Supplemental digital content is available in the text.
Key Words: gemcitabine, liposomal irinotecan, metastatic pancreatic cancer, nab-paclitaxel, serum albumin, topoisomerase inhibitor
Objectives
Liposomal irinotecan (nal-IRI) is a topoisomerase inhibitor proven to improve survival in metastatic pancreatic cancer (mPC). This study describes real-world characteristics of patients treated with nal-IRI for mPC.
Methods
Patients 18 years or older diagnosed with stage IV mPC and treated with nal-IRI were selected retrospectively from a deidentified electronic health record database of more than 2 million US cancer patients. Demographics, clinical and dosing characteristics, and treatment outcomes were collected.
Results
Of 257 total patients, 145 (57%) received nal-IRI as first- or second-line therapy. Median nal-IRI treatment duration was 51 days, longer when nal-IRI was used as first/second versus as third-line therapy or later (62 vs 44.5 days). Seventy patients (27.2%) experienced dose modification. Median time to treatment discontinuation was 2.3 versus 1.6 months for first-/second- versus third-line therapy or later, respectively. Median overall survival from nal-IRI initiation was 5.6 versus 4.1 months for first-/second- versus third-line therapy or later, respectively. Prior irinotecan treatment, baseline serum albumin less than 40 g/L, and baseline neutrophil-to-lymphocyte ratio greater than 5 were associated with reduced overall survival.
Conclusions
This is the first large US study of real-world US mPC patients treated with nal-IRI. These results, comparable to the NAPOLI-1 trial, can help inform future studies and the efficacy of nal-IRI in mPC therapy.
Pancreatic adenocarcinoma is an aggressive and devastating disease. In 2019, roughly 56,770 new cases of pancreatic cancer are expected to be diagnosed.1 Pancreatic cancer accounts for approximately 3% of all cancers and is the third leading cause of cancer death in the United States.2 By the year 2030, pancreatic cancer is projected to be the second leading cause of cancer death in the United States behind lung cancer.3
Among patients with a new diagnosis of pancreatic cancer, more than 60% are diagnosed at stages III and IV and have incurable disease.4 Cases diagnosed at an early stage carry a high risk of recurrence despite surgery and adjuvant therapy.5–7 Because of the lack of early diagnostic signs and symptoms, the aggressive nature of the disease, and limited treatment options, 5-year survival for patients remains very low at 9%.1 There are limited options for the treatment of advanced pancreatic cancer, and liposomal irinotecan (nal-IRI) is the only Food and Drug Administration–approved drug after gemcitabine treatment providing a treatment sequence option.
Liposomal irinotecan is a topoisomerase inhibitor indicated for the treatment of patients with metastatic pancreatic ductal adenocarcinoma in combination with fluorouracil and leucovorin after disease progression following gemcitabine-based therapy.8 Efficacy of this combination was evaluated in NAPOLI-1, a randomized, open-label, multicenter, phase 3 trial in 417 patients with locally advanced or metastatic pancreatic ductal adenocarcinoma with documented disease progression after gemcitabine or gemcitabine-based therapy.9 The results demonstrated a significant improvement in overall survival (OS) among patients treated with nal-IRI in combination with 5-fluorouracil and leucovorin (6.1 months) compared with leucovorin and fluorouracil without nal-IRI (4.2 months; P = 0.014).9 Reinforcing the trial results, in a retrospective chart review carried out in a single center, patients treated with nal-IRI + 5 U/LV had an OS of 5.3 months.10
The objective of the current study was to describe real-world patient characteristics, dosing patterns, and outcomes of patients with metastatic pancreatic cancer (mPC) treated with nal-IRI in the United States.
MATERIALS AND METHODS
Data Source
Patient data were acquired from the nationwide Flatiron Health database. The Flatiron Health database is a longitudinal, demographically and geographically diverse database derived from deidentified electronic health record data. Patient-level data include structured and unstructured data, curated via technology-enabled abstraction. At the time of data collection, the database included information from more than 265 community-based cancer treatment clinics and academic hospital centers (~800 sites of care) representing 2 million US cancer patients available for analysis. Institutional review board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent. Data provided to third parties were deidentified, and provisions were in place to prevent reidentification in order to protect patients' confidentiality.
Study Population
The study sample included patients at least 18 years of age and diagnosed with pancreatic cancer (International Classification of Diseases, Ninth Revision [ICD-9] code 157.xx or ICD-10 code C25.xx) with pathology consistent with adenocarcinoma of the pancreas and evidence of stage IV or progressive/recurrent disease on or after January 1, 2014. Patients were also required to have at least 2 documented clinical visits on or after January 1, 2014, and received nal-IRI treatment from an administration or noncanceled order at least 90 days prior to data cutoff (August 31, 2017). The index date was defined as the start date of the initial nal-IRI–containing treatment regimen in the metastatic setting for each patient, and the baseline period was defined starting from the date of mPC diagnosis until the day prior to the index date. The follow-up period was defined as the index date until either patient death or last activity date, whichever occurred first.
Lines of Therapy
Oncologist-defined rule-based lines of therapy in the metastatic setting were derived from therapies administered after or up to 14 days prior to mPC diagnosis. All drugs given within 28 days of an initial therapy were considered part of the same regimen. The addition of a new therapy after 28 days was considered a switch and the start of a subsequent regimen. The following exceptions were made to these rules: substitution of fluorouracil for capecitabine or vice versa, substitution of leucovorin for levoleucovorin or vice versa, and the addition of leucovorin or levoleucovorin to a regimen did not advance the line of therapy; the addition of protein-bound paclitaxel to a gemcitabine regimen (or vice versa) within 90 days from the start of the line did not advance the line. These are operational rules informed by clinical input and are applied to the data post hoc. Based on these rules, first-line treatment in the metastatic setting may not reflect that these patients have received treatment soon after adjuvant therapy and may not necessarily be first line clinically.
Outcome Measures
Baseline demographics and clinical characteristics of patients receiving nal-IRI in the metastatic setting at the index date were documented. The most recent height, weight, Eastern Cooperative Oncology Group (ECOG) performance status, serum albumin, neutrophil count, and lymphocyte count prior to initiating nal-IRI were identified. Characteristics of nal-IRI treatment and dosing patterns were also collected. These included the 6-week dose intensity (total dose of nal-IRI, measured in mg/m2) and dose density (percent of total expected 6-week dose intensity assuming treatment with the indicated dose of 70 mg/m2, free base, equivalent to 80 mg/m2 salt-based dosing, every 2 weeks) within the first 6 weeks of initiating a nal-IRI containing regimen. Frequency, timing, and patterns of dose modification (defined as a difference of at least 7 mg/m2 between consecutive administrations of nal-IRI) were also analyzed. Length of therapy was defined as the number of days between the date of initiation and last date of last treatment cycle; no censoring was employed. Use of granulocyte colony-stimulating factor (G-CSF) prior to and during treatment with nal-IRI was defined as a documented administration or noncanceled order of G-CSF. A separate analysis of patients who were previously treated with gemcitabine-based therapy was also performed.
Treatment outcomes were assessed, including median OS and real-world time to treatment discontinuation (rwTTD), defined as time from treatment initiation to treatment discontinuation for any reason. Proportion of patients with 3 prespecified real-world adverse effects: neutropenia, thrombocytopenia, and diarrhea, were stratified by line of therapy and dose for the baseline period (from metastatic diagnosis until start of nal-IRI) and from treatment initiation until the follow-up end date, with no censoring employed. Neutropenia and thrombocytopenia were identified directly from electronic medical records using ICD-9 and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes. The presence of diarrhea was abstracted from unstructured data in individual health records as well as identified via ICD-9/10-CM codes from the structured data. When available in the health records, diarrhea was attributed to the treatment regimen, nal-IRI specifically, or mPC. When laboratory values were available, patients were stratified by grade of neutropenia based on the National Cancer Institute Common Terminology Criteria for Adverse Events grading system for reduced neutrophil count: grade 1: <lower limit of normal–1.5 × 109/L, grade 2: <1.5–1.0 × 109/L, grade 3: <1.0–0.5 × 109/L, and grade 4: <0.5 × 109/L. Patients could be at risk of each grade separately. The rationale for nal-IRI discontinuation (disease progression, disease-related symptoms, and treatment toxicity) was identified through abstraction of unstructured data.
Statistical Analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). Baseline demographics were analyzed by descriptive statistics. Kaplan-Meier methods were used to estimate the median OS and rwTTD from treatment initiation. For the OS analyses, patients who died were assigned the 15th day of their month of death, and patients who did not die during the study were censored at their last visit or administration date. For rwTTD analyses, patients who did not die during the study period with evidence of planned treatment with nal-IRI on or after the study end were censored on August 31, 2017; otherwise, patients were considered to have discontinued treatment on the last administration or order for nal-IRI or death date, whichever occurred first. Cox proportional hazards models were used to estimate the differences in survival between subgroups of interest. The log-rank test was used to assess differences in survival, and the Wald χ2 test was used to assess differences in hazards.
RESULTS
Baseline Characteristics
A total of 257 patients with mPC who were treated with a nal-IRI regimen between August 1, 2015, and August 31, 2017, were identified and included in the study. The median patient age was 68 years, and the sex distribution was almost evenly divided (51.4% male). All patients had a Charlson Comorbidity Index score of 2 or greater, with a median score of 3. The primary tumor location was the head of the pancreas in 51.4% of patients, followed by the body of the pancreas (26.5%; Table 1). Among patients with ECOG data recorded on or prior to nal-IRI initiation (n = 189), 15% had a most recent score of 2 or greater. The most common nal-IRI–based treatment regimen was nal-IRI in combination with fluorouracil and leucovorin (n = 230 [89.5%]; Table 1).
TABLE 1.
Baseline Characteristics
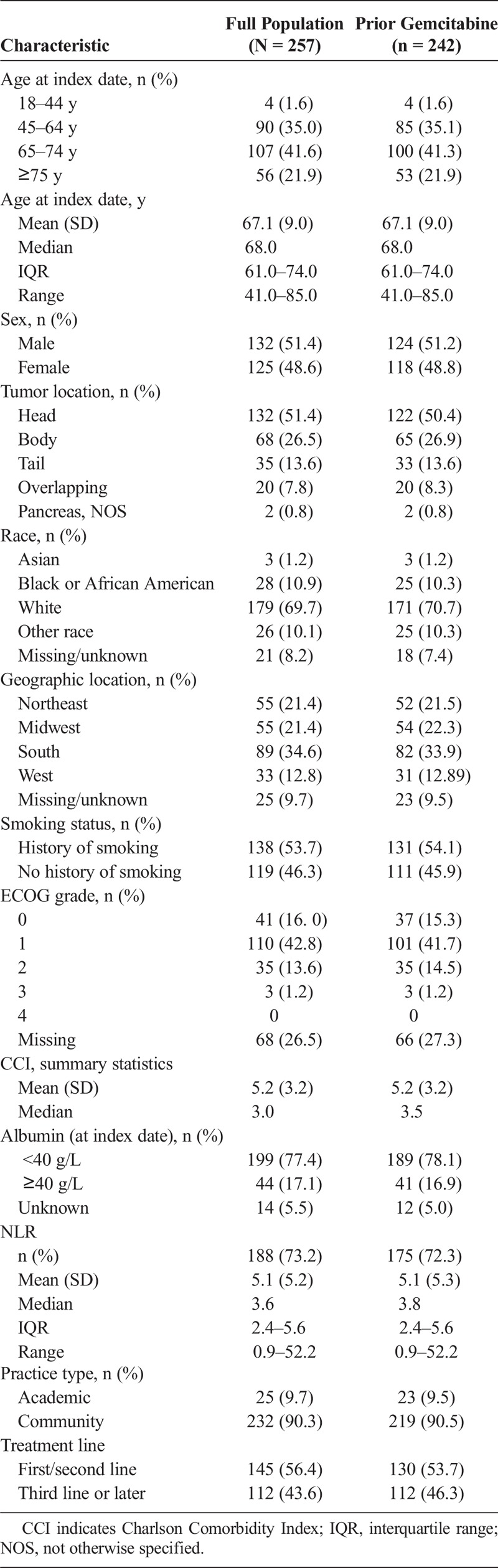
The majority of patients (94.2%, n = 242) were treated with gemcitabine therapy any time prior to nal-IRI. Following diagnosis of metastatic or recurrent pancreatic cancer, 56.4% (n = 145) of all patients received nal-IRI as first- or second-line therapy; of these patients 89.7% (n = 130) were treated with gemcitabine prior to treatment with nal-IRI therapy (Table 1). First-line patients received neoadjuvant, adjuvant, or locally advanced treatment, but no previous therapy for metastatic disease.
Dose Modifications and Outcomes
The median dose of nal-IRI at treatment initiation was 69.4 mg/m2, and at 6 weeks, the median dose intensity was 190 mg/m2. There were 152 patients (59.1%) who initiated nal-IRI treatment at the indicated dose (65 to <75 mg/m2). Patients were stratified as either being below (n = 115) or at/above (n = 116) median dose intensity. Patients below median dose intensity were older compared with those at/above median dose intensity (P = 0.003). A total of 44.5% of patients below median dose intensity were initiated at a lower dose of nal-IRI (30–65 mg/m2), compared with 13.8% of patients at or above median dose intensity. The mean (standard deviation [SD]) nal-IRI dose intensity at 6 weeks was 177.8 (74.9) mg/m2, whereas dose density was 84.7% (35.7%) (Table 2). Mean dose intensity was similar for patients who initiated nal-IRI as first-/second-line therapy (176.4 mg/m2; n = 131) compared with later lines (179.6 mg/m2; n = 100).
TABLE 2.
Dose Intensity at 6 Weeks (Total mg/m2)
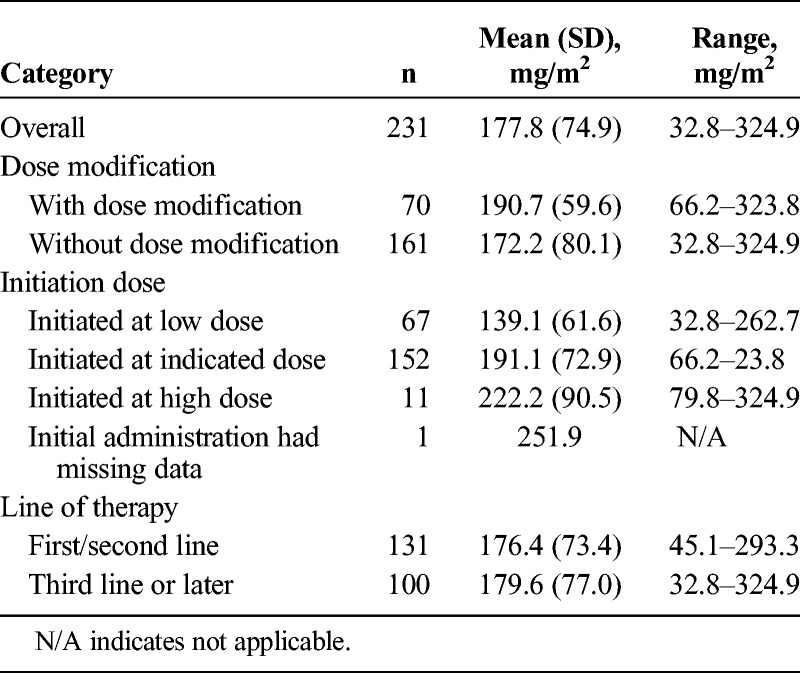
The median length of nal-IRI therapy was 7.3 weeks (51 days; see Supplemental Table 1, Length of Liposomal Irinotecan Therapy, http://links.lww.com/MPA/A768). Among patients who received nal-IRI as first-/second-line therapy, the median duration of exposure (DOE) was 8.9 weeks compared with 6.3 weeks in patients who received it in the third-line setting and beyond (P = 0.027). The median length of therapy for patients initiated at the recommended dose of nal-IRI (n = 152) was 8.1 weeks compared with 7.1 and 6.1 weeks for patients initiated at a lower dose (n = 67) or higher dose (75–90 mg/m2; n = 11), respectively. Patients above median dose intensity had a median DOE of 13.1 weeks compared with 3.1 weeks for patients under median dose intensity (P < 0.0001).
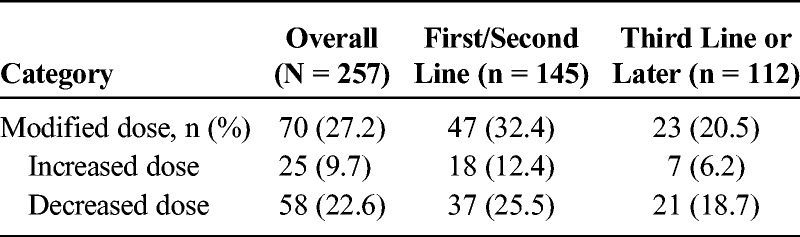
In total, 70 patients (27.2%) experienced at least 1 dose modification: 32.4% while receiving first-/second-line nal-IRI therapy plus 20.5% receiving nal-IRI third line or later (Table 3). Patients with a dose modification had a median length of therapy of 13.1 versus 6.1 weeks among patients without a dose modification.
TABLE 3.
Liposomal Irinotecan Dose Modifications
Nearly half of the study population (47.9%) had evidence of G-CSF use while undergoing treatment with nal-IRI, and 63.8% of patients received G-CSF prior to initiating nal-IRI.
Treatment Outcomes
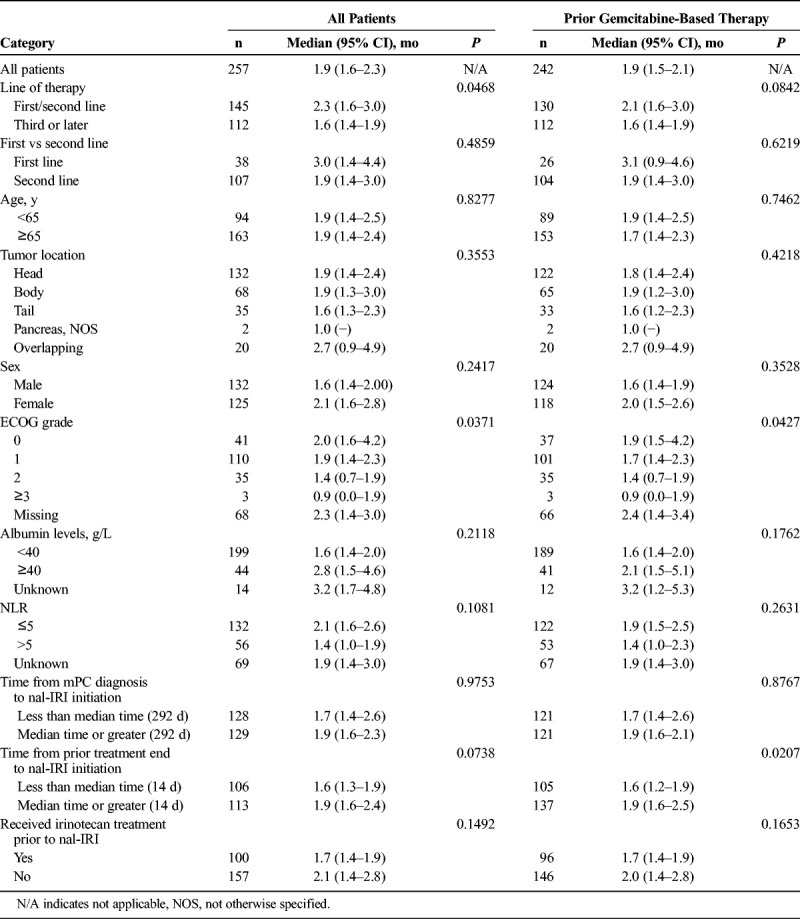
The median rwTTD was 2.3 months for patients who were treated with nal-IRI as first- or second-line treatment compared with 1.6 months as third line or later (P = 0.047; Table 4). Among patients previously treated with gemcitabine-based therapy before nal-IRI, rwTTD was 2.1 versus 1.6 months third line or later (P = 0.084). Outcomes were similar between patients treated in the first and second line, 3.0 versus 1.9 months (P = 0.4859), respectively.
TABLE 4.
Real World Time to Treatment Discontinuation
Median OS from initiation of nal-IRI was 5.6 months (95% confidence interval [CI], 4.8–7.3 months) for patients who received nal-IRI as first-line (n = 38) or second-line (n = 107) treatment (total n = 145) compared with 4.1 months (95% CI, 3.4–4.9 months) for patients treated with nal-IRI third line or later (n = 112; Table 5). Median OS was similar between patients treated in the first and second line, 5.9 versus 5.4 months (P = 0.3926), respectively. When analyzing survival, we found that patients who received nal-IRI third line or later had 57% higher mortality risk compared with patients who received nal-IRI as first-/second-line therapy (hazard ratio [HR], 1.6; 95% CI, 1.2–2.1; Fig. 1A). Adjusting for age, sex, serum albumin, prior exposure to irinotecan, and neutrophil-to-lymphocyte ratio (NLR) patients who received nal-IRI third line or later had 32% higher mortality risk compared with patients who received nal-IRI as first-/second-line therapy (adjusted HR, 1.3; 95% CI, 0.9–1.9). Among patients previously treated with gemcitabine-based therapy, median OS was 5.5 months (95% CI, 4.7–7.5 months; Table 5).
TABLE 5.
Overall Survival From Liposomal Irinotecan Initiation Date

FIGURE 1.
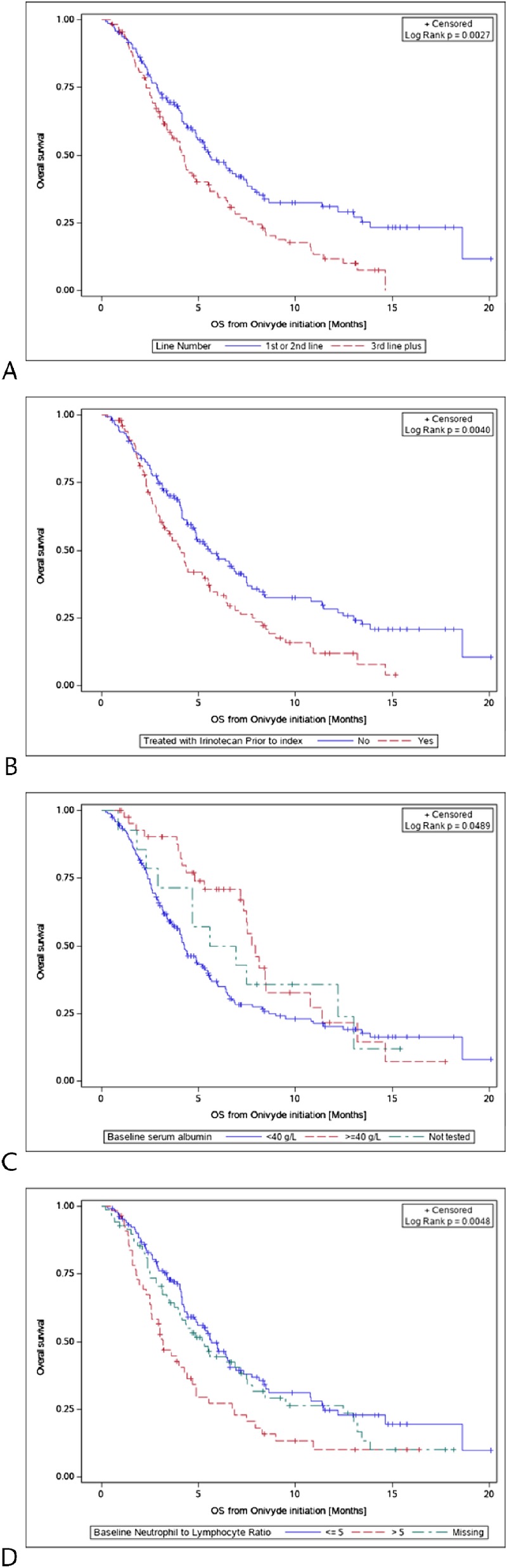
Overall survival curves among patients by (A) nal-IRI as first-/second-line therapy compared with third-line-or-later therapy, (B) prior treatment with irinotecan, (C) baseline serum albumin level, and (D) baseline NLR.
We performed additional survival analyses to identify other characteristics that may have affected survival in this patient population. Irinotecan-containing treatment prior to nal-IRI was associated with a lower OS compared with no prior irinotecan treatment (HR, 1.6; 95% CI, 1.2–2.1; Fig. 1B). A serum albumin level of 40 g/L or greater was associated with a higher OS compared with albumin less than 40 g/L (HR, 0.6; 95% CI, 0.4–0.9; Fig. 1C). Finally, patients with an NLR of greater than 5 had shorter OS compared with an NLR of 5 or less (HR, 1.8; 95% CI, 1.3–2.6; Fig. 1D).
Real-Word Adverse Effects
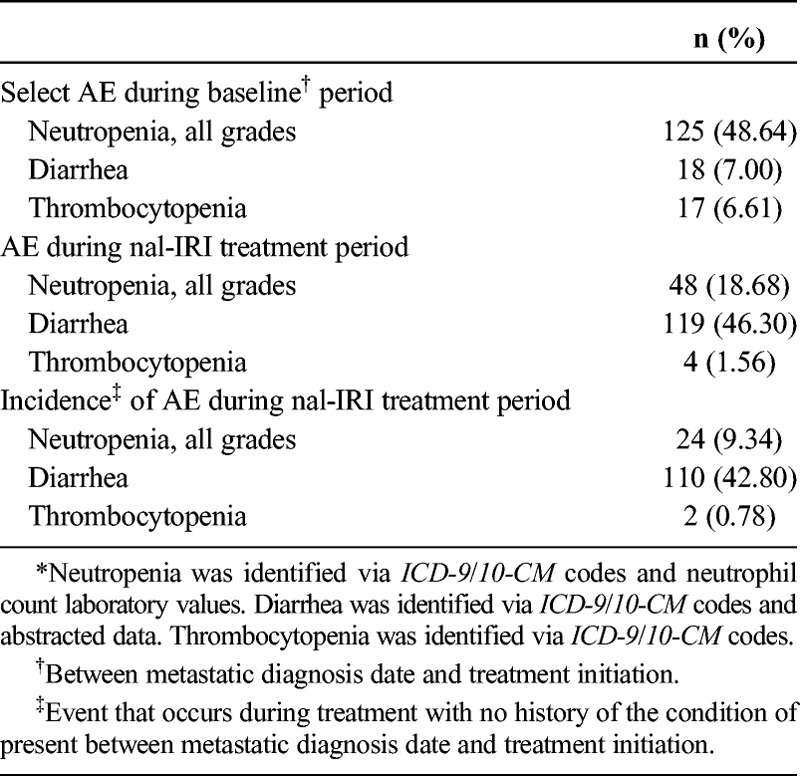
The safety evaluation revealed that 18.7% of patients experienced neutropenia (all grades) during their treatment with nal-IRI, compared with 48.6% of patients who experienced neutropenia between mPC diagnosis and the start of nal-IRI (baseline; Table 6). During treatment with nal-IRI, 18 patients (7.0%) experienced grade 3 neutropenia, and 5 patients (1.95%) experienced grade 4 neutropenia. Diarrhea was documented for 46.3% of patients while receiving treatment with nal-IRI. Diarrhea was attributed to the disease in 2.0% of patients, treatment regimen in 30.7% of patients, and to nal-IRI in 10.9% of patients (data not shown); patients could be classified under multiple categories. Only 4 patients (1.6%) experienced thrombocytopenia throughout their nal-IRI treatment period compared with 17 patients (6.6%) who exhibited the condition during the baseline period.
TABLE 6.
Select Adverse Effects* (N = 257)
Among patients for whom reasons for discontinuation were available (n = 186), disease progression (57%), disease-related symptoms (17%), and treatment toxicity (14%) were most often reported.
DISCUSSION
In this report, we present results of a large retrospective study designed to describe real-world characteristics, treatment patterns, and clinical outcomes of patients with mPC treated with nal-IRI. We analyzed a large database of patients with mPC who were treated during the 2.5 years following nal-IRI regulatory approval in the United States, yielding a robust analysis that provided a broad range of baseline characteristics, treatment activity patterns, and outcomes among these patients in clinics around the United States. These data could be useful for both establishing realistic eligibility criteria when designing future clinical trials and identifying attributes that may contribute to treatment success in the clinic.
Overall, this analysis provided important insights about the characteristics of patients treated with nal-IRI in the United States in predominantly community oncology settings, thus providing a better aspect of real-world practice. We show that recipients of nal-IRI after approval were generally older (median age of 63 years in NAPOLI-1 vs 68 years in this study) and had more comorbidities compared with the trial population. The proportion of patients from North America in the NAPOLI-1 trial was 16%, which limits its generalizability to the US population.
The median OS in patients who received nal-IRI in first- and second-line setting was 5.6 months (95% CI, 4.8–7.3 months) and comparable to the reported survival outcomes in the NAPOLI trial (6.1 months; 95% CI, 4.8–8.9 months). According to the prescribing information, nal-IRI is indicated, in combination with fluorouracil and leucovorin, for the treatment of patients with mPC after disease progression following gemcitabine-based therapy.8 Although we identified 257 patients with mPC who were treated with nal-IRI, 230 of those patients were confirmed to have received the indicated combination regimen; 27 patients (10.5%) were treated with alternative nonindicated nal-IRI combinations. Moreover, when comparing the numbers of patients who were treated with nal-IRI as first- or second-line therapy based on line-of-therapy rules (n = 145), there was a difference of 15 patients between the full population versus those documented as having been previously treated with gemcitabine. Therefore, when evaluating the outcomes in these patients, we must consider the possible influence of the subset of patients who were treated with nal-IRI “off label,” by using either a nonindicated drug combination or sequence of therapy. However, given the small sample size, this was unlikely to influence our findings related to patient outcomes treated with nal-IRI.
This study identified patient characteristics that may serve as prognostic factors. Patients previously treated with irinotecan-based therapies (n = 100) had an OS of 4.1 months (95% CI, 3.0–5.3 months) compared with 5.6 months (95% CI, 4.7–6.9 months) irinotecan-naive patients (n = 157). Glassman et al10 identified a similar trend in survival outcomes when comparing irinotecan-naive patients to those previously exposed and reported a median OS of 9.0 months in prior irinotecan patients with no progression, 3.9 months among prior irinotecan patients with documented progression, and 7.7 months among irinotecan-naive patients. Data on disease progression were not available in our study, which may influence the impact of prior irinotecan on survival outcomes and limit the ability to determine sensitivity to irinotecan. In line with other studies, patients with a lower NLR at treatment initiation (NLR ≤5, n = 132) had an OS of 5.7 months (95% CI, 4.4–6.6 months) than those with an elevated NLR (NLR >5, n = 56) who had an OS of 3.2 months (95% CI, 2.5–4.4 months).11–13 Serum albumin levels have been associated with survival in cancer, with higher levels associated with better survival.14 Patients with serum albumin at or greater than 40 g/L at treatment initiation (n = 44) experienced better survival outcomes, OS of 7.9 months (95% CI, 7.2–10.7 months) compared with those with lower albumin levels (n = 199), OS of 4.3 months (95% CI, 3.8–5.2 months).
Our findings revealed that patients who were treated with nal-IRI in the first- or second-line had an OS of 5.6 months and rwTTD of 2.3 months from initiation of nal-IRI therapy. For patients treated with nal-IRI third line or later, these results were 4.1 and 1.6 months, respectively. Improved outcomes in earlier lines of therapy would be expected, considering that the latter patients are more heavily pretreated as the gemcitabine and nab-paclitaxel regimen was approved in 2013, prior to the NAPOLI-1 study. The NAPOLI-1 study, where treatment regimens were tightly controlled (first-line gemcitabine therapy prior to entering the study) followed by second-line nal-IRI plus fluorouracil and leucovorin during the study, demonstrated a median OS and time to treatment failure of 6.1 and 2.3 months, respectively.9 Similar results were observed in a retrospective chart review that also examined real-world nal-IRI outcomes, albeit from a single clinical site.10 In that study, both patient baseline characteristics and outcomes were similar to our findings (the observed median OS was 5.3 months).
Dosing information suggested that the mean dose exposure at 6 weeks was 177.8 mg/m2 in our cohort, slightly higher than that in the NAPOLI trial (167.5 mg/m2). Patients who experienced dose modifications were able to receive a higher dose intensity at 6 weeks (190.7 mg/m2) and were on therapy longer (13.1 vs 6.1 weeks), suggesting that dose modifications in the real world are practical for sustaining patients on treatment longer. Furthermore, the DOE to treatment was marginally lower in this cohort (7.3 weeks in our cohort vs 8.7 weeks in the NAPOLI trial). However, in patients receiving first- and second-line therapy, based on rwTTD estimates, the median duration of nal-IRI was 2.3 months, matching the NAPOLI trial. Additionally, we found that patients who received lower doses of nal-IRI were older (median, 70 years) than those who received higher doses (median, 65 years), suggesting that providers adjust the starting dose based on an assessment of patient characteristics.
With regard to treatment-related toxicities, neutropenia is among the significant toxicities of nal-IRI. Although G-CSF prophylaxis was not part of the NAPOLI trial, 37% of the trial population experienced neutropenia. Interestingly, nearly half of the population in our cohort received G-CSF after initiating nal-IRI treatment, which is in line with the guidelines for use of G-CSF in regimens with more than 20% risk of neutropenia.15
There are several limitations present in this study. Age is capped at 85 years for deidentification reasons, so the true age of some older patients with mPC who received nal-IRI and their associated clinical outcomes and treatment-related toxicities are unknown. Diagnosis codes from the structured data from an oncology clinic may not capture all the patients' comorbid conditions, particularly those less likely to be relevant in an oncology setting. This may lead to an underestimate of the true comorbidity burden of patients included in the study. Entry errors may be present in the structured data, leading to extreme values. Abstraction of certain variables such as diarrhea from unstructured data was exploratory and may be underreported in the data. This study utilized time-to-event data analyses, which may be subject to bias based on the frequency of data collection. These results may not be generalizable outside the community oncology setting because most patients were treated at community clinics.
CONCLUSIONS
This was the first large-scale study that examined the real-world patient characteristics, treatment patterns, and outcome of patients with mPC and treated with nal-IRI in the United States, bearing in mind that the trial enrolled patients from 14 countries. The analysis demonstrates that effectiveness of nal-IRI in the real world may mirror the efficacy findings of the pivotal, phase 3, NAPOLI-1 trial, despite differences in the patient characteristics and dosing patterns. Although safety analysis was limited in this study, trends were similar to those observed in the clinical trial. Contrary to reports of fast adoption of immunotherapy treatment for approved indications, adoption of nal-IRI in the community had been low.16 We hope that our findings encourage the providers to consider this treatment in the appropriate patient population, where survival differences based on patient attributes such as prior irinotecan therapy, serum albumin levels, and NLR can be used as association biomarkers for appropriate patients.
Supplementary Material
Footnotes
This study was sponsored by Ipsen Biopharmaceuticals, Inc. The sponsor was involved in the design of the study, analysis, and interpretation as well as final approval of the manuscript.
D.A. serves in an advisory role to Eisai Inc. R.M. and A.Z.T. are employees of Flatiron, a member of the Roche Group, and report a consulting/advisory role with Ipsen Biopharmaceuticals, Inc. A.S. and F.A.C. are employees of Genesis Research and report an advisory/consulting role with Ipsen. S.W. is formerly an employee of Genesis Research and worked in a consulting/advisory role with Ipsen. K.M. is an employee of Glean Insights, LLC, and reports and advising/consulting role with Ipsen Biopharmaceuticals, Inc. S.P. and A.V. are formerly employees of Ipsen Biopharmaceuticals. T.B.-S. serves in consulting/advisory role with Ipsen. A.B. declares no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer Facts and Figures 2017. Atlanta, GA: American Cancer Society; 2018: Available at: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html. Accessed July 31, 2018. [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 4.Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975–2015 [SEER website]. 2018. Available at: https://seer.cancer.gov/csr/1975_2015/. Accessed July 28, 2018.
- 5.Neureiter D, Jäger T, Ocker M, et al. Epigenetics and pancreatic cancer: pathophysiology and novel treatment aspects. World J Gastroenterol. 2014;20:7830–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 8.Onivyde [package insert]. Basking Ridge, NJ: Ipsen Biopharmaceuticals; 2017. Available at: www.fda.gov/medwatch. Accessed July 28, 2018. [Google Scholar]
- 9.Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. [DOI] [PubMed] [Google Scholar]
- 10.Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer. 2018;18:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. [DOI] [PubMed] [Google Scholar]
- 12.Piciucchi M, Stigliano S, Archibugi L, et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci. 2017;18 pii: E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4:e180798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


