Abstract
Gaze following plays a role in parent–infant communication and is a key mechanism by which infants acquire information about the world from social input. Gaze following in Deaf infants has been understudied. Twelve Deaf infants of Deaf parents (DoD) who had native exposure to American Sign Language (ASL) were gender-matched and age-matched (±7 days) to 60 spoken-language hearing control infants. Results showed that the DoD infants had significantly higher gaze-following scores than the hearing infants. We hypothesize that in the absence of auditory input, and with support from ASL-fluent Deaf parents, infants become attuned to visual-communicative signals from other people, which engenders increased gaze following. These findings underscore the need to revise the ‘deficit model’ of deafness. Deaf infants immersed in natural sign language from birth are better at understanding the signals and identifying the referential meaning of adults’ gaze behavior compared to hearing infants not exposed to sign language. Broader implications for theories of social-cognitive development are discussed.
Keywords: deaf, gaze following, sign language, social cognition, visual attention
1 |. INTRODUCTION
Gaze following entails an observer looking where another person is looking. It is a crucial component of nonverbal communication and social cognition. Little is known about gaze following in Deaf infants, but this topic presents an important test for theories of developmental science and has societal implications. Here, we report the first experimental study of gaze following in Deaf infants of Deaf parents (DoD) who had native exposure to American Sign Language (ASL).1
Work with hearing infants shows that gaze following is an important aspect of infant social-cognitive development (e.g. Baldwin & Moses, 1996; Brooks & Meltzoff, 2015; Butterworth & Jarrett, 1991; Carpenter, Nagell, & Tomasello, 1998) and predicts infant word learning (e.g. Brooks & Meltzoff, 2008; Mundy et al., 2007). Hearing infants integrate auditory and visual information as they interact with caregivers. For example, if a parent turns to look at a book and says, ‘Let’s read this book’, the child might follow the parent’s gaze, visually encounter the object, and (nearly) simultaneously hear the linguistic label. A good deal of empirical work has been done on such auditory-visual social interactions and their contribution to the early stages of language acquisition (Bornstein, Tamis-LeMonda, Hahn, & Haynes, 2008; Carpenter et al., 1998; Conboy, Brooks, Meltzoff, & Kuhl, 2015; Harris, 2000; Rowe & Goldin-Meadow, 2009; Tomasello & Farrar, 1986). Research with children with autism spectrum disorder has shown that they have deficits in gaze following, which are correlated with slowed language acquisition (e.g. Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Mundy, 2018; Toth, Munson, Meltzoff, & Dawson, 2006; Yoder, Watson, & Lambert, 2015).
Some studies have reported that deaf children lag behind their hearing peers in measures of visual attention and gaze shifting between people and objects (Cejas, Barker, Quittner, & Niparko, 2014; Tasker, Nowakowski, & Schmidt, 2010). Crucially, however, there is notable variability among deaf children, some of which can be traced to their language input experience. Many deaf children raised by hearing parents (DoH) have a low quality and quantity of exposure to language, at least early in development (which usually lacks sign-language input, see Humphries et al., 2012 for a review). When early language input is diminished, there are consequences for language development as well as social and cognitive development (Mayberry, 2003; Mayberry & Eichen, 1991; Niparko et al., 2010; Peterson & Siegal, 2000).
By contrast, Deaf children of Deaf parents (DoD) often have full exposure to language via sign language and have robust language, cognitive, and social development (Loots, Devisé, & Jacquet, 2005; MacDonald, LaMarr, Corina, Marchman, & Fernald, 2018; Meadow-Orlans, Spencer, & Koester, 2004; Newport & Meier, 1985; Peterson & Siegal, 2000; Petitto, 2005; Rinaldi, Caselli, Di Renzo, Gulli, & Volterra, 2014). These positive patterns consistently emerge across small to modest samples of DoD children (who are difficult to recruit, inasmuch as only 5%–10% of deaf children have deaf parents, Mitchell & Karchmer, 2004). Thus, although small in number, the DoD group is informative to theory, because they uniquely have natural exposure to language and other communicative behavior primarily through the visual modality rather than auditory modality.
Deaf parents who are fluent signers actively engage their Deaf infants with specific visual (and tactile) strategies that attract, maintain, and guide their infants’ visual attention (Corina & Singleton, 2009; Harris, Clibbens, Chasin, & Tibbitts, 1989). With continued input and support, DoD infants seem to learn to shift their gaze from their own ongoing activity to look at their parent for information. Seminal observational studies of DoD infants and toddlers suggest that they develop distinctive patterns of gaze behavior characterized by more frequently looking back and forth between the parent and an object compared to hearing children of hearing parents (HoH) (Lieberman, Hatrak, & Mayberry, 2014) and DoH infants (Spencer, 2000). This pattern of looking back and forth from parent to object potentially enables DoD infants to glean information from their signing parents about objects and events, because both the communicative signal and the referent are visually perceived and typically not co-located in space.
These groundbreaking observational studies are intriguing; but they have not adopted strict experimental designs, and many have focused on older toddlers or preschool children, possibly missing important issues about developmental onset. Also, these observational studies have primarily examined a single type of gaze behavior – infants’ gaze shifts from an object to their parent (or from parent to object). The findings show that DoD infants are facile at disengaging attention from objects they are manipulating to shift to look at their parent, but such studies do not address the behavior of gaze following per se. Gaze following has its own rich and widely replicated literature with HoH infants, and has chiefly focused on adults turning to look at an object and infants turning to look at the same target.2
The difference between gaze following and looking back and forth between the physical object and the adult gazer (gaze shifting) is important. Although both behaviors emerge in the first year of life for hearing infants (HoH), these two behaviors are not typically correlated with each other, and they make distinct contributions to development (Brune & Woodward, 2007; Mundy et al., 2007). For example, infants’ gaze shifts can help maintain parent–child interaction but do not rely on detecting the direction of their parent’s eye gaze. Findings from neuroscience also suggest a distinction between these behaviors, inasmuch as they recruit different brain regions (Mundy, 2018; Redcay, Kleiner, & Saxe, 2012).
1.1 |. Rationale for the Current Study
Corina and Singleton (2009) hypothesized that early immersion in a signed language may provide DoD infants rich experiences with adult gaze behaviors and suggested that this might lead to advanced development in infant gaze-following behavior. The general idea that gaze following is malleable and that special experiences can change infant gaze behavior has been supported by experiments with hearing infants. Two sets of studies suggest that the development and deployment of gaze following is sensitive to experiential input. In one line of work, specific laboratory interventions were designed to provide infants self-experience with how opaque physical barriers block their own vision of external objects. The intervention was shown to enhance infants’ understanding and processing of the gaze of others (Meltzoff & Brooks, 2008). Another line of work provided evidence that aspects of daily viewing experiences at home are associated with infant gaze behaviors in subsequent laboratory testing (e.g. Peña, Arias, & Dehaene-Lambertz, 2014; Senju et al., 2015; Xiao et al., 2018).
Here, we propose that comparing the gaze following of DoD infants (exposed to fluent signers of ASL from birth) with HoH infants (exposed to fluent speakers of language from birth) provides a natural experiment that can inform theories in developmental science. Both groups of infants in the study have early and rich language experience, but they differ in the modality of their primary language input. Their different experiences could influence their response to adult looking behavior, including how consistently infants follow the adult’s gaze to a peripheral target.
Three different predictions are possible about the gaze-following behaviors in deaf compared to hearing infants. First, it could be that DoD infants are advanced in gaze following because of the particular, intensive experience that Deaf parents provide Deaf infants (including exposure to a natural visual language and scaffolded interactions that emphasize visual attention to other people’s communicative bodily actions). This prediction emerges in part from prior studies with native signing deaf adults that have shown enhancements in certain aspects of visual attention, such as greater attention to peripheral information (Bavelier et al., 2001; Proksch & Bavelier, 2002).
Second, it is possible that DoD infants are delayed compared to HoH infants in gaze following. For example, some researchers have suggested that when audition is absent there are difficulties in other areas of development including visual attention (Conway, Pisoni, & Kronenberger, 2009; Quittner, Smith, Osberger, Mitchell, & Katz, 1994); however, these findings have been debated (Dye, Hauser, & Bavelier, 2008; Tharpe, Ashmead, & Rothpletz, 2002) and largely draw on data for older deaf children and adults with diminished language experience.3
Finally, a third possibility is that the development of gaze following is an ‘experience expectant’ behavior of evolutionary importance, which primarily follows a maturational timetable. If so, there may be no measurable difference in gaze following between age-matched HoH and DoD infants.
The overall goal of the study was to examine gaze-following behaviors of DoD and HoH infants. The age range for the infants was 7–20 months to allow for an assessment of gaze-following behavior (which is commonly evaluated between 6 and 24 months of age, e.g. Carpenter et al., 1998; Morales et al., 2000; Mundy et al., 2007) and to test for possible group variation (advanced, delayed, or no difference). This is the first experimentally controlled test of gaze following with DoD infants and used well-established procedures: Infants faced an adult who then silently turned to look at objects in the room, while the infants’ behavior was video recorded for subsequent scoring.
We recruited five hearing gender and age-matched infants for each Deaf infant. This oversampling of the control participants is a standard practice in experimental work with low-incidence pediatric or clinical populations. More specifically, the ratio of control to experimental participants (indicated by x:y) is as follows for the following studies: For children with autism spectrum disorder: Adamson, Bakeman, Deckner, & Nelson, 2012 (3:1), Dawson et al., 1998 (2:1); for blind individuals: Landau, Gleitman, & Spelke, 1981 (5:1), Senju et al., 2013 (10:1); for deaf children: Loots et al., 2005 (3:1), Peterson, Wellman, & Liu, 2005 (6:1); and for William’s syndrome: Hocking et al., 2013 (2:1), Järvinen et al., 2015 (3:1). Crucially, for the current study, we closely matched age, such that each hearing control was within ±7 days of the age of a Deaf infant.
2 |. METHODS
2.1 |. Participants
The participants were 72 infants in the age range of 7.73–20.09 months. For all infants, there were no reported cognitive or medical problems by the parents. The Deaf infants were recruited in five cities through parent–infant programs serving deaf/hard-of-hearing infants. The hearing infants were recruited as matched controls by contacting parent volunteers. The recruitment and experimental procedures were approved by the Institutional Review Boards of University of Washington and Georgia Institute of Technology, and all parents gave informed consent before the study.
2.1.1 |. Deaf
Each of the 12 Deaf infants (7 boys and 5 girls) had one or more Deaf parents. The parents reported that 11 of the 12 infants also had non-parental Deaf relatives (siblings or others in their extended families). All parents were fluent signers; nine infants had two Deaf parents using ASL; and three infants’ Deaf parent had a hearing spouse/partner fluent in ASL. No infant had a cochlear implant or wore a hearing aid in the test session. All the Deaf infants had been exposed to ASL from birth.
2.1.2 |. Hearing
The controls were 60 hearing infants who were age- and gender-matched at an individual level to the Deaf infants, such that there were five controls matched (±7 days) to each Deaf infant. All hearing infant controls (35 boys, 25 girls) had hearing parents. All hearing parents primarily spoke English and none used ASL (although some used five or less ‘baby signs’). Additional hearing infants were excluded because of extreme fussiness (n = 1), parent interference (n = 1), and procedural problems (n = 4).
2.2 |. Procedure
For the experimental test, infants sat on their parent’s lap across the table from an experimenter in an area surrounded by tall, plain curtains in a quiet room (at a laboratory or school). The experimenter sat at approximately the infant’s eye level. Two cameras recorded the experiment with one focused on the frontal view of the infant (face and upper body) and the other focused on the experimenter. Synchronized time codes (every 1/30th s, each video frame) were inserted on each recording for subsequent video scoring.
During the warm-up (and also between test trials), the Deaf or hearing experimenter used the primary language of the parent (ASL or English) as she played with the infant and toys. After warm-up (about 3 min) and prior to the onset of the test trials, the experimenter sequentially placed two identical targets on pedestals at the infant’s eye level. The two targets (plastic toys: 9-cm diameter × 16-cm tall) were silent and colorful, with one placed to the left and the other to the right side of the infant (with targets in the periphery 75° off-mid-line and 135 cm away from the infant). Immediately prior to each test trial, the experimenter briefly (about 1 s) made eye contact with the infant while displaying a neutral and slightly positive facial expression, which ensured that all infants started in the same location at midline looking at the adult’s face. The experimenter then silently turned her head and eyes in a natural way toward one of the two targets. The experimenter visually fixated on the target with a neutral, relaxed facial expression until the end of the trial. Each test trial lasted 7.5 s starting from the onset of the experimenter’s head movement. For each infant, four test trials were randomly assigned to a Left/Right order of LRLR, RLRL, LRRL, or RLLR (although due to experimenter error one infant was tested in each of the following orders: RRLL, LLRR).
2.3 |. Scoring
Infant looking behavior was scored from the video recording of the infant only. This allowed for the objective scoring of infant gaze behavior with the coder kept blind to which direction the adult was turning. All scoring was done by a coder who was kept uninformed about the hypotheses. The coder identified the onset and the offset of infant looks.
2.3.1 |. Gaze-following score
Each trial began with the infant looking at the adult’s face at midline. A target look was defined as occurring when the infant turned to look at one of the peripheral targets and the infant’s eyes aligned with that target for at least 10 video frames (0.33 s). For each trial, the first target look was scored as a correct look if the infant looked at the same target as the experimenter, or an incorrect look if the infant looked at the opposite target from the experimenter (as commonly scored in the gaze-following literature, e.g. Brooks & Meltzoff, 2002; Corkum & Moore, 1995). A summary score was calculated based on an approach used with infants of blind parents (Senju et al., 2013, 2015). Specifically, the ‘gaze-following score’ was a proportion, composed of the number of trials of correct looking minus the number of trials of incorrect looking, divided by the total number of trials with any target looking (zero assigned to infants without any target looks), with positive scores indicating more correct than incorrect looks and negative scores indicating more incorrect than correct looks.
2.3.2 |. Checking-back score
Because of observational studies reporting that Deaf children show enhanced looking back and forth between the person and object, we also scored such behavior. However, as pointed out in the peer-review process, this measure is not wholly independent from the gaze-following measure (because infants need to look at an object in order to look back from it); therefore, we present the results in the Supporting Information to make them available to clinicians and researchers working with Deaf infants, without claiming that they are independent from gaze following.
2.3.3 |. Initial facial-fixation score
By design, each infant had to look at the experimenter’s face before the test trial began (ensuring that all infants were equated for the start point at the midline). Once the trial started, infants could vary how long they continued to look at the experimenter’s face in an uninterrupted manner (even though the experimenter was now looking to the side at one of the targets). The ‘initial facial-fixation score’ was the mean duration of the first facial fixation across the four trials.
2.3.4 |. Scoring agreement
For 25% of the sample, the infant behaviors were scored by a second coder who was uninformed of the direction of the adult’s head turns. The interscorer agreement was excellent for gaze following (κ = 0.98), checking back (κ = 0.90), and initial facial fixation (κ = 0.90). The intrascorer agreement (also 25% of the sample) was also excellent (κ = 1.00, 0.95, 0.93, respectively).
3 |. RESULTS
3.1 |. Preliminary analyses
The effect of infant gender was not significant for the gaze following or initial facial-fixation scores (ps > .25). Trial order was also not significant (ps > .15). Therefore, the scores were collapsed across gender and order for analyses.
3.2 |. Main analyses
The difference between HoH and DoD infant groups was statistically evaluated using t-tests with the Satterthwaite method for unequal variances and bootstrapping to estimate 95% confidence intervals (CI) (Howell, 2013). The use of bootstrapping is increasingly common in psychological science, because it has few statistical assumptions and is appropriate with unequal group sizes (Mooney & Duval, 2011). The bootstrapping procedure took 10,000 random samples (Monte Carlo simulation) with replacement from the raw data to obtain the bias-corrected 95% CI of the mean group difference (i.e. to show whether it differs from 0).
3.2.1 |. Initial facial fixation
For the initial facial-fixation score, we found that Deaf infants looked at the experimenter’s face (M = 2.59 s, SD = 1.55) for a similar duration as hearing infants (M = 2.92 s, SD = 1.60), suggesting that both groups were attentive to the experimenter at the start of the test trials. The effect of group was not significant, t(16.1) = 0.66, p = .52, d = 0.21, Mdifference = −0.32, 95% CI [−1.14, 0.73].
3.2.2 |. Gaze following
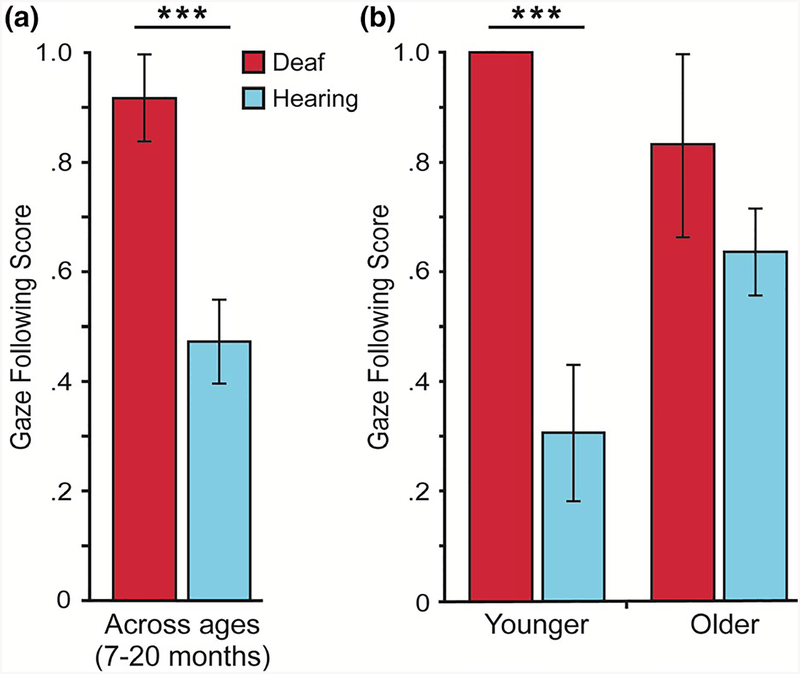
The dependent measure of gaze following was tested for group differences between the Deaf and the hearing infants. As shown in Figure 1a, Deaf infants (M = 0.92, SD = 0.29) had significantly higher gaze-following scores than hearing infants (M = 0.47, SD = 0.59). This effect of group was significant, t(32.9) = 3.93, p = .0004, d = 0.80, Mdifference = 0.44, 95% CI [0.20, 0.64]. Because infants’ ages ranged from 7 to 20 months, infant age was also tested as a covariate with group as the main effect in an analysis of covariance (ANCOVA, after determining there was no age by group interaction). The ANCOVA yielded a significant effect of group, F(1, 69) = 6.76, p = .011, partial η2 = 0.09, and age, F(1, 69) = 5.07, p = .028, partial η2 = 0.07. These results show that Deaf infants had higher gaze-following scores than hearing infants, even after controlling for infant age. The significant age effect suggests that gaze following increases as a function of age, as previously reported with hearing infants (Morales et al., 2000; Mundy et al., 2007). A scatter plot showing the gaze-following scores for each of the individual 72 infants is provided in Supporting Information (Figure S1).
FIGURE 1.
Gaze following: mean gaze-following scores for Deaf (dark red) and hearing (light blue) infants: (a) across ages; and (b) younger infants (7.7–14.1 months) and older infants (14.2–20.1 months) split at median age (of 14.12 months of age). Error bars show ± 1 SE. ***p < .0005
To provide a further illustration of age and group differences in gaze following, we subdivided the age range at the median of the sample (Mdn = 14.12 months) and explored group patterns for younger and older infants with the t-test approach (described above). This 14-month-old age is cited repeatedly in the literature as an average age for significant changes in infant gaze behaviors (e.g. Bornstein et al., 2008; Brooks & Meltzoff, 2002; Walden & Ogan, 1988). Two results emerged. First, gaze-following scores for ‘younger infants’ (7.7–14.1 months) were significantly higher for Deaf infants than hearing infants, t(29.0) = 5.57, p = .000005, d = 1.10, Mdifference = 0.69, 95% CI [0.47, 0.95] (Figure 1b). Second, gaze-following scores for ‘older infants’ (14.2–20.1 months) were numerically higher for Deaf infants than hearing infants, but were not significantly different, p = .32, Mdifference = 0.19, 95% CI [−0.21, 0.47]. Future researchers may want to examine these developmental issues and consider that the robust enhancement in gaze following for Deaf infants seems to occur at the earliest ages, perhaps at the ‘onset’ of gaze following.
For completeness, we also re-analyzed the data using a mixed-model approach based on the helpful suggestion of a reviewer. The results led to the same basic conclusions as already described, showing that the effect of group was significant. More specifically, the four test trials were analyzed with a linear mixed model using restricted maximum likelihood with a Kenward-Roger correction, because it is a powerful approach for repeated measures (trial-by-trial data) and smaller samples (Howell, 2013; McNeish, 2017). Using SAS Version 9.4 (proc mixed), the linear mixed model nested test trials within infant (four trials per infant, with each trial categorized as correct looking [+1], nonlooking [0], and incorrect looking [−1]) with an autoregressive covariance structure (to fit the correlations observed between adjacent trials). The model tested the fixed effect of group (Deaf vs. hearing) with infant age as a covariate. The model yielded significant effects for age: b = 0.05 (SE = 0.01), F(1, 101) = 14.47, p = .0002, and group: b = 0.28 (SE = 0.21), F(1, 97.4) = 3.97, p = .049, with higher scores for Deaf infants (M = 0.60, SD = 0.54) than hearing infants (M = 0.38, SD = 0.69). Thus, multiple strategies for analyzing infant gaze following revealed a significant effect of group, with Deaf infants having higher scores than hearing infants.
4 |. DISCUSSION
The current study is the first experimentally controlled test of DoD infant gaze following. We ensured that both the DoD infants and the HoH infants had exposure to language from birth–ASL for Deaf infants and spoken language for hearing infants. By design, the infants in the Deaf group were carefully matched in terms of age (±7 days) and gender to the hearing infants.
This experiment makes several novel contributions to the literature. We found that Deaf infants had significantly higher gaze-following scores than hearing infants. The gaze-following advantage was manifest in the full sample (7–20 months) and was significant for younger Deaf infants (7–14 months). Deaf infants were highly attuned to the adult looking behavior and readily turned toward the external targets. There may be many reasons why the hearing infants with lower scores chose not to follow the gaze of the experimenter (e.g. no feedback during the test trial) and why Deaf infants did gaze follow. A reasonable hypothesis is that the social-linguistic ecologies of the Deaf infants entrained them from an early age to attend to the adult’s gaze–inasmuch as gaze direction is a prominent visual signal that singles out interesting people, things, and events, especially in the absence of audition.
After infants initially looked at the target, we also observed an interesting pattern of infants disengaging from the target and checking back to look at the adult. Deaf infants especially at the older ages showed a pronounced tendency for this checking-back behavior (see Figure S2), which complements patterns reported in observational studies of DoD children (e.g. Lieberman et al., 2014). By following the adult’s gaze and then looking back to the adult’s face, infants glean useful information, which in everyday life consists of linguistic descriptions or emotional reactions by the adult (Baldwin & Moses, 1996). In hearing infants, such checking back is described as a developmentally advanced behavior (Desrochers, Morissette, & Ricard, 1995; Walden & Ogan, 1988). We hypothesize that being reared by fluent signers gives DoD infants extra experience with visual ‘comments’ by adults about the target objects. HoH infants can look to the target and simultaneously perceive a verbal label or emotional vocalization through audition. Deaf infants cannot pick up the adults’ reactions by ear and must use vision to seek out adults’ input. DoD infants would have daily practice in looking back and forth between the gazer and the target object (referent) for further communicative information, which is delivered through the visual modality. This is consistent with Spencer’s (2000) report that infants of Deaf parents spend more time looking at their parents than HoH infants, and also with Dye et al.’s (2008) suggestion that changes in visual attention in Deaf children can be framed as adaptive, attentional strengths. Multiple other studies likewise provide examples of rapid, effortless, and adaptive learning by infants based on interactions with other people (Meltzoff & Marshall, 2018).
The present findings differ from a historically common (although misleading) stereotype that deaf children have broad delays and deficits defined by their ‘deafness’. The current work with DoD infants aligns with other findings demonstrating that DoD children of fluent signers have notable strengths (e.g. Lederberg, Schick, & Spencer, 2013; Newport & Meier, 1985; Peterson et al., 2005; Petitto, 2005). Although DoH infants raised by non-fluent signers or non-signers are reported to show delays in language and social cognition (e.g. Cejas et al., 2014; Peterson & Siegal, 2000), DoD children exposed to fluent sign language from birth are reported in several studies to be fully on track for language and social cognition (including theory of mind), especially in studies that use appropriately matched controls (e.g. Hall, Eigsti, Bortfeld, & Lillo-Martin, 2018; Petitto et al., 2016; Schick, de Villiers, de Villiers, & Hoffmeister, 2007). Clearly, deaf individuals are not a homogenous group–and the use of natural sign language by Deaf parents and caregivers offers Deaf infants a visual learning ecology that supports social, cognitive, and linguistic development (Meadow-Orlans et al., 2004).
We began this inquiry with three broad possibilities: DoD infants could be the same, delayed, or advanced at gaze following compared to their HoH age- and gender-matched peers. Based on the current research, it appears that DoD infants of fluent signers are advanced. A key question now concerns the mechanisms of change that lead to these effects. We offer three interrelated hypotheses. These are not mutually exclusive alternatives, and the relative weight and contribution of each can only be discerned through further empirical work.
Hypothesis-1 holds that deafness itself could lead to increased emphasis on the visual modality. The absence of input in the auditory modality may lead infants to expand their ‘visual vigilance’. Hearing infants can learn to anticipate an approaching person based on audition, which brings order and predictability to the psychological world. Deaf infants may adapt to an absence of auditory input by expanding reliance on the visual modality. This could lead them to notice subtle, visual-social signals such as directional changes in the eyes or head, engendering increased gaze following.
Hypothesis-2 is that there is additional visual information provided to DoD infants during their everyday experiences. Deaf parents show infants a plethora of facial and manual acts in order to attract and maintain their infant’s attention and to foster communication. Deaf parents often rely on the visual modality (e.g. hand movements made within infant’s line of sight), whereas hearing parents are likely to use the auditory modality (Koester & Lahti-Harper, 2010). Thus, Hypothesis-2 proposes that it is not the ‘deafness’ (the lack of audition) per se, but rather the added visual input provided to DoD infants that leads them to become very attentive and attuned to the social bodily signals of others that are perceived through the visual modality. This added input could help infants pay attention to eye gaze, head orientation, or both to support the gaze following reported here. Stated more generally, the experiences of DoD infants in the visual modality could lead them to devote special attentional resources to others’ faces and bodily acts.
Hypothesis-3 is that the sign language from caregivers provides specific socializing and scaffolding behavior that may play a role over and above the Deaf infant’s absence of hearing (H-1) or their increased experience with attending to visual bodily signals (H-2). Hypothesis-3 holds that Deaf parents actively engage in specific communicative and linguistic behaviors that are highly adaptive in the Deaf culture and may scaffold gaze-following development (Corina & Singleton, 2009; Harris, 2000; Lieberman, Hatrak, & Mayberry, 2011; Meadow-Orlans et al., 2004; Spencer, 2000).
For example, Deaf parents often seek to optimize their infant’s perception of the parent’s face, a manual sign, and the referent object within the same visual field. Parents accomplish this in a variety of ways: (a) by actively moving the target object to their own face, (b) by placing their signing hands close to the object, (c) by re-positioning the infant so that the parent and object are both viewable, or (d) waiting to sign until the infant has connected gaze with them. Over time, Deaf parents gradually and purposely increase the distance between the referent object, the parent’s face, and the manual sign, thus entraining the child to gaze check back and forth. It is as if there is intentional socializing of gaze behaviors, which facilitates communication without audition. Deaf infants of fluent signers could be motivated to devote special attention to facial expressions and bodily acts because these are the sources of their linguistic information. Evidence for the influence of sign-language experiences (as opposed to deafness per se) on infant behavior is also suggested in a study of the real-time comprehension of sign-language stimuli by older infants (MacDonald et al., 2018). In that study, both Deaf and hearing ASL-exposed infants demonstrated similar eye gaze patterns, including rapid gaze-shifting ability.
4.1 |. Limitations, future directions, and broader theoretical implications
This study is not without limitations. One is that the sample size of DoD infants was modest (but this was expected because only 5% of Deaf infants have Deaf parents). That said, the sample size for DoD infants was comparable in size to other prominent studies of language processing and social cognition with DoD children (e.g. MacDonald et al., 2018; Peterson et al., 2005) and other low-incidence populations (e.g. Williams Syndrome: Hocking et al., 2013; Järvinen et al., 2015; blind children: Iverson, 1999; Landau et al., 1981). Future work could strive to include not only more DoD infants, but also to recruit other populations that could provide further theoretically driven tests.
It would be especially informative to test deaf infants of hearing parents (DoH) to assess whether deafness itself influences gaze following (Hypothesis-1), while also tracking differences in the age at which the infants are first exposed to a natural sign language (early exposure may lead to a different impact on gaze following than later exposure). It is also of interest to test hearing infants of Deaf parents (HoD) who are fluent signers (similar to MacDonald et al., 2018; Spencer, 2000). HoD are exposed to the early, rich visual language and social patterns of their signing parents while having access to auditory information. These types of comparisons will help assess the degree to which the three hypotheses (deafness per se, increased visual experience with bodily movements, or parental socialization and scaffolding provided during natural sign-language learning) contribute to the enhanced gaze following reported here.
The current work also has more general implications for developmental theory. The kinds of enhancements reported here may extend beyond gaze behavior to other aspects of social cognition. A domain worthy of study concerns the development of infants’ acquisition of emotion categories. Fourteen- to 18-month-old hearing infants readily distinguish happy from sad visual expressions (positive vs. negative emotions), but often confuse the fear and disgust categories, both high-arousal, negative emotions (e.g. Lindquist & Gendron, 2013; Ruba, Meltzoff, & Repacholi, 2019; Widen, 2013). An interesting experiment might be to test whether Deaf infants are accelerated in their understanding of the categories of visual emotional expressions, which could occur based on a heightened attention and analysis of visual-social signals. Based on a study of older DoD children’s acquisition of ASL, Reilly McIntire and Bellugi (1994) suggested that ‘affective facial expressions’ are acquired before ‘ASL facial expressions’ (used for grammatical purposes), and that early experience may help a Deaf child understand that examining the details of the facial expressions of others is important and relevant to language. Still, we do not yet know whether, and in what ways, the processing of facial expressions in DoD might be enhanced by the rich experiences of both affective and linguistic facial expressions of their caregivers. Knowing this would begin to assess the generality of the kinds of experience-based enhancement effects reported in this paper.
There are also societal implications. Professionals in the field of early intervention often mention deaf infants’ differences and delays, but the current study shows that deafness does not destine an individual to blanket deficits. To the contrary, DoD infants may be accelerated compared to HoH infants in passing certain developmental milestones involving gaze following and disengaging from the target object to check back to the adult communicator. This strongly suggests that early sign-language experience is not harming Deaf children, but rather is providing them with richly structured input that not only contributes to language development but also to gaze-following behavior. The social-cognitive flexibility of infants based on input from other people allows them to become well-adapted to their particular sociocultural and linguistic ecologies.
The enhanced processing of social-visual signals by DoD parent–infant dyads underscores that there are multiple routes to building interpersonal communication and social cognition. The current findings highlight the fundamental human capacity to learn socially and build communicative connections with our fellow human beings through a variety of perceptual modalities.
Supplementary Material
Research Highlights.
This study is the first experimentally controlled test of gaze following with Deaf infants.
Deaf infants of Deaf parents (DoD) were matched in age (±7 days) and gender to hearing infants of hearing parents (HoH).
DoD infants showed significantly enhanced gaze-following behavior compared to the controls, suggesting that they devote special attention to analyzing the visual-communicative bodily signals of others.
We hypothesize that enhanced gaze following derives from the sociocultural and linguistic experiences of DoD infants, revealing striking malleability in gaze following based on input.
Acknowledgments:
This work was supported by the National Science Foundation Science of Learning program, SBE-0835854 (to the LIFE Center at the University of Washington) and SBE-0541953 (to the VL2 Center at Gallaudet University); by the National Institute of Child Health & Human Development, U54HD083091 (to the Center for Human Development & Disabilities); by the Virginia Merrill Bloedel Hearing Research Center; and by the University of Washington I-LABS Innovative Research Fund. We thank the participants and the many cooperative organizations in the Deaf community
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors have no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author (Rechele Brooks, recheleb@uw.edu).
We adopt the standard convention of capitalizing the term Deaf to refer to individuals who identify with the Deaf Community as a linguistic and cultural identity. Because all of the parents of the Deaf infants tested in this study were part of the Deaf community, we followed this convention to describe the participants in this study. We also used parent report of their child’s deafness rather than tests of hearing loss. For shorthand, we sometimes refer to our participants as DoD infants, but it is noteworthy that all parents of the Deaf infants in this sample were fluent signers of ASL and had exposed their infants to ASL from birth.
In tests of gaze following, the adult behavior shown to infants is typically an adult turning the head and eyes to fixate on a location (e.g. Brooks & Meltzoff, 2002; Carpenter et al., 1998), but some researchers have dissected this act to eye direction alone (with head stationary, e.g. Butterworth & Jarrett, 1991) or head direction alone (without shifting eye gaze, e.g. Corkum & Moore, 1995). The current work uses the most standard case of congruent head and eye turn because it is the most common in experimental studies.
Neither Quittner nor Conway are specifically looking at deafness as it relates to gaze behavior, but we use their work as exemplars of researchers who have argued for possible delays or differences resulting from minimal access to audition. Quittner et al., (1994) argue that individuals without access to hearing have poor multimodal sensory integration that in turn affects visual attention. Conway et al. (2009) make a similar argument, privileging the role that audition plays in the development of the more general cognitive ability of sequential memory. However, these studies are based on older deaf children and deaf adults (DoH) with reduced early language experience. Importantly, a deficit argument cannot be made across all areas of visual attention, nor is it observed in all children with profound deafness (Dye et al., 2008; Tharpe et al., 2002).
REFERENCES
- Adamson LB, Bakeman R, Deckner DF, & Nelson PB (2012). Rating parent–child interactions: Joint engagement, communication dynamics, and shared topics in autism, Down syndrome, and typical development. Journal of Autism and Developmental Disorders, 42, 2622–2635. 10.1007/s10803-012-1520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DA, & Moses LJ (1996). The ontogeny of social information gathering. Child Development, 67, 1915–1939. 10.1111/j.1467-8624.1996.tb01835.x [DOI] [Google Scholar]
- Bavelier D, Brozinsky C, Tomann A, Mitchell T, Neville H, & Liu G (2001). Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. Journal of Neuroscience, 21, 8931–8942. 10.1523/JNEUROSCI.21-22-08931.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS, Hahn C-S, & Haynes OM (2008). Maternal responsiveness to young children at three ages: Longitudinal analysis of a multidimensional, modular, and specific parenting construct. Developmental Psychology, 44, 867–874. 10.1037/0012-1649.44.3.867 [DOI] [PubMed] [Google Scholar]
- Brooks R, & Meltzoff AN (2002). The importance of eyes: How infants interpret adult looking behavior. Developmental Psychology, 38, 958–966. 10.1037/0012-1649.38.6.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, & Meltzoff AN (2008). Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: A longitudinal, growth curve modeling study. Journal of Child Language, 35, 207–220. 10.1017/S030500090700829x [DOI] [PubMed] [Google Scholar]
- Brooks R, & Meltzoff AN (2015). Connecting the dots from infancy to childhood: A longitudinal study connecting gaze following, language, and explicit theory of mind. Journal of Experimental Child Psychology, 130, 67–78. 10.1016/j.jecp.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune CW, & Woodward AL (2007). Social cognition and social responsiveness in 10-month-old infants. Journal of Cognition and Development, 8, 133–158. 10.1080/15248370701202331 [DOI] [Google Scholar]
- Butterworth G, & Jarrett N (1991). What minds have in common is space: Spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology, 9, 55–72. 10.1111/j.2044-835x.1991.tb00862.x [DOI] [Google Scholar]
- Carpenter M, Nagell K, & Tomasello M (1998). Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development, 63 (Serial No. 255). 10.2307/1166214. [DOI] [PubMed] [Google Scholar]
- Cejas I, Barker DH, Quittner AL, & Niparko JK (2014). Development of joint engagement in young deaf and hearing children: Effects of chronological age and language skills. Journal of Speech, Language, and Hearing Research, 57, 1831–1841. 10.1044/2014_JSLHR-L-13-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy BT, Brooks R, Meltzoff AN, & Kuhl PK (2015). Social interaction in infants’ learning of second-language phonetics: An exploration of brain-behavior relations. Developmental Neuropsychology, 40, 216–229. 10.1080/87565641.2015.1014487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, & Kronenberger WG (2009). The importance of sound for cognitive sequencing abilities: The auditory scaffolding hypothesis. Current Directions in Psychological Science, 18, 275–279. 10.1111/j.1467-8721.2009.01651.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corina D, & Singleton J (2009). Developmental social cognitive neuroscience: Insights from deafness. Child Development, 80, 952–967. 10.1111/j.1467-8624.2009.01310.x [DOI] [PubMed] [Google Scholar]
- Corkum V, & Moore C (1995). Development of joint visual attention in infants In Moore C & Dunham PJ (Eds.), Joint attention: Its origins and role in development (pp. 61–83). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, & Brown E (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28, 479–485. 10.1023/A:1026043926488 [DOI] [PubMed] [Google Scholar]
- Desrochers S, Morissette P, & Ricard M (1995). Two perspectives on pointing in infancy In Moore C & Dunham PJ (Eds.), Joint attention: Its origins and role in development (pp. 85–101). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Dye MWG, Hauser PC, & Bavelier D (2008). Visual attention in deaf children and adults: Implications for learning environments In Marschark M & Hauser PC (Eds.), Deaf cognition: Foundations and outcomes (pp. 250–263). New York, NY: Oxford University Press; 10.1093/acprof:oso/9780195368673.003.0009 [DOI] [Google Scholar]
- Hall ML, Eigsti I-M, Bortfeld H, & Lillo-Martin D (2018). Auditory access, language access, and implicit sequence learning in deaf children. Developmental Science, 21, e12575 10.1111/desc.12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M (2000). Social interaction and early language development in deaf children. Deafness and Education International, 2, 1–11. 10.1179/146431500790561260 [DOI] [Google Scholar]
- Harris M, Clibbens J, Chasin J, & Tibbitts R (1989). The social context of early sign language development. First Language, 9, 81–97. 10.1177/014272378900902507 [DOI] [Google Scholar]
- Hocking DR, Thomas D, Menant JC, Porter MA, Smith S, Lord SR, & Cornish KM (2013). The interplay between executive control and motor functioning in Williams syndrome. Developmental Science, 16, 428–442. 10.1111/desc.12042 [DOI] [PubMed] [Google Scholar]
- Howell DC (2013). Statistical methods for psychology (8th edn.). Belmont, CA: Wadsworth, Centage Learning. [Google Scholar]
- Humphries T, Kushalnagar P, Mathur G, Napoli DJ, Padden C, Rathmann C, & Smith SR (2012). Language acquisition for deaf children: Reducing the harms of zero tolerance to the use of alternative approaches. Harm Reduction Journal, 9, 16 10.1186/1477-7517-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM (1999). How to get to the cafeteria: Gesture and speech in blind and sighted children’s spatial descriptions. Developmental Psychology, 35, 1132–1142. 10.1037/0012-1649.35.4.1132 [DOI] [PubMed] [Google Scholar]
- Järvinen A, Ng R, Crivelli D, Neumann D, Grichanik M, Arnold AJ, … Bellugi U (2015). Patterns of sensitivity to emotion in children with Williams syndrome and autism: Relations between autonomic nervous system reactivity and social functioning. Journal of Autism and Developmental Disorders, 45, 2594–2612. 10.1007/s10803-015-2429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester LS, & Lahti-Harper E (2010). Mother-infant hearing status and intuitive parenting behaviors during the first 18 months. American Annals of the Deaf, 155, 5–18. 10.1353/aad.0.0134 [DOI] [PubMed] [Google Scholar]
- Landau B, Gleitman H, & Spelke E (1981). Spatial knowledge and geometric representation in a child blind from birth. Science, 213, 1275–1278. 10.1126/science.7268438 [DOI] [PubMed] [Google Scholar]
- Lederberg AR, Schick B, & Spencer PE (2013). Language and literacy development of deaf and hard-of-hearing children: Successes and challenges. Developmental Psychology, 49, 15–30. 10.1037/a0029558 [DOI] [PubMed] [Google Scholar]
- Lieberman AM, Hatrak M, & Mayberry RI (2011). The development of eye gaze control for linguistic input in deaf children In Danis N, Mesh K, & Sung H (Eds.), Proceedings of the 35th annual Boston University conference on language development (Vol. 35, pp. 391–403). Somerville, MA: Cascadilla Press. [Google Scholar]
- Lieberman AM, Hatrak M, & Mayberry RI (2014). Learning to look for language: Development of joint attention in young deaf children. Language Learning and Development, 10, 19–35. 10.1080/15475441.2012.760381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, & Gendron M (2013). What’s in a word? Language constructs emotion perception. Emotion Review, 5, 66–71. 10.1177/1754073912451351 [DOI] [Google Scholar]
- Loots G, Devisé I, & Jacquet W (2005). The impact of visual communication on the intersubjective development of early parent-child interaction with 18- to 24-month-old deaf toddlers. Journal of Deaf Studies and Deaf Education, 10, 357–375. 10.1093/deafed/eni036 [DOI] [PubMed] [Google Scholar]
- MacDonald K, LaMarr T, Corina D, Marchman VA, & Fernald A (2018). Real-time lexical comprehension in young children learning American Sign Language. Developmental Science, 21, e12672 10.1111/desc.12672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry RI (2003). Cognitive development in deaf children: The interface of language and perception in neuropsychology In Segalowitz SJ and Rapin I (Eds.), Handbook of neuropsychology: Child neuropsychology (Vol. 8, Part II, 2nd ed., pp. 71–107). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Mayberry RI, & Eichen EB (1991). The long-lasting advantage of learning sign language in childhood: Another look at the critical period for language acquisition. Journal of Memory and Language, 30, 486–512. 10.1016/0749-596X(91)90018-F [DOI] [Google Scholar]
- McNeish D (2017). Small sample methods for multilevel modeling: A colloquial elucidation of REML and the Kenward-Roger correction. Multivariate Behavioral Research, 52, 661–670. 10.1080/00273171.2017.1344538 [DOI] [PubMed] [Google Scholar]
- Meadow-Orlans KP, Spencer PE, & Koester LS (Eds.). (2004). The world of deaf infants: A longitudinal study. New York, NY: Oxford University Press. [Google Scholar]
- Meltzoff AN, & Brooks R (2008). Self-experience as a mechanism for learning about others: A training study in social cognition. Developmental Psychology, 44, 1257–1265. 10.1037/a0012888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, & Marshall PJ (2018). Human infant imitation as a social survival circuit. Current Opinion in Behavioral Sciences, 24, 130–136. 10.1016/j.cobeha.2018.09.006 [DOI] [Google Scholar]
- Mitchell RE, & Karchmer MA (2004). Chasing the mythical ten percent: Parental hearing status of deaf and hard of hearing students in the United States. Sign Language Studies, 4, 138–163. 10.1353/sls.2004.0005 [DOI] [Google Scholar]
- Mooney CZ, & Duval RD (2011). Bootstrapping: A nonparametric approach to statistical inference. Newbury Park, CA: Sage; 10.4135/9781412983532 [DOI] [Google Scholar]
- Morales M, Mundy P, Delgado CEF, Yale M, Messinger D, Neal R, & Schwartz HK (2000). Responding to joint attention across the 6- through 24-month age period and early language acquisition. Journal of Applied Developmental Psychology, 21, 283–298. 10.1016/S0193-3973(99)00040-4 [DOI] [Google Scholar]
- Mundy P (2018). A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. European Journal of Neuroscience, 47, 497–514. 10.1111/ejn.13720 [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, & Parlade MV (2007). Individual differences and the development of joint attention in infancy. Child Development, 78, 938–954. 10.1111/j.1467-8624.2007.01042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport EL, & Meier RP (1985). The acquisition of American Sign Language In Slobin DI (Ed.), The crosslinguistic study of language acquisition: Vol. 1. The data (pp. 881–938). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang N-Y, Quittner AL, & Fink NE (2010). Spoken language development in children following cochlear implantation. Journal of the American Medical Association, 303, 1498–1506. 10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M, Arias D, & Dehaene-Lambertz G (2014). Gaze following is accelerated in healthy preterm infants. Psychological Science, 25, 1884–1892. 10.1177/0956797614544307 [DOI] [PubMed] [Google Scholar]
- Peterson CC, & Siegal M (2000). Insights into theory of mind from deafness and autism. Mind & Language, 15, 123–145. 10.1111/1468-0017.00126 [DOI] [Google Scholar]
- Peterson CC, Wellman HM, & Liu D (2005). Steps in theory-of-mind development for children with deafness or autism. Child Development, 76, 502–517. 10.1111/j.1467-8624.2005.00859.x [DOI] [PubMed] [Google Scholar]
- Petitto LA (2005). How the brain begets language In McGilvray J (Ed.), The Cambridge companion to Chomsky (pp. 84–101). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Petitto LA, Langdon C, Stone A, Andriola D, Kartheiser G, & Cochran C (2016). Visual sign phonology: Insights into human reading and language from a natural soundless phonology. Wiley Interdisciplinary Reviews: Cognitive Science, 7, 366–381. 10.1002/wcs.1404 [DOI] [PubMed] [Google Scholar]
- Proksch J, & Bavelier D (2002). Changes in the spatial distribution of visual attention after early deafness. Journal of Cognitive Neuroscience, 14, 687–701. 10.1162/08989290260138591 [DOI] [PubMed] [Google Scholar]
- Quittner AL, Smith LB, Osberger MJ, Mitchell TV, & Katz DB (1994). The impact of audition on the development of visual attention. Psychological Science, 5, 347–353. 10.1111/j.1467-9280.1994.tb00284.x [DOI] [Google Scholar]
- Redcay E, Kleiner M, & Saxe R (2012). Look at this: The neural correlates of initiating and responding to bids for joint attention. Frontiers in Human Neuroscience, 6, 169 10.3389/fnhum.2012.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JS, McIntire ML, & Bellugi U (1994). Faces: The relationship between language and affect In Volterra V & Erting CJ (Eds.), From gesture to language in hearing and deaf children (pp. 128–141). Washington, DC: Gallaudet University Press. [Google Scholar]
- Rinaldi P, Caselli MC, Di Renzo A, Gulli T, & Volterra V (2014). Sign vocabulary in deaf toddlers exposed to sign language since birth. Journal of Deaf Studies and Deaf Education, 19, 303–318. 10.1093/deafed/enu007 [DOI] [PubMed] [Google Scholar]
- Rowe ML, & Goldin-Meadow S (2009). Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science, 323, 951–953. 10.1126/science.1167025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruba AL, Meltzoff AN, & Repacholi BM (2019). How do you feel? Preverbal infants match negative emotions to events. Developmental Psychology, 55, 1138–1149. 10.1037/dev0000711 [DOI] [PubMed] [Google Scholar]
- Schick B, de Villiers P, de Villiers J, & Hoffmeister R (2007). Language and theory of mind: A study of deaf children. Child Development, 78, 376–396. 10.1111/j.1467-8624.2007.01004.x [DOI] [PubMed] [Google Scholar]
- Senju A, Tucker L, Pasco G, Hudry K, Elsabbagh M, Charman T, & Johnson MH (2013). The importance of the eyes: communication skills in infants of blind parents. Proceedings of the Royal Society B: Biological Sciences, 280, 20130436 10.1098/rspb.2013.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Vernetti A, Ganea N, Hudry K, Tucker L, Charman T, & Johnson MH (2015). Early social experience affects the development of eye gaze processing. Current Biology, 25, 3086–3091. 10.1016/j.cub.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PE (2000). Looking without listening: Is audition a prerequisite for normal development of visual attention during infancy? Journal of Deaf Studies and Deaf Education, 5, 291–302. 10.1093/deafed/5.4.291 [DOI] [PubMed] [Google Scholar]
- Tasker SL, Nowakowski ME, & Schmidt LA (2010). Joint attention and social competence in deaf children with cochlear implants. Journal of Developmental and Physical Disabilities, 22, 509–532. 10.1007/s10882-010-9189-x [DOI] [Google Scholar]
- Tharpe AM, Ashmead DH, & Rothpletz AM (2002). Visual attention in children with normal hearing, children with hearing aids, and children with cochlear implants. Journal of Speech, Language, and Hearing Research, 45, 403–413. 10.1044/1092-4388(2002/032) [DOI] [PubMed] [Google Scholar]
- Tomasello M, & Farrar MJ (1986). Joint attention and early language. Child Development, 57, 1454–1463. 10.2307/1130423 [DOI] [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, & Dawson G (2006). Early Predictors of Communication Development in Young Children with Autism Spectrum Disorder: Joint Attention, Imitation, and Toy Play. Journal of Autism and Developmental Disorders, 36, 993–1005. 10.1007/s10803-006-0137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden TA, & Ogan TA (1988). The development of social referencing. Child Development, 59, 1230–1240. 10.2307/1130486 [DOI] [PubMed] [Google Scholar]
- Widen SC (2013). Children’s interpretation of facial expressions: The long path from valence-based to specific discrete categories. Emotion Review, 5, 72–77. 10.1177/1754073912451492 [DOI] [Google Scholar]
- Xiao NG, Wu R, Quinn PC, Liu S, Tummeltshammer KS, Kirkham NZ, … Lee K (2018). Infants rely more on gaze cues from own-race than other-race adults for learning under uncertainty. Child Development, 89, e229–e244. 10.1111/cdev.12798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder P, Watson LR, & Lambert W (2015). Value-added predictors of expressive and receptive language growth in initially nonverbal preschoolers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45, 1254–1270. 10.1007/s10803-014-2286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.