Abstract
Objective
The definite diagnosis of esophageal achalasia is established using manometry, which is performed in patients with suspected achalasia based on the findings of screening examinations, such as upper gastrointestinal endoscopy, chest computed tomography (CT), or a barium swallow test. However, the exact values of test characteristics in these supportive diagnostic examinations remain unclear.
Methods
We estimated the sensitivity and specificity of characteristic findings in the supportive diagnostic examinations for achalasia by comparing the data of a large number of achalasia patients and others with digestive symptoms.
Patients
Achalasia patients (n=119) and non-achalasia patients with suspected achalasia and repeated feelings of chest discomfort (n=37) who were treated in a single university hospital.
Results
Characteristic findings on chest CT (i.e., dilated esophagus, air-fluid level formation) and barium swallow tests were observed in more than 80% of achalasia patients but in less than 10% of non-achalasia patients. In contrast, conventional characteristic findings of upper gastrointestinal endoscopy (i.e., intra-esophageal food debris, feeling of resistance at the esophagogastric junction) were seen in only 40-70% of achalasia patients. In particular, the feeling of resistance at the esophagogastric junction was observed by the examiner in only 30-50% of patients.
Conclusion
Intra-esophageal food debris or resistance at the esophagogastric junction on upper gastrointestinal endoscopy will be positive in only about half of patients with achalasia. Other supportive diagnostic examinations, such as chest CT or barium fluoroscopy, should therefore be included in order to avoid overlooking the disease.
Keywords: barium swallow test, chest CT, esophageal achalasia, sensitivity, specificity, upper gastrointestinal endoscopy
Introduction
Achalasia is an esophageal disorder based on disturbed relaxation of the lower esophageal sphincter muscle of unknown cause (1,2). Patients usually present with difficulty swallowing with or without chest discomfort or pain (3). If the symptoms are prolonged, without proper treatment, patients often experience weight loss (4). Although the disorder itself is not usually fatal, it can significantly and negatively impact the quality of life (5,6).
Making the diagnosis of achalasia is not always easy; in fact, it can be delayed for several years (7). The definite diagnosis of achalasia is now established using manometry results (8,9). However, the availability of manometry at facilities is limited; thus, most clinicians who first examine patients must suspect achalasia from the clinical course and perform supportive diagnostic examinations other than manometry (9,10). Such examinations include upper gastrointestinal (GI) endoscopy, chest CT, and the barium swallow test. In upper GI endoscopy, findings characteristic of achalasia include a dilated intra-esophageal space with retained food debris or a feeling of resistance observed by the examiner when the endoscope passes through the esophagogastric junction (EGJ) (11,12). On chest CT, a dilated esophagus with air-fluid formation inside and the absence of gastric air are considered characteristic findings (13). In the barium swallow test, a dilated esophagus with a “bird's beak” appearance and the retention of swallowed barium in the esophagus for more than several minutes are considered characteristic findings (14). The combination pattern or timing of these supportive diagnostic examinations was recently reported to significantly affect the time required from the first hospital visit to the diagnosis (15). However, the sensitivity and specificity of each supportive diagnostic examination are currently unclear.
We retrospectively assessed the prevalence of the above-described diagnostic examinations in 119 consecutive achalasia patients who had been treated in our university hospital in the last 10 years. In addition, we studied their prevalence in patients with digestive disorders other than achalasia in order to estimate the specificity of each supportive diagnostic examination for achalasia.
Materials and Methods
Enrolled patients and diagnostic examinations performed before manometry
Patients with achalasia in our university hospital were followed in the Department of Surgery (n=38) or the Department of Psychosomatic Medicine (n=81) from 2006-2015. These 119 patients with achalasia are referred to as the “achalasia group” in this study. As reported previously, the definite diagnosis of achalasia was established using manometry or high-resolution manometry before treatment (15).
A total of 37 consecutive patients with repeated digestive symptoms (e.g., chest discomfort, feeling of regurgitation) without achalasia as diagnosed at the Department of Psychosomatic Medicine were also enrolled to estimate the specificity of the diagnostic examinations. These 37 patients are referred to as the “non-achalasia group” in this study.
Among the 119 patients in the achalasia group, before the definite diagnosis based on manometry, upper GI endoscopy was performed in 76 patients (63.9%), chest CT was performed in 70 (58.8%), and a barium swallow test was performed in 88 (73.9%). Among the 37 patients in the non-achalasia group, upper GI endoscopy was performed in 19 (51.4%), chest CT was performed in 18 (48.6%), and a barium swallow test was performed in 31 (83.8%). The diagnostic examinations performed after the definite diagnosis of achalasia based on manometry were excluded from this study because diagnostic bias might have affected the results. Diagnostic examinations that were performed after the treatment for achalasia were also excluded from this study.
Upper GI endoscopy was performed using a GIF-H260, GIF-H260Z, or GIF-Q260J endoscope (Olympus, Tokyo, Japan). The endoscopy tip diameters were 9.8 mm, 10.8 mm, and 9.9 mm, respectively. Among the 76 achalasia patients who underwent upper GI endoscopy, 73 underwent the procedure with a GIF-H260, 2 with a GIF-H260Z, and 1 with a GIF-Q260J. Non-contrast chest CT was performed with a SOMATOM Definition (Siemens, Munich, Germany), SOMATOM Definition flash (Siemens), or Aquilion One Vision Edition (Toshiba Medical Systems, Otawara, Japan) with a tube voltage of 120 kV and a tube current using CT-automatic exposure control optimization technique. CT images were evaluated with 2 slice thicknesses (thin: 1 mm; thick: 5 or 10 mm). The barium swallow test was performed using a Medites Crea Fluoroscopy System (Hitachi, Tokyo, Japan).
Characteristic findings of each diagnostic examination
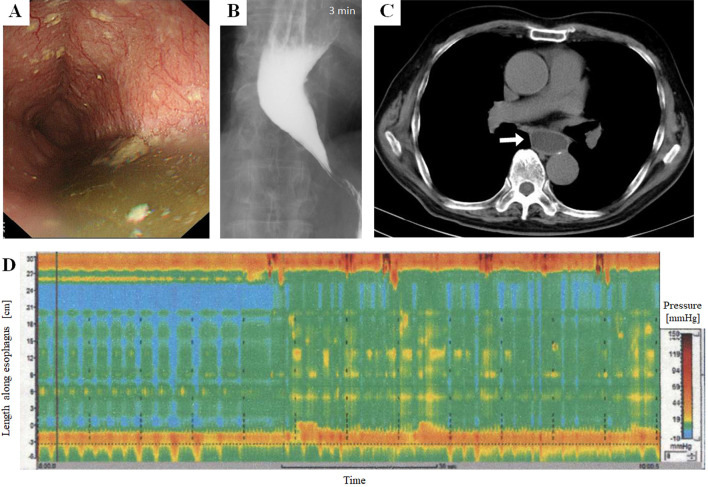
Regarding upper GI endoscopy, the following characteristic findings for achalasia were evaluated: 1) intra-esophageal retention of food debris and 2) feeling of resistance by the examiner when the endoscope passed the EGJ. Regarding chest CT, the following characteristic findings for achalasia were evaluated: 1) esophageal dilatation ≥3.0 cm in the major axis; 2) intra-esophageal air-fluid formation by the retained liquid; and 3) absence of a gastric air bubble. Regarding the barium swallow test, the presence of a dilated esophagus with a “bird's beak” appearance was evaluated. In addition, the level of dilatation on barium fluoroscopy was divided into the following three grades: Grade I (φ<3.5 cm), Grade II (3.5 cm≤φ<6.0 cm), and Grade III (6.0 cm≤φ). Actual images of the diagnostic examinations with the above-described characteristic findings from a patient with achalasia are shown in Figure.
Figure.
Images of the diagnostic examinations in a patient with achalasia. (A) Upper gastrointestinal endoscopy showing intra-esophageal retention of food debris within the extended esophagus. (B) Barium swallow test showing a dilated esophagus with a “bird’s beak” appearance. (C) Chest CT showing a dilated esophagus with the retained liquid inside (white arrow). (D) High-resolution manometry showing aperistalsis and absent esophageal pressurization, which is compatible with the criteria of Type I achalasia in the Chicago classification.
The positivity of these findings was retrospectively established based on the description in the medical records and the results of the performed examinations. Regarding chest CT, the absence of gastric air was equivocal in 1 patient of the achalasia group (3.2%); this patient was therefore excluded from the prevalence estimation of this characteristic finding. Regarding upper GI endoscopy, the presence of intra-esophageal food debris was not described in the report of 9 patients (11.8%), and the presence of resistance felt by the examiner at the EGJ was not described in the report of 18 patients (23.7%) in the achalasia group. These patients were therefore also excluded from the prevalence estimation of these characteristic findings.
Among the 119 enrolled achalasia patients, 26 underwent 32-channel high-resolution manometry (HRM) with pressure topography as a diagnostic examination before the therapeutic interventions; subsequently, they were divided into the following three categories based on Chicago classification version 3.0: type I (aperistalsis and absent esophageal pressurization), type II (increased pan-esophageal pressure), and type III (spastic achalasia) (16).
Statistical analyses
Statistical analyses in this study were performed using the SPSS Statistics Base 22 (IBM, Armonk, USA) and MATLAB R2015a (MathWorks, Natick, USA) software programs. Because multiple characteristic findings were simultaneously analyzed, p values less than 0.01 were considered statistically significant in simultaneous multiple comparisons of the chi-squared tests and Fisher's exact tests based on the concept of Bonferroni correction. Based on the same concept, 99% confidence intervals were calculated for the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of each characteristic finding.
Institutional review board approval
This retrospective study was approved by the Institutional Review Board of Tohoku University Graduate School of Medicine (IRB-2018-1-325).
Results
Characteristics of the enrolled patients
Among the 119 patients with achalasia, 62 were men, and 57 were women. The mean and standard deviation (SD) of the onset age in these achalasia patients was 47.8±17.2 years. The median and 1st-3rd quartile range (25th-75th percentiles) of the disease duration at the first diagnostic examination before treatment was 12 months (1st-3rd quartile range, 2-36 months). In the 88 achalasia patients who underwent barium fluoroscopy, 22 were categorized into Grade I (φ<3.5 cm), 49 into Grade II (3.5 cm≤φ<6.0 cm), and 17 into Grade III (6.0 cm≤φ). Among the 26 achalasia patients who underwent HRM with pressure topography before the treatment, 8 (30.8%) were classified into type I, 15 (57.7%) into type II, and 3 (11.5%) into type III.
Among the 37 non-achalasia patients, 16 were men, and 21 were women. The mean±SD of the onset age was 57.1±15.0 years. Details of the diagnosis in the non-achalasia group were as follows: psychosomatic disorder (n=7), esophageal dysmotility (n=6), eating disorder (n=4), systemic scleroderma (n=4), functional dyspepsia (n=3), pharyngolaryngeal paresthesia (n=3), gastroenteritis (n=3), and other (n=7).
Prevalence of characteristic findings in the achalasia and non-achalasia groups
The prevalence of each characteristic finding in the diagnostic examinations for achalasia by group are listed in Table 1. All of the evaluated findings were significantly more prevalent in the achalasia group, but the prevalence was relatively low (40-60%) on upper GI endoscopy. Among the three diagnostic examinations, barium fluoroscopy appeared to be the most powerful method for discriminating between patients with and without achalasia.
Table 1.
Prevalence of Characteristic Findings on Diagnostic Examinations of Achalasia.
| Achalasia (n=119) | Non-achalasia (n=37) | p value | ||||
|---|---|---|---|---|---|---|
| Upper gastrointestinal endoscopy | ||||||
| Food debris in the esophagus | 36/67 (53.7%) | 1/19 (5.3%) | 0.0001 | |||
| Resistance at the EGJ | 23/58 (43.1%) | 0/19 (0.0%) | 0.0004 | |||
| Non-contrast chest CT scan | ||||||
| Esophageal dilatation | 62/70 (88.6%) | 1/18 (5.6%) | <0.0001 | |||
| Air-fluid level | 59/70 (84.3%) | 1/18 (5.6%) | <0.0001 | |||
| Absence of gastric air | 44/69 (63.8%) | 0/18 (0.0%) | <0.0001 | |||
| Esophageal fluoroscopy (barium swallow) | ||||||
| Dilation, retention, or bird beak | 83/88 (94.3%) | 2/31 (6.5%) | <0.0001 | |||
The prevalence of characteristic findings suggestive of achalasia was the lowest on upper gastrointestinal endoscopy. Shown p values are the results of a Fisher exact test.
EGJ: esophagogastric junction, CT: computed tomography
Based on the prevalence of each characteristic finding in each group, we estimated the diagnostic characteristics of each finding (i.e., sensitivity, specificity, likelihood ratio, odds ratio) (Table 2). Barium fluoroscopy was the most powerful diagnostic examination for achalasia, while upper GI endoscopy was the least powerful.
Table 2.
Diagnostic Characteristics of Each Screening Test for Achalasia.
| Sensitivity (99% CI) | Specificity (99% CI) | LR+(99% CI) | LR- (99% CI) | OR (99% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upper gastrointestinal endoscopy | ||||||||||
| Food debris | 0.537 (0.380-0.694) | 0.947 (0.815-1.00) | 10.2 (0.8-127.4) | 0.49 (0.34-0.71) | 20.9 (1.4-317.6) | |||||
| Resistance at the EGJ | 0.397 (0.231-0.562) | 1.00 (0.757-1.00) | N.A. | 0.60 (0.46-0.79) | N.A. | |||||
| Non-contrast chest CT scan | ||||||||||
| Dilation | 0.886 (0.788-0.984) | 0.944 (0.805-1.00) | 15.9 (1.3-195.3) | 0.12 (0.05-0.29) | 223.2 (12.2-4,077) | |||||
| Air-fluid level | 0.843 (0.731-0.955) | 0.944 (0.805-1.00) | 15.2 (1.2-186.1) | 0.17 (0.08-0.35) | 132.8 (7.9-2,225) | |||||
| Absence of gastric air | 0.638 (0.489-0.787) | 1.00 (0.745-1.00) | N.A. | 0.36 (0.24-0.55) | N.A. | |||||
| Esophageal fluoroscopy (barium swallow) | ||||||||||
| Dilation, retention, bird beak | 0.943 (0.880-1.00) | 0.935 (0.822-1.00) | 14.6 (2.5-85.2) | 0.06 (0.02-0.19) | 240.7 (26.0-2,229) | |||||
Barium fluoroscopy was the most powerful screening test for achalasia, whereas upper gastrointestinal endoscopy was the weakest.
CI: confidence interval, EGJ: esophagogastric junction, LR+: positive likelihood ratio, LR-: negative likelihood ratio, N.A.: not applicable, OR: odds ratio
For reference, the maximum esophageal diameter on barium fluoroscopy (i.e., grade) was not significantly different between the achalasia patients with resistance at the EGJ on upper GI endoscopy and those without resistance (46.1±11.5 mm vs. 44.2±16.4 mm, respectively; p=0.64, Student's t-test).
Discussion
In this study, we estimated the sensitivity and specificity of conventional characteristic findings on supportive diagnostic examinations for achalasia. The results were reliable because they were derived from a large number of achalasia patients (n=119) and non-achalasia patients (n=37). Almost all of the achalasia patients in our tertiary medical care zone (population ≥2 million) underwent consultations at our university hospital during the study period. Therefore, any kind of biases including achalasia type or severity are unlikely to have significantly affected the results of this study.
One of the most notable findings of this study was that less than half of the upper GI endoscopy results mentioned resistance felt by the examiner at the EGJ. Intra-esophageal food debris was observed on upper GI endoscopy in only around half of the achalasia patients. Some newly introduced endoscopic findings have increased the detection level, such as the presence of an esophageal rosette or pinstripe pattern (17,18). However, if we rely solely on conventional characteristic findings on upper GI endoscopy, about half of achalasia patients may be overlooked when this examination is used exclusively.
The suggested sensitivity of chest CT and the barium swallow test for achalasia exceeded 80-90%. Therefore, chest CT and the barium swallow test may be more suitable examinations for screening patients with achalasia than upper GI endoscopy. However, 10-20% of achalasia patients, especially those of Type III Chicago classification, may show negative findings on chest CT or the barium swallow test. Therefore, although these two modalities are very useful supportive diagnostic tools for screening patients with achalasia, manometry should be performed in order to definitely establish a diagnosis of achalasia, as many patients with achalasia may be overlooked otherwise. Of note, upper GI endoscopy should not be omitted for patients suspected of having achalasia based on the argument that the test may have a reduced sensitivity for achalasia. When diagnosing achalasia, conditions of pseudo-achalasia, such as esophageal cancer, must be ruled out. In cases of pseudo-achalasia, the patient will show abnormal findings similar to those seen in achalasia cases on chest CT and barium fluoroscopy. Therefore, during discrimination, upper GI endoscopy would be the most powerful examination for ruling out such cases of pseudo-achalasia with organic lesions. While we must bear in mind that many patients with achalasia may be overlooked if only upper GI endoscopy is used as a screening test, the examination should still be performed in all patients with suspected achalasia in order to reach a correct diagnosis.
Finally, in the primary care setting, many clinicians other than gastroenterologists do not usually perform endoscopy (19,20). However, chest CT and/or a barium swallow test should be considered as reliable as or much more reliable than endoscopy for ruling out achalasia. These two examinations can be easily performed by primary care doctors in the primary care setting if the facility has such imaging machines.
This study has some limitations. First, for some achalasia patients, upper GI endoscopy reports did not describe the feeling of resistance at the EGJ [18/76 patients (23.7%)]. Among these excluded patients, the feeling of resistance at the EGJ may simply have been absent. If so, the estimated sensitivity of resistance at the EGJ may be lower than that shown in this study (39.7%). Second, less than half of the enrolled patients underwent HRM with pressure topography before treatment. The relationship between the evaluated characteristic findings of the supportive diagnostic examinations and the Chicago classification subtype is therefore unknown.
Conclusion
Chest CT or a barium swallow test appears to be a more useful and reliable diagnostic examination tool than upper GI endoscopy when screening for achalasia. However, while upper GI endoscopy alone may be insufficient to rule out achalasia, the examination should still be performed in order to enable the correct diagnosis in patients with suspected achalasia.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by JSPS KAKENHI (grant number JP15K15486).
References
- 1. O'Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 19: 5806-5812, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richter JE. Achalasia - an update. J Neurogastroenterol Motil 16: 232-242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stavropoulos SN, Friedel D, Modayil R, Parkman HP. Diagnosis and management of esophageal achalasia. BMJ 354: i2785, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Moonen A, Boeckxstaens G. Current diagnosis and management of achalasia. J Clin Gastroenterol 48: 484-490, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Liu XJ, Tan YY, Yang RQ, et al. The outcomes and quality of life of patients with achalasia after peroral endoscopic myotomy in the short-term. Ann Thorac Cardiovasc Surg 21: 507-512, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meshkinpour H, Haghighat P, Meshkinpour A. Quality of life among patients treated for achalasia. Dig Dis Sci 41: 352-356, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Gockel I, Muller M, Schumacher J. Achalasia--a disease of unknown cause that is often diagnosed too late. Dtsch Arztebl Int 109: 209-214, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis 16: 297-303, 2007. [PubMed] [Google Scholar]
- 9. Uppal DS, Wang AY. Update on the endoscopic treatments for achalasia. World J Gastroenterol 22: 8670-8683, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muller M, Eckardt AJ, Wehrmann T. Endoscopic approach to achalasia. World J Gastrointest Endosc 5: 379-390, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lujan-Sanchis M, Suarez-Callol P, Monzo-Gallego A, et al. Management of primary achalasia: The role of endoscopy. World J Gastrointest Endosc 7: 593-605, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 383: 83-93, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Rabushka LS, Fishman EK, Kuhlman JE. CT evaluation of achalasia. J Comput Assist Tomogr 15: 434-439, 1991. [DOI] [PubMed] [Google Scholar]
- 14. Ates F, Vaezi MF. The pathogenesis and management of achalasia: current status and future directions. Gut Liver 9: 449-463, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishii T, Akaishi T, Abe M, et al. Importance of barium swallow test and chest CT for correct diagnosis of achalasia in the primary care setting. Tohoku J Exp Med 247: 41-49, 2019. [DOI] [PubMed] [Google Scholar]
- 16. Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 27: 160-174, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minami H, Isomoto H, Miuma S, et al. New endoscopic indicator of esophageal achalasia: “pinstripe pattern”. PLoS One 10: e0101833, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iwakiri K, Hoshihara Y, Kawami N, et al. The appearance of rosette-like esophageal folds (“esophageal rosette”) in the lower esophagus after a deep inspiration is a characteristic endoscopic finding of primary achalasia. J Gastroenterol 45: 422-425, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Day LW, Siao D, Inadomi JM, Somsouk M. Non-physician performance of lower and upper endoscopy: a systematic review and meta-analysis. Endoscopy 46: 401-410, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hilsden RJ, Tepper J, Moayyedi P, Rabeneck L. Who provides gastrointestinal endoscopy in Canada? Can J Gastroenterol 21: 843-846, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]