Abstract
The extensive use of silver nanoparticles (AgNPs) in manufactured products will inevitably increase environmental exposure, highlighting the importance of accurate toxicity assessments. A frequent strategy to estimate AgNP cytotoxicity is to use absorbance or fluorescent-based assays. In this study we report that AgNPs – with or without surface functionalizations (polyvinyl pyrrolidone or gum arabic), and of different sizes (2–15 nm) – can interfere with the spectrometric quantification of different dyes commonly used in cytotoxicity assays, such as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), neutral red (NR), Hoechst, and Resazurin. Some AgNP types caused more interference than others, which was dependent on the assay. Overall most AgNPs caused the direct reduction of MTT, as well as Hoechst and NR fluorescence quenching, and absorbed light at the same wavelength as NR. None of the AgNPs tested caused the direct reduction of Resazurin; however, depending on AgNP characteristics and concentration, they may still promote fluorescence quenching of this dye. Our results show that AgNPs with different size and coatings can have interfere with spectroscopy-based assays to different degrees, suggesting that their cytotoxicity may be underestimated or overestimated. We suggest that when using any spectroscopy-based assay it is essential that each individual nanoparticle formulation be tested first for potential interferences at all intended concentrations.
Keywords: nanomaterials, cellular viability, Alamar Blue
Graphical Abstract
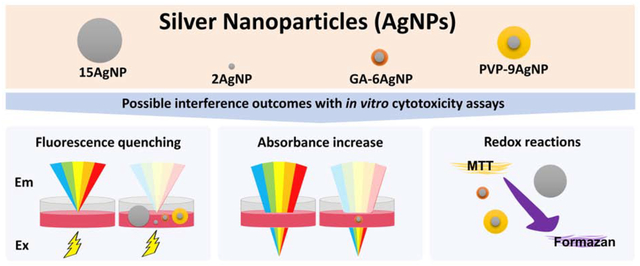
1. Introduction
Silver nanoparticles (AgNPs) are among the most widely produced nanoparticles due to their unique physicochemical and broad-spectrum antimicrobial properties. Currently, AgNPs are used in consumer products, medicinal devices, biotechnology, pharmacology, engineering, electronics, and environmental remediation [1,2]. This increase in applications of AgNPs raises the concern of potential effects on environmental and human health.
Adoption of in vitro spectroscopy-based assays as alternative methods to animal testing for toxicity assessment is ongoing and growing [3,4], including for nanomaterials [5]. Thus, the reliability of standard assays is critical. A few publications have specifically addressed the concern that several types of nanomaterial may interfere with these assays [6,7]. Nonetheless, to this date, many researchers continue to publish nanotoxicity studies using these dyes without performing proper interference controls. According to Ong et al. [7], in 2012, 90 % of the papers that performed some type of toxicity assay based on absorbance or fluorescence measurements did not mention any type of interference control. A look at more recent publications indicates that this reality is unchanged to this date. Although some studies have already demonstrated that AgNPs are among nanomaterials that can significantly interfere with a number of spectrometric-based assays [8–12], to our knowledge, none of them have compared the interference of AgNPs with different physicochemical characteristics along with different spectroscopy-based assays side-by-side. Concomitant comparison of these assays and different AgNPs has the advantage of identifying which assays may promote the most or least interference, and whether the physicochemical characteristics of AgNPs affect interference levels. Thus, the purpose of this study was to specifically test for AgNP interference using a range of size and coatings of AgNPs: uncoated 2 nm and 15 nm AgNPs (2AgNP and 15AgNP, respectively), gum arabic (GA) coated 6 nm AgNP and polyvinylpyrrolidone (PVP) coated 9 nm AgNP (GA-6AgNP and PVP-9AgNP, respectively) and four dyes classically used in in vitro assays: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Neutral Red (NR), Hoechst, and Resazurin. We also included dissolved ionic silver (from AgNO3 stocks) in all analyses. Our findings revealed that detectable fluorescence or absorbance interference was observed with all assays tested, however, Resazurin interference levels were negligible. Furthermore, different types of AgNPs can cause distinct levels of interference; therefore, if interference is not adequately assessed, this has direct implications for comparing the safety of different AgNPs. For these studies, in which we intended to examine AgNP effects on mitochondria, we chose AML12 cells. Because these are non-tumorigenic mouse hepatocytes, they allow us to circumvent the Crabtree effect that usually occurs with cancer cell lines.
2. Materials and Methods
2.1. Silver nanoparticles (AgNPs)
The gum arabic (GA) and polyvinylpyrrolidone (PVP) coated AgNPs (GA-6AgNP and PVP-9AgNP, respectively) were produced by the laboratory of Professor Mark Wiesner, as part of the Center for the Environmental Implications of Nanotechnology (CEINT). The synthesis and characterization of these coated AgNPs were previously described [13]. The 2 nm and 15 nm uncoated AgNP (2AgNP and 15AgNP, respectively) were purchased from US Research Nanomaterials, Inc. (Houston, USA) as aqueous dispersions. Transmission electron microscopy (TEM) images are available on the company’s website (https://www.us-nano.com/inc/sdetail/891 for the 2 nm AgNP; https://www.usnano.com/inc/sdetail/808 for the 15 nm AgNP). Hydrodynamic diameters and zeta potential of AgNPs suspended in ultrapure water (18.2 MΩ – cm) were measured using the Zetasizer Nano (Malvern Instruments Ltd.). The absorbance spectrum (300–700 nm) was performed using a Spectramax M5 plate reader (Molecular Devices). Total silver concentration was quantified in AgNPs stock solutions. AgNPs solutions (15–70 μl) were digested in triplicate in 5 ml of concentrated trace metal grade nitric acid (Fisher Chemical) using a hot block set at 70° C for one hour. After one hour, the digestates were diluted with 18.2 MΩ ultrapure water to a final volume of 50 ml. For analysis, the solutions were further diluted into a 2% (v/v) trace metal grade nitric acid and a 0.5% (v/v) trace metal grade hydrochloric acid solution made with 18.2 MΩ ultrapure water. The samples were measured on an Agilent 7900 ICP-MS in helium mode. The signal of 107Ag isotope was normalized to a 115Rh internal standard.
2.2. Cell culture
Mouse hepatocyte AML12 cells were purchased from the Duke University Cell Culture Facility. AML12 cells were maintained in complete growth medium (CGM) which consisted of DMEM/F12 (US Biological) supplemented with 10% non-heat activated fetal bovine serum (FBS, GE Life Sciences Hyclone), penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively, Gibco), 10 μg/ml insulin, 5 μg/ml transferrin, 7 ng/ml selenium (1:100 dilution of ITS, Millipore), and 100 nM dexamethasone (Sigma). Cells were grown in a humidified tissue culture incubator (Symphony, VWR) at 37 °C with 5% CO2. For experiments, cells were harvested using trypsin (0.25%) –ethylenediaminetetraacetic acid and seeded into 96-well plates (5×104 cells/well) in 200 μl of CGM and allowed to settle and grow for approximately 18 h at 37 °C with 5% CO2.
2.3. Interference assays
2.3.1. MTT
The yellow tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) was directly incubated in 96-well plates with various concentrations of AgNPs. The MTT final concentration was the same as used for cell viability measurements (0.5 mg/ml) in Dulbecco’s Phosphate Buffered Saline (DPBS, Sigma). Incubation was performed in the dark, at 37°C for 1 h. Supernatant was removed and the precipitated formazan crystals were then solubilized with pure dimethyl sulfoxide. Absorbance was assessed at 560 nm using a plate reader (FLUOstar OPTIMA, BMG Labtech).
2.3.2. Neutral red
AML12 cells acclimated into 96-well plates (Section 2.2) were washed twice with DPBS then incubated with 0.005% Neutral Red (NR) Solution (Sigma) in DPBS in the dark, at 37°C for 1 h. Superrnatant was removed, cells were washed once with DPBS, and NR was extracted from cells with 100 μl of acidified ethanol (1% glacial acetic acid, 50% ethanol). AgNPs were added to the NR at this point at various concentrations to each well; therefore, the results will reflect exclusively nanoparticle interference with the NR dye, but not cytotoxicity. Absorbance (450–650 nm) and fluorescence (excitation: 530 nm; emission: 550–750 nm) spectra were assessed using a Spectramax M5 plate reader (Molecular Devices).
2.3.3. Hoechst
AML12 cells acclimated into 96-well plates (Section 2.2) were washed twice with DPBS and then incubated with 100 μl of 1 μg/ml Hoechst 33342 (Sigma) in DPBS in the dark, at 37°C for 30 min. After incubation, AgNPs were added to assess nanoparticle interference with the Hoechst dye, but not cytotoxicity. Fluorescence spectrum (excitation: 352 nm; emission: 400–550 nm) was assessed using Spectramax M5 plate reader (Molecular Devices).
2.3.4. Resazurin
To determine a possible direct reduction of Resazurin by AgNPs, Resazurin (Sigma) was directly incubated in 96-well plates with various concentrations of AgNPs. The Resazurin final concentration was the same as used for cell viability measurements (25 μg/ml) in DPBS. Incubations were performed in the dark, at 37°C for 1 h. The fluorescence spectrum (excitation: 560 nm; emission: 570–750 nm) was assessed using Spectramax M5 plate reader (Molecular Devices).
3. Results and Discussion
3.1. AgNP characterization
TEM characterization of the coated AgNPs was previously published in Ma et al [13], and TEM micrographs of uncoated AgNPs are provided through the company’s website (see Materials and Methods section). Absorbance spectra (Figure 1), hydrodynamic diameters and zeta potentials (Table 1) were measured for all AgNPs. All AgNPs besides 2AgNPs presented an absorbance peak near 400 nm. All AgNPs presented a negative charge and each of their hydrodynamic sizes, assessed by dynamic light scattering (DLS), is provided, except for 2AgNPs as we were unable to obtain accurate DLS measurements for this formulation (Table 1). This raises the concern of whether the 2AgNPs are really nanoparticles, as claimed by the company that produced it. Nonetheless, we decided to continue using 2AgNPs for interference testing, as this formulation is available on the market and likely to be applied in consumer products, which implies that its cytotoxicity should be assessed.
Figure 1.
Absorbance spectra of silver nanoparticles and silver nitrate in ultrapure water.
Table 1.
Hydrodynamic diameter and zeta potential of silver nanoparticles.
| hydrodynamic diameter (nm) | zeta potential (mV) | |
|---|---|---|
| 2AgNP | n.a.* | −15.2 ± 10.2 |
| 15AgNP | 37.7 | −4.8 ± 1.3 |
| GA-6AgNP | 10.5 | −47 ± 13 |
| PVP-9AgNP | 25 | −24 ± 13 |
not available due to equipment limitation.
3.2. Interference assays
MTT reduction
The yellow tetrazolium salt MTT is one of the most widely used dyes to assess the cytotoxicity of a variety of compounds, including nanoparticles [6]. Despite reports of interference of several nanoparticles with the reduction reaction of tetrazolium salts [6,7,14], studies using MTT to assess AgNP cytotoxicity continue to surge without use of interference controls. Using cell-free conditions we observed a concentration-dependent MTT reduction by all AgNPs tested, except for 2AgNPs (Figure 2). Despite the odd behavior of these 2AgNPs, this reaction was most likely catalyzed by silver, as AgNO3 was also capable of reducing MTT in a dose-dependent manner (Figure 2A). Indeed, redox-active metals can catalyze the reduction of tetrazolium salts [6], and AgNPs are capable of catalyzing redox reactions [15]. Size and coating altered the magnitude of the reaction kinetics, indicating that different AgNPs can cause distinct levels of interference.
Figure 2.
Silver nanoparticles (AgNPs) interfere with the MTT assay. Silver nitrate (AgNO3) (A), uncoated 2 nm (B) and 15 nm (C) AgNPs, gum arabic (GA) coated 6 nm AgNP (D), and polyvinylpyrrolidone (PVP) coated 9 nm AgNP (E) were incubated using a cell-free system with MTT for 1h. (F) Formazan can be generated either in the presence of cellular NAD(P)H or AgNPs. The purple formazan product absorbance was measured at 540nm. Blank values, with AgNPs in each concentration but no MTT, were subtracted. Graphs represent mean ± standard deviation (n=3). Here and elsewhere, AgNPs representative images have proportional core sizes among each other based on TEM mean core sizes. Coating size is proportional to the core size and was based on the hydrodynamic diameter.
Fluorescence quenching
In addition to having a significant redox catalytic activity that could affect the dyes, AgNPs also have inherent optical properties. We next tested the ability of AgNPs to interfere with the fluorescence of other dyes commonly used to assess cytotoxicity: neutral red (NR), Hoechst and Resazurin. The NR assay is based on the ability of viable cells to import and retain the NR dye in the lysosomes and is commonly used to evaluate the cytotoxicity of a variety of xenobiotics [16]. Hoechst is a classic DNA stain used in numerous applications including cell proliferation [17] and is also commonly used in combination with other dyes [18]. As NR needs to be taken up by live cells, and Hoechst fluorescence is negligible when not bound to DNA, we used a different strategy to assess AgNP interference with these dyes. We performed the assays using viable non-exposed cells, and added AgNPs immediately before spectroscopic quantification; therefore, differences in fluorescence does not reflect AgNP toxicity. Our results revealed a fluorescence quenching property of some of the AgNPs tested. Hoechst fluorescence was clearly affected, in a dose-dependent manner by the uncoated 15AgNP and the GA-coated 6AgNP (Figure 3). The other AgNPs, as well as AgNO3, presented minimal or negligible interference (Figure 3).
Figure 3.
Some silver nanoparticles (AgNPs) interfere with the Hoechst assay. Silver nitrate (AgNO3) (A), uncoated 2 nm (B) and 15 nm (C) AgNPs, gum arabic (GA) coated 6 nm AgNP (D), and polyvinylpyrrolidone (PVP) coated 9 nm AgNP (E) were added after viable, untreated mouse hepatocyte (AML12) cells’ nuclei were stained with Hoechst 33342 for 30 min. Hoechst fluorescence was assessed by excitation at 352 nm and emission at 400–550 nm. Graphs represent means of n=3 and Ag concentrations are in μg/ml.
Similar results were observed with NR, in which 15AgNPs and GA-6AgNPs showed the highest levels of fluorescence quenching; however, some interference was also observed with 2AgNPs at the highest concentrations tested (Figure 4). Again, PVP-9AgNP and AgNO3 showed negligible effect (Figure 4). As NR uptake can be quantified by either fluorescence or absorbance, we also assessed the possible interference of AgNPs with NR absorbance signals. In this case, GA-6AgNPs also showed high levels of interference, but instead of decreased levels as seen for the fluorescence signals, there was a dose-dependent increase in absorbance, which was not evident with PVP-9AgNP and AgNO3 (Figure 5).
Figure 4.
Some silver nanoparticles (AgNPs) interfere with the Neutral red (NR) fluorescence assay. Silver nitrate (AgNO3) (A), uncoated 2 nm (B) and 15 nm (C) AgNPs, gum arabic (GA) coated 6 nm AgNP (D), and polyvinylpyrrolidone (PVP) coated 9 nm AgNP (E) were added to NR dye extracted from viable untreated mouse hepatocyte (AML12) cells after 1h incubation. NR fluorescence was assessed by excitation at 530 nm and emission at 550–750 nm. Graphs represent means of n=3 and Ag concentrations are in μg/ml.
Figure 5.
Silver nanoparticles (AgNPs) interfere with the neutral red (NR) absorbance assay. Silver nitrate (AgNO3) (A), gum arabic (GA) coated 6 nm AgNP (B), and polyvinylpyrrolidone (PVP) coated 9 nm AgNP (C) were added to NR dye extracted from viable untreated mouse hepatocyte (AML12) cells after 1h incubation. NR absorbance was assessed at 540 nm. Graphs represent means of n=3 and Ag concentrations are in μg/ml.
Finally, we also tested Resazurin –another dye commonly used to assess cytotoxicity. Resazurin (which is the basic reagent in the Alamar Blue assay) is a cell permeable dye that can be reduced by viable cells with active metabolism into the resorufin product, which is pink and fluorescent [19]. Although Resazurin undergoes redox reaction in vivo similar to MTT, reacting primarily with NADH, there was no direct reduction of Resazurin by any of the AgNPs tested (Figure 6A–D). There was some fluorescence quenching observed with 15AgNP and GA-6AgNP, although it was much less significant than for the other dyes (Figure 6E, F). For example, fluorescence levels using the highest GA-6AgNP concentration were approximately 7, 25 and 62 % lower than no AgNP controls for Resazurin, Hoechst and NR, respectively. Although overall Resazurin interference seems minimal even with the AgNP types that presented high interference in all assays (15AgNP and GA-6AgNP), this may not be true of all AgNP types. It is important to note that the reason Resazurin dye causes negligible interference with these AgNPs is not clear at present; and other nanoparticles, such as titanium dioxide and cadmium selenide, were shown to interfere with this assay [7].
Figure 6.
Silver nanoparticles (AgNPs) cause negligible interference with the Resazurin assay. Silver nitrate (AgNO3) (A), uncoated 2 nm (B) and 15 nm (C) AgNPs, and gum arabic (GA) 6 nm AgNP (D) were incubated with Resazurin for 1h. Fluorescence was assessed by excitation at 560 nm and emission at 600–750 nm. Graphs represent means of n=3 and Ag concentrations are in μg/ml. (E, F) Relative fluorescence quenching comparison between the Resazurin, Hoechst and neutral red (NR) assays. %Control (Ctrl) values were calculated based on fluorescence levels obtained from the peak of the spectrum curve of each dye, corresponding to 635, 455 and 610 nm for Resazurin, Hoechst and NR, respectively.
Our results clearly show that some AgNPs more than others can significantly interfere with spectroscopy-based assays. This finding is relevant not only to accurate toxicity assessment of each AgNP, which can be either under or over-estimated, but also to safety comparisons of different AgNPs with different sizes and coatings. It is important to note that, in most assays, cells previously exposed with AgNPs are washed and excess AgNP present in the medium is removed. In these cases, interference is likely to be minimized. Nonetheless, the remaining AgNP within cells or associated with the cell membrane may still show interference. As the AgNP content remaining will most likely be unknown in these cases, we do not advise researchers to simply use interference controls to normalize the spectrophotometric or spectrofluorimetric readings. Instead, we recommend as the best approach finding a cytotoxicity assay that has minimal AgNP interference. In this study, that was Resazurin. In another study, Fluoro-Jade C was used to assess cytotoxicity, with minimal interference by a citric acid coated AgNP observed [20]. However, care must still be taken when using these dyes, especially when using high AgNP concentrations, and proper interference assessments should be performed.
4. Conclusion
In this study, we demonstrated that AgNPs have the potential to cause absorbance increase, fluorescence quenching, and MTT reduction. Interference level is dependent on the physicochemical characteristics (size and functionalization) of each AgNP type. We also found that the Resazurin assay caused negligible interference with the AgNPs tested in this study when compared to the other assays. Nonetheless, we suggest that it is essential that each individual nanoparticle formulation be tested for compatibility with all spectroscopy-based assays. Nanotechnology is an exponentially growing field, which makes it imperative that nanomaterials’ impact on environmental and human health be accurately estimated. Quality control practices such as these are critical to produce reliable cytotoxicity assays, which will inform and complement other types of assays (gene expression, metabolism, activation of signaling pathways, etc.), ultimately contributing to the appropriate evaluation of the potential risk and regulation of AgNPs.
Most of the AgNPs tested and silver ions caused the direct reduction of MTT.
Some AgNPs caused fluorescence quenching of Hoechst and Neutral red dyes.
All AgNPs tested showed negligible interference with Resazurin dye.
Interference is dependent on the AgNP type and dye.
We recommend interference testing of AgNP with spectroscopy-based assays.
Acknowledgments
This work was supported by the collaborative multi-institutional Center for the Environmental Implications of Nanotechnology (CEINT) funded by the U.S. National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under NSF Cooperative Agreement EF-0830093 and DBI-1266252, and by the National Institute of Environmental Health Sciences (NIH; 1R21ES026743). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF, EPA or the NIH. This work has not been subjected to EPA review and no official endorsement should be inferred. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Du J, Tang J, Xu S, et al. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol [Internet] 98, 231–239 (2018). Available from: https://www.sciencedirect.com/science/article/pii/S0273230018302149#bib97. [DOI] [PubMed] [Google Scholar]
- 2.McGillicuddy E, Murray I, Kavanagh S, et al. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ [Internet] 575, 231–246 (2017). Available from: https://www.sciencedirect.com/science/article/pii/S0048969716322070. [DOI] [PubMed] [Google Scholar]
- 3.Basketter D, Clewell H, Kimber I, et al. A roadmap for the development of alternative (non-animal) methods for systemic toxicity testing. ALTEX [Internet] 29(1), 3–91 (2012). Available from: http://www.ncbi.nlm.nih.gov/pubmed/22307314. [DOI] [PubMed] [Google Scholar]
- 4.Daneshian M, Kamp H, Hengstler J, Leist M, van de Water B. Highlight report: Launch of a large integrated European in vitro toxicology project: EU-ToxRisk. Arch. Toxicol [Internet] 90(5), 1021–4 (2016). Available from: http://www.ncbi.nlm.nih.gov/pubmed/27017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nel AE, Nasser E, Godwin H, et al. A Multi-Stakeholder Perspective on the Use of Alternative Test Strategies for Nanomaterial Safety Assessment. ACS Nano [Internet] 7(8), 6422–6433 (2013). Available from: http://pubs.acs.org/doi/10.1021/nn4037927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J [Internet] 20(1), 1–11 (2016). Available from: http://www.ncbi.nlm.nih.gov/pubmed/26286636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong KJ, MacCormack TJ, Clark RJ, et al. Widespread Nanoparticle-Assay Interference: Implications for Nanotoxicity Testing. PLoS One [Internet] 9(3), e90650 (2014). Available from: http://dx.plos.org/10.1371/journal.pone.0090650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riaz Ahmed KB, Nagy AM, Brown RP, Zhang Q, Malghan SG, Goering PL. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr [Internet] 38, 179–192 (2017). Available from: http://ac.els-cdn.com/S088723331630220X/1-s2.0-S088723331630220X-main.pdf?_tid=e40a9fae-7c7b-11e7-bb5e-00000aacb362&acdnat=1502225901_929ea77b065e75baf4c11e11b49a85e6. [DOI] [PubMed] [Google Scholar]
- 9.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health Perspect [Internet] 118(3), 407–13 (2010). Available from: http://www.ncbi.nlm.nih.gov/pubmed/20064793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar G, Degheidy H, Casey BJ, Goering PL. Flow cytometry evaluation of in vitro cellular necrosis and apoptosis induced by silver nanoparticles. Food Chem. Toxicol [Internet] 85, 45–51 (2015). Available from: https://www.sciencedirect.com/science/article/pii/S0278691515002008. [DOI] [PubMed] [Google Scholar]
- 11.Sabatini CA, Pereira RV., Gehlen MH. Fluorescence Modulation of Acridine and Coumarin Dyes by Silver Nanoparticles. J. Fluoresc [Internet] 17(4), 377–382 (2007). Available from: http://link.springer.com/10.1007/s10895-007-0204-2. [DOI] [PubMed] [Google Scholar]
- 12.Guadagnini R, Halamoda Kenzaoui B, Walker L, et al. Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology [Internet] 9(sup1), 13–24 (2015). Available from: http://www.tandfonline.com/doi/full/10.3109/17435390.2013.829590. [DOI] [PubMed] [Google Scholar]
- 13.Ma R, Levard C, Marinakos SM, et al. Size-Controlled Dissolution of Organic-Coated Silver Nanoparticles. Environ. Sci. Technol [Internet] 46(2), 752–759 (2012). Available from: http://pubs.acs.org/doi/10.1021/es201686j. [DOI] [PubMed] [Google Scholar]
- 14.Holder AL, Goth-Goldstein R, Lucas D, Koshland CP. Particle-induced artifacts in the MTT and LDH viability assays. Chem. Res. Toxicol [Internet] 25(9), 1885–92 (2012). Available from: http://www.ncbi.nlm.nih.gov/pubmed/22799765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallick K, Witcomb M, Scurrell M. Silver nanoparticle catalysed redox reaction: An electron relay effect. Mater. Chem. Phys [Internet] 97(2–3), 283–287 (2006). Available from: https://www.sciencedirect.com/science/article/pii/S025405840500550X. [Google Scholar]
- 16.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc [Internet] 3(7), 1125–1131 (2008). Available from: http://www.nature.com/doifinder/10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 17.Richards WL, Song M-K, Krutzsch H, Evarts RP, Marsden E, Thorgeirsson SS. Measurement of cell proliferation in microculture using hoechst 33342 for the rapid semiautomated microfluorimetric determination of chromatin DNA. Exp. Cell Res [Internet] 159(1), 235–246 (1985). Available from: https://www.sciencedirect.com/science/article/pii/S0014482785800525?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- 18.Chan LL-Y, McCulley KJ, Kessel SL. Assessment of Cell Viability with Single-, Dual-, and Multi-Staining Methods Using Image Cytometry [Internet] Humana Press, New York, NY, 27–41 (2017) [cited 2018 Dec 29]. Available from: http://link.springer.com/10.1007/978-1-4939-6960-9_3. [DOI] [PubMed] [Google Scholar]
- 19.Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel) [Internet] 12(9), 12347–60 (2012). Available from: http://www.ncbi.nlm.nih.gov/pubmed/23112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Q, Cuevas E, Ali SF, et al. An Alternative In Vitro Method for Examining Nanoparticle-Induced Cytotoxicity. Int. J. Toxicol [Internet] 38(5), 385–394 (2019). Available from: http://journals.sagepub.com/doi/10.1177/1091581819859267. [DOI] [PubMed] [Google Scholar]








