Abstract
Post-traumatic patellofemoral osteoarthritis is prevalent after anterior cruciate ligament reconstruction (ACLR) and early cartilage degradation may be especially common in the femoral trochlear cartilage. Determining the presence of and factors associated with early femoral trochlear cartilage degradation, a precursor to osteoarthritis, is a critical preliminary step in identifying those at risk for patellofemoral osteoarthritis development and designing interventions to combat the disease. Early cartilage degradation can be detected using quantitative magnetic resonance imaging measures, such as tissue T2 relaxation time. The purposes of this study were to 1) compare involved (ACLR) versus uninvolved (contralateral) femoral trochlear cartilage T2 relaxation times 6 months after ACLR, and 2) determine the relationship between walking speed and walking mechanics 3 months after ACLR and femoral trochlear cartilage T2 relaxation times 6 months after ACLR. Twenty-six individuals (age 23±7 years) after primary, unilateral ACLR participated in detailed motion analyses 3.3±0.6 months after ACLR and quantitative magnetic resonance imaging 6.3±0.5 months after ACLR. There were no between limb differences in femoral trochlear cartilage T2 relaxation times. Slower walking speed was related to higher (worse) femoral trochlear cartilage T2 relaxation times in the involved limb (Pearson r: −.583, p=.002) and greater interlimb differences in trochlear T2 relaxation times (Pearson r: −.349, p=.080). Walking mechanics were weakly related to trochlear T2 relaxation times.
Keywords: anterior cruciate ligament reconstruction (ACLR), gait mechanics, magnetic resonance imaging (MRI), patellofemoral osteoarthritis, trochlear cartilage
Introduction
Post-traumatic patellofemoral osteoarthritis (OA) exists in at least one-third of individuals 10 years after anterior cruciate ligament reconstruction (ACLR).1 While many have assessed the tibiofemoral compartments for preliminary manifestations of OA development after ACLR, recent reports suggest that early signs of OA may be more common in the patellofemoral joint compared to the tibiofemoral compartments.2 Moreover, features of patellofemoral OA, but not tibiofemoral OA, one year after ACLR predict symptoms and quality of life two years later.3 Identifying potentially modifiable factors related to early patellofemoral OA pathogenesis is a critical early step to develop targeted interventions and rehabilitation strategies to combat this disease.
One potentially modifiable factor that may be related to post-traumatic OA development is aberrant walking mechanics.4–9 Most studies investigating the association between walking mechanics and post-traumatic OA, however, have examined the tibiofemoral joint, rather than the patellofemoral joint. These studies have found that unloading the tibiofemoral joint, especially its medial compartment, early after ACLR is associated with future tibiofemoral OA.7–10 One study by Culvenor et al. found that those with established, radiographic patellofemoral OA approximately nine years after ACLR walked with altered mechanics at that time.11 The altered walking mechanics may have been a result of the patellofemoral OA, rather than its cause. Among those with idiopathic patellofemoral OA and no history of ACLR, Teng et al. found that sagittal plane knee kinetics (knee flexion moment and impulse) during gait were correlated to patellofemoral joint degeneration as measured using quantitative magnetic resonance imaging (MRI) measures (i.e., T1rho and T2 relaxation times) in both the patellar and femoral trochlear cartilage.12 Investigations performed early after ACLR using precursors to symptomatic or radiographic OA are needed to determine the relationship between walking mechanics early after ACLR and post-traumatic patellofemoral OA development and progression.
One sensitive precursor to radiographic or clinical OA is cartilage T2 relaxation time. Cartilage T2 relaxation time mapping is a quantitative MRI measure that may be an early indicator of OA.12–15 Higher T2 relaxation times may indicate higher water content and greater cartilage collagen matrix degradation,12 a precursor to OA. Cartilage T2 relaxation times may detect early post-traumatic cartilage degradation at a time-frame when patients are still in rehabilitation and are perhaps more amenable to change. While there are several articular cartilage regions that may be analyzed within the knee (e.g., weight-bearing femur and tibia, patella, femoral trochlea, etc.), early degenerative changes (i.e., bone marrow lesions, cartilage lesions, and osteophytes) may be most common in the femoral trochlear cartilage,2 and cartilage thinning in the femoral trochlea is common after ACLR.16
Following ACLR, patients walk with smaller knee flexion-extension excursion and peak flexion, while also demonstrating lower knee flexion moments, in the involved compared to contralateral knee.17–20 These altered mechanics likely influence the direction and magnitude of the forces exerted on the patellofemoral cartilage during walking. Knee flexion angles and quadriceps muscle forces are correlated positively to patellofemoral joint contact force,21 influencing patellofemoral cartilage homeostasis. Key biomechanical variables of interest include sagittal plane knee kinematics (peak knee flexion angle and knee flexion and extension excursions), sagittal plane knee kinetics (peak knee flexion moment), and quadriceps muscle forces. Walking speed, the “sixth vital sign,”22 is an important indicator of function22 that influences walking mechanics23,24 and also merits consideration as a key predictor of cartilage health.
The purpose of this study was to explore early patellofemoral OA development after ACLR by investigating the relationship between walking mechanics 3 months after ACLR and femoral trochlear cartilage T2 relaxation times 6 months after ACLR. Specifically, the study aimed to 1) compare femoral trochlear cartilage T2 relaxation times between the involved (ACLR) and uninvolved (contralateral) limb 6 months after ACLR; 2) determine the relationship between walking speed 3 months after ACLR and femoral trochlear cartilage T2 relaxation times 6 months after ACLR; and 3) determine the relationship between walking mechanics 3 months after ACLR and femoral trochlear T2 relaxation times 6 months after ACLR. We hypothesized that 1) femoral trochlear cartilage T2 relaxation times would be higher (worse) in the involved limb compared to the uninvolved limb, 2) slower walking speeds would be related to higher (worse) femoral trochlear cartilage T2 relaxation times in the involved limb, and 3) walking mechanics would be related to femoral trochlear cartilage T2 relaxation times.
Methods
Participants
This is a prospective, analytic cohort study (level of evidence: 2). Twenty-six participants (Table 1) from an ongoing, prospective cohort study (R01-HD087459) were included in this study. All participants who had completed motion analysis (biomechanical) testing 3 months after ACLR and quantitative MRI acquisitions (using the same parameters) 6 months after ACLR by April 2019 were included. Participants also met the following inclusion/exclusion criteria: age 16–45 years, primary ACLR with no prior history of ACL injury or surgery to either knee, no other serious leg surgery, no concomitant grade III knee ligament sprains, no repairable meniscus injury, and no contraindications for MRI (i.e., metallic implants or components, extreme claustrophobia, pacemaker, metal in the body, aneurysm clip, or ear or eye implants).
Table 1.
Demographic characteristics of the participants 3 months after ACLR (n = 26).
| Variable | Mean ± Standard Deviation (SD) or Number (%) |
|---|---|
| Age | 23 ± 7 years |
| Sex | 10 women (38.5%), 16 men (61.5%) |
| Height | 1.74 ± 0.08 meters |
| Weight | 81 ± 14 kilograms |
| Meniscus Involvement | 3 partial lateral meniscectomy, 4 partial medial meniscectomy, 1 total medial meniscectomy, 2 medial and lateral partial meniscectomies, 11 none, 5 unknown/not reported |
| Graft Type | 5 (19.2%) soft-tissue allograft*, 13 (50.0%) bone-patellar tendon-bone autograft, 5 (19.2%) hamstring autograft, 3 (11.5%) other/unknown |
| Walking speed | 1.61 ± .15 meters/second |
Note: of the five soft-tissue allografts, there was one using posterior tibialis tendon with extra-articular anterolateral ligament (ALL) reconstruction using gracilis allograft; the other four were unknown.
The study was performed between June 2016 and April 2019 at the University of Delaware, which granted Institutional Review Board approval. All participants provided written informed consent; parental consent and minor assent were provided for all individuals under age 18 years at enrollment.
Walking Mechanics
All participants completed motion analysis testing of over-ground walking approximately 3 months (3.3 ± 0.6 months) after ACLR. Prior to walking, electromyography (EMG) electrodes (MA-300 EMG System, Motion Lab Systems, Baton Rouge, LA) were placed on 7 muscles (medial and lateral gastrocnemii, medial and lateral hamstrings, vastus medialis, vastus lateralis, and rectus femoris) on each lower extremity after shaving, cleaning, and abrading the skin. Participants completed maximal volitional isometric contractions (MVICs) for each muscle group (gastrocnemii, hamstrings, and quadriceps),25 and the highest physiological values for each muscle group during any trial were used for EMG normalization. EMG data were collected at 1080 Hz; EMG post-processing included a high-pass, 2nd order Butterworth filter at 30 Hz, rectification, and a low-pass filter at 6 Hz to create a linear envelope. Following EMG placement and MVICs, 39 retroreflective markers were placed on the bilateral lower extremities and pelvis.26 Participants then walked over an embedded force platform (Bertec Corporation, Columbus, OH) in the motion analysis laboratory while kinetic data (1080 Hz) and kinematic data (120 Hz) were captured using an 8 camera motion analysis system (VICON, Oxford, UK). Participants walked at a self-selected speed; once participants walked at a consistent speed (typically within 5 practice trials), walking trials were recorded for analysis and only trials with walking speeds that were within ± 5% of one another were used in the analyses.
Commercial software (Visual3D, C-Motion, Germantown, MD) was used to calculate kinematic and kinetic variables.25 A validated,27 EMG-driven, patient-specific musculoskeletal model, described previously in detail,28 was used to estimate quadriceps muscle forces during gait. Biomechanical data were normalized to the stance phase of gait for each participant and averaged across 3 trials per limb. Kinetic variables were normalized by mass*height (kg*m) and quadriceps muscle forces were normalized by body weight (BW).29
Biomechanical variables of interest included walking speed, peak knee flexion angle (PKFA) and moment, knee flexion excursion during weight acceptance (i.e., the loading response phase of gait from initial contact to PKFA, approximately 0–25% of stance),30 knee extension excursion during the midstance phase of gait (occurring from PKFA to peak knee extension angle),30 and peak quadriceps muscle forces during gait (constrained to the first 50% of stance phase).
Femoral Trochlear Cartilage T2 Relaxation Times
All participants also underwent supine bilateral knee imaging using a 3 Tesla, Siemens MRI scanner (Washington, D.C.) approximately 6 months (6.3 ± 0.5 months) after ACLR using a 2-dimensional sagittal T2 mapping sequence. The scan parameters for all participants were: scan duration = 7 minutes and 17 seconds, field of view = 150 mm, resolution = 0.6 mm/pixel, slice thickness = 3 mm, repetition time = 3090 ms, echo time = 10, 20, 30, 40, 50, 60, and 70 ms.
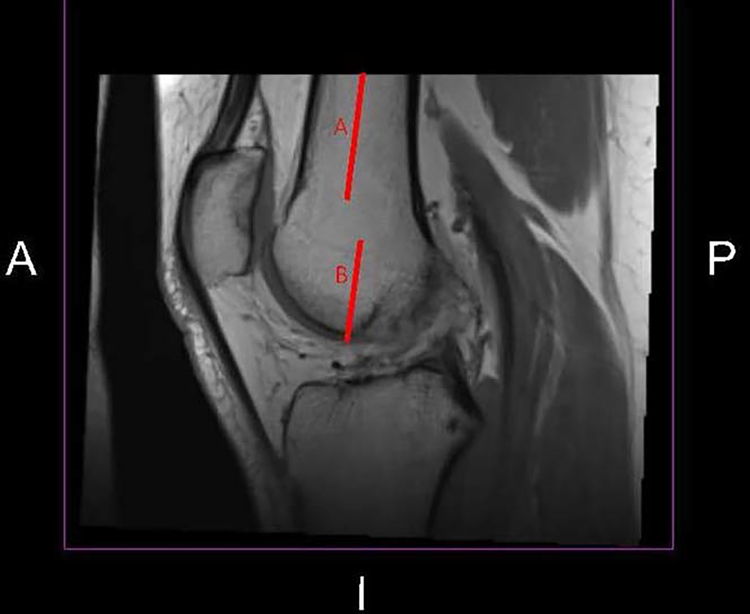
T2 relaxation maps were calculated using mono-exponential fitting on a pixel-by-pixel basis (3DSlicer, National Institutes of Health [NIH])31,32 after registering each individual’s involved knee to the uninvolved knee.33 The first echo was skipped to reduce stimulated echo artifacts.13 The entire trochlear cartilage for each knee (involved and uninvolved) of every participant was manually segmented at 3mm increments by one reader (LD). The trochlea was subdivided into medial and lateral regions of interest, with the medial trochlea defined as the deepest aspect of the trochlear sulcus and all cartilage medial to it; the lateral trochlear cartilage consisted of all trochlear cartilage lateral to the sulcus. The inferior boundary of the trochlea was defined as the anterior aspect of the visible meniscus34–36 or the line parallel to the shaft of the femur through the trochlear notch (when neither meniscus was visible, Figure 1).37,38 The medial, lateral, and superior boundaries of the trochlea corresponded to the edge of the cartilage. Mean T2 relaxation time values for the total, medial, and lateral trochlea were calculated using a volume-weighted mean.
Figure 1.
This figure illustrates the inferior boundary of the trochlear cartilage in MRI slices when neither meniscus was visible (centrally) on the image. The top red line (A) represents a line parallel to the shaft of the femur, while the inferior red line (B) is the line parallel to the shaft of the femur through the trochlear notch.37,38 Femoral cartilage antero-superior to the inferior aspect of this line (B) was defined as trochlear cartilage in images where neither meniscus was visible. Image produced in 3DSlicer (National Institutes of Health [NIH]).31,32 (Abbreviations: A, anterior; I, inferior; P, posterior.)
Trochlear T2 Relaxation Time Reliability Analyses
We established inter- and intra-rater reliability using intraclass correlation coefficients (ICC). For inter-rater reliability, two authors (JJC and LD) each analyzed 16 knees. Inter-rater reliability was excellent for the total (ICC = .94), medial (ICC = .94) and lateral (ICC = .98) femoral trochlear cartilage T2 relaxation times. For intra-rater reliability, the author (LD) who performed all trochlear cartilage T2 relaxation time segmentations for this study completed 10 additional knees twice. These 10 knees were selected randomly by the first author (JJC) using a random number generator, then coded so that the reader (LD) was blinded to participant. We established excellent intra-rater reliability for the total (ICC = .97), medial (ICC = .96), and lateral (ICC = .97) trochlear cartilage T2 relaxation times.
Statistical Analysis
Paired sample t-tests were used to compare femoral trochlear cartilage T2 relaxation times (total, medial, and lateral) between the involved and uninvolved limbs of each participant. Pearson correlation and simple linear regression were used to determine the relationship between each biomechanical predictor variable and the total trochlear T2 relaxation time. Alpha was set at 0.05 for all analyses. Due to the exploratory nature of the study, no statistical adjustments were made for multiple comparisons.39 Statistical analyses were performed using Microsoft Excel (Redmond, WA) and SPSS version 25.0 (IBM Corporation, Armonk, New York, USA). R2 effect sizes were described as small (R2 > .0196), medium (R2 > .13), and large (R2 > .26).40
Results
Results for Hypothesis 1: Trochlear Cartilage T2 Relaxation Times in the Involved vs. Uninvolved Limb
There were no differences between the involved limb and the uninvolved limb for the total, medial, or lateral femoral trochlear T2 relaxation times 6 months after ACLR (Table 2).
Table 2.
There were no between limb differences in total, medial, or lateral femoral trochlear T2 relaxation times 6 months after ACLR.
| Measure | Involved | Uninvolved | P-Value |
|---|---|---|---|
| Total Trochlear T2 Relaxation Time (ms) | 47.9 ± 3.1 | 47.9 ± 3.2 | .993 |
| Medial Trochlear T2 Relaxation Time (ms) | 47.9 ± 3.7 | 47.6 ± 4.5 | .732 |
| Lateral Trochlear T2 Relaxation Time (ms) | 48.0 ± 3.5 | 48.0 ± 4.2 | .942 |
Results for Hypothesis 2: Walking Speed and Trochlear Cartilage T2 Relaxation Times
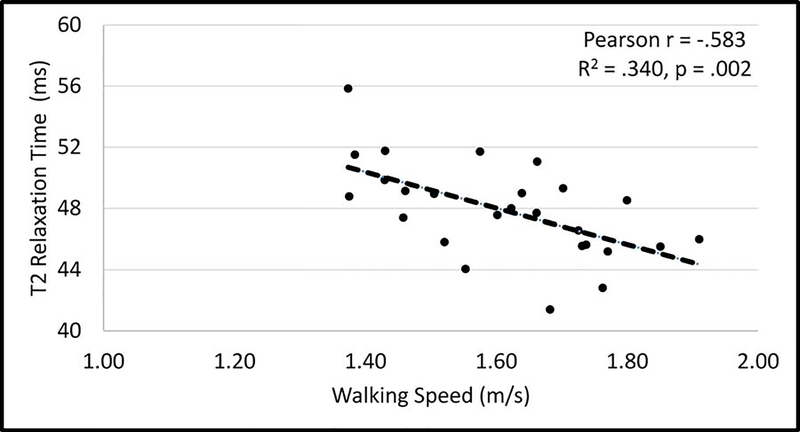
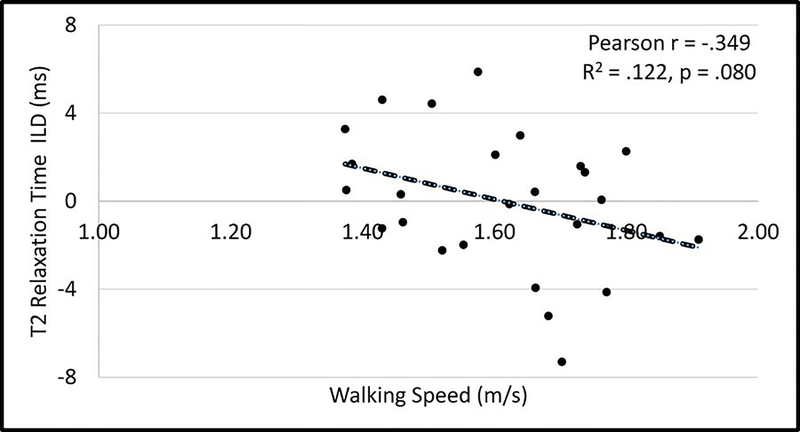
Slower walking speed was the strongest, significant predictor of higher (worse) femoral cartilage T2 relaxation time in the involved limb (Figure 2, Pearson r = −.583, p = .002), but not in the uninvolved limb (Pearson r = −.226, p = .267). Walking speed tended to be related to the interlimb difference (ILD: involved minus uninvolved limb) in femoral trochlear T2 relaxation times (Figure 3, Pearson r = −.349, p = .080). There was a large effect40 for the relationship between walking speed and femoral trochlear cartilage T2 relaxation time in the involved limb (R2 = .340) and a small-to-medium effect40 for the relationship between walking speed and femoral trochlear T2 relaxation time ILDs (R2 = .122). A strong, significant negative relationship remained in a secondary analyses removing the 2 participants with involved limb femoral cartilage trochlear T2 relaxation times that were more than 2 standard deviations from the mean (Supplemental Figure).
Figure 2.
Relationship between walking speed and involved limb femoral trochlear cartilage T2 relaxation time.
Figure 3.
Relationship between walking speed and interlimb differences (ILD: involved minus uninvolved) in femoral trochlear cartilage T2 relaxation times.
Results for Hypothesis 3: Walking Mechanics and Trochlear Cartilage T2 Relaxation Times
There were no statistically significant relationships between walking mechanics and femoral trochlear cartilage T2 relaxation times in the involved limb (Table 3) or in the uninvolved limb (Table 4). There were, however, small effects40 for the relationships between knee flexion and extension excursion and trochlear T2 relaxation times in both the involved limb and in the uninvolved limb. A small effect40 was observed for peak quadriceps muscle forces and trochlear T2 relaxation time in the involved limb only. There were no statistically significant relationships between interlimb differences (ILDs) in walking mechanics and ILDs in trochlear T2 relaxation times (Table 5). The relationship between the ILD for knee extension excursion and the ILD for trochlear T2 relaxation time had a small effect.40
Table 3.
Relationship between walking mechanics and femoral trochlear cartilage T2 relaxation times in the involved limb.
| Walking Mechanics Variable (Involved Limb) | Pearson r | Effect Size (R2) | P-value |
|---|---|---|---|
| Peak Knee Flexion Angle | .027 | <.001 | .898 |
| Knee Flexion Excursion | −.282 | .080 | .162 |
| Knee Extension Excursion | −.129 | .021 | .529 |
| Peak Knee Flexion Moment | −.043 | .002 | .835 |
| Peak Quadriceps Muscle Forces | −.272 | .074 | .221 |
Table 4.
Relationship between walking mechanics and femoral trochlear cartilage T2 relaxation times in the uninvolved limb.
| Walking Mechanics Variable (Uninvolved Limb) | Pearson r | Effect Size (R2) | P-value |
|---|---|---|---|
| Peak Knee Flexion Angle | −.094 | .009 | .649 |
| Knee Flexion Excursion | −.349 | .122 | .081 |
| Knee Extension Excursion | −.314 | .099 | .118 |
| Peak Knee Flexion Moment | −.102 | .010 | .620 |
| Peak Quadriceps Muscle Forces | −.039 | .002 | .864 |
Table 5.
Relationship between interlimb differences (ILDs) in walking mechanics and ILDs in femoral trochlear cartilage T2 relaxation times.
| Walking Mechanics Variable (ILD) | Pearson r | Effect Size (R2) | P-value |
|---|---|---|---|
| Peak Knee Flexion Angle | −.026 | .001 | .901 |
| Knee Flexion Excursion | −.129 | .017 | .530 |
| Knee Extension Excursion | −.207 | .043 | .310 |
| Peak Knee Flexion Moment | −.031 | .001 | .881 |
| Peak Quadriceps Muscle Forces | −.107 | .011 | .636 |
Discussion
The purpose of this study was to determine the relationship between walking mechanics and potential markers of early patellofemoral OA (cartilage collagen matrix degradation), assessed using femoral trochlear cartilage T2 relaxation times 6 months after ACLR. The first hypothesis, that trochlear cartilage T2 relaxation times would be higher (worse) in the involved limb compared to the uninvolved limb, was not supported. The second hypothesis, that slower walking speeds would be related to higher (worse) trochlear cartilage T2 relaxation times in the involved limb, was supported. The third hypothesis, that walking mechanics would be related to trochlear cartilage T2 relaxation times, was partially supported. Our findings suggest slower walking speed 3 months after ACLR is a strong predictor of femoral trochlear cartilage degradation 6 months after ACLR. Aberrant walking mechanics, especially truncated sagittal plane knee excursions and perhaps smaller quadriceps muscle forces, may also be weakly related to femoral trochlear cartilage degradation; however, this study was not able to confirm this. The findings of this exploratory analysis suggest slower walking speed and perhaps aberrant sagittal plane walking mechanics are related to early trochlear cartilage degradation, a precursor of patellofemoral OA, 6 months after ACLR.
To our knowledge, this is the first study to investigate the relationship between walking mechanics and quantitatively assessed patellofemoral cartilage degradation among individuals early after ACLR. While the study was exploratory in nature, our finding that walking speed was such a strong40 predictor of trochlear cartilage health adds to evidence supporting the use of walking speed as a functional vital sign.22 Slower self-selected walking speed has previously been associated with more severe OA among older adults with knee OA41 and with more severe pathology among those with articular cartilage defects.42 Slower walking speed has also been associated with greater serum biomarkers of cartilage breakdown (collagen type II cleavage product) among individuals after ACLR.43 Walking speed is easy to measure clinically and monitor over time. The present study suggests that evaluating walking speed during rehabilitation after ACLR may help clinicians identify patients who are at greater risk for developing patellofemoral OA.
Walking speed was strongly and significantly correlated to femoral trochlear cartilage T2 relaxation times in the involved limb but only weakly and non-significantly correlated to femoral trochlear T2 relaxation times in the uninvolved limb. Given that cartilage breakdown and OA are much more likely to develop in the involved than uninvolved limb, cartilage in the involved limb may be more susceptible to degradation and more strongly influenced by gait biomechanics. Alternatively, underlying, preliminary cartilage degradation in the involved limb could be influencing walking speed or some combination thereof.
Despite the prospective nature of the present study, cause and effect cannot be determined. Participants who walked more slowly 3 months after ACLR may have done so due to more severe underlying knee pathology or preclinical knee OA, although we did control for severity of initial injury and excluded people with previous or concomitant lower extremity injury/surgery. Alternatively, walking more slowly, coupled with other associated biomechanical changes,24,41,44 may contribute to the pathogenesis of patellofemoral OA by changing the direction, magnitude, and duration of forces (thereby also altering pressure) acting on the patellofemoral articular cartilage.45–47 Given that lower medial tibiofemoral joint contact forces are associated with tibiofemoral OA after ACLR,7–10 it is not surprising that slower walking speeds and, to a lesser degree, reduced knee excursions and lower quadriceps muscle forces—which collectively cause lower patellofemoral joint contact forces21,41—were related to femoral trochlear cartilage degradation after ACLR. Cartilage responds to cyclic loading and may need an appropriate loading stimulus to maintain homeostasis.
While the present study is unable to determine whether slower walking drives cartilage degradation or vice versa, it does inform future study designs and clinical practice. We cannot conclude from this study that walking faster will prevent patellofemoral OA development after ACLR, but future studies could explore this idea. Our study does, however, inform who may be at high risk for subsequent patellofemoral OA: those who walked more slowly early after ACLR were more likely to have higher trochlear cartilage T2 relaxation times, a sensitive precursor to established or symptomatic OA.12–15,48,49 Simply put, while instructing patients to “walk faster” after ACLR may not prevent patellofemoral OA (and could potentially even exacerbate symptoms), clinicians may identify patients who walk more slowly as having greater risk for post-traumatic patellofemoral OA.
Our study indicates femoral trochlear cartilage T2 relaxation times may not be higher among individuals 6 months after ACLR. Kim et al. recently found that femoral trochlea T2 relaxation times were significantly higher in the involved limb compared to the uninvolved limb among individuals who were 3 years (rather than 6 months) after ACLR.50 The study by Kim et al. also differed from the present study in that the participants were older (mean 34 years vs. mean 23 years) and the trochlear cartilage of each knee was subdivided into superficial and deep sub-regions within medial, lateral, and central trochlear cartilage regions of interest.50 Cartilage degradation (detectable via T2 relaxation time) may occur in only a subset of individuals 6 months after ACLR.
The present study has cartilage T2 relaxation times and walking speeds that fall within the range found in the literature.13,17,19,20,37,50–55 Cartilage T2 relaxation time values, however, vary greatly in the literature, from approximately 23 milliseconds in the deep layers of the medial femoral cartilage of ACL-injured knees at baseline54 to higher than 60 milliseconds in the superficial central trochlear cartilage 3 years after ACLR50. T2 relaxation time values are typically higher in the patellar and trochlear cartilage than the weight-bearing tibiofemoral cartilage.37,53 Walking speeds also vary both within and across studies, and our mean walking speed (1.61 m/s) is very similar to previous studies in separate cohorts from our lab (means of 1.5 to 1.6 m/s),19,20,51 albeit similar to or faster than other cohorts.17 Our study sample was young and free of any serious previous or concomitant injury.
There are limitations to consider when interpreting the results of this study. Individuals with multiple graft types were included. A bone-pateller tendon-bone autograft may present image segmentation challenges for certain regions of the patellar cartilage; however, this does not impact segmentation of the trochlear cartilage across graft types. The study is also exploratory in nature and limited in sample size (26 participants). Only one region of interest (femoral trochlea) and one quantitative MRI variable (T2 relaxation time) were used in the present study. Patellofemoral joint contact forces were not evaluated. Future studies should calculate patefellomoral joint contact forces and examine multiple quantitative and semi-quantitative precursors to clinical OA in both the patellar and trochlear cartilage of the patellofemoral joint, in addition to long-term radiographic follow-up. Longitudinal followup and controlling for intra-operative findings (e.g., arthroscopic cartilage or meniscus findings) would also strengthen future studies.
In conclusion, our findings suggest slow walking speeds 3 months after ACLR were strongly related to higher (worse) femoral trochlear cartilage T2 relaxation times 6 months after ACLR. Altered gait biomechanics, including truncated knee excursions and lower quadriceps muscle forces, may also be weakly related to higher femoral trochlear cartilage T2 relaxation times. Slower walking speed was by far the strongest predictor of femoral trochlear cartilage degradation, suggesting walking speed may be an early clinical indicator of future patellofemoral OA after ACLR.
Supplementary Material
Supplemental Figure. Relationship between walking speed and involved limb femoral trochlear cartilage T2 relaxation time after removing 2 participants whose involved knee trochlear cartilage T2 values were more than 2 standard deviations from the mean.
Statement of Clinical Significance:
Slower walking speed was by far the strongest predictor of worse femoral trochlear cartilage health, suggesting slow walking speed may be an early clinical indicator of future patellofemoral osteoarthritis after ACLR.
Acknowledgments
Funding was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of General Medical Sciences: R01-HD087459, F30-HD096830, R37-HD037985, T32-HD007490, and U54-GM104941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JJC received funding from the University of Delaware: University Doctoral Fellowship Award and University of Dissertation Fellowship Award. JJC’s work was supported in part by Promotion of Doctoral Studies (PODS) - Level I and Level II Scholarships from the Foundation for Physical Therapy Research. Thank you to the National Institutes of Health (NIH); the Foundation for Physical Therapy Research; Martha Callahan and the Delaware Rehabilitation Institute Research Core; Angela H. Smith and the University of Delaware Physical Therapy Clinic; and Bryn Bonner for her assistance with data processing.
Footnotes
Disclosure Statement: No competing financial interests exist for any of the authors.
References
- 1.Culvenor AG, Cook JL, Collins NJ, Crossley KM. 2013. Is patellofemoral joint osteoarthritis an under-recognised outcome of anterior cruciate ligament reconstruction? A narrative literature review. Br. J. Sports Med 47(2):66–70 Available from: http://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2012-091490. [DOI] [PubMed] [Google Scholar]
- 2.Culvenor AG, Collins NJ, Guermazi A, et al. 2015. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol. 67(4):946–55. [DOI] [PubMed] [Google Scholar]
- 3.Culvenor AG, Collins NJ, Guermazi A, et al. 2016. Early Patellofemoral Osteoarthritis Features One Year after Anterior Cruciate Ligament Reconstruction: Symptoms and Quality of Life at Three Years. Arthritis Care Res. 68(6):784–792. [DOI] [PubMed] [Google Scholar]
- 4.Morgenroth DC, Medverd JR, Seyedali M, Czerniecki JM. 2014. The relationship between knee joint loading rate during walking and degenerative changes on magnetic resonance imaging. Clin. Biomech. (Bristol, Avon) 29(6):664–70 Available from: http://www.sciencedirect.com/science/article/pii/S026800331400093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari AMW, Briant PL, Bevill SL, et al. 2008. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med. Sci. Sports Exerc 40(2):215–222. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Kothari A, Souza RB, et al. 2014. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: A pilot study. Knee 21(5):881–885 Available from: 10.1016/j.knee.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellsandt E, Gardinier ES, Manal K, et al. 2016. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am. J. Sports Med 44(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrosimone B, Loeser RF, Blackburn JT, et al. 2017. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J. Orthop. Res 35(10):2288–2297 Available from: http://doi.wiley.com/10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxby DJ, Bryant AL, van Ginckel A, et al. 2019. Greater magnitude tibiofemoral contact forces are associated with reduced prevalence of osteochondral pathologies 2–3 years following anterior cruciate ligament reconstruction. Knee Surgery, Sport. Traumatol. Arthrosc 27(3):707–715 Available from: 10.1007/s00167-018-5006-3. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer SJ, Spang J, Nissman D, et al. 2019. Gait Mechanics and T1ρ MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction. Med. Sci. Sports Exerc 51(4):630–639. [DOI] [PubMed] [Google Scholar]
- 11.Culvenor AG, Schache AG, Vicenzino B, et al. 2014. Are knee biomechanics different in those with and without patellofemoral osteoarthritis after anterior cruciate ligament reconstruction? Arthritis Care Res. 66(10):1566–1570. [DOI] [PubMed] [Google Scholar]
- 12.Teng HL, Calixto NE, MacLeod TD, et al. 2016. Associations between patellofemoral joint cartilage T1ρ and T2 and knee flexion moment and impulse during gait in individuals with and without patellofemoral joint osteoarthritis. Osteoarthr. Cartil 24(9):1554–1564 Available from: 10.1016/j.joca.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum T, Stehling C, Joseph GB, et al. 2012. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: Data from the osteoarthritis initiative. J. Magn. Reson. Imaging 35(2):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum T, Joseph GB, Karampinos DC, et al. 2013. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr. Cartil 21(10):1474–1484 Available from: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckstein F, Guermazi A, Gold G, et al. 2014. Imaging of cartilage and bone: Promises and pitfalls in clinical trials of osteoarthritis. Osteoarthr. Cartil 22(10):1516–1532 Available from: 10.1016/j.joca.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frobell RB. 2011. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: A two-year prospective MRI study of sixty-one subjects. J. Bone Jt. Surg. - Ser. A 93(12):1096–1103. [DOI] [PubMed] [Google Scholar]
- 17.Kaur M, Ribeiro DC, Theis JC, et al. 2016. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sport. Med 46(12):1869–1895. [DOI] [PubMed] [Google Scholar]
- 18.Hart HF, Culvenor AG, Collins NJ, et al. 2016. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br. J. Sports Med 50(10):597–612 Available from: http://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 19.Capin JJ, Zarzycki R, Ito N, et al. 2019. Gait Mechanics in Women of the ACL-SPORTS Randomized Control Trial: Interlimb Symmetry Improves Over Time Regardless of Treatment Group. J. Orthop. Res :Epub May 1, 2019 ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capin JJ, Khandha A, Zarzycki R, et al. 2018. Gait Mechanics and Tibiofemoral Loading in Men of the ACL-Sports Randomized Control Trial. J. Orthop. Res 36(9):2364–2372 Available from: 10.1002/jor.23895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesfar W, Shirazi-Adl A. 2005. Biomechanics of the knee joint in flexion under various quadriceps forces. Knee 12(6):424–434. [DOI] [PubMed] [Google Scholar]
- 22.Middleton A, Fritz SL, Lusardi M. 2015. Walking speed: the functional vital sign. J. Aging Phys. Act 23(2):314–22 Available from: https://journals.humankinetics.com/doi/pdf/10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velasques Stoelben KJ, Pappas E, Mota CB. 2019. Lower Extremity Joint Moments Throughout Gait At Two Speeds More Than 4 Years After Acl Reconstruction. Gait Posture 70:347–354 Available from: 10.1016/j.gaitpost.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Winter DA. 1984. Kinematic and kinetic patterns in human gait: Variability and compensating effects. Hum. Mov. Sci 3(1–2):51–76. [Google Scholar]
- 25.Capin JJ, Khandha A, Zarzycki R, et al. 2017. Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J. Orthop. Res 35(9):1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Stasi S, Hartigan EH, Snyder-Mackler L. 2012. Unilateral Stance Strategies of Athletes With ACL Deficiency. J Appl Biomech 28(4):374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manal K, Buchanan TS. 2013. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. J. Biomed. Eng 135(2):021014 Available from: http://biomechanical.asmedigitalcollection.asme.org/article.aspx?articleid=1666667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan TS, Lloyd DG, Manal K, Besier TF. 2004. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech 20(4):367–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moisio KC, Sumner DR, Shott S, Hurwitz DE. 2003. Normalization of joint moments during gait: A comparison of two techniques. J. Biomech 36(4):599–603. [DOI] [PubMed] [Google Scholar]
- 30.Capin JJ, Zarzycki R, Arundale A, et al. 2017. Report of the Primary Outcomes for Gait Mechanics in Men of the ACL-SPORTS Trial: Secondary Prevention With and Without Perturbation Training Does Not Restore Gait Symmetry in Men 1 or 2 Years After ACL Reconstruction. Clin. Orthop. Relat. Res 475(10):2513–2522 Available from: http://link.springer.com/10.1007/s11999-017-5279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.2019. 3DSlicer. [cited 2019 May 6] Available from: https://www.slicer.org/.
- 32.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 2012. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30(9):1323–1341 Available from: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Chav R, Cresson T, et al. 2016. 3D knee segmentation based on three MRI sequences from different planes. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2016–Octob:1042–1045. [DOI] [PubMed] [Google Scholar]
- 34.Behzadi C, Welsch GH, Laqmani A, et al. 2017. Comparison of T2* relaxation times of articular cartilage of the knee in elite professional football players and age-and BMI-matched amateur athletes. Eur. J. Radiol 86:105–111 Available from: 10.1016/j.ejrad.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Bolbos RI, Link TM, Benjamin Ma C, et al. 2009. T1ρ relaxation time of the meniscus and its relationship with T1ρ of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthr. Cartil 17(1):12–18 Available from: 10.1016/j.joca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza RB, Kumar D, Calixto N, et al. 2014. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr. Cartil 22(10):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culvenor AG, Wirth W, Maschek S, et al. 2017. Longitudinal change in patellofemoral cartilage thickness, cartilage T2 relaxation times, and subchondral bone plate area in adolescent vs mature athletes. Eur. J. Radiol 92:24–29 Available from: 10.1016/j.ejrad.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Eckstein F, Heudorfer L, Faber SC, et al. 2002. Long-term and resegmentation precision of quantitative cartilage MR imaging (qMRI). Osteoarthr. Cartil 10(12):922–928. [DOI] [PubMed] [Google Scholar]
- 39.Rothman K 1990. No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46. [PubMed] [Google Scholar]
- 40.Cohen J 1988. Statistical Power Analysis for the Behavioral Sciences, 2nd Edition Hillsdale, New Jersey: Lawrence Erlbaum Associates; 567 p. [Google Scholar]
- 41.Zeni JA, Higginson JS. 2009. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: A result of altered walking speed? Clin. Biomech 24(4):372–378 Available from: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thoma LM, McNally MP, Chaudhari AM, et al. 2017. Differential knee joint loading patterns during gait for individuals with tibiofemoral and patellofemoral articular cartilage defects in the knee. Osteoarthr. Cartil 25(7):1046–1054 Available from: 10.1016/j.joca.2017.02.794. [DOI] [PubMed] [Google Scholar]
- 43.Pietrosimone B, Troy Blackburn J, Harkey MS, et al. 2016. Walking Speed As a Potential Indicator of Cartilage Breakdown Following Anterior Cruciate Ligament Reconstruction. Arthritis Care Res. 68(6):793–800. [DOI] [PubMed] [Google Scholar]
- 44.Astephen Wilson JL. 2012. Challenges in dealing with walking speed in knee osteoarthritis gait analyses. Clin. Biomech 27(3):210–212 Available from: 10.1016/j.clinbiomech.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Andriacchi TP, Koo S, Scanlan SF. 2009. Gait Mechanics Influence Healthy Cartilage Morphology and Osteoarthritis of the Knee. J. Bone Jt. Surg 91:95–101 Available from: http://jbjs.org/cgi/doi/10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andriacchi TP, Dyrby CO. 2005. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech 38(2):293–298. [DOI] [PubMed] [Google Scholar]
- 47.Khandha A, Manal K, Wellsandt E, et al. 2017. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J. Orthop. Res 35(3):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joseph GB, McCulloch CE, Nevitt MC, et al. 2017. Medial femur T2 Z-scores predict the probability of knee structural worsening over 4–8 years: Data from the osteoarthritis initiative. J. Magn. Reson. Imaging 46(4):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph GB, Baum T, Alizai H, et al. 2012. Baseline mean and heterogeneity of MR cartilage T2are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3years - data from the Osteoarthritis Initiative. Osteoarthr. Cartil 20(7):727–735 Available from: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim CW, Hosseini A, Lin L, et al. 2018. Quantitative analysis of T2 relaxation times of the patellofemoral joint cartilage 3 years after anterior cruciate ligament reconstruction. J. Orthop. Transl 12:85–92 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khandha A, Manal K, Capin J, et al. 2018. High Muscle Co-Contraction Does Not Result in High Joint Forces During Gait in Anterior Cruciate Ligament Deficient Knees. J. Orthop. Res :E-pub ahead of print on Sept 19, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souza RB, Baum T, Wu S, et al. 2012. Effects of Unloading on Knee Articular Cartilage T1rho and T2 Magnetic Resonance Imaging Relaxation Times: A Case Series. J. Orthop. Sport. Phys. Ther 42(6):511–520 Available from: http://www.jospt.org/doi/10.2519/jospt.2012.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teng H-L, Wu D, Su F, et al. 2017. Gait Characteristics Associated With a Greater Increase in Medial Knee Cartilage T 1ρ and T 2 Relaxation Times in Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med 45(14):3262–3271. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Kuo D, Theologis A, et al. 2011. Cartilage in Anterior Cruciate Ligament–Reconstructed Knees: MR Imaging T1 ρ and T2—Initial Experience with 1-year Follow-up. Radiology 258(2):505–514 Available from: http://pubs.rsna.org/doi/10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stehling C, Liebl H, Krug R, et al. 2010. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology 254(2):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Relationship between walking speed and involved limb femoral trochlear cartilage T2 relaxation time after removing 2 participants whose involved knee trochlear cartilage T2 values were more than 2 standard deviations from the mean.