Abstract
The evolution of viviparity in therian mammals, i.e. marsupials and “placental” mammals, occurred by retention of the conceptus in the female reproductive tract and precocious “hatching” from the shell coat. Both eutherian embryo implantation and the opossum embryo attachment reaction are evolutionarily derived from and homologous to a defensive inflammatory process induced after shell coat hatching. However, both lineages, marsupials and placental mammals, have modified the inflammatory response substantially. We review the induction, maintenance, and effects of inflammation throughout pregnancy, with special attention to the role of prostaglandins and the mucosal inflammatory response, both of which likely had roles in early mammalian viviparity. We propose that the key step was not only suppression of the inflammatory response after implantation in placental mammals, but also the transfer of the inflammatory cell-cell communication network to a different set of cell types than in generic inflammation. To support this conclusion we discuss evidence that pro-inflammatory signal production in the opossum is not limited to maternal cells, as expected in bona fide defensive inflammation, but also includes fetal tissues, in a process we term cooperative inflammation. The ways in which the inflammatory reaction was independently modified in these two lineages helps explain major life history differences between extant marsupials and eutherians.
Keywords: Inflammation, Implantation, Gene Expression Recruitment, Monodelphis domestica, Theria
1. Introduction
Stem therian mammals evolved viviparity from an ancestral egg-laying animal between 176 and 220 million years ago (Madsen, 2009). Pre-partum erosion of the eggshell barrier brought fetal and maternal tissues into direct physical contact, resulting in new cell-cell interactions from which the evolution of the placenta proceeded (Griffith and Wagner, 2017). Embryonic attachment to the uterus likely stimulated an inflammatory response due to tissue damage (Chavan et al., 2017) and an adaptive immune response to paternally-derived fetal antigens (Medawar, 1953). How mammalian lineages addressed these challenges set the stage for their subsequent divergent reproductive evolution. This review focuses specifically on how maternal-fetal interactions following attachment were modified to reach their current state in marsupials and placental mammals.
Two extant clades of viviparous mammals diverged after the evolution of viviparity, the marsupials and the so-called “placental” mammals (Marshall, 1979; Killian et al., 2001). Marsupials consist primarily of Australian endemic fauna such as kangaroos and koalas, as well as the opossums which remain in the New World. “Placental” mammals (hereafter referred to by their phylogenetic branch-based name, eutherians, to avoid confusion with the placental organ) include humans, mice, elephants, and all of the species that descend from their most recent common ancestor. Marsupial gestation is in most cases shorter than a sterile sexual cycle, and yields highly altricial young which must complete development attached to the mother’s nipple. On the other hand, crown eutherian lineages have evolved extended pregnancy longer than a single sexual cycle, which can yield precocial young (Chavan et al., 2016).
Comparison of uterine gene expression during pregnancy suggests that this fundamental life history difference between marsupials and eutherians is due to independent modification of the inflammatory response that likely was ancestrally induced upon contact of fetal and maternal tissues (Griffith et al., 2017). Invasive embryo implantation and placentation evolved in the eutherian stem lineage (Wildman et al., 2006; Mess and Carter, 2006; Elliot and Crespi, 2009). It has been proposed that eutherians modified the ancestral inflammatory response into embryo implantation (Finn, 1986; Griffith et al., 2017), and coupled it with a long anti-inflammatory period that extends pregnancy (Mor and Cardenas, 2010; Mor et al., 2011; Chavan et al., 2017). The decidual stromal cell type, responsible for decidualization of the endometrium to support an implanting embryo, also evolved in the stem of Eutheria (Mess and Carter, 2006). The original functional role of the decidual cells likely was suppression of inflammation caused by the attachment reaction (Chavan et al., 2016). Early within the crown group of Eutheria, the major lineages independently evolved recognition of pregnancy (Chavan et al., 2016).
Marsupials lack the anti-inflammatory period after embryo contact, and attachment of opossum embryos to the uterus coincides with the initiation of an inflammatory cascade that is followed quickly by parturition (Griffith et al., 2017; Hansen et al., 2017), although whether there is a role for inflammation in the highly derived macropods (wallabies, kangaroos and their relatives) has not been investigated. The model marsupial for laboratory research, the gray short-tailed opossum Monodelphis domestica, retains a similar reproductive strategy to the reconstructed marsupial ancestral state (Zeller and Freyer, 2001; Freyer et al., 2003). The therian common ancestor, too, likely underwent a gestation similar to that of the opossum (Chavan et al., 2016).
Attachment-induced inflammation was therefore suggested to be a developmental constraint on the duration of marsupial gestation, which eutherians have overcome (Griffith et al., 2017). In this review we come to the conclusion that in both, eutherians and marsupials, the attachment-induced inflammation underwent independent modifications by redistribution of signaling roles to different cell types to serve different purposes. In marsupials, this redistribution of signaling roles reinforces inflammatory processes and likely utilizes them to induce parturition, rather than suppressing them. We review evidence that the inflammatory processes in opossum pregnancy are not solely performed by maternal cells, as expected in a bona fide inflammatory response, but instead they depend upon the cooperation of fetal and maternal cells. The modification of the innate immune response into distinct physiological processes – induction of parturition in the case of marsupials, or embryo implantation in the case of eutherians – suggests that inflammation was not only a constraint to be overcome but also a source of evolutionary innovation.
2. Evolution of Viviparity
The three major mammalian clades, the monotremes, marsupials and eutherians, diverged at different points in the evolution of pregnancy. This is illustrated by their differential deposition and shedding of egg coats: the outer layers are deposited and maintained in monotremes until hatching, in marsupials they are deposited but shed within the body, and in eutherians the shell coat is absent except for the zona pellucida. The characteristic egg of the Amniota, which include reptiles and mammals, is surrounded by three acellular egg coats. The first layer, the zona (zona pellucida in mammals), is a glycoprotein matrix deposited by the ovarian follicular cells around the oocytes, and is present in all mammals (Hughes, 1977). Additional egg coats are secreted around the conceptus during passage through the reproductive tract. These consist of the mucoid coat, an acidic glycoprotein layer, and the proteinaceous shell coat comprised of ovokeratin, which is rich in disulfide bonds (Hughes, 1977; Selwood, 2000). The shell coat is primarily deposited in monotremes and marsupials within the upper uterus (Selwood, 2000; Zeller and Freyer, 2001). This part of the uterus is homologous to the shell gland which deposits calcified shell coats around bird and lizard eggs (Lombardi, 1998). Erosion of the shell coat during embryonic development evolved in the stem lineage of therian mammals, and in the eutherians it is no longer deposited at all.
Viviparity evolved in mammals by a heterochronic shift of shell coat hatching to earlier in development when the fetus is still inside the mother (Figure 1). Monotreme development involves an intrauterine egg incubation period of 15–24 days (Rismiller and McKelvey, 2000; Hawkins and Battaglia, 2009), followed by oviposition and around 10 days of external incubation, in a nest in the case of the platypus, or in the mother’s skin pouch in the case of the echidna (Griffiths et al., 1969). This pattern is thought to resemble the reproductive mode of the egg-laying mammalian common ancestor. Marsupial gestation is ancestrally short as well, approximately the length of monotreme intrauterine gestation, with the opossum having a total gestation of 14 days (Harder et al., 1993), although the macropods have evolved gestations of up to 38 days (Hughes, 1962; Poole, 1975). Breakdown of the shell coat in marsupials occurs at the somitogenesis stage of embryonic development (day 12 in the opossum; Zeller and Freyer, 2001). Marsupial neonates and monotreme hatchlings are altricial in similar ways, with underdeveloped hindlimbs but precociously formed forelimbs and claws necessary for maneuvering to the lactational areas (Griffiths, 1978:252; Hughes and Hall, 1998).
Figure 1.
Evolution of pregnancy within crown mammals. Mammalian MRCA The mammalian most recent common ancestor (MRCA) was likely oviparous with inflammatory internal insemination, deposition of a mucoid coat and leathery shell coat, oviposition, external egg incubation, and hatching. This condition is approximately maintained in living monotremes. Therian MRCA Shift of the shell coat hatching event from after partition to before partition resulted in the evolution of viviparity in therian mammals, and an inflammatory attachment stage. Opossum The rapid growth phase in marsupials is derived from the ancestral attachment-induced inflammatory reaction, but quickly leads to parturition. Human Eutherians evolved embryo implantation, fetal invasiveness, and the anti-inflammatory growth phase of pregnancy. Parturition remains an inflammatory process. Opossum silhouette © Sarah Werning CC BY 3.0 (https://creativecommons.org/licenses/by/3.0).
After shell coat hatching, embryo attachment and formation of the yolk sac placenta mark the beginning of the rapid growth phase characteristic of marsupial development, during which fetal size increases exponentially (Rose, 1989). The rapid growth phase involves increased nutrient transfer from maternal to fetal blood (Freyer et al., 2006). This allows the marsupial fetus to complete the same amount of growth that would encompass the entire egg incubation period of monotremes in a fraction of the time, only the last two days of gestation in the opossum. The observation that this phase of development occurs under inflammatory conditions (Griffith et al., 2017) suggests that certain components of inflammation may be beneficial to fetal growth rather than pathological or coincidental.
Eutherian embryos can implant into the endometrium and undergo extended gestation. Eutherian gestation ranges from less than a month in some rodents, including the golden hamster Mesocricetus aurutus with a gestation as short as 16 days (Purdy and Hillemann, 1950), to humans at 9 months, and elephants at 22 months (Perry, 1953), unheard of for marsupials. Crucial to extended gestation is the ability of eutherian embryos to implant into the endometrium. While much of human pregnancy is considered anti-inflammatory, such a characterization applies only to the growth phase of eutherian pregnancy, which is evolutionarily derived (Griffith et al., 2017; Chavan et al., 2017). This anti-inflammatory phase is sandwiched between two stages, implantation and parturition, which are pro-inflammatory processes (Figure 1) (Mor and Cardenas, 2010). The evolution of extended gestation therefore involved two related evolutionary events, the modification of the pro-inflammatory attachment reaction into implantation to establish the fetus within the uterus, and the creation of an anti-inflammatory phase of pregnancy (Chavan et al., 2017). The common ancestor of therian mammals likely experienced inflammation upon insemination (Paulesu et al., 2008), embryo attachment (Finn, 1986; Finn, 1996; Griffith et al., 2017) and parturition (Hansen et al., 2017), but did not have sustained embryo invasion or an extensive anti-inflammatory growth phase as humans and other eutherian mammals do.
3. Inflammation Induced by Normal Pregnancy
The question of how viviparity could have evolved in the presence of an immune system that evolved to attack foreign bodies has long puzzled scientists (Billington, 2003). From an immunobiological perspective, loss of the shell coat barrier exposes the uterus to direct physical contact with the fetus, and by extension any tissue injury or alloantigen presence resulting from the apposition of an invasive semi-foreign body. An immune response to the fetus would presumably have been induced as a consequence of this transition in the timing of hatching in the first viviparous mammals, as the immune system evolved long before viviparity. The version of the adaptive immune system known from humans is shared among gnathostomes, i.e. all vertebrates derived from the most recent common ancestor of humans and sharks (Litman et al., 2010), whereas innate immune responses such as inflammation are of even more ancient origin, at least as old as metazoans [multicellular animals] (Rowley, 1996; Ashley et al., 2012). While providing resilience against perturbations throughout life, such defenses also have consequences for the sustainability of a semi-allogeneic offspring within the mother. The anti-inflammatory phase of gestation does not simply involve temporary suppression of the immune response, as the compromise of host defense in such a critical time for organismal fitness would presumably carry an enormous fitness cost (Mor et al., 2011). Rather, immune processes spatially specific to the uterus and temporally restricted to pregnancy must have diverged from the generic inflammatory response, and evolved along an independent evolutionary trajectory from normal defensive inflammation in order to enable viviparity. In the following sections we discuss points in therian mammal reproduction that might elicit immune or inflammatory responses, and whether these processes resemble or appear to be modified from normal host defense.
3.1. Antigen-Specific Immune Responses as a Consequence of Viviparity
The immune system responds to pathogenic non-self organisms or to tissue damage and stress. It acts through the antigen-specific responses of the adaptive immune system, as well as pattern recognition in the form of conserved non-self structural feature recognition, and detection of loss of homeostasis (damage) or danger through functional feature recognition (Iwasaki and Medzhitov, 2015). In the first viviparous vertebrates, the embryo may have induced the immune response through the same pathways as a non-self intruder. Here, we review potential non-self and damage induction mechanisms for the inflammatory response upon fetal-maternal contact as it likely took place in the first viviparous mammals (summarized in Table 1).
Table 1.
Comments on potential immunogenic stimuli behind the inflammation observed upon embryo attachment in therian mammals.
| Inducer | Potential Example | Ancestral Counterpart | Embryo Mimics | Comments |
|---|---|---|---|---|
| antigen (allograft) | alloantigen detected by T cell receptor | adaptive immunity | extracellular fungus or bacterium | internal fertilization should also induce reaction, adaptive immune response is too slow if shell coat hatching is a prerequisite |
| Pathogen-associated molecular pattern | evolved response to serine protease (allergy-like, Th2) | innate immunity, inflammation, detoxification | macro-parasite | mast cell response weak, anti-helminthic Th2 response is derived in eutherians |
| damage-associated molecular pattern | endometrial necrosis/apoptosis, downstream of invasion or protease | tissue repair/ inflammation | tissue damage | likely therian ancestral state |
| signal | embryo hijacks maternal signaling (cytokines, hormones, prostaglandins) | maternal homeostasis, physiology, estrus | mother | proposed here as cooperative inflammation in a marsupial (M. domestica) |
The question of maternal tolerance of the fetus has traditionally been posed in terms of the adaptive immune system’s response to paternally-derived antigens, particularly cellular (T-cell mediated) immunity by analogy to graft-host interaction in tissue transplantation (Medawar, 1953). Such an allograft rejection-like reaction was posited to be a constraint responsible for the short duration of marsupial gestation (Moors, 1974; Lillegraven, 1975). In many marsupials the duration of the post-attachment intrauterine development, when the fetus establishes intimate contact with the uterus, is shorter than the experimentally determined onset of the graft rejection response suggesting that the marsupial gestation is constained to be shorter than the time delay between antigen presentation and adaptive immune response. For example, in Monodelphis domestica skin graft rejection begins 19 days after transplantation and is complete at 31 days (Stone et al., 1997), whereas the post-attachment period is only 2 to 3 days (Griffith et al., 2017). In the Tasmanian devil Sarcophilus harrisii, the graft rejection occurs after 14 days (Kreiss et al., 2011) but the post-attachment period is 2 to 7 days (Rose, 1989), and in the wallaby Macropus eugenii graft rejection takes 10–16 days whereas the post-attachment period is around 7.5 days (Walker and Tyndale-Biscoe, 1978; Renfree and Shaw, 2014). This timing is consistent with the necessity for marsupials to give birth before the onset of an antigen-specific immune response, but not with such a response being the proximate cause for parturition. Furthermore, several predictions from the graft rejection model have not been supported. If the reason for marsupial short gestation is the onset of an antigen-specific reaction, it would be predicted that immunological memory from previous exposure to paternal antigens would cause complications or make parturition occur more quickly upon shell coat rupture in successive pregnancies, such as in multiple pregnancies with the same partner or under artificial conditions akin to vaccination. Attempts to induce graft rejection-like pregnancy failure by active immunization to paternal antigens have not been successful in marsupials (Rodger et al., 1985), just as in eutherians (Lanman and Herod, 1965; Beer and Billingham, 1976; Wegmann et al., 1979). Overall, the attachment reaction in marsupials appears to consist of non-antigen-specific inflammation, rather than an antigen-specific T cell-mediated response.
Another potential factor in the immune tolerance of the fetus is the presence of regulatory T cells, which are elevated during pregnancy in mice and humans (Somerset et al., 2004). These consist of natural regulatory T cells produced in the thymus, and an induced regulatory T (iTreg) cell population that differentiates in peripheral tissues. Although iTreg cells have antigen-specific T cell receptors, upon activation through these receptors they dampen local immune responses through bystander suppression (Kahn and Baltimore, 2010). Outside of pregnancy, iTreg cells form a homeostatic counterbalance to pro-inflammatory Th17 cells, and aid in peripheral tolerance of commensal microorganisms in the gut-associated lymphoid tissues, among other functions (Littman and Rudensky, 2010). The generation of iTregs at the maternal-fetal interface is thought to be a derived trait only in eutherians, made possible by the insertion, in the stem eutherian lineage of eutherians, of a TGFβ- and retinoic acid-response element CNS1 into the FOXP3 locus, a gene crucial for Treg differentiation (Samstein et al., 2012). The ablation of this promoter in mice leads to increased embryo resorption in allogeneic matings, but not complete sterility (Samstein et al., 2012). The phylogenetic placement of this insertion coincides with the evolution of implantation and invasion, suggesting that Tregs may play important functions in implantation, even if their presence is not absolutely essential for successful pregnancy.
Several explanations for the lack of an adaptive immune response to the fetus in eutherians have been proposed. Classical major histocompatibility complex class I (MHC-I) proteins, expressed in most nucleated cells, present peptides to T cells, whereas non-classical MHC-I have immunomodulatory effects on uterine natural killer cells, CD8+ T cells, and dendritic cells at the fetal-maternal interface (Clark et al., 2010). In humans, expression of classical MHC-I genes HLA-A and HLA-B by the trophoblast is suppressed, which may allow these cells to hide from allorecognition by reduced presentation of self peptides (Moffett and Loke, 2006). While human cytotrophoblast and syncytiotrophoblast are reported to lack MHC expression (Sunderland et al., 1981; Tilburgs et al., 2015), HLA-C as well as non-classical HLA-E and secreted HLA-G are expressed in extravillous trophoblast (Tilburgs et al., 2015). There, these MHC molecules may play roles in suppression of natural killer cell cytotoxicity or CD8+ T cell activation (Fournel et al., 2000), or act as positive regulators of inflammation that aid in implantation (Rajagopalan et al., 2005; Trowsdale and Betz, 2006). However, these facts do not answer the question how immune tolerance first evolved because extravillous trophoblast cells are only known to exist in great apes (Crosley et al., 2013) and a somewhat similar invasive cell type in horses that ultimately forms the endometrial cups (Noronha and Antczak, 2010). In addition, both classical and non-classical MHC are expressed in the trophoblast of mice (Madeja et al., 2011), of bovines in late pregnancy (Davies et al., 2006) and of wallabies (Buentjen et al., 2015), so this state likely represents the ancestral condition for therian mammals. Therefore, the suppression of HLA-A and HLA-B in trophoblast cells cannot explain the origin of extended gestation, but may instead be linked to traits such as deep invasion and the role of extravillous trophoblast in vascular remodeling.
Suppression of antigen-specific effector mechanisms is also thought to play a role in successful viviparity. The trophoblast produces various immunomodulatory factors. Placental prostaglandin E2, among other functions, has been shown to inhibit proliferation of mouse cytotoxic T cells (Kvirkvelia et al., 2002) and is associated with T cell anergy by defective phosphorylation of the CD3 chain of the T cell receptor (Volumenie et al., 1997; Eblen et al., 2002). Human T cell activity and antigen presentation following allotransplantation differ from T cell activity during fetal-maternal interaction (Collins et al., 2009; Mor et al., 2017). This led to the interpretation that local tolerance, rather than systemic suppression of the immune system, occurs during human pregnancy (Moffett and Loke, 2006), and that the fetal role is more akin to a tumor than an allograft due to its own contribution to tolerance (Beaman et al., 2016; Mor et al., 2017; Costanzo et al., 2018). This distinction makes clear that the presence of an adaptive immune system with the capability to reject allografts does not preclude the evolution of viviparity. The pregnancy of viviparous sharks, for instance, can last from several months to 2 years, and yet their adaptive immune system rejects skin grafts in a matter of one month or less (Good and Finstad, 1964; Chaouat, 2016). As sharks and mammals are separated by substantial evolutionary divergence in both reproductive and immune biology, they achieved such prolonged gestation independently and likely through different mechanisms. Whether the lack of fetal rejection in these species is due to functions of the fetus that suppress it or because rejection is not induced in the first place has yet to be demonstrated.
Putting this work on maternal tolerance towards the semi-allogenic fetus into phylogenetic context suggests that the modulation of the adaptive immune response was a trait that evolved as the gestational length increased after the most recent common ancestor of eutherian mammals. The ancestral eutherian mammal was a small animal that gave birth to small neonates relative to the size of the mother, and thus likely had a short gestation period on the order of Monodelphis pregnancy (Chavan et al., 2016). As noted above, the gestational length of basally branching marsupials, such as the opossum, is shorter (14 days) than the time it takes for an allograft rejection to develop in the same species (19 days), and the time of direct fetal-maternal contact is even shorter (2 days). The immunological processes in the uterus of ancestral therian and eutherian animals were thus more likely related to the activation of damage- and stress-induced inflammation than the activation of the adaptive immune response. To this issue we are turning in the next section.
3.2. Non-Antigen-Specific Responses as Consequences of Viviparity
A potential inducer of inflammation in viviparity is uterine tissue damage caused by direct contact with the fetal membranes, for instance due to continued secretion of proteases that play a role in hatching form the shell coat (Griffith et al., 2007). Damaged and dying cells release damage-associated molecular patterns, or “hidden self,” which trigger inflammatory and immune responses (Matzinger, 1994; Kono and Rock, 2008). Danger cues produced by dead and dying cells include membrane depolarization and extracellular presence of DNA, ATP, and other compounds, and detection of these cues triggers inflammatory responses in a wide range of multicellular eukaryotes (Heil and Land, 2014). Contact between the fetal tissues and the uterus, accompanied by tissue turnover in the placenta, mean that the damage response could be a component of the immunological milieu of pregnancy (Rovere-Querini et al., 2007), particularly the highly invasive pregnancy of humans, as tissue damage is more likely to result if the trophoblast is invasive. During human pregnancy, the tissue damage marker high mobility group box 1 protein (HMGB1) is released from the fetal membranes as well as from decidual cells (Holmlund et al., 2007). HMGB1 is normally localized to the nucleus but released upon damage or necrosis, or actively secreted by monocytes and macrophages (Scaffidi et al., 2002). HMGB1 binds to the receptor for advanced glycation end-products (RAGE), or toll-like receptors 2 and 4, and stimulates further inflammatory signaling (Park et al., 2004). HMGB1 promotes angiogenesis when applied to the extraembryonic membranes of the chick (Mitola et al., 2006), suggesting that this damage marker may also promote growth and vascularization of the placenta and the endometrium. The immune response to tissue damage promotes growth, and in doing so is suggested to nurture the fetus, rather than attacking it (Moffett and Loke, 2004).
Even in the opossum, tissue damage occurs upon fetal-maternal contact. Evidence for the invasive nature of the opossum trophoblast comes from the observation that shortly before birth, the trophoblast cells can be seen to penetrate the uterine epithelium and extend cytoplasmatic processes into the endometrial stroma (Enders and Enders, 1969; Wagner, unpublished observation), in spite of the fact that opossum placentation is classified as epitheliochorial. Nevertheless, the invasive phenotype is only seen on the last day of pregnancy and certainly does not lead to sustained fetal implantation as in many eutherian mammals. Instead, we have proposed that this quasi-invasion may function, too, to accelerate parturition in the opossum (Griffith et al., 2017).
Fetal protease secretion is another potential early inducer of inflammation. Secretion of proteases is a conserved feature of the therian trophoblast, and has been documented in human, rabbit and mouse blastocysts (Denker, 1977; Brosens et al., 2014) and protease activity from the trophoblast has also been shown in marsupials such as the wallaby (Denker and Tyndale-Biscoe, 1986) and Monodelphis (Griffith et al., 2017). We have previously proposed that serine protease secreted by the attaching blastocyst after hatching from the shell coat may be the ancestral trigger for the inflammatory attachment reaction (Griffith et al., 2017). This protease could have induced inflammation through endometrial tissue damage, or by triggering pattern recognition. The hatching of eukaryotic parasite eggs also involves serine protease secretion (Young et al., 1999), and as a consequence the mammalian innate immune system has evolved to recognize protease as a conserved pattern indicating helminth presence and induce an anti-helminthic Th2 immune response (Sokol et al., 2007). However, the anti-helminthic response is a type 2 immune response, whereas the inflammation induced after shell coat hatching in the opossum (Chavan et al., 2017) or during implantation in the human (Mor and Cardenas, 2010) is type 1. Therefore, while serine proteases have the ability to cause pro-inflammatory tissue damage and to activate the type 2 immune response through pattern recognition, the lack of a type 2 profile in the post-attachment opossum suggests that has been lost in marsupials, which is a hint that the opossum post-attachment reaction may be evolutionarily modified.
Eutherians arrive in the uterus surrounded by the zona pellucida. While and after the blastocyst hatches from the zona pellucida the trophoblast of eutherians secretes serine protease. This has been shown in the mouse (Ruan et al., 2012) and human (Brosens et al., 2014). Serine proteases are thought to stimulate epithelial sodium channels (ENaC) in the uterine lumen, leading to calcium influx, prostaglandin production, and ultimately decidualization of the endometrial stromal fibroblasts. In humans, the ability of the embryo to secrete serine proteases has been suggested to function during embryo selection as a quality signal detectable by the endometrium (Brosens et al., 2014; Macklon and Brosens, 2014). If so, this is likely a secondarily acquired function of protease secretion, with the original function having been disruption of the shell coat and the zona pellucida.
We thus suggest that the immunological problem for the evolutionary origin of eutherian pregnancy should be seen primarily from the perspective of an inflammatory damage response, while the dimensions of non-self exposure gained relevance only after the inflammatory challenge was overcome and gestational period could be extended.
Inflammatory signals have taken on physiological roles at several events throughout the reproductive cycle – post-copulation, implantation, and parturition. These events are of different phylogenetic ages and degrees of conservation across therian mammals, so they are considered separately. For each stage, the questions of whether inflammation is induced by the fetus or mother, and whether it represents an adaptation or a constraint, can be asked.
3.3. Inflammation Associated with Copulation
Uterine inflammation following copulation is reported across therian mammals, including horses, pigs, and cattle (Katila, 2012), as well as humans and mice (De et al., 1991; Robertson et al., 2003; Shima et al., 2015), and opossums (Baggott et al., 1987). A study of amphibians found that the cytokine IL-1β and its receptor IL-1R1 are expressed more broadly in the uterine mucosa in species with internal fertilization compared to species with external fertilization (Jantra et al., 2007). This inflammation recruits granulocytes such as neutrophils. In mice, neutrophil recruitment following insemination coincides with elevated expression of the mucosal inflammatory cytokine IL-17A, which is upstream of neutrophil chemoattractants (Song et al., 2016). This IL-17A is thought to be derived from γδ T cells, as seminal fluid has been shown to increase IL-17A production by γδ T cells in vitro (Song et al., 2016). Seminal fluid is a plausible inducer for inflammation after copulation, but it cannot be the only component. Inflammation, including recruitment of effector granulocytes to the uterus, also occurs in sterile sexual cycles in several species: neutrophils and eosinophils have been found to fluctuate throughout the estrous cycle in mice, peaking at estrus and metestrus (Diener et al., 2016), and in the opossum, granulocytes infiltrate the uterus at the time of copulation, but also during the times of maximum behavioral receptivity in sterile cycles (Baggott et al., 1987). These findings suggest that the inflammation occurring after copulation is in part endogenously controlled, and may have an anticipatory role, i.e. activate the immune system in anticipation of the arrival of sperm and seminal fluid.
3.4. Inflammation and Implantation, Decidualization, and Post-Attachment Phases
Eutherian blastocyst implantation occurs by shedding of the zona pellucida, orientation, apposition, attachment, and invasion (Spencer et al., 2004). In species with invasive placentation, the final invasion stage of the implantation process establishes the conceptus within the maternal tissues, although it has been suggested that perhaps a more appropriate term than invasion is “embedding,” due to the roles of both fetal and maternal tissues in orchestrating this interaction (Macklon and Brosens, 2014). In species with non-invasive placentation, such as pigs, and bovines and their relatives, as well as most marsupials, invasion does not occur.
Physiological roles for inflammation in normal pregnancy were first recognized in the late 19th century by noting similarities between the eutherian decidual reaction and inflammatory granulation (Creighton, 1878; summarized in Finn, 1986). In the majority of species with invasive placenta, decidualization transforms the endometrium in response to an implanting embryo. In menstruating species such as humans, decidualization is induced by maternal hormones, and occurs even in the absence of an embryo (Finn, 1998). However, phylogenetic inference suggests that decidualization was ancestrally induced by the fetus, as it still is in the majority of eutherian species (Emera et al., 2011). As decidualization is a derived character that evolved in eutherian mammals (Mess and Carter, 2006), it likely arose as a modification of, the ancestral maternal reaction to the fetus (Finn, 1998; Erkenbrack et al., 2018). The decidual reaction can be induced in the guinea pig uterus by physical damage to the endometrium (Loeb, 1907, 1908), or in the rat and mouse uterus with administration of an oil droplet or a plastic bead (Finn and Pope, 1991), suggesting that inflammation or stress is sufficient to induce decidualization. The decidua orchestrates the transition of implantation-associated inflammation into the Th2-dominated growth phase in eutherians whereas in opossums, embryo attachment initiates the rapid growth phase followed by parturition in short order (Chavan et al., 2017).
The eutherian implantation reaction, complete with intrauterine embedding, is therefore proposed to have evolved from previously existing inflammatory pathways (Finn, 1986; Griffith et al., 2017). In the opossum, the post-attachment phase involves an increase in inflammatory signaling, including type 1 cytokines IL1A, IL6, tumor necrosis factor (TNF), and neutrophil chemotactic factor (CXCL8) (Griffith et al., 2017; Hansen et al., 2017). Several of these genes, such as IL6, TNF, and CXCL8, are also expressed during implantation in humans (Mor et al., 2011). Inflammatory marker expression in the opossum immediately follows the degradation of the shell coat, suggesting that inflammation is induced only upon attachment of the trophoblast to the uterus (Griffith et al., 2017; Chavan et al., 2017). Precocious shell coat hatching and induction of inflammation in response to embryo attachment are reconstructed as traits shared between opossums and early viviparous mammals, as shared cytokine expression between eutherian implantation and marsupial attachment illustrates (Griffith et al., 2017).
Furthermore, inflammation also modulates expression of adhesion molecules, which have imortant roles in placentation and implantation (Chaouat, 2013).The integrin- and L-selectin-mediated adhesion of the blastocyst to the endometrium resembles diapedesis, the process whereby leukocytes bind to endothelial cell adhesion molecules and transverse blood vessels during inflammation (Genbacev et al., 2003; Dominguez et al., 2005). Therefore, it has been argued that the machinery supporting transendothelial migration of leukocytes was evolutionarily coopted to support migration across the luminal epithelium during embryo implantation (Liu, 2018). Diapedesis, like implantation, is a cooperative reaction between the migrating cell or blastocyst and the tissue, and can only occur properly under inflammatory conditions: as a consequence, eutherian embryo implantation is dependent upon the presence of inflammatory signaling.
3.5. Inflammation at Parturition
Inflammatory signals play important roles at the end of gestation. In addition to acting as inflammatory mediators prostaglandins, particularly PGF2α, induce “partition,” defined as either parturition/birth or oviposition, in a wide range of species. PGF2α can be produced directly from PGH2 (Kabututu et al., 2009) or from PGE2 (Phillips et al., 2011). PGF2α induces oviposition in oviparous reptiles (Guillette Jr. et al., 1991; Hargrove and Ottinger, 1992; Kupittayanant et al., 2009), and elicits myometrial contractions during labor in viviparous mammals (Karim, 1968; Challis et al., 2000; McKeown et al., 2000). PGF2α binds to the prostaglandin F receptor, whose activation leads to release of intracellular calcium which in turn binds calmodulin; calcium-calmodulin then activates myosin light chain kinase to phosphorylate myosin light chains and cause muscle contraction (Challis et al., 2000). This mechanism has been identified in bird oviposition, showing that it is not unique to viviparity (Kupittayanant et al., 2009). PGF2α surges at the time of parturition in the tammar wallaby (Renfree et al., 1994) and is sufficient to induce birthing behavior in the opossum (Rose and Fadem, 2000) and tammar wallaby (Hinds et al., 1990). These observations suggest that its role at parturition is shared among marsupials. Uterine PGF2α also can elicit luteolysis in a wide range of amniotes, including turtles (Mahmoud et al., 1988), squamates (Guillette Jr. et al., 1984), tammar wallabies (Hinds et al., 1990), and sheep (Douglas and Ginther, 1973). Thus, during the inflammatory end phase to gestation, prostaglandins promote expulsion of the fetus, after which inflammation can resolve.
Cytokines such as IL6, IL1B and CXCL8 are upregulated in the human myometrium at labor (Bollopragada et al., 2009). Labor also involves the cytokine-mediated recruitment of effector immune cells to the uterus: neutrophils are recruited at term (Kelly, 1996; Romero et al., 2006) and in cases of pre-term labor (Elovitz and Mrinalini, 2004; Romero et al., 2006; Goldenberg et al., 2008). On the maternal side, granulocytes infiltrate the decidua and myometrium (Thomson et al., 1999; summarized in Gomez-Lopez et al., 2010). In mice, Fas ligand, which binds to and promotes apoptosis of Fas+ leukocytes, is expressed by uterine epithelia and stroma during early gestation, and by the trophoblast in late gestation, and in its absence neutrophils and macrophages prematurely infiltrate the fetal-maternal interface, impairing fertility (Hunt et al., 1997). Neutrophils infiltrate the uterus before parturition in various marsupials, including opossums (Chavan et al., 2017), and several diprotodont species (Sharman, 1961). Recruited granulocytes produce pro-inflammatory signals such as TNF and eicosanoids, the group of compounds that includes prostaglandins (Cassatella, 1995; Gomez-Lopez et al., 2010). They also express proteases that digest extracellular matrix such as neutrophil elastase, collagenase, and matrix metalloproteinase. On the fetal side, inflammation also occurs in the extraembryonic membranes during parturition (Halgunset et al., 1994), and extracts from fetal membranes of women during labor have neutrophil chemotactic properties (Gomez-Lopez et al., 2009).
This raises the question of what purpose neutrophils serve in the uterus during parturition. Neutrophils are specialized to carry out effector functions, primarily the removal of pathogens by phagocytosis and release of cytotoxic reactive oxygen species. The high cost of neutrophil recruitment due to collateral damage makes their recruitment beneficial only in specific circumstances: for this reason, there are no tissue-resident neutrophils, and the threshold for summoning them is high (Okabe and Medzhitov, 2016). The function of neutrophil recruitment in parturition, however, is not entirely clear. They may be part of the system activating uterine contractions, or may function afterwards in repair and cleanup (Keski-Nisula et al., 2000; Timmons et al., 2009; Shynlova et al., 2013). If neutrophils promote expulsion of the fetus, then their presence following embryo attachment in marsupials but conspicuous absence in eutherian implantation (Chavan et al., 2017) is noteworthy: prevention of neutrophil recruitment was a necessary prerequisite to the evolution of extended gestation. Hence neutrophil recruitment at parturition could be a phylogenetic rudiment of an ancestral acute inflammatory reaction that is suppressed during the growth phase in eutherians, and then gets released to aid in parturition.
4. Eutherian and Marsupial Inflammatory Signaling Networks during Gestation
Two of the pro-inflammatory events in eutherian pregnancy, implantation and parturition, are similar to and likely homologous to the inflammatory attachment reaction of marsupial pregnancy, and both have been proposed to be derived from the ancestral therian attachment-induced inflammatory response (Griffith et al., 2017). While the marsupial (in particular the opossum) condition may approximate the therian ancestral state with its lack of eutherian- or macropod-specific extended gestation (Freyer et al., 2003) more than 170 million years of independent evolution (Madsen, 2009) mean that the physiology of the opossum has likely diverged from that of the first viviparous mammals. Nevertheless, as no other extant mammalian lineage diverged after the evolution of viviparity and before the split of therian mammals, we must consider the ancestral inflammatory signaling network using insights from the opossum, as well as from eutherian mammals and defensive inflammation outside of the context of pregnancy.
4.1. A Hypothetical Early Viviparous Mammal Inflammatory Network
Two classes of inflammatory mediators relevant to immune responses at mucosal surfaces are eicosanoids (prostaglandins) and cytokines (particularly those in the interleukin 17 pathway). Prostaglandins have been discussed above.
IL-17-mediated immunity predates the evolution of the adaptive immune system in gnathostomes. IL-17 homologs have been discovered in lampreys, where they are expressed in VLR lymphocytes of the convergently evolved agnathan adaptive immune system (Guo et al., 2009). IL-17 homologs are also expressed in the mucosal epithelial cells of echinoderms in response to extracellular bacteria (Buckley et al., 2017), suggesting that IL-17 has deep evolutionary roots as a cell-autonomous inflammatory mediator, not unlike its expression in innate immune cells and mucosal epithelia in humans (Cua and Tato, 2010). If these functions are homologous, IL-17 functioned in mucosal epithelial defense before the divergence of deuterostomes. Thus, given resemblance of the embryo to an extracellular pathogen, IL-17 would have been a component of the original attachment-induced inflammatory response (Chavan et al., 2017).
4.2. The Inflammatory Network in the Marsupial Post-Attachment Inflammation
There is little evidence for maternal recognition of pregnancy in marsupials outside of the macropods (Renfree, 2000), suggesting that ancestrally, marsupial pregnancy did not elicit systemic physiological changes in the mother. On the local scale of the uterus, local changes in response to the presence of fetuses do occur, such as the activation of uterine glands and replacement of the sub-epithelial stroma by a network of capillaries (Griffith et al., 2019). That said, the inflammatory process is not solely maternal, but is the result of contributions from both the fetus and mother.
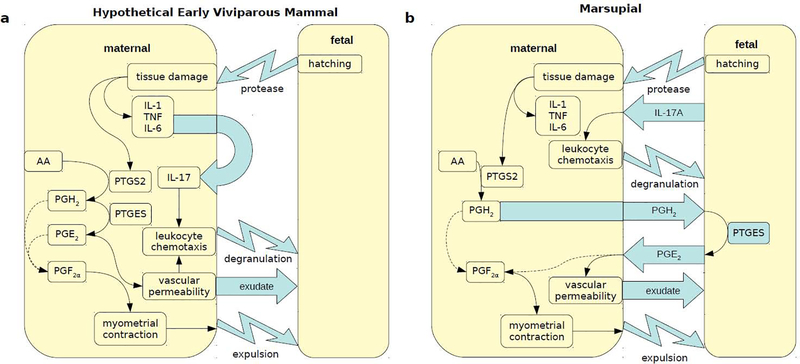
Expression of genes in the prostaglandin synthesis pathway are highly upregulated in the opossum uterus during the attachment reaction, e.g. PTGS2 aka COX2 (Griffith et al., 2017). In the opossum, PTGS2 is localized to the maternal uterine luminal epithelium, whereas the downstream enzyme for the production of PGE2, PTGES, is localized to the trophoblast, i.e. expressed in cells of fetal origin (Figure 2b) (Griffith et al., 2017). This suggests that PGE2 synthesis during opossum pregnancy is cooperative: based on this expression pattern, in order for PGE2 to be produced from arachidonic acid, both the fetal and maternal tissues would have to be in close enough proximity for the intermediate PGH2 to travel between them, although such a shuttle mechanism of PGH2 to neighboring cells has yet to be demonstrated. If this scenario is correct, it would constitute a mechanism of maternal recognition of pregnancy in the opossum, albeit not one where the embryo prolongs the function of corpus luteum, and if anything, one that brings about parturition. Furthermore, it suggests that the opossum fetus is not just incidentally setting off inflammatory signaling, but rather the fetus itself is instrumental in the production of inflammatory mediators, likely to effect its own birth.
Figure 2.
Types of inflammatory signals produced at the maternal-fetal interface upon initial contact. a. An inflammatory response was mounted to the fetus in the first viviparous mammals, possibly due to protease production during an early intrauterine hatching reaction. Inflammatory signals are maternally produced: Tissue damage triggers inflammatory pathways such as prostaglandin production, type 17 mucosal inflammation recruits neutrophils to the uterus that degranulate, and ultimately the fetus is expelled. b. Inflammatory signals are produced at the fetal-maternal interface of the opossum at embryo attachment, but the fetus plays a role in production of inflammatory mediators, such as IL-17A and PGE2 production, in addition to initial induction of an immune response.
After embryo attachment in the opossum, expression of IL17A is elevated, particularly during the last day before parturition (Griffith et al., 2017; Hansen et al., 2017). However, marsupial IL-17 signaling during pregnancy is not identical to typical mucosal defense against an extracellular pathogen. We found that the source of IL17A expression at the end of opossum pregnancy is the trophoblast (Chavan et al., in prep.). This suggests that IL-17 mediated inflammation, like prostaglandin metabolism, results from fetal as well as maternal components in opossum pregnancy. Production of IL17A by the opossum trophoblast suggests recruitment of expression of this inflammatory signal from maternal leukocytes or epithelia, the producers of IL17A in mucosal defense. Its expression, like prostaglandin synthesis, may contribute to expulsion of the embryo in parturition.
4.3. Modified Inflammatory Networks in Eutherian Pregnancy
Redistribution of expression of maternally-produced signals to the fetus and vice versa can be explained as a process of enhancing beneficial fetal-maternal interactions and pruning detrimental ones (Griffith and Wagner, 2017).
The evolution of extended gestation in eutherians was enabled by the gain of an anti-inflammatory period of pregnancy following implantation. Decidual stromal cells have been shown to suppress type 1 inflammation and wound healing pathways in the decidua until parturition (Nancy et al., 2018). As the decidual stromal cell type is absent in marsupials (Kin et al., 2014), these immunomodulatory functions represent divergent evolution in the eutherian lineage from the ancestral inflammatory response. However, the ancestral role of decidual stromal cells is reconstructed to have been at implantation, as their presence later in gestation is not universal within eutherians (Chavan et al., 2016).
While the presence of prostaglandins in mammalian reproductive physiology is ubiquitous, the localization of enzymes in the prostaglandin pathway is variable within eutherians. Maternal prostaglandin production plays an important role in human implantation to increase receptivity of the endometrium (Achache et al., 2010). In the mouse it has been shown that the pre-implantation blastocyst itself expresses PTGS2 (Liu, 2018). When inflammation recurs at parturition, complementary expression of enzymes catalyzing different steps in the prostaglandin synthesis pathway is reported in humans (Pavličev et al., 2017). At term, the upstream enzyme involved in liberating arachidonic acid, phospholipase A2 (PLA2G16), is expressed in both fetal and maternal tissues, as is prostaglandin E synthase (PTGES) (Pavličev et al., 2017), although prostaglandin E synthase protein levels are elevated in the trophoblast relative to the decidua (Phillips et al., 2014). On the other hand, other enzymes appear to be more exclusively maternal, such as prostaglandin F synthase (FAM213B) which is localized to the decidua (Pavličev et al., 2017). This suggests that after widespread PGH2 synthesis, PGE2 production, which increases blood flow and nutrient delivery to the fetus, is more under the control of the fetal membranes, whereas PGF2α production, which more directly elicits contractions and parturition, occurs in the decidua and acts locally on the myometrium. Such a situation may reflect a legacy of gene expression recruitment.
5. Conclusions
5.1. Cooperative Inflammation as an Alternative Form of Viviparity
The brevity of marsupial pregnancy has provoked the theory that its duration is constrained by an overwhelming allograft rejection reaction (Moors, 1974; Lillegraven, 1975). Molecular studies (Griffith et al., 2017; Hansen et al., 2017) revise the notion of immune rejection substantially to focus on inflammation, a component of the innate immune system, rather than the lymphocyte-mediated process of graft rejection, which in opossum has been shown to take 19 days, much longer than the duration of fetal-maternal attachment of two days. These all point to a model of developmental constraint by the induction of an ancestral defensive inflammatory response to the embryo that forces parturition (Figure 3a). In this model, marsupials respond to the fetus in the same way that they would to an extracellular pathogen, i.e. by inflammation and neutrophil recruitment.
Figure 3.
Models for evolution of inflammation during pregnancy in therian mammals. a. In the constraint model, marsupial short gestation is a result of developmental constraint, stemming from the induction of an unmodified instance of the same inflammatory response that originally evolved to respond to mucosal damage or pathogens (mucosal inflammation, red) against the embryo. Eutherian embryo implantation evolved by the individualization and divergence of inflammation into a form specific to the pregnant uterus, which became embryo implantation (blue), enabling subsequent evolution of extended gestation. b. In the cooperative inflammation model (proposed in this paper), the first viviparous mammals reacted to the hatching of the fetus from the shell coat with defensive inflammation, but this physiological character individualized into a uterine-specific form and was modified independently in both the metatherian and eutherian lineages. In the metatherian lineage, inflammation was reinforced and expression of signals was redistributed to rely upon cooperation of the fetus and mother. Eutherian embryo implantation evolved by the modification proposed in (a). Opossum silhouette © Sarah Werning CC BY 3.0 (https://creativecommons.org/licenses/by/3.0).
In the light of findings that the expression of some inflammatory mediators is not limited to maternal cells, as expected in a bona fide inflammatory response, but instead results from both fetal and maternal cells, the constraint model needs to be amended. We propose that a fetal-maternal communication network has emerged out of a generic inflammatory response by gene expression recruitment to fetal cells. In marsupials, this has the effect of reinforcing the inflammatory response at the end of pregnancy, whereas in eutherians the outcome was the evolution of embryo implantation and the transition to an anti-inflammatory phase of pregnancy (Figure 3b). In both cases, what was ancestrally an antagonistic response of host versus inflammatory inducer was modified into a cooperative fetal-maternal communication network. In the case of marsupials, this cooperative network is pro-inflammatory and promotes parturition. We term this process cooperative inflammation. In the case of eutherians, fetus and mother cooperate to orchestrate the embedding of the fetal tissues within the mother, the process of blastocyst implantation. Both of these processes resulted from individualization of an uterine- or pregnancy-specific process from the generic inflammatory process, and the resulting individualized fetal-maternal networks each had their own evolutionary trajectory. Marsupials and eutherians have modified their inflammatory networks independently in ways that lead to divergent reproductive modes, implantation and cooperative inflammation, and predispose these clades to disparate life history patterns.
5.2. Clinical Implications
Understanding the ancestral female reproductive tract biology of therian mammals and how inflammatory networks were remodeled during the evolution of pregnancy has relevance to medical and veterinary practice. When exclusively viewed from the allograft perspective, the implication would be that suppression of inflammatory and immune responses would be beneficial to pregnancy. If, however, a good part of implantation and parturition is an evolutionarily modified inflammatory process then interventions affecting parts of this process are expected to be deleterious. Understanding the therian ancestral state as well as the independent modifications of the ancestral network in the marsupial lineage as compared to the human lineage should allow for better targeting of specific signaling interactions to improve implantation success in the assisted reproduction clinic and treat implantation failure.
In addition, inflammation arising from bacterial infection during pregnancy increases the risk of preterm delivery, in part because inflammatory signaling has evolved to be integrated into the control of parturition, resulting in vulnerability to interferences from other sources of inflammatory stimuli. Better understanding of ways in which inflammation at parturition has been modified from defensive inflammation may help in the development of clinical interventions to prevent pre-term birth. The evolution of innate immune activation, maternal-fetal signaling and diversity between species is a promising field for reciprocal illumination between the basic and applied sciences.
Highlights.
Inflammatory stages of pregnancy in therian mammals evolved by the transfer of a cell-cell communication network to a different set of cell types of both maternal and fetal origin.
Recruitment of inflammatory signals into reproductive physiology likely occurred independently in marsupials and eutherian mammals.
Marsupials evolved cooperative inflammation at term pregnancy which reinforced the ancestral inflammatory response induced by embryo attachment and likely elicits parturition.
Eutherian mammals modified the inflammatory response induced by embryo attachment into the implantation process, and postponing the inflammation associated with parturition and resulting in prolonged gestation.
Acknowledgments
Feedback from Carla Staver and members of the 2018 Yale EEB 725 seminar improved the writing quality of this manuscript.
Funding Sources
Research in the Wagner lab is supported by NCI grant U54CA209992, and John Templeton Foundation grant #61329. DJS is supported by the NIH Predoctoral Training Program in Genetics (T32 GM 007499).
Abbreviations
- HMGB1
high mobility group box 1 protein
- MHC
major histocompatibility complex
- MRCA
most recent common ancestor
- PG
prostaglandin
- PTGES
prostaglandin E synthase
- PTGS
prostaglandin-endoperoxide synthase (cyclooxygenase)
- Th2
type 2 helper T cell
- TNF
tumor necrosis factor
- Treg
regulatory T cell
Footnotes
Competing Interests
The authors have no competing interests to declare.
I declare that I participated in the study by assisting with conceptual development and literature review, writing the initial draft of the manuscript, and assisting with editing, and that I have seen and approved the final version. I have no conflicts of interest to declare.
Materials and Methods
No experiments were conducted explicitly for this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achache H, Tsafrir A, Prus D, Reich R, Revel A, 2010. Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertil. Steril 94 (4), 1271–1278. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Weil ZM, Nelson RJ, 2012. Inflammation: mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. S 43, 385–406. [Google Scholar]
- Baggott LM, Davis-Butler S, Moore HDM, 1987. Characterization of oestrus and timed collection of oocytes in the grey short-tailed opossum, Monodelphis domestica. J. Reprod. Fertil 79, 105–144. [DOI] [PubMed] [Google Scholar]
- Beaman KD, Jaiswal MK, Katara GK, Kulshreshta A, Pamarthy S, Ibrahim S, Kwak-Kim J, Gilman-Sachs A, 2016. Pregnancy is a model for tumors, not transplantation. Am. J. Reprod. Immunol 76, 3–7. [DOI] [PubMed] [Google Scholar]
- Beer AE, Billingham RE The immunobiology of mammalian reproduction. New Jersey: Prentice Hall; 1976. [Google Scholar]
- Billington WD, 2003. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J. Reprod. Immunol 60 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- Bollopragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S, 2009. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol 200 (1), 104.e1–104.e11. [DOI] [PubMed] [Google Scholar]
- Blackburn DG, 1995. Saltationist and punctuated equilibrium models for the evolution of viviparity and placentation. J. Theor. Biol 174, 199–216. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, Steel JH, Christian M, Chan Y-W, Boomsma CM, Moore JD, Harthshorne GM, Šućurovic S, Mulac-Jericevic B, Hejinen CJ, Quenby S, Koerkamp MJG, Holstege FCP, Shmygol A, Macklon NS, 2014. Uterine selection of human embryos at implantation. Sci. Rep.-UK 4, 3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KM, Ho ECH, Hibino T, Schrankel CS, Schuh NW, Wang G, Rast JP, 2017. IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. eLife 6, e23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buentjen I, Drews B, Frankenberg SR, Hildebrandt TB, Renfree MB, Menzies BR, 2015. Characterisation of major histocompatibility complex class I genes at the fetal-maternal interface of marsupials. Immunogenetics 67, 385–393. [DOI] [PubMed] [Google Scholar]
- Cassatella MA, 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16 (1), 21–26. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ, 2000. Endocrine and paracrine regulation of birth at term and preterm. Endocr. Rev 21 (5), 514–550. [DOI] [PubMed] [Google Scholar]
- Chaouat G, 2013. Inflammation, NK cells and implantation: friend and foe (the good, the bad and the ugly?): replacing placental viviparity in an evolutionary perspective. J. Reprod. Immunol 97, 2–13. [DOI] [PubMed] [Google Scholar]
- Chaouat G, 2016. Reconsidering the Medawar paradigm placental viviparity existed for eons, even in vertebrates; without a “problem”: why are Tregs important for preeclampsia in great apes? J. Reprod. Immunol 114, 48–57. [DOI] [PubMed] [Google Scholar]
- Chavan AR, Bhullar B-AS, Wagner GP, 2016. What was the ancestral function of decidual stromal cells? A model for the evolution of eutherian pregnancy. Placenta 40, 40–51. [DOI] [PubMed] [Google Scholar]
- Chavan AR, Griffith OW, Wagner GP, 2017. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr. Opin. Genet. Dev 47, 24–32. [DOI] [PubMed] [Google Scholar]
- Chavan AR, Griffith OW, Stadtmauer D, Maziarz J, Pavličev M, Fishman R, Koren L, Romero R, Wagner GP, in preparation. Evolution of embryo implantation was enabled by the origin of decidual stromal cells in eutherian mammals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R, 2010. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am. J. Reprod. Immunol 63, 93–103. [DOI] [PubMed] [Google Scholar]
- Collins MK, Tay C-S, Erlebacher A, 2009. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest 119 (7), 2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo V, Bardelli A, Siena S, Abrignani S, 2018. Exploring the links between cancer and placenta development. Open Biol. 8: 180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, 1977. Why marsupials can’t win. Nature 265, 14–15. [Google Scholar]
- Creighton C, 1878. The formation of the placenta in the guinea-pig. J. Anat. Physiol 12, 534–590. [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM, 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol 10, 478–489. [DOI] [PubMed] [Google Scholar]
- Davies CJ, Eldridge JA, Fisher PJ, Schlafer DH, 2006. Evidence for expression of both classical and non-classical major histocompatibility complex class I genes in bovine trophoblast cells. Am. J. Reprod. Immunol 55, 188–200. [DOI] [PubMed] [Google Scholar]
- De M, Choudhuri R, Wood GW, 1991. Determination of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from mating through implantation. J. Leukocyte Biol 50 (3), 252–262. [DOI] [PubMed] [Google Scholar]
- Diener KR, Robertson SA, Hayball JD, Lousberg EL, 2016. Multi-parameter flow cytometric analysis of uterine immune cell fluctuations over the murine estrous cycle. J. Reprod. Immunol 113, 61–67. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Yáñez-Mó M, Sanchez-Madrid F, Simón C, 2005. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 19 (9), 1056–1060. [DOI] [PubMed] [Google Scholar]
- Douglas RH, Ginther OJ, 1973. Luteolysis following a single injection of prostaglandin F2α in sheep. J. Anim. Sci 37 (4), 990–993. [DOI] [PubMed] [Google Scholar]
- Eblen AC, Gercel-Taylor C, Nakajima ST, Taylor DD, 2002. Modulation of T-cell CD3-zeta chain expression in early pregnancy. Am. J. Reprod. Immunol 47 (3), 167–173. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C, 2004. Animal models of preterm birth. Trends Endocrin. Met 15 (10), 479–487. [DOI] [PubMed] [Google Scholar]
- Emera D, Romero R, Wagner G, 2011. The evolution of menstruation: a new model for genetic assimilation. Bioessays 34, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkenbrack EM, Maziarz JD, Griffith OW, Liang C, Chavan AR, Nnamani MC, Wagner GP, 2018. The mammalian decidual cell evolved from a cellular stress response. PLoS Biol. 16 (8), e2005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn CA, 1986. Implantation, menstruation and inflammation. Biol. Rev 61, 313–328. [DOI] [PubMed] [Google Scholar]
- Finn CA, Pope MD, 1991. Infiltration of neutrophil polymorphonuclear leucocytes into the endometrial stroma at the time of implantation of ova and the initiation of the oil decidual cell reaction in mice. J. Reprod. Fertil 91, 365–369. [DOI] [PubMed] [Google Scholar]
- Finn CA, 1996. Why do women menstruate? Historical and evolutionary review. Eur. J. Obstet. Gyn. R. B 70, 3–8. [DOI] [PubMed] [Google Scholar]
- Finn CA, 1998. Menstruation: a nonadaptive consequence of uterine evolution. Q. Rev. Biol 73 (2), 163–173. [DOI] [PubMed] [Google Scholar]
- Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Boutellier P, 2000. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J. Immunol 164 (12), 6100–6104. [DOI] [PubMed] [Google Scholar]
- Freyer C, Zeller U, Renfree MB, 2003. The marsupial placenta: a phylogenetic analysis. J. Exp. Zool 299a, 59–77. [DOI] [PubMed] [Google Scholar]
- Freyer C, Zeller U, Renfree MB, 2006. Placental function in two distantly related marsupials. Placenta 28, 249–257. [DOI] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang Z-Q, Kiessling LL, Rosen SD, Fisher SJ, 2003. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 299 (5605), 405–408. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R, 2008. Epidemiology and causes of preterm birth. Lancet 371 (9606), 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F, 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol 80, 122–131. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Guilbert LJ, Olson DM, 2010. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J. Leukocyte Biol 88, 625–633. [DOI] [PubMed] [Google Scholar]
- Good RA, Finstad J, 1964. Phylogenetic development of transplantation immunity. Ann. NY Acad. Sci 120, 15–20. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP, 2017. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, E6566–E6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP, 2018. Inflammation before implantation both in evolution and development. Proc. Natl. Acad. Sci. USA 115 (1), E3–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Wagner GP, 2017. The placenta as a model for understanding the origin and evolution of vertebrate organs. Nat. Ecol. Evol 1, 0072. [DOI] [PubMed] [Google Scholar]
- Griffiths M, McIntosh DL, Coles REA, 1969. The mammary gland of the echidna, Tachyglossus aculeatus, with observations on the incubation of the egg and on the newly-hatched young. J. Zool. Lond 158, 371–386. [Google Scholar]
- Griffiths M The biology of the monotremes. New York: Academic Press; 1978. [Google Scholar]
- Guillette LJ Jr., Lavia LA, Walker NJ, Roberts DK, 1984. Luteolysis induced by prostaglandin F2α in the lizard, Anolis carolinensis. Gen. Comp. Endocr 56 (2), 271–277. [DOI] [PubMed] [Google Scholar]
- Guillette LJ Jr., Masson GR, DeMarco V, 1991. Effects of prostaglandin F2α prostaglandin E2 and arachidonic acid on the induction of oviposition in vivo and in vitro in oviparous lizards. Prostagl. 42 (6), 533–540. [DOI] [PubMed] [Google Scholar]
- Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD, 2009. Dual nature of the adaptive immune system in lampreys. Nature 459, 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgunset J, Johnsen H, Kjøllesdal AM, Qvigstad E, Espevik T, Austgulen R, 1994. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur. J. Obstet. Gynecol. R. B 56, 153–160. [DOI] [PubMed] [Google Scholar]
- Hansen VL, Faber LS, Salehpoor AA, Miller RD, 2017. A pronounced uterine pro-inflammatory response at parturition is an ancient feature in mammals. P. R. Soc. B 284, 20171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen VL, Schilkey FD, Miller RD, 2016. Transcriptomic changes associated with pregnancy in a marsupial, the gray short-tailed opossum Monodelphis domestica. PLoS One 11 (9), e0161608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JD, Stonerook MJ, Pondy J, 1993. Gestation and placentation in two new world opossums: Didelphis virginiana and Monodelphis domestica. J. Exp. Zool 266 (5), 463–479. [DOI] [PubMed] [Google Scholar]
- Hargrove TL, Ottinger MA, 1992. Induced oviposition of precalcified eggs following prostaglandin administration. Poultry Sci. 71 (3), 548–552. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Battaglia A, 2009. Breeding behavior of the platypus (Ornithorhynchus anatinus) in captivity. Aust. J. Zool 57 (4), 283–293. [Google Scholar]
- Heil M, Land WG, 2014. Danger signals – damaged-self recognition across the tree of life. Front. Plant Sci 5, 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds LA, Tyndale-Biscoe CH, Shaw G, Fletcher TP, Renfree MB, 1990. Effects of prostaglandin and prolactin on luteolysis and parturient behaviour in the non-pregnant tammar, Macropus eugenii. Reproduction 88 (1), 323–333. [DOI] [PubMed] [Google Scholar]
- Holmlund U, Wähämaa H, Bachmayer N, Bremme K, Sverremark-Ekström E, Palmblad K, 2007. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology 122, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RL, 1962. Reproduction in the macropod marsupial Potorous tridactylus (Kerr). Aust. J. Zool 10 (2), 193–224. [Google Scholar]
- Hughes RL Egg membranes and ovarian function during pregnancy in monotremes and marsupials In: Calab JH, Tyndale-Biscoe CH, editors: Reproduction and Evolution. Canberra: Australian Academy of Sciences; 1977. p. 281–291. [Google Scholar]
- Hughes RL, Hall LS, 1998. Early development and embryology of the platypus. Philos. T. Roy. Soc. B 353, 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JS, Vassmer D, Ferguson TA, Miller L, 1997. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and conceptus. J. Immunol 158, 4122–4128. [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R, 2015. Control of adaptive immunity by the innate immune system. Nat. Immunol 16 (4), 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantra S, Bigliardi E, Brizzi R, Ietta F, Bechi N, Paulesu L, 2007. Interleukin 1 in oviductal tissues of viviparous, oviarpous, and ovuliparous species of amphibians. Biol. Reprod 76, 10091015. [DOI] [PubMed] [Google Scholar]
- Kabututu Z, Manin M, Pointud J-C, Maruyama T, Nagata N, Lambert S, Lefrançois-Martinez A-M, Martinez A, Urade Y, 2009. Prostaglandin F2α synthase activities of aldo-keto reductase 1B1, 1B3 and 1B7. J. Biochem 145 (2), 161–168. [DOI] [PubMed] [Google Scholar]
- Kahn DA, Baltimore D, 2010. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc. Natl. Acad. Sci. USA 107 (20), 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim SMM, Trussell RR, Patel RC, Hillier K, 1968. Response of pregnant human uterus to prostaglandin-F2α-induction of labour. Brit. Med. J 4, 621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katila T, 2012. Post-mating inflammatory responses of the uterus. Reprod. Domest. Anim 47(5), 31–41. [DOI] [PubMed] [Google Scholar]
- Kelly RW, 1996. Inflammatory mediators and parturition. J. Reprod. Fertil 106, 89–96. [DOI] [PubMed] [Google Scholar]
- McKeown KJ, Challis JRG, Small C, Adamson L, Bocking AD, Fraser M, Rurak D, Riggs KW, Lye SJ, 2000. Altered fetal pituitary-adrenal function in the ovine fetus treated with RU486 and meloxicam, an inhibitor of prostaglandin synthase-II. Biol. Reprod 63, 1899–1904. [DOI] [PubMed] [Google Scholar]
- Keski-Nisula L, Aalto M-L, Katila M-L, Kirkinen P, 2000. Intrauterine inflammation at term: a histopathologic study. Hum. Pathol 31 (7), 841–846. [DOI] [PubMed] [Google Scholar]
- Keys JL, King GJ, Kennedy TG, 1986. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biol. Reprod 34, 405–411. [DOI] [PubMed] [Google Scholar]
- Killian JK, Buckley TR, Stewart N, Munday BL, Jirtle RL, 2001. Marsupials and eutherians reunited: genetic evidence for the Theria hypothesis of mammalian evolution. Mamm. Genome 12, 513–517. [DOI] [PubMed] [Google Scholar]
- Kin K, Maziarz J, Wagner GP, 2014. Immunohistological study of the endometrial stromal fibroblasts in the opossum, Monodelphis domestica: evidence for homology with eutherian stromal fibroblasts. Biol. Reprod 90 (5):111, 1–12. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL, 2008. How dying cells alert the immune system to danger. Nat. Rev. Immunol 8, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, Medzhitov R, 2015. Homeostasis, inflammation, and disease susceptivility. Cell 160, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss A, Cheng Y, Kimble F, Wells B, Donovan S, Belov K, Woods GM, 2011. Allorecognition in the Tasmanian devil (Sarcophilus harrisii), an endangered marsupial species with limited genetic diversity. PLoS One 6 (7), e22402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupittayanant S, Kupittayanant P, Suwannachat C, 2009. Mechanisms of uterine contractility in laying hens. Anim. Reprod. Sci 115, 215–224. [DOI] [PubMed] [Google Scholar]
- Kvirkvelia N, Vojnovic I, Warner TD, Athie-Morales V, Free P, Rayment N, Chain BM, Rademacher TW, Lund T, Roitt IM, Delves PJ, 2002. Placentally derived prostaglandin E2 acts via the EP4 receptor to inhibit IL-2-dependent proliferation of CTLL-2 T cells. Clin. Exp. Immunol 127, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanman JT, Herod L, 1965. Homograft immunity in pregnancy: the placental transfer of cytotoxic antibody in rabbits. J. Exp. Med 122 (3), 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegraven JA, 1975. Biological considerations of the marsupial-placental dichotomoy. Evolution 29, 707–722. [DOI] [PubMed] [Google Scholar]
- Litman GW, Rast JP, Fugmann SD, 2010. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol 10, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY, 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858. [DOI] [PubMed] [Google Scholar]
- Liu J-L, 2018. Implantation in eutherians: which came first, the inflammatory reaction or attachment? Proc. Natl. Acad. Sci. USA 115 (1), E1–E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L, 1907. Ueber die experimentelle Erzeugung von Knoten von Deciduagewebe in dem Uterus des Meerschweinchens nach stattgefundener. Copulation Zentralbl. Allg. Pathol. Pathol. Anat 18, 563–565. [Google Scholar]
- Loeb L, 1908. The production of deciduomata and the relation between the ovaries and the formation of the decidua. J. Am. Med. Assoc 50 (3), 1897–1901. [Google Scholar]
- Lombardi J Comparative vertebrate reproduction. Boston: Kluwer Academic Publishers; 1998. [Google Scholar]
- Lynch VJ, Nnamani MC, Kapusta A, Brayer K, Plaza SL, Mazur EC, Emera D, Sheikh SZ, Grutzner F, Bauersachs S, Graf A, Young SL, Lieb JD, DeMayo FJ, Feschotte C, Wagner GP, 2015. Ancient transposable elements transformed the uterine regulatory landscape and transcriptome during the evolution of mammalian pregnancy. Cell Rep. 10, 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon NS, Brosens JJ, 2014. The human endometrium as a sensor of embryo quality. Biol. Reprod 91 (4), 98, 1–8. [DOI] [PubMed] [Google Scholar]
- Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, Moffett A, Colucci F, Hemberger M, 2011. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl. Acad. Sci. USA 108, 4012–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen O,. Mammals (Mammalia) In: Kumar SB, editor. The Timetree of Life. New York: Oxford University Press; 2009. p. 459–461. [Google Scholar]
- Mahmoud IY, Cyrus RV, McAsey ME, Cady C, Woller MJ, 1988. The role of arginine vasotocin and prostaglandin F2α on oviposition and luteolysis in the common snapping turtle Chelydra serpentina. Gen. Comp. Endocr 69, 56–64. [DOI] [PubMed] [Google Scholar]
- Marshall LG, 1979. Evolution of metatherian and eutherian (mammalian) characters: a review based on cladistic methodology. Zool. J. Linn. Soc.-Lond 66, 369–410. [Google Scholar]
- Masters SL, Simon A, Aksentijevich I, Kastner DL, 2009. Horror Autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol 27, 621–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P, 1994. Tolerance, danger, and the extended family. Annu. Rev. Immunol 12, 991–1045. [DOI] [PubMed] [Google Scholar]
- Medawar PB, 1953. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Sym. Soc. Exp. Biol 7, 320–388. [Google Scholar]
- Mess A, Carter AM, 2006. Evolutionary transformations of fetal membrane characters in Eutheria with special reference to Afrotheria. J. Exp. Zool. Part B 306 (2), 140–163. [DOI] [PubMed] [Google Scholar]
- Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M, 2006. Cutting edge: extracellular high mobility grou box-1 protein is a proangiogenic cytokine. J. Immunol 176, 12–15. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke YW, 2004. The immunological paradox of pregnancy: a reapprisal. Placenta 25, 1–8. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C, 2006. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol 6, 584–594. [DOI] [PubMed] [Google Scholar]
- Moors PJ, 1974. The foetal-maternal relationship and its significance in marsupial reproduction: a unifying hypothesis. Aust. Mammal 1, 263–266. [Google Scholar]
- Moriuchi H, Koda N, Okuda-Ashitaka E, Daiyasu H, Ogasawara K, Toh H, Ito S, Woodward DF, Watanabe K, 2008. Molecular characterization of a novel type of prostamide/prostaglandin F synthase, belonging to the thioredoxin-like superfamily. J. Biol. Chem 283 (2), 792–801. [DOI] [PubMed] [Google Scholar]
- Mor G, Aldo P, Alvero AB, 2017. The unique immunolgical and microbial aspects of pregnancy. Nat. Rev. Immunol 17, 469–482. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I, 2010. The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol 63, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S, 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. NY Acad. Sci 1221, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancy P, Siewiera J, Rizzuto G, Tagliani E, Osokine I, Manandhar P, Dolgalev I, Clementi C, Tsirigos A, Erlebacher A, 2018. H3K27me3 dynamics dictate evolving uterine states in pregnancy and parturition. J. Clin. Invest 128 (1), 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha LE, Antczak DF, 2010. Maternal immune responses to trophoblast: the contribution of the horse to pregnancy immunology. Am. J. Reprod. Immunol 64, 231–244. [DOI] [PubMed] [Google Scholar]
- Novy MJ, Piasecki G, Jackson BT, 1974. Effect of prostaglandins E2 and F2α on umbilical blood flow and fetal hemodynamics. Prostagl. 5 (6), 545–555. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R 2016. Tissue biology perspective on macrophages. Nat. Immunol 17 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- Okin D Medzhitov R, 2012. Evolution of inflammatory diseases. Curr. Biol 22, R733–R740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A, Abraham E, 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem 279 (9), 7370–7377. [DOI] [PubMed] [Google Scholar]
- Paulesu L, Janta S, Ietta F, Brizzi R, Bigliardi E, 2008. Interleukin-1 in reproductive strategies. Evol. Dev 10 (6), 778–788. [DOI] [PubMed] [Google Scholar]
- Pavličev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, Kallapur SG, Muglia L, Jones H, 2017. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. 27, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JS, 1953. The reproduction of the African elephant, Loxodonta africana. Philos. T. Roy. Soc. B 237 (643), 93–149. [Google Scholar]
- Purdy DM, Hillemann HH, 1950. Prenatal growth in the golden hamster (Cricetus auratus). Anat. Rec 106 (4), 591–597. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Al-Zamil H, Hunt LP, Fortier MA, López Bernal A, 2011. Genes for prostaglandin synthesis, transport and inactivation are differentially expressed in human uterine tissues, and the prostaglandin synthase AKR1B1 is induced in myometrial cells by inflammatory cytokines. Mol. Hum. Reprod 17 (1), 1–13. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Fortier MA, López Bernal A, 2014. Prostaglandin pathway gene expression in human placenta, amnion, and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childb. 14, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole WE, 1975. Reproduction in the two species of grey kangaroos, Macropus giganteus (Shaw) and M. fulinginosus (Desmarest). II. Gestation, parturition, and pouch life. Aust. J. Zool 23 (3), 333–353. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, Long EO, 2005. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PloS Biol. 4 (1), e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin JH, Phernetton TM, 1976. Effect of prostaglandin E2 on ovine maternal placental blood flow. Am. J. Physiol 231 (3), 754–759. [DOI] [PubMed] [Google Scholar]
- Rauk PN, Chiao J-P, 2000. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am. J. Reprod. Immunol 43 (3), 152–159. [DOI] [PubMed] [Google Scholar]
- Renfree MB, 2000. Maternal recognition of pregnancy in marsupials. Rev. Reprod 5, 6–11. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Shaw G, Fletcher TP, 1994. Evidence for the essential role of prostaglandins for parturition in a marsupial, Macropus eugenii. J. Reprod. Fertil 102, 433–436. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Shaw G, 2014. Embryo-endometrial interactions during early development after embryonic diapause in the marsupial tammar wallaby. Int. J. Dev. Biol 58, 175–181. [DOI] [PubMed] [Google Scholar]
- Rismiller PD, McKelvey MW, 2000. Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus. J. Mammal 81 (1), 1–17. [Google Scholar]
- Robertson SA, Bromfield JJ, Tremellen KP, 2003. Seminal “priming” for protection from pre-eclampsia – a unifying hypothesis. J. Reprod. Immunol 59 (2), 253–265. [DOI] [PubMed] [Google Scholar]
- Rodger JC, Fletcher TP, Tyndale-Biscoe CH, 1985. Active anti-paternal immunization does not affect the success of marsupial pregnancy. J. Reprod. Immunol 8, 249–256. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK, 2006. Inflammation in preterm and term labor and delivery. Sem. Fetal Neonat. M 11 (5), 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RW, 1989. Embryonic growth rates of marsupials with a note on monotremes. J. Zool 218 (1), 11–16. [Google Scholar]
- Rose R, Fadem BH, 2000. The hormonal control of birth behavior in the gray short-tailed opossum (Monodelphis domestica). Horm. Behav 37, 163–167. [DOI] [PubMed] [Google Scholar]
- Rovere-Querini P, Castiglioni MT, Sabbadini MG, Manfredi AA, 2007. Signals of cell death and tissue turnover during physiological pregnancy, pre-eclampsia, and autoimmunity. Autoimmunity 40 (4), 290–294. [DOI] [PubMed] [Google Scholar]
- Rowley AF, 1996. The evolution of inflammatory mediators. Mediat. Inflamm 5, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YC, Guo JH, Liu X, Zhang R, Tsang LT, Dong JD, Chen H, Yu MK, Jiang X, Zhang XH, Fok KL, Chung YW, Huang H, Zhou WL, Chan HC 2012. Activation of the epithelial Na+ channel triggers prostaglandin E2 release and production required for embryo implantation. Nat. Med 18 (7), 1112–1118. [DOI] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME, 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195. [DOI] [PubMed] [Google Scholar]
- Selwood L, 2000. Marsupial egg and embryo coats. Cells Tissues Organs 166, 208–219. [DOI] [PubMed] [Google Scholar]
- Sharman GB, 1961. The embryonic membranes and placentation in five genera of diprotodont marsupials. P. Zool. Soc. Lond 137, 197–220. [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, Lye SJ, 2013. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J. Cell. Mol. Med 17 (2), 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC, 2004. Implantation mechanisms: insights from the sheep. Reproduction 128 (6), 657–668. [DOI] [PubMed] [Google Scholar]
- Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O, Saito S, 2015. Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasmapriming in allogeneic mouse pregnancy. J. Reprod. Immunol 108, 72–82. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R, 2007. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol 9 (3), 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT, 2004. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunol. 112, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z-H, Li Z-Y, Li D-D, Fang W-N, Liu H-Y, Yang D-D, Meng C-Y, Yang Y, Peng J-P, 2016. Seminal plasma induces inflammation in the uterus through the γδ/IL-17 pathway. Sci. Rep 6, 25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, 1983. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos 41, 173–187. [Google Scholar]
- Stone WH, Manis GS, Hoffman ES, Saphire DG, Hubbard GB, VandeBerg JL, 1997. Fate of allogeneic skin transplantations in a marsupial (Monodelphis domestica). Lab. Anim. Sci 47 (3), 283–287. [PubMed] [Google Scholar]
- Sunderland CA, Naiem M, Mason DY, Redman CWG, Stirrat GM, 1981. The expression of major histocompatibility antigens by human chorionic villi. J. Reprod. Immunol 3 (6), 323–331. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJR, Cameroon IT, Greer IA, Norman JE, 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum. Reprod 14 (1), 229–236. [PubMed] [Google Scholar]
- Tilburgs T, Crespo AC, van der Zwan A, Rybalov B, Raj T, Stranger B, Gardner L, Moffet A, Strominger JL, 2015. Human HLA-G+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proc. Natl. Acad. Sci. USA 112 (23), 7219–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons BC, Fairhurst A-M, Mahendroo MS, 2009. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J. Immunol 182, 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J, Betz AG, 2006. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol 7, 241–246. [DOI] [PubMed] [Google Scholar]
- Volumenie JL, Mognetti B, de Smedt D, Menu E, Chaouat G, 1997. Induction of transient murine T cell anergy by a low molecular weight compound obtained from supernatants of human placental cultures is linked to defective phosphorylation of TCR CD3 chain. Am. J. Reprod. Immunol 40 (1), 47–62. [DOI] [PubMed] [Google Scholar]
- Waddington CH 1942. Canalization of development and the inheritance of acquired characters. Nature 3811, 563–565. [DOI] [PubMed] [Google Scholar]
- Waddington CH, 1953. Genetic assimilation of an acquired character. Evolution 7 (2), 118–126. [Google Scholar]
- Walker KZ, Tyndale-Biscoe CH, 1978. Immunological aspects of gestation in the tammar wallaby, Macropus eugenii. Aust. J. Biol. Sci 31, 173–182. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Kin K, Muglia L, Pavličev M, 2014. Evolution of mammalian pregnancy and the origin of the decidual stromal cell. Int. J. Dev. Biol 58, 117–126. [DOI] [PubMed] [Google Scholar]
- Wegmann TG, Waters CA, Drell DW, Carlson GA, 1979. Pregnant mice are not primed but can be primed to fetal alloantigens. Proc. Natl. Acad. Sci. USA 76 (5), 2410–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R, 2006. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl. Acad. Sci. USA 103 (9), 3203–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Mancuso N, Bowles VM, 1999. Biochemical aspects of egg hatch in endo- and ectoparasites: potential for rational drug design. Int. J. Parasitol 29, 861–867. [DOI] [PubMed] [Google Scholar]
- Zeller U, Freyer C 2001. Early ontogeny and placentation of the grey short-tailed opossum, Monodelphis domestica (Didelphidae: Marsupialia): contribution to the reconstruction of the marsupial morphotype. J. Zool. Syst. Evol. Res 39, 137–158 [Google Scholar]