Abstract
Photoexcitation is a common strategy for initiating radical reactions in chemical synthesis. We found that photoexcitation of flavin-dependent ‘ene’-reductases changes their catalytic function, enabling these enzymes to promote an asymmetric radical cyclization. This reactivity enables the construction of 5-, 6-, 7-, and 8-membered lactams with stereochemical preference conferred by the enzyme active site. After formation of a prochiral radical, the enzyme guides the delivery of a hydrogen atom from flavin, a challenging feat for small molecule chemical reagents. The initial electron transfer occurs by direct excitation of an electron donor-acceptor complex that forms between the substrate and the reduced flavin cofactor within the enzyme active site. Photoexcitation of promiscuous flavoenzymes has thus furnished a previously unknown biocatalytic reaction.
Radical enzymes catalyze radical-mediated reactions with exquisite chemo-, regio-, and enantioselectivity (1). These catalysts, however, exploit strategies for radical formation that do not translate well outside of the natural setting, and thus do not lend themselves readily to be harnessed for robust synthetic organic chemistry, in which operational simplicity, generality, and ease of reaction execution are desirable (2). To achieve these features, it would be attractive to develop biocatalytic reactions that exploit mechanisms of radical formation commonly used in chemical synthesis. If these mechanisms were to occur within active sites of established enzymatic platforms known to be substrate promiscuous, general catalysts for asymmetric radical reactions could be accessed. Moreover, by repurposing known enzymes, new biocatalytic functions can be achieved while retaining desirable characteristics such as ease of handling, substrate promiscuity, and evolvability already associated with these catalysts (3).
Photoexcitation is widely used in organic synthesis to facilitate radical reactions (4) but less common in enzyme catalysis. Light has been used to activate enzymes through conformational changes (5), drive charge separation in multiprotein systems (6), and facilitate cofactor turnover or substrate epimerization (7). Direct use of photonic energy to drive native, biological reactions is much more limited, but has been documented for protochlorophyllide reductase (8), fatty acid photodecarboxylase (9), and DNA photolyase (10). We recently found that nicotinamide-dependent ketoreductases (KREDs) and double bond reductases (DBRs) can exploit photoinduced electron transfer to affect asymmetric radical hydrodehalogenation and hydrodeacetoxylation reactions (11,12). These examples illustrate that irradiation of common oxidoreductases can draw out a non-natural reaction mode for catalysis, a feature we anticipate can be exploited to address challenges in chemical synthesis.
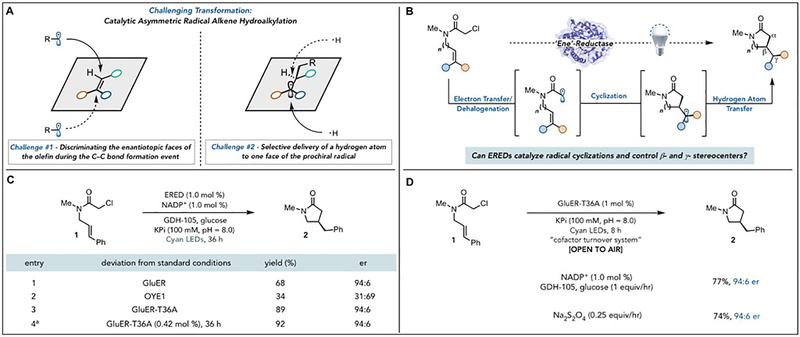
The stereoselective coupling of electrophilic radicals and unactivated alkenes enables the preparation of structural motifs found in agrochemical and pharmaceutical agents (13). Catalytic strategies for rendering this type of transformation stereoselective remain elusive (14). This is primarily due to the challenge of maintaining association between radical species and chiral catalysts throughout the stereoselectivity determining step and the lack of reagents capable of supplying a hydrogen atom to one face of a prochiral radical (Fig 1A) (15). Given these challenges, we imagined that an enzyme would be an ideal catalyst for this transformation because of their ability to precisely control the conformational landscape of reactive species. As a model for this family of reactivity, we targeted the development of a biocatalytic radical cyclization of α-chloroamides to afford β-stereogenic lactams (Fig 1B). The lactam motif is prevalent in medicinally valuable molecules (16), and the proposed synthesis would be distinct from existing biocatalytic approaches for generating N-heterocycles (17). While this cyclization is well-known in the radical literature, it is plagued by preferential formation of the hydrodehalogenated and oligomerized product and there are no known catalytic asymmetric variants (18,19). Enzymatic Csp3-Csp3 bond forming reactions are desired in biocatalysis (20,21).
Fig. 1. Strategy for achieving a biocatalytic C–C bond formation mediated by radicals.
(A) Challenges associated with achieving a catalytic asymmetric radical hydroalkylation (B) Proposed catalytic cycle for the biocatalytic radical cyclization to set β- and γ- positions. (C) Enzyme screen and optimization via mutagenesis. a Reaction run using cell free lysates on 1 gram scale (D) Reaction conditions and results for running this reaction open to air.
We looked to flavin-dependent ‘ene’-reductases (EREDs) as a potential catalyst family for the proposed cyclization of α-chloroamides. As EREDs have large, substrate promiscuous active sites, we hypothesized that these enzymes would be able to bind the substrate in a conformation to enable cyclization (22,23). Moreover, our recent studies demonstrated that these enzymes are able to control the stereochemical outcome of radical hydrodehalogenation reactions (24). Unfortunately, flavin hydroquinone (FMNhq) is a modest single electron reductant (E1/2 = −0.45 V vs SCE) making electron transfer to α-chloroamides (Ep/2red = −1.65 V vs SCE) thermodynamically challenging (Fig. S23). The excited state of the flavin hydroquinone (FMNhq*) (E1/2* = −2.26 V vs SCE), however, should be capable of facilitating this initial electron transfer (25). Previous work has investigated the fundamental photophysics of the FMNhq* in flavin-dependent enzymes (26,27). If this excited state could be used for catalysis, it would transform flavin-dependent enzymes into chiral photocatalysts, significantly expanding their synthetic utility.
We began by testing the cyclization of α-chloroacetamide 1 to afford γ-lactam 2 under visible light irradiation (Table S1). An ERED from Gluconobacter oxydans (GluER) was effective when irradiated with a near UV light (390 nm), furnishing the desired cyclization product with modest yield and enantioselectivity (Table S2). Optimization of the lighting source revealed a cyan light (497 nm) to provide product with improved yields and enantioselectivity while producing less than 2% of hydrodehalogenation product (Fig. 1C, entry 1). The opposite enantiomer is favored by Old Yellow Enzyme 1 (OYE1) (Figure 1C, entry 2). Control experiments confirm that light, ERED, NADP+, glucose, and glucose dehydrogenase (GDH-105) are required for reactivity (Table S1). To increase the activity of GluER for this non-natural function, we conducted mutagenesis and found that mutation of T36, a residue located at the surface of the protein, to alanine (T36A) furnished improved yields of product and only trace quantities of the hydrodehalogenated product (Fig. 1C, entry 3). We solved crystal structures of GluER and GluER-T36A and found no differences in the structure which would explain the improved yield (backbone RMSD of 0.53 Å; Fig. S48–52). Importantly, this mutation does not have a detrimental effect on the native function of this enzyme (Fig. S45) and allows the catalyst loading to be decreased to 0.5 mol % without compromising yield or enantioselectivity (Fig. S46). Moreover, this reaction can be conducted with lyophilized cell-free lysates. This feature enabled the model cyclization to be run on gram-scale, providing product with no change in yield or enantioselectivity (Fig. 1C, entry 4). The reaction could be run open to air with slow addition of glucose over 8 hours, providing comparable yields and enantioselectivities to reactions run under inert atmosphere (Fig. 1D). Alternatively, GDH-105, NADP+, and glucose could be replaced with periodic addition of sodium dithionite and degassed buffer; when run open to air, there was no observable change in the reaction outcome (Fig. 1D).
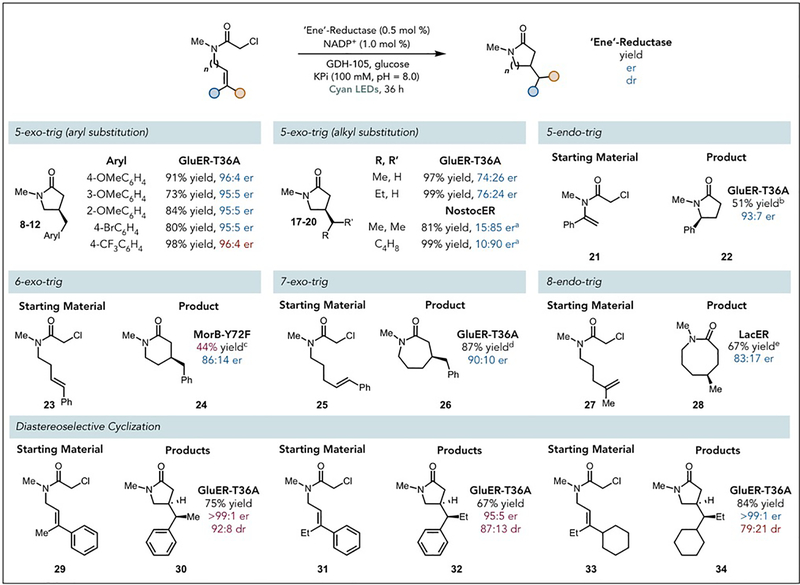
With optimized conditions in hand, we explored the scope and limitations of this reaction (Fig. 2). Aromatic substituted alkenes are tolerated for 5-exo-trig cyclizations, affording product in high yield and enantioselectivity with substituents at the para-, meta-, and ortho-positions (Fig. 2, 8–12). The electronic characteristics of these substituents had only a modest effect on reaction efficiency. Alkyl substituents on the olefin are also tolerated, affording product in nearly quantitative yield in all cases, albeit with diminished enantioselectivities (Fig. 2, 17–20). As this mode of reactivity should be accessible across the entire ERED family, we suspected other members might provide improved levels of enantioselectivity. An ERED from Nostoc sp. (NostocER) accordingly provided improved enantioselectivities for the more sterically demanding alkyl-substituted substrates (Fig. 2, 19, 20).
Fig. 2. Scope of biocatalytic radical cyclization.
Reaction conditions: GluER-T36A (0.5 mol %), NADP+ (1 mol %), GDH-105 (0.2 mg/mg starting material), glucose (6 equiv.), KPi (pH = 8.0, 100 mM, with 10% glycerol), substrate (1 equiv). 50W Cyan LED. Average temperature 35 °C. a Results obtained using NostocER and favors the opposite product enantiomer. b 12% formation of the hydrodehalogenated product. c 1 mol % enzyme loading (18 hours). d 2 mol % enzyme loading (18 hours). e 4 mol % enzyme loading (72 hours).
We next shifted our attention to other cyclization modes. GluER-T36A catalyzes a 5-endo-trig cyclization, wherein the stereogenic center is created via hydrogen atom transfer (HAT) to yield the 5-substituted γ-lactam (Fig. 2. 22). The ERED is thus capable of controlling the delivery of a hydrogen atom to sites distal to the carbonyl, a feat largely unknown in the small molecule literature (10,28). A 6-exo-trig cyclization to furnish δ-lactams occurs with GluER-T36C (Fig 2. 24). Improved levels of enantioselectivity and yield can be achieved using a variant of Morphinone reductase (MorB-Y72F). GluER-T36A can also catalyze a 7-exo-trig cyclization (Fig 2. 26), albeit with increased catalyst loadings. Finally, an 8-endo-trig cyclization is also possible using GluER-T36A, affording product in high yields but with essentially no enantioselectivity (Fig 2. 28). An ERED from Lactobacillus casei (LacER) provides significantly improved levels of enantioselectivity but slightly diminished yields, with the remaining mass balance being unreacted starting material. The latter examples are particularly exciting because they are underrepresented cyclization modes in the small molecule literature (29).
Inspired by the selectivity of the HAT event, we considered the possibility of ERED-controlled hydrogen atom delivery to exocyclic prochiral radicals. As these radicals are conformationally flexible, controlling HAT is challenging. We began by testing substrates possessing trisubstituted alkenes for the 5-exo-trig cyclization mode. Substrates containing phenyl/methyl and phenyl/ethyl substituents at the terminal position of the olefin afforded lactams with excellent enantio- and diastereoselectivities (Fig 2. 30 and 32). As these substrates provides an equimolar mixture of diastereomers when prepared with photoredox catalysts, there does not appear to be a thermodynamic preference for one diastereomer suggesting the enzyme is responsible for controlling the delivery of the hydrogen atom. The cyclohexyl/Et substituted alkene substrate 33, which lacks an aromatic group, was nonetheless a substrate for this reaction with diastereoselectivity only slightly diminished (Fig 2. 34). Substrates containing substituents with steric bulk may thus be ideal for achieving high levels of diastereoselectivity.
We conducted a series of initial rate experiments to better understand how the kinetic profile of the reaction compares to native alkene reduction. We found that the model reaction follows Michaelis-Menten kinetics with a Km = 9.7 mM, a kcat = 0.012 s−1 and a kcat/Km of 0.0012 mM−1 s−1 (Fig. S27). Based on our observation that increased light intensities afford increased rates and higher yields, we hypothesize that the decreased kcat is due to the reaction being photon limited (Fig. S28). A quantum yield of 7.8% is observed.
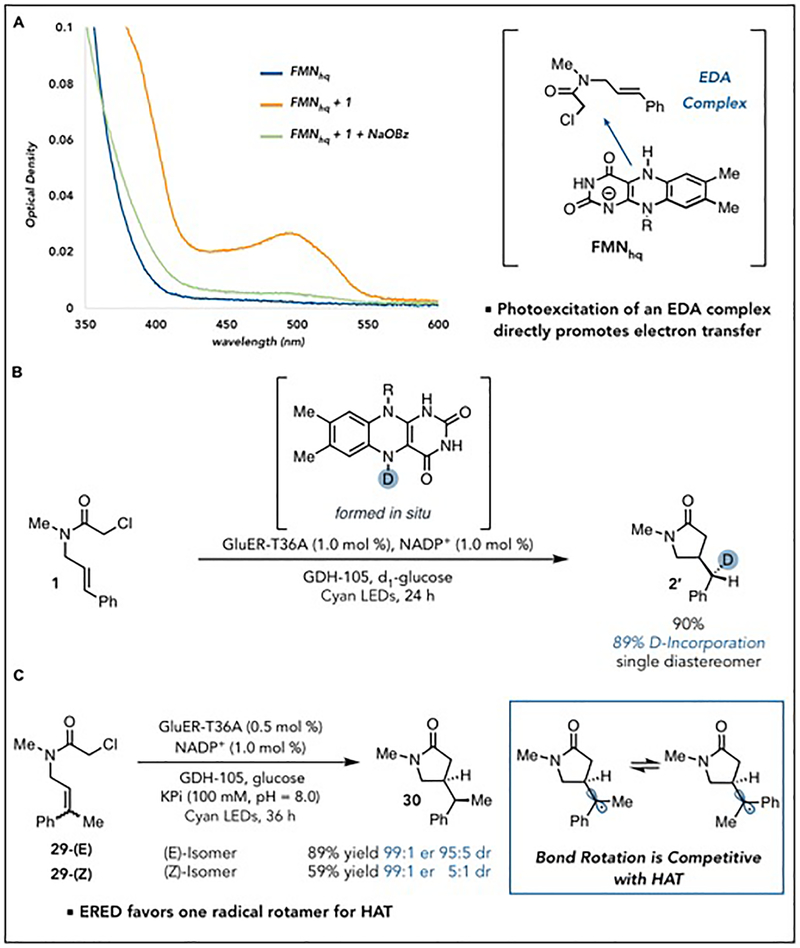
We conducted a series of mechanistic experiments to better understand the nuances of this reaction, including the superior reactivity with cyan LEDs (𝜆max = 497 nm). Reduction of GluER to the FMNhq oxidation state with dithionite revealed formation of the diagnostic FMNhq signature with minimal absorption at 497 nm, suggesting direct excitation of the cofactor is not responsible for the observed wavelength preference (Fig 3A, FMNhq). Addition of 100 equivalents of chloroamide 1 resulted in formation of a new broad absorption band at 𝜆max = 500 nm (Fig 3A, FMNhq + 1). As this absorption feature is not lost upon addition of sodium dithionite, we do not attribute it to FMNsq. It is lost, however, upon addition of a 150 equivalents of sodium benzoate (Fig 3A, FMNhq + 1 + NaOBz). These data are consistent with the formation of an electron donor-acceptor complex between the substrate and FMNhq within the enzyme active site. We hypothesize that excitation of this charge transfer band promotes the initial electron transfer from FMNhq to 1.
Fig. 3. Mechanistic experiments.
(A) UV-visible spectrum of reduced GluER-T36A (FMNhq) in the presence of substrate (FMNhq + 1) and in the presence of substrate and sodium benzoate (FMNhq + 1 + NaOBz) (B) Isotope labeling. (C) Conformation-selective HAT.
Next, we sought evidence for the existence of radical intermediates by preparing a substrate containing a cyclopropyl ring as a radical clock. GluER-T36A produced only products of cyclopropane ring opening, supporting the existence of a radical intermediate (Fig. S9). To determine the terminal hydrogen atom source, we used labeled FMNDhq generated in situ from D-glucose-1-d1 (Fig. 3A). Deuterium was incorporated exclusively at the benzylic carbon with an exquisite level of diastereocontrol. These experiments support a reaction mechanism where substrate is initially reduced by one electron following irradiation of the electron donor-acceptor complex formed between the substrate and FMNhq within the enzyme active site. The α-acyl radical can react with the pendent olefin to afford an exocyclic radical which is terminated via hydrogen atom transfer from neutral flavin semiquinone (FMNsq) to the afford the product and oxidized flavin (Fig S47).
Based on this mechanistic hypothesis, we reasoned that the configuration of the alkene may be responsible for the observed levels of diastereoselectivity if HAT is faster than rotation of the exocyclic C-C bond. We thus performed the reaction on starting material 29 with (Z)-alkene geometry rather than the (E)-isomer. Both alkene geometries favor the same diastereomer and produce no change in the enantioselectivity (Fig. 3C). The enzyme thus favors HAT from one rotamer of the prochiral radical at rates that are competitive with bond rotation. The diminished levels of diastereoselectivity observed with the (Z)-alkene isomer are presumably due to a small degree of hydrogen atom transfer prior to bond rotation.
Transient absorption spectroscopy with olefin isomers of 29 provides additional support for this mechanistic hypothesis. Initial excitation at 370 nm followed by a broad-band probe pulse reveals underlying dynamics of the flavin redox states. We used a sequential kinetic scheme to fit the data by global analysis. We found three time constants that describe the temporal evolution of excited state flavin species through evolutionary associated difference spectra (EADS). When (E)-29 is used as the substrate, a signal corresponding to the charge transfer state decays in 10 ps to the FMNsq. This time scale in consistent with previously reported rates of decomposition for the α-chloroacetophenone ketyl radical anion (30). The neutral FMNsq decays on the time scale of 250 ps to the flavin quinone (FMNox) (Fig S42 and S43). This decay likely corresponds to the rate of cyclization and termination of the radical via hydrogen atom transfer from FMNsq. When the same experiment is conducted on (Z)-29, the FMNsq lifetime increases to 700 ps (Fig. S39 and S40). These data are consistent with post-cyclization rotation of the exo-cyclic radical to a conformation in which HAT from the FMNsq to the substrate-centered radical is kinetically feasible.
We anticipate that light-promoted reactivity demonstrated here will be replicable widely in EREDs, KREDs and other classes of flavoenzymes known in biocatalysis for their promiscuity and adaptability. These flavoenzymes may serve as stereoselective catalysts for unexpected radical transformations beyond those demonstrated here, depending on the active site organization, provided starting material, and properties of the excited state flavin. By more widely investigating and exploiting photoexcited states of cofactors, it should be possible to photoinitiate radical-based reactions within enzymes that are otherwise inaccessible with cofactors in the ground-state.
Supplementary Material
Acknowledgments
We thank Mike Souza for preparing glassware for spectroscopy studies, Christina Kraml and Laura Wilson at Lotus Separations, LLC for compound purification and Phil Jeffrey for assistance with x-ray structure determination, Kenith Conover for assistance in photoNMR data acquisition, as well as the staff of NSLS-II beamline FMX (17-ID-2) for help with data collection.
Funding: Research reported in this publication was supported by the NIH National Institute of General Medical Sciences (R01 GM127703), the Searle Scholars Award (SSP-2017-1741), Sloan Research Fellowship, the Princeton Catalysis Initiative, and Princeton University. D.G.O acknowledges support from the Postgraduate Scholarships Doctoral Program of the NSERC. D.G.O and G.D.S acknowledge support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant No. DE-SC0019370. The AMX (17-ID) beamline of The Life Science Biomedical Technology Research (LSBR) resource is primarily supported by the National Institute of Health, National Institute of General Medical Sciences (NIGMS) through a Biomedical Technology Research Resource P41 grant (P41GM111244), and by the DOE Office of Biological and Environmental Research (KP1605010). As a National Synchrotron Light Source II facility resource at Brookhaven National Laboratory, work performed at the LSBR is supported in part by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences Program under contract number and DE-SC0012704 (KC0401040).
Footnotes
Competing interests: The authors declare no conflicts of interest.
Data and materials availability: All data is available in the main text or the supplementary materials. Crystallographic models and structure factors have been deposited in the Protein Data Bank with accession numbers 60O8 and 6MYW for GluER and GluER-T36A, respectively. X-Ray crystallographic data for lactam 2 have been deposited in the Cambridge Crystallographic Data Centre (1878605).
References
- 1.Jäger CM, Croft AK, Anaerobic Radical Enzymes for Biotechnology ChemBioEng. 5, 143–162 (2018). [Google Scholar]
- 2.Studer A, Curran DP, Catalysis of Radical Reactions: A Radical Chemistry Perspective, Angew. Chem. Int. Ed 55, 58–102 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Bornscheuer UT, Kazlauskas RJ, Catalytic Promiscuity in Biocatalysis: Using Old Enzymes to Forge New Bonds and Follow New Pathways. Angew. Chem. Int. Ed 43, 6032–6040 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Romero NA, Nicewicz DA, Organic Photoredox Catalysis. Chem. Rev. 116, 10075–10166 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Seifert S, Brakmann S, LOV Domains in the Design of Photoresponsive Enzymes. ACS Chem. Biol. 13, 1914–1920 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Wang H, Yuan Q, Feng Y, Structure and Function of the Photosystem Supercomplexes. Front. Plant Sci 9, Article 357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmermund L, Jurkas V, Özgen FF, Barone GD, Büchsenschültz HC, Winkler CK, Schmidt S, Kourist R, Kroutil W. Photo-Biocatalysis: Biotransformations in the Presense of Light. ACS Catal. 9, 4115–4144 (2019). [Google Scholar]
- 8.Gabruk M, Mysliwa-Kurdziel B, Light-dependent protochlorophyllide oxidoreductase: Phylogeny, regulation, and catalytic properties. Biochemistry 54, 5255–5262 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Sorigué D et al. An Algal photoenzyme converts fatty acids to hydrocarbons. Science. 357, 903–907 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Sancar A, Structure and Function of DNA Photolyase and Cryptochrome Blue-Light Photoreceptors. Chem. Rev 103, 2203–2238 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Emmanuel MA, Greenberg NR, Oblinsky DG, Hyster TK, Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Biegasiewicz KF, Cooper SJ, Emmanuel MA, Miller DC, Hyster TK, Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases. Nat. Chem. 10, 770–775 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Hung K, Hu X, Maimone TJ, Total synthesis of complex terpenoids employing radical cascade processes. Nat. Prod. Rep 35, 174–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibi MP, Manyem S, Zimmerman J, Enantioselective Radical Processes, Chem. Rev 103, 3262–3296 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Roberts BP, Tocher DA, Carbohydrate-derived thiols as protic polarity-reversal catalysts for enantioselective radical-chain reactions, J. Chem. Soc., Perkin Trans 1, 1376–1386 (2002). [Google Scholar]
- 16.Vitaku E, Smith DT, Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U. S. FDA Approved Pharmaceuticals. J. Med. Chem 57, 10257–10274 (2014). [DOI] [PubMed] [Google Scholar]
- 17.France SP, Hussain S, Hill AM, Hepworth LJ, Howard RM, Mulholland KR, Flitsch SL, Turner NJ, One-Pot Cascade Synthesis of Mono- and Disubstituted Piperidines and Pyrrolidines using Carboxylic Acid Reductase (CAR), ω-transaminase (ω-TA), and Imine Reductase (IRED) Biocatalysis. ACS Catal. 6, 3753–3759 (2016). [Google Scholar]
- 18.Curran DP, Tamine J, Effects of Temperature on Atom Transfer Cyclization Reaction of Allylic α-iodo esters and amides. J. Org. Chem 56, 2746–2750 (1991). [Google Scholar]
- 19.Hiroi K, Ishii M, Asymmetric induction in the inter- and intramolecular radical carbon-carbon bond forming reactions of N-arylsulfonyl-α-bromocarboxamides with chiral ligands: the use of chiral sulfoxide ligands with radical chemistry. Tetrahedron Lett. 41, 7071–7074 (2000). [Google Scholar]
- 20.Schmidt NG, Eger E, Kroutil W, Building Bridges, Biocatalytic C–C Bond Formation Toward Multifunctional Products. ACS Catal. 6, 4286–4311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner NJ, O’Reilly E, Biocatalytic Retrosynthesis. Nat. Chem. Biol 9, 285–288 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Toogood HS, Scrutton NS, Discovery, Characterization, Engineering, and Applications of Ene-Reductases for Industrial Biocatalysis. ACS Catal. 8, 3532–3549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heckenbichler K, Schweiger A, Brandner LA, Binter A, Toplak M, Macheroux P, Gruber K, Breinbauer R, Asymmetric Reductive Carbocyclization Using Engineered Ene Reductases. Angew. Chem. Int. Ed 57, 7240–7244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval BA, Meichan AJ, Hyster TK, Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc 139, 11313–11316 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Warren JJ, Ener ME, Vlček A, Winkler JR, Gray HB, Electron hopping through proteins. Coord. Chem. Rev 256, 2478–2487 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghisla S, Massey V, Lhoste J-M, Mayhew SG, Fluorescence and Optical Characteristics of Reduced Flavines and Flavoproteins. Biochemistry. 13, 589–597 (1974). [DOI] [PubMed] [Google Scholar]
- 27.Massey V, Hemmerich P, Photoreduction of Flavoproteins and Other Biological Compounds Catalyzed by Deazaflavins. Biochemistry 17, 9–17 (1978). [DOI] [PubMed] [Google Scholar]
- 28.Brill ZG, Grover HK, Maimone TJ, Enantioselective Synthesis of an ophiobolin sesterterpene via a programed radical cascade. Science 352, 1078–1082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yet L, Free Radicals in the synthesis of medium-sized rings. Tetrahedron 55, 9349–9403 (1999). [Google Scholar]
- 30.Tanner DD, Chen JJ, Chen L, Luelo C, Fragmentaiton of Substituted Acetophenone and Halobenzophenone ketlys. Calibration of a Mechanistic Probe. J. Am. Chem. Soc 113, 8074–8081 (1991). [Google Scholar]
- 31.Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith Nat HO. Methods 6, 343–345 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Aliverti A, Curti B, Vanoni MA Indentifying and Quantitating FAD and FMN in Simple and in Iron-Sulfur-Containing Flavoproteins In Flavoprotein Protocols, Chapman SK; Reid GA, Eds.; Humana Press: Totowa, New Jersey, 131, 9 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Fava E, Nakajima M, Nguyen ALP, Rueping J M. Org. Chem 81, 6959–6964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Q, Liu D, W. Zhang. Org. Lett 19, 1144–1147 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Halgren TA, Roberts JD, Horner JH, Martinez FN, Tronche C, Newcomb M. J. Am. Chem. Soc 122, 2988–2994 (2000). [Google Scholar]
- 36.Sherer EC, Lee CH, Shpungin J, Cuff JF, Da CX, Ball R, Bach R, Crespo A, Gong XY, Welch CJ, J. Med. Chem 57, 477–494 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Liu ZQ, Shultz CS, Sherwood CA, Krska S, Dormer PG, Desmond R, Lee C, Sherer EC, Shpungin J, Cuff J, Xu F Tetrahedron Lett. 52, 1685–1688 (2011). [Google Scholar]
- 38.Miehlich B, Savin A, Stoll H, Preuss Chem H. Phys. Lett. 157, 200–206 (1989). [Google Scholar]
- 39.Petersson GA, Allaham J MA. Chem. Phys 94, 6081–6090 (1991). [Google Scholar]
- 40.Petersson GA, Bennett A, Tensfeldt TG, Allaham MA, Shirley WA, Mantzaris J J. Chem. Phys. 89, 2193–2218 (1988). [Google Scholar]
- 41.Rassolov VA, Pople JA, Ratner MA, Windus TL, J. Chem. Phys 109, 1223–1229 (1998). [Google Scholar]
- 42.Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA, J. Comput. Chem 22, 976–984 (2001). [Google Scholar]
- 43.Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, Defrees DJ, Pople J JA. Chem. Phys 77, 3654–3665 (1982). [Google Scholar]
- 44.Hehre WJ, Ditchfield R, Pople J JA. Chem. Phys 56, 2257–2261 (1972). [Google Scholar]
- 45.Becke J AD. Chem. Phys 98, 5648–5652 (1993). [Google Scholar]
- 46.Lee CT, Yang WT, Parr Phys RG. Rev. B 37, 785–789 (1988). [DOI] [PubMed] [Google Scholar]
- 47.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, and Fox DJ, Gaussian, Inc., Wallingford CT, 2016. [Google Scholar]
- 48.Cheeseman JR, Frisch MJ, Devlin FJ, Stephens P, J. Chem. Phys. Lett 252, 211–220 (1996). [Google Scholar]
- 49.Shen JA, Zhu CY, Reiling S, Vaz R, Spectrochim. Acta Part A 76, 418–422 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Ji Y, DiRocco DA, Hong CM, Wisner MK, Reibarkh M, Org. Lett. 20, 2156–2159 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Massey V, Palmer G, On the Existence of Spectrally Distinct Classes of Flavoprotein Semiquinones. A New Method for the Quantitative Production of Flavoprotein Semiquinones. Biochemistry. 5, 3181–3189 (1966). [DOI] [PubMed] [Google Scholar]
- 52.Mehling K, Hansen K, Hedén M, Lassesson A, Bulgakov AV, B Campbell EE, Energy distributions in multiple photon absorption experiments. J. Chem. Phys, 120, 4281 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Kabsch W XDS. Acta Cryst. D66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans PR and Murshudov GN Acta Cryst. D69, 1204–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC and Read RJ Phaser crystallographic software. J. Appl. Cryst 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murshudov GN, Vagin AA and Dodson EJ Refinement of Macromolecular Structures by the Maximum-Likelihood method, Acta Cryst. D53, 240–255 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.