Abstract
A patient with underlying Hashimoto's thyroiditis developed amiodarone-induced thyrotoxicosis type 1 that was successfully treated using methimazole in combination with potassium iodide. A 35-year-old woman admitted for perinatal care of twin-to-twin transfusion syndrome was given amiodarone for 7 days for paroxysmal ventricular contraction following pulseless ventricular tachycardia 1 day after delivery. She developed thyrotoxicosis one month after the discontinuation of amiodarone therapy and was negative for thyroid-stimulating hormone receptor antibody. An increased peak velocity of the superior thyroid artery suggested amiodarone-induced thyrotoxicosis type 1. Her thyroid function recovered after combination therapy with methimazole and potassium iodide.
Keywords: amiodarone, amiodarone-induced thyrotoxicosis, color flow Doppler sonography, methimazole, potassium iodide
Introduction
Amiodarone-induced thyrotoxicosis (AIT) type 1, a form of iodine-induced hyperthyroidism, typically develops in patients with underlying Graves' disease or nodular goiter (1). The onset timing of AIT type 1 has been reported to be a median three months after beginning amiodarone therapy (2). Antithyroid drugs in the presence of a 4- to 6-week course of sodium perchlorate are recommended for the treatment of AIT type 1, but higher doses (e.g., 40-60 mg/day of methimazole) and longer periods of treatment are often needed (3,4). Because AIT type 1 is very rare in iodine-sufficient areas, such as Japan, the clinical course of such cases has been rarely described (5-7).
We herein report a case of AIT type 1 in a patient with underlying Hashimoto's thyroiditis that developed one month after the discontinuation of amiodarone treatment and was successfully resolved with methimazole (15 mg/day) in combination with potassium iodide.
Case Report
A 35-year-old woman with a history of Hashimoto's thyroiditis was referred to our hospital for perinatal care of a monochorionic diamniotic twin pregnancy at 24 weeks and 5 days of gestation. She had been euthyroid before her pregnancy [free thyroxin (FT4) 1.1 ng/dL, free triiodothyronine (FT3) 2.5 pg/mL, thyroid-stimulating hormone (TSH) 1.72 μIU/mL, anti-thyroglobulin antibody (TgAb) 138 IU/mL, and anti-thyroperoxidase antibody (TPOAb) >1,300 IU/mL at the diagnosis of Hashimoto's thyroiditis (28 years old)]. She was started on 50 μg L-thyroxine per day at 4 weeks of gestation as her TSH level was >2.5 μIU/mL (FT4 1.2 ng/dL, FT3 2.4 pg/mL, and TSH 3.91 μIU/mL) (8), and she was euthyroid during her pregnancy with a maintenance dose of 200 μg L-thyroxine per week. She was also diagnosed with gestational diabetes at seven weeks of gestation, and her blood glucose levels were well controlled after starting basal and bolus insulin therapy. She denied any family history of thyroid disorders. Her iodine intake during hospitalization was thought to be comparable to that of other healthy Japanese people.
Fetoscopic laser photocoagulation of communicating vessels was performed for twin-to-twin transfusion syndrome (TTTS) at 25 weeks of gestation. Frequent paroxysmal ventricular contractions (PVCs) including bigeminy were observed during and after the procedure, and X-ray the next day showed cardiomegaly, pulmonary congestion and minor pleural effusion. Echocardiography showed normal left ventricular contraction. Based on the diagnosis of heart failure and possible Mirror syndrome (9), noninvasive positive pressure ventilation and medical therapy including bisoprolol tape 1 mg, intravenous furosemide and lidocaine were started. Her heart failure was resolved, so these treatments were stopped within a week. However, given that the symptomatic PVCs, including bigeminy but not ventricular tachycardia (VT), continued throughout the pregnancy, she started taking mexiletine at 27 weeks of pregnancy and switched to propranolol at 32 weeks, continuing this regimen until delivery; however, this ultimately failed to resolve her PVCs.
She delivered healthy twin male babies at 34 weeks and 2 days (1,745 g and 1,767 g) by planned Caesarean section, and L-thyroxine was discontinued after delivery because she had been euthyroid before her pregnancy. She developed sustained pulseless VT with transient loss of consciousness at one day after delivery. Immediate cardiac resuscitation with chest compression and the use of a cardiac defibrillator restored her to a sinus rhythm. A temporary pacemaker was inserted the same day to maintain an increased heart rate in order to suppress the PVCs that triggered VT. She was also started on amiodarone intravenously with a loading dose (125 mg over 10 minutes, 300 mg over 6 hours and 450 mg over 18 hours on the first day; 600 mg over 24 hours on the second and third days, and 400 mg/day thereafter), but the PVCs were soon resolved, so the amiodarone was stopped one week later.
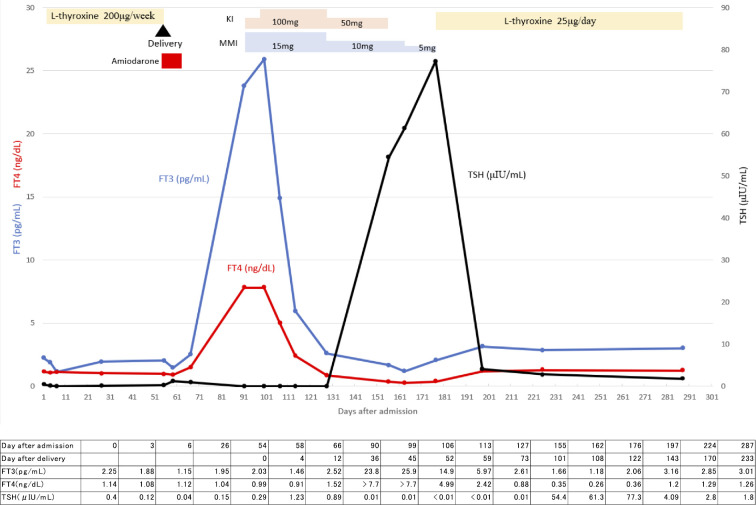
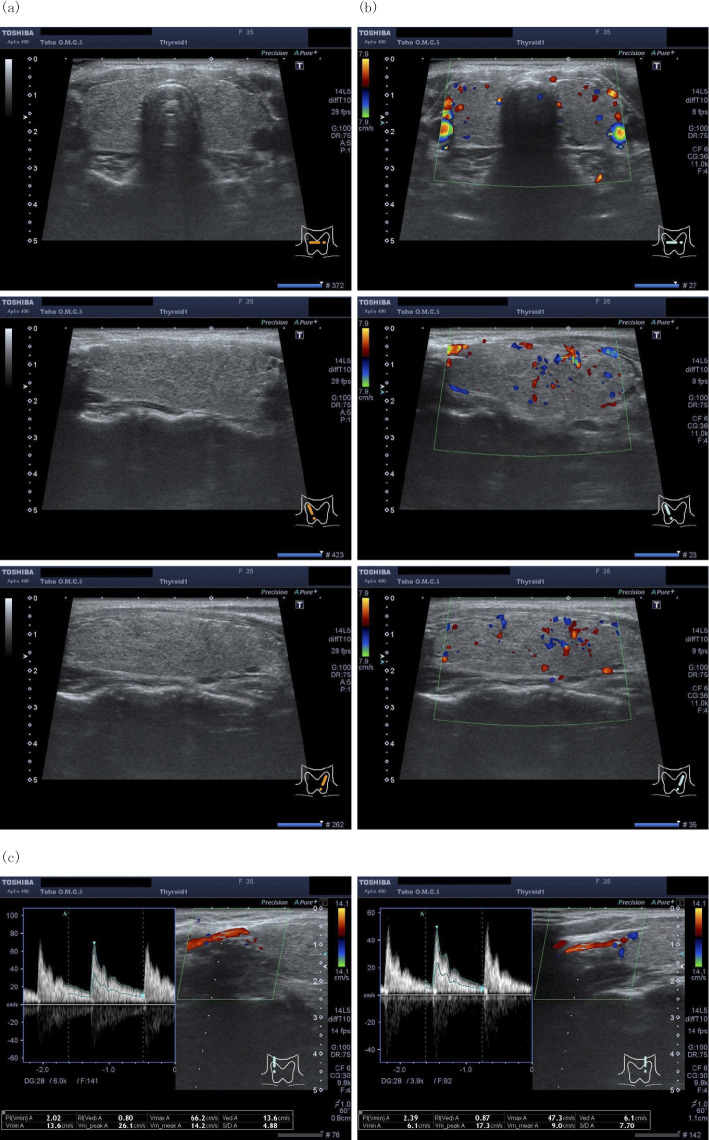
At 36 days after delivery, she was found to have thyrotoxicosis on a thyroid function test (Fig. 1). On an examination, the patient was afebrile, her blood pressure was 92/56 mmHg and her pulse rate was 82/min. Her thyroid gland was soft and had neither increased in size nor was tender; no nodules were palpable. There were no symptoms or signs of Graves' ophthalmopathy. During a careful interview, she reported palpitation and breathlessness on exacerbation, although she initially thought these were normal physiological responses after delivery. She also had mild diarrhea one week before she was diagnosed with thyrotoxicosis. She was initially suspected of having developed painless thyroiditis because of its high incidence among women two to four months after delivery (10,11), especially in those with underlying Hashimoto's thyroiditis. However, color flow Doppler sonography (CFDS) at 38 days after delivery showed an increased peak velocity of the superior thyroid artery (66.2 cm/s in the right and 47.3 cm/s in the left) (Fig. 2), findings that were not compatible with painless thyroiditis. In addition, repeated thyrotropin receptor antibody (TRAb) tests were negative (Table), suggesting that AIT type 1 rather than Graves' disease or painless thyroiditis was the most likely diagnosis. No nodules were detected in her thyroid glands, which suggested that autonomously functioning thyroid nodules or toxic multinodular goiter were unlikely. She was therefore started on 15 mg of methimazole and 50 mg of potassium iodide, the latter of which was increased to 100 mg 1 week later (Fig. 1).
Figure 1.
The clinical state and hormone concentration in the patient. FT3: free triiodothyronine, FT4: free thyroxine, KI: potassium iodide, MMI: methimazole, TSH: thyroid-stimulating hormone
Figure 2.
Ultrasound images of the thyroid and the measurement of the peak systolic velocity of the superior thyroid artery. (a) Ultrasound showed diffuse enlargement of the thyroid gland with nonuniform echogenicity without nodular lesions. The estimated volume of the thyroid calculated by the ellipsoid formula was 19.1 mL. (b) Doppler imaging showed no marked increase in the vascularity in the right or left thyroid glands. (c) Color flow Doppler sonography showed an increased peak velocity of the superior thyroid artery (66.2 cm/s in the right and 47.3 cm/s in the left).
Table.
Laboratory Findings
| On admission | Reference range | 6 days after admission | Reference range | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 6.8×103 | /μL | 3.3-8.6×103 | Thyroglobulin | 0.96 | ng/mL | <32.7 | |||||
| Hb | 12.7 | g/dL | 11.6-14.8 | TgAb | 75.5 | IU/mL | <28 | |||||
| Plt | 22.4×104 | /μL | 15.8-34.8×104 | TPOAb | 93.0 | IU/mL | <16 | |||||
| AST | 19 | IU/L | 13-30 | |||||||||
| ALT | 10 | IU/L | 7-23 | 90 days after admission | Reference range | |||||||
| ALP | 234 | IU/L | 106-322 | TRAb | <0.80 | IU/L | <2 | |||||
| LDH | 219 | IU/L | 124-222 | TSAb | 100 | % | <120 | |||||
| CK | 54 | IU/L | 41-153 | |||||||||
| BUN | 10 | mg/dL | 8-20 | |||||||||
| Cr | 0.51 | mg/dL | 0.46-0.79 | |||||||||
| TP | 6.4 | g/dL | 6.6-8.1 | |||||||||
| Alb | 2.8 | g/dL | 4.1-5.1 | |||||||||
| Na | 139 | mEq/L | 138-145 | |||||||||
| K | 3.8 | mEq/L | 3.6-4.8 | |||||||||
| CRP | 0.2 | mg/dL | 0.0-0.2 | |||||||||
| Free T3 | 2.25 | pg/mL | 2.26-4.15 | |||||||||
| Free T4 | 1.14 | ng/dL | 1.01-1.67 | |||||||||
| TSH | 0.4 | μIU/mL | 0.32-4.12 | |||||||||
Alb: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BUN: blood urea nitrogen, CK: creatine kinase, Cr: creatinine, CRP: C-reactive protein, Hb: hemoglobin, K: potassium, LDH: lactate dehydrogenase, Na: sodium, Plt: platelet, T3: triiodothyronine, T4: thyroxine, TgAb: anti-thyroglobulin antibody, TP: total protein, TPOAb: anti-thyroperoxidase antibody, TRAb: anti-TSH receptor antibody, TSH: thyroid-stimulating hormone, WBC: white blood cell count
At five weeks after starting these drugs, her FT4 and FT3 levels returned to the normal range or below, and both palpitation and breathlessness disappeared, at which point the potassium iodide and methimazole dosages were reduced. Potassium iodide was stopped at 9 weeks, and methimazole was stopped at 12 weeks, since she displayed hypothyroidism after 5 weeks of the treatment. After 25 μg L-thyroxine per day was started, she remained euthyroid.
Discussion
Amiodarone, a benzofuranic iodine-rich anti-arrhythmic drug, is widely used to treat ventricular and atrial arrhythmia (1). However, it causes thyroid dysfunction, including both hyper- and hypothyroidism, in 15-20% of cases (1,12). There are two types of AIT: type 1, a form of increased synthesis of thyroid hormone that often develops in patients with underlying nodular goiter or Graves' disease; and type 2, a form of destructive thyroiditis (4,13). AIT type 1 frequently develops in iodine-deficient areas, whereas AIT type 2 develops in iodine-sufficient areas (3,4,13). Uchida et al. reported that AIT develops in 6% of amiodarone-treated Japanese patients, all of whom are classified as AIT type 2 (7). Since AIT type 1 is very rare in Japan, the clinical course has rarely been described in detail. This is the first case report of AIT type 1 that developed in a patient with underlying Hashimoto's thyroiditis. The present case suggests important clinical points concerning the management of AIT type 1 in terms of its diagnosis and treatment.
Differentiating the two types of AIT is crucial because the treatment approach differs between the two types; however, such differentiation is sometimes difficult, since some patients may have an overlapping condition of both types (3,14). Given the onset of thyrotoxicosis after delivery in the present case, painless thyroiditis, which often develops 2-4 months after delivery, and Graves' disease, which often develops 4-10 months after delivery, were other differential diagnoses (10,11). The 24-h radioactive iodine uptake is helpful for differentiating mild Graves' disease (increased uptake) from painless thyroiditis (decreased uptake) (15). A thyroid ultrasound is also a useful non-invasive examination; Graves' disease is often associated with hypervascularity in the thyroid gland with an increased systolic blood-flow velocity in the superior thyroid artery, whereas painless thyroiditis does not show hypervascularity (16). Hypervascularity in the thyroid gland is also shown in AIT type 1 while being absent in AIT type 2 (17). In the present case, CFDS was useful for differentiating AIT type 1 from painless thyroiditis. At a cut-off value of 43 cm/s for the average peak systolic blood-flow velocity in the superior thyroid artery, the sensitivity and specificity for discriminating Graves' disease from painless thyroiditis have been reported to be 0.87 and 1.00, respectively (16). Therefore, in the present case, AIT type 1 rather than painless thyroiditis or Graves' disease was the most likely diagnosis based on the combination of negative TRAb findings and an increased peak velocity of the superior thyroid artery (66.2 cm/s in the right and 47.3 cm/s in the left).
The urinary iodine concentration has been reported to be useful for differentiating destructive thyroiditis from Graves' disease (18). In the present case, however, it was not tested because elevated urine iodine excretion after amiodarone treatment negates its diagnostic value (18,19). Although the FT3/FT4 ratio also has been reported to be helpful for differentiating painless thyroiditis from Graves' disease (20), the present patient's severe comorbid conditions, including lethal arrhythmia and heart failure, would have modified the FT3/FT4 ratio in this case.
Antithyroid drugs with a 4- to 6-week course of sodium perchlorate are recommended as the treatment of most cases of AIT type 1, but a higher dose of antithyroid drugs (e.g., 40-60 mg/day of methimazole) and longer periods of treatment are often needed (3,4). Oral glucocorticoids are recommended as the first-line treatment for AIT type 2 (3,4). Sodium perchlorate, which decreases the iodine uptake to the thyroid, has synergetic therapeutic effects with antithyroid drugs on AIT type 1 (3). However, sodium perchlorate is not available in Japan. Potassium iodide has also been used to treat hyperthyroidism, especially when rapid clinical or biochemical improvement is required (e.g., in patients with thyroid storm or before urgent thyroidectomy) (15). Inorganic iodide suppresses thyroid hormone secretion (21). In addition, excess iodine has inhibitory effects on iodine organification in the thyroid, known as the Wolff-Chaikoff effect (12). Combination therapy of antithyroid drugs with potassium iodide has been reported to achieve euthyroid status for patients with Graves' disease more effectively and rapidly than antithyroid drugs alone (22). Such combination therapy can also be applied to AIT type 1, as in the present case. To our knowledge, this is the first report of AIT type 1 successfully being treated with methimazole in combination with potassium iodide. Because the rapid restoration of the thyroid function was necessary in order to prevent lethal arrythmia in the present case, combination therapy was immediately started, although painless thyroiditis, for which no medication is usually required, was not completely excluded.
Thyroid function tests are recommended before amiodarone therapy and at three- to four-months intervals during treatment because amiodarone-induced thyroid dysfunction is not a rare condition (23). The onset time is short (median 3 months) in AIT type 1 after the beginning of amiodarone, whereas it is long (median 30 months) in AIT type 2 (2). However, after starting amiodarone, sooner or more frequent tests need to be considered when a patient has a history of thyroid diseases, since the present case developed thyrotoxicosis shortly (one month) after the discontinuation of amiodarone treatment with minimal symptoms. A careful medical interview or a physical examination is needed to diagnose AIT, as bed rest and the beta-blocking effects of amiodarone may mask palpitations and tachycardia caused by hyperthyroidism (1), which often worsen during physical activities.
One possible mechanism underlying the development of AIT type 1 with underlying Hashimoto's thyroiditis in this case is that she discontinued L-thyroxine after delivery, which can alter the iodine uptake to the thyroid gland, while also starting to take the high-iodine-content drug amiodarone for ventricular arrhythmia; this enhanced the total iodine uptake to her thyroid gland, resulting in increased thyroid hormone synthesis. The 24-h radioactive iodine uptake might have been useful for proving this hypothesis, but it was not assessed in the present case because the continued administration of the anti-thyroid drug was necessary in order to prevent hyperthyroidism-induced heart sensitivity or lethal arrhythmia.
One limitation of this report is that the diagnosis of AIT type 1 was made based on the combination of ultrasound findings and negative TRAb results without the uptake of 24-h radioactive iodine. However, the diagnosis and classification of AIT is often challenging, as demonstrated by the heterogeneous responses of expert thyroidologists to recent surveys (24,25). Only one definite AIT type 1 case, diagnosed based on the combination of ultrasound and the 24-h radioactive iodine uptake findings, has been reported in Japan to date (6); many other AIT cases have been reported as either AIT type 2 or possible mixed type (type 1 and type 2) (5,6). To our knowledge, this is the first case report of AIT type 1 with positive antibodies related to Hashimoto's thyroiditis.
In conclusion, we experienced a rare case of AIT type 1 in a patient with underlying Hashimoto's thyroiditis successfully treated using methimazole in combination with potassium iodide. This case demonstrates two clinical important issues. First, the careful monitoring of thyroid hormones along a medical interview and a physical examination are necessary in patients with an underlying thyroid condition (not only Graves' disease or nodular goiter but also Hashimoto's thyroiditis) who start amiodarone therapy. Second, potassium iodide in combination with antithyroid drugs might be an effective treatment for AIT type 1 in countries where sodium perchlorate is not available.
Author's disclosure of potential Conflicts of Interest (COI).
Naoki Kumashiro: Honoraria, Novo Nordisk Pharma, Takeda Pharmaceutical and Sanofi. Takahisa Hirose: Honoraria, Sanofi, Eli Lilly Japan, Novo Nordisk Pharma, Takeda Pharmaceutical, MSD, Sumitomo Dainippon Pharma, Novartis Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, AstraZeneca, Daiichi Sankyo, Mitsubishi Tanabe Pharma and Kissei Pharmaceutical; Research funding, Ono Pharmaceutical and Mitsubishi Tanabe Pharma.
References
- 1. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med 118: 706-714, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Tomisti L, Rossi G, Bartalena L, Martino E, Bogazzi F. The onset time of amiodarone-induced thyrotoxicosis (AIT) depends on AIT type. Eur J Endocrinol 171: 363-368, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European Thyroid Association (ETA) Guidelines for the Management of Amiodarone-Associated Thyroid Dysfunction. Eur Thyroid J 7: 55-66, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab 95: 2529-2535, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Sato K, Miyakawa M, Eto M, et al. Clinical characteristics of amiodarone-induced thyrotoxicosis and hypothyroidism in Japan. Endocr J 46: 443-451, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Sato K, Omi Y, Kodama H, et al. Differential diagnosis and appropriate treatment of four thyrotoxic patients with Graves' disease required to take amiodarone due to life-threatening arrhythmia. Intern Med 47: 757-762, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Uchida T, Kasai T, Takagi A, et al. Prevalence of amiodarone-induced thyrotoxicosis and associated risk factors in Japanese patients. Int J Endocrinol 2014: 534904, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 27: 315-389, 2017. [DOI] [PubMed] [Google Scholar]
- 9. Braun T, Brauer M, Fuchs I, et al. Mirror syndrome: a systematic review of fetal associated conditions, maternal presentation and perinatal outcome. Fetal Diagn Ther 27: 191-203, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Amino N, Tada H, Hidaka Y. Postpartum autoimmune thyroid syndrome: a model of aggravation of autoimmune disease. Thyroid 9: 705-713, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Ide A, Amino N, Kang S, et al. Differentiation of postpartum Graves' thyrotoxicosis from postpartum destructive thyrotoxicosis using antithyrotropin receptor antibodies and thyroid blood flow. Thyroid 24: 1027-1031, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Cohen-Lehman J, Dahl P, Danzi S, Klein I. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol 6: 34-41, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 22: 240-254, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Khan A, Puttanna A, Raskauskiene D. Amiodarone-induced thyrotoxicosis: type 1 or type 2? BMJ Case Rep 2014: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper DS. Hyperthyroidism. Lancet 362: 459-468, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Hiraiwa T, Tsujimoto N, Tanimoto K, Terasaki J, Amino N, Hanafusa T. Use of color Doppler ultrasonography to measure thyroid blood flow and differentiate graves' disease from painless thyroiditis. Eur Thyroid J 2: 120-126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bogazzi F, Bartalena L, Brogioni S, et al. Color flow Doppler sonography rapidly differentiates type I and type II amiodarone-induced thyrotoxicosis. Thyroid 7: 541-545, 1997. [DOI] [PubMed] [Google Scholar]
- 18. Sugimoto T, Momotani N, Iino S, Ito K. [Clinical significance of the measurement of the urinary concentration of iodine in differentiating silent thyroiditis from Graves' disease]. Nihon Naibunpi Gakkai Zasshi (Jpn J Endocrinol) 70: 1083-1092, 1994(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 19. Giovacchini G, Giovanella L, Haldemann A, Staub U, Fuchsel FG, Koch P. Potentiometric measurement of urinary iodine concentration in patients with thyroid diseases with and without previous exposure to non-radioactive iodine. Clin Chem Lab Med 53: 1753-1760, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Shigemasa C, Abe K, Taniguchi S, et al. Lower serum free thyroxine (T4) levels in painless thyroiditis compared with Graves' disease despite similar serum total T4 levels. J Clin Endocrinol Metab 65: 359-363, 1987. [DOI] [PubMed] [Google Scholar]
- 21. Wartofsky L, Ransil BJ, Ingbar SH. Inhibition by iodine of the release of thyroxine from the thyroid glands of patients with thyrotoxicosis. J Clin Invest 49: 78-86, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takata K, Amino N, Kubota S, et al. Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves' disease. Clin Endocrinol 72: 845-850, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Trip MD, Wiersinga W, Plomp TA. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med 91: 507-511, 1991. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed S, Van Gelder IC, Wiesfeld AC, Van Veldhuisen DJ, Links TP. Determinants and outcome of amiodarone-associated thyroid dysfunction. Clin Endocrinol 75: 388-394, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Raghavan RP, Taylor PN, Bhake R, et al. Amiodarone-induced thyrotoxicosis, an overview of UK management. Clin Endocrinol 77: 936-937, 2012. [DOI] [PubMed] [Google Scholar]