Abstract
Incorporation of asparaginase (ASNase) and pegylated asparaginase (PEG-ASP) into pediatric-inspired regimens for adults with acute lymphoblastic leukemia (ALL) has led to improved treatment outcomes albeit with increased toxicities. This study compared the efficacy and safety of the Children’s Oncology Group standard PEG-ASP (SD) dosing (>1000, median 2500 IU/m2/dose) in adult Philadelphia chromosome-negative ALL patients receiving multiagent chemotherapy vs reduced dose PEG-ASP (RED) (≤1000, median 500 IU/m2/dose) during induction. 51 patients were included, 26 in RED and 25 in SD (median age 49 vs 37 years, p=0.027). Median day 7 ASNase activity level for RED was 0.16 IU/mL. All 11 patients who received PEG-ASP 1000 IU/m2 and 9/11 patients who received 500 IU/m2 achieved an ASNase level ≥0.1 IU/mL. Patients receiving RED experienced fewer total grade 3/4 toxicities during induction compared to SD (p=0.02) while still attaining therapeutic ASNase levels. RED permits safer ASNase use in adults with ALL and should be tested in a larger cohort prospectively.
Keywords: Acute Lymphoblastic Leukemia, Philadelphia Chromosome Negative, Asparaginase, AYA, Adolescent Young Adult
Introduction
Pediatric-inspired regimens in the treatment of ALL in the adult and young adolescent (AYA) population have consistently resulted in an overall survival (OS) advantage. In an analysis of 16 nonrandomized studies examining AYA patients treated according to either a pediatric or adult ALL regimen, 13 of them favored the use of pediatric regimens [1]. A meta-analysis of a subset of these comparisons also revealed a lower all-cause mortality rate at 3 years (RR 0.58; 95% CI 0.51–0.67) [2]. Several prospective trials investigating the use of pediatric-inspired regimens for adults with ALL have demonstrated promising outcomes as well, with overall survival (OS) ranging from 67–85% [3–5]. These findings have in turn led to an increase in the use of pediatric regimens to treat adults with ALL.
One of the key distinguishing factors of pediatric regimens is the intensive use of L-asparaginase (ASNase) in several of the intensive phases of these pediatric regimens. ASNase rapidly catalyzes and depletes extracellular L-asparagine, effectively starving the ALL cells which are unable to synthesize the essential amino acid due to the lack of the L-asparagine synthase enzyme; in turn, this results in inhibition of protein synthesis, cell cycle arrest, and subsequent apoptotic death in lymphoblasts [6]. ASNase has been used in pediatric regimens since the 1960s, with several studies showing a superiority of ASNase-containing regimens over non-ASNase regimens [7–10].
Native ASNase was first derived from Escherichia coli, which led to profound hypersensitivity and led to the development of alternative forms of ASNase that did not share cross-reactivity[11]. A pegylated (PEG) E. coli enzyme was created (PEG-asparaginase), which has since supplanted the E. coli derivative (no longer available commercially in the United States) as the standard of care for pediatric regimens. PEG-asparaginase (PEG-ASP) has the advantage of reduced immunogenicity, longer half-life (7 days vs. 34–49 hours), and flexibility in the route of administration (IV or IM) [10]. Available evidence suggests that PEG-ASP is at least as efficacious as native ASNase, with some arguing that the longer half-life of PEG-ASP results in improved asparagine depletion [12,13]. The shorter half-life of ASNase may be preferred if a toxicity does occur, as its clearance will be faster compared to PEG-ASP. A native ASNase derived from Erwinia chrysanthemi is also commercially available for patients who have developed hypersensitivity reactions to PEG-ASP.
The biggest barrier to incorporation of ASNase of any formulation has been toxicity. ASNase’s dose-limiting toxicities include hepatotoxicity, pancreatitis, hypertriglyceridemia, hypersensitivity reactions and thrombosis, which are more common in adolescents and young adults (AYAs) compared to younger patients [1]. Development of these toxicities can result in delays in post-remission induction therapy and lead to potential dose reductions of other antileukemic drugs with overlapping toxicity. This is particularly problematic in the AYA and older patient population where age alone is an independent risk factor for treatment delay, significantly impacting leukemia free survival and overall survival [14]. Moreover, adult ALL patients who received fewer PEG-ASP doses (<3) experienced a higher relapse risk compared to those who received 3 or more [15]. There does not appear to be a difference in grade 3–4 toxicity between PEG-ASP and native ASNase in adult ALL patients; however, incidence of toxicity was strongly associated with pre-treatment obesity and hypofibrinogenemia [16].
PEG-ASP doses used in adult and pediatric patients include 500, 1000, 2000, and 2500 IU/m2, with the 2500 IU/m2 dose being used most commonly in the pediatric population in the United States [10,17]. The Children’s Oncology Group (COG) AALL0232 study used a PEG-ASP dose of 2500 IU/m2, which served as the foundation for PEG-ASP dosing in the CALGB 10403 study in adults [5,18]. Pharmacodynamic studies in adults suggest that a dose of 2000 IU/m2 is sufficient to cause complete asparagine deamination within 2 hours and maintain 100% asparagine depletion up to 14 days after administration [19]. Testing of actual asparagine levels has been difficult and is not commercially available; therefore, target ASNase activity has been used as a surrogate to define therapeutic ASNase activity, with levels above a cutoff of ≥0.1 IU/mL resulting in complete asparagine depletion [20,21]. It remains unknown whether lower doses of PEG-ASP are capable of achieving adequate ASNase levels and asparagine depletion in adults with ALL.
In an effort to address this uncertainty, we present an investigation into the use of dose-reduced PEG-ASP as part of a modified CALGB 10403 regimen for adult ALL patients [22]. We compared a historical cohort of adult ALL patients receiving multi-agent chemotherapy with traditional PEG-ASP dosing (median 2500 IU/m2/dose, IQR 1900–2500 IU/m2/dose) to a group who received reduced dose PEG-ASP (median 500 IU/m2/dose, IQR 500–1000 IU/m2/dose) during induction; we hypothesized that the dose-reduced regimen during induction would be associated with less induction-related toxicity while still achieving acceptable goal asparaginase activity levels.
Methods
This study is a single-center IRB-approved retrospective study that evaluated consecutive patients age ≥ 18 years old with a diagnosis of Philadelphia chromosome (Ph) negative ALL who received at least one dose of PEG-ASP during induction of five drug pediatric-inspired induction regimen (CALGB 10403, )[22] between January 2008 and September 2018 (see box 1 for the remission induction regimen and supplemental figure for detailed dosing regimen). Dosing groups were defined based on the first dose of PEG-ASP during induction: the COG standard dose (SD) PEG-ASP (>1000 IU/m2) and reduced dose (RED) PEG-ASP (≤ 1000 IU/m2). Patients in the SD PEG-ASP group were treated from January 2008 to August 2014. Starting in August 2014, a programmatic change was made such that all non-study patients ≥ 18 years old with Ph-negative ALL received PEG-ASP 1000 IU/m2 during induction with the CALGB 10403 regimen. Patients may have received a further dose reduction of PEG-ASP to 500 IU/m2 or 250 IU/m2 if any of the following comorbidities were present at baseline: age > 50 years old, baseline liver dysfunction, diabetes, or obesity defined as a Body Mass Index (BMI) ≥ 30 kg/m2. The distinction between 250 and 500 IU/m2 was per prescriber discretion based on the constellation of comorbidities. All patients were pre-medicated with hydrocortisone, diphenhydramine, and acetaminophen per institutional policy. For patients whose ASNase activity was < 0.1 IU/mL seven days after administration of PEG-ASP and did not experience grade 3 or 4 toxicity, an additional dose of PEG-ASP was administered as soon as the low value resulted at a dose equivalent to the initial dose; in subsequent cycles they received double the original dose. For those patients who did experience grade 3 or 4 toxicity, ASNase activity was repeated at day 14. Patients continued to receive post-remission therapy according to CALGB 10403 with continued modification of PEG-ASP dosing according to their assigned dosing group.
Box 1: CALGB 10403 Remission Induction (Course I) Regimen As Used in This Study.
| Agent | Route | Dose | Schedule |
|---|---|---|---|
| Daunorubicin | IV | 25 mg/m2 | Days 1, 8, 15, 22 |
| Vincristine | IV | 1.5 mg/m2 (maximum dose 2 mg) | Days 1, 8, 15, 22 |
| Peg-Asparaginase* | IM/IV | 2500 IU/m2 (SD) 250–1000 IU/m2 (RED) | Day 4 |
| Dexamethasone | PO/IV | 10 mg/m2 | Days 1–7, 15–21 |
| Cytarabine | IT | 70 mg | Day 1 |
| Methotrexate | IT | 15 mg | Days 8, 29 |
Premedicated with hydrocortisone, diphenhydramine, and acetaminophen
Abbreviations: RED = Reduced-dose group; SD = Standard dose group.
The primary objective of this study was to determine the rate of achieving target ASNase activity (≥0.1 IU/mL) one week after administration of reduced PEG-ASP during induction therapy. Target ASNase activity has previously been used in several protocols to define therapeutic ASNase activity, with levels above a cutoff of ≥0.1 IU/mL resulting in complete asparagine depletion [20,21]. Secondary objectives included the comparison of incident grade 3/4 toxicities of hepatotoxicity, pancreatitis, hypertriglyceridemia, and venous thromboembolism between the two dosing groups. Grade 3/4 toxicity during induction (course I) was determined per CTCAE v4.0 criteria. Grade 3/4 elevation in alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) was defined as ALT>5x upper limit of normal and/or AST>5x ULN. Grade 3/4 total bilirubin elevation was defined as total bilirubin >3x ULN. Grade 3/4 amylase or lipase elevation was defined as lipase > 2x ULN and amylase > 2x ULN. A clinical diagnosis of pancreatitis was characterized by an elevated amylase or lipase accompanied by severe pain, emesis, or requiring medical intervention. Hypertriglyceridemia was defined as triglycerides > 500 mg/dL, and venous thromboembolism (VTE) was defined as either a DVT or PE by diagnostic testing. Progression free survival and overall survival were also compared between the two groups. Progression was defined according to the criteria from the Center for International Blood and Marrow Transplant Research (CIBMTR). Patients with minimal residual disease were not considered to have progressed.
Fisher’s exact test was used for comparisons of categorical variables, the 2-sided independent sample t-test was used for normally distributed continuous variables, and the Mann-Whitney U test was used for non-normally distributed continuous variables. Survival analyses was carried out using the log-rank test and Cox regression analysis to estimate hazard ratios along with 95% confidence intervals. Analyses were performed using Stata software, version 15.0 (StataCorp). The data cutoff date was September 1, 2018.
Results
Baseline Characteristics
Fifty-one consecutive patients with Ph-negative ALL were included, 26 in the RED PEG-ASP group and 25 in the SD PEG-ASP group. Median age in the SD PEG-ASP group was 36.6 years (range 18–64) compared to 49 years (range 19–76) in the RED PEG-ASP group (p=0.027). The SD PEG-ASP group had only one patient (4%) who was age 60 years or older, compared to 8/26 (30.8%) in the RED PEG-ASP group (p=0.024). There were no appreciable differences in gender, ethnicity, comorbidities, disease status, or ALL phenotype at the time of treatment (see table 1). The incidence of pre-treatment diabetes mellitus was 3/25 (12%) in the SD PEG-ASP group compared to 8/26 (31%) in the RED PEG-ASP group (p=0.17). A pre-treatment BMI ≥ 25 kg/m2 (cutoff for overweight/obesity) was present in 21 (81%) patients in the SD PEG-ASP group and in 19 (76%) patients in the RED PEG-ASP group (p=0.74). Pre-treatment liver disease (as indicated by prior diagnosis in electronic medical record or disease noted on imaging) was noted in 1 (4%) patient in the SD PEG-ASP group and in 5 (19%) in the RED PEG-ASP group (p=0.19). Seven (28%) patients in the SD PEG-ASP group and four (15%) patients in the RED PEG-ASP underwent allogeneic stem cell transplantation while in complete remission (p=0.32).
Table 1:
Baseline Characteristics
| COG Standard PEG-ASP (>1000 units/m2) | Reduced PEG-ASP (≤1000 units/m2) | p-value | |
|---|---|---|---|
| Patients, n | 25 | 26 | |
| Age, median (range) | 36.6 (18–64) | 49.0 (19–76) | 0.0275 |
| AYA (age 18–39), n (%) | 13 (52%) | 10 (38.5%) | 0.245 |
| Age ≥ 60, n(%) | 1 (4%) | 8 (30.8%) | 0.024 |
| Male gender, n (%) | 16 (64%) | 17 (65%) | 1 |
| Ethnicity, n (%) | 0.564 | ||
| Caucasian | 16 (64%) | 14 (53.9%) | |
| African American | 2 (8%) | 6 (23.1%) | |
| Hispanic | 4 (16%) | 3 (11.5%) | |
| Other | 3 (12%) | 3 (11.5%) | |
| Disease Status, n (%) | 0.703 | ||
| Newly diagnosed | 21 (84%) | 23 (88.5%) | |
| Relapsed/Refractory | 4 (16%) | 3 (11.5%) | |
| Phenotype, n (%) | 1 | ||
| B-cell | 18 (72%) | 17 (65.4%) | |
| T-cell | 5 (20%) | 6 (23.1%) | |
| Mixed | 2 (8%) | 3 (11.5%) | |
| Initial WBC count (x109/L), median | 7.5 | 6.8 | 0.899 |
| BMI (kg/m2) during induction, median | 28.7 | 28.7 | 0.914 |
| History of Liver Disease, n (%) | 1 (4%) | 5 (19.2%) | 0.191 |
| History of Pancreatitis, n (%) | 0 (0%) | 0 (0%) | 1 |
| History of Diabetes, n (%) | 3 (12%) | 8 (30.8%) | 0.173 |
| History of VTE, n (%) | 2 (8%) | 5 (19.2%) | 0.419 |
| Received allogeneic SCT in CR, n (%) | 7 (28%) | 4 (15.4%) | 0.324 |
| Induction PEG-ASP dose, units, median | 4450 | 1537 | <0.001 |
| Induction PEG-ASP dose, units/m2, median (IQR) | 2500 (1900–2500) | 500 (500–1000) | <0.001 |
| Day +7 ASNase activity level - IU/mL, median (range) | - | 0.16 (0.037–0.85) | - |
| Achieved Guideline Adherent Day +7 ASNase Induction Level, n (%) | - | 22 (84.6%) | - |
Abbreviations: ASNase = Asparaginase, AYA = Adolescent and young adult, BMI = body mass index, COG = Children’s Oncology Group, CR = complete remission, PEG-ASP = Pegylated asparaginase, SCT = stem cell transplant, VTE = venous thromboembolism, WBC = white blood cell
Achievement of Target Asparaginase Activity During Induction
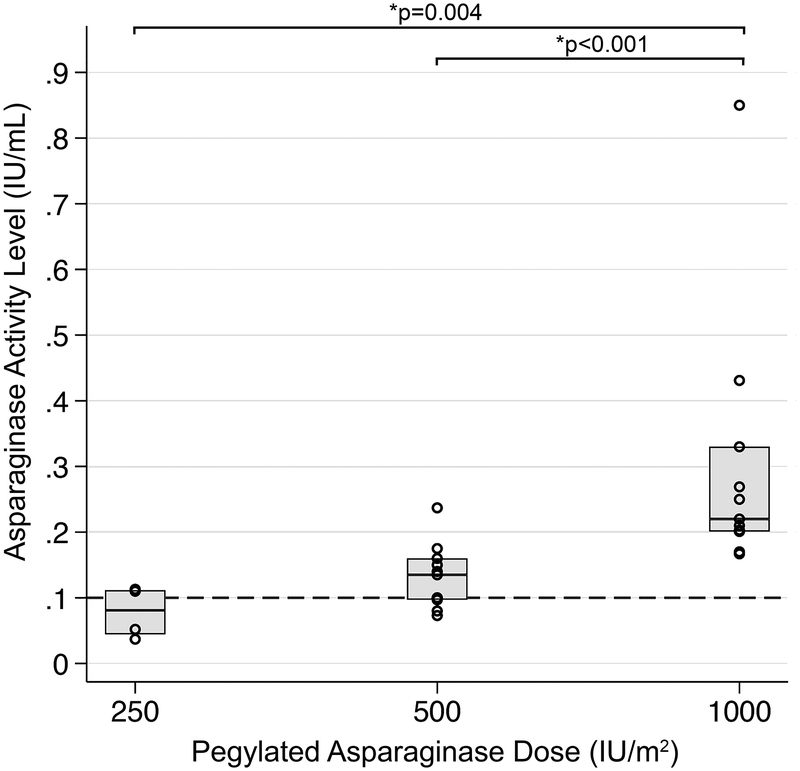
The median 7-day post-PEG-ASP ASNase activity level for patients in the RED PEG-ASP group was 0.16 IU/mL (range 0.037–0.85 IU/mL), with 22/26 (84.6%) having achieved an ASNase activity level ≥ 0.1 IU/mL (see figure 1). Median ASNase activity level was higher for patients receiving PEG-ASP 1000 IU/m2 compared to 500 IU/m2 (0.22 vs. 0.14 IU/mL, p=0.0006) or 250 IU/m2 (0.22 vs. 0.081 IU/mL, p=0.004). All 11 patients who received PEG-ASP 1000 IU/m2 reached a guideline adherent induction ASNase level ≥0.1 IU/mL, compared to 9/11 in those who received PEG-ASP 500 IU/m2 and 2/4 in those who received PEG-ASP 250 IU/m2.
Figure 1:
Day +7 asparaginase (ASNase) activity of reduced dose induction pegylated asparaginase (RED PEG-ASP). Dashed line corresponds to an ASNase activity level of 0.1 IU/mL, indicating achievement of ‘guideline adherent’ dosing of PEG-ASP. 2/4 patients in the 250 IU/m2, 9/11 in the 500 IU/m2, and 11/11 in the 1000 IU/m2 groups achieved an ASNase activity level ≥0.1 IU/mL. The 1000 IU/m2 dose group achieved significantly higher ASNase levels compared to either group.
Toxicity During Induction
Of the CTCAE toxicities that were evaluated and described in the methods section, at least one ≥ grade 3 toxicity during induction was reported per patient in 46% of the RED PEG-ASP cohort compared to 80% of the SD PEG-ASP cohort (p=0.02). For patients age >40 years old and for those with a BMI ≥ 25kg/m2, patients in the RED PEG-ASP group had fewer total grade 3 and 4 toxicities (p=0.014 and p=0.017, respectively). Details regarding individual grade 3 and 4 toxicities during induction are found in Table 2.
Table 2:
Grade 3/4 Toxicities During Induction by Treatment Arm
| COG Standard (>1000 units/m2) n=25 |
Reduced (≤1000 units/m2) n=26 |
p-value | |
|---|---|---|---|
| Any grade 3/4 toxicity, n (%) | 20 (80%) | 12 (46.2%) | 0.02 |
| AST or ALT elevation, n (%) | 8 (32%) | 11 (42.3%) | 0.565 |
| Total bilirubin elevation, n (%) | 11 (44%) | 6 (23.1%) | 0.144 |
| Amylase or lipase elevation, n (%) | 4 (16%) | 3 (11.5%) | 0.703 |
| Clinical pancreatitis, n (%) | 4 (16%) | 2 (7.7%) | 0.419 |
| Triglyceride elevation, n (%) | 2 (8%) | 4 (15.4%) | 0.668 |
| Fibrinogen < 100 mg/dL, n (%) | 15 (60%) | 10 (38.5%) | 0.165 |
| New venous thromboembolism, n (%) | 5 (20%) | 4 (15.4%) | 0.726 |
| Age | |||
| BMI | |||
COG = Children’s Oncology Group
Relapse Free Survival and Overall Survival
The median duration of follow-up was 16.3 months (range 1–55 months) in the RED PEG-ASP group and 53.8 months (range 1–125 months) in the SD PEG-ASP group (p<0.001). Disease relapse or death occurred in 21 patients (13 in the SD PEG-ASP group and 8 in the RED PEG-ASP group).
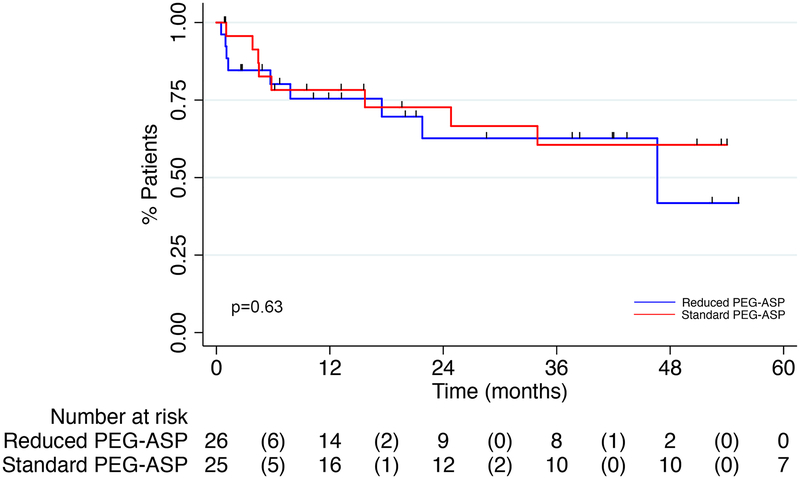
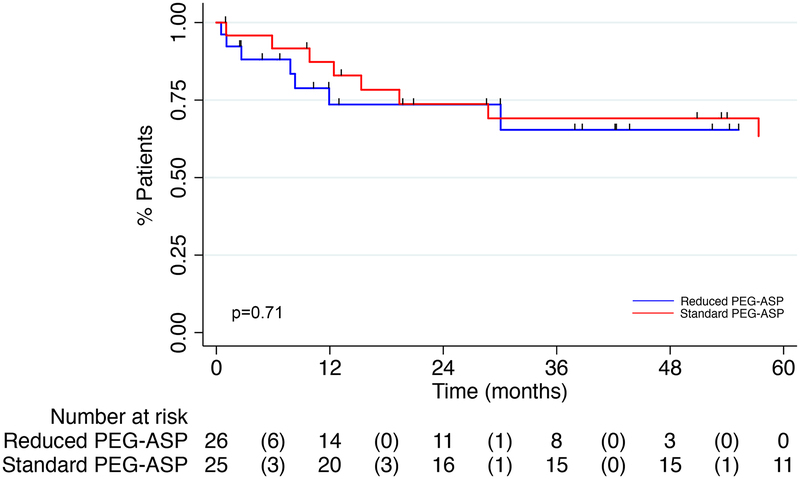
Median relapse free survival (RFS) was 46.6 months in the RED PEG-ASP group and not reached in the SD PEG-ASP group (HR=0.79, 95% CI 0.30–2.07, p=0.63) (figure 2). Median OS was not reached for the RED PEG-ASP group and 107 months for the SD PEG-ASP group (HR 0.82, 95% CI 0.29–2.34, p=0.71) (figure 3). Estimated 2-year RFS was 62.7% in the RED PEG-ASP group compared to 72.7% in the SD PEG-ASP group (p=0.45); estimated 2-year OS was 73.6% and 73.7%, respectively (p=0.99).
Figure 2:
Kaplan-Meier Curve for Relapse Free Survival
Figure 3:
Kaplan Meier Curve for Overall Survival
Discussion
ASNase is a critical component of the modern day treatment of pediatric ALL. The introduction of PEG-ASP results in more prolonged exposure to ASNase and has been demonstrated in a number of recent studies to further improve treatment outcomes. ASPase doses exceeding 2000 IU/m2 are common in pediatric ALL (according to dosing in COG AALL0232), but have been more challenging to deliver in older adolescents and in the older adult population due to an increased rate of treatment related toxicities, including thrombotic, hepatic, pancreatic and neurologic complications [1]. Given the importance of this agent in treatment of ALL, we have questioned whether administration of lower doses of PEG-ASP can effectively reduce treatment related toxicities and still achieve therapeutic drug levels in older adults with ALL and/or younger adults with co-morbid conditions, including obesity. Thus, we assessed whether reduced dosing of PEG-ASP (≤1000 IU/m2) as part of a pediatric inspired regimen for ALL was capable of achieving guideline adherent ASNase activity levels during treatment induction. Twenty two of twenty six patients in the RED PEG-ASP group achieved a post-PEG-ASP ASNase activity level > 0.1 IU/mL seven days after administration. Of this group, 100% of patients who received a PEG-ASP dose of 1000 IU/m2 and nearly 85% of patients who received 500 IU/m2 achieved guideline adherent plasma ASNase activity levels. Importantly, RED PEG-ASP resulted in significantly fewer patients experiencing incident grade 3 or 4 toxicities compared to SD PEG-ASP despite the RED PEG-ASP group representing an older population. Small patient numbers likely explain why individual toxicities between the two groups were not significantly different. While previous reports have concluded that the threshold level of plasma ASNase activity necessary to achieve asparagine depletion is between 0.1–0.4 IU/mL [19,21], a recent pediatric report suggests that the threshold level may be as low as 0.02 IU/mL [23]. All patients in this study, even at doses of 250 IU/m2, achieved ASNase activity levels >0.02 IU/mL at seven days after administration. Day 7 ASNase activity levels were obtained to allow an opportunity to intervene quickly if discovered to be low. Based on the seven-day half-life of PEG-ASP, one would expect the majority (if not all) of patients to have a ASNase level >0.02 IU/mL at day 14. This is also supported by data that suggests PEG-ASP activity lasts 22–29 days [23].
Silent inactivation of ASNase caused by neutralizing anti-drug antibodies without the development of overt allergy symptoms is a known phenomenon [20,24]. Given that 22 of 26 patients in the RED PEG-ASP group achieved an ASNase activity level ≥ 0.1 IU/mL, it is unlikely that silent inactivation played a key role. Of the 4 patients who did not achieve ASNase level ≥ 0.1 IU/mL, 2 did not achieve that ASNase level with a second induction dose; both of these patients were in the 250 IU/m2 group, which may indicate that this is not a reliably effective dose.
These data suggest that RED PEG-ASP to as low as 500 IU/m2 not only results in therapeutic asparaginase levels during induction therapy for ALL but may be better tolerated than SD PEG-ASP and might provide an opportunity to increase adherence to pediatric regimens where PEG-ASP is administered multiple times throughout the intensive portion of treatment [1,3,5,25,26]. As this was a retrospective study focused on the tolerance of induction PEG-ASP it was difficult to demonstrate a connection between PEG-ASP tolerance (i.e. reduced toxicity) and treatment adherence due to many factors including lack of data capture or first remission transplantation. There were significantly fewer toxicities in overweight/obese patients (BMI ≥ 25) with RED PEG-ASP; this is promising in light of the known association of pre-treatment obesity and ASNase-related hypofibrinogenemia, and that reduced tolerance of PEG-ASP may have been in part responsible for the high early failure rate in obese patients enrolled on the CALGB 10403 protocol [5,16]. A comparison of toxicities from the CALGB 10403 in adult and young adolescent (AYA) patients age 17–39 and the older adolescents enrolled in the pediatric AALL0232 revealed that rates of hepatotoxicity, pancreatitis, and thrombosis were higher in the CALGB 10403 patients compared to those in AALL0232 during induction but not during post-remission therapy[5,27]. This lends credence to the idea that mitigating early toxicity during the induction period can enhance tolerability and in turn efficacy. L-carnitine has recently been reported to have a therapeutic benefit in PEG-ASP related hepatotoxicity, which many also serve to reduce the extent of toxicities [28,29].
Other trials have used reduced dose PEG-ASP and/or omitted PEG-ASP during induction therapy, including the NOPHO ALL2008, GRAAL-2005, and DFCI 01–175 studies [3,4,30]. Interestingly, the pediatric inspired regimen used in the NOPHO ALL2008 was tolerable and effective for patients up to the age of 45 years. In this study, PEG-ASP administration was delayed until the second course of treatment for the standard and intermediate risk groups and the dose of PEG-ASP was lower (1000 IU/m2 at 2 week intervals) than the doses used in the Children’s Oncology Group AALL0232 study upon which CALGB 10403 was based [4]. While BMI was not evaluated as a prognostic factor in the NOPHO publication, one wonders whether lower rates of obesity and/or other differences in the study populations between northern Europe and the United States might account for some of these differences. In a recent publication, a polymorphism in the SOD2 gene was found to be more common in US patients of self-reported Hispanic descent and has been associated with higher rates of asparaginase related hepatotoxicity [31]. Similar to the CALGB 10403 study, the DFCI 01–175 employed an intensive pediatric ASNase dosing schedule in adults with ALL up to the age of 50 years; 37% of patients did not complete all doses of PEG-ASP due to toxicity. The GRAAL-2005 study used an age cutoff of 59 years, and was notable not only for lower compliance in patients over 55 years of age but also for lower CR, EFS, and OS [30]; in total, this led to the conclusion that intense asparaginase dosing may not be feasible for adults over the age of 45–55 years.
Our preliminary results suggest that the improved tolerance of lower doses of PEG-ASP may allow for increased schedule adherence, improved dose intensity of additional chemotherapies, and longer exposure to PEG-ASP in post-remission cycles, which may in turn lead to improved long-term outcomes. Baseline characteristics between the two groups were similar except most notably for age, with the RED PEG-ASP group being significantly older and having a larger proportion of patients ≥ 60 years old. It should be noted that the median follow-up duration was significantly shorter for the RED PEG-ASP group, owing to a programmatic shift in August 2014 to dose all patients ≥ 18 years old with reduced dose PEG-ASP. Given the small number of patients, the age and disease heterogeneity and short follow-up of patients who received the dose reduced PEG-ASP, it is impossible for us to comment here on the potential for improvements in survival. Nevertheless, there is no evidence thus far of a survival difference between the two groups. Dose reduction of PEG-ASP also comes with the potential added benefit of cost savings; with an average cost of approximately $4,350 per 1000 units of PEG-ASP [32], there is also a small financial incentive to dose reduction, particularly in areas of the world where cost creates prohibitive restrictions to the use of PEG-ASP. Furthermore, patients who develop significant toxicities from treatment may experience longer hospital lengths of stay or require the services of the intensive care unit, both of which may drive up the total cost of care.
In conclusion, our pilot study suggests that for adults age 40 years and older or any patient with comorbidities such as obesity, diabetes, liver disease, or venous thromboembolism, reducing the dose of PEG-ASP to 1000 IU/m2 can achieve therapeutic asparaginase levels with reduced toxicity than the standard doses employed in many pediatric regimens. While many exciting new agents including blinatumomab and inotuzumab ozogamicin are now being studied in frontline trials of both younger and older adults with ALL with initially promising results [33–35], it may still be worthwhile to pursue improvements in standard regimens given the exorbitantly high costs and uncertain response durability of these newer agents. A larger prospective study of dose-adjusted PEG-ASP in an intensive pediatric backbone such as CALGB 10403 will be required to determine whether this approach would result in improved adherence, safety and efficacy for adults with ALL.
Supplementary Material
Acknowledgements:
An earlier version of this study was published in abstract form at ASCO 2017. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.e18501
Footnotes
Declaration of Interest Statement:
Disclosures and Competing Interests:
BAD is supported by NIH Training Grant 2T32CA009566–31.
MS, JW, TNC, RWK: Report no conflict of interest
EC receives research funding from Merck, Incyte, and Gilead.
WS serves on the advisory boards of Amgen, Pfizer, Astellas, Jazz, Agios, Kite, Daiichi Sankyo, and receives honoraria from Research to Practice, UptoDate, along with a service honorarium from the American Society of Hematology.
Data Availability Statement:
The authors confirm that the data supporting the findings of this study are available within the article.
References
- [1].Siegel SE, Stock W, Johnson RH, et al. Pediatric-Inspired Treatment Regimens for Adolescents and Young Adults With Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Review. JAMA Oncol. 2018;4:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ram R, Wolach O, Vidal L, et al. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am. J. Hematol 2012;87:472–478. [DOI] [PubMed] [Google Scholar]
- [3].DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–615. [DOI] [PubMed] [Google Scholar]
- [5].Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;blood-2018. –10–881961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. HO 2009;7:600–606. [PubMed] [Google Scholar]
- [7].Sallan SE, Gelber RD, Kimball V, et al. More is better! Update of Dana-Farber Cancer Institute/Children’s Hospital childhood acute lymphoblastic leukemia trials. Haematol. Blood Transfus 1990;33:459–466. [DOI] [PubMed] [Google Scholar]
- [8].Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. [DOI] [PubMed] [Google Scholar]
- [9].Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol 2010;32:554–563. [DOI] [PubMed] [Google Scholar]
- [10].Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk. Lymphoma 2011;52:2237–2253. [DOI] [PubMed] [Google Scholar]
- [11].Asselin BL. L-asparaginase for treatment of childhood acute lymphoblastic leukemia: What have we learned? (Commentary on Schrey et al., page 378). Pediatr. Blood Cancer 2011;57:357–358. [DOI] [PubMed] [Google Scholar]
- [12].Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia colil-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. [DOI] [PubMed] [Google Scholar]
- [13].Wetzler M, Sanford BL, Kurtzberg J, et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood. 2007;109:4164–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kumar AJ, Gimotty PA, Gelfand JM, et al. Delays in postremission chemotherapy for Philadelphia chromosome negative acute lymphoblastic leukemia are associated with inferior outcomes in patients who undergo allogeneic transplant: An analysis from ECOG 2993/MRC UK ALLXII. Am. J. Hematol 2016;91:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aldoss I, Pullarkat V, Martinez D, et al. The Number Of Peg-Asparaginase Doses Administered Is a Determinant Of Relapse Risk In Adult ALL Treated With a Pediatric-Like Regimen. Blood. 2013;122:3915–3915. [Google Scholar]
- [16].Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract 2018;24:299–308. [DOI] [PubMed] [Google Scholar]
- [17].Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2010;115:1351–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Larsen EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J. Clin. Oncol 2016;34:2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007;109:2744–2750. [DOI] [PubMed] [Google Scholar]
- [20].van der Sluis IM, Vrooman LM, Pieters R, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin. Pharmacokinet 2005;44:367–393. [DOI] [PubMed] [Google Scholar]
- [22].Stock W, Luger SM, Advani AS, et al. Favorable Outcomes for Older Adolescents and Young Adults (AYA) with Acute Lymphoblastic Leukemia (ALL): Early Results of U.S. Intergroup Trial C10403. Blood. 2014;124:796–796. [Google Scholar]
- [23].Schore RJ, Devidas M, Bleyer A, et al. Plasma asparaginase activity and asparagine depletion in acute lymphoblastic leukemia patients treated with pegaspargase on Children’s Oncology Group AALL07P4. Leuk. Lymphoma 2019;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salzer W, Bostrom B, Messinger Y, et al. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk. Lymphoma 2018;59:1797–1806. [DOI] [PubMed] [Google Scholar]
- [25].Ribera J-M, Oriol A, Sanz M-A, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 2008;26:1843–1849. [DOI] [PubMed] [Google Scholar]
- [26].Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 2009;27:911–918. [DOI] [PubMed] [Google Scholar]
- [27].Advani AS, Sanford B, Luger S, et al. Frontline-Treatment Of Acute Lymphoblastic Leukemia (ALL) In Older Adolescents and Young Adults (AYA) Using a Pediatric Regimen Is Feasible: Toxicity Results of the Prospective US Intergroup Trial C10403 (Alliance). Blood. 2013;122:3903–3903. [Google Scholar]
- [28].Alshiekh-Nasany R, Douer D. L-Carnitine for Treatment of Pegasparaginase-Induced Hepatotoxicity. Acta Haematol. 2016;135:208–210. [DOI] [PubMed] [Google Scholar]
- [29].Wieduwilt MJ, Goodman A, Jonas BA, et al. L-carnitine for pegylated-l-asparaginase induced hepatotoxicity. J. Clin. Oncol 2017;35:e21626–e21626. [Google Scholar]
- [30].Huguet F, Chevret S, Leguay T, et al. Intensified Therapy of Acute Lymphoblastic Leukemia in Adults: Report of the Randomized GRAALL-2005 Clinical Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 2018;36:2514–2523. [DOI] [PubMed] [Google Scholar]
- [31].Alachkar H, Fulton N, Sanford B, et al. Expression and polymorphism (rs4880) of mitochondrial superoxide dismutase (SOD2) and asparaginase induced hepatotoxicity in adult patients with acute lymphoblastic leukemia. Pharmacogenomics J. 2017;17:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oncaspar Prices, Coupons & Patient Assistance Programs [Internet]. Drugs.com. [cited 2019 Jan 18]. Available from: https://www.drugs.com/price-guide/oncaspar.
- [33].Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Short NJ, Jabbour EJ, Ravandi F, et al. Chemoimmunotherapy with Inotuzumab Ozogamicin Combined with Mini-Hyper-CVD, with or without Blinatumomab, for Newly Diagnosed Older Patients with Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: Results from a Phase II Study. Blood. 2018;132:36–36. [Google Scholar]
- [35].Richard-Carpentier G, Kantarjian HM, Short NJ, et al. A Phase II Study of the Hyper-CVAD Regimen in Sequential Combination with Blinatumomab As Frontline Therapy for Adults with B-Cell Acute Lymphoblastic Leukemia (B-ALL). Blood. 2018;132:32–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.