Abstract
Objective
Urinary angiotensinogen (AGT) is a surrogate marker for intrarenal renin-angiotensin system (RAS) activity that plays an important role in the development of renal damage. Urinary AGT levels are determined by the filtration of plasma AGT through the damaged glomeruli and production of AGT in the proximal tubules. However, the relative merits of the filtration and production of urinary AGT levels in chronic kidney diseases (CKD) have not been clarified. Therefore, we investigated them in CKD patients.
Methods
We recruited 41 biopsy-proven patients diagnosed with IgA nephropathy (IgAN) in 31, membranous nephropathy (MN) in 5, and tubulointerstitial nephritis (TIN) in 5. The patients taking RAS blockers were excluded.
Results
The urinary albumin levels in MN patients were significantly higher and those in TIN patients significantly lower than in IgAN patients, and the urinary AGT levels in the MN and TIN patients were significantly higher than those in IgAN patients. Conversely, the urinary AGT-to-urinary albumin (urinary AGT/Alb) ratios were the same for IgAN and MN patients, and those of TIN patients were significantly higher than those of IgAN and MN patients. A multiple linear regression analysis revealed that the urinary AGT/Alb ratios had a significant positive association with IgAN and TIN after adjustments (β=0.75, and p<0.01).
Conclusion
These data suggest that the origins of urinary AGT may differ according to the etiology of renal damage [i.e. glomerular damage (such as IgAN and MN) or tubulointerstitial damage (such as TIN)], and a higher urinary AGT/Alb ratio, as in TIN, may reflect AGT production in the kidney.
Keywords: chronic kidney disease, intrarenal renin-angiotensin system, urinary angiotensinogen to urinary albumin ratio
Introduction
It has been known for many years that the circulating renin-angiotensin system (RAS) plays a critical role in the regulation of arterial pressure and sodium homeostasis (1). Furthermore, a tissue-specific RAS exists in some organs, independent of the circulating RAS. Among the tissue-specific RASs, the intrarenal RAS plays a role in sodium reabsorption, inflammation, and fibrosis in the kidney and is one of the most important contributors to the pathophysiology in patients and animal models with chronic kidney disease (CKD) and hypertension (2-10).
Angiotensinogen (AGT) is the only known substrate for renin, the rate-limiting enzyme in the RAS. The AGT levels influence RAS activation since they are close to the Michaelis-Menten constant for renin (11,12), and urinary AGT is reported to be a useful biomarker that reflects the intrarenal RAS activity and CKD severity (2-5,13,14).
The mechanism underlying intrarenal RAS activation depends on the condition of the kidney disease. Urinary AGT levels are reportedly determined by the filtration of plasma AGT through the damaged glomeruli and production of AGT in the proximal tubules in some animal models (15,16). However, the relative merits of filtration and production of AGT for each CKD condition have not been clarified. Therefore, we investigated them in patients with several types of CKDs.
Materials and Methods
Patients
This study was approved by the ethics committee of Hamamatsu University School of Medicine and adhered to the principles of the Declaration of Helsinki.
We recruited 41 patients with CKD who were admitted to our hospital for a renal biopsy and diagnosed with IgA nephropathy (31 patients), membranous nephropathy (5 patients), and tubulointerstitial nephritis (5 patients) consecutively from February 2012 to November 2016. Because IgA nephropathy is the most common chronic glomerulonephritis, and membranous nephropathy is a representative glomerular disease that excretes massive albuminuria through the damaged glomeruli, we selected these patients for this study. In contrast, because tubulointerstitial nephritis is a representative tubulointerstitial disease without glomerular damage, we also selected patients with this condition.
Written informed consent was obtained from all patients. We excluded patients taking RAS blockers [i.e., angiotensin II (Ang II) receptor blockers, angiotensin-converting enzyme inhibitors, mineralocorticoid receptor blockers, or direct renin inhibitors], as these are known to suppress the intrarenal RAS activity (2,6,7,17).
Study protocols
Ambulatory blood pressure (BP) monitoring (ABPM) was conducted at 30-minute intervals for 24 hours using an automatic device (TM-2431; A and D, Tokyo, Japan). Urine samples were collected for 24 hours, and blood samples were drawn at 6:00 AM the next day, after the patients had rested in the supine position for at least 15 minutes. Thereafter, the samples were centrifuged at 3,000 rpm for 10 minutes at 4℃ and stored at −80℃ until the analysis, as described previously (18-21).
Clinical data
The patients' clinical data, such as age, sex, height, body weight, and body mass index (BMI) were recorded at the time of admission. During 24-hour ABPM, the BP and heart rate were measured noninvasively every 30 minutes as described above (18-21). Serum creatinine concentrations and urinary excretion levels of creatinine, albumin (Alb) and α1 microglobulin (α1MG), a surrogate marker of tubulointerstitial damage, were measured in the clinical laboratory of the Hamamatsu University School of Medicine, University Hospital. The levels of urinary AGT, known to be a surrogate marker of intrarenal RAS activity, were measured using an enzyme-linked immunosorbent assay (ELISA), as described previously (2,17,22).
The plasma Ang II level, known to be an effector of circulating RAS activity, was determined using a radioimmunoassay without special pretreatments (SRL, Tokyo, Japan). The serum creatinine concentrations were measured from blood, and the estimated glomerular filtration rate (eGFR) was calculated based on the serum creatinine concentrations using the Japanese eGFR equation (23).
The evaluation of tubulointerstitial lesions
A renal biopsy specimen was fixed in formalin and embedded in paraffin. Tissue sections (3 μm) were stained with Masson's trichrome for the histopathological evaluation of tubulointerstitial lesions. The percentage of tubulointerstitial fibrosis was evaluated in microscopic fields observed at ×100 magnification. All microscopic fields were evaluated for each patient, using a point-counting method, and the mean values were calculated. All quantitative analyses were performed by a blinded operator to avoid bias.
Statistical analyses
The results were expressed as the means ± standard deviation. Nonparametric data were expressed as the median (interquartile range). The significance of differences among the diseases was determined using an analysis of variance (ANOVA) or Mann-Whitney U test according to the distribution. Urinary daily Alb and AGT levels, the urinary daily AGT-to-Alb ratio (urinary AGT/urinary Alb), urinary α1-MG levels, and the ratios of urinary Alb to creatinine (urinary Alb/Cr) and urinary AGT to creatinine (urinary AGT/Cr) did not show a normal distribution, so logarithmic transformation was applied to them prior to the ANOVA.
Multiple linear regression analyses were conducted in order to evaluate the relationships between the category of CKD (IgA nephropathy vs. membranous nephropathy and IgA nephropathy vs. tubulointerstitial nephritis) and urinary AGT/urinary Alb. The age, sex, BMI, and eGFR were selected as independent variables because these parameters are commonly used to perform multiple linear regression analyses. Because systolic BP is positively associated with urinary AGT excretion levels, systolic BP was selected as an independent variable (24). The category of CKD was used as a dummy variable for multiple linear regression analyses. The receiver-operator characteristic (ROC) curves of the levels of urinary AGT/urinary Alb excretion ratio, urinary AGT excretion, and urinary Alb excretion of tubulointerstitial nephritis were examined. We considered a p value of <0.05 to be statistically significant. Statistical analyses were performed using the IBMⓇSPSSⓇ software program, version 23 (IBM Corporation, Armonk, USA).
Results
Patient characteristics
We recruited 41 patients with CKD who were admitted to our hospital for a renal biopsy and diagnosed with IgA nephropathy (31 patients), membranous nephropathy (5 patients), and tubulointerstitial nephritis (5 patients). We excluded patients taking RAS blockers because these drugs are known to suppress the intrarenal RAS activity.
The baseline characteristics are shown in Table 1. Although 8 patients, including 4 calcium channel blocker recipients, suffered from hypertension, their BP was well-controlled (117.2±15.5/72.1±10.3 mmHg, respectively). The patients' renal function was maintained, as follows: serum creatinine, 0.99±0.42 mg/dL and eGFR, 62.3±23.0 mL/min/1.73 m2. The median daily urinary Alb and urinary AGT excretion levels were 268.7 (110.5-848.8) mg/day and 59,279.3 (30,700.2-192,933.6) ng/day, respectively. Furthermore, the logarithmic transformation of daily urinary Alb and urinary AGT excretion levels as well as the urinary AGT/urinary Alb showed 2.48±0.60 mg/day, 4.96±0.76 ng/day, and 2.49±0.79 ng/mg, respectively, and the logarithmic transformation of urinary Alb/Cr and urinary AGT/Cr showed 2.47±0.64 μg/mg and 1.96±0.82 ng/mg, respectively.
Table 1.
Patient Characteristics.
| Age, year | 48.6±18.0 | |
| Sex | Male 13 / Female 28 | |
| Height (cm) | 161.5±9.5 | |
| Body weight (kg) | 54.9±10.0 | |
| Body mass index (kg/m2) | 21.0±2.9 | |
| Systolic BP (mmHg) | 117.2±15.5 | |
| Diastolic BP (mmHg) | 72.1±10.3 | |
| Heart rate (/min) | 66.2±7.3 | |
| sCr (mg/dL) | 0.99±0.42 | |
| eGFR (mL/min/1.73m2) | 62.3±23.0 | |
| CKD stage | Stage 1: 5/Stage 2: 17/Stage 3: 14/Stage 4: 5/Stage 5: 0 | |
| Plasma Ang II (pg/mL) | 11.7±5.8 | |
| Urinary Alb/day (mg/day) | 268.7 [110.5-848.8] | |
| Log u-Alb/day (mg/day) | 2.48±0.60 | |
| Log u-Alb/Cr (μg/mg) | 2.47±0.64 | |
| Urinary AGT/day (ng/day) | 59,279.3 [30,700.2-192,933.6] | |
| Log u-AGT/day (ng/day) | 4.96±0.76 | |
| Log u-AGT/Cr (ng/mg) | 1.96±0.82 | |
| Log u-AGT/Alb (ng/mg) | 2.49±0.79 | |
| Urinary α1MG (mg/L) | 7.60 [3.03-17.03] | |
| Log u-α1MG (mg/L) | 0.93±0.53 | |
| Interstitial fibrosis (%) | 25.7±20.9 |
BP: blood pressure, sCr: serum creatinine, eGFR: estimated glomerular filtration rate, CKD: chronic kidney disease, Ang II: angiotensin II, u-Alb/day: urinary albumin/day, u-Alb/Cr: urinary albumin to creatinine ratio, u-AGT/day: urinary angiotensinogen/day, u-AGT/Cr: urinary angiotensinogen to creatinine ratio, u-AGT/Alb: urinary angiotensinogen to albumin ratio, u-α1MG: urinary alpha 1 microglobulin
Patient characteristics by IgA nephropathy, membranous nephropathy, and tubulointerstitial nephritis
Table 2 shows the patient characteristics by IgA nephropathy, membranous nephropathy and tubulointerstitial nephritis. The patients with membranous nephropathy and tubulointerstitial nephritis tended to be older than those with IgA nephropathy. However, no significant differences in the sex, body mass index, blood pressure, or heart rate were found among the groups.
Table 2.
Patient Characteristics in IgA Nephropathy (IgAN), Membranous Nephropathy (MN) and Tubulointerstitial Nephritis (TIN).
| IgAN | MN | TIN | ||||
|---|---|---|---|---|---|---|
| Age, year | 48.6±18.0 | 68.8±12.6* | 66.4±7.1** | |||
| Sex (Male / Female) | 12 / 19 | 1 / 4 | 0 / 5 | |||
| Height (cm) | 162.8±10.4 | 159.0±4.3 | 156.0±3.8* | |||
| Body weight (kg) | 56.4±10.5 | 52.6±6.9 | 48.6±7.3 | |||
| Body mass index (kg/m2) | 21.2±3.0 | 20.8±2.2 | 20.0±3.6 | |||
| Systolic BP (mmHg) | 116.2±13.1 | 117.8±17.6 | 123.4±27.4 | |||
| Diastolic BP (mmHg) | 72.3±10.0 | 67.8±12.7 | 75.2±11.1 | |||
| Heart rate (/min) | 65.7±6.1 | 65.6±8.3 | 69.2±13.0 | |||
| sCr (mg/dL) | 0.88±0.22 | 0.90±0.36 | 1.82±0.51*, # | |||
| eGFR (mL/min/1.73m2) | 69.1±18.6 | 58.0±20.5 | 24.1±7.6**, # | |||
| Plasma Ang II (pg/mL) | 11.8±4.5 | 7.6±3.1 | 14.8±11.8 | |||
| Log u-Alb/day (mg/day) | 2.48±0.43 | 3.29±0.48* | 1.64±0.49*, ## | |||
| Log u-Alb/Cr (μg/mg) | 2.45±0.50 | 3.35±0.51** | 1.74±0.49*, ## | |||
| Log u-AGT/day (ng/day) | 4.69±0.51 | 5.61±1.04 | 6.00±0.60* | |||
| Log u-AGT/Cr (ng/mg) | 1.66±0.56 | 2.67±1.05** | 3.10±0.61** | |||
| Log u-AGT/Alb (ng/mg) | 2.21±0.35 | 2.32±0.58 | 4.36±0.19**, ## | |||
| Log u-α1MG (mg/L) | 0.73±0.35 | 1.27±0.48 | 1.83±0.33** | |||
| Interstitial fibrosis (%) | 21.4±15.3 | 18.0±16.8 | 60.0±25.2# |
BP: blood pressure, sCr: serum creatinine, eGFR: estimated glomerular filtration rate, Ang II: angiotensin II, u-Alb/day: urinary albumin/day, u-Alb/Cr: urinary albumin to creatinine ratio, u-AGT/day: urinary angiotensinogen/day, u-AGT/Cr: urinary angiotensinogen to creatinine ratio, u-AGT/Alb: urinary angiotensinogen to urinary albumin ratio, u-α1MG: urinary alpha 1 microglobulin
*p<0.05 and **p<0.01 versus IgA nephropathy and#p<0.05 and##p<0.01versus membranous nephropathy
There were also no significant differences in the renal function and tubulointerstitial fibrosis between the patients with IgA nephropathy and membranous nephropathy. However, the levels of serum creatinine and tubulointerstitial fibrosis were significantly higher in the patients with tubulointerstitial nephritis than in those with IgA nephropathy and membranous nephropathy.
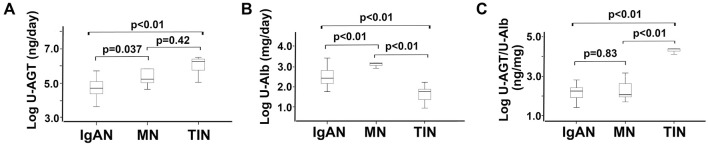
The mean levels of daily urinary Alb excretion and urinary Alb/Cr in the patients with membranous nephropathy and tubulointerstitial nephritis were significantly higher and lower, respectively, than those in patients with IgA nephropathy. In contrast, the mean levels of daily urinary AGT excretion and urinary AGT/Cr in the patients with tubulointerstitial nephritis were significantly higher than those in the patients with IgA nephropathy. Furthermore, the mean levels of urinary AGT/urinary Alb excretion ratios were roughly the same between the patients with IgA nephropathy and membranous nephropathy. In contrast, the mean levels of urinary AGT/urinary Alb excretion ratios of patients with tubulointerstitial nephritis were significantly higher than those of the patients with IgA nephropathy and membranous nephropathy.
Fig. 1A, B and Supplementary material show the distribution of daily urinary AGT and Alb excretion levels and urinary AGT- and Alb-to-creatinine ratios, and Fig. 1C shows the urinary AGT/urinary Alb excretion ratios in all three CKDs. Although the distributions of the daily urinary AGT and Alb excretion (urinary AGT; p=0.037 and urinary Alb; p<0.01, respectively) and urinary AGT- and Alb-to-creatinine ratios (urinary AGT/Cr; p=0.014 and urinary Alb/Cr; p<0.01, respectively) were significantly different between IgA nephropathy and membranous nephropathy, the distribution of the urinary AGT/urinary Alb excretion ratio between IgA nephropathy and membranous nephropathy was not markedly different (p=0.83). However, the distributions of the daily urinary AGT and Alb excretion (urinary AGT; p<0.01 and urinary Alb; p<0.01, respectively) and urinary AGT- and Alb-to-creatinine ratios (urinary AGT/Cr; p<0.01 and urinary Alb/Cr; p<0.01, respectively) between IgA nephropathy and tubulointerstitial nephritis were significantly different, as was the distribution of the urinary AGT/urinary Alb excretion ratio between IgA nephropathy and tubulointerstitial nephritis (p<0.01).
Figure 1.
The comparison of urinary daily angiotensinogen (U-AGT) levels, urinary albumin (U-Alb) levels, and U-AGT to U-Alb ratios (U-AGT/U-Alb) among the categories of chronic kidney disease. The box plots represent the 25th percentile, median, and 75th percentile of each group. Error bars denote the 10th and 90th percentiles. IgAN: IgA nephropathy, MN: membranous nephropathy, TIN: tubulointerstitial nephritis
Multiple linear regression analyses of the urinary AGT/urinary Alb ratio and the clinical parameters, including the causative disorders of CKD (IgA nephropathy, membranous nephropathy, and tubulointerstitial nephritis)
We performed multiple linear regression analyses to evaluate the relationship between urinary AGT/urinary Alb excretion ratios and the causative disorders of CKD (IgA nephropathy, membranous nephropathy, and tubulointerstitial nephritis). Multiple linear regression analyses revealed that the urinary AGT/urinary Alb excretion ratio had a significant positive association with the causative disorders between IgA nephropathy and tubulointerstitial nephritis but not between IgA nephropathy and membranous nephropathy after adjusting for the age, sex, BMI, eGFR, and systolic BP (Model 3; between IgA nephropathy and membranous nephropathy: β=−0.015, p=0.85; and between IgA nephropathy and tubulointerstitial nephritis: β=0.75, p<0.01, respectively) (Table 3).
Table 3.
Multiple Linear Regression Analyses of Urinary Angiotensinogen to Urinary Albumin Ratio (urinary AGT / Urinary Alb Ratio) and the Clinical Parameters Including the Causative Disorders of Chronic Kidney Diseases.
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r=0.91 p<0.01 | r=0.91, p<0.01 | r=0.93, p<0.01 | ||||||||||
| β | p | β | p | β | p | |||||||
| Age | 0.18 | 0.063 | 0.089 | 0.45 | 0.045 | 0.69 | ||||||
| Sex | 0.059 | 0.45 | 0.069 | 0.38 | 0.16 | 0.065 | ||||||
| Body mass index (kg/m2) | 0.020 | 0.79 | 0.010 | 0.14 | 0.013 | 0.85 | ||||||
| eGFR (mL/min/1.73m2) | -0.15 | 0.22 | -0.089 | 0.45 | ||||||||
| Systolic BP (mmHg) | 0.19 | 0.034 | ||||||||||
| Causative disorders of CKD | ||||||||||||
| IgAN vs. MN | -0.044 | 0.61 | -0.027 | 0.75 | -0.015 | 0.85 | ||||||
| IgAN vs. TIN | 0.81 | <0.01 | 0.74 | <0.01 | 0.75 | <0.01 | ||||||
eGFR: estimated glomerular filtration rate, BP: blood pressure, CKD: chronic kidney disease, IgAN: IgA nephropathy, MN: membranous nephropathy, TIN: tubulointerstitial nephritis
Finally, we examined the area under the ROC curve of the urinary AGT/urinary Alb excretion ratio, urinary AGT excretion levels, and urinary Alb excretion levels of tubulointerstitial nephritis to demonstrate the diagnostic efficacy. The area under the individual ROC curve was 1.00 for the urinary AGT/urinary Alb excretion ratio, 0.91 for the urinary AGT excretion levels, and 0.072 for the urinary Alb excretion levels, making the area under the ROC curve of the urinary AGT/urinary Alb excretion ratio highest among the parameters (Fig. 2).
Figure 2.
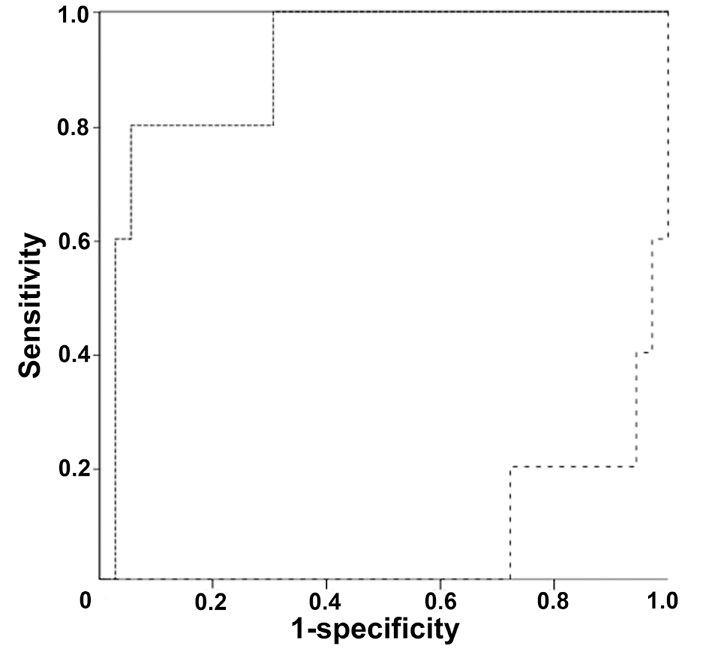
Receiver-operator characteristic (ROC) curves of the urinary angiotensinogen (AGT) /urinary albumin (Alb) excretion ratio, urinary AGT excretion levels, and urinary Alb excretion levels of tubulointerstitial nephritis. The area under the individual ROC curve was 1.00 for the urinary AGT/urinary Alb excretion ratio, 0.91 for the urinary AGT excretion levels, and 0.072 for the urinary Alb excretion levels. The solid line, dotted line, and dashed line indicate the ROC curves of the urinary AGT/urinary Alb excretion ratio, urinary AGT excretion levels, and urinary Alb excretion levels of tubulointerstitial nephritis, respectively.
Discussion
The mechanisms underlying intrarenal RAS activation depend on the type of kidney disease in some animal models.
One mechanism underlying AGT production involves the proximal tubules. Nakano et al. reported the glomerular permeability of Atto565-labeled human AGT (Atto565-hAGT) using multiphoton microscopy in intact mouse and rat kidneys. In healthy mice and Munich-Wistar-Frömter rats at the early stage of glomerulosclerosis, the glomerular sieving coefficient of systemically infused Atto565-hAGT was only 25% of the glomerular sieving coefficient of albumin, and its urinary excretion was undetectable. Furthermore, they showed that the glomerular permeability of Atto565-hAGT was slightly higher but still very low in a more advanced phase of kidney disease. The significantly higher urinary excretion of endogenous rat AGT did not correlate with either the Atto565-hAGT or Atto565-albumin glomerular sieving coefficients. Given these results, the authors concluded that most urinary AGT originates from the tubules rather than glomerular filtration (15). In addition, Kobori et al. investigated whether or not urinary AGT originates from production in the kidney. They used the following three groups: 1) single transgenic mice expressing human AGT only in the kidney (group A), 2) double-transgenic mice expressing human renin systemically in addition to human AGT only in the kidney (group D), and 3) wild-type mice (group W). They found that the kidney Ang II levels were higher in group D than in groups A and W, and that endogenous renal mouse AGT mRNA and protein were significantly higher in group D than in groups A or W. As a result, they concluded that the selective stimulation of intrarenal production of Ang II from human AGT augments endogenous intrarenal mouse AGT mRNA and protein expression (25).
Another mechanism of AGT production is the filtration of plasma AGT through the damaged glomeruli. Matsusaka et al. investigated the mechanism underlying the enhanced renal Ang II generation in glomerular diseases using kidney- or liver-specific AGT gene knockout mice with inducible podocyte injury. They demonstrated that kidney AGT knockout mice had attenuated renal AGT mRNA but not renal Ang II, renal, or urinary AGT protein. Furthermore, liver AGT knockout mice had markedly reduced renal Ang II, renal and urinary AGT protein but not renal AGT mRNA. Given these findings, they concluded that the increased renal Ang II generation could be attributed to increased filtered AGT of liver origin, resulting from the loss of the macromolecular barrier function of the glomerular capillary wall upon severe podocyte injury (16). In addition, although it is difficult to clarify causal relationships in clinical research, most clinical studies have shown that the urinary AGT levels significantly and positively correlate with urinary Alb levels (2,17,24). These data show that the majority of urinary AGT excretion reflects plasma AGT filtered through damaged glomerular capillaries.
However, Nishiyama et al. reported that even though the levels of proteinuria in patients with minor glomerular abnormalities are much higher than those in patients with IgA nephropathy, the levels of urinary AGT excretion in patients with minor glomerular abnormalities are much lower than those in patients with IgA nephropathy. In addition, they showed that the urinary AGT excretion levels are significantly and positively associated with the expression of AGT mRNA and Ang II in the kidney. They concluded that AGT in the kidney is produced by positive feedback of Ang II in the kidney (4). In addition, Saito et al. reported that urinary AGT excretion precedes microalbuminuria and suggested that the intrarenal RAS is activated in the early phase of type 1 diabetes mellitus nephropathy (5). We also clarified that the levels of urinary AGT excretion but not urinary Alb excretion were significantly increased and that no significant correlations were found between urinary AGT excretion and urinary albumin excretion in kidney transplant donors immediately after kidney donation. We therefore concluded that AGT in the kidney is produced by activation in the kidney and not from the filtration of plasma AGT (19).
In the present study, although the distributions of the daily urinary AGT and Alb excretion and urinary AGT- and Alb-to-creatinine ratios were significantly different between IgA nephropathy and membranous nephropathy, the distribution of the urinary AGT/urinary Alb excretion ratio between IgA nephropathy and membranous nephropathy was not markedly different. In addition, we also showed that the distribution of the daily urinary AGT and Alb excretion differed significantly between IgA nephropathy and tubulointerstitial nephritis, as did the distribution of the urinary AGT/urinary Alb excretion ratio between IgA nephropathy and tubulointerstitial nephritis. These relationships were confirmed after adjusting for the age, sex, BMI, eGFR, and systolic BP. These data suggest that urinary AGT excretion in patients with membranous nephropathy reflects filtration through the glomerular capillaries and that urinary AGT excretion in patients with tubulointerstitial nephritis reflects the production in the kidney.
Why the intrarenal RAS activation was increased in patients with tubulointerstitial nephritis is unclear. However, inflammation and reactive oxygen species (ROS) in tubulointerstitial nephritis are well known to be upregulated, and inflammation and ROS augment intrarenal RAS activation (9,26-31). For example, Satou et al. showed that interleukin-6 (IL-6) augments AGT in primary cultured renal proximal tubular cells (27). In addition, Ozawa et al. found that inflammatory pathway activators, such as nuclear factor-kappa B, play a pivotal role in Ang II-induced renal injury (28). We have also shown the sequential activation of the ROS/AGT/RAS axis in some animal models, such as type 2 diabetic nephropathy, IgA nephropathy, and crescentic glomerulonephritis (9,29,30). Ogawa et al. additionally demonstrated in diabetic nephropathy patients that Ang II receptor blocker treatment reduced the blood pressure and urinary levels of AGT, the albumin-to-creatinine ratio, oxidative stress markers such as 8-hydroxydeoxyguanosine (8-OHdG) and 8-epi-prostaglandin F2α (8-epi-PGF2α), and key inflammatory factors such as monocyte chemoattractant protein (MCP)-1 and IL-6. They also found that the reduction rate of urinary AGT correlated with the reduction rates of BP and the urinary albumin to creatinine ratio (ACR), 8-OHdG, 8-epi-PGF2alpha, MCP-1, and IL-6 levels (31). These data suggest that tubulointerstitial nephritis upregulates the intrarenal RAS activity via inflammation and ROS activation aggressively in the kidney.
Several limitations associated with the present study warrant mention. First, it is possible that the urinary AGT excretion levels in tubulointerstitial nephritis are higher than those in IgA nephropathy due to the development of renal damage. Therefore, the urinary AGT excretion levels should be compared between severely damaged IgA nephropathy and tubulointerstitial nephritis in this study or between IgA nephropathy in this study and mildly damaged tubulointerstitial nephritis. However, most cases of IgA nephropathy are detected because of abnormal urinalysis findings at a medical checkup in Japan. As a result, a renal biopsy is performed in the early phase of IgA nephropathy. In contrast, because abnormal urinalysis findings are uncommon in tubulointerstitial nephritis, severe renal damage develops in most cases. Therefore, it is very difficult to compare similar degrees of renal damage between IgA nephropathy and tubulointerstitial nephritis in the usual clinical setting. Consequently, we performed multiple linear regression analyses, which showed that the urinary AGT excretion levels had a significant positive association with the causative disorders between IgA nephropathy and tubulointerstitial nephritis after adjusting for the eGFR (β=0.75, p<0.01, data not shown). As a result, we believe that the differences in the urinary AGT excretion levels are due to the causative disorders and not the degrees of renal damage.
Second, the sample size was relatively small, because this study was conducted in our single-center cohort, and patients who took RAS blockers, which are used broadly for CKD patients as renal protective agents, were excluded. However, as shown in Fig. 1 and Supplementary material, the distributions of urinary AGT, urinary Alb, and urinary AGT/urinary Alb ratio in IgA nephropathy, membranous nephropathy and tubulointerstitial nephritis were remarkably different, and the fact that the origin of urinary AGT differs among kidney diseases has been clearly shown. A large study is expected to be conducted in the future to set cut-off values of the urinary AGT/urinary Alb ratio in order to distinguish between filtration through the damaged glomerulus and production in the proximal tubules.
Finally, we were unable to evaluate the expression of AGT mRNA in the proximal tubules by kidney disease because bleeding from the kidney is one of the most serious side effects, and additional punctures for research are not permitted in our hospital. In addition, AGT is abundantly localized in the proximal tubules rather than in the glomeruli and small vessels. Therefore, when the sample size is small, the percentage of proximal tubules in each specimen changes markedly. As a result, it is difficult to evaluate the expression of AGT mRNA using renal biopsy samples. For these reasons, we were unable to evaluate the expression of AGT mRNA in this clinical study.
In conclusion, the findings from the present study suggest that the origin of urinary AGT may differ according to the etiology of renal damage (glomerular or tubulointerstitial damage), and a higher urinary AGT/urinary Alb ratio, such as that seen in TIN, may reflect AGT production in the kidney.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
The comparison of urinary angiotensinogen and albumin levels to urinary creatinine levels among the categories of chronic kidney disease
References
- 1. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251-287, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Kobori H, Alper AB Jr, Shenava R, et al. . Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 53: 344-350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamoto T, Nakagawa T, Suzuki H, et al. . Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558-1565, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Nishiyama A, Konishi Y, Ohashi N, et al. . Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant 26: 170-177, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci 338: 478-480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohashi N, Katsurada A, Miyata K, et al. . Activation of reactive oxygen species and the renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol 36: 509-515, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Isobe S, Ohashi N, Ishigaki S, et al. . Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res 39: 312-320, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592-597, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohashi N, Katsurada A, Miyata K, et al. . Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol 36: 750-755, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohashi N, Yamamoto T, Huang Y, et al. . Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Renal Physiol 295: F1512-F1518, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res 5: 86-89, 1971. [DOI] [PubMed] [Google Scholar]
- 12. Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension 27: 465-475, 1996. [DOI] [PubMed] [Google Scholar]
- 13. Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579-585, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobori H, Navar LG. Urinary angiotensinogen as a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. Int Rev Thromb 6: 108-116, 2011. [PMC free article] [PubMed] [Google Scholar]
- 15. Nakano D, Kobori H, Burford JL, et al. . Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol 23: 1847-1856, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int 85: 1068-1077, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobori H, Ohashi N, Katsurada A, et al. . Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens 2: 349-354, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuyama T, Ohashi N, Ishigaki S, et al. . The relationship between the intrarenal dopamine system and intrarenal renin-angiotensin system depending on the renal function. Intern Med 57: 3241-3247, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohashi N, Isobe S, Matsuyama T, et al. . The intrarenal renin-angiotensin system is activated immediately after kidney donation in kidney transplant donors. Intern Med 58: 643-648, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohashi N, Isobe S, Ishigaki S, et al. . The Effects of unilateral nephrectomy on blood pressure and its circadian rhythm. Intern Med 55: 3427-3433, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukuda M, Mizuno M, Yamanaka T, et al. . Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension 52: 1155-1160, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Katsurada A, Hagiwara Y, Miyashita K, et al. . Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 293: F956-F960, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, et al. . Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Isobe S, Ohashi N, Fujikura T, et al. . Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol 19: 231-239, 2015. [DOI] [PubMed] [Google Scholar]
- 25. Kobori H, Ozawa Y, Satou R, et al. . Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938-F945, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ni XJ, Xu ZQ, Jin H, Zheng SL, Cai Y, Wang JJ. Ginsenoside Rg1 protects human renal tubular epithelial cells from lipopolysaccharide-induced apoptosis and inflammation damage. Braz J Med Biol Res 51: e6611, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Satou R, Gonzalez-Villalobos RA, Miyata K, et al. . IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol 311: 24-31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappaB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol 293: F100-F109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol 35: 922-927, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urushihara M, Ohashi N, Miyata K, Satou R, Acres OW, Kobori H. Addition of angiotensin II type 1 receptor blocker to CCR2 antagonist markedly attenuates crescentic glomerulonephritis. Hypertension 57: 586-593, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogawa S, Kobori H, Ohashi N, et al. . Angiotensin II Type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with Type 2 diabetic nephropathy. Biomark Insights 4: 97-102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The comparison of urinary angiotensinogen and albumin levels to urinary creatinine levels among the categories of chronic kidney disease