ABSTRACT
Purpose
This trial aimed to demonstrate the feasibility of high-intensity interval training (HIIT) in postmenopausal, overweight/obese women at high risk of invasive breast cancer and to explore HIIT on changes in cardiorespiratory fitness (CRF), body weight, and body mass index (BMI) compared with moderate-intensity continuous training (MICT) and usual care (UC).
Methods
Forty-four women were randomized to HIIT, MICT, or UC for a 12-wk, thrice weekly, supervised exercise intervention. HIIT included a 5-min warm-up at 50%–70% HRpeak, four cycles of 4 min at 90%–100% HRpeak, followed by 3 min at 50%–70% HRpeak. MICT consisted of 41 min at 60%–70% HRpeak. Feasibility was assessed by consent, adherence, compliance, and retention rates. CRF, body weight, and BMI were measured at baseline and end of study. Repeated-measures linear mixed models were used to assess within- and between-group differences.
Results
Average age was 63.9 ± 8.8 yr. BMI was 30.9 ± 5.7 kg·m−2. Participants completed 90% and 89% of HIIT and MICT workouts, respectively, with 100% compliance to the exercise prescriptions. No serious adverse events were reported. Compared with MICT and UC, HIIT exhibited improvements in change in treadmill time (101 s greater than MICT, and 125 s greater than UC, respectively, P < 0.001). Compared with UC, HIIT exhibited improvement in changes in absolute and relative V˙O2peak (a 0.15-L·min−1 increase, P = 0.005, and a 2.3-mL·kg−1⋅min−1 increase, P = 0.004). There were no significant differences between groups for body weight or BMI (P > 0.05).
Conclusions
HIIT is feasible, safe, and seems to promote greater improvements in CRF compared with MICT and UC in women at high risk for breast cancer.
Key Words: EXERCISE DOSE, PRESCRIPTION, DISEASE RISK, CANCER
Obesity is a key public health concern given its high prevalence in the United States and its link to multiple diseases, including postmenopausal breast cancer (1). Postmenopausal women are a particularly vulnerable population, given that they can experience significant changes in weight and body composition with increasing age, leading to overweight/obesity and unfavorable alterations in the release of adipokines, proinflammatory cytokines, and growth factors, all of which are implicated in breast carcinogenesis (2–4). Previous epidemiological studies have observed that regular exercise and subsequent improvements in cardiorespiratory fitness (CRF) are associated with a reduction in incident breast cancer and breast cancer mortality independent of weight status (i.e., normal weight, overweight, or obese) (5–8). For example, in a study conducted by Fournier and colleagues (6), ≥12 MET·h·wk−1 of recreational physical activity versus <12 MET·h·wk−1 was linked with a 10% reduction in invasive breast cancer risk among postmenopausal women (HR = 0.90, 95% confidence interval [CI] = 0.82–0.99) adjusting for body mass index (BMI). Furthermore, Peel and colleagues (8) observed a 55% reduction in breast cancer mortality among women in the highest category for CRF compared with the lowest CRF category (HR = 0.45, 95% CI = 0.22–0.95) after BMI adjustment. However, the optimal exercise “dose” (e.g., exercise frequency, intensity, and duration) to be recommended within an exercise prescription to optimize CRF for overweight/obese postmenopausal women at heightened risk of developing breast cancer is untested in the clinical setting.
Exercise intensity (e.g., low, moderate, and high) is a core component of an exercise prescription and is a key factor in the overall “dose” of exercise achieved. High-intensity interval training (HIIT) involves bursts of high-intensity exercise (≥80% HRmax) separated by periods of rest or active recovery; this is in contrast to the traditionally prescribed moderate-intensity continuous training (MICT), performed at constant intensity (60%–75% HRmax) (9). HIIT, when matched or similar to MICT in total energy expenditure, has demonstrated greater improvements in CRF compared with MICT (10–13).
As a first step in assessing HIIT in the breast cancer prevention setting, we conducted a 12-wk supervised exercise trial comparing HIIT to MICT and usual care (UC) among overweight/obese postmenopausal women considered at heightened risk of developing invasive breast cancer. We defined heightened risk as postmenopausal women with overweight or obesity who had a diagnosis of ductal carcinoma in situ (DCIS), a benign high-risk breast disease, or an elevated 5-yr Gail or lifetime risk score. Because HIIT remains untested in this clinical population, our primary aim was to assess the feasibility of HIIT within this patient population. Our secondary aim was to explore the effect of HIIT compared with MICT and UC on changes in CRF (including associated CRF measures such as peak METs and total exercise time) and body weight, two important risk factors associated with invasive breast cancer risk. We hypothesized that HIIT would be feasible in women at heightened risk for breast cancer.
METHODS
Study Design
This 12-wk supervised exercise trial was a randomized controlled trial. Participants were randomized in a 1:1:1 ratio to HIIT, MICT, or UC. If a participant was assigned to the HIIT arm but experienced a hypertensive responsive during the cardiopulmonary exercise testing (CPET), reassignment to the MICT arm was permitted (n = 1). This investigation was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board and registered in clinicaltrials.gov (NCT02923401).
Participant Recruitment
Participants were recruited from the Cancer Prevention Center at The University of Texas MD Anderson Cancer Center. Inclusion criteria consisted of the following: postmenopausal, as defined by provider discretion and notated in the medical record; at high risk of developing breast cancer, defined as a history of DCIS or benign high-risk breast disease (e.g., lobular carcinoma in situ, ductal or lobular atypia); Gail 5-yr risk score >1.66% (14,15) or lifetime risk score of >20%; BMI ≥ 25 kg·m−2; oriented to person, place, and time; and speaks and reads English. Exclusion criteria consisted of the following: pre- or perimenopausal status, underlying medical problems that contraindicate unsupervised exercise (16); previous history of cardiovascular disease; use of a walker, wheelchair, or scooter; currently being treated for diabetes or autoimmune disease; lives outside the greater Houston area; currently pregnant as determined by self-report; currently taking risk reduction therapy such as tamoxifen; and blood pressure of ≥140/90 at the time of baseline testing.
Approximately 1 to 2 wk in advance, study personnel screened clinic schedules to identify potentially eligible patients. All eligible patients received an e-mail in advance of their scheduled clinic visit containing the study brochure and contact information of the study team for interested patients. Patients who contacted the study team in advance of their scheduled clinic visit received details regarding the study over the phone. If they were still interested in participating, they were approached in clinic for the informed consent interview. If the study team did not hear from the patients who were e-mailed the study brochure before their scheduled clinic visit, a member of the team approached these patients during their upcoming scheduled clinic visit to follow up on the e-mailed study brochure and inquire about interest in participating in the trial.
Flow of Participants
Participants were recruited between November 2016 and January 2018. Figure 1 presents the CONSORT diagram of the trial. A total of 1066 patients met the inclusion criteria, 690 were no longer eligible at the time of clinic visit (e.g., initiated risk reduction therapy, BMI < 25 kg·m−2, etc.), 87 canceled their clinic appointment, and 47 did not present to their scheduled appointment. Therefore, 242 patients were approached by study personnel. Of these 242 patients, 190 patients (78%) declined participation and 52 consented. Of the 52 patients who consented, two were screen failures and six withdrew consent before initiating baseline testing procedures because of personal reasons. A total of 44 patients were randomized to HIIT, MICT, or the UC group. One patient was reassigned from HIIT to MICT before starting the exercise intervention because of a hypertensive response to exercise during the CPET. The most common reason for discontinuing participation after randomization was personal reasons. In all, a total of 33 participants completed the 12-wk trial.
FIGURE 1.
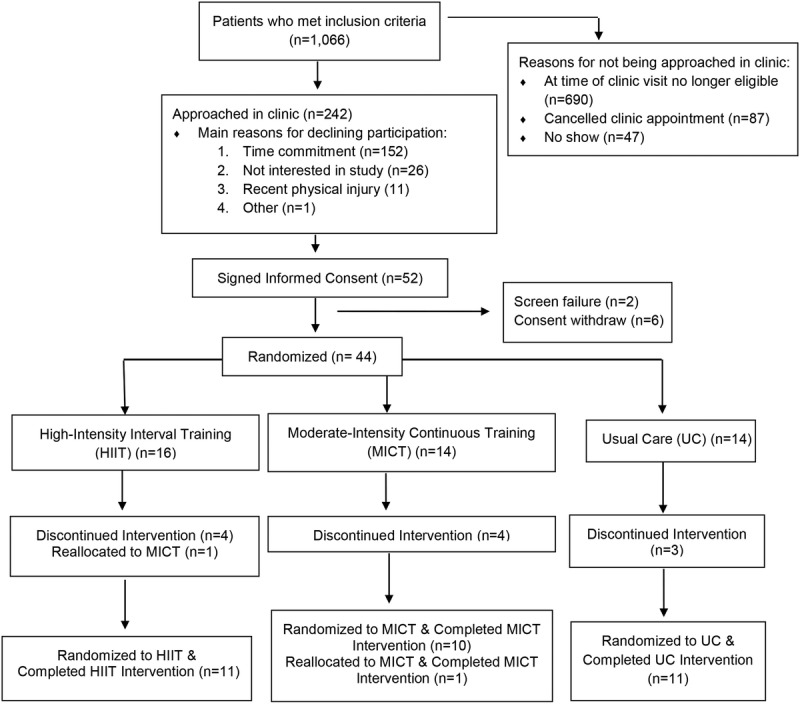
CONSORT diagram.
Assessment Sessions—Procedures and Protocols
Figure 2 consists of a study diagram of assessment and intervention procedures. All participants presented at baseline, 6 wk, and 12 wk (end of study) for assessment sessions. Resting HR and blood pressure were measured at all assessment sessions. Demographic information and heart health history were completed at baseline. At baseline and 12 wk, participants completed a fasted blood draw, anthropometric measurements, CPET, whole-body dual-energy x-ray absorptiometry scan, and quality-of-life questionnaires. A midpoint CPET was completed at 6 wk. At 12 wk, participants in the exercise groups completed an exit interview related to the exercise protocol they followed and plans to continue exercise poststudy.
FIGURE 2.
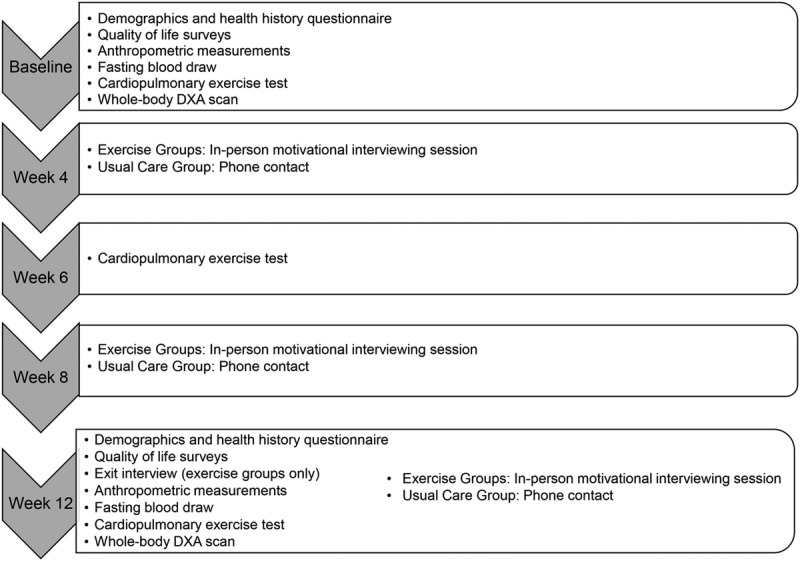
Flow diagram of assessment and intervention procedures.
Anthropometric measurements
Height was measured at baseline, and body weight was measured at baseline and 12 wk following standard procedures (16). These measurements were conducted on scales available in the Cancer Prevention Center. Body mass index (BMI) was calculated from these measurements (weight [kg]/height squared [m2]).
Baseline activity assessment
Participants completed the Godin Leisure Time Activity questionnaire to determine baseline activity levels. The Godin questionnaire has a reliability coefficient of 0.81, along with significant correlations with CRF (17).
CPET
To measure CRF (V˙O2peak), all participants performed a maximal CPET following the modified Balke protocol using standard procedures (16). Peak MET and total exercise time were also collected within this test. All tests were conducted on a treadmill and included a 2-min cooldown. Expired ventilation and respiratory gases were monitored continuously using a calibrated ParvoMedics TrueMax 2400 Metabolic Measurement System (Parvomedics Inc., Sandy, UT). A 12-lead electrocardiogram was used to continuously monitor HR and rhythm (Quinton Cardiology Systems, Bothell, WA). HR, blood pressure, and RPE as assessed by the Borg revised category–ratio scale (0–10 scale) were recorded at rest, at the end of the warm-up, at the end of every stage during the exercise test, at peak aerobic capacity, and every 2 min during the cooldown.
Intervention—Procedures and Protocols
Exercise groups
HR training was used for the exercise protocols. HR measured at peak aerobic capacity (HRpeak) was used to calibrate HR training zones from baseline to 6 wk. After the CPET at week 6, the training zones were recalibrated based on the newly measured HRpeak. Participants randomized to one of the exercise groups were instructed to present to the Cancer Prevention Center three times per week for 12 wk to complete their workouts. Participants were not required to complete their workouts at the same time of day on the same days every week and were able to schedule their workouts as their schedules allowed on a weekly basis with study personnel. All workouts were completed on a treadmill, and both exercise protocols were matched in estimated total energy expenditure. Participants in the exercise groups also completed monthly in-person motivational interviewing sessions with study personnel and received text messages related to the intervention three times per week.
HIIT and MICT
Participants randomized to HIIT completed a 5-min warm-up at 50%–70% HRpeak, followed by four 4-min intervals at 90%–100% HRpeak. Each 4-min high-intensity interval was followed by 3 min of active recovery at 50%–70% HRpeak. The final 3-min active recovery interval served as the cooldown. The total exercise time of the HIIT protocol was 33 min. We measured HR and RPE at the end of the warm-up and each high-intensity and active recovery interval. The MICT protocol consisted of a continuous 41-min workout at 60%–70% HRpeak. We measured HR and RPE in 5-min increments throughout the duration of the workout.
Monthly motivational interviewing sessions
Individuals randomized to the exercise groups participated in monthly one-on-one, in-person motivational interviewing sessions with trained study personnel. All sessions were approximately 15–30 min in length. These sessions were held before or after the participant’s supervised exercise session. Within these sessions, strategies for overcoming barriers to attending exercise sessions were discussed along with strategies to continue physical activity upon completion of the program.
Text messaging
Participants randomized to the exercise groups received a total of 36 text messages throughout the study, three messages per week. The content of the text messages was based on the self-determination theory (18) and was used to both help motivate participants to complete their workouts and remind participants to come in for their scheduled workouts.
UC Group
Participants randomized to the UC group received written material related to healthy eating and engaging in regular physical activity. These materials were the same materials administered by providers and health coaches within the Cancer Prevention Center. Participants also received monthly phone calls, approximately 15 min in length, by study personnel to discuss progress regarding their personal goals toward healthy lifestyle change.
Feasibility Assessment
Feasibility was determined based on the consent rate, adherence, compliance, and retention to the exercise intervention. Consent rate was defined as the number of individuals who met the inclusion criteria and consented after being approached by study personnel in clinic about the study. Adherence was defined as attendance to 80% or more of the planned number of supervised exercise sessions (29 or more of the total 36 sessions), and compliance was the duration (time) of each session participated and maintaining HR within the target HR zones. Retention was defined as the proportion of participants who participated until study completion.
Sample Size and Power Calculation
We aimed to recruit a total of 36 (n = 12 per arm) evaluable participants randomized in a 1:1:1 ratio to HIIT, MICT, or UC. Assuming a 45% eligibility rate and a 50% consent rate, we needed to screen 320 patients to reach 144 patients who met the inclusion criteria, with half of them (72 patients) consenting to participate in the study and 36 of the 72 participants completing all study assessments (baseline to 12 wk). The half width values of a 90% CI for the eligibility and consent rates were 0.046 and 0.069, respectively. The half width values of a 90% CI for the adherence and retention rates (assuming 80% underlying adherence and 50% retention rates) were 0.078 and 0.097, respectively, if 72 participants were recruited and 36 evaluable participants were obtained. The 50% retention rate estimate was based on previous retention rates obtained for diet and/or exercise interventions (19–21).
The secondary objective of the study was to estimate the effects of HIIT on CRF, as compared with those of the MICT. This objective of between-group comparisons (of changes from baseline to 12-wk follow-up) is acknowledged as underpowered, such that a sample size of 12 participants per group at 12 wk had 80% power to detect a large effect size of 1.34 between HIIT and MICT at a two-sided 0.025 significance level.
Data Analysis
Data were analyzed based on the intention-to-treat principle. Summary statistics were provided for participants’ baseline demographic and clinical characteristics, as well as for the outcome variables overall and in each intervention group. We performed repeated-measures analyses using linear mixed models to examine the between- and within-group differences, as well as the group–time interaction effects, for the six secondary outcomes. All models included study group (HIIT, MICT, and UC; reference group: HIIT), time (12-wk T2 vs baseline T1; reference: T1), and their interaction (group–time). Our primary interest in the analysis of the secondary outcomes lies in the test of the group–time interaction effects, which represent the group differences of the change in each outcome from T1 to T2 (as our power analysis for the secondary outcomes is based). Thus, a significant interaction effect would indicate a significant intervention effect. We also tested for the overall between- and within-group differences, as well as specific differences between HIIT and UC, and between HIIT and MICT groups. Findings from these analyses should be interpreted as hypothesis generating that require confirmatory evaluation in a future larger trial. All the results were based on random-intercept models with parameters estimated using the restricted maximum likelihood method. Procedure MIXED in Stata 15.1 was used to obtain the results.
RESULTS
Baseline characteristics
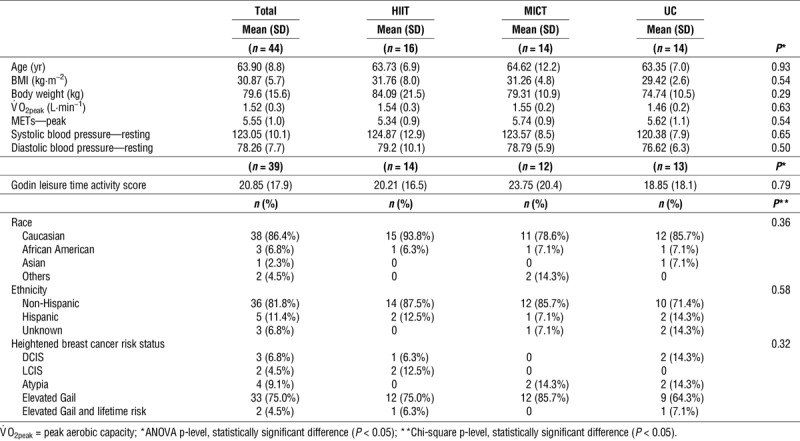
Table 1 presents the summary statistics of demographic and clinical factors measured at baseline among all participants collectively and by intervention group. ANOVA revealed no statistically significant differences (P > 0.05) between groups in age, BMI, body weight, peak aerobic capacity, resting blood pressure, and leisure time activity at baseline. Furthermore, chi-square tests did not reveal statistically significant differences in race, ethnicity, or criteria to define heightened breast cancer risk between groups.
TABLE 1.
Baseline characteristics.
Feasibility
We observed a 22% consent rate. Out of the 44 participants who were randomized to an intervention group, 42 completed all components of baseline testing with the intent to participate in the intervention. Out of these 42 participants, 33 completed the 12-wk trial, yielding a 79% retention rate. Furthermore, the average workout adherence rate among the participants who completed the trial was 90% in the HIIT group and 89% in the MICT group. All participants were compliant to each workout, except two cases where we discontinued exercise because of adverse events. Among all participants in the HIIT group, the mean and SD values for HR and RPE after the warm-up, high-intensity intervals, and active recovery windows were as follows, respectively: 97 ± 6.8 bpm, 1.5 ± 0.5; 144 ± 10.1 bpm, 4.5 ± 1.9; and 109 ± 8.0 bpm, 1.6 ± 0.5. Among all participants in the MICT group, the average HR for the workouts was 107 ± 7.5 bpm and the average RPE was 2.5 ± 0.6.
Adverse events
There were no adverse events reported in the UC group, and statistically significant differences were not observed in the number of adverse events reported between exercise groups (six in HIIT and four in MICT, P = 0.49). There were two adverse events in which we discontinued the training session. The first case was due to shortness of breath, and the second case was due to report of dizziness. Other adverse events possibly related to participation in the trial include reports of muscle soreness and joint pain. There were no serious adverse events reported.
Secondary outcomes
Table 2 presents the results of the repeated-measures analysis using linear mixed models for measures of CRF, body weight, and BMI. The overall main effect for study group was not statistically significant for any of the outcomes examined, nor was any of them significant at baseline, the latter suggesting that the three groups (UC, MICT, and HIIT) were similar at baseline. The overall main effect of time, as well as the time effect in the HIIT group, was statistically significant for all measures of CRF, but not for body weight or BMI. Specifically, there was a significant change from baseline to 12-wk follow-up in absolute and relative peak aerobic capacity (V˙O2peak; P < 0.001), peak METs (P < 0.001), and total exercise time (P < 0.001) in the HIIT group. Figure 3 depicts these changes in CRF variables by study group. The within-group differences were also examined for MICT and UC, and no significant differences were found. The group–time interaction effect (or the overall intervention effect) was significant for all measures of CRF, peak METs, and total exercise time, but not for body weight or BMI. For specific between-group comparisons of the change from baseline, UC had significantly less improvement in all CRF measures than HIIT, but not in body weight or BMI. Similarly, MICT had less improvement in total exercise time compared with HIIT, with no difference between the two groups for the other secondary outcomes.
TABLE 2.
Repeated-measures analysis using linear mixed models for CRF, body weight, and body mass index by study group over the 12-wk intervention.
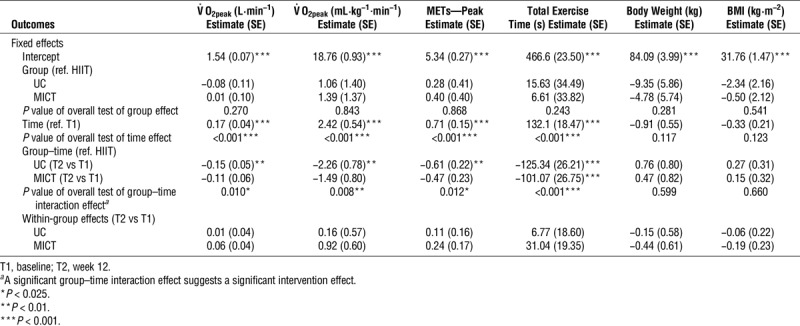
FIGURE 3.
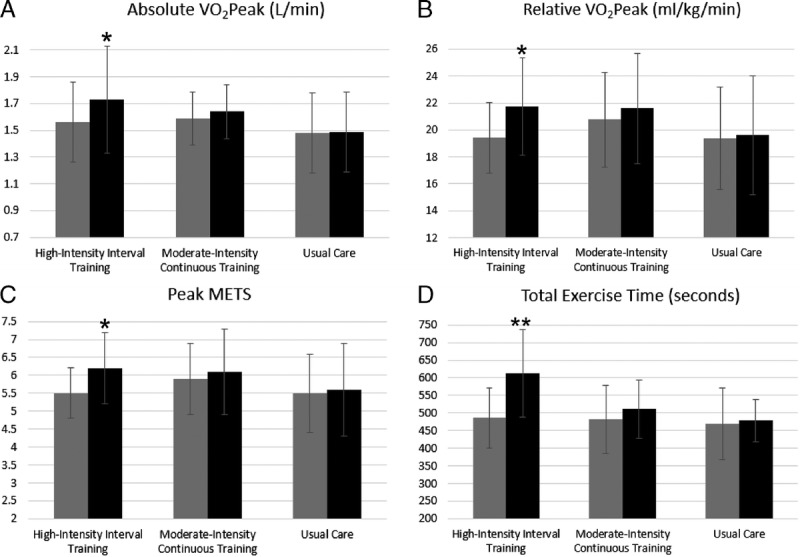
Changes in cardiorespiratory fitness variables (absolute V02peak [A]; relative V02 peak [B]; peak METs [C]; total exercise time [D]) within groups. Gray bars indicate group means at baseline, and black bars indicate group means at 12 wk. *P = 0.001. **P < 0.001.
DISCUSSION
Overall, HIIT is feasible based on the observed high adherence and compliance to the HIIT protocol and retention to the 12-wk intervention within this high-risk patient population. The HIIT group demonstrated significantly greater improvements in CRF compared with MICT and UC. Epidemiological data support the role for exercise in the reduction of invasive breast cancer risk, especially among those who engage in high-intensity exercise (19). Previous work indicates that greater than 6 h·wk−1 of vigorous recreational exercise over a lifetime had a 23% reduction in the odds ratio of invasive breast cancer when compared with women reporting no recreational exercise (20). In the current trial, we are the first, to our knowledge, to test the feasibility of implementing HIIT as an exercise intervention strategy in women at heightened risk for invasive breast cancer.
We observed a similar adherence and compliance rate between HIIT and MICT. Our observed 90% adherence and 100% compliance rates in HIIT are consistent with what has previously been reported in trials among other clinical populations, greater than an 88% adherence rate and 100% compliance rate (21–23). We also observed a 79% retention rate to the trial, with no serious adverse events reported and no significant difference between the number of grade 1 or grade 2 adverse events reported between HIIT or MICT groups. Together, these findings confirm the feasibility and safety of HIIT among women at heightened risk of breast cancer and add to the body of literature demonstrating that HIIT is feasible and safe among various clinical populations (10–13,21–28).
In addition to our adherence and compliance rates, our consent rate was 22% with time for travel to the clinic three times per week for exercise and time to travel for completion of assessment sessions as the top reason for decline in participation. These findings suggest the importance of developing interventions that require minimal travel burden to participants, such as interventions conducted in a home-based setting. Of note, the time required for the exercise session was not a given reason for decline in consent. Anecdotally, participants in the HIIT group expressed that the time needed for HIIT workout was more acceptable than the time needed for the MICT workout. This is important as the time requirement of exercise continues to serve as the biggest reported barrier to exercise (22,29,30).
We observed significant improvements in CRF variables within the HIIT group, but not in MICT or UC. These findings align with previous research conducted in other clinical populations (10–13,21). For example, in a meta-analysis conducted by Hwang and colleagues (12) of six studies among patients with cardiometabolic disorders (e.g., overweight/obesity, metabolic syndrome, and heart disease), HIIT improved CRF by 3.6 mL·kg−1⋅min−1 or 1 MET compared with MICT. Our trial is the first to our knowledge to demonstrate greater improvements in CRF with HIIT compared with MICT or UC among overweight/obese postmenopausal women at heightened risk of invasive breast cancer. These findings are of key importance in the context of breast cancer prevention among this high-risk population, considering the known inverse association between CRF and breast cancer risk as well as all-cause mortality (31–35). For example, a 1-MET increase is associated with an 11% reduction in all-cause mortality and 18% reduction in cardiovascular disease-specific mortality (34). In the current study, we found, on average, a nearly 1-MET increase at peak aerobic capacity in the HIIT group in just 12 wk, a change in CRF that has been demonstrated to be associated with improved breast cancer survival (8).
Evidence from two systematic reviews suggest that the superior improvements in CRF observed with HIIT over MICT may be attributed to the various adaptations that occur in the skeletal muscle, vasculature, and myocardium secondary to the heightened physiological stress induced by HIIT (9,36). At the level of the skeletal muscle, HIIT significantly increases calcium reuptake in the sarcoplasmic reticulum (9,36), up to 50%–73% (9), which improves work capacity and thereby contributes to improvements in CRF. HIIT also increases mitochondrial capacity, a critical component for energy metabolism in aerobic exercise, via increasing a major regulator of muscle mitochondria, peroxisome proliferator-activated receptor, gamma, coactivator 1, and alpha (9,36). Further, increased shear stress posed on the vasculature from HIIT elicits improvements in nitric oxide synthase and flow-mediated dilation (9,36), which facilitates faster oxygen delivery to the working muscle. At the level of the myocardium, HIIT improves fractional shortening and enhanced diastolic filling (9,36), which increases oxygen supply to the working muscles. Taken together, skeletal muscle and cardiovascular adaptations unique to HIIT contribute to superior improvements in CRF with HIIT compared with MICT.
Strengths of the present trial include the use of a randomized controlled trial and prospective nature of the study design. Limitations include a smaller sample size. Future research should consider using a larger sample to assess the efficacy of other factors associated with breast cancer risk, conducting the intervention in a home-based setting, and conducting a prospective trial assessing the incidence of breast cancer and/or changes in biomarkers at the breast tissue level in relation to different exercise doses.
In all, our findings demonstrate the feasibility and safety of HIIT in women at high risk of breast cancer and promise in HIIT as a strategy to significantly improve CRF beyond UC and the typical MICT exercise prescription. An additional benefit of HIIT is that the same dose of exercise achieved by the standard MICT prescription can be achieved in less time. Therefore, HIIT exercise prescription may be an advantageous strategy for breast cancer risk reduction among high-risk women, although further research is required.
Acknowledgments
The authors thank the NCI R25 Cancer Prevention Research Training Program (CA057730, PI: Shine Chang, Ph.D.), the MD Anderson Cancer Center/Energy Balance Assessment Supplemental Funding (PI: Susan Gilchrist MD), and the MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment.
The authors declare that they have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by the American College of Sports Medicine and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teoh SL, Das S. Tumour biology of obesity-related cancers: understanding the molecular concept for better diagnosis and treatment. Tumor Biol. 2016;37(11):14363–80. [DOI] [PubMed] [Google Scholar]
- 3.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Lam KS, Xu A. Adiponectin as a negative regulator in obesity-related mammary carcinogenesis. Cell Res. 2007;17:280–2. [DOI] [PubMed] [Google Scholar]
- 5.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170(19):1758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fournier A, Dos Santos G, Guillas G, et al. Recent recreational physical activity and breast cancer risk in postmenopausal women in the E3N cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1893–902. [DOI] [PubMed] [Google Scholar]
- 7.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA. 2003;290(10):1331–6. [DOI] [PubMed] [Google Scholar]
- 8.Peel JB, Sui X, Adams SA, Hébert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc. 2009;41(4):742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–34. [DOI] [PubMed] [Google Scholar]
- 10.Devin JL, Jenkins DG, Sax AT, et al. Cardiorespiratory fitness and body composition responses to different intensities and frequencies of exercise training in colorectal cancer survivors. Clin Colorectal Cancer. 2018;17(2):e269–79. [DOI] [PubMed] [Google Scholar]
- 11.Devin JL, Sax AT, Hughes GI, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv. 2016;10(3):467–79. [DOI] [PubMed] [Google Scholar]
- 12.Hwang CL, Wu YT, Chou CH. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J Cardiopulm Rehabil Prev. 2011;31:378–85. [DOI] [PubMed] [Google Scholar]
- 13.Sijie T, Hainai Y, Fengying Y, JianXiong W. High-intensity interval exercise training in overweight young women. J Sports Med Phys Fitness. 2012;52:255–62. [PubMed] [Google Scholar]
- 14.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–8. [DOI] [PubMed] [Google Scholar]
- 15.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. [DOI] [PubMed] [Google Scholar]
- 16.Pescatello S. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. pp. 52–125. [Google Scholar]
- 17.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6. [PubMed] [Google Scholar]
- 18.Teixeira PJ, Carraca EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act. 2012;9(78):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–57. [DOI] [PubMed] [Google Scholar]
- 20.Sprague BL, Trentham-Dietz A, Newcomb PA, Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational physical activity and risk of in situ and invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(2):236–43. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy S, Thoma C, Hallsworth K, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2016;59(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher G, Brown AW, Bohan Brown MM, et al. High intensity interval- vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One. 2015;10(10):e0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjønna AE, Lee SJ, Rognmo Ø, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutin B, Barbeau P, Owens S, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr. 2002;75:818–26. [DOI] [PubMed] [Google Scholar]
- 25.Moholdt TT, Amundsen BH, Rustad LA, et al. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158(6):1031–7. [DOI] [PubMed] [Google Scholar]
- 26.Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216–22. [DOI] [PubMed] [Google Scholar]
- 27.Wallman K, Plant LA, Rakimov B, Maiorana AJ. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight population. Res Sports Med. 2009;17(3):156–70. [DOI] [PubMed] [Google Scholar]
- 28.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94. [DOI] [PubMed] [Google Scholar]
- 29.Godin G, Desharnias R, Valois P, Lepage P, Jobin J, Bradet R. Differences in perceived barriers to exercise between high and low intenders: observations among different populations. Am J Health Promot. 1994;8:279–85. [Google Scholar]
- 30.Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of difference racial/ethnic groups. Women Health. 2000;30(4):61–76. [DOI] [PubMed] [Google Scholar]
- 31.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–10. [PubMed] [Google Scholar]
- 32.Frisch RE, Wyshak G, Albright NL, et al. Lower lifetime occurrence of breast cancer and cancers of the reproductive system among former college athletes. Am J Clin Nutr. 1987;45(1 Suppl):328–35. [DOI] [PubMed] [Google Scholar]
- 33.Lakoski SG, Willis BL, Barlow CE, et al. Midlife cardiorespiratory fitness, incident cancer, and survival after cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol. 2015;1(2):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlow CE, Defina LF, Radford NB, et al. Cardiorespiratory fitness and long-term survival in “low-risk” adults. J Am Heart Assoc. 2012;1(4):e001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6):e183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassidy S, Thoma C, Houghton D, Trenell MI. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia. 2017;60:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


