Abstract
Background:
Sarcoidosis is a granulomatous inflammatory disease with limited blood markers to predict outcomes. The interferon-gamma (IFN-γ)-inducible chemotactic cytokines (chemokines), CXCL9 and CXCL10, are both increased in sarcoidosis patients, yet they possess important molecular differences. Our study determined if serum chemokines correlated with different aspects of disease severity.
Methods:
We measured CXCL9 and CXCL10 serum levels at initial study visits and longitudinally in sarcoidosis subjects using ELISA. We examined these chemokines’ relationships with pulmonary, and organ involvement outcomes, their gene expression, peripheral blood immune cell populations, and immunosuppression use.
Results:
Higher CXCL10 levels negatively correlated with FVC, TLC, and DLCO at subjects’ initial visit and when measured repeatedly over two years. CXCL10 also positively correlated with longitudinal respiratory symptom severity. Additionally, for every log10(CXCL10) increase, the risk of longitudinal pulmonary function decline increased 8.8 times over the 5-year study period (95% CI 1.6 – 50, p = 0.014, log10(CXCL0) range 0.84 – 2.7). In contrast, CXCL9 levels positively correlated with systemic organ involvement at initial study visit (1.5 additional organs involved for every log10(CXCL9) increase, 95% CI 1.1 – 2.0, p = 0.022, log10(CXCL9) range 1.3 – 3.3). CXCL10, not CXCL9, positively correlated with its own blood gene expression and monocyte level. Immunosuppressive treatment was associated with lower levels of both chemokines.
Conclusions:
In sarcoidosis subjects, serum CXCL9 levels correlated with systemic organ involvement and CXCL10 levels strongly correlated with respiratory outcomes, which may ultimately prove helpful in clinical management. These differing associations may be due to differences in cellular regulation and tissue origin.
Keywords: Sarcoidosis, CXCL9, CXCL10, CXCL11, Interferon-gamma, Chemokine
1. Introduction
Sarcoidosis is a systemic inflammatory disease characterized by non-necrotizing granulomas occurring in adults of any age, sex, or race [1–3]. Although any organ can be affected, pulmonary involvement occurs in over 90% of patients, which can lead to abnormal lung function and debilitating respiratory symptoms [4]. Two important clinical challenges in the disease include predicting which patients will develop progressive pulmonary and multi-organ disease, and deciding who to treat and when to stop since treatment is not curative [5–7]. These clinical challenges persist partly due to the paucity of detailed longitudinal sarcoidosis studies.
IFN-gamma (IFN-γ) is one of the main cytokines in sarcoidosis-associated inflammation [8–13], and several studies have identified upregulation of interferon-inducible chemotactic cytokines (chemokines) in the blood of sarcoidosis patients compared to control groups [14–23]. These chemokines, CXCL9, CXCL10, and CXCL11 all bind to the CXCR3 receptor, and are responsible for homing of CD4+ T cells, monocytes, and other inflammatory cells to sites of inflammation including granulomas [16, 17, 24–29]. These chemokines also participate in other functions related to angiogenesis and cell proliferation [30–32]. Results from studies in mice suggest that these chemokines have nonredundant functions in vivo [24, 33]. For example, CXCL11 has unique properties related to T cell function and can also bind to the CXCR7 receptor [34, 35], which led us to examine it in a previous study where we found its relationships to clinical outcomes of lung function and organ involvement [23].
The other two chemokines, CXCL9 and CXCL10, can also bind and signal through the CXCR3 receptor, but with different binding affinities [36, 37]. They also differ in how they are induced, their ability to antagonize other receptors [38], and spatial expression in vivo. For example, CXCL9 is solely induced by IFN-γ, whereas CXCL10 can also be induced by TNF-α, INF-a2, and LPS [24, 39–47]. Furthermore, CXCL9 and CXCL10 are expressed in different tissues in humans [46, 47], and are produced by different cellular sources in mouse models directly comparing their production in vivo [40]. These prior observations led us to hypothesize that CXCL9 and CXCL10 may be associated with different clinical outcomes based on their differing biological properties. In this study, we wanted to determine if lung disease severity and multi-organ involvement are differentially influenced by circulating levels of CXCL9 and CXCL10. To understand the relationship between chemokine levels, pulmonary physiology, organ involvement, and other clinical data, we took advantage of longitudinal measurements obtained over a five-year follow-up period within our University of California, San Francisco (UCSF) Sarcoidosis Cohort [48]. Finding unique relationships between chemokines and distinct clinical features may provide more specific markers for clinically meaningful outcomes to inform the care of sarcoidosis patients.
2. Methods
2.1. Study Population and Measurements
We enrolled sarcoidosis subjects who met diagnostic criteria established by the American Thoracic Society [4] at any point in their disease course as part of the UCSF Sarcoidosis Cohort as previously described [48]. The study design required tissue confirmation of granulomatous inflammation and no alternative lung disease at enrollment but individuals did not have to be newly diagnosed to participate. This cohort had follow-up visits every 6 to 12 months for up to 66 months (~5 years). At each visit, blood sampling was performed and clinical data were collected. The following data were used in this study: demographics, organ involvement at the initial visit (as assessed by physician review of medical records) [48], chest X-ray imaging at the initial visit, clinical laboratory tests including complete blood counts, and pulmonary function tests, which included forced expiratory volume in 1 second (FEV1) percent predicted (%pred), forced vital capacity (FVC %pred), diffusing capacity for carbon monoxide (DLCO %pred), and total lung capacity (TLC %pred), and severity of respiratory symptoms as assessed by the UCSD Dyspnea Questionnaire [49–51]. We obtained immunosuppression use history, including dosages of oral corticosteroids or disease-modifying antirheumatic drugs (DMARDs), specifically, methotrexate, azathioprine, mycophenolate, colchicine, hydroxychloroquine, or anti-TNF-α therapy that subjects were actively taking at the time of their study visits. For the current analysis, we included gene transcript levels of CXCL9 and CXCL10 from whole blood RNA samples that we previously described [22].
2.2. Protein Assay
We measured levels of CXCL9 and CXCL10 in serum using Quantikine Colorimetric Sandwich ELISA kits on samples obtained over a two-year time period after enrollment per manufacturer’s instructions (R&D Systems Minneapolis, MN, USA). Samples were thawed, analyzed in duplicate, and processed in one batch. The duplicates per sample were averaged for final interpretation.
2.3. Statistical Analysis
We normalized chemokine levels and dyspnea scores using log10 transformations given their skewed distributions prior to analysis. We used Chi-squared tests for analyzing categorical variables, t-tests for bivariate comparisons of continuously distributed parametric data, and analysis of variance (ANOVA) to analyze variables with more than two groups. For adjusted cross-sectional analyses of data obtained at the initial visit, we used linear regression models for continuous and normally distributed clinical outcome variables; logistic regression for binary outcomes; and Poisson regression for count data (i.e. number of organs involved, where thoracic adenopathy and/or lung parenchymal involvement was considered one organ).
To analyze all follow-up visit data and account for drop-outs, we used mixed effects linear regression models that assessed correlations between changes in predictors and dependent variables measured over multiple visits. The fixed effects were the clinical predictors of interest and the random effects were the subjects. In these mixed effects models, we made conservative assumptions by allowing each subject to have a separate intercept, allowing slopes to vary by subject, and using unstructured covariation matrices [52, 53]. To determine if either chemokine was predictive of clinically significant pulmonary function decline [54–56], we performed a time-to-event analysis as described in the Supplementary Materials.
To identify independent predictors of chemokine levels, we performed similar linear regression and mixed effects models where chemokine level was the outcome and whole blood RNA transcript levels for CXCL9 and CXCL10, blood immune cell populations, or different classifications of immunosuppressionuse (see Supplementary Materials) were the predictors of interest. We calculated correlation coefficients (r values) for these models by taking the square-root of the adjusted R2 from the linear regression equations; for mixed effects linear regression models, the R2 values were initially calculated using methods as described by Snijders and Bosker [57]. Where indicated, we adjusted regression models for several confounders including age, sex, race, binary designations for immunosuppression use (yes/no), and prior smoking history (yes/no). All statistical analyses were done using Stata/SE 15.1 software (StataCorp LLC, College Station, TX) and GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA) was used to construct figures.
3. Results
3.1. Characteristics of Sarcoidosis Subjects
One hundred and eight sarcoidosis subjects had available longitudinal blood and clinical measurements for this analysis. There were 103/108 subjects who had samples available for chemokine measurements from the initial study visit; the remaining 5/108 had measurements at the second and/or later visits (Table 1). Forty-nine percent of subjects were taking systemic immunosuppressive therapy at the initial study visit and the majority of subjects (74%) had extra-thoracic involvement defined by physician assessment of medical records (Table 2).
Table 1.
Demographics at each visit
| All Subjects | Initial Visit | 6-month follow-up | 12-month follow-up | 24-month follow-up | |
|---|---|---|---|---|---|
| Total number per visit | 108 | 103 | 67 | 59 | 36 |
| Age (years ± SD) | 50 ± 10 | 51 ± 10 | 52 ± 11 | 53 ± 11 | 56 ±11 |
| Female (%) | 67 (62) | 64 (62) | 37 (54) | 32 (54) | 19 (53) |
| Race (%) | |||||
| African American | 15 (14) | 15 (15) | 7 (10) | 5 (8) | 5 (14) |
| White | 75 (69) | 71 (69) | 48 (71) | 45 (76) | 25 (69) |
| Hispanic | 8 (7.4) | 8 (7.8) | 7 (10) | 5 (8) | 4 (11) |
| Other Ethnicity | 10 (9.3) | 9 (8.7) | 6 (9) | 4 (7) | 2 (6) |
| Ever Smokers (%) | 50 (46) | 47 (46) | 30 (44) | 23 (39) | 14 (39) |
Table 2.
Clinical characteristics of sarcoidosis subjects at their initial visit
| Imaging: Scadding Stage | N (%) | ||
|---|---|---|---|
| 0 | 11† (10) | ||
| 1 | 13 (12) | ||
| 2 | 50 (46) | ||
| 3 | 10 (9) | ||
| 4 | 24 (22) | ||
| Immunosuppression use | 49 (45) | ||
| Extra-thoracic involvement | 74 (69) | ||
| Pulmonary Function Tests | |||
| N | Mean (SD) | Range | |
| FVC %predicted | 100 | 96 (15) | 59 - 140 |
| FEV1 %predicted | 100 | 90 (18) | 28 - 140 |
| FEV1/FVC | 100 | 0.75 (0.088) | 0.34-0.95 |
| DLCO %predicted | 73 | 71 (14) | 39 - 108 |
| TLC %predicted | 68 | 96 (15) | 59 - 131 |
Two subjects with Scadding stage 0 had prior lung involvement, the other subjects either had neurologic or skin involvement
Abbreviations: DLCO = Diffusing Capacity of the Lungs for Carbon Monoxide, FEV1 = Forced Expiratory Volume in 1 Second, FEV1/FVC = Forced Expiratory Volume in 1 Second to Forced Vital Capacity ratio, FVC = Forced Vital Capacity, TLC = Total Lung Capacity
3.2.1. CXCL10 had Greater Correlation with Pulmonary Function and Respiratory Symptoms Relative to CXCL9
We previously found that CXCL9 and CXCL10 levels were not perfectly correlated with each other at study enrollment (Spearman r =0.72) [23]. Thus, we hypothesized that these chemokines may relate differently to specific outcomes, including lung function. We first correlated lung function with serum levels of each chemokine at initial subject visit while adjusting for prior smoking and immunosuppression use (Fig. 1). We found that %predicted FVC was 17 percentage points lower (~670 mL in this cohort) for each 10-fold increase in CXCL10 (e.g. 1.6 to 2.6 log10 units on the log scale) (Fig. 1A). We found similar results for FEV1, DLCO, and TLC (Fig. 1B–D, Supplementary Table S1).
Fig. 1.

Correlations between pulmonary function measurements and serum CXCL10 levels at initial subject visit. A) FVC, B) FEV1, C) DLCO, and D) TLC. Data are displayed as log10 transformations of serum CXCL10 levels (individual values denoted by open black circles) and fitted lines for predicted pulmonary function values (dashed lines). The β-coefficients show how the average %predicted pulmonary function values vary for every log10-increase in CXCL10 (see Supplementary Table S1) (*p <0.05, **P<0.01). Both fitted lines and β-coefficients were adjusted for prior smoking and immunosuppression use. Abbreviations: FEV1 = Forced Expiratory Volume in 1 Second, FVC= Forced Vital Capacity, DLCO = Diffusing Capacity for Carbon Monoxide.
Our primary analysis goal was to examine the relationships between repeated measurements of lung function and chemokine levels over the initial two-year follow-up period. We used mixed effects models and adjusted for prior smoking and immunosuppression use. Higher levels of CXCL10 were associated with lower FVC, TLC and DLCO values (Table 3). This indicates that in a given individual, the average %predicted FVC was lower by 4.7 percentage points for every 10-fold increase in CXCL10 at any visit within the first two years of the study. In contrast, CXCL9 was only correlated with DLCO (Table 3 and Supplementary Table S1). Of note, the total ranges for log10(CXCL10) and log10(CXCL9) in this study were 0.84 – 2.7 and 1.3 – 3.3, respectively.
Table 3.
Results from mixed effects regression models of respiratory variables measured repeatedly over two years
| Outcome | Main Predictor† | β-coefficient | 95% CI | p-value |
|---|---|---|---|---|
| FVC %pred | CXCL9 | 1.1 | (−2.5, 4.8) | 0.54 |
| CXCL10 | −4.6 | (−9.1, 0.68) | 0.047 | |
| FEV1 %pred | CXCL9 | 1.9 | (−1.6, 5.4) | 0.28 |
| CXCL10 | −1.6 | (−6.0, 2.8) | 0.48 | |
| DLCO %pred | CXCL9 | −12 | (−19, 5.4) | 4.5x10−4 |
| CXCL10 | −15 | (−24, 6.7) | 5.0x10−4 | |
| TLC %pred | CXCL9 | −2.2 | (−7.9, 3.6) | 0.46 |
| CXCL10 | −9.5 | (−17, −2.5) | 0.0076 | |
| Dyspnea Score | CXCL9 | 17%‡ | (15%, 61%) | 0.34 |
| CXCL10 | 58% | (5.0%, 140%) | 0.028 |
Chemokine variables analyzed as log10 transformations; models adjusted for immunosuppression use and prior smoking
Coefficients for dyspnea score are shown as the % change in score for each 10-fold increase in chemokine level
Abbreviations: CI = Confidence Interval, DLCO = Diffusing Capacity of the Lungs for Carbon Monoxide, FEV1 = Forced Expiratory Volume in 1 Second, FVC = Forced Vital Capacity, %pred = % predicted, TLC = Total Lung Capacity
To further assess pulmonary involvement by chest radiography obtained at initial subject visit, we compared chemokine levels by Scadding stage [48]. In analyses that compared chemokines between Scadding stages 1 through 4, there were no differences in chemokine levels between stages in either unadjusted (ANOVA) or adjusted (linear regression) models. Also, neither of the chemokines differed between subjects with or without fibrosis on chest radiography.
We also assessed whether CXCL10 and CXCL9 correlated longitudinally with respiratory symptoms as assessed by the UCSD Dyspnea score, where a higher score indicates more dyspnea [49–51]. Using a similar mixed effects model as for the pulmonary function measurements, we found that for every 10-fold increase in CXCL10, the average shortness of breath score increased by 58% (Table 3). To investigate whether this effect on symptom severity was mediated by lower pulmonary function, we also included a model with % predicted FVC and DLCO. After this adjustment, CXCL10 was still statistically significantly correlated with shortness of breath score (122% increase for every 10-fold increase in CXCL10, 95% CI 6.4% - 370%, p = 0.038). The correlation between longitudinal dyspnea scores and CXCL9 was lower and not statistically significant in similar analyses (Table 3).
3.2.2. CXCL10 had Greater Predictive value for Pulmonary Function Declines
To assess whether chemokine levels measured during the first two years of the study were associated with pulmonary function declines at any point in the ~5 years (66 months) of total follow-up, we performed a time to event analysis. First, we identified subjects with a decline in absolute FVC or DLCO values of ≥10% or ≥15%, respectively [54, 55], at any time after subjects’ initial visit. Next, we performed analyses to determine if the risk of pulmonary function decline was increased with higher chemokine levels (represented as either binary variables designating those above or below the median chemokine value or continuous chemokine level variables). In unadjusted analysis, subjects with a CXCL10 level above the median (176 pg/mL) had a higher risk of eventual decline (log-rank p = 0.037). In the Cox proportional hazards models, we included age, sex, race, and prior smoking as co-variates, but we stratified by immunosuppression use since this variable violated the proportional hazards assumption based on the Schoenfeld test [56]. Subjects with CXCL10 levels above the median had 4.1 times the risk of pulmonary function decline (HR = 4.1, 95% CI 1.5 – 12, p = 0.0078) (Fig. 2A). Modeled as a continuous variable, we found that each 10-fold increase in CXCL10 was associated with 8.8 times the risk of experiencing a decline (HR for log10(CXCL10) = 8.8, 95% CI 1.6 – 50, p = 0.014). This relationship did not meet statistical significance for CXCL9 (log rank p = 0.40, adjusted HR = 1.9 with p = 0.17 for CXCL9 dichotomized at the median (119 pg/mL); adjusted HR for log10(CXCL9) = 2.2, p = 0.15) (Fig. 2B). As a sensitivity analysis to address subjects censored prior to complete follow-up, we tested different assumptions about event rates in those censored. Whether we assumed all censored subjects made it to the five-year follow-up without a decline, all censored subjects had decline at the time of censoring, or the same proportion of randomly chosen censored subjects had a decline at the time of censoring as those who made it to five-year follow-up, the direction and magnitude of the effects of our predictors on the hazard ratios or log-rank statistics as reported above did not change.
Fig. 2.
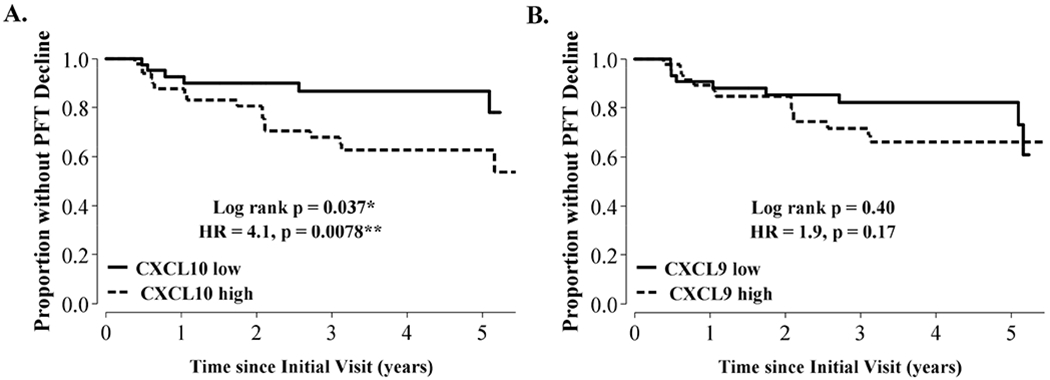
Relationship between serum CXCL10 and CXCL9 and longitudinal decline in lung function.
We defined lung function decline as a percent change in absolute forced vital capacity or diffusing capacity of 10% or 15%, respectively, over the study period (~5 years). Total time at risk was 291 person-years. We used Cox proportional hazards models adjusted for age, race, sex, prior smoking, and immunosuppression use and dichotomized subjects based on chemokine levels above or below median values to assess the predictive value of these chemokines for lung function decline. Abbreviations: HR = hazard ratio.
3.2.4. CXCL9, but not CXCL10, Positively Correlated with Organ Involvement
Our second main analytic goal was to assess relationships between total systemic organ involvement and levels of CXCL9 and CXCL10 at subjects’ initial visit [23]. First, we compared chemokine levels in subjects with one versus greater than one organ involved, and then determined if total organ number increased incrementally with higher levels of each chemokine. In unadjusted analyses, we found higher CXCL9 levels in subjects who had greater than one organ involved compared to only one organ (mean CXCL9 ± SD was 258 ± 360 versus 123 ± 71 pg/mL, p = 0.034, N = 37 vs. 64, respectively) (Fig. 3A). After adjusting for age, sex, race, and immunosuppression use, we found that for every 10-fold increase in CXCL9, the odds of having more than one organ involved increased 5.7 times (odds ratio (OR) = 5.7, p = 0.017, 95% CI 1.4 – 24). Next, using a Poisson regression adjusted for age, sex, race, and immunosuppression, we found a statistically significant increase in organ number with higher CXCL9 levels (log10(CXCL9) β = 1.5, 95% CI 1.1 – 2.0, p = 0.022), indicating that for every 10-fold increase in CXCL9, between 1 – 2 additional organs were involved (Fig. 3B). In contrast, there were no differences in CXCL10 levels between those with one versus greater than one organ involved in unadjusted (p = 0.13) or adjusted (OR 95% CI 0.41 – 9.4, p = 0.43) analyses and there was no statistically significant correlation between organ involvement and CXCL10 using Poisson regression (95% CI 0.74 – 1.9, p = 0.47).
Fig. 3.
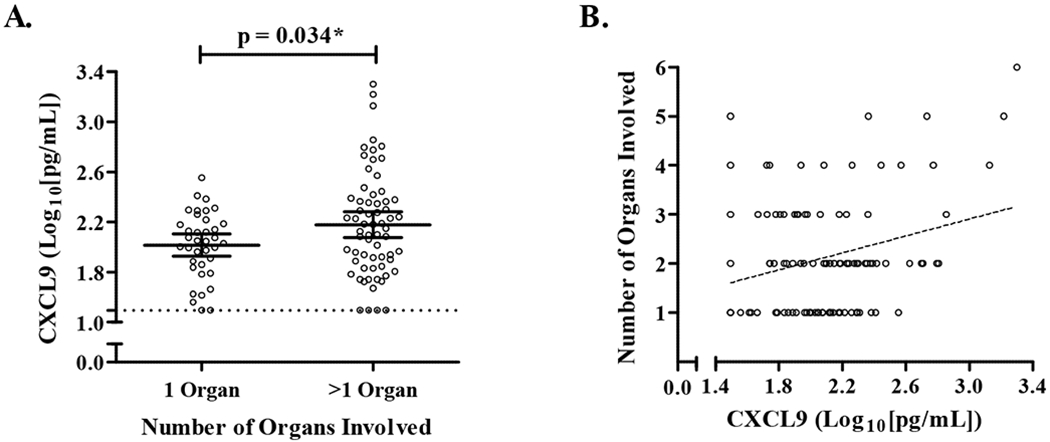
Relationship between serum CXCL9 levels and the number of organs involved with sarcoidosis. Organ assessments were performed by study physician review of the patient’s records at initial subject visit. A) Subjects categorized as having one or more than one organ involved (*p<0.05). B) Relationship between total organ involvement and CXCL9. Data are displayed as log10 transformations of CXCL9 levels (individual values denoted by open black circles). The lower limits of assay detection denoted by the dashed line in A). The dashed line in B) represents the fitted line for organ number adjusted for age, sex, race, and immunosuppression use.
Finally, we used logistic regression to assess if either chemokine level was higher in subjects with specific organ system involvement. In these analyses, we required 10 or more subjects to have that organ involved to provide a sufficient sample size. Higher CXCL9 levels increased the odds of extra-thoracic lymph node involvement (OR log10(CXCL9) = 6.7, 95% CI 1.6 – 28, p = 0.0090) and ocular disease (OR log10(CXCL9) = 12, 95% CI 2.3 – 58, p = 0.0029). In contrast, CXCL10 levels were not significantly associated with either extra-thoracic lymph node involvement (OR 95% CI 0.30 – 9.2, p = 0.57) or ocular involvement (OR 95% CI 0.74 – 150, p = 0.083).
3.3.1. CXCL10, not CXCL9, Correlated with its own Gene Expression in Peripheral Blood and Blood Monocyte Counts
Because we found that CXCL9 and CXCL10 were predictive of different clinical sarcoidosis outcomes, we wanted to explore potential reasons for these differences, specifically if there was evidence that the two chemokines were differentially produced by cells in the blood. Given that several blood immune cell populations can produce these chemokines, including monocytes and T cells [58–60], we analyzed gene expression data previously generated from the same blood samples used to measure the chemokine proteins [22]. In cross-sectional and longitudinal mixed effects linear regression analyses adjusted for, age, sex, race, and immunosuppression use, we found that CXCL10 protein level was positively correlated with CXCL10 expression, but the degree of correlation between CXCL9 expression and CXCL9 protein was lower and did not meet statistical significance (Table 4 and Fig. 4). For every doubling of CXCL10 expression, CXCL10 protein level increases by 32% (p = 1.0x10−6), suggesting that peripheral blood could be a significant source of CXCL10, whereas the main source of CXCL9 may be from other tissues.
Table 4.
Results from regression models for CXCL9 or CXCL10 levels with either their respective mRNA transcript levels or peripheral blood monocyte concentrations
| Cross-sectional Models at Initial Visit† | ||||
|---|---|---|---|---|
| Outcome | Main Predictor | |||
| Corresponding CXCL9 or CXCL10 Level (Log2[Relative Expression])‡ | ||||
| β-coefficient§ | 95% CI | p-value | r value | |
| CXCL9 | 16% | (6.5, 43) | 0.18 | 0.15 |
| CXCL10 | 32% | (19, 47) | 1.0x10−6 | 0.54 |
| Longitudinal Models¶ | ||||
| Outcome | Main Predictor | |||
| Corresponding CXCL9 or CXCL10 Level (Log2[Relative Expression])‡ | ||||
| β-coefficient§ | 95% CI | p-value | r value | |
| CXCL9 | 6.7% | (−0.30, 13) | 0.058 | 0.31 |
| CXCL10 | 16% | (9.2, 24) | 2.9x10−6 | 0.54 |
| Monocytes (106 cells/mL)†† | ||||
| β-coefficient | 95% CI | p-value | r value | |
| CXCL9 | −3.7% | (−35, 44) | 0.86 | 0.29 |
| CXCL10 | 47% | (5.6, 110) | 0.022 | 0.43 |
Linear regression models with measurements obtained at initial subject visits and adjusted for age, race, sex, and immunosuppression use
CXCL9 expression range: −1.9 – 5.6; CXCL10 expression range −2.8 – 3.0
Non-linear combinations of β-coefficients performed to show the %change in chemokine level for every unit increase in predictor
Mixed effects models with measurements obtained over two years and adjusted for age, race, sex, and immunosuppression use
Monocytes concentrations had a total range of 1.5x106 cells/mL
Abbreviations: CI = Confidence Interval
Fig. 4.
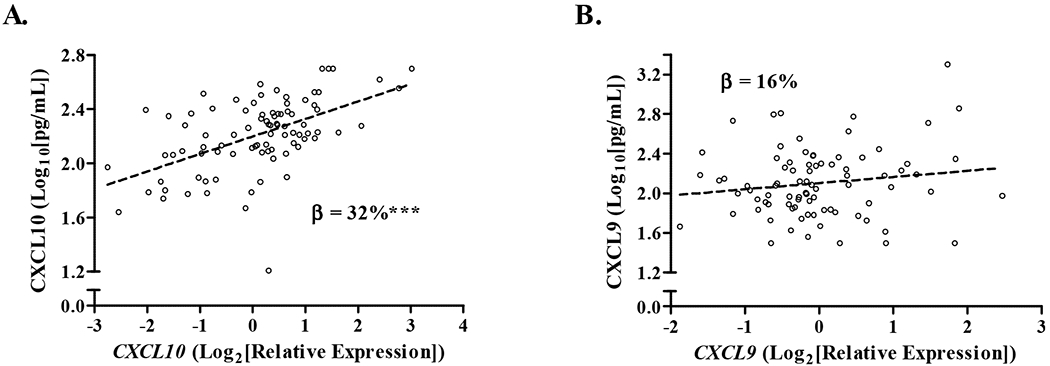
Relationship between serum chemokines and their respective whole blood mRNA gene transcript level at initial subject visit.
We analyzed paired chemokine and mRNA gene transcript levels measured from the same blood sample and obtained at subjects’ initial visit using linear regression analysis. Data for A) CXCL10 and B) CXCL9 are displayed as log10 transformations of chemokine values and log2 transformations of relative gene expression values (individual values denoted by open black circles) with the fitted lines for the chemokines adjusted for age, sex, race, and immunosuppression. The β-coefficients show the % increase in chemokine level for every log2-unit increase in gene expression adjusted for age, sex, race, and immunosuppression use (See Table 4); CXCL10 **p = 1.0x10−6, CXCL9 p = 0.18.
To identify a potential immune cellular source of CXCL10 in the blood, we assessed the relationships between CXCL10 chemokine levels and concentrations of blood immune cell populations as measured by clinical laboratory testing. In analyses using repeated measures of the blood markers, we found that the counts of peripheral blood monocytes (but not white blood cells, lymphocytes, or neutrophils) were positively associated with serum CXCL10 level suggesting that monocytes could be a source of CXCL10 in the blood (Table 4). Congruent with the correlations of CXCL9 protein to CXCL9 gene expression, we found no relationship between serum levels of CXCL9 and blood monocytes.
3.4.1. CXCL9 and CXCL10 are both Negatively Correlated with Immunosuppression Use
Because immunosuppression use could influence CXCL9 and CXCL10 levels through reducing inflammation, we controlled for immunosuppression as part of our analysis plan using a binary variable (any immunosuppression use or none at a given visit). However, we also wanted to determine how immunosuppression itself influenced chemokine levels. Using the observational data collected from this cohort on the types and amounts of immunosuppression at each blood draw, we performed separate mixed effects models where either CXCL9 or CXCL10 was the dependent variable and immunosuppression use was the predictor (using binary or continuous metrics). Controlling for age, sex, and race in these models, we found that CXCL9 and CXCL10 levels were 28-29% lower in subjects taking any immunosuppression at a given blood draw (Table 5). Using a different model that included two separate terms for immunosuppression use with prednisone dose in mg/day as a continuous variable and DMARD use (e.g. methotrexate or azathioprine) as present or not, we found that higher prednisone levels resulted in lower CXCL10 levels and DMARD use was associated with lower levels of both chemokines (Table 5).
Table 5.
Results from mixed effects regression analysis of CXCL9 or CXCL10 obtained over two years and immunosuppression use at time of measurement modeled in two different ways
| Outcome | Main Predictor(s)† | |||||
|---|---|---|---|---|---|---|
| Model 1: Binary Immunosuppression Use (Yes/No) | ||||||
| β-coefficient‡ | (95% CI) | p-value | ||||
| CXCL9 | −29% | (−42, −14) | 4.9x10−4 | |||
| CXCL10 | −28% | (−37, −17) | 6.4x10−6 | |||
| Model 2: Prednisone Dose (mg/day)§ | DMARD Use (Yes/No) | |||||
| β-coefficient | (95% CI) | p-value | β-coefficient | (95% CI) | p-value | |
| CXCL9 | −0.70% | (−1.6, 0.17) | 0.12 | −35% | (−47, −20) | 5.0x10−5 |
| CXCL10 | −9.6% | (−1.7, −0.33) | 0.0034 | −22% | (−34, −11) | 5.1x10−4 |
Models adjusted for age, race, and sex
Non-linear combinations of β-coefficients performed to show the %change in chemokine level for every unit increase in continuous predictor or if binary variable = Yes
Prednisone dose in units of 1 mg/day
Abbreviations: CI = Confidence Interval, DMARD = disease-modifying antirheumatic drug
4. Discussion
Sarcoidosis is a systemic disease involving granulomatous inflammation with upregulation of immune pathways related to IFN-γ [11, 12, 20, 61]. In this study, our goals were to compare serum levels of two interferon-induced chemokines, CXCL9 and CXCL10, with respect to important clinical outcomes in sarcoidosis. Because in vivo and in vitro observations have shown differences in the types of inflammatory stimuli that can induce these chemokines [24, 39–47], we were interested in assessing whether the circulating levels of each chemokine were differentially associated with specific clinical outcomes. For CXCL10, we found negative correlations with lung function measurements both at entry into the cohort and over time and we found that higher CXCL10 levels during the first two years of follow up increased the risk of having a future decline in % predicted FVC or DLCO during the 5-year study period. Higher CXCL0 levels also correlated with greater dyspnea scores in longitudinal analyses. There was less association of CXCL9 with these same outcomes. In contrast, when examining the endpoint of organ involvement, we found that CXCL9 was positively associated with the total number of organs involved as well as for specific organs, such as ocular or extra-thoracic lymph node involvement, while CXCL10 was not. In a prior study, we examined the relationship of these same outcomes with levels of CXCL11 [23]. Interestingly, we found that higher CXCL11 levels at enrollment increased the risk of future DLCO and FVC decline and positively correlated with number of organs involved. We speculate that a reason for these different associations between the three chemokines and clinical outcomes may be related to differences in receptor recognition and cellular sources of production [24, 33–37]. Taken together, the findings suggest that each chemokine could be used to help predict specific clinical outcomes in sarcoidosis patients.
Our findings are consistent with prior sarcoidosis studies that found elevated levels of CXCL9 and/or CXCL10 in the lung (lavage fluid, lavage cells, or tissue) [29, 62–66] or the lung and blood [14–17, 67]. Most of these studies found higher chemokine levels when comparing all sarcoidosis subjects to healthy controls. Some studies compared Scadding stage I or II subjects to healthy controls [64, 65, 68, 69]. Two studies found lower levels of these chemokines in subjects with Löfgren’s syndrome relative non-Löfgren sarcoidosis [64, 65]. These studies were cross-sectional in design, except one that measured lung lavage chemokine levels and did not find these levels to be predictive of radiographic remission at two-year follow-up [65]. The strength of our study was the fact that it included a longitudinal study design to correlate pulmonary physiology and chemokine levels, with both values measured repeatedly. We also carried out detailed organ phenotyping amongst sarcoidosis subjects, allowing us to assess relationships between chemokine levels and organ involvement at entry into the cohort.
We also observed that higher immunosuppression usage was associated with lower chemokine levels. Our finding that DMARD use was associated with lower levels of both chemokines, whereas only CXCL10 levels were lower with higher doses of prednisone could potentially be due to differing effects of prednisone on the sources of CXCL10 and CXCL9. However, given that these were correlative analyses, there could be other non-causal explanations. We acknowledge that our study was not a randomized clinical trial and was not designed to differentiate patients with active versus resolved sarcoidosis or assess the effect of treatment on these protein levels, however our findings suggest that these serum chemokine levels have potential as prognostic markers for both pulmonary outcomes and response to therapy. There is precedent for using CXCL9 and CXCL10 as markers of disease activity in other granulomatous diseases. CXCL9 and CXCL10 levels have been found to be predictive of disease progression and response to therapy in tuberculosis [70–72]. Chung et al., showed that protein levels of CXCL9 and CXCL10 were increased in those with confirmed tuberculosis infection compared to those without active infection and both protein levels decreased after successful treatment [72]. Thus, these chemokines could be potentially used in several granulomatous diseases to assess prognosis and treatment efficacy.
This study was not designed to understand the mechanisms for why these chemokines relate to different clinical endpoints. However, to explore ideas for why CXCL10 was more predictive for lung outcomes and CXCL9 was more correlated with systemic organ involvement, we compared serum protein levels to their blood mRNA transcript levels. CXCL10 protein was more strongly correlated with CXCL10 mRNA transcript level as well as with monocyte levels in the blood, while CXCL9 was not correlated with its respective mRNA transcript or monocyte levels. Prior studies have shown that both monocytes and macrophages can express CXCL9 and CXCL10 gene transcripts [47, 59, 73–75], although human monocytes cultured ex vivo have shown greater secretion of CXCL10 protein compared to CXCL9 after stimulation with IFN-α2a [60]. Given that our data are observational, we can only speculate on potential explanations for our findings in the context of the clinical observations we found. One possibility is that major sources of CXCL10 protein in the blood are peripheral circulating monocytes and cells in the lung, while blood CXCL9 protein levels are influenced more by activities of IFN-γ on fibroblasts, endothelial cells, and macrophages in affected tissues throughout the body. Future understanding of the cellular regulation of these chemokines in vivo will further our understanding of the roles of these proteins in the disease pathogenesis.
Our study was limited by the lack of longitudinal data related to organ involvement and chest radiography, which prevented us from assessing these outcomes over time. The fact that we did not find an association between these chemokines and fibrosis on chest imaging is likely due to a combination of low power (only 22% of subjects had fibrosis), the lack of serial imaging to identify those who may have developed fibrosis during the follow-up period, and the lack of PET scan imaging to differentiate those subjects with fibrosis who do not have evidence of granulomatous inflammation from those with persistent inflammation. To address the dropout in our cohort, we used mixed effects modeling methodology for our longitudinal analyses, which allowed us to account for variable follow-up [53]. Another important limitation relates to generalizability since our cohort was heterogeneous, therefore our study design did not allow us to extrapolate the prognostic value CXCL10 or CXCL9 levels at initial diagnosis and is also was not designed to address the question of whether these chemokine levels can predict the likelihood of spontaneous remission. Additionally, while we did not find any differences in CXCL9 or CXCL10 based on race, our cohort is demographically composed of greater numbers of white subjects than other racial or ethnic groups. Some of these limitations can be addressed in future studies that take advantage of existing biorepositories from other large U.S.-based cohorts such as those from the GRADS and ACCESS studies [55, 76, 77].
5. Conclusions and Future Directions
In summary, we provide evidence showing that serum CXCL10 levels correlated with a greater number of lung function measures, pulmonary function decline, and respiratory symptoms as compared to CXCL9, which had greater correlation with systemic organ involvement. These differences may be related to each chemokine’s cellular source, which is supported by our analyses using levels of mRNA transcripts and circulating immune cells. Future goals include determining how CXCL9 and CXCL10 correlate with outcomes when measured at time of diagnosis and how they change in response to treatment. With this information, we may be able to leverage biological data taken at the time of sarcoidosis diagnosis to inform patient prognosis and guide clinical decision making.
Supplementary Material
Highlights.
Serum CXCL10 was negatively associated with FVC, DLCO, and TLC in sarcoidosis subjects
Subjects with increased levels of CXCL10 had increased risk of PFT declines.
Serum CXCL9 positively correlated with organ involvement.
Subjects with increased CXCL11 levels suffered declines in PFTs more quickly.
CXCL10 gene expression and blood monocyte levels positively correlated with CXCL10 protein levels, whereas CXCL9 did not.
Both CXCL9 and CXCL10 were lower with higher immunosuppression usage.
Acknowledgements
The authors thank the following individuals for their specific contributions: Michelle Nguyen, Joris Ramstein, Christine Nguyen, Sara Sun, and Zoe Lehman for assistance with sample acquisition, analysis, and management of the database; and Owen Solberg, Ph.D., for database programming. We would also like to thank all of the participants who volunteered their time for this study.
Funding This work was supported by the National Institutes of Health (R56IO87652 and T32HL007185).
Abbreviations:
- ANOVA
Analysis of Variance
- DLCO
Diffusing Capacity of the Lungs for Carbon Monoxide
- DMARD
Disease Modifying Antirheumatic Drug
- ELISA
Enzyme-Linked Immunosorbent Assay
- FEV1
Forced Expiratory Volume in 1 Second
- FVC
Forced Vital Capacity
- FEV1/FVC
Forced Expiratory Volume in 1 Second to Forced Vital Capacity ratio
- HR
Hazard Ratio
- IFN-γ
Interferon-Gamma
- OR
Odds Ratio
- %pred
Percent Predicted
- PET
Positron Emission Tomography
- TLC
Total Lung Capacity
- TNF-α
Tumor Necrosis Factor Alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: none
Conflict of interest All the other authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Research Involving Animal Studies This article does not contain any studies with animals performed by any of the authors.
References
- [1].Chappell AG, Cheung WY, Hutchings HA. Sarcoidosis: a long-term follow up study. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2000;17(2):167–73. https://www.ncbi.nlm.nih.gov/pubmed/10957765. [PubMed] [Google Scholar]
- [2].Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. The New England journal of medicine. 2007;357(21):2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- [3].Rabin DL, Richardson MS, Stein SR, Yeager H, . Sarcoidosis severity and socioeconomic status. The European respiratory journal. 2001;18(3):499–506. [DOI] [PubMed] [Google Scholar]
- [4].Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 1999;16(2):149–73. https://www.ncbi.nlm.nih.gov/pubmed/10560120. [PubMed] [Google Scholar]
- [5].Gerke AK. Morbidity and mortality in sarcoidosis. Current opinion in pulmonary medicine. 2014;20(5):472–8. doi: 10.1097/mcp.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gerke AK, Judson MA, Cozier YC, Culver DA, Koth LL. Disease Burden and Variability in Sarcoidosis. Annals of the American Thoracic Society. 2017;14(Supplement_6):S421–s8. doi: 10.1513/AnnalsATS.201707-564OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Shirude S, Naghavi M, Mokdad AH, Murray CJL. Trends and Patterns of Differences in Chronic Respiratory Disease Mortality Among US Counties, 1980-2014. Jama. 2017;318(12):1136–49. doi: 10.1001/jama.2017.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985;75(5):1488–95. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prasse A, Georges CG, Biller H, Hamm H, Matthys H, Luttmann W, Virchow JC. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4(+) and CD8(+) T cells. Clinical and experimental immunology. 2000;122(2):241–8. doi: DOI 10.1046/j.1365-2249.2000.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Inui N, Chida K, Suda T, Nakamura H. TH1/TH2 and TC1/TC2 profiles in peripheral blood and bronchoalveolar lavage fluid cells in pulmonary sarcoidosis. The Journal of allergy and clinical immunology. 2001;107(2):337–44. doi: 10.1067/mai.2001.112273. [DOI] [PubMed] [Google Scholar]
- [11].Mollers M, Aries SP, Dromann D, Mascher B, Braun J, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax. 2001;56(6):487–93. https://www.ncbi.nlm.nih.gov/pubmed/11359967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wahlstrom J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. American journal of respiratory and critical care medicine. 2001;163(1):115–21. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- [13].Kriegova E, Fillerova R, Tomankova T, Hutyrova B, Mrazek F, Tichy T, Kolek V, du Bois RM, Petrek M. T-helper cell type-1 transcription factor T-bet is upregulated in pulmonary sarcoidosis. European Respiratory Journal. 2011;38(5): 1136–44. doi: 10.1183/09031936.00089910. [DOI] [PubMed] [Google Scholar]
- [14].Antoniou KM, Tzouvelekis A, Alexandrakis MG, Sfiridaki K, Tsiligianni I, Rachiotis G, Tzanakis N, Bouros D, Milic-Emili J, Siafakas NM. Different angiogenic activity in pulmonary sarcoidosis and idiopathic pulmonary fibrosis. Chest. 2006;130(4):982–8. doi: 10.1378/chest.130.4.982. [DOI] [PubMed] [Google Scholar]
- [15].Sugiyama K, Mukae H, Ishii H, Kakugawa T, Ishimoto H, Nakayama S, Shirai R, Fujii T, Mizuta Y, Kohno S. Elevated levels of interferon gamma-inducible protein-10 and epithelial neutrophil-activating peptide-78 in patients with pulmonary sarcoidosis. Respirology (Carlton, Vic). 2006;11(6):708–14. doi: 10.1111/j.1440-1843.2006.00933.x. [DOI] [PubMed] [Google Scholar]
- [16].Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, Sone S. CXCL9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clinical and experimental immunology. 2007;149(2):317–26. doi: 10.1111/j.1365-2249.2007.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nureki S, Miyazaki E, Ando M, Ueno T, Fukami T, Kumamoto T, Sugisaki K, Tsuda T. Circulating levels of both Th1 and Th2 chemokines are elevated in patients with sarcoidosis. Respiratory medicine. 2008;102(2):239–47. doi: 10.1016/j.rmed.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [18].Nagata K, Maruyama K, Uno K, Shinomiya K, Yoneda K, Hamuro J, Sugita S, Yoshimura T, Sonoda KH, Mochizuki M, Kinoshita S. Simultaneous analysis of multiple cytokines in the vitreous of patients with sarcoid uveitis. Investigative ophthalmology & visual science. 2012;53(7):3827–33. doi: 10.1167/iovs.11-9244. [DOI] [PubMed] [Google Scholar]
- [19].Takeuchi M, Oh IK, Suzuki J, Hattori T, Takeuchi A, Okunuki Y, Usui Y, Usui M. Elevated serum levels of CXCL9/monokine induced by interferon-gamma and CXCL10/interferon-gamma-inducible protein-10 in ocular sarcoidosis. Investigative ophthalmology & visual science. 2006;47(3):1063–8. doi: 10.1167/iovs.05-0966. [DOI] [PubMed] [Google Scholar]
- [20].Koth LL, Solberg OD, Peng JC, Bhakta NR, Nguyen CP, Woodruff PG. Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. American journal of respiratory and critical care medicine. 2011;184(10):1153–63. doi: 10.1164/rccm.201106-1143OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Su R, Nguyen ML, Agarwal MR, Kirby C, Nguyen CP, Ramstein J, Darnell EP, Gomez AD, Ho M, Woodruff PG, Koth LL. Interferon-inducible chemokines reflect severity and progression in sarcoidosis. Respir Res. 2013;14:121. doi: 10.1186/1465-9921-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Su R, Li MM, Bhakta NR, Solberg OD, Darnell EP, Ramstein J, Garudadri S, Ho M, Woodruff PG, Koth LL. Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. The European respiratory journal. 2014;44(4):985–93. doi: 10.1183/09031936.00039714. [DOI] [PubMed] [Google Scholar]
- [23].Arger NK, Ho M, Woodruff PG, Koth LL. Serum CXCL11 correlates with pulmonary outcomes and disease burden in sarcoidosis. Respiratory medicine. 2019;152:89–96. doi: 10.1016/j.rmed.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology. 2011;89(2):207–15. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grimm MC, Doe WF. Chemokines in Inflammatory Bowel Disease Mucosa: Expression of RANTES, Macrophage Inflammatory Protein (MIP)-1alpha, MIP-1beta, and gamma-Interferon-Inducible Protein-10 by Macrophages, Lymphocytes, Endothelial Cells, and Granulomas. Inflammatory bowel diseases. 1996;2(2):88–96. [PubMed] [Google Scholar]
- [26].Kishi J, Nishioka Y, Kuwahara T, Kakiuchi S, Azuma M, Aono Y, Makino H, Kinoshita K, Kishi M, Batmunkh R, Uehara H, Izumi K, Sone S. Blockade of Th1 chemokine receptors ameliorates pulmonary granulomatosis in mice. The European respiratory journal. 2011. ;38(2):415–24. doi: 10.1183/09031936.00070610. [DOI] [PubMed] [Google Scholar]
- [27].Aranday-Cortes E, Bull NC, Villarreal-Ramos B, Gough J, Hicks D, Ortiz-Pelaez A, Vordermeier HM, Salguero FJ. Upregulation of IL-17A, CXCL9 and CXCL10 in early-stage granulomas induced by Mycobacterium bovis in cattle. Transboundary and emerging diseases. 2013;60(6):525–37. doi: 10.1111/j.1865-1682.2012.01370.x. [DOI] [PubMed] [Google Scholar]
- [28].Torraca V, Cui C, Boland R, Bebelman JP, van der Sar AM, Smit MJ, Siderius M, Spaink HP, Meijer AH. The CXCR3-CXCL11 signaling axis mediates macrophage recruitment and dissemination of mycobacterial infection. Disease models & mechanisms. 2015;8(3):253–69. doi: 10.1242/dmm.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Qin S, Luster AD, Semenzato G. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161(11):6413–20. [PubMed] [Google Scholar]
- [30].Aksoy MO, Yang Y, Ji R, Reddy PJ, Shahabuddin S, Litvin J, Rogers TJ, Kelsen SG. CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. American journal of physiology Lung cellular and molecular physiology. 2006;290(5):L909–18. doi: 10.1152/ajplung.00430.2005. [DOI] [PubMed] [Google Scholar]
- [31].Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16(6):593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [32].Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Current topics in developmental biology. 2005;68:149–81. doi: 10.1016/s0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- [33].Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. The Journal of experimental medicine. 2001;193(8):975–80. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. The Journal of experimental medicine. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, Barsheshet Y, Karp CL, Karin N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. 2014;124(5):2009–22. doi: 10.1172/JCI71951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. The Journal of biological chemistry. 2004;279(29):30219–27. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- [37].Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. The Journal of experimental medicine. 1998;187(12):2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xanthou G, Duchesnes CE, Williams TJ, Pease JE. CCR3 functional responses are regulated by both CXCR3 and its ligands CXCL9, CXCL10 and CXCL11. European journal of immunology. 2003;33(8):2241–50. doi: 10.1002/eji.200323787. [DOI] [PubMed] [Google Scholar]
- [39].Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, Hershenson MB. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183(11):6989–97. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Amichay D, Gazzinelli RT, Karupiah G, Moench TR, Sher A, Farber JM. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157(10):4511–20. [PubMed] [Google Scholar]
- [41].Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, Farber JM, Luster AD. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol. 2006;176(11):7087–95. doi: 10.4049/jimmunol.176.11.7087. [DOI] [PubMed] [Google Scholar]
- [43].Ohmori Y, Wyner L, Narumi S, Armstrong D, Stoler M, Hamilton TA. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. The American journal of pathology. 1993;142(3):861–70. [PMC free article] [PubMed] [Google Scholar]
- [44].Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M. TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion. European journal of immunology. 2002;32(7):2037–45. doi:. [DOI] [PubMed] [Google Scholar]
- [45].Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315(6021):672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- [46].Proost P, Verpoest S, Van de Borne K, Schutyser E, Struyf S, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. Journal of leukocyte biology. 2004;75(5):777–84. doi: 10.1189/jlb.1003524. [DOI] [PubMed] [Google Scholar]
- [47].Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, Wuyts A, Opdenakker G, Van Damme J. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. European journal of immunology. 2003;33(11):3146–53. doi: 10.1002/eji.200324136. [DOI] [PubMed] [Google Scholar]
- [48].Benn BS, Lehman Z, Kidd SA, Ho M, Sun S, Ramstein J, Arger NK, Nguyen CP, Su R, Gomez A, Gelfand JM, Koth LL. Clinical and Biological Insights from the University of California San Francisco Prospective and Longitudinal Cohort. Lung. 2017. doi: 10.1007/s00408-017-0037-y. [DOI] [PubMed] [Google Scholar]
- [49].Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998; 113(3):619–24. https://www.ncbi.nlm.nih.gov/pubmed/9515834. [DOI] [PubMed] [Google Scholar]
- [50].Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, Brown KK, Fischer A. Assessing dyspnea and its impact on patients with connective tissue disease-related interstitial lung disease. Respiratory medicine. 2010;104(9):1350–5. doi: 10.1016/j.rmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Swigris JJ, Han M, Vij R, Noth I, Eisenstein EL, Anstrom KJ, Brown KK, Fairclough D. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respiratory medicine. 2012;106(10):1447–55. doi: 10.1016/j.rmed.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vittinghoff E, V. Glidden D, C. Shiboski S, E. McCulloch C. Statistics for Biology and Health. 2012. p. 7–26. [Google Scholar]
- [53].McLean RA, Sanders WL, Stroup WW. A Unified Approach to Mixed Linear Models. The American Statistician. 1991. ;45(1):54–64. doi: 10.1080/00031305.1991.10475767. [DOI] [Google Scholar]
- [54].Keir G, Wells AU. Assessing pulmonary disease and response to therapy: which test? Seminars in respiratory and critical care medicine. 2010;31(4):409–18. doi: 10.1055/s-0030-1262209. [DOI] [PubMed] [Google Scholar]
- [55].Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R. Clinical characteristics of patients in a case control study of sarcoidosis. American journal of respiratory and critical care medicine. 2001;164(10 Pt 1):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- [56].Schoenfeld D Partial Residuals for The Proportional Hazards Regression Model. Biometrika. 1982;69(1):239–41. doi: 10.2307/2335876. [DOI] [Google Scholar]
- [57].Snijders TAB, Bosker RJ. Modeled Variance in Two-Level Models. Sociological Methods & Research. 1994;22(3):342–63. doi: 10.1177/0049124194022003004. [DOI] [Google Scholar]
- [58].Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). The Journal of experimental medicine. 1987;166(4):1084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zipfel PF, Bialonski A, Skerka C. Induction of members of the IL-8/NAP-1 gene family in human T lymphocytes is suppressed by cyclosporin A. Biochemical and biophysical research communications. 1991;181(1): 179–83. [DOI] [PubMed] [Google Scholar]
- [60].Padovan E, Spagnoli GC, Ferrantim M, Heberer M. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. Journal of leukocyte biology. 2002;71(4):669–76. [PubMed] [Google Scholar]
- [61].Prior C, Haslam PL. Increased levels of serum interferon-gamma in pulmonary sarcoidosis and relationship with response to corticosteroid therapy. The American review of respiratory disease. 1991;143(1):53–60. doi: 10.1164/ajrccm/143.1.53. [DOI] [PubMed] [Google Scholar]
- [62].Miotto D, Christodoulopoulos P, Olivenstein R, Taha R, Cameron L, Tsicopoulos A, Tonnel AB, Fahy O, Lafitte JJ, Luster AD, Wallaert B, Mapp CE, Hamid Q. Expression of IFN-gamma-inducible protein; monocyte chemotactic proteins 1, 3, and 4; and eotaxin in TH1- and TH2-mediated lung diseases. The Journal of allergy and clinical immunology. 2001;107(4):664–70. doi: S0091-6749(01)23352-1[pii]. [DOI] [PubMed] [Google Scholar]
- [63].Cui A, Anhenn O, Theegarten D, Ohshimo S, Bonella F, Sixt SU, Peters J, Sarria R, Guzman J, Costabel U. Angiogenic and angiostatic chemokines in idiopathic pulmonary fibrosis and granulomatous lung disease. Respiration; international review of thoracic diseases. 2010;80(5):372–8. doi: 10.1159/000245332. [DOI] [PubMed] [Google Scholar]
- [64].Arakelyan A, Kriegova E, Kubistova Z, Mrazek F, Kverka M, du Bois RM, Kolek V, Petrek M. Protein levels of CC chemokine ligand (CCL)15, CCL16 and macrophage stimulating protein in patients with sarcoidosis. Clinical and experimental immunology. 2009;155(3):457–65. doi: 10.1111/j.1365-2249.2008.03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Piotrowski WJ, Mlynarski W, Fendler W, Wyka K, Marczak J, Gorski P, Antczak A. Chemokine receptor CXCR3 ligands in bronchoalveolar lavage fluid: associations with radiological pattern, clinical course, and prognosis in sarcoidosis. Polskie Archiwum Medycyny Wewnetrznej. 2014;124(7-8):395–402. https://www.ncbi.nlm.nih.gov/pubmed/24859629. [DOI] [PubMed] [Google Scholar]
- [66].Li H, Zhao X, Wang J, Zong M, Yang H. Bioinformatics analysis of gene expression profile data to screen key genes involved in pulmonary sarcoidosis. Gene. 2017;596:98–104. doi: 10.1016/j.gene.2016.09.037. [DOI] [PubMed] [Google Scholar]
- [67].Pignatti P, Brunetti G, Moretto D, Yacoub MR, Fiori M, Balbi B, Balestrino A, Cervio G, Nava S, Moscato G. Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis. American journal of respiratory and critical care medicine. 2006;173(3):310–7. doi: 10.1164/rccm.200502-244OC. [DOI] [PubMed] [Google Scholar]
- [68].Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, Huang C, Zisman DA, Fishbein M, Lynch JP 3rd, Strieter RM, Elashoff RM, Belperio JA. CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis. The European respiratory journal. 2009;34(3):676–86. doi: 10.1183/09031936.00157508. [DOI] [PubMed] [Google Scholar]
- [69].Schnerch J, Prasse A, Vlachakis D, Schuchardt KL, Pechkovsky DV, Goldmann T, Gaede KI, Muller-Quernheim J, Zissel G. Functional Toll-Like Receptor 9 Expression and CXCR3 Ligand Release in Pulmonary Sarcoidosis. American journal of respiratory cell and molecular biology. 2016;55(5):749–57. doi: 10.1165/rcmb.2015-0278OC. [DOI] [PubMed] [Google Scholar]
- [70].Kim S, Lee H, Kim H, Kim Y, Cho JE, Jin H, Kim DY, Ha SJ, Kang YA, Cho SN, Lee H. Diagnostic performance of a cytokine and IFN-gamma-induced chemokine mRNA assay after Mycobacterium tuberculosis-specific antigen stimulation in whole blood from infected individuals. The Journal of molecular diagnostics : JMD. 2015;17(1):90–9. doi: 10.1016/j.jmoldx.2014.08.005. [DOI] [PubMed] [Google Scholar]
- [71].Lee K, Chung W, Jung Y, Kim Y, Park J, Sheen S, Park K. CXCR3 ligands as clinical markers for pulmonary tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(2):191–9. doi: 10.5588/ijtld.14.0525. [DOI] [PubMed] [Google Scholar]
- [72].Chung W, Lee K, Jung Y, Kim Y, Park J, Sheen S, Lee J, Kang D, Park K. Serum CXCR3 ligands as biomarkers for the diagnosis and treatment monitoring of tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(12): 1476–84. doi: 10.5588/ijtld.15.0325. [DOI] [PubMed] [Google Scholar]
- [73].Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26(3):311–27. doi: 10.1016/j.cytogfr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- [74].Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(14):5238–42. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, Fraeyman A, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J, Proost P. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Laboratory investigation; a journal of technical methods and pathology. 2006;86(9):902–16. doi: 10.1038/labinvest.3700453. [DOI] [PubMed] [Google Scholar]
- [76].Moller DR, Koth LL, Maier LA, Morris A, Drake W, Rossman M, Leader JK, Collman RG, Hamzeh N, Sweiss NJ, Zhang Y, O’Neal S, Senior RM, Becich M, Hochheiser HS, Kaminski N, Wisniewski SR, Gibson KF. Rationale and Design of the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) Study. Sarcoidosis Protocol. Annals of the American Thoracic Society. 2015; 12( 10):1561–71. doi: 10.1513/AnnalsATS.201503-172OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, Yeager H, Jr., McLennan G, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki BA, Weinberger SE, Knatterud GL, Cherniak R. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2003;20(3):204–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


