a higher level of protection with daily iron taking compared to less-than-daily practice. These findings are significant for NHW in both the ferritin <12 and <26 ng/mL models and are significant for Asians in the ferritin <26 ng/mL model (borderline in the <12 ng/mL model), with similar magnitudes of effect. In contrast, for African American and Hispanic donors, the observed association with daily iron supplementation appears relatively weak and does not achieve significance, while less-than-daily supplementation is associated with no significant effect or, counterintuitively, is associated with an OR value of more than 1, indicating increased risk rather than a protective effect (OR, 1.8 for Hispanic; 95% CI, 1.1-2.9 in the ferritin <26 ng/mL model).
Models including all subjects (Table 5) allow for formal comparisons of risk for ferritin levels of less than 12 or less than 26 ng/mL across race/ethnicity and for assessment of covariates of relatively lower frequency in the study population. After other risk factors were controlled for, race/ethnicity remains a significant contributor to iron status. Both African American (OR, 0.81; 95% CI, 0.68-0.96) and Asian (OR, 0.76; 95% CI, 0.62-0.91) subjects are at moderately lower risk for ferritin levels of less than 12 ng/mL than NWH donors, while Hispanic donors are at elevated risk (OR, 1.25; 95% CI, 1.04-1.52). Similar results are obtained for ferritin levels of less than 26ng/mL.
TABLE 5.
Adjusted ORs (95% CIs) for ferritin levels of less than 12 and less than 26 ng/mL in all subjects
| Risk factor | Ferritin < 12 ng/mL | Ferritin < 26 ng/mL | |||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 3.46 (2.64, 4.54) | 5.30 (4.23, 6.62) | |||
| Male | Ref | Ref | |||
| Age group (years) | Female | Male | Female | Male | |
| 18-19 | 2.77 (2.09-3.67) | 1.60 (0.98-2.62) | 2.50 (1.91-3.27) | 1.56 (1.08-2.27) | |
| 20-29 | 1.92 (1.55-2.38) | 1.13 (0.82-1.55) | 1.67 (1.37-2.03) | 1.11 (0.87,1.41) | |
| 30-39 | 1.24 (0.99-1.55) | 1.31 (0.96-1.77) | 1.07 0.88-1.31) | 1.11 (0.88-1.41) | |
| 40-49 | Ref | Ref | Ref | Ref | |
| 50-59 | 0.56 (0.46-0.69) | 0.96 (0.74-1.25) | 0.41 (0.34-0.50) | 1.05 (0.86-1.28) | |
| 60+ | 0.39 (0.32-0.49) | 0.89 (0.69-1.15) | 0.37 (0.30-0.44) | 1.14 (0.94-1.39) | |
| Race | |||||
| African American | 0.81 (0.68-0.96) | 0.86 (0.75-0.99) | |||
| Asian | 0.76 (0.62-0.91) | 0.66 (0.57-0.77) | |||
| Hispanic | 1.25 (1.04-1.52) | 1.19 (1.01-1.41) | |||
| NHW | Ref | Ref | |||
| Donation frequency | |||||
| 0 | Ref | Ref | |||
| 1-2 | 1.86 (1.58-2.19) | 2.22 (1.96-2.52) | |||
| 3-4 | 3.64 (3.03-4.38) | 5.02 (4.33-5.83) | |||
| 5-6 | 6.68 (5.46-8.18) | 9.66 (8.15-11.43) | |||
| 7+ | 10.21 (8.47-12.30) | 17.04 (14.60-19.89) | |||
| Weight (per pound) | 0.993 (0.992-0.995) | 0.994 (0.993-0.995) | |||
| Iron supplement | |||||
| Daily | 0.59 (0.52-0.66) | 0.67 (0.61-0.75) | |||
| Less than daily | 0.81 (0.70-0.94) | 0.95 (0.83-1.08) | |||
| None | Ref | Ref | |||
| Smoking | |||||
| Yes | 0.66 (0.54-0.81) | 0.72 (0.61-0.84) | |||
| Missing | 1.10 (0.94-1.29) | 1.16 (1.01-1.34) | |||
| No | Ref | Ref | |||
| Antacids | |||||
| Yes | 1.24 (1.08-1.42) | 1.21 (1.08-1.37) | |||
| No | Ref | Ref | |||
| Hormones | |||||
| Female | 0.85 (0.72-1.0) | 0.78 (0.67-0.90) | |||
| Male | 2.29 (1.35-3.89) | 1.49 (0.92-2.41) | |||
| None | Ref | Ref | |||
Reference group is 40-year-old male with 0 donations in last 24 months, non-smoker, weight 180 pounds, not taking supplemental iron, hormones, or antacids.
Smoking, reported by 8% of all subjects, with rates ranging from 4% in Asian females to 16% in Hispanic males, was associated with reduced risk for LF (Table 5) compared to those reporting not having smoked in the prior 30 days. Odds for a ferritin level of less than 12 ng/mL were approximately one-third less than those in smokers (OR, 0.66; 95% CI, 0.54-0.81) and of similar magnitude for a ferritin level of less than 26 ng/mL. Use of prescription antacid medications—reported by 14% of subjects—was associated with a modest increase in risk for a ferritin level of less than 12 ng/mL (OR, 1.24; 95% CI, 1.08-1.42) and a ferritin level of less than 26 ng/mL (OR, 1.21; 95% CI, 1.08-1.37). Use of exogenous hormone supplements, reported by 1% to 2% of males and 15% to 20% of female donors, was associated with opposite effects in the two sexes: use of oral contraceptives or estrogen in females was associated with a moderately lower risk for ID that was only significant in the model for a ferritin level of less than 26 ng/mL (OR, 0.78; 95% CI, 0.67-0.90). In males, testosterone was associated with an elevated risk that was only significant in the model for a ferritin level of less than 12 ng/mL (OR, 2.29; 95% CI, 1.35-3.89).
Plots of observed and modeled risk for ferritin levels of less than 12 and less than 26 ng/mL indicate that observed prevalence and intrinsic risk might differ in subpopulations of donors. In Fig. 1A, plots of measured prevalence of ferritin levels of less than 12 ng/mL in NHW donors show little variation across age groups in men and a decline in females from approximately 40% in females younger than 20 years old to 25% in those 60 or older. In Fig. 1B, which plots model residuals to show the “unexplained” risk attached to each age-sex pairing, the heightened risk for young females is accentuated, whereas females age 60 or older have risks virtually indistinguishable from male donors. Also, while empirical observations reflect a growing prevalence of ferritin levels of 26 ng/mL in Caucasian males as age increases (Fig. 1C), the model residuals shown in Fig. 1D indicate that the adjusted risk is stable across age groups.
Fig. 1.
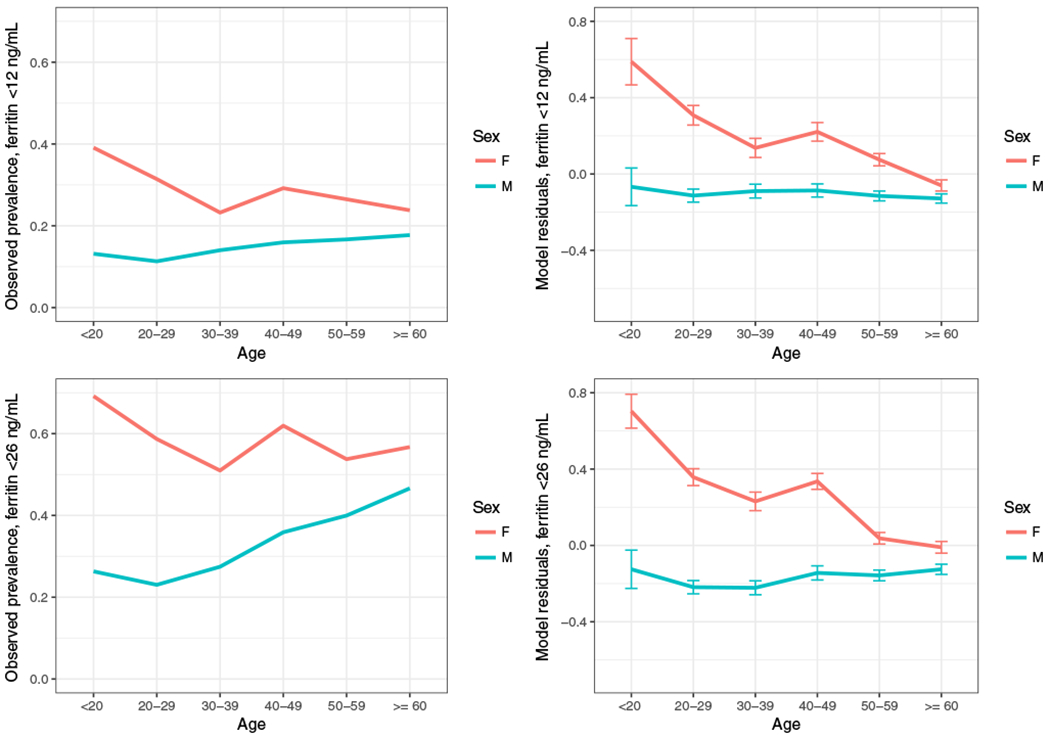
Observed and modeled risk for ferritin levels of less than 12 and less than 26 ng/mL in NHW subjects. Plots show the observed prevalence of ferritin levels of less than 12 (A) and less than 26 ng/mL (C) for NHW subjects, stratified by sex. Residuals from logistic regression models that omit age and sex show the unexplained variability for ferritin levels of less than 12 (B) and less than 26 ng/mL (D) for each pairing of sex and age. [Color figure can be viewed at wileyonlinelibrary.com]
In similar fashion, Figs. 2A and 2B, 2C and D, and 2E and 2F show the observed and modeled risk for ferritin levels of less than 12 ng/mL in African American, Asian, and Hispanic groups, respectively. While the general trends of higher risk in females than males, and a convergence of observed and modeled risk in females toward those of males in older age groups was uniformly observed, these curves differ from each other and from the much larger group of NHW in some details. In both Fig. 2A and 2B, for example, a higher risk for young African American males was evident. In Figs. 2C and 2D, the observed and modeled risk curves for male and female Asian donors cross such that male Asians 60 or older were indicated as having greater risk then their female counterparts. Fig. 2D also indicates, uniquely across the four groups, a risk for a ferritin level of less than 12 ng/mL that increased with age in males, even if not monotonically. Plots for measured and modeled ferritin levels of less than 26 ng/mL for African American, Asian, and Hispanics are shown in Fig. S1.
Fig. 2.
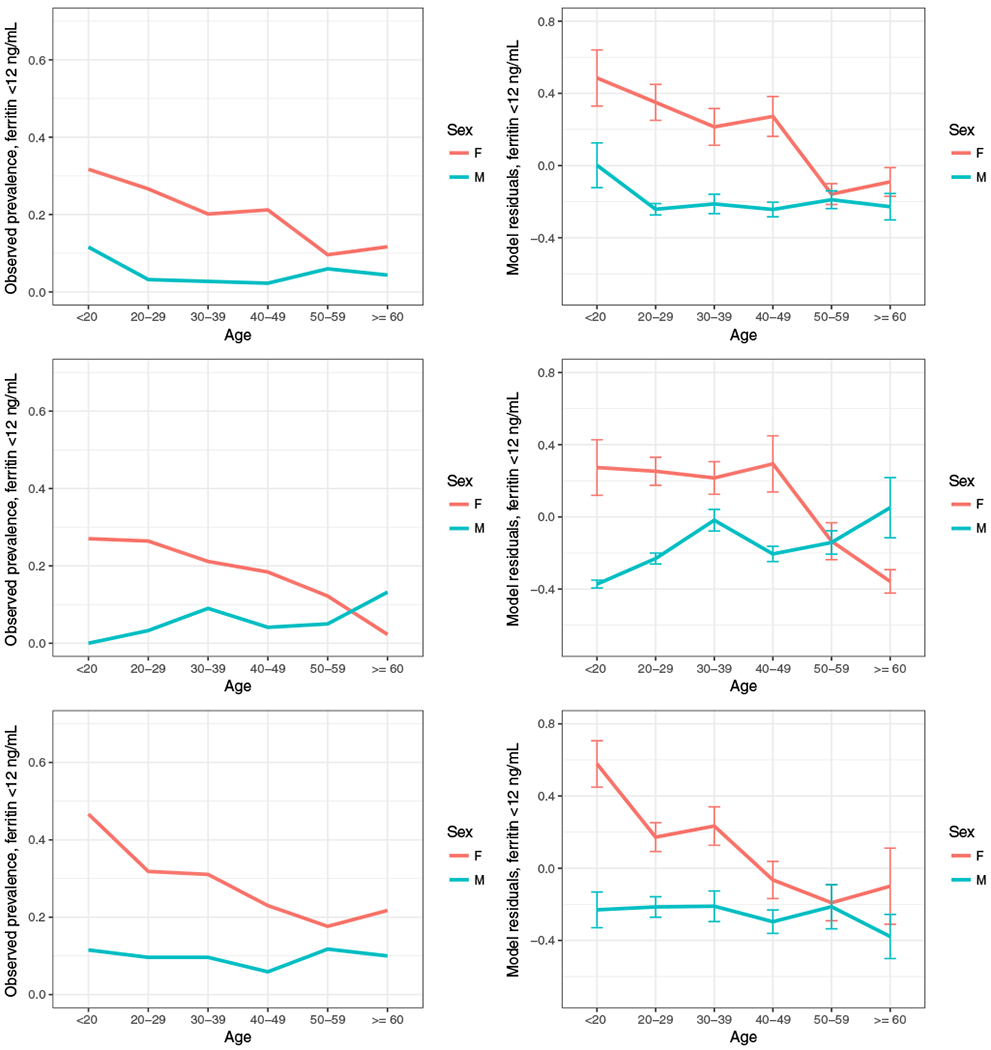
Observed and modeled risk for ferritin levels of less than 12 ng/mL in African American, Asian, and Hispanic subjects. Plots show the observed prevalence of ferritin levels of less than 12 ng/mL (A, C, E) for African American, Asian, and Hispanic subjects, stratified by sex. Residuals from logistic regression models that omit age and sex show the unexplained variability for ferritin levels of less than 12 ng/mL (B, D, F) for each pairing of sex and age. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
The iron status of blood donors has become a prominent issue in recent years both in the United States12,17 and internationally.6,7 This study of 12,683 racially and ethnically diverse blood donors affirms a high prevalence of ID in US blood donors and characterizes risk factors beyond age, sex, and donation frequency. Excluding the high-frequency cohort in our study, the proportion of donors with ferritin levels of less than 12 ng/mL was 17% while 38% had ferritin levels of less than 26 ng/mL. In multivariable analysis, African American and Asian donors had odds for ferritin levels of less than 12 ng/mL approximately 20% to 25% lower than those of NHW donors, while those for Hispanic donors were 25% greater. Smokers, representing approximately 8% of the participants, had odds for ferritin levels of less than 12 ng/mL approximately one-third lower than those of nonsmokers, of comparable magnitude to the protective effect observed for daily iron supplementation, which was reported by 25% of donors. Use of antacid medications, known to inhibit intestinal absorption of dietary iron, raised the odds for LF by approximately 20%, while use of exogenous hormones was associated with slightly lower risk in females and with elevated risk in males.
These results extend those from the REDS-II RISE study8,9 and complement a simulation study based on RISE analysis outputs.1 Although the RISE study design precluded direct extrapolation of its results to the US donor population, the logistic regression results allowed for development of simulation models estimating overall prevalence of ferritin levels of less than 12 and less than 26 ng/mL of 13 and 33% in presenting donors with a successful donation, respectively.1 This study’s findings of 17 and 38% in unselected donors with ferritin levels of less than 12 and less than 26 ng/mL, respectively, provide additional evidence that a sizable proportion of US donors donate blood with completely depleted storage iron and twice as many donate with at least an intermediate stage of ID. Continuation of existing efforts to protect donors from the consequences of ID is justified, and Hispanic donors might benefit from additional attention. These results serve as a benchmark for future studies on the prevalence of iron deficiency in US donors and the outcome of potential mitigation efforts.
Based on carefully designed surveys of the general US population and the results from RISE, the approximately 15% to 20% reduction in risk for LF or AIS, respectively, observed in African Americans compared to Caucasian donors was unexpected. The National Health and Nutrition Examination Survey from 1988 to 1994 (NHANES-III) found that 8% of Caucasian and 15% of African American women age 20 to 49 years old had iron deficiency, based on abnormal laboratory values from two of three indicators (erythrocyte protoporphyrin, transferrin saturation, and serum ferritin).15 Comparable differentials were more recently found between African American and Caucasian females age 12 to 49 years for ferritin levels of less than 12 ng/mL in surveys spanning 1999 to 2006 and for negative iron balance (body iron <0 mg/kg) between 2003 and 2006.16 In a multivariable analysis of the 2003 to 2006 NHANES data, Pfeiffer and colleagues18 found that 10 sociodemographic and lifestyle variables accounted for only 4% to 13% of the variation in iron status for women age 20 to 49 years, with ferritin levels of non-Hispanic blacks 7% lower than NHW donors. The RISE study found black donors at elevated risk for intermediate iron deficiency, but at equivalent risk for a ferritin level of 12 ng/mL.8 A plausible, but unproven, explanation for the lower iron deficiency risk for African American donors in this study could be the differing distributions of Hb in Caucasian and African American individuals. While a minimum Hb level of 12.5 g/dL is required for eligibility to donate blood in the United States (increased to 13.0 g/dL for male donors in May 2016) independent of race/ethnicity, the lower limit of healthy African American females is suggested as 11.5 g/dL and that of African American males at 12.8 g/dL, thus 0.5 to 0.8 g/dL lower than their Caucasian counterparts.19 Hence, whereas this cutoff would permit donation from Caucasians near or below the lower limit of normal, it would enrich for African American donors above the low bound of normal and who would accordingly be less likely to have LF or AIS. Further, African American blood donors may not be representative of all African Americans sampled in the NHANES study.
Prior research of non-blood donors found that smokers have increased ferritin,18 while previous studies of the effect of smoking on ferritin in blood donors have been mixed. Our results, indicating a reduction in odds for LF and AIS of approximately 30%, are consistent with the Danish results in older females and with RISE findings.5,8,9 Our findings also reinforce those of the NHANES study by Pfeiffer and colleagues,18 which showed a 24% increase in ferritin in smokers. Although confounding by alcohol use, which is positively correlated with both smoking20 and ferritin values,21,22 might explain in part our findings, the Pfeiffer study, which controlled for alcohol, showed that smoking and alcohol had independent effects on ferritin of comparable magnitude.18 While the mechanism of how smoking impacts iron homeostasis is under active investigation,23,24 ferritin’s being an acute-phase reactant responsive to inflammation, infection, and other stimuli is relevant. A large study of the Danish cohort confirms low-grade inflammation, as measured by C-reactive protein, in donors who smoked, were obese, and used oral contraceptives.25 Our study did not measure inflammatory markers, but the multivariable model results for female hormone use, smoking, and body weight are consistent with ferritin elevation due to inflammatory stimulus. Future studies might productively evaluate the accuracy of ferritin and other biomarkers of iron status in donors with increased likelihood of inflammation.
Antacid medications, known to inhibit intestinal iron absorption, are commonly used in the adult population and were reported by 14% of this study’s subjects. One recent study, based on longitudinal analysis of total body iron from the STRIDE study, estimated that antacid use in frequent donors was associated with a reduction of 57 mg of iron in the form of ferritin and a decrease of 88 mg in total body iron (storage iron plus that in RBCs).26 The latter quantity, equivalent roughly to one-third of the iron content of a whole blood donation in the United States, can now be correlated with an attendant increase in risk in antacid users for intermediate or advanced ID of 20%, controlling for other risk factors.
Exogenous hormone use showed differing effects on male and female donors in our study. Females reporting oral contraceptives or estrogen use had 15% to 22% lower odds for ferritin levels of less than 12 and less than 26 g/dL, respectively, consistent with hormonal contraception’s reduction of menstrual bleeding as well as low-grade inflammation. Use of testosterone in male donors, in contrast, was associated with a significant doubling of odds for ferritin levels of 12 ng/mL. Although reported by only 1.5% of the male donors in this study, testosterone therapy has emerged as an area of growing scrutiny. Testosterone prescriptions have increased sharply in the US population recently,27 and concerns exist that some donors on testosterone therapy might be motivated to donate by the desire to receive a therapeutic benefit.28 Polycythemia is a common adverse event associated with testosterone replacement, and frequent phlebotomy is the recommended treatment, occurring under physician’s orders in some cases. Testosterone use is not typically reported by donors, however, nor part of the usual health history questions. ID is commonly found in patients diagnosed with polycythemia vera;29 we also found ID in allogeneic blood donors reporting exogenous testosterone use.
While this study contributes to the growing literature on iron status of blood donors by enrolling a racially/ethnically diverse cohort of donors and analyzing potentially actionable risk factors, it has some limitations. As a cross-sectional study, causal relationships may not be ascertainable. This might be seen, most notably, in the inconsistent associations across race/ethnicity (Table 4 and Table S3) between iron supplementation and ferritin levels. It may be that some, or even many, donors within a given subgroup are taking iron due to knowledge of poor iron status or low Hb, which could confound these results. Likewise, donors taking antacids may have LF due to occult gastrointestinal blood loss rather than or in addition to reduced iron absorption. The cross-sectional data collection does not allow for identifiability of potential confounding by indication, and we did not elicit donor motivations for iron supplementation or gastric acid reducing medications. It is possible that some donors had recently initiated iron supplementation in response to blood center messaging, but available evidence indicates a large majority of donors either do not supplement with iron (80%) or report motivations for supplementation unrelated to blood donation (10%), suggesting negligible to modest impact.30 Finally, these findings do not address the clinical relevance of specific ferritin levels in the absence of anemia. The clinical impact of nonanemic iron deficiency remains the subject of debate,31,32 leading to controversy about how strongly to intervene to mitigate potentially adverse outcomes.
Nonetheless, the findings here reinforce our understanding of the major factors associated with ID, namely, donation frequency, sex, weight, and age, to which race/ethnicity can now be added. Additionally, iron supplementation, lower use of antacids, and use of oral contraceptives/estrogen when clinically indicated may improve iron status. Of these factors, iron replacement and/or decreasing donation frequency appear to be the only actionable factors that can be translated into practical strategies that can be implemented at blood centers to reduce ID, while age, sex, race/ethnicity, and weight can help individualize or tailor these strategies to protect donor health and enhance donor retention.
Supplementary Material
Fig. S1: Observed and modeled risk for ferritin <26 ng/mL in African-American, Asian, and Hispanic subjects. Plots show the observed prevalence of ferritin <26 ng/mL (S1a, S1c, S1e) for AA, As, and Hisp subjects, stratified by sex. Residuals from logistic regression models that omit age and sex show the unexplained variability for ferritin <26 ng/mL (S1b, S1d, S1f) for each pairing of sex and age.
TABLE S1: Donor demographics, donation behavior, risk factors.
TABLE S2: Prevalence and unadjusted risk for ferritin <12 and < 26 ng/mL by demographic and behavioral characteristics.
TABLE S3: Adjusted ORs for ferritin <26 ng/mL in reduced models, stratified by race/ethnicity.
TABLE 3.
Laboratory values by race/ethnicity and sex for all donors
| Lab measure | African American (n = 1605) |
Asian (n = 1616) |
Hispanic (n = 1023) |
Caucasian non-Hispanic (n = 8439)* |
||||
|---|---|---|---|---|---|---|---|---|
| Female (n = 862) | Male (n = 743) | Female (n = 735) | Male (n = 881) | Female (n = 614) | Male (n = 409) | Female (n = 4141) | Male (n = 4298) | |
| Hb (mean ± SD) | 12.9 ± 0.8 | 14.4 ± 1.1 | 13.3 ± 0.9 | 15.0 ± 1.1 | 13.1 ± 1.2 | 14.8 ± 1.0 | 13.3 ± 0.9 | 14.6 ± 1.1 |
| Ferritin (ng/mL) | ||||||||
| Geometric mean | 28.98 | 60.98 | 27.3 | 67.76 | 19.1 | 55.63 | 21.01 | 33.95 |
| <12 (%) | 19.84 | 4.44 | 20.95 | 5.68 | 30.46 | 9.54 | 26.83 | 15.94 |
| <26 (%) | 42.58 | 17.63 | 42.72 | 15.66 | 59.12 | 19.8 | 56.65 | 37.99 |
Includes high-intensity donors and unselected NHW donors.
ACKNOWLEDGMENTS
The authors acknowledge NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). The authors would like to express their deep gratitude to the RBC-Omics research staff at the participating blood centers and testing lab for its exceptional performance and contribution to this project.
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), domestic component, is the responsibility of the following persons:
Hubs:
A.E. Mast and J.L. Gottschall, BloodCenter of Wisconsin (BCW), Milwaukee, WI
D.J. Triulzi and J.E. Kiss, The Institute for Transfusion Medicine (now Vitalant Northeast Division), Pittsburgh, PA
E.L. Murphy and E.M. St Lezin, University of California, San Francisco (UCSF), and Laboratory Medicine, Department of Veterans Affairs Medical Center, San Francisco, CA
E.L. Snyder, Yale University School of Medicine, New Haven, CT
R.G. Cable, American Red Cross Blood Services, Farmington, CT
Data coordinating center:
D.J. Brambilla and M.T. Sullivan, Research Triangle International, Rockville, MD
Central laboratory:
M.P. Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, CA
Publication Committee chairman:
R.Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
Steering Committee chairman:
S.H. Kleinman, University of British Columbia, Victoria, British Columbia, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health:
S.A. Glynn and K.B. Malkin, Bethesda, MD
This work was supported by NHLBI contracts NHLBI HHSN2682011-00001I, HHSN2682011-00002I, HHSN2682011-00003I, HHSN2682011-00004I, HHSN2682011-00005I, HHSN2682011-00006I, HHSN2682011-00007I, HHSN2682011-00008I, and HHSN2682011-00009I.
CONFLICT OF INTEREST
BRS serves on the advisory board of HemaStrat. AEM receives research grant funding from Novo Nordisk. The other authors have disclosed no conflicts of interest.
ABBREVIATIONS:
- AIS
absent iron stores
- ID
iron depletion
- LF
low ferritin
- NHW
non-Hispanic white
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Spencer BR, Johnson B, Wright DJ, et al. Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals. Transfusion 2016;56:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman M, Uzicanin S, Osmond L, et al. A large national study of ferritin testing in Canadian blood donors. Transfusion 2017;57:564–70. [DOI] [PubMed] [Google Scholar]
- 3.Salvin HE, Pasricha SR, Marks DC, et al. Iron deficiency in blood donors: a national cross-sectional study. Transfusion 2014;54:2434–44. [DOI] [PubMed] [Google Scholar]
- 4.O’Meara A, Infanti L, Stebler C, et al. The value of routine ferritin measurement in blood donors. Transfusion 2011;51:2183–8. [DOI] [PubMed] [Google Scholar]
- 5.Rigas AS, Sorensen CJ, Pedersen OB, et al. Predictors of iron levels in 14,737 Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2014;54:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman M, Magnussen K, Gorlin J, et al. International forum regarding practices related to donor haemoglobin and iron. Vox Sang 2016;111:449–73. [DOI] [PubMed] [Google Scholar]
- 7.Vuk T, Magnussen K, de Kort W, et al. International forum: an investigation of iron status in blood donors. Blood Transfus 2017;15:20–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2011;51: 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2012;52:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer BR, Bialkowski W, Creel DV, et al. Elevated risk for iron depletion in high-school age donors. Transfusion 2019;59: 1706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Angelantonio E, Thompson SG, Kaptoge S, et al. Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): a randomised trial of 45000 donors. Lancet 2017; 390:2360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AABB. Updated strategies to limit or prevent iron deficiency in blood donors [Internet]. Association Bulletin #17-02 Bethesda: AABB; 2017. [cited 2019 Jan 28]. Available from: http://www.aabb.org/programs/publications/bulletins/Docs/ab17-02.pdf. [Google Scholar]
- 13.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat, and tears: red blood cell-omics study objectives, design, and recruitment activities. Transfusion 2019;59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone M, Brambilla D, Murcia K, et al. Feasibility of routine ferritin testing for donor management: validation of delayed processing and demonstration of within donor reproducibility over time. Transfusion 2016;56:2422–5. [DOI] [PubMed] [Google Scholar]
- 15.Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA 1997;277(12):973–6. [DOI] [PubMed] [Google Scholar]
- 16.United States Center for Disease Control and Prevention. National Center for Environmental Health, Division of Laboratory Sciences. Second national report on biochemical indicators of diet and nutrition in the U.S. population [Internet]. Atlanta: Centers for Disease Control and Prevention; 2012. [cited 2017 Jan 28]. Available from: https://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf. [Google Scholar]
- 17.AABB. Donor iron deficiency risk-based decision-making assessment report: report of the ad hoc iron-deficiency working group [Internet]. Bethesda: AABB; 2018. [cited 2019 Jan 28]. Available from: http://www.aabb.org/tm/Documents/AABB-Donor-Iron-Deficiency-RBDM-Assessment-Report.pdf. [Google Scholar]
- 18.Pfeiffer CM, Sternberg MR, Caldwell KL, et al. Race-ethnicity is related to biomarkers of iron and iodine status after adjusting for sociodemographic and lifestyle variables in NHANES 2003–2006. J Nutr 2013;143:977S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006;107:1747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drobes DJ. Concurrent alcohol and tobacco dependence: mechanisms and treatment. Alcohol Research and Health 2002;26:136–42. [Google Scholar]
- 21.Whitfield JB, Zhu G, Heath AC, et al. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res 2001;25:1037–45. [PubMed] [Google Scholar]
- 22.Whitfield JB, Heath AC, Madden PAF. Metabolic and biochemical effects of low-to-moderate alcohol consumption. Alcohol Clin Exp Res 2013;37:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghio AJ, Hilborn ED, Stonehuerner JG, et al. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am J Respir Crit Care Med 2008;178:1130–8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang WZ, Butler JJ, Cloonan SM. Smoking-induced iron dysregulation in the lung. Free Radic Biol Med 2019;133:238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen CJ, Pedersen OB, Petersen MS, et al. Combined oral contraception and obesity are strong predictors of low-grade inflammation in healthy individuals: results from the Danish Blood Donor Study. PLoS One 2014;9:e88196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bialkowski W, Kiss JE, Wright DJ, et al. Estimates of total body iron indicate 19 mg and 38 mg oral iron are equivalent for the mitigation of iron deficiency in individuals experiencing repeated phlebotomy. Am J Hematol 2017;92:851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002–2016. JAMA 2018;320:200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AABB. FDA Liaison Meeting Minutes 2014. May 16 [cited 2018 January 28]. Available from: http://www.aabb.org/advocacy/government/fdaliaison/bloodcomponents/Pages/flm051614.aspx.
- 29.Ginzburg YZ, Feola M, Zimran E, et al. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia 2018;32:2105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cable RG, Spencer BR. Iron supplementation by blood donors: demographics, patterns of use, and motivation. 10.1111/trf.15407 Epub 2019 Mar 29. [DOI] [PubMed]
- 31.Vassallo RR. Donor iron depletion: beneficial or burdensome? Transfusion 2019;59(7):2184–2186. 10.1111/trf.15282 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Spencer BR. Iron depletion in adult and teenage blood donors: prevalence, clinical impact, options for mitigation. (In press). Hematol Oncol Clin North Am 2019;33(5). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Observed and modeled risk for ferritin <26 ng/mL in African-American, Asian, and Hispanic subjects. Plots show the observed prevalence of ferritin <26 ng/mL (S1a, S1c, S1e) for AA, As, and Hisp subjects, stratified by sex. Residuals from logistic regression models that omit age and sex show the unexplained variability for ferritin <26 ng/mL (S1b, S1d, S1f) for each pairing of sex and age.
TABLE S1: Donor demographics, donation behavior, risk factors.
TABLE S2: Prevalence and unadjusted risk for ferritin <12 and < 26 ng/mL by demographic and behavioral characteristics.
TABLE S3: Adjusted ORs for ferritin <26 ng/mL in reduced models, stratified by race/ethnicity.


