Abstract
Purpose
Between 19% and 58% of oncology patients experience chemotherapy-induced nausea (CIN). In a sample of outpatients with breast, gastrointestinal (GI), gynecological, and lung cancer, the study purposes were to evaluate for inter-individual differences in the severity of CIN over two cycles of chemotherapy (CTX) and to determine which demographic and clinical characteristics and GI symptoms were associated with higher initial levels as well as with the trajectories of CIN severity.
Methods
Patients completed study questionnaires at six time points over two cycles of CTX. These questionnaires provided information on demographic and clinical characteristics, as well as the occurrence of twelve GI symptoms. Hierarchical linear modeling based on full maximum likelihood estimation was performed.
Results
Of the 1251 patients, 47.2% reported CIN. Across two cycles of CTX, lower functional status scores and higher levels of comorbidity were associated with higher initial levels of CIN. Younger age and emetogenicity of the CTX regimen were associated with higher initial levels as well as worse trajectories of CIN. The occurrence of five GI symptoms (i.e., vomiting, lack of appetite, constipation, feeling bloated and difficulty swallowing) were associated with higher initial levels of CIN. The occurrence of mouth sores was associated with higher initial levels as well as with worst trajectories of CIN.
Conclusions
This study is the first to identify distinct demographic, clinical, and GI symptom characteristics associated with CIN severity. These findings suggest that the etiology of CIN is complex and may warrant interventions beyond standard antiemetics.
Keywords: nausea, chemotherapy, antiemetics, gastrointestinal symptoms, cancer, hierarchical linear modeling
INTRODUCTION
Uncontrolled chemotherapy-induced nausea (CIN) is a significant clinical problem [14,20]. In a recent network analysis [24], nausea occurred in 47.5% of the patients receiving chemotherapy (CTX) and was a structurally important node (i.e., connection point) across all three symptom dimensions evaluated (i.e., occurrence, severity, and distress). Persistent CIN can lead to decreases in nutritional status and quality of life and can result in discontinuation of cancer treatment [7,25].
Only three cross-sectional studies have evaluated for relationships between demographic, clinical, and gastrointestinal (GI) symptom characteristics and the occurrence of CIN [5,26,36]. The most common phenotypic characteristics associated with an increased risk of CIN included: female gender [26], having a lower level of education [36], having child care responsibilities [36], emetogenicity of CTX of the regimen [26], receiving CTX on a 14-day cycle [36], occurrence of anticipatory nausea [26], and having a lower functional status [36]. In terms of GI symptoms, in a study of ovarian cancer patients receiving CTX [5], the severity of abdominal bloating, bowel disturbances, lack of appetite, vomiting, and weight loss were associated with an increased risk for CIN.
Two studies have evaluated for associations among demographic and clinical characteristics and changes over time in the occurrence of CTX-induced nausea and vomiting (CINV) [6,17]. In one study of patients who received moderately or highly emetogenic CTX and were given guideline-consistent or guideline-inconsistent CINV prophylaxis [17], multivariate logistic regression analyses were used to evaluate for risk factors associated with the occurrence of CINV at the first, second, and third cycle of CTX. No single risk factor was associated with the occurrence of CINV at all three timepoints. Risk factors associated with higher occurrence rates for CINV across the timepoints included: younger age, higher emetogenicity of the CTX regimen, occurrence of anticipatory nausea, use of an antiemetic regimen that was not consistent with international guidelines, and persistent nausea in the previous CTX cycle.
In the second study, risk factors associated with the occurrence of ≥2 grade CINV were evaluated using generalized estimation equation modeling [6]. The majority of patients received moderately or highly emetogenic CTX as well as routine pre- and post-CTX antiemetics. The occurrence of CINV was evaluated from initiation through 5 days after CTX administration. Risk factors identified included: age <60 years, occurrence of anticipatory CIN, sleep duration of <7 hours on the night before CTX, history of morning sickness, use of non-prescribed antiemetics, receipt of platinum or anthracycline-based CTX, nausea or vomiting in the previous cycle, and a higher number of CTX cycles.
While both of these longitudinal studies provide important information [6,17], neither evaluated for risk factors that are unique to the occurrence of CIN. Despite the use of guideline-based antiemetic prophylaxis, between 30% and 60% of patients experience unrelieved CIN [35,36]. In addition, neither of these studies evaluated the relative contribution of other GI symptoms to the occurrence or severity of CIN. Of note, in our recent analysis of differences in gene expression in patients with and without CIN [34], perturbations in pathways involved in mucosal inflammation and the gut microbiome were identified. Our findings, as well as one descriptive study [5], suggest that an evaluation of the associations between GI symptoms and CIN occurrence and severity is warranted.
Given the paucity of research on changes in and characteristics associated with CIN severity, the purposes of this study, in a sample of outpatients with breast, GI, gynecological (GYN), and lung cancer (n=1251) were to evaluate for inter-individual differences in the severity of CIN across two cycles of CTX and to determine which demographic and clinical characteristics, as well as GI symptoms were associated with higher initial levels as well as with the trajectories of CIN severity.
METHODS
Patients and Settings
This analysis is part of a larger, longitudinal study, funded by the National Cancer Institute, of the symptom experience of oncology outpatients receiving CTX [16,41]. Eligible patients were ≥18 years; had a diagnosis of breast, GI, GYN, or lung cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were not receiving concomitant radiation therapy; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs.
Study Procedures
The study was approved by the Institutional Review Board at each of the study sites. Of the 2234 patients approached, 1343 consented to participate and 1251 had evaluable data for this analysis. The major reason for refusal was being overwhelmed with their cancer treatment. Eligible patients were approached in the infusion unit during their first or second cycle of CTX to discuss participation in the study.
Patients completed study questionnaires in their homes, a total of six times over two cycles of CTX, namely: prior to CTX administration (i.e., recovery from previous CTX cycle; Assessments 1 and 4), approximately 1 week after CTX administration (i.e., acute symptoms; Assessments 2 and 5), and approximately 2 weeks after CTX administration (i.e., potential nadir; Assessments 3 and 6). Medical records were reviewed for disease and treatment information.
Instruments and Coding of Drug Regimen
Demographic and clinical characteristics
Patients completed the demographic questionnaire, Karnofsky Performance Status (KPS) scale [12], and Self-Administered Comorbidity Questionnaire (SCQ) [32].
Assessment of nausea severity
One item from the 41-item Quality of Life-Patient Version (QOL-PV) asked patients to rate the severity of their nausea using a 0 (no problem) to 10 (severe problem) numeric rating scale (NRS). The QOL-PV has well established validity and reliability [8,23].
Assessment of GI symptoms
A modified version of the Memorial Symptom Assessment Scale (MSAS) was used to evaluate the occurrence of twelve GI symptoms: dry mouth, nausea, feeling bloated, vomiting, diarrhea, lack of appetite, abdominal cramps, difficulty swallowing, mouth sores, weight loss, constipation, and change in the way food tastes. The validity and reliability of the MSAS are well established [27].
Coding of the emetogenicity of the CTX regimens
Using the Multinational Association for Supportive Care in Cancer guidelines [31], each CTX drug was classified as having: minimal, low, moderate, or high emetogenic potential. Emetogenicity of the regimen was categorized into one of three groups (i.e., low/minimal, moderate, high) based on the CTX drug with highest emetogenic potential.
Coding of the antiemetic regimens
Each antiemetic was coded as either a neurokinin-1 (NK-1) receptor antagonist, a serotonin receptor antagonist, a dopamine receptor antagonist, prochlorperazine, lorazepam, or a steroid. Antiemetic regimens were coded into four groups: none; steroid alone or serotonin receptor antagonist alone; serotonin receptor antagonist and steroid; or NK-1 receptor antagonist and two other antiemetics.
Data Analyses
Descriptive statistics and frequency distributions were generated for sample characteristics and GI symptom occurrence rates at enrollment using the Statistical Package for the Social Sciences (SPSS) version 25 [10]. Fisher Exact tests were used to evaluate for differences in the occurrence of GI symptoms between patients who did and did not report CIN.
Hierarchical linear modeling (HLM), based on full maximum likelihood estimation, was used to evaluate for inter-individual variability in initial levels and trajectories of nausea severity [29]. The HLM methods are described in detail elsewhere [1,4]. First, intra-individual variability in nausea severity over time was examined. A piecewise model strategy was employed to evaluate the pattern of change in nausea over time. The six assessments were coded into two pieces. Assessments 1, 2, and 3 comprised the first piece (PW1) that was used to model changes over time during the first CTX cycle. Assessments 4, 5, and 6 comprised the second piece (PW2) that was used to model changes over time during the second CTX cycle [22].
Second, inter-individual differences in the piecewise trajectories of nausea were examined by modeling the individual change parameters (i.e., intercept and slope parameters) as a function of proposed predictors at level 2. Supplementary Table 1 lists the potential predictors for nausea that were evaluated based on a review of literature [5,6,17,25,26,36].
Exploratory level 2 analyses were completed in which each potential predictor was assessed to determine whether it would result in a better fitting model if it alone were added as a level 2 predictor. Predictors with a t value of <2.0 were excluded from subsequent model testing. All potential significant predictors from the exploratory analyses were entered into the model to predict each individual change parameter. Demographic characteristics were entered first in a backward stepwise approach, in which the potential predictor variables that were not statistically significant were deleted from the model one by one. Next, clinical characteristics, followed by GI symptom occurrence variables, were entered into the model using the same backward stepwise approach. Only predictors that maintained a statistically significant contribution in conjunction with other predictors (i.e., p-value <.05) were retained in the final model.
RESULTS
Sample Characteristics
Sample characteristics are summarized in Table 1. Patients were predominately female (78.0%), had a mean age of 57.00 (±12.23) years; an average of 16.23 (±3.00) years of education, a body mass index of 26.24 (±5.69), and a KPS score of 80.14 (±12.33). Majority of patients were treated with 21-day CTX cycles (50.4%), moderately emetogenic CTX (60.6%), and an antiemetic regimen that included a serotonin receptor antagonist and a steroid (46.5%).
Table 1:
Demographic and Clinical Characteristics of the Patients (n=1251)
| Demographic Characteristics | |
|---|---|
| Age (years; mean (SD)) | 57.00 (12.23) |
| Gender (% female (n)) | 78.0 (976) |
| Ethnicity (% (n)) | |
| White | 70.1 (866) |
| Black | 7.2 (89) |
| Asian/Pacific Islander | 12.2 (151) |
| Hispanic/Mixed/Other | 10.5 (130) |
| Education (years; mean (SD)) | 16.23 (3.00) |
| Married or partnered (% yes (n)) | 64.5 (795) |
| Lives alone (% yes (n)) | 21.7 (268) |
| Currently employed (% yes (n)) | 35.6 (441) |
| Child care responsibilities (% yes (n)) | 22.1 (271) |
| Income (% yes (n)) | |
| Less than $30,000 | 17.7 (199) |
| $30,000 to <$70,000 | 21.4 (240) |
| $70,000 to < $100,000 | 16.8 (189) |
| More than $100,000 | 44.0 (494) |
| Clinical Characteristics | |
| Number of comorbidities (mean (SD)) | 2.39 (1.43) |
| Self-administered Comorbidity Questionnaire score (mean (SD)) | 5.46 (3.22) |
| Body mass index (kg/m2; mean (SD)) | 26.24 (5.69) |
| Karnofsky Performance Status score (mean (SD)) | 80.14 (12.33) |
| Have you ever considered yourself a smoker (% yes (n)) | 34.7 (427) |
| Exercise on a regular basis (% yes (n)) | 71.0 (868) |
| Cancer diagnosis (% yes (n)) | |
| Breast | 40.2 (503) |
| Gastrointestinal | 30.7 (384) |
| Gynecological | 17.8 (223) |
| Lung | 11.3 (141) |
| Time since cancer diagnosis (years; mean (SD)) | 1.96 (3.84) |
| Time since cancer diagnosis (years; median) | 0.42 |
| Any prior cancer treatments (% yes (n)) | 75.2 (935) |
| Number prior cancer treatments (mean (SD)) | 1.59 (1.50) |
| Chemotherapy cycle length (% (n)) | |
| 14 days | 42.2 (528) |
| 21 days | 50.4 (631) |
| 28 days | 7.3 (91) |
| Emetogenicity of CTX regimen (% (n)) | |
| Minimal/Low | 19.6 (245) |
| Moderate | 60.6 (758) |
| High | 19.8 (248) |
| Antiemetic regimen (% (n)) | |
| None | 6.9 (86) |
| Steroid alone or serotonin receptor antagonist alone | 20.1 (252) |
| Serotonin receptor antagonist & steroid | 46.5 (582) |
| NK-1 receptor antagonist & two other antiemetics | 24.1 (301) |
| Presence of metastatic disease (% yes (n)) | 67.7 (841) |
| Number of metastatic sites including lymph node involvement (mean (SD)) | 1.25 (1.23) |
| Number of metastatic sites excluding lymph node involvement (mean (SD)) | 0.79 (1.05) |
Abbreviations: CTX = chemotherapy; kg/m2 = kilograms per meters squared; NK-1 = neurokinin-1; SD = standard deviation
Differences in GI Symptom Occurrence
Overall occurrence rates for the GI symptoms ranged from 12.7% (i.e., vomiting) to 48.9% (i.e., change in the way food tastes, Table 2). Compared to the no nausea group, patients in the nausea group reported significantly higher occurrence rates for: change in the way food tastes, dry mouth, constipation, lack of appetite, feeling bloated, diarrhea, weight loss, abdominal cramps, mouth sores, difficulty swallowing, and vomiting (all, p<0.001).
Table 2.
Occurrence Rates for Gastrointestinal Symptoms in the Total Sample (n=1251) and Differences in These Occurrence Rates Between Patients With and Without Chemotherapy-Induced Nausea
| Gastrointestinal Symptom | Highest to Lowest Occurrence in Total Sample % (n) | No Nausea 52.8% (n = 660) | Nausea 47.2% (n = 591) | Statistics |
|---|---|---|---|---|
| % (n) | % (n) | |||
| Change in the way food tastes | 48.9 (612) | 37.7 (249) | 61.4 (363) | FE, p < 0.001 |
| Dry mouth | 45.6 (570) | 33.5 (221) | 59.1 (349) | FE, p < 0.001 |
| Constipation | 43.2 (541) | 32.6 (215) | 55.2 (326) | FE, p < 0.001 |
| Lack of appetite | 41.3 (517) | 24.1 (159) | 60.6 (358) | FE, p < 0.001 |
| Feeling bloated | 33.1 (414) | 24.8 (164) | 42.3 (250) | FE, p < 0.001 |
| Diarrhea | 29.9 (374) | 21.7 (143) | 39.1 (231) | FE, p < 0.001 |
| Weight loss | 24.9 (312) | 16.7 (110) | 34.2 (202) | FE, p < 0.001 |
| Abdominal cramps | 22.4 (280) | 13.5 (89) | 32.3 (191) | FE, p < 0.001 |
| Mouth sores | 21.3 (267) | 15.5 (102) | 27.9 (165) | FE, p < 0.001 |
| Difficulty swallowing | 13.6 (170) | 7.4 (49) | 20.5 (121) | FE, p < 0.001 |
| Vomiting | 12.7 (159) | 1.7 (11) | 25.0 (148) | FE, p < 0.001 |
Abbreviation: FE = Fisher’s Exact test
Changes in CIN Severity Over Time
The first HLM analysis examined how nausea scores changed within the two cycles of CTX. The estimates for the initial piecewise model are presented in Table 3. The linear and quadratic trends for both cycles of CTX were significant (all p<.0001). Since the model was unconditional, the intercept represents the average nausea score at enrollment (i.e., 2.697 on a 0 to 10 NRS). Estimated linear rates of change in nausea were 0.685 and 0.910 (both p<.0001) for PW1 and PW2, respectively. Estimated quadratic rates of change in nausea were −0.489 and −0.312 (both p<.0001) for PW1 and PW2, respectively.
Table 3.
Hierarchical Linear Model for the Severity of Chemotherapy-Induced Nausea
| Nausea | Coefficient (SE) | |
|---|---|---|
| Unconditional Model | Final Model | |
| Fixed effects | ||
| Intercept | 2.697 (.083)+ | 2.697 (.072)+ |
| Piecewise 1 - linear rate of change | 0.685 (.117)+ | 0.682 (.116)+ |
| Piecewise 1 - quadratic rate of change | −0.489 (.055)+ | −0.487 (.054)+ |
| Piecewise 2 - linear rate of change | 0.910 (.081)+ | 0.902 (.079)+ |
| Piecewise 2 - quadratic rate of change | −0.312 (.026)+ | −0.308 (.025)+ |
| Time invariant covariates | ||
| Intercept | ||
| Age | −0.027 (.005)+ | |
| SCQ score | 0.054 (.021) | |
| Karnofsky Performance Status score | −0.027 (.006)+ | |
| Feeling bloated | 0.351 (.138) | |
| Vomiting | 1.531 (.192)+ | |
| Lack of appetite | 0.856 (.135)+ | |
| Difficulty swallowing | 0.425 (.187)* | |
| Mouth sores | 0.443 (.159)* | |
| Constipation | 0.523 (.131)+ | |
| CTX Emetogenicity | ||
| Moderately vs minimal/low emetogenic CTX | 0.448 (.189) | |
| Highly vs minimal/low emetogenic CTX | 1.042 (.235)+ | |
| Piecewise 1 - linear rate of change | ||
| CTX Emetogenicity | ||
| Moderately vs minimal/low emetogenic CTX | 1.051 (.304)* | |
| Highly vs minimal/low emetogenic CTX | 1.689 (.372)+ | |
| Piecewise 1 - quadratic rate of change | 1.689 (.372)+ | |
| CTX Emetogenicity | ||
| Moderately vs minimal/low emetogenic CTX | −0.540 (.142)+ | |
| Highly vs minimal/low emetogenic CTX | −0.888 (.175)+ | |
| Piecewise 2 - linear rate of change | ||
| Age | −0.021 (.006)+ | |
| Child care responsibilities | −0.380 (.169)* | |
| Mouth sores | −0.420 (.162)* | |
| CTX Emetogenicity | ||
| Moderately vs minimal/low emetogenic CTX | 0.471 (.208)* | |
| Highly vs minimal/low emetogenic CTX | 1.175 (.256)+ | |
| Piecewise 2 - quadratic rate of change | ||
| Age | 0.007 (.002)* | |
| Child care responsibilities | 0.096 (.058) | |
| Mouth sores | 0.118 (.055) | |
| CTX Emetogenicity | ||
| Moderately vs minimal/low emetogenic CTX | −0.173 (.067) | |
| Highly vs minimal/low emetogenic CTX | −0.380 (.082)+ | |
| Variance components | ||
| Intercept | 6.466+ | 4.323+ |
| Piecewise 1 - linear rate of change - slope | 2.726+ | 2.309+ |
| Piecewise 1 - quadratic rate of change - slope | 0.460* | 0.347 |
| Piecewise 2 - linear rate of change - slope | 1.907+ | 1.511+ |
| Piecewise 2 - quadratic rate of change - slope | 0.182+ | 0.144+ |
| Goodness-of-fit deviance (parameters estimated) | 28588.996 (21)** | 28131.581 (46) |
| Model comparison x2 (df) | 457.385 (25)** | |
p<.05
p<.001
p<.0001
Abbreviations: CTX = chemotherapy; df = degrees of freedom; SCQ = Self-administered Comorbidity Questionnaire; SE = standard error
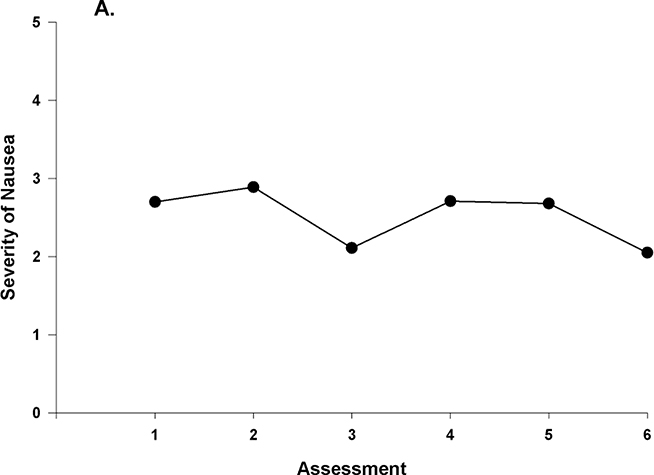
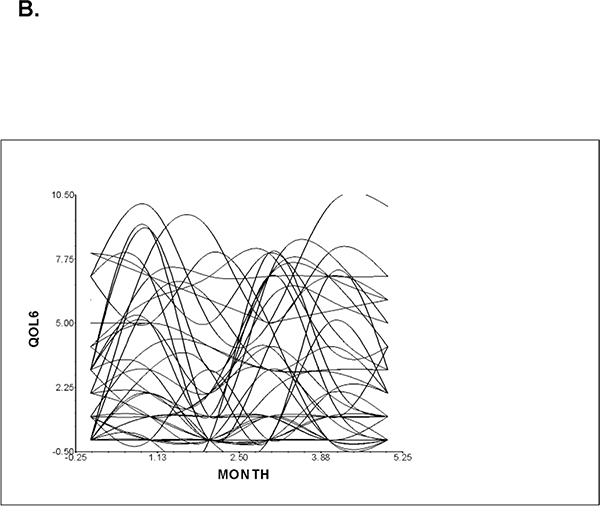
Figure 1A displays the mean nausea severity scores over the two cycles of CTX. Nausea severity peaked at assessment 2 and decreased at assessment 3, rose slightly at assessment 4, and then decreased over assessments 5 and 6. These results indicate a sample-wide change in nausea scores over time. However, they do not indicate that all of the patients’ nausea severity scores changed at the same rate over time. The variance components (Table 3) suggest that considerable inter-individual variability existed in the trajectories of nausea. A spaghetti plot of a random sample of 50 patients demonstrates the inter-individual variability in nausea severity (Figure 1B). These results supported additional analyses of predictors of inter-individual differences in initial levels as well as in the trajectories of nausea severity scores.
Figure 1 A –
Piecewise model of mean nausea scores for six assessments over two cycles of chemotherapy (CTX).
Figure 1 B -.
Spaghetti plot of individual nausea trajectories (QOL6) for a random sample of 50 patients over two cycles of CTX.
Phenotypic Characteristics Associated with CIN Severity
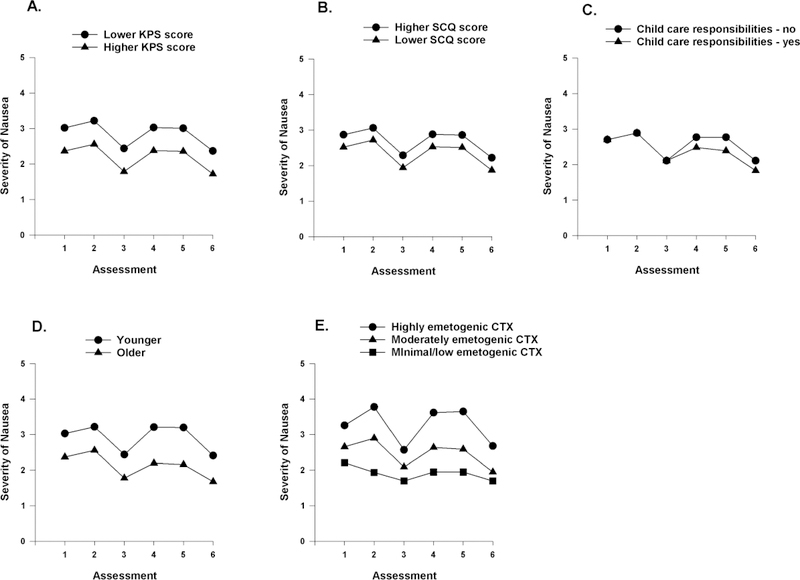
The two clinical characteristics that were associated with the inter-individual differences in initial levels of nausea were the KPS and SCQ scores (Table 3). To illustrate the effects of these scores, Figures 2A and 2B display the adjusted change curves for nausea that were estimated based on differences in KPS and SCQ scores (i.e., lower/higher calculated as one standard deviation (SD) above and below the mean KPS and SCQ scores, respectively).
Figure 2.
A-E- Influence of enrollment scores for Karnofsky Performance Status (KPS) score (A) and Self-Administered Comorbidity Questionnaire (SCQ) (B) on inter-individual differences in the intercept for nausea; the influence of child care responsibilities (C) on inter-individual differences in the slope parameter for nausea; and the influence of age (D) and emetogenicity of the chemotherapy regimen (E) on inter-individual differences in the intercept and slope parameters for nausea.
Having child care responsibilities predicted inter-individual differences only in the linear and quadratic components of PW2 (Figure 2C). Age predicted inter-individual differences in both initial levels as well as in the linear and quadratic components of PW2. Figure 2D displays the adjusted change curves for nausea that were estimated based on differences in age (i.e, younger/older calculated as one SD above and below the mean age). The emetogenicity of the CTX regimen predicted inter-individual differences in initial levels as well as in the trajectories of nausea (Figure 3F).
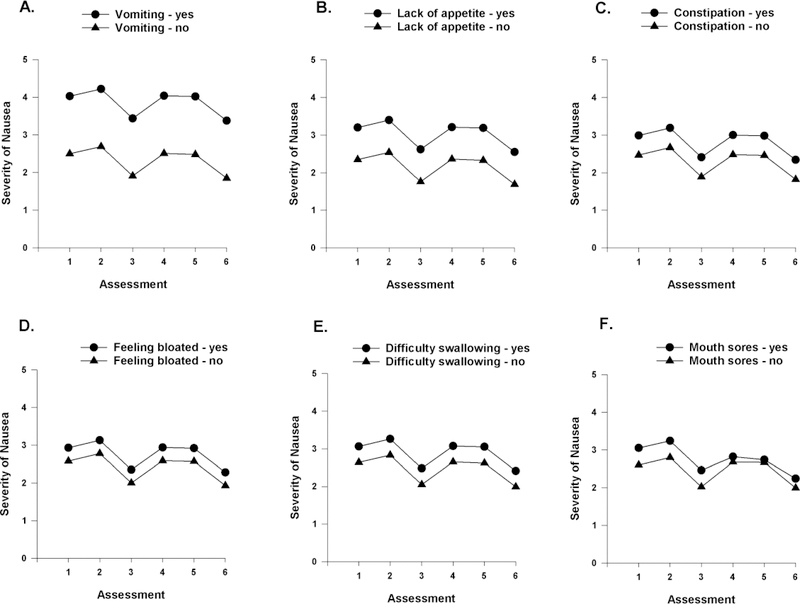
Figure 3.
A-E Influence of vomiting (A), lack of appetite (B), constipation (C), feeling bloated (D), and difficulty swallowing (E) on inter-individual differences in the intercept for nausea and influence of mouth sores (F) on inter-individual differences in the intercept and slope parameters for nausea.
GI Symptoms Associated with CIN Severity
As shown in the final model (Table 3), five GI symptoms (i.e., vomiting, lack of appetite, constipation, feeling bloated, difficulty swallowing) were associated with inter-individual differences in initial levels of nausea. To illustrate the effects of the various GI symptoms, Figures 3A–E display the adjusted change curves for nausea severity that were estimated based on the occurrence (i.e., yes or no) of each GI symptom. As shown in Figure 3F, the occurrence of mouth sores predicted inter-individual variability in both initial levels as well as in the linear and quadratic components of PW2.
DISCUSSION
This study is the first to evaluate for inter-individual differences in the severity of CIN and to identify GI symptoms that were associated with these differences in a large sample of oncology patients receiving CTX. Across two cycles of CTX, the occurrence of five GI symptoms (i.e., vomiting, lack of appetite, constipation, feeling bloated and difficulty swallowing) were associated with higher initial levels of CIN. The occurrence of mouth sores was associated with higher initial levels as well as with worst trajectories of CIN. In addition, a number of phenotypic characteristics were associated with inter-individual differences in initial levels (i.e., higher SCQ and lower KPS scores), trajectories (i.e., having child care responsibilities), or both initial levels and trajectories (i.e., younger age, emetogenicity of the CTX regimen) of CIN.
GI Symptoms Associated with CIN Severity
Consistent with previous reports [5,18], in our initial analyses (Table 2), eleven GI symptoms are associated with the occurrence of CIN. This finding is not surprising given that the administration of CTX results in inflammatory responses along the entire GI tract [38]. In addition, in studies that evaluated for symptom clusters in patients receiving CTX [18,39,40,42], nausea was a consistent symptom in the GI cluster.
While in the exploratory analyses, all eleven GI symptoms were associated with either initial levels or the trajectories of CIN severity, six remained significant in the final model (i.e., vomiting, lack of appetite, constipation, feeling bloated, difficulty swallowing, mouth sores). The association between GI symptoms and CIN may be partially explained by the fact that these symptoms share common biological mechanisms. For example, in addition to nausea and vomiting, CTX can cause delayed gastric emptying that manifests as abdominal bloating and lack of appetite [21]. CTX-induced oral mucositis can cause difficulty swallowing [28]. Finally, one of the major side effects of the serotonin and tachykinin receptor antagonists that are used to prevent and treat CINV is constipation [20]. Taken together, these findings suggest that the mechanisms that underlie variations in CIN severity are complex and multifactorial. Our findings may explain the persistently high occurrence rates and severity of CIN despite the administration of antiemetics.
In terms of the relative contribution of GI symptoms to CIN severity, vomiting had the largest intercept coefficient. As noted in a systematic review [35], a few studies provide evidence for an association between the occurrence of vomiting and CIN severity. Patients who do not experience complete relief from CINV at the beginning of CTX are more likely to experience increased CIN severity [17]. Several factors may explain this association including: poor adherence with the antiemetic regimen [17], emetogenicity of CTX [17], and/or alterations in the cytochrome P450 gene which influences the metabolism of CTX and antiemetic drugs [35].
Consistent with previous reports [2,19], lack of appetite was associated with increased CIN severity. In addition, in a prospective longitudinal study [9], compared to patients who received one day of antiemetic prophylaxis, patients whose CIN severity was controlled by a multi-day regimen of anti-emetic prophylaxis did not experience lack of appetite.
Another potential explanation for the co-occurrence of multiple GI symptoms and increased CIN severity is that the administration of CTX results in the release of proinflammatory cytokines [3,37]. In one study [15], increases in cytokine levels correlated with increases in severity of nausea, abdominal symptoms, as well as delayed gastric emptying. In another study [3], patients who had elevated levels of TNF-α prior to CTX were more likely to report more severe CIN and mucositis. Finally, in one study [18], abdominal bloating during the second cycle of CTX was associated with more severe CIN. Taken together, these findings suggest that clinicians need to assess not only for CIN but for a cluster of GI symptoms that may warrant interventions.
Phenotypic Characteristics Associated with CIN
In our previous cross-sectional study [36], while younger age was associated with the occurrence of CIN in the univariate analysis, this association did not remain significant in the multivariate model. Findings regarding the association between age and CINV are inconsistent with two studies reporting no association [9,26] and four studies [6,13,17,33] reporting that age younger than 65 years was a predictor of CINV occurrence. These inconsistent findings may be related to the dimension of the symptom experience evaluated (i.e., occurrence vs severity) and/or whether the outcome was CIN or the combination of CINV. Future studies should assess risk factors for CIN and CIV separately.
In our cross-sectional study [36], patients who had child care responsibilities had a 1.4 increase in the odds of reporting the occurrence of CIN in the week prior to their next cycle of CTX. In our current study, having child care responsibilities was associated with a decrease in CIN severity during the second cycle of CTX. Explanations for these associations are not readily apparent and warrant additional investigation.
Consistent with our previous study [36] and two additional studies [9,30], in the current study, lower functional status was associated with higher initial levels of CIN (Figure 2A). While the mean KPS score of this sample was 80.1 (i.e., patients were able to carry out daily activities and work without special care needs), at a KPS score of 67.8 (i.e., 1 SD below the mean), patients are unable to work and require assistance to meet their needs [11]. In a study that used the Eastern Cooperative Oncology group performance status (PS) score to assess functional status [9], patients with a PS score of ≥1 (i.e., worse function) had a 2.2 increase in the odds of belonging to the CINV group. In another study of changes in CIN severity [30], every 10-point decrease in KPS score was associated with a 0.2 point increase in CIN severity. These findings suggest that patients’ physical function should be assessed prior to the initiation of CTX.
While not associated with CIN occurrence in our previous study,[36] in this analysis higher levels of comorbidity were associated with higher CIN severity at enrollment. While no studies have reported this association, one can hypothesize that medications used to treat other comorbid conditions may have nausea as one of their adverse effects.
While in our previous cross-sectional study, emetogenicity of the CTX was not associated with the occurrence of CIN in the multivariate analysis [36], in another study [17], the emetogenicity of the CTX regimen was associated with CINV occurrence during the first but not during subsequent cycles of CTX. The association we observed may be partially explained by repeated exposure to moderately or highly emetogenic CTX and/or ineffective antiemetic prophylaxis.
Limitations
Several limitations need to be acknowledged. At enrollment, patients were not CTX naïve. Future studies should confirm our findings with assessments done prior to the initiation of CTX. Assessments of CIN severity were done on a weekly basis. Future studies can use a daily diary to obtain more detailed information on inter-individual variability in CIN and assess for different types of CIN occurrence/severity (e.g., anticipatory, acute, delayed). While a large number of potential predictors were assessed, future studies should include an evaluation of additional risk factors (e.g., motion sickness, morning sickness, history of CIN). Given that patients were predominately female, White, college educated, and had metastatic disease, our findings may not generalize to all oncology patients receiving CTX. Finally, information on changes in the antiemetic regimen and patients’ adherence with the analgesic regimen need to be evaluated in future studies.
Conclusions
Despite these limitations, our study is the first to describe significant associations between the occurrence of GI symptoms, as well as between phenotypic characteristics and inter-individual variability in CIN severity over two cycles of CTX. These findings can assist clinicians to identify high risk patients and to initiate more targeted interventions to decrease this extremely adverse effect of CTX. While most studies investigated risk factors for nausea AND vomiting as the “combined symptom” of CINV, our previous [36] and current findings suggest that separate evaluations of the occurrence and severity of each symptom (i.e., CIN and CIV) are warranted to determine common and unique risk factors for these two adverse effects. This recommendation is supported by the fact that while CIV appears to be effectively managed with current antiemetic regimens (i.e., only 12.7% of our total sample reported vomiting), persistent nausea remains a significant clinical problem [18]. In addition, the mechanisms that underlie increased levels of CIN, as well as the co-occurring GI symptoms warrant investigations to develop more effective interventions.
Supplementary Material
Acknowledgments
Conflicts of interest - This study was supported by a grant from the National Cancer Institute (NCI, CA134900). Dr. Miaskowski is an American Cancer Society Clinical Research Professor and is funded by a K05 award from the NCI (CA168960). Komal Singh is supported by a T32 grant (NR016920) from the National Institute of Nursing Research. The authors had full control of all of the primary data and will allow the journal to review the data if requested to do so. The authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Wara W, Swift P, Dunn LB, Miaskowski C (2009) Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue Biol Res Nurs 11: 27–41 [DOI] [PubMed] [Google Scholar]
- 2.Balaban CD, Yates BJ (2017) What is nausea? A historical Analysis of changing views Auton Neurosci 202: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen JM, White I, Smith L, Tsykin A, Kristaly K, Thompson SK, Karapetis CS, Tan H, Game PA, Irvine T, Hussey DJ, Watson DI, Keefe DM (2015) Pre-therapy mRNA expression of TNF is associated with regimen-related gastrointestinal toxicity in patients with esophageal cancer: a pilot study Support Care Cancer 23: 3165–3172 [DOI] [PubMed] [Google Scholar]
- 4.Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C (2010) Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy Cancer Nurs 33: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donovan HS, Hagan TL, Campbell GB, Boisen MM, Rosenblum LM, Edwards RP, Bovbjerg DH, Horn CC (2016) Nausea as a sentinel symptom for cytotoxic chemotherapy effects on the gut-brain axis among women receiving treatment for recurrent ovarian cancer: an exploratory analysis Support Care Cancer 24: 2635–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, Jordan K, Young A, Aapro M (2017) The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting Ann Oncol 28: 1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell C, Brearley SG, Pilling M, Molassiotis A (2013) The impact of chemotherapy-related nausea on patients’ nutritional status, psychological distress and quality of life Support Care Cancer 21: 59–66 [DOI] [PubMed] [Google Scholar]
- 8.Ferrell BR, Dow KH, Grant M (1995) Measurement of the quality of life in cancer survivors Qual Life Res 4: 523–531 [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Shimokawa M, Matsuo K, Miyoshi T, Toriyama Y, Yokota C, Taniguchi J, Hanada K, Tsumagari K, Okubo N, Koutake Y, Sakata K, Kawamata Y, Goto T, Tsurusaki Y, Koyabu M (2018) Risk factors for delayed chemotherapy-induced nausea and vomiting with low-emetic-risk chemotherapy: a prospective, observational, multicenter study Cancer Manag Res 10: 4249–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IBM (Released 2017) IBM SPSS Statistics for Windows, Version 25. IBM Corp, City. [Google Scholar]
- 11.Karnofsky D (1977) Performance scale. Plenum Press, New York [Google Scholar]
- 12.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH (1948) The use of nitrogen mustards in the palliative treatment of carcinoma Cancer 1: 634–656 [Google Scholar]
- 13.Kim HK, Hsieh R, Chan A, Yu S, Han B, Gao Y, Banos A, Ying X, Burke TA, Keefe DM (2015) Impact of CINV in earlier cycles on CINV and chemotherapy regimen modification in subsequent cycles in Asia Pacific clinical practice Support Care Cancer 23: 293–300 [DOI] [PubMed] [Google Scholar]
- 14.Kuchuk I, Bouganim N, Beusterien K, et al. (2013) Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat 142: 101–107 [DOI] [PubMed] [Google Scholar]
- 15.Liebregts T, Adam B, Bredack C, Gururatsakul M, Pilkington KR, Brierley SM, Blackshaw LA, Gerken G, Talley NJ, Holtmann G (2011) Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia Am J Gastroenterol 106: 1089–1098 [DOI] [PubMed] [Google Scholar]
- 16.Miaskowski C, Cooper BA, Melisko M, Chen LM, Mastick J, West C, Paul SM, Dunn LB, Schmidt BL, Hammer M, Cartwright F, Wright F, Langford DJ, Lee K, Aouizerat BE (2014) Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy Cancer 120: 2371–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molassiotis A, Aapro M, Dicato M, Gascon P, Novoa SA, Isambert N, Burke TA, Gu A, Roila F (2014) Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational study J Pain Symptom Manage 47: 839–848 e834 [DOI] [PubMed] [Google Scholar]
- 18.Molassiotis A, Farrell C, Bourne K, Brearley SG, Pilling M (2012) An exploratory study to clarify the cluster of symptoms predictive of chemotherapy-related nausea using random forest modeling J Pain Symptom Manage 44: 692–703 [DOI] [PubMed] [Google Scholar]
- 19.Morita M, Kishi S, Ookura M, Matsuda Y, Tai K, Yamauchi T, Ueda T (2017) Efficacy of aprepitant for CHOP chemotherapy-induced nausea, vomiting, and anorexia Curr Probl Cancer 41: 419–425 [DOI] [PubMed] [Google Scholar]
- 20.NCCN (2018) Antiemetics. In: Editor (ed)^(eds) Book Antiemetics, City. [Google Scholar]
- 21.Nelson K, Walsh D, Sheehan F (2002) Cancer and chemotherapyrelatedupper gastrointestinal symptoms: the role of abnormal gastric motor function and its evaluation in cancer patients. Supportive Care in Cancer 10: 455–461 [DOI] [PubMed] [Google Scholar]
- 22.Osborne C, Berger LM, Magnuson K (2012) Family structure transitions and changes in maternal resources and well-being Demography 49: 23–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padilla GV, Ferrell B, Grant MM, Rhiner M (1990) Defining the content domain of quality of life for cancer patients with pain Cancer Nurs 13: 108–115 [PubMed] [Google Scholar]
- 24.Papachristou N, Barnaghi P, Cooper B, Kober KM, Maguire R, Paul SM, Hammer M, Wright F, Armes J, Furlong EP, McCann L, Conley YP, Patiraki E, Katsaragakis S, Levine JD, Miaskowski C (2019) Network analysis of the multidimensional symptom experience of oncology patients Sci Rep 9: 2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirri C, Bayliss E, Trotter J, Olver IN, Katris P, Drummond P, Bennett R (2013) Nausea still the poor relation in antiemetic therapy? The impact on cancer patients’ quality of life and psychological adjustment of nausea, vomiting and appetite loss, individually and concurrently as part of a symptom cluster Support Care Cancer 21: 735–748 [DOI] [PubMed] [Google Scholar]
- 26.Pirri C, Katris P, Trotter J, Bayliss E, Bennett R, Drummond P (2011) Risk factors at pretreatment predicting treatment-induced nausea and vomiting in Australian cancer patients: a prospective, longitudinal, observational study Support Care Cancer 19: 1549–1563 [DOI] [PubMed] [Google Scholar]
- 27.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L, et al. (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress Eur J Cancer 30A: 1326–1336 [DOI] [PubMed] [Google Scholar]
- 28.Rahnama M, Madej-Czerwonka B, Jastrzebska-Jamrogiewicz I, Jamrogiewicz R (2015) Analysis of the influence of parenteral cancer chemotherapy on the health condition of oral mucosa Contemp Oncol 19: 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raudenbush SW, Bryk A (2002) Hierarchical linear models: Applications and data analysis methods. Sage Publications, Thousand Oaks, CA [Google Scholar]
- 30.Rohrl K, Guren MG, Smastuen MC, Rustoen T (2019) Symptoms during chemotherapy in colorectal cancer patients Support Care Cancer [DOI] [PubMed] [Google Scholar]
- 31.Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M, participants of the MECcC (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients Ann Oncol 27: v119–v133 [DOI] [PubMed] [Google Scholar]
- 32.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN (2003) The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research Arthritis Rheum 49: 156–163 [DOI] [PubMed] [Google Scholar]
- 33.Sekine I, Segawa Y, Kubota K, Saeki T (2013) Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis Cancer Sci 104: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh K, Dhruva A, Flowers E, Paul S, Hammer M, Wright F, Cartwright F, Conley Y, Melisko M, Levine J, Miaskowski C, Kober K (2019) Alterations in patterns of gene expression and perturbed pathways in the Gut-Brain Axis are associated with chemotherapy-induced nausea BMC Cancer [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh KP, Dhruva AA, Flowers E, Kober KM, Miaskowski C (2018) A review of the literature on the relationships between genetic polymorphisms and chemotherapy-induced nausea and vomiting Critical Reviews in Oncology/Hematology 121: 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh KP, Kober KM, Dhruva AA, Flowers E, Paul SM, Hammer MJ, Cartwright F, Wright F, Conley YP, Levine JD, Miaskowski C (2018) Risk factors associated with chemotherapy-induced nausea in the week prior to the next cycle and impact of nausea on quality of life outcomes J Pain Symptom Manage 56: 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stockhorst U, Steingrueber HJ, Enck P, Klosterhalfen S (2006) Pavlovian conditioning of nausea and vomiting Auton Neurosci 129: 50–57 [DOI] [PubMed] [Google Scholar]
- 38.Stringer AM, Gibson RJ, Bowen JM, Keefe DM (2009) Chemotherapy-induced modifications to gastrointestinal microflora: evidence and implications of change. Curr Drug Metab 10: 79–83 [DOI] [PubMed] [Google Scholar]
- 39.Sullivan CW, Leutwyler H, Dunn LB, Cooper BA, Paul SM, Levine JD, Hammer M, Conley YP, Miaskowski CA (2018) Stability of symptom clusters in patients with breast cancer receiving chemotherapy J Pain Symptom Manage 55: 39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward Sullivan C, Leutwyler H, Dunn LB, Miaskowski C (2018) A review of the literature on symptom clusters in studies that included oncology patients receiving primary or adjuvant chemotherapy J Clin Nurs 27: 516–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Predictors and trajectories of morning fatigue are distinct from evening fatigue J Pain Symptom Manage 50: 176–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yates P, Miaskowski C, Cataldo JK, et al. (2015) Differences in composition of symptom clusters between older and younger oncology patients. Journal of Pain and Symptom Management 49: 1025–1034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.