Abstract
Between 2016 and 2017, several U.S. diplomats in Havana, Cuba, experienced perplexing vestibular and neurological symptoms attributed to an unknown source. They presented with significant vestibular and headache symptoms similar to individuals who experience vestibular migraine (VM). As such, we hypothesize that VM may be a possible explanation for the Havana cohort’s presenting symptoms. To evaluate this hypothesis, we compared symptoms reported by the affected individuals in Havana, Cuba, to symptoms reported by corresponding patients from a tertiary academic neurotology clinic with a chief complaint of vertigo or disequilibrium, who met the International Classification of Headache Disorders criteria for VM. The prevalence of the Havana subjects experiencing various symptomatic domains was compared with the VM cohort, leading to 26 unique domains and statistical comparisons between the cohorts. Of the 26 domains compared between the two cohorts, 18 were not significantly different. This included the two most important components of meeting criteria for VM, namely the co-existence of headache and vestibular symptoms. On regression analysis, the only feature which kept its significant difference between the two cohorts was acute intense head pressure (P = 0.007). The domains with similar occurrence ratios included dizziness, headache, light sensitivity, hearing reduction, and tinnitus. In other words, multiple headache and vestibular symptoms, consistent with VM criteria, were similar between the two cohorts. The considerable similarities across various domains between VM patients and Havana subjects could be due to migraine as a possible common etiology for both groups. We propose VM as a potential etiology for the symptomatology in the U.S. diplomats in Cuba.
Keywords: migraine, brain injury, vestibular migraine, neurological symptom, vestibular symptom, Havana, U.S. diplomats
Introduction
Between 2016 and 2017, several U.S. diplomats stationed in Havana, Cuba, experienced perplexing vestibular and neurological symptoms that have been attributed to exposure to an auditory and/or sensory source. These individuals identified during the initial triage were evaluated by the University of Pennsylvania’s Center for Brain Injury and Repair, and the preliminary results were recently published (1). Following assessment of various cognitive, vestibular, headache, and oculomotor symptoms, it was suggested that injury to the widespread brain networks was the best-fitting etiology that justified the phenomena. Hoffer et al. performed further testing on a portion of the cohort and found that the single commonality among all sufferers was damage to the inner ear organs responsible for balance (2). More recently, a study commissioned by the Canadian government found changes consistent with acquired brain injury, theorized to be caused by cholinesterase inhibitor intoxication from mosquito fumigation (3). Given this Havana cohort’s especially predominant vestibular and headache symptoms and damage to the inner ear provoked by an unknown source, we theorize that vestibular migraine (VM) could be the underlying diagnosis in many of these individuals. To investigate this hypothesis, we compared the reported symptoms experienced by the Havana cohort to those experienced by a patient cohort diagnosed with VM.
Hypothesis
We hypothesize that the considerable similarities found between the Havana cohort and a VM patient cohort may be due to “migrainous phenomenon” acting as a possible common etiology for both groups. In order to test this hypothesis, symptoms reported by the affected individuals were compared to symptoms reported by a large VM cohort who presented to our tertiary neurotology clinic.
Evaluation of the hypothesis
Data regarding the affected Havana cohort’s symptoms were extracted from tables, text, and supplementary materials provided by Swanson et al.’s retrospective manuscript reporting preliminary information of this cohort (1). The corresponding VM patients presented to our tertiary academic neurotology clinic from 2014 to 2018 with a chief complaint of vertigo or disequilibrium and met the International Classification of Headache Disorders (ICHD) criteria for VM (4). At the time of presentation, these VM patients completed a comprehensive questionnaire about their symptoms. The prevalence of the Havana subjects experiencing various symptomatic domains was then compared with our cohort. This led to 26 unique domains and statistical comparisons between the two cohorts. Univariate analyses including the chi-square or Fisher’s exact tests were performed to compare the categorical variables between the two cohorts. Variables with statistically significant P values of less than 0.05 were extracted for second-step analysis. These variables were included in a multivariate binary logistic regression, where their independent effects were determined.
There were 21 Havana cohort’s individuals (52% female) and 104 VM patients (68% female) with an average age of 43 ± 8 years and 49 ± 16 years, respectively. Of the 26 domains compared between the two cohorts, 8 were initially significantly different and 18 were not significantly different (Table 1). On regression analysis, the only feature that remained significantly different between the two cohorts was acute intense head pressure (P = 0.007). The domains with similar occurrence ratios included dizziness, headache, light sensitivity, hearing reduction, and tinnitus.
Table 1.
The results of univariate analysis comparing the prevalence of domains between the Havana and vestibular migraine cohorts.
| Domain | Havana cohort (%) [N = 21] |
VM cohort (%) [N = 104] |
P value |
|---|---|---|---|
| Persistent nausea | 7 (33) | 61 (59) | 0.034 |
| Visual problems | 16 (76) | 42 (40) | 0.003 |
| Ear pain at days-to-weeks | 5 (24) | 68 (65) | <0.001 |
| Persistent ear pressure | 8 (38) | 68 (65) | 0.019 |
| Sensorineural hearing loss | 3 (14) | 27/57 (47) | 0.008 |
| Acute intense head pressure* | 5 (24) | 58/99 (59) | 0.004 |
| Headache due to photophobia | 9 (43) | 77 (74) | 0.005 |
| Headache due to phonophobia | 6 (29) | 75 (72) | <0.001 |
| Sex (female) | 11 (52) | 71 (68) | 0.162 |
| Persistent dizziness | 13 (62) | 67/97 (69) | 0.524 |
| Memory problems | 16 (76) | 70 (67) | 0.423 |
| Mental fog | 16 (76) | 69/102 (68) | 0.440 |
| Concentration impairment | 15 (71) | 68/101 (67) | 0.714 |
| Feeling cognitively slow | 14 (67) | 73/102 (72) | 0.653 |
| Irritability | 14 (67) | 59 (57) | 0.399 |
| Nervousness | 12 (57) | 59 (57) | 0.972 |
| Light sensitivity | 13 (62) | 77 (74) | 0.259 |
| Persistent tinnitus | 12 (57) | 71 (68) | 0.325 |
| Hearing change at days-to-weeks | 7 (33) | 27/57 (47) | 0.268 |
| Persistent sound sensitivity | 14 (67) | 75 (72) | 0.615 |
| Persistent hearing reduction | 9 (43) | 44 (42) | 0.963 |
| Sleep problems | 18 (86) | 64/99 (65) | 0.059 |
| Drowsiness or fatigue | 16 (76) | 70 (67) | 0.423 |
| Headache days-to-weeks | 17 (81) | 57/92 (62) | 0.099 |
| Persistent headache | 16 (76) | 57/92 (62) | 0.218 |
| Headache improved with medication | 12 (67) | 71/100 (68) | 0.214 |
VM, vestibular migraine.
Denominators noted if not 104 in the VM cohort.
Persistent defined as presence more than 3 months after exposure in Havana cohort and presence more than 3 months before presented to our clinic in VM cohort.
The only domain that was found to be significantly different between the two cohorts after multivariate regression analysis.
On univariate analysis, 18 of 26 domains had similar incidences in the VM and Havana cohorts. This included the most important component of meeting criteria for VM: the co-existence of headache and vestibular symptoms. None of the eight domains that had significantly different ratios were among those required for VM diagnosis based on the ICHD criteria. Additionally, after multivariate regression analysis only intense head pressure remained significantly different between the two cohorts and the rest of the seven features lost their significance. Furthermore, symptoms such as hearing reduction, light sensitivity, and tinnitus which occur more commonly in patients with migraine (5), were not significantly different between the two cohorts.
Discussion
Our analyses suggest that the Havana cohort did not present with a significantly different symptomatology, particularly in regards to meeting the VM diagnostic criteria, compared to a cohort with confirmed VM diagnosis. As such, we suspect that the Havana cohort’s reported symptoms may be consistent with an underlying migrainous etiology. Furthermore, individuals in the Havana cohort reported a fluctuating pattern of their symptoms for several days as well as cognitive symptoms, imbalance, headache, and exacerbation of symptoms by exercise, all of which are also consistent with a migraine etiology. Interestingly, Swanson et al. showed that the headaches in certain Havana individuals were improved with known migraine medications (n = 12, 57%) (1). Accordingly, the reason that not all exposed individuals (namely, the “housemates” of the diplomats) developed symptoms may be due to genetic differences that alter the individual’s susceptibility to migraine and VM. Those in the Havana cohort who were symptomatic may have been so due to greater genetic susceptibility to provoking factors such as those discussed in detail below. This is contrary to Hoffer et al.’s suggestion that susceptibility was based on precise space/time exposure of the stimuli (2).
For reasons including an absence of blunt head trauma, the injury pattern seen in the Havana cohort may not be entirely compatible with the patterns reported in concussion or mild traumatic brain injury (mTBI) as hypothesized by Swanson and colleagues (1). Diffusion tensor imaging of the Havana cohort showed lower diffusivity and higher functional anisotropy compared to demographically similar healthy controls which is in contrast to the increased diffusivity and decreased functional anisotropy observed in TBI (6). These individuals also showed significantly lower functional connectivity in the auditory and visuospatial networks but not in the executive control network. These functional imaging differences were supported by the lower tissue integrity measures of mean diffusivity, radial and axial diffusivity, and free water volume fraction in the inferior colliculi, which are linked to auditory and vestibular function (6).
In regard to what ultimately provoked the Havana cohort’s symptoms, the true cause may never be discovered. However, Swanson et al. summarized the likely inciting factor as an auditory and sensory stimulus from a directional source (1). Some of the affected individuals described the inciting episode as “a few minutes of a high-pitched noise, often accompanied by a high-pressure sensation, described as a force field” (1). It is known that low frequency noise (e.g., infrasound) can be extremely distressing, and given the right parameter, can cause headache, dizziness, and problems with sleep and concentration in 29-67% of exposed patients (7). Sound has been found to trigger a headache in 79% of subjects with chronic migraine or headache disorder (8), and there are reports in the literature regarding the utilization of tone-burst stimuli or low-frequency fluctuation to potentiate or provoke symptoms of VM (9, 10). Via the utilization of amplitude of low-frequency fluctuation, Xue et al. also demonstrated that migraine episodes can be associated with spontaneous neuronal activity in specific pain processing areas (10). Considering these, we suspect that the Havana phenomenon was potentially provoked by a similar low-frequency infrasound stimulus, leading to concurrent headache and vestibular symptoms consistent with a diagnosis of VM.
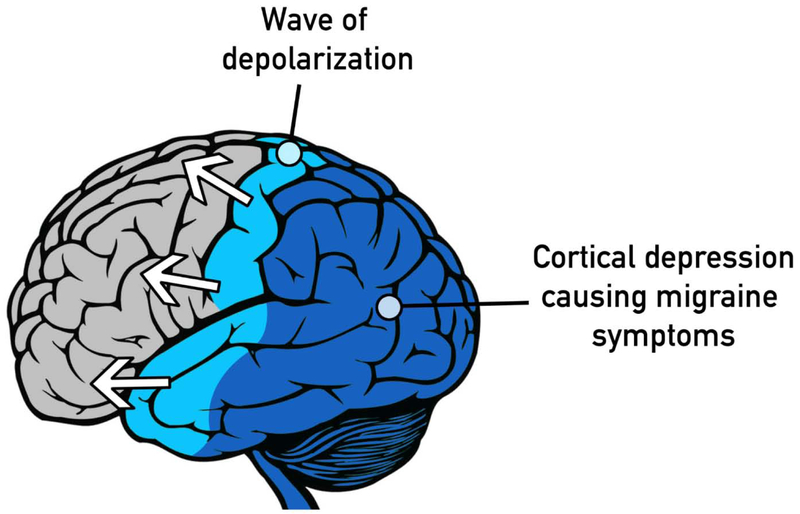
Other theories beyond provocation by aural phenomena exist as well. More recently, Friedman et al. reported the presence of neural changes consistent with acquired brain injury and of increased permeability of the blood-brain barrier in Canadian diplomats who visited Havana and developed similar symptoms as American diplomats (3). The study suggested that the symptoms were likely caused by neurotoxins, specifically cholinesterase inhibitors from insect fumigation, rather than from sound-based sources. This is not in disagreement with our hypothesis, because it is possible that this neural damage from pesticide may have also provoked VM. It is now theorized that migraine, including VM, begins with cortical spreading depression (CSD), a wave of depolarization that can cause aura, pain, and vestibular symptoms (Fig. 1) (11). The intricacies of the triggering factors for CSD are not completely known; however injury to neurons in the brain, inflammation, and breakdown of the blood-brain barrier have all been shown to provoke CSD in animal models (12). It is possible that the use of pesticides caused these aforementioned long-lasting changes in the brains of those who were exposed, making them more susceptible to CSD events which then further manifested as VM. This could explain why these cohorts can experience persisting symptoms even after the termination of exposures.
Fig 1.
Schematic representation of cortical spreading depression, a wave of depolarization that can cause aura, pain, and vestibular symptoms.
Friedman’s investigation of the respective Canadian diplomats’ symptoms was summarized with an emphasis on fatigue, headache, sleep disturbance, concentration, memory, blurry vision, tinnitus, feeling off balance, and sensitivity to sound (3). This multi-faceted spectrum of symptoms is not uncommon among patients with VM, which are also claimed to be underdiagnosed despite being relatively common (13, 14). Magnetic resonance imaging of these patients demonstrated significant vascular pathology, which is not in disagreement with several reports exploring the underlying vascular etiology of migraine with resulting brain changes (15-18). Coupled with the reported gradual onset of symptoms with no clear precipitating event (3), these findings support that VM remains a possible explanation for the spectrum of symptoms experienced by the Havana diplomats.
Despite the explorative nature of this hypothesis manuscript, our study is limited by a lack of extensive information concerning the specific duration of dizziness episodes and the frequency of headaches, as well as other details pertaining to VM criteria, experienced by the Havana cohort. Furthermore, complete data regarding exposure and potential symptom development in all other exposed individuals who stayed in the same work and housing environment, such as family members and other diplomats, was classified and could not be evaluated. Additionally, some of the statistical differences of the investigated domains may stem from the Havana cohort’s small sample size. If the cohort of affected individuals was as large a cohort as our VM patients, some differences could become insignificant. Regardless, given our large VM cohort, we urge readers to consider VM as a potential etiology for the symptomatology in the U.S. diplomats in Cuba.
Conclusion
This manuscript is the first to propose VM as a possible underlying etiology for U.S. Havana diplomats suffering from unknown vestibular and neurologic symptoms. In addition to demonstrating great similarities in presenting symptoms between the Havana cohort and a patient cohort with a confirmed diagnosis of VM, our hypothesis was further strengthened by discussing how the hypothesized sources of injury, including pesticides or infrared sound sources, can provoke VM. Reports of certain diplomats’ responsiveness to migraine medication as well as the susceptibility of certain individuals to not become affected were further in-line with an underlying migrainous phenomena. As such, we encourage the readers to regard VM as one of the possible differential diagnoses of the Havana diplomats.
Acknowledgements
Mehdi Abouzari, MD, PhD; is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant TL1TR001415-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Swanson RL 2nd, Hampton S, Green-McKenzie J, Diaz-Arrastia R, Grady MS, Verma R, et al. Neurological Manifestations Among US Government Personnel Reporting Directional Audible and Sensory Phenomena in Havana, Cuba. JAMA. 2018;319(11):1125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffer ME, Levin BE, Snapp H, Buskirk J, Balaban C. Acute findings in an acquired neurosensory dysfunction. Laryngoscope Investig Otolaryngol. 2019;4(1):124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman AC, Calkin C, Bowen C. Havana Syndrome: Neuroanatomical and Neurofunctional Assessment in Acquired Brain Injury Due to an Unknown Etiology [Internet]. 2019. [cited October 14, 2019]. Available from: https://www.scribd.com/document/426438895/Etude-du-Centre-de-traitement-des-lesions-cerebrales-de-l-Universite-de-Dalhousie. [Google Scholar]
- 4.Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia; 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JH, Tsai SJ, Liu TC, Chen YC, Lai JT. Association of Tinnitus and Other Cochlear Disorders With a History of Migraines. JAMA Otolaryngol Head Neck Surg. 2018;144(8):712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma R, Swanson RL, Parker D, Ould Ismail AA, Shinohara RT, Alappatt JA, et al. Neuroimaging Findings in US Government Personnel With Possible Exposure to Directional Phenomena in Havana, Cuba. JAMA. 2019;322(4):336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moller H, Lydolf M. A questionnaire survey of complaints of infrasound and low-frequency noise. J Low Freq Noise V A. 2002;21(2):53–63. [Google Scholar]
- 8.Martin PR, Todd J, Reece J. Effects of noise and a stressor on head pain. Headache. 2005;45(10):1353–64. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RL, Zagami AS, Gibson WPR, Black DA, Watson SRD, Halmagyi GM, et al. Vestibular evoked myogenic potentials to sound and vibration: characteristics in vestibular migraine that enable separation from Meniere's disease. Cephalalgia; 2012;32(3):213–25. [DOI] [PubMed] [Google Scholar]
- 10.Xue T, Yuan K, Cheng P, Zhao L, Zhao LM, Yu DH, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. Nmr Biomed. 2013;26(9):1051–8. [DOI] [PubMed] [Google Scholar]
- 11.Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9(11):637–44. [DOI] [PubMed] [Google Scholar]
- 12.Harriott AM, Takizawa T, Chung DY, Chen SP. Spreading depression as a preclinical model of migraine. J Headache Pain. 2019;20(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connell Ferster AP, Priesol AJ, Isildak H. The clinical manifestations of vestibular migraine: A review. Auris Nasus Larynx. 2017;44(3):249–52. [DOI] [PubMed] [Google Scholar]
- 14.Neuhauser HK, Radtke A, von Brevern M, Feldmann M, Lezius F, Ziese T, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028–33. [DOI] [PubMed] [Google Scholar]
- 15.Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM, et al. Structural brain changes in migraine. JAMA. 2012;308(18):1889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shevel E. The extracranial vascular theory of migraine--a great story confirmed by the facts. Headache. 2011;51(3):409–17. [DOI] [PubMed] [Google Scholar]
- 17.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwedt TJ, Dodick DW. Advanced neuroimaging of migraine. Lancet Neurol. 2009;8(6):560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]