Abstract
Methylphenidate (MP) is a commonly prescribed psychostimulant to individuals with Attention Deficit Hyperactivity Disorder, and is often used illicitly among healthy individuals with intermittent breaks to coincide with breaks from school. The present study examined how intermittent abstinence periods impact the physiological and behavioral effects of chronic oral MP self-administration in rats, and whether these effects persist following prolonged abstinence from the drug. Rats were treated orally with water, low dose (LD), or high dose (HD) MP, beginning at PND 28. This daily access continued for 3 consecutive weeks followed by a one-week abstinence; after three repeats of this cycle, there was a 5-week abstinence period. Throughout the study, we examined body weight, food intake, locomotor activity, and anxiety- and depressive-like behaviors. During the treatment phase, HD MP decreased body weight, food intake, and depressive- and anxiety-like behaviors, while it increased locomotor activity. During intermittent abstinence, the effects of MP on locomotor activity were eliminated. During prolonged abstinence, most of the effects of HD MP were ameliorated to control levels, with the exception of weight loss and anxiolytic effects. These findings suggest that intermittent exposure to chronic MP causes physiological and behavioral effects that are mostly reversible following prolonged abstinence.
Keywords: Methylphenidate, Ritalin, Psychostimulant, Attention Deficit Hyperactivity Disorder, Anxiety
Introduction
Methylphenidate (MP, Ritalin) is one of the major stimulant drugs prescribed to individuals with Attention Deficit Hyperactivity Disorder (ADHD) (Lakhan and Kirchgessner, 2012). As the rate of prescriptions has increased over the past decades, so has the likelihood of misuse and abuse (Piper et al., 2018). Reportedly, 16.2% of healthy college students illicitly use stimulants and 96% of these same students specified using MP (White et al., 2006), while 4% of healthy 8th and 10th graders use non-prescribed stimulant medications (León and Martínez, 2017). Primary reasons for illicit MP use among healthy students were to enhance concentration, increase alertness, and “get high” (Teter et al., 2006). With the increasing illicit use of MP, it is important to properly study and understand the consequences of MP use in healthy individuals and how different schedules of exposure affect these consequences.
In individuals with ADHD, MP ameliorated attention deficit, hyperactivity, and impulsivity (Konrad-Bindl et al., 2016). It also enhanced cognitive performance such as executive and non-executive memory as well as reaction time in adolescents (Coghill et al., 2014). MP also reportedly reduced depressive- and anxiety-like symptoms, while increasing social behaviors in children with ADHD (Sobanski et al., 2008; Golubchik et al., 2017). While MP has generally been known to disrupt sleep in children with ADHD (Storebø et al., 2015), some studies suggest that MP may help to enhance sleep in adults with ADHD (Sobanski et al., 2008). Similar to ADHD patients, MP enhanced response inhibition and memory (Peloquin and Klorman, 1986; Vaidya et al., 1998), improved working memory and attention, and reduced distractibility in healthy individuals (Elliott et al., 1997; Volkow et al., 2002).
Rather than a regular daily pattern of chronic MP administration, healthy individuals may consume MP only during peak periods when its cognitive-enhancing effects are desired (Franke et al., 2012; Beyer et al., 2014; Urban and Gao, 2017; Juárez-Portilla et al., 2018). These patterns of use are also evident in individuals with ADHD who are prescribed MP. Reportedly, 25% to 75% of individuals prescribed MP take intentional breaks from medicine-taking, otherwise known as “drug holidays” (Ibrahim and Donyai, 2014). “Drug holidays” often coincide with weekends and seasonal breaks from school, with fewer prescriptions of MP being written in the summer compared to spring months (Martins et al., 2004; Cascade et al., 2008; Shyu et al., 2016). The American Academy of Child and Adolescent Psychiatry (AACAP) recommends these breaks in order to test the need for continued pharmacotherapy in children and adolescents with ADHD (Pliszka, 2007; van de Loo-Neus et al., 2011; Ibrahim and Donyai, 2014), and parents tend to utilize these breaks in order to manage the negative side effects observed in their children, including suppressed appetite and stunted growth (Ibrahim and Donyai, 2014; Shyu et al., 2016). However, few clinical and preclinical studies on the behavioral and physiological impact of “drug holidays” have been conducted (Martins et al., 2004; Griggs et al., 2010; Carias et al., 2019). Therefore, it is important to further understand the behavioral and developmental effects of interrupted schedules of MP use, in addition to the existing knowledge of uninterrupted chronic daily MP use (Thanos et al., 2015; Robison et al., 2017a; Robison et al., 2017b; Martin et al., 2018; Uddin et al., 2018).
The goal of this study was to use a rat model to examine the long-lasting physiological, behavioral and developmental effects of three consecutive cycles of a three-week daily MP consumption followed by one-week abstinence, and the reversal of such effects during a five-week abstinence period. We hypothesized that chronic MP exposure would be associated with hyperactivity and decreased anxiety, depressive-like symptoms, and social interaction as previously reported following weekday-only (Carias et al., 2019) and uninterrupted chronic 13-week daily MP use (Robison et al., 2017b; Martin et al., 2018) in adolescent rats. The current schedule of administration was chosen in order to supplement our previous report on weekday-only administration (Carias et al., 2019). We expected these effects to persist regardless of the intermittent 1 week abstinence from MP, but to be reversed during the prolonged 5-week abstinence period. The findings from this study may provide further insight on the behavioral and developmental consequences of different schedules of MP use that occur, including intermittent and prolonged abstinence periods.
Materials and Methods
Animals
Adolescent male (n=72) Sprague Dawley rats were obtained from Taconic Farms (Germantown, NY). On arrival, rats were single housed in a temperature- and humidity-controlled room on a reverse 12-hour light cycle (lights off at 08:00 h). Rat chow (Teklad, Indianapolis, IN) was provided ad libitum, and animals had access to water for 8 hours daily. Animals were single-housed in order to accurately monitor individual fluid and food intake, which were recorded daily and weekly, respectively. Body weight was also recorded daily throughout the experiment. Experiments were conducted in conformity with the National Academy of Science’s Guide for the Care and Use of Laboratory Animals (NAS and NRC, 1996) and approved by the University at Buffalo’s Institutional Animal Care and Use Committee.
Experimental Design and Methylphenidate Consumption Paradigm
MP was delivered to rats as methylphenidate hydrochloride (Sigma-Aldrich, St Louis, MO) in water (vehicle) beginning on PND 28. The present study employed a previously described 13-week MP dosing paradigm (Thanos et al., 2015; Robison et al., 2017a; Robison et al., 2017b; Martin et al., 2018; Uddin et al., 2018; Carias et al., 2019) with slight modifications as follows. Rats were assigned to either a low dose MP (LD), high dose MP (HD), or a water group (n=24 per group) that had limited 8-hour daily access to the respective MP dose or water on treatment weeks 1–3, 5–7, 9–11, and 13. The LD and HD treated rats received MP orally as previously described (Thanos et al. 2015). Briefly, rats had access to either a 4 mg/kg or 30 mg/kg MP solution, respectively, during the first hour of access followed by a 10 mg/kg or 60 mg/kg MP solution, respectively, for the following 7 hours of access (Thanos et al. 2015). This dosing paradigm has been previously reported to produce peak serum concentrations of 8 ng/ml and 30 ng/ml in the LD and HD treated rats (respectively) with concentration of drug in fluid adjusted daily based on prior day’s consumption (Thanos et al. 2015). This paradigm ensured that dosing was consistent independent of fluid consumption. MP was provided for an 8 hour period (0900 h-1700 h) during the dark phase (0800 h-2000 h). Weeks 4, 8, and 12 were each 1-week abstinence periods for the MP-treated groups as all animals had access to water during the 8-hour access period. After 13 weeks of treatment, half of the animals were sacrificed, while the other half received a 5-week abstinence period (weeks 14–18) for the MP-treated groups when all animals had access to water.
Behavioral Tests
Open field locomotor activity
Locomotor activity in an open field arena was assessed during treatment weeks 1, 3, 5, 7, 9, and 11, and during abstinence weeks 4, 8, 12, 15, 16, and 18. Rats were tested for 90 minutes during the dark cycle (1100 h-1700 h) in an open-field arena (dimensions 40.64 cm × 40.64 cm × 40.64 cm, 2.54 cm beam space and 1.27 cm spatial resolution) equipped with a photo beam activity monitoring system (Coulbourn Instruments, Allentown, PA). Open field locomotor activity data was acquired with TruScan v2.0 software. Distance traveled in the floor (horizontal) and vertical planes, floor plane velocity, time spent moving, number of entries into and time spent in the vertical plane, and both distance and time spent in the center of the chamber were analyzed.
Circadian activity
24-hour circadian locomotor activity was assessed as previously described (Robison et al., 2017b) during treatment weeks 1, 2, 3, and 11, and during abstinence weeks 4, 8, 12, 15, 16, and 18. Circadian locomotor activity, designated by beam breaks, was measured using a photo beam activity monitor (Starr Life Sciences Corp, VitalView software 1.1; Oakmont, Pennsylvania) attached to the cage top of each animal’s original home cage (50 cm × 25 cm × 30 cm high). Throughout the 24-hour experiment, ad libitum access to chow and the 8-hour limited access-drinking paradigm were maintained.
Elevated Plus Maze
Anxiety-like behavior was assessed on an elevated plus maze as previously described (Thanos et al., 2015) on week 10 of the 13-week treatment phase and week 2 and 5 of the 5-week abstinence phase. The test lasted for 5 minutes during the dark cycle (1100 h-1700 h) and anxiety-like behavior was measured as a proportion of time spent in the open arms to the total test time. Test sessions were recorded using D-Link cameras and software (D-Link Corporation Taipei, Taiwan) and the results were analyzed using TopScan behavioral analysis software (Clever Sys Inc. Reston, Virginia).
Forced Swim Test
Depressive-like behavior was measured using the forced swim test during week 10 of the 13-week treatment phase and week 1 of the 5-week abstinence phase. Rats were placed in a bucket measuring 39cm x 27cm (height by diameter) filled with water at room temperature. This test consisted of a 15-minute habituation run and a 5-minute test run that took place 24 hours after the habituation run. All testing occurred during the dark cycle (1100h-1700h). Depressive-like behaviors were measured as the latency to immobility, time spent in high activity, and time spent immobile. Test sessions were recorded using D-Link cameras and software (D-Link Corporation Taipei, Taiwan) and the results were analyzed using TopScan behavioral analysis software (Clever Sys Inc. Reston, Virginia).
Social Interaction
Social interaction was assessed during week 10 of the treatment phase and week 2 of the 5-week abstinence phase. The testing apparatus consisted of 3 chambers measuring 20cm x 60cm (width x height). One cage measuring 21.59cm x 12.7cm x 11.43cm (length x width x height) was placed in opposite corners of each of the outer chambers. There was a 3-minute habituation run followed by a 5-minute test run taking place 30 minutes later. During the habituation run, the rat freely explored all chambers while both cages were empty. During the test run, the rat freely explored all chambers while a social rat was located in one of the apparatus cages to allow for the option of social interaction. All testing occurred during the dark cycle (1100h-1700h). The discrimination index was calculated as the ratio of time spent interacting with the social cage to the total time spent interacting with both cages. The testing was recorded using D-Link cameras and software (D-Link Corporation Taipei, Taiwan) and the results were analyzed using TopScan behavioral analysis software (Clever Sys Inc. Reston, Virginia).
Statistical Analysis
Body weight, food and fluid consumption, and open field locomotor activity were analyzed separately during the 13-week treatment and 5-week abstinence phases using two-way repeated measures ANOVAs, with treatment group and time (i.e. week of assessment) as the between-and within-subject variables, respectively. This same ANOVA design was used to analyze circadian activity during the dark phase of the light cycle compared across weeks. Hourly circadian activity on week 11 of the 13-week treatment phase and week 2 of the 5-week abstinence phase were each analyzed using a two-way repeated measures ANOVA with time of day and treatment group as the within- and between-subject variables, respectively. Elevated plus maze, forced swim test and social interaction data during the 13-week treatment phase as well as forced swim test data during the 5-week abstinence phase were analyzed using a one-way ANOVA with treatment group as the independent variable. EPM and SI data during the 5-week abstinence phase were analyzed using a two-way repeated measures ANOVA with treatment group as the between-subject variable and week of treatment as the within-subjects variable. When appropriate, follow-up Tukey’s HSD post-hoc analyses were conducted for pair-wise comparisons. All statistical analyses were run using the SigmaPlot 11.0 statistical program.
Results
Physiological effects of chronic MP and abstinence
Fluid consumption
A two-way repeated measures ANOVA of average weekly fluid intake revealed no significant effect [F (2, 896) = 1.530; p=0.224] when controlling for body weight, consistent with what was reported in our original dosing paradigm paper (Thanos et al., 2015) (data not shown).
Food consumption
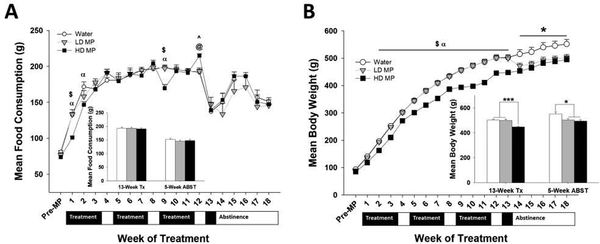
During the 13-week treatment phase, a two-way repeated measures ANOVA revealed a significant effect of time [F(12,827) = 199.640; p<.001] and interaction between treatment and time [F24,827)=4.061; p<.001], while the effect of treatment was not significant. Tukey’s post hoc analysis revealed that food intake was lower in the HD MP group during weeks 1, 2, and 9 compared to the water group (p<.01). Food intake was also higher in the LD MP group during weeks 1 and 9 compared to the HD MP group (p<.01). In contrast, food intake was higher in the HD MP group during week 12 compared to the water and LD MP groups (p<.05) (See Figure 1A).
Figure 1.
Physiological measures (n=24 per group). (A) Weekly food consumption presented as Mean + SEM (α - HD < W, p<.01; $ - HD < LD, p<.01; ^ - HD > W, p<.05; @ - HD > LD, p<.05). Insert: Weekly food consumption during week 11 (treatment) compared to week 18 (abstinence) presented as Mean + SEM. (B) Weekly body weight presented as Mean + SEM (α - HD < W, p<.01; $ - HD < LD, p<.01; * - W > HD & LD (main effect only), p<.05). Insert: Body weight during week 11 (treatment) compared to week 18 (abstinence) presented as Mean + SEM (*p<.05, ***p<.001).
During the 5-week abstinence period, a two-way repeated measures ANOVA revealed a significant main effect of time [F(4,131)=27.951; p<.001]. There was not a significant main effect of treatment nor a significant interaction between treatment and time (See Figure 1A).
Body weight
During the 13-week treatment phase, a two-way repeated measures ANOVA of average weekly weight revealed significant main effects of treatment [F(2,896)=18.578; p<.001], time [F(13,896)=6,365.193; p<.001], and a significant interaction between treatment and time [F(26,896)=15.337; p<.001]. Tukey’s post hoc analysis revealed that the HD MP group weighed less than both the LD MP (p<.01) and water (p<.01) groups during weeks 2–13 (See Figure 1B).
During the 5-week abstinence period, a two-way repeated measures ANOVA revealed significant main effects of treatment [F(2,128)=5.817; p<0.01] and time [F(32,128)=326.373; p<.001], while the interaction between treatment and time was not significant. Tukey’s post hoc analysis revealed that, overall during abstinence, the water group weighed more than both the LD MP (p<.05) and HD MP (p<.01) groups (See Figure 1B).
Behavioral effects of chronic MP and abstinence
Locomotor Activity
Floor Plane Activity
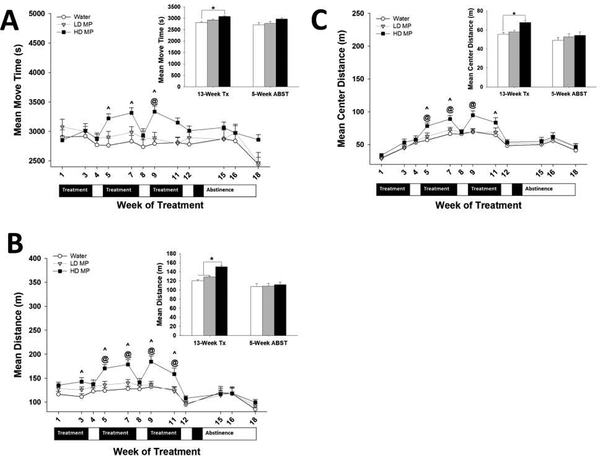
During the 13-week treatment phase, a two-way repeated measures ANOVA of the duration of motion also revealed a significant main effect of time [F (8,497) = 2.751; p<0.01] and a significant interaction between treatment and time [F(16,497) = 3.155; p<.001]. There was not a significant main effect of treatment. Tukey’s post hoc analysis revealed that the HD MP group spent significantly more time moving during weeks 5, 7, and 9 compared to the water group (p<.01), and during week 9 compared to the LD MP group (p<.01) (See Figure 2A) Also during the 13-week treatment phase, a two-way repeated measures ANOVA of floor plane distance traveled revealed significant main effects of treatment [F (2,497) =7.866; p<.001], time [F(8,497)=24.435; p<.001], and a significant interaction between treatment and time [F(16,497)=3.494; p<.001]. Tukey’s post hoc analysis revealed that the HD MP group traveled longer distance during weeks 3, 5, 7, 9, and 11 compared to the water group (p<.01), and during weeks 5, 7, 9, and 11 compared to the LD MP group (p<.01) (See Figure 2B).
Figure 2:
Open Field (n=24 per group). (A) Move time in the open field presented as Mean + SEM (^ - HD > W, p<.01; @ - HD > LD, p<.01). Insert: Move time during treatment (week 11) and abstinence (week 18) presented as Mean + SEM (*p<.05). (B) Distance traveled in the open field presented as Mean + SEM (^ - HD > W, p<.01; @ - HD > LD, p<.01). Insert: Distance traveled during treatment (week 11) and abstinence (week 18) presented as Mean + SEM (*p<.05). (C) Center distance traveled in the open field presented as Mean + SEM (^ - HD > W, p<.01; @ - HD > LD, p<.05). Insert: Center distance traveled during treatment (week 11) and abstinence (week 18) presented as Mean + SEM (*p<.05).
During the 5-week abstinence period, a two-way repeated measures ANOVA of the duration of motion revealed a significant main effect of time [F (2, 64) = 12.518; p<.001] but neither a main effect of treatment nor a significant interaction between treatment and time (See Figure 2A). A two-way repeated measures ANOVA of distance traveled also revealed a significant main effect of time [F (2, 64) = 15.771; p<.001]. There was not a significant main effect of treatment nor a significant interaction between treatment and time (See Figure 2B).
Center Activity
During the 13-week treatment phase, a two-way repeated measures ANOVA of center distance traveled revealed significant main effects of treatment [F (6, 497) = 3.659; p<0.05], time [F(8,497) = 67.879; p<.001], and a significant interaction between treatment and time [F(16,497) = 2.640; p<.001]. Tukey’s post hoc analysis revealed that the HD MP group traveled greater center distance during weeks 5, 7, 9, and 11 compared to the water group (p<.01), and during weeks 5, 7, and 9 compared to the LD MP group (p<.05) (See Figure 2C).
During the 5-week abstinence phase, a two-way repeated measures ANOVA revealed a significant main effect of time [F (2, 64)=14.749; p<.001] but neither a main effect of treatment nor a significant interaction between treatment and time (See Figure 2C).
Circadian sleep-wake activity
Hourly Activity
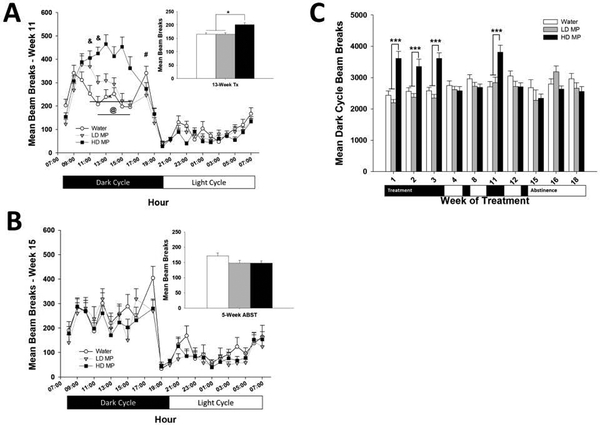
A two-way repeated measures ANOVA of hourly activity during week 11 of treatment revealed significant main effects of treatment [F (2, 1518) =5.081; p<0.01], hour [F(22,1518)=75.135; p<.001], and a significant interaction between treatment and hour [F(44,1518)=5.917; p<.001]. Tukey’s post hoc analysis revealed that the HD MP group had greater circadian activity from 11:00h to 16:00h compared to the water group (p<.001), and from 12:00h to 16:00h compared to the LD MP group (p<.05). Additionally, the LD MP group had greater circadian activity from 11:00h to 12:00h compared to the water group (p<.05). In contrast, the LD MP group had lower circadian activity during 18:00h compared to the water group (p<.05) (See Figure 3A).
Figure 3:
Circadian Activity (n=24 per group). (A) Hourly circadian locomotor activity during week 11 of treatment presented as Mean + SEM (# - LD < W, p<.05; ^ - HD > W, p<.001; @ - HD > LD, p<.05; & - LD > W, p<.05). Insert: Total activity during week 11 of treatment presented as Mean + SEM (*p<.05). (B) Hourly circadian locomotor activity during the second week of prolonged abstinence (week 15) presented as Mean + SEM. Insert: Total activity during week 15 presented as Mean + SEM. (C) Beam breaks during the dark phase of the light cycle presented as Mean + SEM (***p<.001).
During week 2 of abstinence (week 15), a two-way repeated measures ANOVA revealed a significant main effect of hour [F (22,704) = 25.995; p<.001] but neither a main effect of treatment nor a significant interaction between treatment and hour (See Figure 3B).
Circadian Weekly Dark Phase Activity
Across the 13-week treatment phase, a two-way repeated measures ANOVA of average weekly activity during the dark phase of the light cycle revealed significant main effects of treatment [F(2,413)=10.821; p<.001], time [F(6,413) =5.110; p<.001], and a significant interaction between treatment and time [F(12,413) =10.722; p<.001]. Tukey’s post hoc analysis revealed that the HD MP group had greater circadian activity during the dark phase of the light cycle during weeks 1, 2, 3, and 11 compared to the water (p<.001) and LD MP (p<.001) groups. Tukey’s post hoc analysis further revealed that there was no difference between groups during weeks 4 and 8, which were intermittent abstinence periods (See Figure 3C).
During the 5-week abstinence phase, a two-way repeated measures ANOVA revealed a significant main effect of time [F (2, 64) = 1.373; p<0.05] but neither significant main effect of treatment nor a significant interaction between treatment and time (See Figure 3C).
Elevated Plus Maze
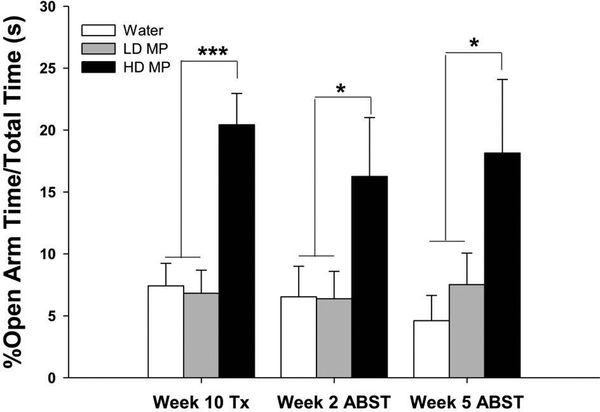
A one-way ANOVA of the percent time spent in the open arms during the treatment phase revealed a significant main effect of treatment [F (2, 68) = 13.484; p<0.001]. Tukey’s post-hoc analysis revealed that the HD group spent more time in the open arms than both the LD group (p<0.001) and the water group (p<0.001) (See Figure 4). During the abstinence phase, a two-way repeated measures ANOVA revealed a significant main effect of treatment [F (2, 63) = 4.827; p<0.05] but neither an effect of time nor an interaction between time and treatment. Tukey’s post hoc analysis revealed that the main effect of treatment was driven by a trend toward a significant difference between the HD MP group and both the water (p = 0.05) and LD MP (p = 0.1) groups (See Figure 4).
Figure 4:
Elevated Plus Maze (n=24 per group). Percent of time spent in open arms relative to total test time (+SEM) shown for testing conducted during week 10 of the treatment phase and weeks 2 and 5 of the abstinence phase. The HD group spent a greater proportion of time in the open arms compared to the LD and water groups during MP treatment and abstinence (***p<0.001).
Forced Swim Test
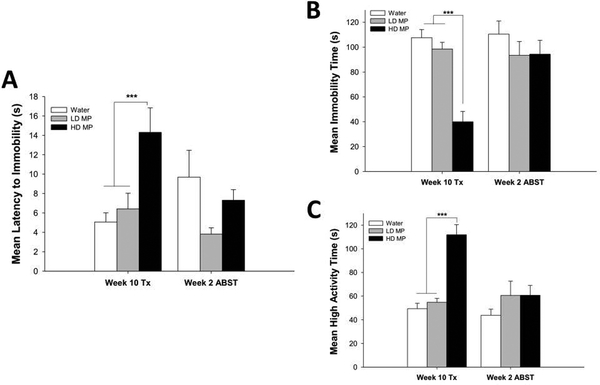
During the treatment phase, a one-way ANOVA of latency to immobility revealed a significant main effect of treatment [F (2, 66) = 7.796; p<0.001]. Tukey’s post-hoc analysis revealed that the HD group displayed greater latency to immobility than both the LD (p<0.01) and water (p<0.001) groups (See Figure 5A). A one-way ANOVA of time spent immobile also revealed a significant main effect of treatment [F (2, 66) = 28.584; p<0.001]. Tukey’s post-hoc analysis revealed that the HD group spent less time immobile than both the LD (p<0.001) and water (p<0.001) groups (See Figure 5B). A one-way ANOVA of time spent displaying high activity revealed a significant main effect of treatment [F (2, 66) = 34.448; p<0.001]. Tukey’s post-hoc analysis revealed that the HD group spent more time in high activity than both the LD (p<0.001) and the water (p<0.001) groups (See Figure 5C). During the abstinence phase, a one-way ANOVA revealed no significant differences between treatment groups for either latency to immobility, time spent immobile or time spent in high activity.
Figure 5:
Forced Swim Test (n=24 per group). (A) Latency to Immobility is shown for testing conducted during week 10 of treatment phase and week 2 of the abstinence phase. During treatment, the HD group had a higher latency to immobility compared to both the LD (p<0.01) and water groups (p<0.001). (B) Immobility time is shown for testing conducted during week 10 of treatment phase and week 2 of the abstinence phase. The HD MP group had a significantly lower time spent immobile than the LD and water groups (p<0.001) during MP treatment. (C) High Activity time is shown for testing conducted during week 10 of treatment phase and week 2 of the abstinence phase. During the MP treatment phase, the HD group spent significantly more time in high activity than both the LD and water groups (p<0.001).
Social Interaction
During MP treatment and during the abstinence phase, there was no significant difference between treatment groups on how much time was spent interacting with the social object (p < .05) (data not shown).
Discussion
The present study examined the developmental and behavioral effects of three consecutive cycles of a three-week daily MP exposure with each cycle followed by a one week abstinence period. We further assessed the reversibility of such effects during a five-week abstinence period after the third treatment cycle. Chronic HD MP decreased body weight and sporadically decreased food consumption during some weeks of treatment. Chronic HD MP exposure also decreased anxiety- and depressive-like effects, increased locomotor and center time effects in the open field, and had no effect on social interaction. The anxiolytic effects persisted after five weeks of prolonged abstinence, while the locomotor and anti-depressive-like effects dropped to control levels following abstinence.
Stimulants, including MP, are known to decrease food consumption and body weight in individuals with and without ADHD (Davis et al., 2007; Elfers and Roth, 2011; Danilovich et al., 2014; Thanos et al., 2015; Diez-Suarez et al., 2017; Robison et al., 2017b; Martin et al., 2018; Carias et al., 2019), and one of the reasons given for choosing to take “drug holidays” among ADHD patients is to ameliorate these effects (Ibrahim and Donyai, 2014). The knowledge from the present study is consistent with the findings in the present previous reports that HD MP exposure generally decreased food consumption and body weight (Thanos et al., 2015; Robison et al., 2017b; Martin et al., 2018; Carias et al., 2019), and provides preclinical evidence that “drug holidays” do not protect against decreases in body weight. The observed decrease in body weight in the HD MP-treated rats when compared to the LD MP-treated rats corroborates previous reports of certain dose-dependent effects of MP (Charles et al., 1978; Cooper et al., 2005; Cheng et al., 2014). Prolonged abstinence did not ameliorate the associated weight loss in animals treated with HD MP, but did lead to a suspected withdrawal-induced weight loss in animals treated with LD MP. Collectively, these findings reveal that chronic HD MP exposure with intermittent abstinence periods decreases body weight while sporadically decreasing food consumption.
Previous studies reported a dose-related locomotor-increasing characteristic of chronic MP exposure (Kuczenski and Segal, 2001; Penner et al., 2001; Yang et al., 2006; Griggs et al., 2010). Indeed, similar to our previous reports following uninterrupted 13-week daily MP exposure (Robison et al., 2017b; Martin et al., 2018) and 13-week daily MP exposure with weekend abstinence periods (Carias et al., 2019), chronic MP consumption increased locomotor activity in the present study. This effect was evident from the increased floor-plane distance, floor plane move time, and center distance in the open field in the HD MP-treated group compared to both LD and water groups during the treatment phase. Notably, the MP-induced increase in locomotor activity during the treatment phase was absent during the intermittent and prolonged abstinence periods. These findings suggest that the locomotor activity-enhancing effects of chronic MP exposure are short-lived and may only be induced in the physiological presence of MP.
Similar to our previous findings following chronic uninterrupted exposure (Robison et al., 2017b; Martin et al., 2018) and chronic exposure with weekend abstinence periods (Carias et al., 2019), we observed attenuated anxiety-like behaviors in the HD compared to both LD and water groups during the treatment phase. This finding corroborates existing evidence that MP administration exerts an anxiolytic effect on pre-pubertal mice (Normand Carrey, 2009), adult mice prenatally exposed to MP (McFadyen-Leussis et al., 2004) and on adult rats (Britton and Bethancourt, 2009). The results across these studies collectively indicate that MP exposure in healthy individuals can exert anxiolytic effects regardless of age, while our present findings suggest that such effects can be dose-dependent. Interestingly, the HD MP treated rats in the present study continued to demonstrate attenuated anxiety-like behaviors during the prolonged abstinence period. This suggests that the anxiolytic effects of chronic MP exposure in healthy subjects may persist following chronic treatment with intermittent abstinence periods.
Previous clinical studies have provided evidence for an anti-depressive effect of MP (Lazarus et al., 1994; Homsi et al., 2001; Kerr et al., 2012; Golubchik et al., 2017). Consistent with such studies, we also observed attenuated depressive-like behaviors in the HD MP-treated animals in the present study. As expected, this effect was eliminated during the prolonged abstinence from the drug, suggesting that the anti-depressive effects of chronic MP exposure in healthy individuals are reversible following prolonged abstinence from the drug.
The reported effects of chronic MP exposure on social interaction in patients with ADHD have been inconsistent. For instance, while one study reported decreased social interaction in boys (Barkley and Cunningham, 1979), multiple studies have associated MP treatment with improved social interaction (Whalen et al., 1987; Smith et al., 1998; Abikoff et al., 2004; Jahromi et al., 2009). Unlike these studies but consistent with our previous report (Robison et al., 2017b), we observed no effect of chronic MP exposure on social interaction in the present study during both treatment and abstinence phases. Therefore, our findings suggest that while chronic MP exposure may ameliorate impaired social behavior in individuals with ADHD (Brown et al., 1984; Smith et al., 1998), the drug had no effect on social behavior in healthy rats.
One limitation to this study that merits acknowledgement is the individual-housing of the animals. While social isolation may lead to altered behavior, in this study single cage housing was necessary to accurately monitor and quantify MP consumption. Future studies in our lab will investigate the impact of chronic MP exposure on group-housed animals.
In conclusion, the present study demonstrates that an altered schedule of chronic MP consumption consistent with real-world use (Ibrahim and Donyai, 2014) attenuates body weight, and anxiety- and depressive-like behaviors but increases locomotor activity and exploratory behaviors. However, while certain effects of MP treatment in healthy individuals may be short-lived, other behavioral and developmental effects may persist even following prolonged cessation of MP treatment. In summary, chronic non-prescribed MP use with intermittent breaks can yield significant developmental and behavioral consequences, which need further evaluation particularly since the rate of non-prescribed MP use continues to rise.
Acknowledgements:
This work was supported by the National Institute of Child Health and Human Development [RO1HD70888].
Footnotes
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abikoff H, Hechtman L, Klein RG, Gallagher R, Fleiss K, Etcovitch J, Cousins L, Greenfield B, Martin D and Pollack S (2004) Social functioning in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry 43:820–829. [DOI] [PubMed] [Google Scholar]
- Barkley RA and Cunningham CE (1979) The effects of methylphenidate on the mother-child interactions of hyperactive children. Arch Gen Psychiatry 36:201–208. [DOI] [PubMed] [Google Scholar]
- Beyer C, Staunton C and Moodley K (2014) The implications of methylphenidate use by healthy medical students and doctors in South Africa. BMC Med Ethics 15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton GB and Bethancourt JA (2009) Characterization of anxiety-related responses in male rats following prolonged exposure to therapeutic doses of oral methylphenidate. Pharmacol Biochem Behav 93:451–459. [DOI] [PubMed] [Google Scholar]
- Brown RT, Wynne ME and Slimmer LW (1984) Attention deficit disorder and the effect of methylphenidate on attention, behavioral, and cardiovascular functioning. J Clin Psychiatry 45:473–476. [PubMed] [Google Scholar]
- Carias E, Fricke D, Vijayashanthar A, Smith L, Somanesan R, Martin C, Kalinowski L, Popoola D, Hadjiargyrou M, Komatsu DE and Thanos PK (2019) Weekday-only chronic oral methylphenidate self-administration in male rats: Reversibility of the behavioral and physiological effects. Behav Brain Res 356:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascade E, Kalali AH, Weisler RH and Lenderts S (2008) Seasonality and the Changing Adult/Child Prescription Ratios in ADHD Therapy. Psychiatry (Edgmont) 5:23–25. [PMC free article] [PubMed] [Google Scholar]
- Charles L, Zelniker T and Schain RJ (1978) Dose Dependent Effects of Methylphenidate on Hyperactive-Children. Pediatr Res 12:369–369. [PubMed] [Google Scholar]
- Cheng J, Xiong Z, Duffney LJ, Wei J, Liu A, Liu S, Chen GJ and Yan Z (2014) Methylphenidate exerts dose-dependent effects on glutamate receptors and behaviors. Biol Psychiatry 76:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Pedroso S, Usala T, Currie J and Gagliano A (2014) Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry 76:603–615. [DOI] [PubMed] [Google Scholar]
- Cooper NJ, Keage H, Hermens D, Williams LM, Debrota D, Clark CR and Gordon E (2005) The dose-dependent effect of methylphenidate on performance, cognition and psychophysiology. Journal of integrative neuroscience 4:123–144. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Mastrandrea LD, Cataldi L and Quattrin T (2014) Methylphenidate decreases fat and carbohydrate intake in obese teenagers. Obesity (Silver Spring) 22:781–785. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Patte K and Kennedy JL (2007) Dopamine transporter gene (DAT1) associated with appetite suppression to methylphenidate in a case-control study of binge eating disorder. Neuropsychopharmacology 32:2199–2206. [DOI] [PubMed] [Google Scholar]
- Diez-Suarez A, Vallejo-Valdivielso M, Marin-Mendez JJ, de Castro-Manglano P and Soutullo CA (2017) Weight, Height, and Body Mass Index in Patients with Attention-Deficit/Hyperactivity Disorder Treated with Methylphenidate. J Child Adolesc Psychopharmacol 27:723–730. [DOI] [PubMed] [Google Scholar]
- Elfers CT and Roth CL (2011) Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Front Endocrinol (Lausanne) 2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J and Robbins TW (1997) Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 131:196–206. [DOI] [PubMed] [Google Scholar]
- Franke AG, Lieb K and Hildt E (2012) What users think about the differences between caffeine and illicit/prescription stimulants for cognitive enhancement. PLoS One 7:e40047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubchik P, Rapaport M and Weizman A (2017) The effect of methylphenidate on anxiety and depression symptoms in patients with Asperger syndrome and comorbid attention deficit/hyperactivity disorder. Int Clin Psychopharmacol 32:289–293. [DOI] [PubMed] [Google Scholar]
- Griggs R, Weir C, Wayman W and Koeltzow TE (2010) Intermittent methylphenidate during adolescent development produces locomotor hyperactivity and an enhanced response to cocaine compared to continuous treatment in rats. Pharmacol Biochem Behav 96:166–174. [DOI] [PubMed] [Google Scholar]
- Homsi J, Nelson KA, Sarhill N, Rybicki L, LeGrand SB, Davis MP and Walsh D (2001) A phase II study of methylphenidate for depression in advanced cancer. Am J Hosp Palliat Care 18:403–407. [DOI] [PubMed] [Google Scholar]
- Ibrahim K and Donyai P (2014) Drug Holidays From ADHD Medication: International Experience Over the Past Four Decades. Journal of Attention Disorders 19:551–568. [DOI] [PubMed] [Google Scholar]
- Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, McDougle CJ, Scahill L, Tierney E, Arnold LE, Vitiello B, Ritz L, Witwer A, Kustan E, Ghuman J and Posey DJ (2009) Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord 39:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-Portilla C, Molina-Jiménez T, Morin J, Roldán-Roldán G and Zepeda RC (2018) Influence of Drugs on Cognitive Functions. Health and Academic Achievement Blandina Bernal-Morales, IntechOpen. [Google Scholar]
- Kerr CW, Drake J, Milch RA, Brazeau DA, Skretny JA, Brazeau GA and Donnelly JP (2012) Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 43:68–77. [DOI] [PubMed] [Google Scholar]
- Konrad-Bindl DS, Gresser U and Richartz BM (2016) Changes in behavior as side effects in methylphenidate treatment: review of the literature. Neuropsych Dis Treat 12:2635–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R and Segal DS (2001) Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther 296:876–883. [PubMed] [Google Scholar]
- Lakhan SE and Kirchgessner A (2012) Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav 2:661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus LW, Moberg PJ, Langsley PR and Lingam VR (1994) Methylphenidate and nortriptyline in the treatment of poststroke depression: a retrospective comparison. Arch Phys Med Rehabil 75:403–406. [DOI] [PubMed] [Google Scholar]
- León KS and Martínez DE (2017) To Study, to Party, or Both? Assessing Risk Factors for Non-Prescribed Stimulant Use among Middle and High School Students. Journal of Psychoactive Drugs 49:22–30. [DOI] [PubMed] [Google Scholar]
- Martin C, Fricke D, Vijayashanthar A, Lowinger C, Koutsomitis D, D. P, Hadjiargyrou M, Komatsu DE and Thanos PK (2018) Recovery from behavior and developmental effects of chronic oral methylphenidate following an abstinence period. Pharmacology, Biochemistry and Behavior 172:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM and Rohde LA (2004) Weekend Holidays During Methylphenidate Use in ADHD Children: A Randomized Clinical Trial. Journal of Child and Adolescent Psychopharmacology 14:195–206. [DOI] [PubMed] [Google Scholar]
- McFadyen-Leussis MP, Lewis SP, Bond TL, Carrey N and Brown RE (2004) Prenatal exposure to methylphenidate hydrochloride decreases anxiety and increases exploration in mice. Pharmacol Biochem Behav 77:491–500. [DOI] [PubMed] [Google Scholar]
- Normand Carrey MPM, and Richard E. Brown (2009) Effects of Subchronic Methylphenidate Hydrochloride Administration on the Locomotor and Exploratory Behavior of Prepubertal Mice. Journal of Child and Adolescent Psychopharmacology 10:277–286. [DOI] [PubMed] [Google Scholar]
- Peloquin LJ and Klorman R (1986) Effects of Methylphenidate on Normal Childrens Mood, Event-Related Potentials, and Performance in Memory Scanning and Vigilance. J Abnorm Psychol 95:88–98. [DOI] [PubMed] [Google Scholar]
- Penner MR, McFadyen MP, Carrey N and Brown RE (2001) Effects of chronic and acute methylphenidate hydrochloride (Ritalin) administration on locomotor activity, ultrasonic vocalizations, and neuromotor development in 3- to 11-day-old CD-1 mouse pups. Dev Psychobiol 39:216–228. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Ogden CL, Simoyan OM, Chung DY, Caggiano JF, Nichols SD and McCall KL (2018) Trends in use of prescription stimulants in the United States and Territories, 2006 to 2016. PloS one 13:e0206100–e0206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S (2007) Practice Parameter for the Assessment and Treatment of Children and Adolescents With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry 46:894–921. [DOI] [PubMed] [Google Scholar]
- Robison LS, Ananth M, Hadjiargyrou M, Komatsu DE and Thanos PK (2017a) Chronic Oral Methylphenidate Treatment Reversibly Increases Striatal Dopamine Transporter and Dopamine Type 1 Receptor Binding in Rats. Journal of neural transmission (Vienna, Austria : 1996) 124:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LS, Michaelos M, Gandhi J, Fricke D, Miao E, Lam CY, Mauceri A, Vitale M, Lee J, Paeng S, Komatsu DE, Hadjiargyrou M and Thanos PK (2017b) Sex Differences in the Physiological and Behavioral Effects of Chronic Oral Methylphenidate Treatment in Rats. Front Behav Neurosci 11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu Y-C, Lee S-Y, Yuan S-S, Yang C-J, Yang K-C, Lee T-L and Wang L-J (2016) Seasonal Patterns of Medications for Treating Attention-Deficit/Hyperactivity Disorder: Comparison of Methylphenidate and Atomoxetine. Clinical Therapeutics 38:595–602. [DOI] [PubMed] [Google Scholar]
- Smith BH, Pelham WE, Evans S, Gnagy E, Molina B, Bukstein O, Greiner A, Myak C, Presnell M and Willoughby M (1998) Dosage effects of methylphenidate on the social behavior of adolescents diagnosed with attention-deficit hyperactivity disorder. Exp Clin Psychopharmacol 6:187–204. [DOI] [PubMed] [Google Scholar]
- Sobanski E, Schredl M, Kettler N and Alm B (2008) Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: A controlled polysomnographic study. Sleep 31:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, Rosendal S, Groth C, Magnusson FL, Moreira‐Maia CR and et al. (2015) Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA and Boyd CJ (2006) Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy 26:1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Robison LS, Steier J, Hwang YF, Cooper T, Swanson JM, Komatsu DE, Hadjiargyrou M and Volkow ND (2015) A pharmacokinetic model of oral methylphenidate in the rat and effects on behavior. Pharmacol Biochem Behav 131:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin SMZ, Robison LS, Fricke D, Chernoff E, Hadjiargyrou M, Thanos PK and Komatsu DE (2018) Methylphenidate regulation of osteoclasts in a dose- and sex-dependent manner adversely affects skeletal mechanical integrity. Scientific Reports 8:1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban KR and Gao WJ (2017) Psychostimulants As Cognitive Enhancers in Adolescents: More Risk than Reward? Front Public Health 5:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH and Gabrieli JD (1998) Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A 95:14494–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo-Neus GHH, Rommelse N and Buitelaar JK (2011) To stop or not to stop? How long should medication treatment of attention-deficit hyperactivity disorder be extended? European Neuropsychopharmacology 21:584–599. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Ding Y and Gatley SJ (2002) Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord 6 Suppl 1:S31–43. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Henker B, Swanson JM, Granger D, Kliewer W and Spencer J (1987) Natural social behaviors in hyperactive children: dose effects of methylphenidate. J Consult Clin Psychol 55:187–193. [DOI] [PubMed] [Google Scholar]
- White BP, Becker-Blease KA and Grace-Bishop K (2006) Stimulant medication use, misuse, and abuse in an undergraduate and graduate student sample. J Am Coll Health 54:261–268. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC and Dafny N (2006) Chronic methylphenidate modulates locomotor activity and sensory evoked responses in the VTA and NAc of freely behaving rats. Neuropharmacology 51:546–556. [DOI] [PubMed] [Google Scholar]