Abstract
Objective
To prolong the health expectancy, it is important to prevent age-related diseases, such as osteoporosis and cerebrovascular disease, which are major causes of a bedridden state. Early predictable biomarkers for these diseases are urgently required in the clinical setting. Three members of the fibroblast growth factor (FGF) family - FGF19, FGF21, and FGF23 - are designated as endocrine FGFs and play crucial roles in various metabolic processes. We tried to clarify the clinical utility of endocrine FGFs as biomarkers for age-related diseases in elderly patients.
Methods
We examined the serum endocrine FGF levels and analyzed their association with various clinical parameters in 73 outpatients >60 years old as a single-center cross-sectional study.
Results
In a multivariable linear regression analysis, FGF19 was associated with ALT, a history of cardiovascular disease, and medication with active vitamin D3. FGF21 was associated with the estimated glomerular filtration rate (eGFR), triglyceride level, and hypertension. FGF23 was associated with the eGFR and the serum levels of 1,25-dihydroxy vitamin D3 and TRACP5b. In addition, a receiver operating characteristics analysis revealed that the measurement of FGF21 and FGF23 was useful for detecting chronic kidney disease (CKD) and its complications, including cardiovascular disease and metabolic bone disorder.
Conclusion
The measurement of FGF21 and FGF23 may be useful for evaluating CKD and its complications. Using serum endocrine FGFs as biomarkers for age-related conditions may help prevent elderly patients from entering a bedridden state.
Keywords: age-related disease, FGF19, FGF21, FGF23, metabolic syndrome, chronic kidney disease
Introduction
In developed countries, many problems related to aging societies have been emerging. In local communities with residents of advanced age, the increase in age-related diseases, such as osteoporosis, cerebrovascular disease, and cardiovascular disease, which can result in a bedridden state, leads to a shortened healthy life expectancy and worsening medical economy. Therefore, understanding and preventing age-related diseases are critical issues for these communities, and early predictable biomarkers for these complications are urgently needed in the clinical setting.
Among the 22 members of the fibroblast growth factor (FGF) family, FGF19, FGF21, and FGF23 are distinguished in three major respects (1). First, they function not as growth factors but as hormones that regulate various metabolic processes and are thus collectively called endocrine FGFs (2). Second, they lack the heparin-binding domains conserved in many other FGFs (3). The loss of the heparin-binding domains prevents endocrine FGFs from being trapped within the extracellular matrix, which is rich in heparan sulfate, and enables them to migrate into the blood stream and function as hormones. Finally, endocrine FGFs require the Klotho family of transmembrane proteins for binding to and activating canonical FGF receptor (FGFR) tyrosine kinases (4-6). Two members of the Klotho family, αKlotho and βKlotho (7), form constitutive binary complexes to serve as physiological receptors for endocrine FGFs. Specifically, αKlotho binds to either FGFR1c, FGFR3c, or FGFR4 to form binary complexes that function as the FGF23 receptor (6). βKlotho binds to either FGFR1c or FGFR4 to form binary complexes that function as FGF21 or FGF19 receptors, respectively (8,9). Thus, the target organs of endocrine FGFs must express appropriate Klotho-FGFR complexes.
FGF23 is a bone-derived hormone that acts on renal tubular cells expressing both αKlotho and FGFR1c/3c/4 to suppress the reabsorption of phosphate and the activation of vitamin D (10,11). FGF21 is secreted from the liver upon fasting and acts on adipocytes that express both βKlotho and FGFR1c to induce metabolic responses to fasting (12,13). In addition, FGF21 crosses the blood-brain barrier to act on the suprachiasmatic nucleus (SCN) in the hypothalamus and activates the hypothalamus-pituitary-adrenal axis and the sympathetic nervous system to induce stress responses (14). Conversely, FGF19 (the human orthologue of FGF15 in rodents) is secreted from the intestine upon feeding and acts on the liver, which expresses both βKlotho and FGFR4, to induce metabolic responses to feeding, including the promotion of protein and glycogen synthesis and suppression of bile acid synthesis (15,16). Importantly, mice deficient in αKlotho or FGF23 suffer from a disturbed mineral metabolism associated with accelerated aging (17), whereas transgenic mice that overexpress αKlotho (18) or FGF21 (13,19) have longer life spans than normal mice. These findings suggest that the FGF23-αKlotho and FGF21-βKlotho endocrine axes may regulate aging processes. Although direct evidence for the regulation of aging processes by the FGF19-βKlotho endocrine axis has not been reported, the ability of FGF19 to function as a satiety hormone similar to insulin may contribute to aging processes, as the attenuation of insulin/insulin-like growth factor signaling is a common mechanism for life span extension that has been evolutionarily conserved from nematodes through mammals (15).
These findings have raised the possibility that each endocrine FGF may be associated with aging and thus contribute to age-related diseases in humans. As the first step to explore this possibility, we performed a single-center cross-sectional study in a region with a high prevalence of aging (20) and determined whether or not an association exists between the serum levels of endocrine FGFs and clinical parameters relevant to aging and age-related diseases.
Materials and Methods
Study design
This study was conducted with a single-center cross-sectional design. People >60 years old were recruited from among outpatients at Miyashita Hospital between July 2016 and November 2016. All patients lived in Onuma County, in which more than 50% of the residents are elderly (20). A total of 73 patients (58 women and 15 men) agreed to participate in this study, which was approved by the local Ethics Committee at Miyashita Hospital and carried out in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all subjects prior to enrollment.
Patients with apparent infection, active neoplastic diseases, and acute kidney injury were excluded from study participation. Data collected from medical records included patient characteristics, disease history, complications, and history of medication. Laboratory measurements included FGF19, FGF21, and FGF23 in serum and urine samples from the participants. All patients who took active vitamin D3 in this study were diagnosed with osteoporosis before registration.
Definitions of factors
We defined cerebrovascular diseases as a history of cerebral infarction, cerebral hemorrhaging, subarachnoid hemorrhaging and unclassified stroke cardiovascular diseases, and cardiovascular diseases as a history of myocardial infarction, coronary revascularization for angina pectoris, hospitalization for angina pectoris, and hospitalization for heart failure (21). We determined the chronic kidney disease (CKD) stages of each patient according to this definition and classification. The estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine (sCr) or serum cystatin C (sCysC) and age using the following estimation equations: eGFR creatinine (eGFRcreat) (mL/min/1.73 m2)=194×sCr-1.094×Age-0.287(×0.739 for women); eGFRcys for men (mL/min/1.73 m2)=104×sCysC-1.019×0.996age-8; and eGFR cystatin C (eGFRcys) for women (mL/min/1.73 m2)=(104×sCysC-1.019×0.996age×0.929)-8 (22). Hypertension was defined as systolic blood pressure (BP) (sBP) ≥140 mmHg and/or diastolic BP (dBP) ≥90 mmHg in an outpatient clinic. Dyslipidemia was defined as low-density lipoprotein cholesterol (LDL) ≥140 mg/dL or high-density lipoprotein cholesterol (HDL) <40 mg/dL, or triglyceride (TG) ≥150 mg/dL. Osteoporosis was defined as a fragility fracture of a vertebral body or proximal femur, that of other bones with a young adult mean (YAM) of bone mineral density (BMD) <80%, or a YAM <70% even in the absence of fractures. We calculated albumin-corrected calcium according to the following formula: serum calcium+(4.0-Alb) (mg/dL).
Laboratory measurements including endocrine FGFs
Blood and urine samples for laboratory measurements were obtained from patients in the morning in a fasting state at registration (23,24). The blood samples were centrifuged at 3,000×g for 10 minutes at room temperature. We examined the concentrations of FGF19 and FGF21 using a Quantikine ELISA Kit (R & D Systems, Minneapolis, USA), the measurement principle of which is the sandwich enzyme immunoassay. We measured the absorbance of the color of recombinant human FGFs as standard and patient samples by these kits and drew standard curves on log-log graph sheets. We determined the concentrations of unknown samples from the standard curve, confirming a linear relationship between the standard concentrations and the absorbance. The measurement of serum FGF23 levels was performed using chemiluminescent enzyme immunoassay methods at a commercial laboratory (SRL, Tokyo, Japan). All other measurements were performed at another commercial laboratory (Health Sciences Research Institute, Koriyama, Japan).
Statistical analyses
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (25). The Kolmogorov-Smirnov test was used to analyze the normality of the distribution of parameters. Data that did not show a normal distribution, such as endocrine FGFs and the urinary albumin/creatinine ratio, were analyzed after log transformation. We confirmed the normality of their log distributions. All variables with normal distributions were expressed as the mean±standard deviation, and log normal distributions were expressed as the median with the interquartile range (IQR). We used the Jonckheere-Terpstra test to determine the increasing or decreasing tendencies of continuous variables. A univariate correlation analysis was performed by a Pearson's correlation analysis for continuous variables and a linear regression analysis for binary variables. Factors independently associated with endocrine FGF levels were determined using a multivariable linear regression analysis. We also checked the variance inflation factor (VIF) to examine the multicollinearity among variables. The upper limit of the VIF indicating harmful collinearity was set at 10. We used the analysis of covariance (ANCOVA) test for serum FGF21 levels between dyslipidemia-positive and dyslipidemia-negative patients because of the significant correlation between the serum FGF21 levels and the eGFRcys and the lack of any interaction between the eGFRcys and dyslipidemia. We determined the cut-off values of FGF21 and FGF23 for CKD by a receiver operating characteristics (ROC) curve analysis. We then divided the patients according to the cut-off values and performed the statistical comparison of various clinical parameters between two groups using the t-test or Welch's t-test. Furthermore, we constructed ROC curves of both FGF21 and FGF23 for CKD by a multivariable logistic regression analysis to evaluate the accuracy. All statistical tests were two-sided, using a significance level of 0.05.
Results
Baseline patient characteristics
The mean age of the 73 patients included in this study was 76.2 years old, and 79% were women. Their body mass index (BMI), BP, disease and drug history are shown in Table 1. Because patients with CKD are reported to have high FGF23 and FGF21 and low FGF19 levels (14,17), we examined the prevalence of CKD in this population and found that 41% of the patients had CKD as defined by the eGFR and the urinary albumin/creatinine ratio. We calculated the eGFRcreat and eGFRcys using sCr and sCysC.
Table 1.
Baseline Characteristics of Patients.
| Patient characteristics | No. of patients (%) or mean±SD (n=73) | |
|---|---|---|
| Women | 58 (79) | |
| Age, y | 76.2±8.0 | |
| Body mass index; BMI, kg/m2 | 23.6±3.5 | |
| Systolic blood pressure; sBP | 132.5±17.8 | |
| Diastolic blood pressure; dBP | 74.1±9.9 | |
| Bone mineral density (young adult mean; YAM) (%) | ||
| Lumbar spine | Male 95.9±23.8, Female 82.0±17.3 | |
| Hip | Male 85.0±23.0, Female 73.4±14.1 | |
| Disease history | ||
| Cerebrovascular disease | 4 (5.4) | |
| Cardiovascular disease | 10 (14) | |
| Complications | ||
| Hypertension | 52 (71) | |
| Dyslipidaemia | 42 (58) | |
| Diabetes mellitus | 18 (25) | |
| Chronic kidney disease | ||
| Stage G1 | 8 (11) | |
| Stage G2 | 35 (48) | |
| Stage G3a | 18 (25) | |
| Stage G3b | 10 (14) | |
| Stage G4 | 2 (2.7) | |
| Osteoporosis | 33 (45) | |
| Regular alcohol consumption | 6 (8.2) | |
| Drug used | ||
| Calcium channel blocker | 40 (45) | |
| Angiotensin-converting enzyme inhibitor | 1 (1.4) | |
| Angiotensin II receptor blocker | 42 (58) | |
| β-blocker | 5 (6.8) | |
| Diuretic | 17 (23) | |
| Statin | 39 (53) | |
| Dipeptidyl peptidase-4 inhibitor | 6 (8.2) | |
| Antiplatelet drug | 14 (19) | |
| Bisphosphonate | 5 (6.8) | |
| Active vitamin D3 | 12 (16) |
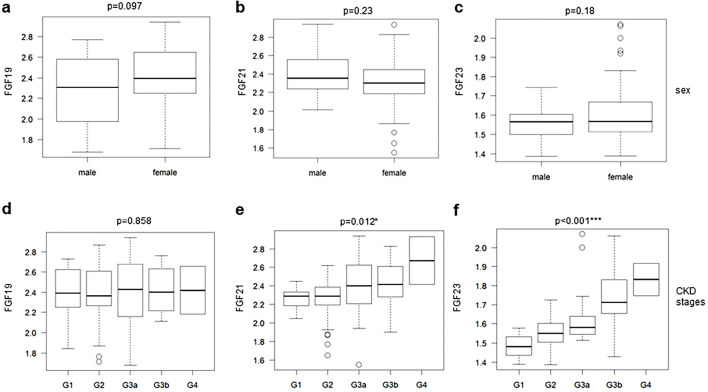
The blood and serum parameters are shown in Table 2. The median FGF19, FGF21, and FGF23 levels were 235.2 (IQR 164.2-427.5) pg/mL, 204.8 (IQR 159.2-281.4) pg/mL, and 37.0 (IQR 32.6-45.3) pg/mL, respectively. Endocrine FGFs did not show significant differences between men and women (Fig. 1a-c). The serum FGF21 and FGF23 levels significantly increased with CKD progression (p=0.012, and p<0.001). However, the FGF19 levels were not correlated with the CKD stages (Fig. 1d-f).
Table 2.
Baseline Characteristics of Laboratory Measurements.
| Patient characteristics | Mean±SD or median and. interquartile range (IQR) (n=73) |
|---|---|
| Liver function | |
| Aspartate aminotransferase; AST (IU) | 22.7±6.8 |
| Alanine aminotransferase; ALT (IU) | 17.7±8.4 |
| Alkaline phosphatase; ALP (IU) | 237.3±61.6 |
| Total bilirubin; T-bil (mg/dL) | 0.76±0.3 |
| Total protein; TP (g/dL) | 7.37±0.6 |
| Albumin; Alb (g/dL) | 4.24±0.3 |
| Renal function | |
| Serum creatinine; sCr (mg/dL) | 0.76±0.2 |
| Estimated glomerular filtration rate by creatinine; eGFRcreat (mL/min/1.73) | 61.7±14.7 |
| Serum cystatin C; sCysC (mg/L) | 1.04±0.3 |
| Estimated glomerular filtration rate by creatinine; eGFRcys (mL/min/1.73) | 66.5±19.1 |
| Sodium (mEq/L) | 141.9±2.0 |
| Potassium (mEq/L) | 4.39±0.3 |
| Chloride (mEq/L) | 105.3±2.2 |
| Albumin-corrected calcium (mg/dL) | 9.50±0.3 |
| Phosphorus (mg/dL) | 3.46±0.7 |
| Uric acid; UA (mg/dL) | 5.14±1.2 |
| Intact parathyroid hormone; iPTH (pg/mL) | 44.2±15.7 |
| 1,25-dihydroxy vitamin D3 (pg/mL) | 70.4±22.0 |
| Urine albumin/creatinine ratio (mg/gCr) | 9.75 (6.6-17.0) |
| Complete blood counts | |
| Haemoglobin; Hb (g/dL) | 13.1±1.5 |
| Platelet; Plt (×103/µL) | 232.2±52.4 |
| White blood cell; WBC (×103/µL) | 5.8±1.6 |
| Lipid metabolism | |
| Total cholesterol; T-chol (mg/dL) | 188.9±23.0 |
| Low density lipoprotein cholesterol; LDL (mg/dL) | 111.0±21.6 |
| High density lipoprotein cholesterol; HDL (mg/dL) | 60.8±12.6 |
| Triglyceride; TG (mg/dL) | 105.5±49.9 |
| Glucose metabolism | |
| Fasting plasma glucose; FPG (mg/dL) | 106.6±18.8 |
| Haemoglobin A1c; HbA1c (%) | 5.9±0.5 |
| Bone metabolism | |
| Bone specific alkaline phosphatase; BAP (μg/L) | 16.1±5.3 |
| Deoxypyridinoline; DPD (nM/mMCr) | 5.7±1.6 |
| Type I procollagen N-terminal propeptide; P1NP (ng/mL) | 46.5±20.0 |
| Tartrate-resistant acid phosphatase 5b; TRACP5b (mU/dL) | 473.7±159.4 |
| Endocrine fibroblast growth factors | |
| FGF19 (pg/mL) | 235.2 (164.2-427.5) |
| FGF21 (pg/mL) | 204.8 (159.2-281.4) |
| FGF23 (pg/mL) | 37.0 (32.6-45.3) |
Figure 1.
The tendency of serum endocrine FGF levels to be associated with general parameters in clinical observations. Endocrine FGF levels stratified by sex (a-c) and CKD stages (d-f) are shown as box plots. The values shown are medians, interquartile ranges (IQR), minimal and maximal values, and outliers (defined as values between 1.5 and 3 times IQR and represented by circles). P values were calculated by Student’s t-test (sex) and the Jonckheere-Terpstra test (CKD stage).
A Pearson's correlation analysis of the serum endocrine FGF levels and clinical parameters
The correlations between endocrine FGFs and various clinical parameters are shown in Table 3. The serum FGF19 levels were positively correlated with age (r=0.28; p=0.018) and negatively correlated with the body weight (BW) (r=-0.32; p=0.007), hip BMD (r=-0.25; p=0.034), aspartate aminotransferase (AST) (r=-0.26; p=0.031), alanine aminotransferase (ALT) (r=-0.34; p=0.003), total protein (TP) (r=-0.36; p=0.002), and albumin (Alb) (r=-0.29; p=0.015), suggesting that serum FGF19 levels may reflect some aspects of the liver function. The serum FGF21 levels were positively correlated with the BMI (r=0.30; p=0.011), sBP (r=0.33; p=0.005), TG (r=0.37 ; p<0.001), and fasting plasma glucose (FPG) (r=0.25; p=0.035), which is consistent with the reported association between high FGF21 levels and metabolic syndrome. Furthermore, the serum FGF21 levels were positively correlated with sCr (r=0.32; p=0.006) and sCysC (r=0.39; p<0.001) and negatively correlated with the eGFRcreat (r=-0.30; p=0.011), eGFRcys (r=-0.34; p=0.003) and phosphorus (r=-0.26; p=0.030). These results are also consistent with the reported association between high FGF21 levels and CKD.
Table 3.
Pearson’s Correlation Coefficients between Endocrine FGF Plasma Levels and Other Clinical Parameters.
| Variables | Serum FGF19 | Serum FGF21 | Serum FGF23 | |||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |||
| Anthropometric parameters | ||||||||
| Age | 0.28 | 0.018* | 0.050 | 0.69 | 0.17 | 0.15 | ||
| BW | -0.32 | 0.007** | 0.21 | 0.078 | 0.056 | 0.64 | ||
| BMI | -0.20 | 0.11 | 0.30 | 0.011* | 0.14 | 0.24 | ||
| sBP | -0.11 | 0.37 | 0.33 | 0.005** | 0.036 | 0.77 | ||
| dBP | -0.16 | 0.19 | 0.18 | 0.12 | -0.20 | 0.088 | ||
| BMD lumbar spine (YAM) | -0.14 | 0.26 | 0.045 | 0.71 | 0.19 | 0.11 | ||
| Hip (YAM) | -0.25 | 0.034* | -0.0024 | 0.98 | 0.12 | 0.30 | ||
| Laboratory measurements | ||||||||
| Liver function | ||||||||
| AST | -0.26 | 0.031* | -0.016 | 0.90 | -0.13 | 0.30 | ||
| ALT | -0.34 | 0.003** | 0.082 | 0.50 | -0.16 | 0.18 | ||
| ALP | 0.12 | 0.36 | 0.008 | 0.95 | -0.24 | 0.055 | ||
| T-bil | 0.17 | 0.16 | -0.18 | 0.15 | -0.16 | 0.20 | ||
| TP | -0.36 | 0.002** | 0.13 | 0.29 | -0.23 | 0.053 | ||
| Alb | -0.29 | 0.015* | 0.077 | 0.52 | -0.27 | 0.020* | ||
| Renal function | ||||||||
| sCr | -0.074 | 0.54 | 0.32 | 0.006** | 0.43 | <0.001*** | ||
| eGFRcreat | -0.039 | 0.75 | -0.30 | 0.011* | -0.53 | <0.001*** | ||
| sCysC | -0.015 | 0.90 | 0.39 | <0.001*** | 0.57 | <0.001*** | ||
| eGFRcys | -0.046 | 0.70 | -0.34 | 0.003 | -0.56 | <0.001*** | ||
| iPTH | 0.058 | 0.63 | 0.043 | 0.72 | -0.085 | 0.48 | ||
| 1,25-dihydroxy vitamin D3 | -0.007 | 0.95 | -0.16 | 0.18 | -0.49 | <0.001*** | ||
| Ca | -0.12 | 0.33 | -0.045 | 0.71 | 0.029 | 0.81 | ||
| P | -0.14 | 0.25 | -0.26 | 0.030* | 0.21 | 0.079 | ||
| Complete blood counts | ||||||||
| Hb | -0.080 | 0.51 | 0.038 | 0.76 | -0.25 | 0.035* | ||
| Plt | -0.12 | 0.33 | 0.24 | 0.041* | -0.016 | 0.089 | ||
| WBC | -0.10 | 0.38 | 0.22 | 0.069 | -0.025 | 0.84 | ||
| Lipid metabolism | ||||||||
| T-Chol | 0.091 | 0.45 | 0.11 | 0.37 | 0.16 | 0.18 | ||
| LDL | 0.087 | 0.47 | 0.18 | 0.14 | 0.16 | 0.20 | ||
| HDL | 0.088 | 0.46 | -0.38 | <0.001** | -0.11 | 0.35 | ||
| TG | -0.070 | 0.56 | 0.37 | <0.001** | 0.13 | 0.29 | ||
| Glucose metabolism | ||||||||
| FPG | -0.058 | 0.63 | 0.25 | 0.035* | 0.087 | 0.48 | ||
| HbA1c | -0.082 | 0.52 | 0.002 | 0.98 | 0.064 | 0.61 | ||
| Bone metabolism | ||||||||
| BAP | 0.017 | 0.89 | -0.005 | 0.97 | -0.36 | 0.002** | ||
| DPD | 0.050 | 0.68 | -0.12 | 0.31 | -0.042 | 0.726 | ||
| P1NP | -0.058 | 0.63 | -0.010 | 0.93 | -0.31 | 0.009** | ||
| TRACP5b | 0.075 | 0.53 | -0.25 | 0.036* | -0.30 | 0.009** | ||
| Endocrine FGFs | ||||||||
| FGF19 | NA | NA | -0.22 | 0.065 | 0.19 | 0.11 | ||
| FGF21 | -0.22 | 0.065 | NA | NA | 0.12 | 0.33 | ||
| FGF23 | 0.19 | 0.11 | 0.12 | 0.33 | NA | NA | ||
*p<0.05, **p<0.01, ***p<0.001
NA: not available
The other parameters significantly associated with the serum FGF21 levels included HDL (r=-0.38; p=0.001), platelets (r=0.24; p=0.041), and tartrate-resistant acid phosphatase 5b (TRACP5b) (r=-0.25; p=0.036). Serum FGF23 levels were positively correlated with sCr (r=0.43; p<0.001), and sCysC (r=0.57; p<0.001) and negatively correlated with eGFRcreat (r=-0.53; p<0.001), eGFRcys (r=-0.56; p<0.001), 1,25-dihydroxy vitamin D3 (r=-0.49; p<0.001), and hemoglobin (Hb) (r=-0.25; p=0.035), which is consistent with the fact that FGF23 is a counter-regulatory hormone for vitamin D and increases with CKD progression. FGF23 was also negatively associated with markers for bone formation and absorption, namely bone-specific alkaline phosphatase (BAP) (r=-0.36; p=0.002), type 1 procollagen N-terminal propeptide (P1NP) (r=-0.31; p=0.009), and TRACP5b (r=-0.30; p=0.009), suggesting that high FGF23 levels may be associated with low bone turnover.
A linear regression analysis between serum levels of endocrine FGFs and history of diseases/medication
The serum FGF19 levels tended to be high in patients with a history of cardiovascular disease (β coefficient 0.23; p=0.053) and anti-platelet drug medication (β coefficient 0.22; p=0.066) (Table 4). The serum FGF19 levels were also significantly higher in patients prescribed active vitamin D3 than in others (β coefficient 0.27; p=0.021). In contrast, the serum FGF19 levels were significantly lower in patients taking metformin than in those not taking it (β coefficient -0.25; p=0.034).
Table 4.
Linear Regression Models between Serum Levels of Endocrine FGFs and Disease/drug History.
| Complication or drug | Number positive (%) | Serum FGF19 | Serum FGF21 | Serum FGF23 | |||||
|---|---|---|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | ||||
| Disease history | |||||||||
| Cerebrovascular disease | 4 (5.4) | 0.10 | 0.40 | -0.010 | 0.93 | 0.062 | 0.60 | ||
| Cardiovascular disease | 10 (14) | 0.23 | 0.053 | -0.022 | 0.86 | -0.085 | 0.48 | ||
| Complications | |||||||||
| Hypertension | 52 (71) | 0.064 | 0.59 | 0.31 | 0.009** | 0.016 | 0.087 | ||
| Dyslipidaemia | 42 (58) | -0.15 | 0.22 | 0.21 | 0.072 | -0.034 | 0.78 | ||
| Diabetes mellitus | 18 (25) | -0.053 | 0.66 | 0.062 | 0.61 | 0.25 | 0.035* | ||
| Osteoporosis | 33 (45) | 0.13 | 0.27 | -0.073 | 0.54 | -0.19 | 0.12 | ||
| Chronic kidney disease | 30 (41) | 0.021 | 0.86 | 0.26 | 0.030 | 0.51 | <0.001*** | ||
| Drug used | |||||||||
| Calcium channel blocker | 40 (45) | 0.022 | 0.85 | 0.27 | 0.021* | 0.14 | 0.25 | ||
| Angiotensin II receptor blocker | 42 (58) | -0.15 | 0.22 | 0.18 | 0.14 | 0.12 | 0.32 | ||
| β-Blocker | 5 (6.8) | -0.13 | 0.27 | 0.0082 | 0.87 | 0.16 | 0.19 | ||
| Diuretic | 17 (23) | -0.05 | 0.68 | 0.032 | 0.79 | 0.075 | 0.53 | ||
| Statin | 39 (53) | -0.16 | 0.19 | 0.18 | 0.13 | -0.045 | 0.71 | ||
| DPP-4 inhibitor | 6 (8.2) | -0.057 | 0.63 | 0.048 | 0.69 | 0.22 | 0.067 | ||
| Metformin | 3 (4.1) | -0.25 | 0.034* | 0.050 | 0.68 | -0.042 | 0.73 | ||
| Antiplatelet drug | 14 (19) | 0.22 | 0.066 | 0.13 | 0.28 | 0.026 | 0.83 | ||
| Bisphosphonate | 5 (6.8) | 0.10 | 0.27 | -0.062 | 0.60 | 0.12 | 0.30 | ||
| Active vitamin D3 | 12 (16) | 0.27 | 0.021* | -0.17 | 0.15 | 0.20 | 0.088 | ||
| Proton pump inhibitor | 45 (62) | -0.067 | 0.576 | 0.30 | 0.011* | 0.099 | 0.41 | ||
*p<0.05, **p<0.01, ***p<0.001
The serum FGF21 levels were significantly higher in patients with CKD (β coefficient 0.26; p=0.030) and hypertension (β coefficient 0.31; p=0.009) than in those without these conditions. In addition, the serum FGF21 levels were higher in patients taking calcium channel blockers (CCBs) (β coefficient 0.27; p=0.021) and proton pump inhibitors (PPIs) (β coefficient 0.30; p=0.011) than in others (Table 4). The serum FGF21 levels also tended to be high in patients with dyslipidemia according to a linear regression analysis (β coefficient 0.21; p=0.072) (Table 4). Furthermore, the serum FGF23 levels were significantly higher in patients with diabetes mellitus (β coefficient 0.25; p=0.035) and CKD (β coefficient 0.39; p<0.001) than in those without these conditions.
A multivariable linear regression analysis to identify factors independently correlated with endocrine FGFs
To identify independent parameters that explain the serum levels of endocrine FGFs, we performed a multivariable linear regression analysis using the parameters with significant correlations in a univariate analysis. No parameters had a VIF greater than 10, suggesting the absence of multicollinearity (22). The serum FGF19 levels were independently correlated with ALT [β coefficient -0.012, 95% confidence interval (CI) -0.015 to -0.0081, p=0.002], cardiovascular disease (β coefficient 0.019, 95% CI 0.10 to 0.28, p=0.036), and active vitamin D3 medication (β coefficient 0.23, 95% CI 0.15 to 0.32, p=0.005). The other parameters, including age, sex, Alb, BW, and metformin medication, were excluded from the regression analysis. The serum FGF21 levels were independently correlated with the eGFRcys (β coefficient -0.0037, 95% CI -0.0053 to -0.0021, p=0.025), TG (β coefficient -0.0018, 95% CI -0.0024 to -0.0012, p=0.005), and hypertension (β coefficient 0.15, 95% CI 0.081 to 0.22, p=0.036). Age, sex, BMI, FPG, HDL and PPI medication were excluded. The serum FGF23 levels were independently correlated with 1,25-dihydroxy vitamin D3 (β coefficient -0.0020, 95% CI -0.0027 to -0.0014, p=0.002), the eGFRcys (β coefficient -0.0031 95% CI -0.0039 to -0.0024, p<0.001), and TRACP5b (β coefficient -0.00019 95% CI -0.00027 to -0.00011, p=0.020). Age, sex, BAP, intact parathyroid hormone (iPTH) and P1NP were excluded (Table 5).
Table 5.
Multivariable Linear Regression Analysis.
| Variables | β | (95% CI) | t | p value | ||||
|---|---|---|---|---|---|---|---|---|
| logFGF19 (adjusted R-squared: 0.19) | ||||||||
| ALT | -0.012 | -0.015 to -0.0081 | -3.2 | 0.002** | ||||
| Cardiovascular disease (complication) | 0.19 | 0.10 to 0.28 | 2.1 | 0.036* | ||||
| Active vitamin D3(medication) | 0.23 | 0.15 to 0.32 | 2.9 | 0.005** | ||||
| logFGF21 (adjusted R-squared: 0.29) | ||||||||
| eGFRcys | -0.0037 | -0.0053 to -0.0021 | -2.3 | 0.025* | ||||
| TG | -0.0018 | -0.0024 to -0.0012 | 2.9 | 0.005** | ||||
| Hypertension | 0.15 | 0.081 to 0.22 | 2.1 | 0.036* | ||||
| logFGF23 (adjusted R-squared: 0.40) | ||||||||
| 1,25-dihydroxy vitamin D3 | -0.0020 | -0.0027 to -0.0014 | -3.2 | 0.002** | ||||
| eGFRcys | -0.0031 | -0.0039 to -0.0024 | -4.4 | <0.001*** | ||||
| TRACP5b | -0.00019 | -0.00027 to -0.00011 | -2.4 | 0.020* |
*p<0.05, **p<0.01, ***p<0.001
FGF19 model included age, sex, osteoporosis, Alb, BW, and metformin; FGF21 model included age, sex, BMI, FPG, HDL and PPI; FGF23 model included age, sex, BAP, intact PTH and P1NP, which were all excluded in the final model by p value stepwise.
Endocrine FGFs are useful biomarkers for detecting various metabolic dysfunctions in CKD patients
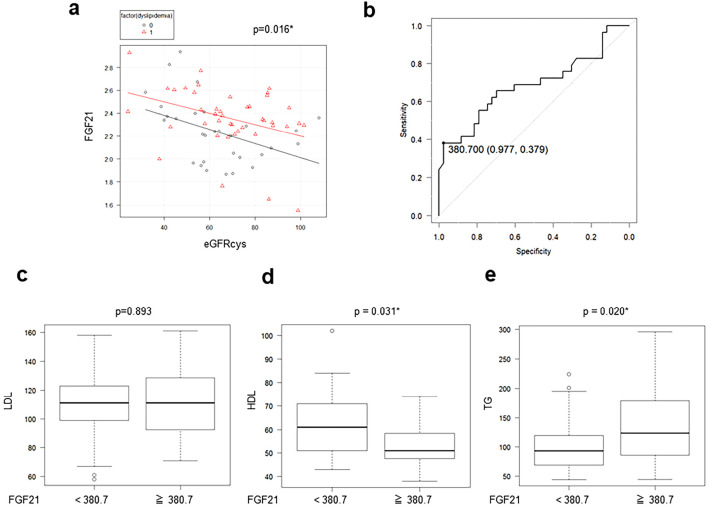
Because our univariate analysis showed that serum FGF21 levels were significantly correlated with the eGFR, we assumed that the FGF21 levels should be adjusted by the eGFR. Therefore, we performed an ANCOVA for the serum FGF21 levels after adjusting for the eGFRcys and found that these levels were significantly higher in patients with dyslipidemia, regardless of statin medication, than in those without it (p=0.016) (Fig. 2a). In addition, we performed an ROC analysis to evaluate the predictive value of the serum FGF21 level for identifying cases of CKD (Fig. 2b). The cut-off value of FGF21 for the diagnosis of CKD was 380.7 pg/mL (sensitivity: 0.379, specificity: 0.977). We divided the patients into two groups according to this cut-off value and analyzed various laboratory parameters. Interestingly, the serum LDL levels showed no significant difference between the two groups (Fig. 2c), whereas the serum HDL and TG concentrations were significantly lower (Fig. 2d) and higher (Fig. 2e), respectively, in patients with high serum FGF21 levels than in those with low levels.
Figure 2.
Patients with elevated serum FGF21 levels remain in a dyslipidemic state, regardless of statin medication. (a) The serum FGF21 levels in patients with or without dyslipidemia are analyzed using an analysis of covariance (ANCOVA) adjusted with the eGFRcys. (b) A receiver operating characteristics (ROC) curve of FGF21 was constructed for the diagnosis of CKD. (c) Serum LDL, (d) HDL, and (e) TG are shown in patients with measurements above or below the cut-off value (380.7 pg/mL) of serum FGF21 determined by an ROC analysis.
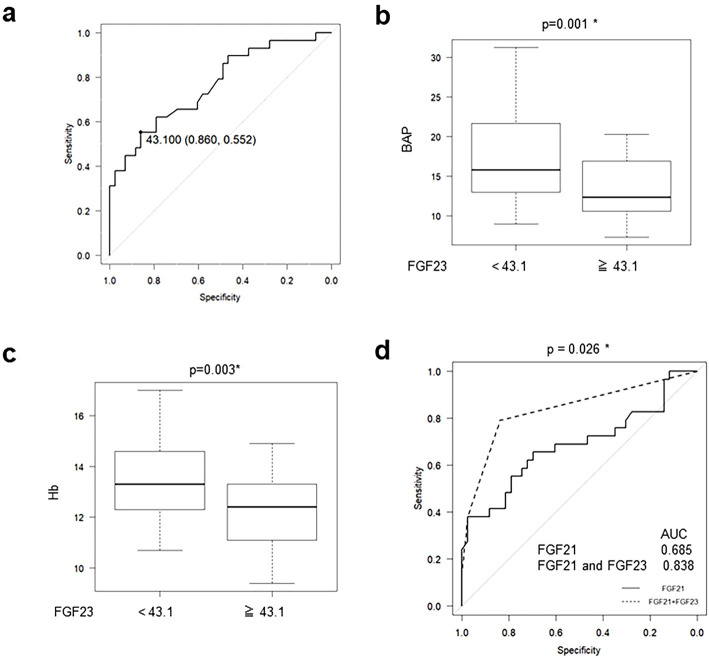
It has been reported that the serum FGF23 levels are correlated with the eGFR and are increased in early CKD stages. The cut-off value of FGF23 for the diagnosis of CKD by an ROC analysis was 43.1 pg/mL (sensitivity: 0.552, specificity: 0.860) (Fig. 3a). We divided the patients according to this cut-off value and found that the BAP (Fig. 3b) and Hb (Fig. 3c) levels were significantly lower in patients with high FGF23 levels than in those with low levels. Finally, we performed a multivariable logistic regression analysis by dividing the patients using the cut-off values of FGF21 and FGF23 levels in CKD patients and constructed ROC curves. The area under the curve (AUC) of the ROC curve generated by FGF21 and FGF23 was 0.838, which was significantly higher than the one generated by FGF21 alone (0.685, p=0.026) (Fig. 3d).
Figure 3.
The serum FGF23 levels are useful for the detection of CKD and its complications. (a) An ROC curve of FGF23 was constructed for the diagnosis of CKD. (b) Serum bone-specific alkaline phosphatase and (c) hemoglobin concentrations are shown in patients with measurements above or below the cut-off value (43.1 pg/mL) of serum FGF23 determined by an ROC analysis. (d) The AUC of the ROC curve generated by a multivariable logistic regression analysis using both FGF21 and FGF23 was significantly larger than the one generated by FGF21 alone.
Discussion
In the present study, we identified correlations between the serum levels of endocrine FGFs and several clinical parameters that have not been reported previously. A multivariable linear regression analysis revealed that serum FGF19 levels were independently correlated with ALT, the history of cardiovascular diseases, and active vitamin D3 medication. The serum FGF21 levels were correlated with TG. The serum FGF23 levels were correlated with 1,25-dihydroxy vitamin D3 and TRACP5b. Because both FGF21 and FGF23 were identified as independent determinants of the eGFR, we constructed a new model for eGFR prediction using FGF21 and FGF23. In addition, the ROC analysis clarified that FGF21 and FGF23 were predictable biomarkers for CKD and associated complications. These results indicated that the measurement of both FGF21 and FGF23 may be useful for evaluating the presence of CKD and its complications, including cardiovascular disease and metabolic bone disorder.
We unexpectedly identified medication with active vitamin D3 as an independent predictor of serum FGF19 levels. In fact, it is not 1,25-dihydroxy vitamin D3 but bile acids that transactivate the FGF19 gene through binding to the nuclear receptor farnesoid X receptor (FXR) (16). Although the mechanism by which the administration of active vitamin D3 increases FGF19 is unclear, all patients taking active vitamin D3 drugs tended to show improved profiles in serum parameters relevant to lipid metabolism, such as high HDL and low TG levels, which can potentially be affected by FGF19. In fact, the overexpression of FGF19 or treatment with recombinant FGF19 was reported to improve lipid metabolism profiles in rodents (26-29). It can also be argued that the ability of active vitamin D3 to alleviate oxidative stress and promote the uptake of glucose via the AMPK cascade may contribute to the improved lipid profiles (30). Further investigations are needed to understand the mechanism underlying the link between FGF19 and active vitamin D3.
FGF21 was reported to reduce the BP in a rat model of hypertension (31,32). In our study, the sBP of the patients with CCBs was higher than the patients without CCBs due to insufficient BP control. In addition, there was a significant correlation between FGF21 and CCB medication in the univariate analysis but not in the multivariable linear regression analysis. These results supported the association between FGF21 levels and hypertension. It was recently reported that high serum FGF21 levels were associated with a high mortality rate in end-stage renal disease (33). Thus, high FGF21 levels may be a response to stress, possibly caused by hypertension and other CKD complications. It can also be argued that the ability of FGF21 to activate the sympathetic nervous system and increase serum glucocorticoid levels may contribute to hypertension.
In addition, our data showed that the serum FGF21 levels of the patients with dyslipidemia were higher than the patients without dyslipidemia, regardless of statin medication. Statins decreased serum LDL concentrations, but the HDL and TG levels were still not improved in these patients. Because FGF21 is known to be induced by various nutritional and environmental stressors (12,32), the FGF21 levels might be affected by remaining dyslipidemia in CKD patients. These patients are considered to be at a higher risk for accelerated arteriosclerosis than those without an increase in FGF21. In addition, 2 out of 18 type 2 diabetes (T2D) patients had diabetic nephropathy (DN) in our cohort. An ROC analysis limited to the T2D patients showed the cut-off value of FGF21 for the diagnosis of DN to be 204 pg/mL (sensitivity: 1.0, specificity: 0.75). This cut-off value is lower than that of all CKD patients in our study. These results suggest that FGF21 may be an independent predictor of the loss of the renal function, especially in DN patients. A further investigation is needed to validate our results.
FGF23 is known to be increased in the early phase of CKD (15,17). However, our data showed that patients with high FGF23 levels had lower BAP and Hb values. Clinically, it is difficult to determine whether bone loss or anemia is a mere consequence of aging, known as age-related osteoporosis or so-called senile anemia, or an active complication of CKD in aged individuals. According to the present study, osteoporosis and anemia are likely to be caused by CKD if the serum FGF23 level is high, although validation with a larger cohort is essential.
Our data suggested that serum endocrine FGFs may serve as biomarkers for age-related diseases, such as CKD and metabolic syndrome, in aged patients; however, there were several limitations associated with this study. First, 79% of this study population were women who were highly concerned about osteoporosis because we published a paper about the correlation between soluble αKlotho and osteoporosis (20). They actively participated in this study. Second, there may be differences in serum endocrine FGF levels among postmenopausal women, younger women, and men. Finally, the correlations between serum endocrine FGFs and medication with active vitamin D3 or complications, such as cardiovascular disease, are preliminary data. To confirm these correlations, we must compare the concentration of serum endocrine FGFs before and after taking medicine or developing disease.
Conclusion
In conclusion, endocrine FGFs levels were found to be significantly associated with various age-related diseases and have the potential to be biomarkers for metabolic dysfunctions particularly in CKD patients. The measurement of FGF21 and FGF23 may be useful for evaluating CKD and its complications, including cardiovascular disease and metabolic bone disorder. Using serum endocrine FGFs as biomarkers for age-related conditions may ultimately lead to preventing elderly patients from entering a bedridden state.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by a research grant from the Fukushima Prefectural Hospitals Bureau (181-1) and Private University Research Branding Project of Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Shogo Yamamoto and Daisuke Koyama contributed equally to this work.
Acknowledgement
We would like to thank Michiko Saito, Masami Niida, Noriko Suzuki, Yumi Sato, Miyuki Ichijo, Saori Saito, Kayo Akagi, Toyoko Kanke, and Eiji Togawa for collecting the patient samples.
References
- 1. Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn 237: 18-27, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8: 235-253, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goetz R, Beenken A, Ibrahimi OA, et al. . Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27: 3417-3428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuro-o M, Matsumura Y, Aizawa H, et al. . Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45-51, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Azuma M, Koyama D, Kikuchi J, et al. . Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J 26: 4264-4274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurosu H, Ogawa Y, Miyoshi M, et al. . Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120-6123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito S, Kinoshita S, Shiraishi N, et al. . Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98: 115-119, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Kurosu H, Choi M, Ogawa Y, et al. . Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282: 26687-26695, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogawa Y, Kurosu H, Yamamoto M, et al. . BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 104: 7432-7437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu S, Tang W, Zhou J, et al. . Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305-1315, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Shimada T, Kakitani M, Yamazaki Y, et al. . Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561-568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angelin B, Larsson TE, Rudling M. Circulating fibroblast growth factors as metabolic regulators--a critical appraisal. Cell Metab 16: 693-705, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Inagaki T, Dutchak P, Zhao G, et al. . Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5: 415-425, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Bookout AL, de Groot MH, Owen BM, et al. . FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19: 1147-1152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503-533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inagaki T, Choi M, Moschetta A, et al. . Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217-225, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9: 650-660, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Kurosu H, Yamamoto M, Clark JD, et al. . Suppression of aging in mice by the hormone Klotho. Science 309: 1829-1833, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie T, Leung PS. Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am J Physiol Endocrinol Metab 313: E292-E302, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koyama D, Sato Y, Aizawa M, et al. . Soluble alphaKlotho as a candidate for the biomarker of aging. Biochem Biophys Res Commun 467: 1019-1025, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Saito I, Kushiro T, et al. . Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension 64: 989-996, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S, Collaborators Developing the Japanese Equation for Estimated GFR . GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis 61: 197-203, 2013. [DOI] [PubMed] [Google Scholar]
- 23. Mizuno H, Hoshide S, Fukutomi M, Kario K. Differing effects of aliskiren/amlodipine combination and high-dose amlodipine monotherapy on ambulatory blood pressure and target organ protection. J Clin Hypertens (Greenwich) 18: 70-78, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mizuno H, Hoshide S, Tomitani N, Kario K. Comparison of ambulatory blood pressure-lowering effects of higher doses of different calcium antagonists in uncontrolled hypertension: the calcium antagonist controlled-release high-dose therapy in uncontrolled refractory hypertensive patients (CARILLON) study. Blood Press 26: 284-293, 2017. [DOI] [PubMed] [Google Scholar]
- 25. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Makishima M, Lu TT, Xie W, et al. . Vitamin D receptor as an intestinal bile acid sensor. Science 296: 1313-1316, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Fu L, John LM, Adams SH, et al. . Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145: 2594-2603, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Tomlinson E, Fu L, John L, et al. . Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143: 1741-1747, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Wu AL, Coulter S, Liddle C, et al. . FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One 6: e17868, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manna P, Achari AE, Jain SK. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch Biochem Biophys 615: 22-34, 2017. [DOI] [PubMed] [Google Scholar]
- 31. He JL, Zhao M, Xia JJ, et al. . FGF21 ameliorates the neurocontrol of blood pressure in the high fructose-drinking rats. Sci Rep 6: 29582, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Z, Xu A, Cheung BMY. The potential role of fibroblast growth factor 21 in lipid metabolism and hypertension. Curr Hypertens Rep 19: 28, 2017. [DOI] [PubMed] [Google Scholar]
- 33. Kohara M, Masuda T, Shiizaki K, et al. . Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease. PLoS One 12: e0178971, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]