Abstract
We tested the hypothesis that exposure to intermittent hypoxia (IH) during pregnancy would prolong the laryngeal chemoreflex (LCR) and diminish the capacity of serotonin (5-hydroxytryptamine; 5-HT) to terminate the LCR. Prenatal exposure to IH was associated with significant prolongation of the LCR in younger, anesthetized, postnatal day (P) rat pups age P8 to P16 compared to control, room air (RA)-exposed rat pups of the same age. Serotonin microinjected into the NTS shortened the LCR in rat pups exposed to RA during gestation, but 5-HT failed to shorten the LCR in rat pups exposed to prenatal IH. Given these observations, we tested the hypothesis that prenatal hypoxia would decrease binding to 5-HT3 receptors in the nucleus of the solitary tract (NTS) where 5-HT acts to shorten the LCR. Serotonin 3 receptor binding was reduced in younger rat pups exposed to IH compared to control, RA-exposed rat pups in the age range P8 to P12. Serotonin 3 receptor binding was similar in older animals (P18-P24) regardless of gas exposure during gestation. The failure of the 5-HT injected into the NTS to shorten the LCR was correlated with a developmental decrease in 5-HT3 receptor binding in the NTS associated with exposure to prenatal IH. In summary, prenatal IH sensitized reflex apnea and blunted processes that terminate reflex apneas in neonatal rat pups, processes that are essential to prevent death following apneas such as those seen in babies who died of SIDS.
Keywords: Laryngeal chemoreflex, serotonin, 5-HT3 receptor, autonomic, neuroscience, SIDS, intermittent hypoxia, autoradiography
Introduction1
We have been investigating the proximate causes of Sudden Infant Death Syndrome (SIDS) and asphyxial deaths by modeling the risk factors for SIDS and asphyxial deaths in neonatal animals. Our investigations are based on a two-step hypothesis in which SIDS occurs in infants who, first, have an unusual propensity for prolonged reflex apneas, and second, lack properly effective mechanisms to terminate apneas, restore regular eupneic breathing, and arouse (Donnelly et al., 2016; Donnelly et al., 2017; Leiter and Böhm, 2007). Our studies related to the first part of this hypothesis - what factors increase the sensitivity and strength of reflex apneas - examined the laryngeal chemoreflex (LCR). The LCR follows stimulation of the larynx by water or acid and consists of a complex set of behaviors, apnea (sometimes profound), swallowing, coughing, sometimes bradycardia and redistribution of blood flow to vital organs (Boggs and Bartlett, 1982; St. Hilaire et al., 2005). Collectively, these behaviors appear to have evolved to remove foreign substances from the airway, protect against further entry of foreign substances into the airway, and enhance survival during the periods of hypoxic stress that can result from apnea associated with this reflex (Leiter and Böhm, 2007; van der Velde et al., 2003). Many investigators feel that the LCR is the first step in a series of physiological events that result in SIDS and asphyxial death in human infants; a process that starts with respiratory inhibition and apnea (Downing and Lee, 1975; Page and Jeffery, 2000; Thach, 2001) and ends with failed autoresuscitation, failed termination of apnea, failed restoration of regular breathing, failed arousal from sleep, and death (Meny et al., 1994; Poets et al., 1999; Poets et al., 1993; Sridhar et al., 2003). Environmental exposures, such as maternal nicotine and tobacco smoke exposure (Xia et al., 2009; Xia et al., 2010) and thermal stress (Xia et al., 2011), and internally generated factors like inflammation (Xia et al., 2016) are known to be risk factors for SIDS, and all these factors increased the strength of the LCR.
More recently, we turned our attention to the second part of the hypothesis – the failure to terminate apnea and restore eupneic breathing. A subset of babies who died of SIDS appear to have deficient serotonergic function (Duncan et al., 2010; Kinney and Haynes, 2019 ; Kinney et al., 2003; Machaalani et al., 2009; Panigrahy et al., 2000; Paterson et al., 2006b). Similar medullary neurochemical abnormalities were present in both infants dying suddenly in circumstances consistent with asphyxia and infants dying suddenly in circumstances without associated evidence of asphyxia (Randall et al., 2013), raising the possibility that infants who die in circumstances consistent with asphyxia may also have difficulty terminating apneas and restoring eupneic breathing. The activity of serotonergic neurons is strongly associated with the waking state (Heym et al., 1982; Jacobs and Fornal, 1999; Veasey et al., 1995) and may be associated with arousal responses in the transition from sleep to wakefulness (Buchanan and Richerson, 2010; Donnelly et al., 2016; Leiter and Böhm, 2007). Thus, any hypothesis about the pathogenesis of SIDS and possible asphyxial death in babies will naturally focus on a potential deficiency of arousal mechanisms and restoration of eupnea (Leiter and Böhm, 2007).
The multiplicity of maternal risk factors for SIDS and asphyxial deaths has led to the hypothesis that prenatal abnormalities may contribute to the occurrence of SIDS (Carlin and Moon, 2017; Hoffman and Hillman, 1992; Leiter and Böhm, 2007). There has been a long standing hypothesis that prenatal hypoxia is the most likely intrauterine event associated with an increased risk of SIDS (Becker, 1990; Kinney et al., 1983; Naeye, 1980; Takashima et al., 1978; Valdes-Dapena, 1986; Valdes-Dapena et al., 1976). In previous studies, 5-HT, originating from the caudal raphe and acting thorough 5-HT3 receptors in the NTS, substantially shortened the LCR and allowed regular breathing to be restored more quickly after the LCR was elicited (Donnelly et al., 2016; Donnelly et al., 2017). Therefore, if prenatal hypoxia is an important risk factor for SIDS and if our two-step hypothesis of the cause of sudden death in infants is correct, then prenatal hypoxia should increase the apparent potency or sensitivity of the LCR, or reduce the power of 5-HT activity in the NTS to terminate apneas and restore eupnea, or both. Therefore, we studied the effect of exposure to intermittent hypoxic (IH) during pregnancy on the LCR and the effect of 5-HT microinjected into the NTS on the LCR in neonatal rat pups between postnatal day P8 and P20, an age range that we believe is behaviorally and developmentally analogous to the period of vulnerability to SIDS in human infants. Because our previous studies demonstrated that 5-HT3 receptors mediated the serotonergic termination of the LCR within the NTS, we also tested the hypothesis that prenatal hypoxia would decrease binding to 5-HT3 receptors in the NTS.
Material and Methods
Ethical Approval
The Institutional Animal Care and Use Committee of Dartmouth College approved all surgical and experimental protocols. We included both male and female Sprague-Dawley rat pups ranging in age from post-natal day 8 (P8) to P24 in all sets of experiments.
Intrauterine hypoxia
We placed Sprague-Dawley pregnant dams carrying fetuses between embryonic age day 4 and day 6 (e4-e6) into either a chamber supplied with normal room air (RA) oxygen levels (21%) or a chamber that we intermittently made hypoxic (IH). The IH chamber was equipped with oxygen sensors, nitrogen and oxygen gas supply lines, and mixing valves (OxyCycler Model A84, Biospherix, Lacona, NY) under software control (WatView Runtime v. 2.5.63, Watflow Anafaze, Watsonville, CA) designed to allow sensor-driven control over chamber oxygen levels. We generated intermittent hypoxia using software written to alternate one hour of RA with one - hour intermittent hypoxia so that there were 12 hours of IH interleaved with 12 hours of RA (See Figure 1). During each hour of IH, there were five -minute hypoxic intervals, during which chamber oxygen dropped rapidly to a nadir of 4.5-7.5% oxygen and during the following 5 minutes, chamber oxygen levels returned to 21% oxygen (see Figure 1). Thus, there were six, 5-minute intervals of hypoxia interleaved with RA in each hour of intermittent hypoxia and 12 hours of this alternating IH interleaved with twelve, one-hour periods of RA. Because the pressure in our nitrogen supply decreased over the course of each hour of hypoxia, the actual nadir of the oxygen concentration became less hypoxic in a relatively linear fashion from ~4.5% fraction inspired oxygen level (FIO2) at the start of each hypoxia hour to ~7.5% FIO2 during the final hypoxic interval of each hour (see lower panel in Figure 1). The control, RA chamber was fed by tubing connected to controllers that opened to allow pressurized air to enter the chamber following a temporal pattern similar to that experienced by animals in the IH chamber so that the noise and gas flows experienced by animals in the IH chamber were identical to those experienced by dams in the control, RA chamber. Dams were kept in the chambers until they delivered and then removed to RA, and postnatal care and development of the rat pups occurred under normoxic conditions. Animals were checked daily in the morning except when a delivery was expected; at which point, we checked the animals twice a day.
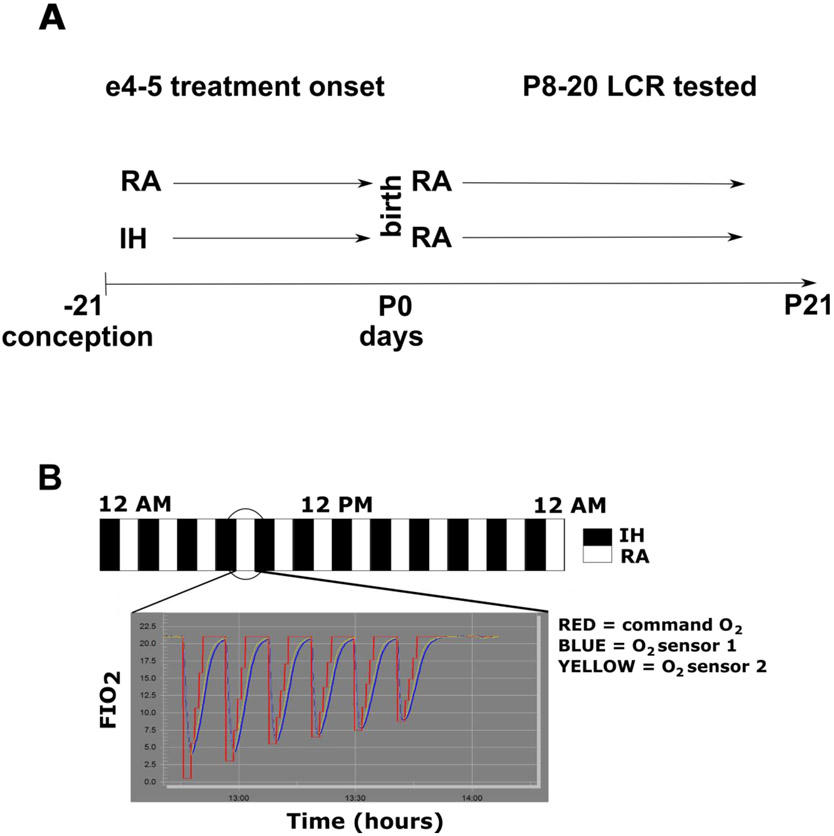
Figure 1:
A schematic of the experimental design is shown above (A). Two groups of pregnant dams were exposed either to room air (RA) or intermittent hypoxia (IH) starting at embryological age 4-5 days. Once the rat pups were born (postnatal day 0), the mothers and pups from both treatment groups were removed from the exposure chamber and each litter and mother were placed in RA in a plexiglass cage with appropriate bedding and food and water ad libitum. Pups were selected for LCR testing between P8 and P20. The scheme of IH is shown in panel B. Intermittent hypoxia, nominally 5% FIO2 (red command O2 line), was administered every other 5-minute interval of every other hour 24 hours per day (so alternate hours of intermittent hypoxia or room air) for the duration of the exposure, which started on e4 or e5 of the pregnancy and ended when the rat pups were born (usually an exposure of 17 days). The RA mothers were housed in RA, but adjacent to the hypoxia-exposure chamber and were exposed to the same environmental noises as the solenoids controlling gas entry into the chambers were activated by the sequence of intermittent gas changes. The exposure chambers are large and accommodated 2-3 pregnant dams, but the size of the chamber meant that the time constant of gas exchange was long. Therefore, 5% FIO2 resulted in an approximately exponential fall in the FIO2 within the hypoxic chamber, and 5% hypoxia was not consistently reached (blue or yellow actual O2 measured by chamber sensors). The inset shows that the nadir of each hypoxic interval was quite brief within each 5 minutes of hypoxia. Moreover, the pressure in the nitrogen supply reservoir decreased over the course of each hour of hypoxia, and therefore, the actual nadir of the oxygen concentration became less hypoxic in a relatively linear fashion from ~4.5% to a value less than 10% in the final hypoxic episode of each hour of IH.
Surgical preparation of rat pups
We determined the sex and weight of each pup, and then administered 1.2-1.5 g/kg urethane (U2500, Sigma-Aldrich, St. Louis, MO) and 10 mg/kg chloralose (C0128, Sigma-Aldrich) in 0.9% saline by intraperitoneal injection. We maintained body temperature at ~34 °C using a rectal temperature probe (RET-4, Physitemp, Clifton, NJ) connected to a Physitemp TCAT-2 DF servo-controller that regulated a heat lamp positioned over the animal. After each animal reached a surgical plane of anesthesia (lack of withdrawal reflex or significant change in heart or respiratory rate following a toe pinch), we inserted two 30-gauge platinum needle electrodes (FE2M, Grass Technologies, West Warwick, RI) subcutaneously on the left upper chest wall to record electrocardiogram (ECG) activity. We amplified (100X) and filtered (low cutoff 10 Hz, high cutoff 500 Hz) signals from the ECG leads using a DAM-80 AC Differential Amplifier (World Precision Instruments, Sarasota, FL). We inserted a second pair of needle electrodes into the intercostal muscles in the region of the ninth through eleventh ribs on the lower right costal margin to monitor respiratory electromyographic (EMG) activity. We secured both electrode pairs with small amounts of cyanoacrylate glue. We amplified (1000X) and filtered (low cutoff 10 Hz, high cutoff 500 Hz, 60 Hz notch) the EMG signals using Iso-DAM8A amplifiers (WPI, Sarasota, FL). We sampled both ECG and EMG signals at 1 kHz using a USB-6009 driven by a custom LabVIEW program (National Instruments, Austin, TX).
We placed each animal supine, exposed the trachea and larynx, incised the anterior wall of the trachea circumferentially between adjacent rings of cartilage and placed a polytetrafluoroethylene breathing tube (19-23 gauge) in the caudal segment. We inserted a length of PE-50 tubing into the rostral trachea and advanced it until its tip was near the caudal border of the larynx; this tubing was used to deliver microliter amounts of distilled water via a syringe pump to the larynx to elicit the LCR. We secured both tubes into the trachea using 4-0 silk sutures and cyanoacrylate adhesive. Next, we placed the animal in the prone position on the edge of an elevated platform and secured its head in a ventri-flexed position using surgical tape. We exposed the foramen magnum by removing the skin and bluntly dissecting the overlying muscle tissue. We used the tip of a 32-gauge needle to incise the membranes covering the foramen. This exposed the cerebrospinal fluid and floor of the fourth ventricle to provide access for focal microinjections. We made 50-100 nanoliter, focal injections through fine, pulled glass pipettes using a Picospritzer II (Parker Hannifin, Cleveland, OH). Bilateral microinjections were made just caudal and lateral (within 1 mm in both directions) to the termination of the floor of the fourth ventricle in the medial portion of the gracile nucleus, and unilateral microinjections were made in the midline just caudal to the termination of the floor of the fourth ventricle. The glass pipettes were held in a micromanipulator perpendicular to the surface of the brainstem to allow precise control over the location and depth of microinjection. We microinjected 4 mM 5-HT diluted in normal saline. We used a high dose of 5-HT to try to saturate 5-HT receptors in the medial caudal NTS. All microinjections included 0.02% of fluorescent 0.4 μ diameter carboxylated latex microspheres (Fluoresbrite, PolySciences, Inc., Warrington, PA) for verification of injection locations.
LCR protocol
After opening the foramen magnum, we delivered small volumes of water rostrally into the larynx via syringe pump to elicit the LCR. We started with 2-4 μL and increased the volume until an LCR-associated apnea of a consistent, moderate duration (usually two to five seconds) was elicited. The water volumes required to elicit the LCR were usually between 8 – 20 μl. Once established in each animal, we held the stimulus volume constant for the entire duration of each experiment in each pup. We gave a minimum of three stimulations using the effective volume to establish a baseline LCR response. Next, we administered 4 mM 5-HT in normal saline by focal microinjection, after which we gave a minimum of three additional LCR stimulations. We waited ten to twenty minutes between stimulations to allow the animal to clear the water from its larynx, to allow breathing to recover and to minimize accommodation to the stimulus. After each experiment, we euthanized the animal by an anesthetic overdose. We decapitated each animal that received a microinjection and carefully removed its brain to determine the injection location.
Neuroanatomy
We fixed the brains of microinjected animals by immersion in approximately twenty volumes of 4% paraformaldehyde in 10 mM phosphate buffered saline for a minimum of 48 hours, and cryoprotected them by immersion in tubes of 30% sucrose in phosphate buffered saline until they no longer floated. We blocked the brains, froze them in an aqueous cutting medium (Tissue-Tek O.C.T., Sakura Finetek, Torrance, CA) and cut 40-0 micron, coronal sections on a cryostat (CM 3050 S, Leica, Buffalo Grove, IL). Each coronal section was examined and photographed immediately using an E800 microscope (Nikon, Melville, NY) with a mercury arc light source with appropriate filters for the fluorescence profile of the beads used. We compared anatomical landmarks visible on each slice with those shown on atlas plates (Paxinos and Watson, 1998) to determine the location of each injection in the brainstem and plotted each location on the atlas plate that corresponded most closely with its position in the brain. Sections from two animals in the IH group could not be analyzed for the location of the microinjections.
5-HT3 autoradiography
From a separate group of dams exposed to IH or RA as described above, we selected pups between ages P7 and P24, and anesthetized each animal before euthanizing it. The brain from each of these animals was removed, frozen and stored at −80 °C until sectioned at 20 microns. Sections were collected serially onto sets of 4 slides such that each slide contained every fourth section. Slides were stored at −80 °C until processed for autoradiography.
Slides were removed from the −80 °C, placed at 4 °C for 2 hours and then dried at room temperature for 1 hour. For each experiment, 1 set of slides was used for total radioligand binding and a second set of slides was processed to determine non-specific binding. The incubation of 5-HT3 receptor antagonist, 3H GR65630, was performed according to Kilpatrick et al. (Kilpatrick et al., 1988), with slight modification. Slides were pre-incubated at room temperature in buffer containing 50 mM HEPES (pH 7.4) for 15 minutes. For total binding, slides were incubated for 2 hours at room temperature in 50 mM HEPES (pH 7.4) containing 0.2 nM 3H GR65630 (Perkin Elmer, Boston, Ma). Non-specific binding was determined with 30 uM metoclopramide (Sigma-Aldrich, St. Louise, MO). Incubated slides were washed 2 times for 2 minutes each in 50 mM HEPES (pH 7.4) followed by a 10 second rinse in water. Slides were air dried overnight and exposed to a Fuji phosphoimaging plate (BAS-TR2025) (GE Healthcare Bio-Sciences; Pittsburgh, PA) for 3 weeks with a 3H standard slide (American Radiolabeled Chemicals, Inc, St. Louise, MO). The standard permitted conversion of optical density of silver grains to femtomoles per mg (fmol/mg) of tissue to quantitate the binding results.
After 3 weeks, the Fuji plates were exposed on a Typhoon FLA 7000 phosphoimager (GE Healthcare Bio-Sciences; Pittsburgh, PA) to obtain a digital autoradiographic image. Quantitative densitometry of autoradiograms was performed using an MCID imaging system (Imaging Research Inc, St. Catharines ON, Canada). For each brain, 3H GR65630 binding was measured at 3 different brainstem levels within the NTS: rostral, middle and caudal. At rostral levels the NTS was bilaterally separate under the 4th ventricle corresponding to atlas levels Bregma −12.90 to −13.50. Middle levels where characterized by the joining of the NTS at the midline over the central canal and below the area postrema at Bregma −13.50 to ™ 14.10. Caudal levels of the NTS were sampled near or caudal to the obex corresponding to atlas levels Bregma −14.10 to −14.70. At each level, 2-4 brainstem sections (depending on availability of sections) were measured and averaged together. Non-specific binding was also measured at each level and subtracted from the total binding to determine specific binding. Due to an increased chance for tissue loss during the incubation process of younger animals, 2 different incubations were performed, and the data from each incubation were averaged together. For the older animals, only one incubation was performed. All quantitative measurements were made blinded to the treatment group.
Statistics and Data Analysis
We measured the effect on respiration of each laryngeal water stimulus in two ways. First, we measured the duration of the apnea, which we defined as the period from the onset of the last breath before the water stimulus was given until the start of the first obvious respiratory activity following the stimulus. Second, we measured the length of the period from the onset of the last breath before the water stimulus until the start of the first of five regular breaths after the stimulus (regular in rhythm, but not necessarily amplitude), a more comprehensive measure of respiratory disruption and instability that we refer to as the LCR. We found in previous studies (Curran et al., 2005; Xia et al., 2007) that this dual set of measurements provided a more robust measurement of respiratory disruption than either would alone.
Data are presented as means ± the standard deviation. To compare apnea and LCR durations before and after focal application of 5-HT, we used a two-way ANOVA in which the effect of prenatal gas exposure (RA versus IH on the LCR was compared between treatment groups, and the LCR responses before and after 5-HT microinjection were compared within each animal (a repeated comparison). The variances of the durations of apnea and the LCR were not homogeneous, and the data were not normally distributed. Therefore, we log-transformed the apnea and LCR durations and performed the statistical analysis in STATA 15.1 (StataCorp, College Station, Texas). We incorporated age as a random effect in some of these analyses. When the ANOVA indicated that significant differences existed among treatments, specific, pre-planned comparisons were made using P-values adjusted by the Bonferroni method for multiple comparisons. We performed regression analysis in STATA 15.1 to determine if the stimulus volume used to elicit the LCR differed between the two treatment groups (RA versus IH) or differed among animals of different post-natal age.
Statistical comparisons of 5-HT3 binding in the NTS were also made in STATA 15.1 using a three-way ANOVA in which the age of the rat pups, the type of prenatal gas exposure, and the rostro-caudal level of the NTS were used as categorical variables. The rostro-caudal levels were treated as a repeated measure within the ANOVA. As above, we performed specific, pre-planned, paired comparisons using P-values adjusted by the Bonferroni method for multiple comparisons only when the ANOVA indicated that significant interactions existed.
Results
Maternal and Neonatal Behavior
The dams and neonatal rat pups exposed to prenatal IH behaved differently from the control, RA-exposed animals. The dams were more agitated and appeared more anxious, especially in the first 24 hours postpartum. The hypoxia-exposed dams tended to neglect the newborn pups and, on occasion, ate them. The dams did not create organized nests and moved restlessly around the cage. All of this occurred even though the dams were placed in a RA environment continuously as soon as we recognized that pups had been born, which usually occurred fewer than 12 hours after delivery in both the IH and the RA groups.
The pups exposed to prenatal IH appeared normal and similar in size to RA, control pups. We cannot compare weights among litters or number of animals within litters because we sometimes discovered dead pups in the morning that had experienced a variable and unknowable degree of dehydration, and some of these pups had been partially or completely eaten by the dam. Although normal in appearance, some pups had no milk in the stomach, and it seemed that the pups exposed to prenatal IH were more likely to become apneic and die during anesthesia or during testing of the LCR. However, when we analyzed the data, these impressions were not confirmed. The level of anesthesia used was identical in the animals, and though a few IH pups died during the LCR testing, the rate of death during LCR testing was not disproportionate to other studies of the LCR that we have conducted in neonatal rat pups in the past. The average age, weight and anesthetic doses for the two groups of animals are shown in Table 1; the treatment groups were similar with respect all three variables.
Table 1.
Comparison of animal age at the time the LCR was tested, body weight at the time of testing and anesthetic dose given prior to LCR testing. None of these variables was different between the RA- and IH-exposed rat pups.
| Maternal exposure: | Room air | Intermittent hypoxia |
p-values |
|---|---|---|---|
| Age at the time of testing (postnatal days) | 13.7 ± 3.9 | 12.9 ± 3.7 | 0.66 |
| Body weight (gms) | 27.8 ± 10.3 | 30.5 ± 9.9 | 0.57 |
| Anesthetic dose (sum of urethane and chloralose in mg/gm body weight) | 4.2 ± 1.2 | 3.6 ± 1.2 | 0.33 |
| Number of animals | 6 | 21 |
Apnea and LCR Durations
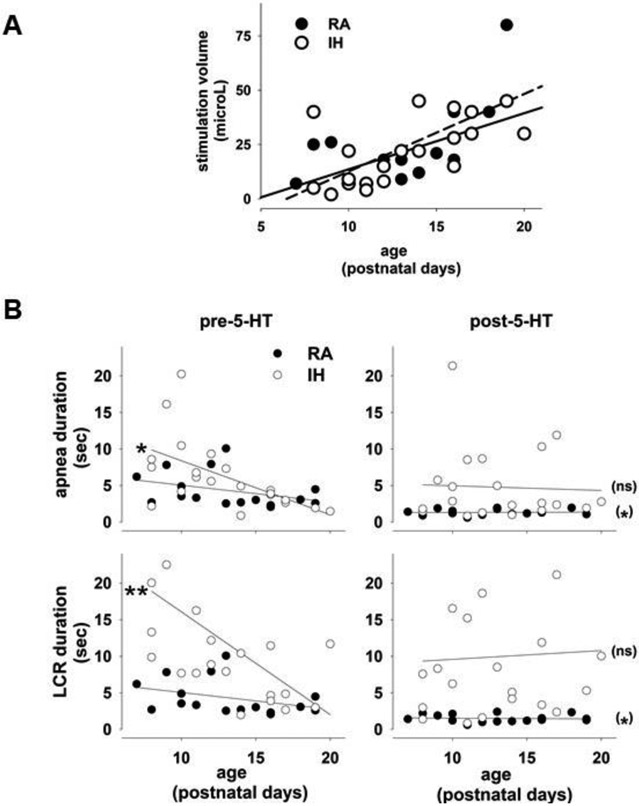
Two examples of the LCR, one from a pup born to a dam exposed to RA throughout gestation, and the other from a pup exposed to IH during gestation, are shown in Figure 2. Note that the RA-exposed pup demonstrated marked shortening of the LCR after 5-HT was injected into the NTS (upper panel). On the other hand, the LCR duration was unchanged before and after 5-HT injected into the NTS in the pup exposed to gestational IH. Among the pups exposed to gestational IH, the LCR durations were equally distributed among longer, unchanged and shorter durations after 5-HT microinjection into the NTS; whereas the LCR was consistently shorter after 5-HT injection in the RA-exposed pups.
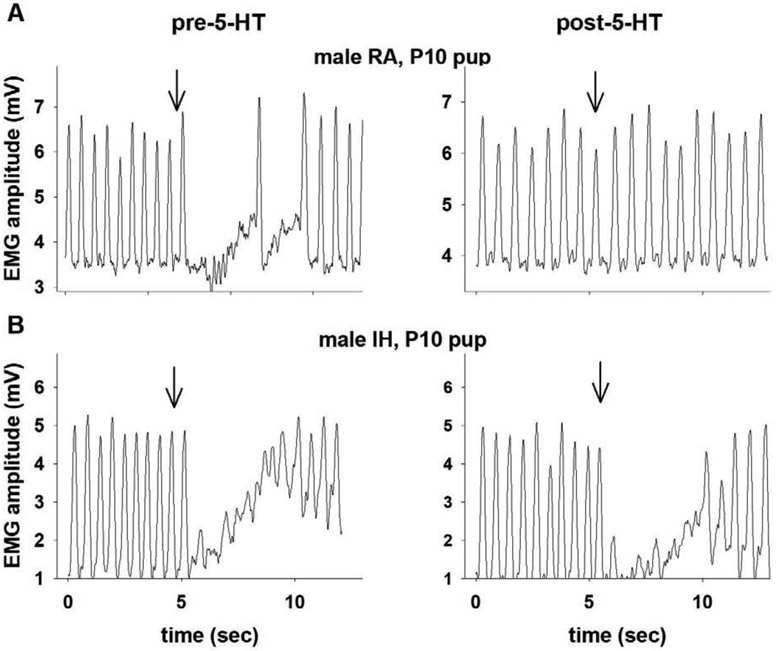
Figure 2:
Examples of integrated diaphragm EMG activity during the LCR from a rat pup born to a dam exposed to RA during gestation (A) and a pup born to a dam exposed to IH during gestation (B). Examples of the LCR before and after receiving a microinjection of 4 mM 5-HT into the caudal NTS in each animal are shown left to right. The downward arrow indicates when the intralaryngeal injection of water was made to elicit the LCR. The LCR was virtually eliminated after microinjection of 5-HT into the NTS in the pup exposed to RA during gestation. The LCR was negligibly affected by 5-HT injected into the NTS in the rat pup exposed gestationally to IH. The time scales vary, but the distance between two tick marks is 5 seconds on every graph.
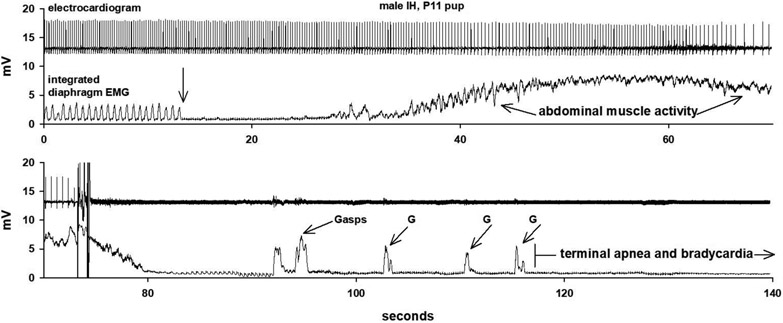
A recording of a pup exposed to IH during gestation that died after the LCR was elicited is shown in Figure 3. The electrocardiogram and integrated diaphragm EMG activity are shown in a split trace that is continuous in time. After the LCR was elicited (downward arrow), there was prolonged apnea, and bradycardia developed as the apnea persisted. The apnea was disrupted by a prolonged period of what appeared to be expiratory abdominal activity (which ‘contaminated’ the diaphragm EMG recording and during which no inspiratory activity was identified). After the abdominal activity ceased, a series of gasps emerged, but eupnea was never restored, and the rat pup died as the heart beat failed (visible in the diaphragm EMG trace in the lower panel of the figure). The animal was anesthetized, and so arousal as a response was not possible. Nevertheless, this chain of events, apnea, bradycardia, gasping without restoration of eupnea and persistent bradycardia, looks remarkably like the tracings of human babies who died of SIDS (Meny et al., 1994; Poets et al., 1999; Poets et al., 1993; Sridhar et al., 2003).
Figure 3:
An example of the electrocardiogram and respiratory activity before and during the LCR in a rat pup exposed to IH during gestation in which the animal failed to recover. The traces are split, but the time is continuous. The downward arrow indicates the point at which water was injected into the larynx to elicit the LCR. Following a prolonged apnea, there was a long burst of abdominal activity (that looked expiratory to the experimenter). Once the abdominal EMG activity ceased, gasping (G) emerged, but autoresuscitation was unsuccessful, and apnea persisted. Note that bradycardia developed slowly (at about the onset of abdominal activity) and became progressively more severe. The electrocardiogram is more apparent in the lower diaphragm EMG tracing late in the apnea. The chain of events recorded resembles events recorded from human babies who died of SIDS (Sridhar et al., 2003).
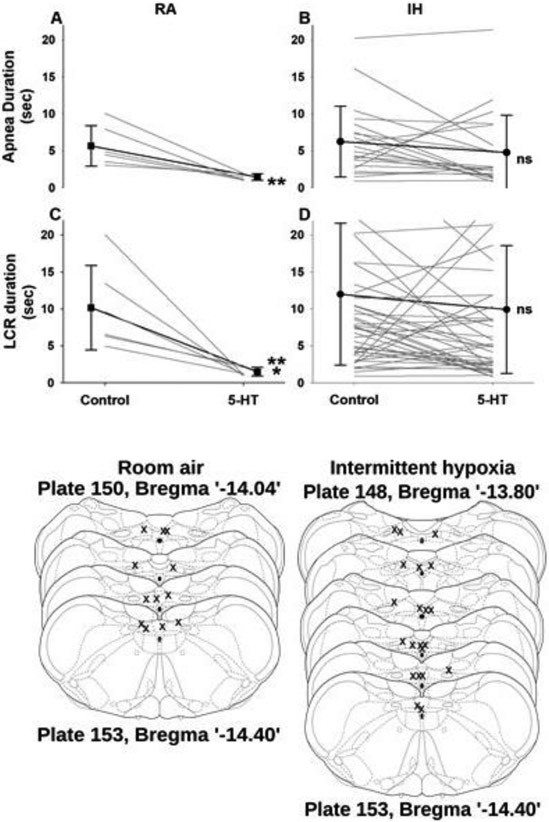
The trends shown above were confirmed in the average responses from the two groups of animals, as shown in Figure 4A. The ANOVA performed to analyze these results revealed a significant two-way interaction between maternal gas exposure (RA versus IH) and the timing of the microinjection with 5-HT (before versus after the microinjection). The interaction indicates that the slope of the response profile of the rat pups to microinjection of 5-HT in the NTS differed significantly – the reduction in the duration of apnea and the duration of the LCR was significantly greater in the animals born to mothers who breathed RA through pregnancy (F =5.10, P =0.033 for apnea duration, and F =9.22, P = 0.006 for the LCR). Based on the presence of a significant interaction, we made three pre-planned comparisons using P-values adjusted by the Bonferroni method. We compared the LCR response before and after microinjection of 5-HT in the RA exposed pups and found that both apnea duration and the LCR duration were significantly shortened after microinjection of 5-HT (P < 0.01 for apnea duration, and P < 0.001 for the LCR duration). Similar comparisons between apnea and LCR responses before and after microinjection of 5-HT in the NTS in the IH-exposed animals failed to reveal a significant effect of the 5-HT microinjection on apnea or the LCR (P > 0.10 for apnea duration and P > 0.05 for the LCR duration). The locations of the microinjections, which are shown in Figure 4B, were not different between the RA- and IH-exposed animals. Thus, prenatal exposure to hypoxia seemed to blunt the ability of 5-HT microinjected into the NTS to shorten the duration of the LCR and the apnea associated with the LCR.
Figure 4:
In upper panel, the individual and average apneic and LCR durations are shown from animals born to mothers exposed to RA during gestation (A and C) and mothers exposed to IH during gestation (B and D). Within each of these treatment conditions, the duration of apnea (upper panels) or the duration of the LCR (lower panels) are shown after a microinjection of 4 mM 5-HT into the caudal region of the NTS. The apnea and LCR durations were significantly less after 5-HT injection in the RA animals, but not different from control values in the IH pups. In the lower part of the figure, a schematic of coronal sections of the NTS are shown with the locations of each microinjection in each animal marked (x) in the location where fluorescent beads included in the microinjection were identified (schematic coronal sections adapted from Paxinos and Watson (2005). Sec – second; ** - P < 0.01; *** - P < 0.001; ns – not significant). The Paxinos and Watson atlas was based on adult rats, and the plate numbers and distances from bregma shown above represent the relative locations and distances in the anatomically smaller and immature brains of the rat pups that were studied.
We have previously performed similar studies using 5-HT to modify the LCR in neonatal rats, and we compared our previous results to the current study in a two-step process. We compared apnea and LCR durations of rat pups exposed to RA throughout gestation in the past, historical RA controls (RAH) (Donnelly et al., 2016), to the current group of pups exposed to RA during gestation (RAC) using a repeated measures ANOVA. We asked whether the LCR responses between RAH and RAC animals differed. They did not – the apnea durations and LCR durations of these two RA-exposed groups were indistinguishable. In the second step of the analysis, we compared the pooled results from the RAH and RAC groups to the apnea and LCR responses in the animals exposed to IH during gestation. This analysis confirmed that the pooled RAH and RAC group demonstrated a significant shortening of apnea and LCR durations after microinjection of 5-HT into the NTS (P < 0.02 for both comparisons), and the animals exposed to IH during gestation did not (P >>0.05). This analysis indicated that the RAC animals were not anomalous in any way that we can detect; RA exposure of the mother in the chamber did not alter the basic characteristics of the LCR responses or the responses to microinjection of 5-HT in the NTS compared to the rat pups that we studied in the past. Moreover, the IH rat pups responded in a way that was quite different from the RAC and RAH animals.
We performed two additional analyses using the pooled RAC and RAH data. The volume of water necessary to elicit the LCR increases as a function of the age of the animal (Donnelly et al., 2016), which is consistent with an age-related decline in the sensitivity of the LCR – it takes a larger laryngeal volume to elicit a similar, consistent LCR response as the animals mature, a maturational profile of the LCR previously described in many animals including humans (Haraguchi et al., 1983; Thach, 2001; Xia et al., 2008; Xia et al., 2010). The results of analyzing the stimulation volume necessary to elicit the LCR as a function of the prenatal gas exposure of the mother and the age of each rat pup are shown in Figure 3A. The volume of water that must be instilled in the larynx to elicit the LCR rose significantly as postnatal age increased (F =26.82, P << 0.001), but there was no difference between the slopes of the increase in stimulation volume as a function of age between animals exposed to RA or IH during gestation F = 0.21, P = 0.65). Thus, the stimulus at each postnatal age was not different between treatment groups.
In the second analysis, we examined the effect of age on the duration of the LCR by adding age as a continuous, random covariate to the two-way ANOVA that we used to investigate the effect of gestational IH and microinjection of 5-HT into the NTS. We found a three-way interaction between age, gestational gas exposure and the timing of the microinjection of 5-HT for both apnea and LCR durations (apnea duration - F = 6.66, P < 0.004; LCR duration - F = 5.53, P < 0.009). The results of this analysis are shown in Figure 3B. Considering the control LCR response in RA-exposed pups as a function of age first, the slopes of the regressions of apnea and LCR durations were not significantly different from zero. Nevertheless, since the volume of water injected in the larynx to elicit the LCR increased as a function of age (Fig. 5A), there was still a clear decrease in the sensitivity of the LCR in RA-exposed pups. In contrast, the IH-exposed pups had longer apnea and LCR durations at younger ages, and the decline in the duration of apnea and LCR responses as animal age increased was accelerated compared to the RA-exposed animals (P = 0.007 for apnea duration and P = 0.011 for LCR duration; left panels in Fig. 5B). After 5-HT microinjection, none of the slopes of the regressions of apnea or LCR duration as a function of age differed from zero (Figure 5B, right panels), indicating that 5-HT in the RA-exposed pups tended to decrease apnea and LCR durations to low and age-invariant values, which were significantly shorter than the control apnea and LCR durations, as shown in Figure 4 and indicated by (*) in Figure 5. The apnea and LCR durations after 5-HT microinjection were also age-invariant, but the intercepts of the regressions for apnea and LCR duration were not significantly different from the pre-5-HT microinjection, control condition, which is also consistent with the findings shown in Figure 4 and indicated in Figure 5 by (ns). Thus, even though the RA and IH rat pups received similar intralaryngeal volumes to elicit the LCR at each postnatal age, the apnea and LCR durations were longer in the younger pups exposed to IH during gestation, and this age-dependent enhanced LCR sensitivity diminished more rapidly in the IH-exposed pups as the postnatal age of the pups increased.
Figure 5.
In A, the LCR stimulation volume has been plotted as a function of age and gestational exposure to RA (RAC and RAH) or IH. The stimulation volume required to elicit the LCR rose significantly as rat pups increased in age (P < 0.001), but at any given age, the same volume of water was sufficient to elicit the LCR in rats exposed to gestational RA or IH. In the lower panels (B), the apnea duration and the LCR have been plotted as a function of postnatal age. As animals mature, the duration of apnea and the LCR diminish, and this change was significantly more pronounced in animals exposed to gestational IH because the LCR was sensitized in the youngest rat pups (P < 0.05 for apnea “*”; P < 0.01 for the LCR “**” – a phenomenon that they appeared to outgrow (note that the LCR diminishes as animals mature, but the rate of decrease was much greater in the IH exposed pups). The apnea and LCR durations were age-invariant and not altered by 5-HT microinjection into the NTS in the IH-exposed pups, as shown in Figure 3 and indicated by (ns). In the RA-exposed pups, apnea and LCR durations were reduced significantly compared to the pre-5-HT microinjection levels, as shown in Figure 3 and indicated by (*), and the apnea and LCR durations were short and age-invariant after 5-HT microinjection.
Serotonin-3 Receptor Binding in the NTS
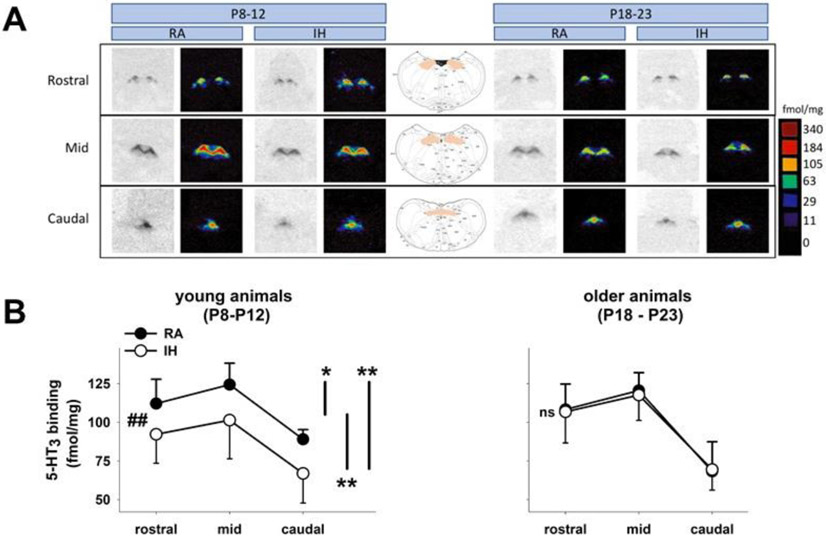
We demonstrated previously that 5-HT microinjected into the NTS shortens the LCR through a 5-HT3 receptor-dependent process (Donnelly et al., 2016). Therefore, we examined the expression of 5-HT3 receptors in the NTS to test the hypothesis that the reason for the reduced shortening of the LCR following 5-HT microinjection in rat pups born to mothers exposed to IH was attributable to decreased expression of 5-HT3 receptors in the NTS. Examples of autoradiographs labeled with a tritiated, selective, 5-HT3 receptor agonist (3H GR65630) from the brainstem of a RA-exposed animal and an animal exposed to intermittent hypoxia are shown in Figure 6A. Note that 5-HT3 receptors are concentrated in the midline in the subcommisural nucleus of the NTS (Jeggo et al., 2005; Miquel et al., 2002) where they act presynaptically on C-fibers to increase glutamate release (Tecott et al., 1993); there is little labeling of 5-HT3 receptors in the medulla outside of the NTS. Average 5-HT3 receptor binding is shown in Figure 6B as a function of the age of the rat pups, the gas exposure during gestation and the levels of the NTS analyzed. The average ages of the animals within each age group were not significantly different between RA and IH animals. There was no three-way interaction (age of pups by prenatal gas exposure by level of the NTS) with respect to levels of 5-HT3 binding, but there was one significant two-way interaction (rat pup age by prenatal gas exposure; F = 9.21, P = 0.003) and a significant main effect (level sampled in the NTS; F = 61.6, P < 0.001). When we performed paired comparisons between young rat pups exposed to gestational IH compared to young rats exposed to RA during gestation, we found a statistically significant difference between 5-HT3 binding averaged across the three NTS levels sampled within each age and prenatal gas exposure group (P < 0.001; indicated by ‘##” in Figure 6B). When we performed a similar comparison among older animals, we found no significant difference (indicated by ‘ns” in Figure 6B). When we compared 5-HT3 binding at different NTS levels to analyze the significant main effect identified by the ANOVA, 5-HT3 receptor binding at each level of the NTS was different from the other two levels across all conditions (IH and postnatal age did not change the relative distribution of binding within the NTS). Thus, 5-HT3 receptor binding averaged across age and prenatal gas exposure was significantly greater in the mid-NTS compared to the rostral NTS (P = 0.022) and to the caudal NTS (P < 0.001), and the 5-HT3 receptor binding in the rostral NTS was greater than the binding in the caudal NTS (P < 0.001). Thus, at a postnatal age at which 5-HT injected into the NTS failed to shorten the LCR in animals exposed to prenatal IH, 5-HT3 receptor binding in NTS was also reduced compared to 5-HT3 receptor binding in rat pups exposed to RA during gestation in which 5-HT injected into the NTS effectively shortened the LCR. On the other hand, prenatal exposure to IH did not change the relative distribution of 5-HT3 binding within the NTS: 5-HT3 binding was greatest in the mid-NTS and lowest in the caudal NTS in all animals studied. Moreover, there was no change in 5-HT3 binding when comparing younger and older pups exposed to RA during gestation.
Figure 6.
Examples of autoradiographs are shown in A. The 2 panels on the left were taken from young pups (P8 - P12) exposed to either RA or IH during gestation. The 2 panels on the right were taken from older pups (P18-P23), exposed to either RA or IH during gestation. Representative sections from the rostral, mid, and caudal levels of the NTS are shown. Stereotaxic coronal images (Paxinos and Watson, 2005) are shown in the middle panel of Figure 6A to denote the anatomical location of 5-HT3 binding. Note that 5-HT3 binding was concentrate in the midline in the NTS in all animals, there is little 5-HT3 binding in the brainstem outside of the NTS. In B, the levels of receptor binding have been plotted as a function of the age of the animals, the gestational gas exposure, and the levels of the NTS. Note that the relative density of 5-HT3 binding in the NTS was similar in all groups of animals. The 5-HT3 binding levels, when averaged across both ages and gas exposure regimens, were significantly different among all three NTS levels sampled (mid-NTS > rostral – P = 0.022 indicated by short vertical line and ‘*’; mid- >> caudal NTS – P < 0.001 indicated by the intermediate length vertical line and ‘**’; and rostral > caudal NTS – P < 0.001 indicated by longest vertical line and ‘**’). The interaction between age and gestational gas exposure is also shown, and the level of 5-HT3 binding was significantly less across all three NTS levels in the young animals exposed to gestational IH compared to the young animals exposed to RA during gestation (P << 0.001; indicated by the ‘##’). In contrast, 5-HT3 receptor binding was not identifiably different in older animals regardless of whether they were exposed to IH or RA during gestation (indicated by ‘ns’). fmol/mg indicates femtomoles per milligram of tissue.
Discussion
We examined the effect of gestational IH experienced by both the dam and fetuses on the LCR measured under normoxic conditions during the postnatal period in rat pups. To assess the impact of gestational IH on the LCR in rat pups, we also tested the hypothesis that 5-HT would shorten the LCR, and we tested the hypothesis that gestational IH would alter 5-HT3 receptor binding in the NTS since the effect of 5-HT microinjection to shorten the LCR is mediated by 5-HT3 receptors (Donnelly et al., 2016; Donnelly et al., 2017). There are three main findings in this study. First, the LCR was enhanced in the younger rat pups exposed to IH during gestation even though all rat pups were raised in RA conditions from the time of birth onward. This enhanced sensitivity of the LCR in pups exposed to IH during gestation waned as the animals matured, and the LCR durations were approximately equal at P18 based on the intersection of regressions of LCR duration as a function of animal age in the RA and IH groups (Figure 5B). Serotonin injected into the NTS shortened the LCR as we have seen previously. However, 5-HT injected into the NTS failed to alter either apnea duration or LCR duration in the rat pups exposed to gestational IH (Figure 4). At the age at which 5-HT injected into the NTS had no effect on the LCR duration in the IH pups (P8 to approximately P16), 5-HT3 receptor binding was also reduced compared to RA pups at the same age and compared to older animals (at which time there was no difference in 5-HT3 binding between the RA and IH pups).
Hypoxic sensitization of the LCR
The LCR is elicited when water, low chloride concentration solutions or acid interact with receptors in the larynx (Boggs and Bartlett, 1982; St. Hilaire et al., 2005). Receptors capable of transducing these varied stimuli within the upper airway and eliciting the LCR send afferent signals via both A- and C-fibers to the brainstem where both fiber types terminate in the nucleus of the solitary tract (NTS) and release glutamate to activate secondary neurons (Anderson et al., 1990; Harding et al., 1978; Hayakawa et al., 2001; Jin et al., 2004; Mutoh et al., 2000; Patrickson et al., 1991; Roulier et al., 2003). The strength and duration of the LCR is modulated by presynaptic receptors on C-fibers responsible for eliciting the LCR (Donnelly et al., 2016; Donnelly et al., 2017; Doyle et al., 2002; Fawley et al., 2014; Hermes et al., 2016; Xia et al., 2011, 2016). Foremost among these presynaptic receptors is the transient receptor potential vanilloid 1 (TRPV1) channel, which is expressed on C-fibers and, which when activated, increases intracellular calcium in the presynaptic neuron and enhances glutamate release. We have shown that activation of TRPV1 receptors in the NTS prolongs the LCR (Xia et al., 2011) and inflammation, acting through the cytokines interleukin 1beta (IL-1β) and IL-6, enhances the LCR and may increase the risk of SIDS (Guntheroth, 1989; Marty et al., 2008; Stoltenberg et al., 1994; Xia et al., 2016). Gestational and postnatal hypoxia promote neuroinflammation (Darnall et al., 2017; Golan and Huleihel, 2006), and sensitization of the LCR by IL-1β or IL-6 associated with neuroinflammation may be one process through which IH prolonged the LCR (Fig. 4). Alternatively, hypoxia is associated with increased levels of reactive oxygen species, and reactive oxygen species also sensitize TRPV1 receptors and increase reflex responses to TRPV1 stimulation (Taylor-Clark, 2016). Thus, the effect of IH-exposure to prolong the LCR, both by primary sensitization of the reflex (which we have called reflex allodynia since it resembles central pain sensitization in the spine mechanistically (Grace et al., 2014), and by delayed development of 5-HT3 receptors, which when activated tend to shorten the LCR, is consistent with our two step hypothesis in which risk factors for SIDS may sensitize apneogenic reflexes and simultaneously blunt responses that terminate apneas and promote eupnea and arousal.
Mechanistic Relationship of 5-HT3 Receptors and Modulation of the LCR
Reduced 5-HT3 binding may indicate reduced receptor affinity or reduced receptor expression presynatically within the NTS, more likely the latter. Regardless of mechanism, there is a functional reduction in 5-HT effectiveness. The LCR causes apnea when glutamate activates second order neurons within the NTS that, in turn, project to the ventral respiratory group in the medulla where apnea is elicited as the activity from the NTS seems to hold the respiratory cycle in an extended post-inspiratory pause, a post-inspiratory apneusis, until the apnea is terminated, and the normal sequence of inspiratory, post-inspiratory and expiratory activity is restored (Czyzyk-Krzeska and Lawson, 1991; Remmers et al., 1986). Apnea and eupnea are, therefore, mutually exclusion; those neurons in the NTS that communicate with and inhibit the post-inspiratory neurons in the ventral medulla to cause the post-inspiratory apneusis prevent the emergence of eupnea. Therefore, the restoration of eupnea after apnea requires the inhibition of those second order neurons in the NTS causing apnea. Activation of 5-HT3 receptors in the NTS seems to participate in the inhibition and termination of apnea (Donnelly et al., 2016; Donnelly et al., 2017), so that eupnea may emerge. We have speculated that 5-HT3 receptors are presynaptic to GABAergic interneurons that inhibit the apnea generating second order neurons (Donnelly et al., 2017). Exposure to IH during gestation was associated with reduced 5-HT3 receptor binding in the NTS at postnatal ages when IH was associated with a reduced apnea-shortening response to 5-HT injected into the NTS. Thus, the reduced 5-HT3 receptor binding is likely mechanistically related to the reduced capacity of 5-HT in the NTS to terminate apnea and shorten the LCR, strengthening a possible link between a 5-HT3 receptor defect and an increased risk of SIDS.
Sensitization of the LCR waned as the animal matured so that by ~ P16, the rat pups exposed to intrauterine IH during development had LCR responses that were similar to animals born to mothers exposed to RA throughout gestation. The rate of normalization of the LCR seemed to be accelerated once the pups were consistently exposed to a RA environment. Moreover, 5-HT3 receptor binding in the NTS was also normalized as the rats grew and matured in a normoxic environment. This acquisition of mature, RA-associated 5-HT3 receptor binding levels and reduced LCR sensitivity, resembles the way in which human infants seem to outgrow the risk for SIDS. However, maturational changes in 5-HT3 receptor binding cannot be the cause of the normal maturational shortening of the LCR seen in the RA-exposed pups because there was no change in 5-HT3 receptor binding in the control, RA pups over the postnatal age range during which the LCR normally shortens (Haraguchi et al., 1983; Thach, 2007). Thus, reduced 5-HT3 receptor binding in the NTS may prolong the LCR, but other factors must also contribute to the normal, age-dependent waning of the LCR.
Impact of Gestational Intermittent Hypoxia on Development of the Caudal Serotonergic system
The ultimate cause of SIDS is unknown. However, Naeye (Naeye, 1980) identified multiple histopathological changes in different organs of infants who died of SIDS and ascribed these pathological findings to a common mechanism of antenatal hypoxia. Subsequent studies confirmed the presence of some of these pathological markers of antenatal hypoxia (Valdes-Dapena, 1986; Valdes-Dapena et al., 1976), and the presence of gliosis in regions of the brainstem associated with autonomic function has been found consistently in multiple investigations (Kinney et al., 1983; Takashima et al., 1978). In addition, numerous epidemiological investigations have confirmed an association between conditions that my compromise fetal oxygen delivery and an increased risk of SIDS. A variety of placental abnormalities, such as maternal smoking, eclampsia, and placental abruption, are risk factors for SIDS (Bulterys et al., 1990; Getahun et al., 2004; Goldstein et al., 2016; Klonoff-Cohen et al., 2002; Li and Wi, 2000; McDonnell-Naughton et al., 2012; Poets et al., 1995). Premature birth is a risk factor for SIDS (Malloy, 2002) and for Sudden Unexpected Infant Death (which combines SIDS and asphyxia-related deaths together) (Ostfeld et al., 2017). Fetal growth retardation and shorter gestation may be risk factors for SIDS (Buck et al., 1989; Oyen et al., 1997) and may originate, in part, from reduced uterine blood flow and intrauterine hypoxia (Naeye, 1989). Maternal anemia during pregnancy increases the risk of SIDS (Klonoff-Cohen et al., 2002; Naeye et al., 1976), especially when combined with cigarette smoke exposure during pregnancy (Bulterys et al., 1990; Poets et al., 1995). All these pathological changes and risk factors associated with SIDS seem to result from chronic, prenatal events that reduce oxygen delivery to the fetus, and so for many infants, SIDS looks like an acquired, congenital, neuropathological disorder, an idea that was endorsed by the National Institute of Child Health and Human Development SIDS Strategic Plan 2001 (Goldwater, 2011).
Pathologists describe the brains of infants who died of SIDS as immature and attribute this to hypoxia-associated developmental delay, and the findings of immature serotonergic neuronal morphology, delayed myelination, and increased numbers of dendritic spines fit this description (Becker, 1990; Bright et al., 2017a; Kinney et al., 1991; Paterson et al., 2006b; Quattrochi et al., 1985; Takashima et al., 1978; Takashima and Becker, 1991; Takashima et al., 1982). Our findings of heightened sensitivity to the LCR, a diminished sensitivity of the LCR to 5-HT, and reduced 5-HT3 receptor binding in younger rat pups exposed to gestational IH can be seen as persistence of fetal patterns of reflex responses and delayed emergence of normal 5-HT3 receptor levels. These findings are consistent with the hypothesis that prenatal hypoxia may cause neurodevelopmental abnormalities in humans that are associated with delayed brain development and a persistent fetal phenotype, including an increased frequency of behaviors that make SIDS more likely (reflex apnea) and a reduced capacity to terminate those apneas (activation of 5-HT3 receptors in the NTS). A variety of neurotransmitters have been examined in infants who died of SIDS (Bright et al., 2017b; Broadbelt et al., 2011; Duncan et al., 2010; Duncan et al., 2008; Hunt et al., 2015; Kinney et al., 1995; Lavezzi et al., 2016; Nachmanoff et al., 1998; Ozawa and Okado, 2002; Ozawa and Takashima, 2002; Panigrahy et al., 1997), but the distribution and density of 5-HT3 receptors have never been examined in any neuropathological study of which we are aware. The results of our previous studies (Donnelly et al., 2016; Donnelly et al., 2017) and the current findings indicate that an examination of the density and distribution of 5-HT3 receptors in infants who died of SIDS may be informative.
Serotonergic Deficits in SIDS: Cause and Effect
A variety of neuropathological abnormalities in the brainstem have been described in babies whose deaths were attributed to SIDS, and these include reduced neurotransmitter receptor binding (Duncan et al., 2010; Kinney et al., 1995; Panigrahy et al., 1997; Paterson et al., 2006a), especially 5-HT1a receptors (Duncan et al., 2010; Panigrahy et al., 2000; Paterson et al., 2006a) and also 5-HT2a receptors(Ozawa and Okado, 2002; Ozawa and Takashima, 2002), increased numbers of immature serotonergic neurons (Bright et al., 2017a; Paterson et al., 2006b), gliosis (Naeye, 1976; Obonai et al., 1996; Takashima et al., 1978; Takashima and Becker, 1985) and increased numbers of dendritic spines (Quattrochi et al., 1985; Takashima and Mito, 1985; Takashima et al., 1994). But what are the functional consequences of these pathological findings? The physiological recordings of infants who died of SIDS demonstrate a sequence of prolonged apneas and bradycardia. In those episodes when recovery is successful, gasping develops late in the apnea; oxygen levels rise as a result of the gasps; eupnea is restored; and the infant arouses. When recovery from apnea and bradycardia is unsuccessful, one or more of these processes (gasping, apnea termination, restoration of eupnea or arousal) fails. Serotonin deficiency reduced the effectiveness of autoresuscitation (Cummings et al., 2011; Erikson et al., 2007; Erikson and Sposato, 2009; Givan and Cummings, 2016), and enhanced serotonergic function restored effective autoresuscitation in 5-HT-deficient mice (Chen et al., 2013). Serotonin stimulates eupnea by acting on 5-HT2 and 5-HT4 receptors in the ventral medulla (Ptak et al., 2009; St. John and Leiter, 2008). Cortical arousal are initiated caudally through an ascending arousal system that originates in the rostral pons, projects through the parabrachial nucleus to the basal forebrain, and ultimately to the cortex (Fuller et al., 2011; Kaur et al., 2013). The parabrachial nucleus receives serotonergic inputs from the serotonergic raphe nuclei (Bang et al., 2012; Miller et al., 2011), and 5-HT acting through 5-HT2A receptors enhances arousal from hypercapnia (Buchanan and Richerson, 2010; Buchanan et al., 2015). The rostral projections from the caudal serotonergic raphe nuclei target excitatory receptor subtypes including 5-HT2A, 5-HT3 and 5-HT4 receptors. Thus, the functional consequences of the pathological abnormalities in the caudal 5-HT system summarized above will be deficient activation of the sequence of events that normally proceed serially from the caudal brainstem, where the mechanisms of gasping, termination of apnea, and restoration of eupnea reside, to the midbrain and cortex where arousal from sleep is organized. The presence of negative feedback in virtually every biological process makes it difficult to identify what is a primary defect and what is a secondary response to the primary defect. Assuming that the primary effect of prenatal hypoxia is to delay development, then the primary serotonergic defect is likely to be delayed emergence of excitatory serotonergic neuronal activity, delayed emergence of 5-HT receptors, and low 5-HT levels (Duncan et al., 2010). If the primary defect in infants at risk for SIDS is deficient excitatory serotonergic activity, then a compensatory downregulation of inhibitory 5-HT1A binding would tend to liberate the endogenous and reflex activity of the serotonergic neurons and restore more neuronal activation. The increased number of immature serotonergic neurons, as reported in SIDS infants (Bright et al., 2017a; Paterson et al., 2006b), might be yet another compensatory mechanism in response to fetal hypoxia and its associated developmental delay and deficiency of excitatory serotonergic activity. A similar line of argument may explain the reduced GABAA receptor binding in medullary nuclei found in infants who died of SIDS (Broadbelt et al., 2011) in that reduced inhibitory activity via GABA receptors acting on serotonergic neurons in a hyposerotonergic system would tend to restore serotonergic activity (Broadbelt et al., 2010), just as a reduction in 5-HT1A receptors would.
The rapid rate at which the pups exposed to IH during gestation outgrew the excessive LCR sensitivity and restored normal 5-HT3 receptor levels is surprising and suggests that the restoration of normoxia was sufficient to allow ‘catch-up’ growth and development of the brain. We did not examine any effects of postnatal hypoxia, but it seems likely that episodes of postnatal hypoxia could delay this catch-up development just as much as intrauterine hypoxia did. Many postnatal risk factors for SIDS, such as prone sleeping, apnea of prematurity, and postnatal cigarette exposure, may cause hypoxia after birth in the infant, and there is biochemical evidence of hypoxia of indeterminate age in many babies who died of SIDS (Butterworth and Tennant, 1989; Jones et al., 2003; Le Cam-Duchez et al., 1999). Therefore, continuing hypoxia in the postnatal period may compound and prolong any developmental delays and prolong the putative hyposerotonergic state that originated from prenatal hypoxia in utero. Hence, hypoxia, whether prenatal or postnatal, may delay the acquisition of more adult reflex, respiratory, and arousal responses appropriate for air breathing and may prolong the period of vulnerability to SIDS.
Limitations of the Methods
Intermittent hypoxic exposure of the dam to simulate intrauterine hypoxia in the fetus does not simulate the usual causes of hypoxia in human neonates. Usually, but not always, fetal hypoxia is caused by functional or anatomical placental insufficiency, and the fetus is hypoxic when the mother generally is not. Moreover, IH altered the behavior of the dams and their pups during the early postnatal period. The mothers appeared more skittish and anxious, and we cannot be sure that litter sizes and birth weights were not changed by exposure to IH. Thus, environmental hypoxic exposures is a weak aspect of the model that we used. It may also be useful to confirm that hypoxia restricted to the fetus created by uterine artery embolization or ligation leads to similar behavioral and neuroanatomical changes.
We tested only one level of hypoxia. The level of hypoxia that we chose arbitrarily did not seem to lead to fetal wastage, but did produce physiological and neuropathological effects. However, it would be useful to test different doses of hypoxia to determine if the severity or duration of hypoxia is correlated with the degree of prolongation of the LCR and the extent to which 5-HT3 receptor binding is reduced. If the severity of intrauterine hypoxia dictates the degree of maturational delay and the severity of the behavioral and neurotransmitter defects that we found, then the greater the degree of intrauterine hypoxia, the greater should be the prolongation of the LCR, the less effectively 5-HT injected into the NTS should shorten the LCR, and the lower level of 5-HT3 receptor binding should be at any postnatal age. In this context, it would be useful to examine in this rat model of prenatal hypoxia the entire phenotypic profile of serotonergic and GABAergic abnormalities described in infants who died of SIDS.
The threshold level and duration of hypoxia to elicit fetal growth retardation (defined as a body weight less than the 10th percentile of the normal population body weight for that gestational age) was an FIO2 less than 14% for 7 or more days in rats (Jang et al., 2015). We did not detect fetal growth retardation, as best we can tell (see Table 1), and the threshold of gestational hypoxia associated with significant behavioral and neurochemical abnormalities may require less severe hypoxia than that required to cause growth retardation (which is consistent with the normal birth weights and postnatal growth patterns of many babies who, nevertheless, died of SIDS). We exposed the rat pups to IH from early in gestation ~P4-5, and we cannot determine if there is a critical time of hypoxia in the rats. The serotonergic system emerges starting at E11.5 in rat pups, so the rats we studied were certainly exposed to IH for days preceding and throughout the intrauterine development of the serotonergic system. In any future hypoxic dose ranging study, different start dates, levels of hypoxia and durations of hypoxic exposure should be explored to define the critical times and level of hypoxia associated with developmental delay and serotonergic deficits.
Conclusions
The findings in the current study are consistent with our two step hypothesis of SIDS (Leiter and Böhm, 2007): we found that exposure to IH during gestation increased the sensitivity and severity of the LCR in younger rat pups (P8 to ~P16), and diminished the capacity of 5-HT to terminate the LCR. Moreover, we demonstrated that prenatal hypoxia may alter the development of a caudal serotonergic function in an animal model that may recapitulate one of the risk factors for SIDS (prenatal hypoxia). The reduction in 5-HT3 receptor binding in the NTS in younger rat pups was temporally correlated with the reduced capacity of 5-HT microinjected into the NTS to shorten the LCR, confirming a novel and unexpected role for activation of 5-HT3 receptors within the NTS by 5-HT that originates, at least in part, from the caudal raphe (Donnelly et al., 2017). This study provides mechanistically specific insight into how delayed development of the serotonergic system associated with prenatal hypoxia might affect important physiological process in both the initiation of apneas and their termination. Infants who died of SIDS demonstrated recurrent prolonged apneas and associated bradycardia, and, ultimately, failure to recover from those apneic episodes. Given the results of the current study, an investigation of 5-HT3 receptor binding in human infants who died of SIDS or in circumstances associated with asphyxia seems warranted.
Highlights:
Intrauterine hypoxia may be a risk factor for Sudden Infant Death Syndrome (SIDS). Reflex laryngeal apneas in infants are thought to be a common initiating event that may lead to sudden infant death. Therefore, we tested the hypothesis that intermittent intrauterine hypoxia (IH) would prolong laryngeal chemoreflex (LCR) apnea.
Prenatal exposure to IH was associated with prolongation of the LCR in younger, anesthetized, postnatal (P) rat pups age P8 to P16, but not older pups.
Serotonin (5-HT) microinjected into the nucleus of the solitary tract (NTS) shortened the LCR in younger rat pups exposed to room air (RA), but 5-HT microinjected into the NTS failed to shorten the LCR in rat pups exposed to prenatal IH.
The 5-HT3 receptor binding was reduced in younger rat pups age P8 to P12 exposed to IH compared to control, RA-exposed rat pups of the same age. Therefore, the failure of the 5-HT injected into the NTS to shorten the LCR seems to reflect a developmental difference in 5-HT3 receptor binding associated with exposure to IH.
Prenatal IH seems to sensitize reflex apnea mechanisms and blunt processes that terminate reflex apneas and facilitate restoration of eupneic breathing in neonatal rats.
Acknowledgments
Funding. This work was funded by grant 36379 from the NICHD.
Footnotes
Analysis of variance – ANOVA; Electrocardiogram – ECG; Electromyography – EMG; Fraction inspired oxygen level - FIO2; Gama-amino butyric acid – GABA; Intermittent Hypoxia – IH; Laryngeal chemoreflex – LCR; Nucleus of the solitary tract – NTS; Postnatal – P; Room air – RA; Serotonin (5-hydroytryptamine) – 5-HT; Sudden Infant Death Syndrome – SIDS; Sudden Unexplained Infant Death – SUID; Transient receptor potential vanilloid 1 – TRPV1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JW, Sant'Ambrosio FB, Mathew OP, Sant'Ambrogio G, 1990. Water-responsive laryngeal receptors in the dog are not specialized endings. Respir. Physiol 79, 33–43. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG, 2012. Projections and interconnections of genetically defined serotonin neurons in mice. The European journal of neuroscience 35, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LE, 1990. Neural maturational delay as a link in the chain of events leading to SIDS. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques 17, 361–371. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Bartlett D Jr., 1982. Chemical specificity of a laryngeal apneic reflex in puppies. J. Appl. Physiol 53, 455–462. [DOI] [PubMed] [Google Scholar]
- Bright FM, Byard RW, Vink R, Paterson DS, 2017a. Medullary Serotonin Neuron Abnormalities in an Australian Cohort of Sudden Infant Death Syndrome. J Neuropathol Exp Neurol 76, 864–873. [DOI] [PubMed] [Google Scholar]
- Bright FM, Vink R, Byard RW, Duncan JR, Krous HF, Paterson DS, 2017b. Abnormalities in substance P neurokinin-1 receptor binding in key brainstem nuclei in sudden infant death syndrome related to prematurity and sex. PloS one 12, e0184958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt KG, Paterson DS, Belliveau RA, Trachtenberg FL, Haas EA, Stanley C, Krous HF, Kinney HC, 2011. Decreased GABAA receptor binding in the medullary serotonergic system in the sudden infant death syndrome. J Neuropathol Exp Neurol 70, 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt KG, Paterson DS, Rivera KD, Trachtenberg FL, Kinney HC, 2010. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton Neurosci 154, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB, 2010. Central serotonin neurons are required for arousal to CO2. Proc. Natl. Acad. Sci. U S A 107, 16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Smith HR, MacAskill A, Richerson GB, 2015. 5-HT2A receptor activation is necessary for CO2-induced arousal. J. Neurophysiol. 114, 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck GM, Cookfair DL, Michalek AM, Nasca PC, Standfast SJ, Sever LE, Kramer AA, 1989. Intrauterine growth retardation and risk of sudden infant death syndrome (SIDS). Am J Epidemiol 129, 874–884. [DOI] [PubMed] [Google Scholar]
- Bulterys MG, Greenland S, Kraus JF, 1990. Chronic fetal hypoxia and sudden infant death syndrome: interaction between maternal smoking and low hematocrit during pregnancy. Pediatrics 86, 535–540. [PubMed] [Google Scholar]
- Butterworth J, Tennant MC, 1989. Postmortem human brain pH and lactate in sudden infant death syndrome. Journal of neurochemistry 53, 1494–1499. [DOI] [PubMed] [Google Scholar]
- Carlin RF, Moon RY, 2017. Risk Factors, Protective Factors, and Current Recommendations to Reduce Sudden Infant Death Syndrome: A Review. JAMA Pediatr 171, 175–180. [DOI] [PubMed] [Google Scholar]
- Chen J, Magnusson J, Karsenty G, Cummings KJ, 2013. Time- and age-dependent effects of serotonin on gasping and autoresuscitation in neonatal mice. J. Appl. Physiol 114, 1668–1676. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE, 2011. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J. Physiol 589, 5247–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D Jr., 2005. Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J. Appl. Physiol 98, 780–786. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Lawson EE, 1991. Synaptic events in ventral respiratory neurones during apnoea induced by laryngeal nerve stimulation in neonatal piglet. J. Physiol 436, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Chen X, Nemani KV, Sirieix CM, Gimi B, Knoblach S, McEntire BL, Hunt CE, 2017. Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism. Pediatric research 82, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly WT, Bartlett D Jr., Leiter JC, 2016. Serotonin in the solitary tract nucleus shortens the laryngeal chemoreflex in anesthetized neonatal rats. Exp. Physiol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly WT, Bartlett D Jr., Leiter JC, 2017. Activation of serotonergic neurons in the medullary caudal raphe shortens the laryngeal chemoreflex in anaesthetized neonatal rats. Exp. Physiol 102, 1007–1018. [DOI] [PubMed] [Google Scholar]
- Downing SE, Lee JC, 1975. Laryngeal chemosensitivity: A possible mechanism of sudden infant death. Pediatrics 55, 640–649. [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Andresen MC, 2002. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J. Neurosci 22, 8222–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg F, Kinney HC, 2010. Brainstem serotonergic deficiency in sudden infant death syndrome. Jama 303, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall B, Habbe D, Mandell F, Welty TK, Iyasu S, Kinney HC, 2008. The effect of maternal smoking and drinking during pregnancy upon (3)H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain pathology 18, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES, 2007. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir. Physiol. Neurobiol 159, 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson JT, Sposato BC, 2009. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J. Appl. Physiol (in press). [DOI] [PubMed] [Google Scholar]
- Fawley JA, Hofmann ME, Andresen MC, 2014. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J. Neurosci 34, 8324–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J, 2011. Reassessment of the structural basis of the ascending arousal system. The Journal of comparative neurology 519, 933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun D, Amre D, Rhoads GG, Demissie K, 2004. Maternal and obstetric risk factors for sudden infant death syndrome in the United States. Obstetrics and gynecology 103, 646–652. [DOI] [PubMed] [Google Scholar]
- Givan SA, Cummings KJ, 2016. Intermittent severe hypoxia induces plasticity within serotonergic and catecholaminergic neurons in the neonatal rat ventrolateral medulla. J. Appl. Physiol. (1985), jap 00048 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan H, Huleihel M, 2006. The effect of prenatal hypoxia on brain development: short- and long-term consequences demonstrated in rodent models. Dev Sci 9, 338–349. [DOI] [PubMed] [Google Scholar]
- Goldstein RD, Kinney HC, Willinger M, 2016. Sudden Unexpected Death in Fetal Life Through Early Childhood. Pediatrics 137, 2015–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater PN, 2011. A perspective on SIDS pathogenesis. the hypotheses: plausibility and evidence. BMC Med. 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR, 2014. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol 14, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntheroth WG, 1989. Interleukin-1 as intermediary causing prolonged sleep apnea and SIDS during respiratory infections. Med. Hypotheses 28, 121–123. [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Fung RQ, Sasaki R, 1983. Effect of hyperthermia on the laryngeal closure reflex. Implications in the sudden infant death syndrome. Ann. Otol. Rhinol. Laryngol 92, 24–28. [DOI] [PubMed] [Google Scholar]
- Harding R, Johnson P, McClelland ME, 1978. Liquid-sensitive laryngeal receptors in the developing sheep, cat and monkey. J. Physiol. (London) 277, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y, 2001. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci. Res 39, 221–232. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Andresen MC, Aicher SA, 2016. Localization of TRPV1 and P2X3 in unmyelinated and myelinated vagal afferents in the rat. Journal of chemical neuroanatomy 72, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heym J, Steinfels GF, Jacobs BL, 1982. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 251, 259–276. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Hillman LS, 1992. Epidemiology of the sudden infant death syndrome: maternal, neonatal, and postneonatal risk factors. Clin. Perinatol 19, 717–737. [PubMed] [Google Scholar]
- Hunt NJ, Waters KA, Rodriguez ML, Machaalani R, 2015. Decreased orexin (hypocretin) immunoreactivity in the hypothalamus and pontine nuclei in sudden infant death syndrome. Acta neuropathologica 130, 185–198. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA, 1999. Activity of serotonergic neurons in behaving animals. Neuropsychopharnacology 21, 9S–15S. [DOI] [PubMed] [Google Scholar]
- Jang EA, Longo LD, Goyal R, 2015. Antenatal maternal hypoxia: criterion for fetal growth restriction in rodents. Frontiers in physiology 6, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo RD, Kellett DO, Wang Y, Ramage AG, Jordan D, 2005. The role of central 5-HT3 receptors in vagal reflex inputs to neurones in the nucleus tractus solitarius of anaesthetized rats. J. Physiol 566, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li B, Schild JH, Andresen MC, 2004. Puringergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J. Neurosci 24, 4709–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Krous HF, Nadeau J, Blackbourne B, Zielke HR, Gozal D, 2003. Vascular endothelial growth factor in the cerebrospinal fluid of infants who died of sudden infant death syndrome: evidence for antecedent hypoxia. Pediatrics 111, 358–363. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB, 2013. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 7627–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB, 1988. The distribution of specific binding of the 5-HT3 receptor ligand [3H]GR65630 in rat brain using quantitative autoradiography. Neurosci. Lett 94, 156–160. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Finkelstein DM, Vawter GF, Mandell F, Gilles FH, 1991. Delayed central nervous system myelination in the sudden infant death syndrome. J. Neuropathol. Exp. Neurol 50, 29–48. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Burger PC, Harrell FE Jr., Hudson RP Jr., 1983. Reactive gliosis in the medulla oblongota of victims of the sudden infant death syndrome. Pediatrics 72, 181–187. [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF, 1995. Decreased muscarinic receptor binding in the arcuate nucleus in Sudden Infant Death Syndrome. Science 269, 1446–1450. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Haynes RL, 2019. The Serotonin Brainstem Hypothesis for the Sudden Infant Death Syndrome. J Neuropathol Exp Neurol 78, 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK, 2003. Serotonergic brainstem abnormalities in Northern Plains Indians with the Sudden Infant Death Syndrome. J. Neuropath. Exp. Neurol 62, 1178–1191. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Srinivasan IP, Edelstein SL, 2002. Prenatal and intrapartum events and sudden infant death syndrome. Paediatr. Perinat. Epidemiol 16, 82–89. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ferrero S, Roncati L, Matturri L, Pusiol T, 2016. Impaired orexin receptor expression in the Kolliker-Fuse nucleus in sudden infant death syndrome: possible involvement of this nucleus in arousal pathophysiology. Neurological research 38, 706–716. [DOI] [PubMed] [Google Scholar]
- Le Cam-Duchez V, Coquerel A, Chevallier F, Vaz E, Menard J, Basset C, Lahary A, Vannier JP, 1999. Erythropoietin blood level is increased in sudden infant death. Biology of the neonate 76, 1–9. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I, 2007. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome (SIDS). Respir. Physiol. Neurobiol 159, 127–138. [DOI] [PubMed] [Google Scholar]
- Li DK, Wi S, 2000. Maternal pre-eclampsia/eclampsia and the risk of sudden infant death syndrome in offspring. Paediatr. Perinat. Epidemiol 14, 141–144. [DOI] [PubMed] [Google Scholar]
- Machaalani R, Say M, Waters KA, 2009. Serotonergic receptor 1A in the sudden infant death syndrome brainstem medulla and associatens with clinical risk factors. Acta Neuropathol. 117, 257–265. [DOI] [PubMed] [Google Scholar]
- Malloy MH, 2002. Trends in postneonatal aspiration deaths and reclassification of Sudden Infant Death Syndrome: Impact of the "Back to Sleep" program. Pediatrics 109, 661–665. [DOI] [PubMed] [Google Scholar]
- Marty V, El Hachmane M, Amedee T, 2008. Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. Eur. J. Neurosci 27, 3132–3150. [DOI] [PubMed] [Google Scholar]
- McDonnell-Naughton M, McGarvey C, O'Regan M, Matthews T, 2012. Maternal smoking and alcohol consumption during pregnancy as risk factors for sudden infant death. Ir. Med. J 105, 105–108. [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH, 1994. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93, 44–49. [PubMed] [Google Scholar]
- Miller RL, Stein MK, Loewy AD, 2011. Serotonergic inputs to FoxP2 neurons of the prelocus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience 193, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, Verge D, 2002. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur. J. Neurosci 15, 449–457. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Kanamaru A, Kojima K, Nishimura R, Sasaki N, Tsubone H, 2000. Effects of perineural capsaicin treatment on cardiopulmonary reflexes elicited by laryngeal instillations of capsaicin and distilled water in sevoflurane-anesthetized dogs. J. Vet. Med. Sci 62, 665–668. [DOI] [PubMed] [Google Scholar]
- Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, Valdes-Dapena M, Krous HF, White WF, Kinney HC, 1998. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J. Neuropath. Exp. Neurol 57, 1018–1025. [DOI] [PubMed] [Google Scholar]
- Naeye RL, 1976. Brain-stem and adrenal abnormalities in the SIDS. Am. J. Clin. Pathol 66, 526–530. [DOI] [PubMed] [Google Scholar]
- Naeye RL, 1980. Sudden infant death. Sci Am. 242, 56–62. [DOI] [PubMed] [Google Scholar]
- Naeye RL, 1989. Pregnancy hypertension, placental evidences of low uteroplacental blood flow, and spontaneous premature delivery. Hum. Pathol 20, 441–444. [DOI] [PubMed] [Google Scholar]
- Naeye RL, Ladis B, Drage JS, 1976. Sudden infant death syndrome. A prospective study. Am. J. Dis. Child 130, 1207–1210. [DOI] [PubMed] [Google Scholar]
- Obonai T, Takashima S, Becker LE, Asanuma M, Mizuta R, Horie H, Tanaka J, 1996. Relationship of Substance P and gliosis in medulla oblongata in neonatal sudden infant death syndrome. Pediatr. Neurol 15, 189–192. [DOI] [PubMed] [Google Scholar]
- Ostfeld BM, Schwartz-Soicher O, Reichman NE, Teitler JO, Hegyi T, 2017. Prematurity and Sudden Unexpected Infant Deaths in the United States. Pediatrics 140. [DOI] [PubMed] [Google Scholar]
- Oyen N, Haglund B, Skjaerven R, Irgens LM, 1997. Maternal smoking, birthweight and gestational age in sudden infant death syndrome (SIDS) babies and their surviving siblings. Paediatric and perinatal epidemiology 11 Suppl 1, 84–95. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N, 2002. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics 33, 142–149. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Takashima S, 2002. Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic science international 130 Suppl, S53–59. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE, 2000. The role of gastro-oesophageal reflux in the aetiology of SIDS. Early Hum. Dev 59, 127–149. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC, 2000. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J. Neuropathol. Exp. Neurol 59, 377–384. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, White WF, Kinney HC, 1997. Decreased kainate receptor binding in the arcuate nucleus of the Sudden Infant Death Syndrome. J Neuropathol Exp Neurol. 56(11), 1253–1261. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Thonpson EG, Kinney HC, 2006a. Serotonergic and glutamatergic neurons at the ventral medullary surface of the human infant: Observations relevant to central chemosensitivity in early human life. Autonmic Neurosci. 124, 112–124. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall RA, Chadwick AE, Krous HF, Kinney HC, 2006b. Multiple serotonergic brainstem abnormalities in the sudden infant death syndrome. J.A.M.A 296, 2124–2132. [DOI] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE, Zhou S-S, 1991. Afferent projections of the superior and recurrent laryngeal nerves. Brain Res. 539, 169–174. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The Rat Brain in Stereotaxic Coordinates, 4th ed. Academic Press, San Diego, CA. [Google Scholar]
- Paxinos G, Watson C, 2005. The Rat Brain in Stereotaxic Coordinates, 5th ed. Academic Press, San Diego. [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE, 1999. Gasping and other cardiorespiratory patterns during sudden infant death. Pediatr. Res 45, 350–354. [DOI] [PubMed] [Google Scholar]
- Poets CF, Samuels MP, Noyes JP, Hewertson J, Hartmann H, Holder A, Southall DP, 1993. Home event recordings of oxygenation, breathing movements, and heart rate and rhythm in infants with recurrent life-threatening events. The Journal of pediatrics 123, 693–701. [DOI] [PubMed] [Google Scholar]
- Poets CF, Schlaud M, Kleemann WJ, Rudolph A, Diekmann U, Sens B, 1995. Sudden infant death and maternal cigarette smoking: results from the Lower Saxony Perinatal Working Group. Eur. J. Pediatr 154, 326–329. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC, 2009. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci 29, 3720–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi JJ, McBride PT, Yates AJ, 1985. Brainstem immaturity in Sudden Infant Death Syndrome: A quantitative rapid golgi study of dendritic spines in 95 infants. Brain Res. 325, 39–48. [DOI] [PubMed] [Google Scholar]
- Randall BB, Paterson DS, Haas EA, Broadbelt KG, Duncan JR, Mena OJ, Krous HF, Trachtenberg FL, Kinney HC, 2013. Potential asphyxia and brainstem abnormalities in sudden and unexpected death in infants. Pediatrics 132, el616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP, 1986. Reflex prolongation of stage I of expiration. Pflügers Arch. 407, 190–198. [DOI] [PubMed] [Google Scholar]
- Roulier S, Arsenault J, Reix P, Dorion D, Praud JP, 2003. Effects of C fiber blockade on cardiorespiratory responses to laryngeal stimulation in concious lambs. Respir. Physiol. Neurobiol 136, 13–23. [DOI] [PubMed] [Google Scholar]
- Sridhar R, Thach BT, Kelly DH, Henslee JA, 2003. Characterization of successful and failed autoresuscitation in human infants. Pediatr. Pulmonol. 36, 113–122. [DOI] [PubMed] [Google Scholar]
- St. Hilaire M, Nsegbe E, Gagnon-Gervais K, Samson N, Moreau-Bussiere F, Fortier P-H, Praud J-P, 2005. Laryngeal chemoreflexes induced by acid, water and saline in non-sedated newborn lambs during quiet sleep. J. Appl. Physiol 102, 1429–1438. [DOI] [PubMed] [Google Scholar]
- St. John WM, Leiter JC, 2008. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of α-1 adrenergic receptors and serotonin 5HT2 receptors J. Appl. Physiol 104, 665–673. [DOI] [PubMed] [Google Scholar]
- Stoltenberg L, Sundar T, Almaas R, Storm H, Rognumm TO, Saugstad OD, 1994. Changes in apnea and autoresuscitation in piglets after intravenous and intrathecal interleukin-1 beta injection. J. Perinat. Med 22, 421–432. [DOI] [PubMed] [Google Scholar]
- Takashima S, Armstrong D, Becker LE, Huber J, 1978. Cerebral white matter lesions in sudden infant death syndrome. Pediatrics 62, 155–159. [PubMed] [Google Scholar]
- Takashima S, Becker LE, 1985. Developmental abnormalities of medullary 'Respiratory centers' in sudden infant death syndrome. Exp. Neurol 90, 580–587. [DOI] [PubMed] [Google Scholar]
- Takashima S, Becker LE, 1991. Delayed dendritic development of catecholaminergic neurons in the ventrolateral medulla of children who died of sudden infant death syndrome. Neuropediatrics 22, 97–99. [DOI] [PubMed] [Google Scholar]
- Takashima S, Becker LE, Chan F-W, 1982. Retardation of neuronal maturation in premature infants compared with term infants of the same postconceptional age. Pediatrics 69, 33–39. [PubMed] [Google Scholar]
- Takashima S, Mito T, 1985. Neuronal development in the medullary reticular formation in sudden infant death syndrome and premature infants. Neuropediatrics 16, 76–79. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mito T, Yamanouchi H, 1994. Developmental brain-stem pathology in sudden infant death syndrome. Acta Paediatr Jpn 36, 317–320. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, 2016. Role of reactive oxygen species and TRP channels in the cough reflex. Cell calcium 60, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D, 1993. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proceedings of the National Academy of Sciences of the United States of America 90, 1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT, 2001. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am. J. Med 111, 69S–77S. [DOI] [PubMed] [Google Scholar]
- Thach BT, 2007. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm. Pharmacol. Therap 20, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Dapena M, 1986. Sudden infant death syndrome. Morphology update for forensic pathologists--1985. Forensic Sci. Int 30, 177–186. [DOI] [PubMed] [Google Scholar]
- Valdes-Dapena MA, Gillane MM, Catherman R, 1976. Brown fat retention in sudden infant death syndrome. Arch. Pathol. Lab. Med 100, 547–549. [PubMed] [Google Scholar]
- van der Velde L, Curran A, Filiano JJ, Darnall RA, Bartlett D Jr., Leiter JC, 2003. Prolongation of the laryngeal chemoreflex after inhibition of the rostroventral medulla in piglets: A role in SIDS? J. Appl. Physiol 94, 1883–1895. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL, 1995. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J. Neurosci 15, 5346–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bartlett D Jr., Leiter JC, 2011. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir. Physiol. Neurobiol 176, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bartlett D Jr., Leiter JC, 2016. Interleukin-1beta and interleukin-6 enhance thermal prolongation fo the LCR in decerebrate piglets. Resp. Physiol. Neurobiol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Crane-Godreau MA, Leiter JC, Bartlett D Jr., 2009. Gestational cigarette smoke exposure and hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir. Physiol. Neurobiol 165, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Damon TA, Leiter JC, Bartlett D Jr., 2007. Focal warming of the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J. Appl. Physiol 102, 54–62. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D Jr., 2008. Nicotine, hyperthermia and the LCR in anesthetized rats. [Google Scholar]
- Xia L, Leiter JC, Bartlett D Jr., 2010. Gestational nicotine exposure exaggerates hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir. Physiol. Neurobiol 171, 17–21. [DOI] [PubMed] [Google Scholar]