Abstract
Objective
Transthyretin amyloidosis, particularly wild-type transthyretin amyloid cardiomyopathy (ATTRwt), has been recognized as an important cause of morbidity and mortality in the aging population. However, it is difficult to manage heart failure itself in patients with cardiac amyloidosis.
Methods
We herein report the management of heart failure in an elderly patient with severe heart failure due to ATTRwt. We also review the clinical situation in an additional seven patients with cardiac amyloidosis who were administered pimobendan in our hospital.
Results
We treated a 71-year-old man with refractory heart failure due to ATTRwt. He was expected to be dependent on dobutamine infusion. We administered pimobendan and successfully improved his symptoms and hemodynamic status to allow his discharge from the hospital. An additional retrospective investigation observed that there were eight patients with ATTR amyloidosis who were administered pimobendan. Although all of the patients at the time of administration of pimobendan were NYHA class III or IV with repeated hospitalization for heart failure, pimobendan seemed to be effective for improving symptoms and enabling patients to be discharged and receive outpatient medical care. Furthermore, focusing on the changes in some biomarkers, we found that the brain natriuretic peptide and estimated glomerular filtration rate values improved after the administration of pimobendan in 5 consecutive patients for whom data were available without additional treatment (p=0.018 and 0.051, respectively).
Conclusion
In clinical practice, pimobendan seems to have beneficial effects in heart failure management for improving physical activities and the quality of life in patients with transthyretin cardiac amyloidosis.
Keywords: transthyretin amyloid cardiomyopathy, heart failure, pimobendan
Introduction
Transthyretin (ATTR) amyloidosis, particularly wild-type ATTR amyloid cardiomyopathy (ATTRwt), has been recognized as not uncommon and an important cause of morbidity and mortality in the aging population (1,2). Management of heart failure in patients with ATTRwt is often difficult because of limited evidence of treatment, few choices for medical therapy (mainstay being only diuretics), its nature of progression, and the fact that it mostly affects elderly people (3-5). Although pimobendan, a positively inotropic agent, is sometimes used to treat end-stage heart failure in clinical practice, there has been no report on its effect in patients with cardiac amyloidosis.
We herein report the dramatic effect of pimobendan on severe heart failure due to ATTRwt. We also review the effects of pimobendan in patients with ATTR amyloidosis in our institute.
Materials and Methods
We first describe a patient who achieved a successful recovery from severe heart failure due to ATTRwt by treatment with pimobendan. We then describe our retrospective investigation of eight consecutive patients with ATTR amyloidosis who were administered pimobendan at Kochi Medical School Hospital from January 2015 to February 2019. In all cases, the diagnosis of ATTR amyloidosis was based on the histological confirmation of TTR amyloid deposition in biopsy specimens. TTR amyloid protein was determined by immunostaining using a monoclonal antibody directed against TTR amyloid. Changes in biomarkers values after the administration of pimobendan were assessed using a paired t-test. A probability value of <0.05 was considered significant. All statistical analyses were performed using the SPSS software program, version 21 (IBM, Armonk, USA). The study was approved by the Ethics Committee on Medical Research of Kochi Medical School.
Results
Case report
A 71-year-old man hospitalized at another hospital for heart failure with a history of heart failure admission several times was transferred to our hospital for the further evaluation and treatment of refractory heart failure. Although he was suspected of having cardiac amyloidosis in the previous hospital, a diagnosis was not made.
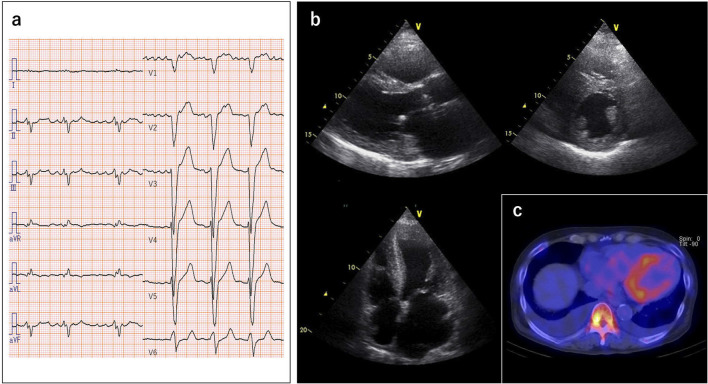
At admission to our hospital, he received oral medications including many types of diuretics, but he still had dyspnea with an NYHA functional class IV. His blood pressure was 106/70 mmHg, and the third heart sound was audible at the apex. Congested jugular veins were observed, but no peripheral edema was found. Chest X-ray showed a high cardiothoracic ratio of 61% with bilateral pleural effusion. Electrocardiographic findings were a heart rate of 65/min with atrial fibrillation, intraventricular conduction delay and left-axis deviation (Fig. 1a). Transthoracic echocardiography revealed diffuse left ventricular (LV) hypertrophy with increased wall thickness of 12 mm, mild hypokinetic LV wall motion (ejection fraction=43%), increased right ventricular (RV) wall thickness of 7 mm with a reduced RV systolic function, and biatrial dilatation (Fig. 1b). Speckle-tracking echocardiography showed an ‘apical sparing’ pattern of longitudinal strain. He had abnormal laboratory test results showing a high brain natriuretic peptide (BNP) level (1,186 pg/mL) and elevated high-sensitivity cardiac troponin T level (0.057 ng/mL). 99mTechnetium pyrophosphate (99mTc-PYP) scintigraphy revealed a grade 3 cardiac uptake on SPECT/CT fusion imaging (Fig. 1c). Bence-Jones protein was not detectable, and the ratio of free light chain κ/λ was normal. Later, we performed an endomyocardial biopsy and genetic analysis, and we finally made a diagnosis of ATTRwt.
Figure 1.
(a) Electrocardiography showing intraventricular conduction delay and left-axis deviation. (b) Echocardiography showing diffuse left ventricular hypertrophy with an increased wall thickness of 12 mm and biatrial dilatation. (c) 99mTechnetium pyrophosphate scintigraphy showing grade 3 cardiac uptake SPECT/CT fusion imaging.
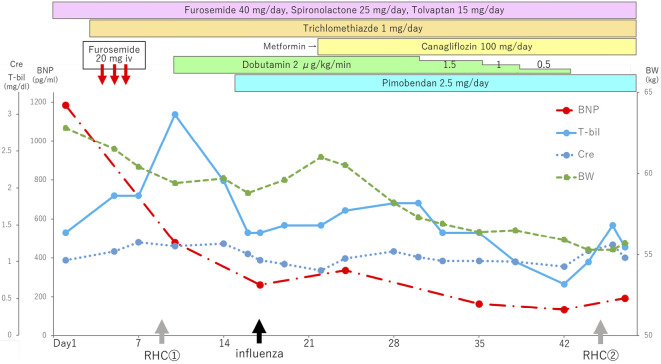
With regard to the management of heart failure, we tried to control his heart failure with oxygen therapy and medical management including intravenous injection of furosemide (Fig. 2), but he still had dyspnea with persistent general fatigue, and his level of bilirubin increased. Right-heart catheterization (RHC) showed a high pulmonary artery wedge pressure and pulmonary hypertension and a low cardiac index of 1.37 L/min/m2 (thermodilution) with a right atrial mean pressure of 8 mmHg (Table 1).
Figure 2.
The clinical course of a 71-year-old man with severe heart failure due to wild-type transthyretin amyloid cardiomyopathy. BNP: brain natriuretic peptide, BW: body weight, Cre: creatinine, T-Bil: total bilirubin, RHC: right-heart catheterization
Table 1.
Right-heart Catheterization Findings.
| ① | ② | |||||
|---|---|---|---|---|---|---|
| PCWP, mmHg | a/v/m | -/42/23 | -/7/6 | |||
| PAP, mmHg | s/d/m | 69/28/41 | 23/11/15 | |||
| RAP, mmHg | a/v/m | -/11/8 | -/4/3 | |||
| CO thermodilution, L/min | 2.34 | 2.96 | ||||
| CI thermodilution, L/min/m2 | 1.37 | 1.89 |
PCWP: pulumonary capillary wedge pressure, PAP: pulumonary artery pressure, RAP: right atrial pressure, CO: cardiac output, CI: cardiac index
Based on these results, we started dobutamine infusion at 2 μg/kg/min for his low cardiac output. His symptoms improved significantly with improvement of creatinine and bilirubin levels. Six days later, we administered pimobendan in order to taper off the dobutamine infusion. During the treatment course, he was basically stable except for temporary deterioration of heart failure due to influenza. After one month of medical therapy and cardiac rehabilitation while admitted, dobutamine infusion was terminated, and RHC showed a dramatic improvement in his hemodynamic status (Table 1). Since his hospital discharge, his condition has remained stable without deterioration of heart failure or arrhythmias for six months.
An additional investigation of the effects of pimobendan in patients with ATTR amyloidosis
Prognostic information was available for 36 patients (30 men and 6 women) with a diagnosis of ATTR amyloidosis based on the histological confirmation of TTR amyloid deposition in biopsy specimens from a variety of tissues in our hospital. The mean age at the diagnosis was 80.1±4.9 years old. During the median follow-up period of 2.1 years, 26 patients died. The prognosis of ATTRwt was poor, with 1-, 3-, and 5-year survival rates after the diagnosis of 69%, 47%, and 31%, respectively.
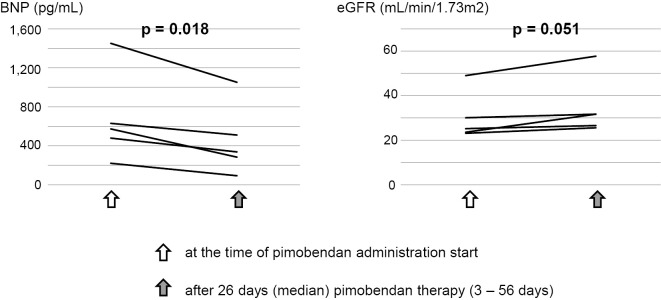
There were eight patients with ATTR amyloidosis who were administered pimobendan at Kochi Medical School Hospital, including the present patient as a case report. All of the patients at the time of the administration of pimobendan were NYHA class III or IV with repeated hospitalization for heart failure (Table 2). Pimobendan seems to be effective for improving symptoms and enabling patients to be discharged and receive outpatient medical care. None of these patients suffered from sustained ventricular tachycardia or sudden death after pimobendan treatment. Fig. 3 shows the changes in the BNP and estimated glomerular filtration rate (eGFR) values after pimobendan therapy in five consecutive patients for whom data were available without additional treatment. Both the BNP and eGFR values significantly improved after the administration of pimobendan.
Table 2.
The Clinical Status in Eight Consecutive Transthyretin Amyloid Cardiomyopathy Patients at the Time of Pimobendan Administration and Their Clinical Course (Present Case Is Patient 1).
| No | Diagnosis | Age | NYHA class | Clinical status | Pimobendan dose | EF (%) | BNP (pg/mL) | eGFR (mL/ min/1.73 m2) |
Clinical course |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ATTRwt | 71 | 4 | In hospital and dobutamine dependent, 3 times HF hospitalization | 2.5 mg | 43 | 479.2 | 49.0 | Free hospitalization and alive for 4 months |
| 2 | ATTRwt | 78 | 3 | In hospital, 10 times HF hospitalization | 2.5 mg | 35 | 630.5 | 30.1 | 3 times HF hospoitalization and HF death after 8 months |
| 3 | ATTRwt | 76 | 3 | Outpatient clinic, 3 times HF hospitalization | 1.25 mg | 35 | 220.9 | 23.1 | 3 times HF hospoitalization and alive for 2 years |
| 4 | ATTRwt | 78 | 4 | In hospital, 3 times HF hospitalization | 1.25 mg | 50 | 1,450.8 | 23.6 | Discharged and HF death after 2 months |
| 5 | ATTRwt | 78 | 4 | In hospital, 3 times HF hospitalization | 2.5 mg | 34 | NA | NA | 2 times HF hospoitalization and HF death after 8 months |
| 6 | ATTRwt | 85 | 4 | In hospital and dobutamine dependent, 5 times HF hospitalization | 1.25 mg | 33 | NA | NA | Temporary going home for 1 day and HF death after 1 week |
| 7 | *ATTR | 84 | 3 | Outpatient clinic, 2 times HF hospitalization | 1.25 mg | 38 | NA | NA | Free hospitalization and alive for 4 months |
| 8 | ATTRv | 66 | 3 | Outpatient clinic, 3 times HF hospitalization | 1.25 mg | 32 | 573.2 | 25.2 | Free hospitalization and alive for 1 year |
*ATTR: no genetic testing.
ATTRwt: wild-type transthyretin amyloid cardiomyopathy, ATTRv: variant (familial) transthyretin amyloid cardiomyopathy, NYHA class: New York Heart Association class, HF: heart failure, EF: ejection fraction, BNP: brain natiriuretic peptide, eGFR: estimated glomerular filtration rate, NA: not available
Figure 3.
Changes in the BNP and eGFR values after pimobendan therapy in five consecutive patients (Patient number 1-4, and 8 in Table 2). BNP: brain natriuretic peptide, eGFR: estimated glomerular filtration rate
Discussion
To our knowledge, this is the first report to describe the clinical significance of pimobendan treatment, a unique inotropic agent, for patients with ATTR amyloidosis. In the present case, dramatic improvements were noted in the hemodynamics and heart failure symptoms due to ATTRwt. Furthermore, based on the outcomes of serial cases with ATTR amyloidosis with pimobendan therapy, we concluded that pimobendan was beneficial in the management of heart failure by extending the life span and improving the quality of life.
A recent study showed that ATTRwt was a frequent cause of heart failure with a preserved ejection fraction (1). With the aging of the population, the prevalence of this disease will increase. Although the prognosis of ATTRwt seems to be better than that of AL amyloidosis, ATTRwt is also a progressive infiltrative disorder, and the prognosis has been reported to be poor, with a median survival period of 47 months after the diagnosis (6). Our retrospective investigation regarding the clinical course of ATTRwt showed a poor prognosis, which is in accordance with the previously reported data obtained from a tertiary center for amyloidosis (6). Recently, tafamidis, which stabilizes the transthyretin tetramer by binding its thyroxin binding site, was reported to be the first treatment for improving the survival in patients with ATTR cardiomyopathy (7). However, there are no guidelines and little evidence concerning the management of cardiac amyloidosis, particularly treatment for heart failure and arrhythmias themselves (3-5). Pimobendan, a benzimidazole pyridazinone derivative, is positively inotropic through its combination of a calcium-sensitizing effect and a cyclic AMP-dependent mode of action [inhibition of phosphodiesterase 3 (PDE3)]. The EPOCH Study revealed that treatment with pimobendan significantly lowered the morbidity and improved the physical activity of patients with mild to moderate chronic heart failure (8).
Regarding the hemodynamics, the infiltration of amyloid deposits results in decreased myocardial compliance, characterized by poor filling with low end-diastolic volume. Furthermore, in the late stage, a reduced LV systolic function results in a decreased stroke volume without the ability of the ventricular cavity to undergo compensatory dilatation. Therefore, excessive diuretics to relieve congestion are not tolerated by patients with hypotension and renal dysfunction due to their low cardiac output. Among the very few potential medical therapies available for cardiac amyloidosis, pimobendan might provide better management than others in the advanced stage. Although the mechanisms underlying this progressive disease remain unknown, impairment of the cardiomyocyte function seems to be partly due to calcium handling. Pimobendan is recognized to increase the calcium sensitivity of human ventricular myofilaments. This results in improving cardiac contractility. Furthermore, pimobendan's PDE3 inhibition in cardiac myocytes may lead to a significant improvement in diastolic relaxation and filling, although most of our ATTRwt patients treated with pimobendan showed a reduced LV ejection fraction (<50%). However, we have misgivings concerning the proarrhythmic effects (increasing lethal ventricular arrythmias) of pimobendane. In our serial cases, we started with a low dose of pimobendan, and no one suffered from sudden death. The dose of pimobendan is probably important for reducing the risk of sudden cardiac death.
As the number of cases in our series was small, a further investigation is needed in order to determine whether or not pimobendan is more effective for patients with cardiac amyloidosis than for those with end-stage heart failure due to other etiologies and to determine whether or not it does not increase the incidence of sudden cardiac death in such heart failure patients in clinical practice. ATTR-targeted therapy including tafamidis is now available, and we believe that combination therapy of pimobendan and tafamidis will provide beneficial effects on the clinical course of ATTR cardiomyopathy.
In conclusions, pimobendan may be an important therapeutic option in heart failure management for improving the physical activity and quality of life in patients with transthyretin cardiac amyloidosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 36: 2585-2594, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2: 113-122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 135: 1357-1377, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gertz MA, Benson MD, Dyck PJ, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 66: 2451-2466, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Izumiya Y, Takashio S, Oda S, Yamashita Y, Tsujita K. Recent advances in diagnosis and treatment of cardiac amyloidosis. J Cardiol 71: 135-143, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Connors LH, Sam F, Skinner M, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation 133: 282-290, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379: 11-21, 2018. [DOI] [PubMed] [Google Scholar]
- 8. The EPOCH Study Group.. Effects of Pimobendan on Chronic Heart Failure Study (EPOCH Study). Effects of pimobendan on adverse cardiac events and physical activities in patients with mild to moderate chronic heart failure: the effects of pimobendan on chronic heart failure study (EPOCH study). Circ J 66: 149-157, 2002. [DOI] [PubMed] [Google Scholar]