ABSTRACT
Background
There is sufficient evidence to show the mortality reduction effect of colorectal cancer (CRC) screening programs using the fecal occult blood test (FOBT). However, we see cases that are found to be advanced CRC despite yearly FOBT screening.
Methods
The aim of this study was to investigate the characteristics of advanced CRC detected by a fecal immunochemical test (FIT) screening program in participants with a negative screening result the previous year, which we call “Negative advanced CRC”. A total of 109,639 participants (10.0% required colonoscopy, of whom 76.9% received one) underwent a CRC screening program using a FIT from fiscal 2009 to 2017. Negative advanced CRC was compared with advanced CRC (First advanced CRC) found at the first visit in a person who had not had a FIT screening history for more than 3 years. In addition, we compared the characteristics of Negative advanced CRC with those of interval cancer: cancer cases detected after a negative screening result and before the date of the next recommended screening.
Results
A total of 339 cases of CRC (175 male: 164 female, 173 early stage: 166 advanced stage) were detected in the nine-year CRC screening period. The rate of right-sided CRCs was significantly higher in female (P < 0.01), advanced stage (P < 0.01), negative result previous year (P < 0.01), and symptom-negative (P < 0.01) participants than in each counterpart, respectively. The ratio of female (22/35; 62.9%) patients in Negative advanced CRCs tended to be high compared with that (40/83; 48.2%) in First advanced CRCs (P = 0.145). Overall, 22 (62.9%) of 35 Negative advanced CRCs and 28 (33.7%) of 83 First advanced CRCs were located in the right-sided colon, and the rate was significantly higher in Negative advanced CRCs (P < 0.01). In addition, the frequency of female patients was significantly higher in right-sided Negative advanced CRCs than in right-sided First advanced CRCs (P = 0.03).
Conclusion
The characteristics of Negative advanced CRC cases (female and right-sided colon) were similar to those of interval cancer reported so far. In the future, it will be necessary to introduce a screening program that is highly sensitive to right-sided CRC.
Keywords: colorectal cancer, fecal immunochemical test, interval cancer, Japanese
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths in the world.1 Survival is strongly related to tumor stage at the time of diagnosis.2, 3 CRC deaths in Japan continue to increase, and in 2017, they were second after lung cancer. The number of cases of CRC is increasing year by year for both men and women, and the number of cases in 2014 was the highest.4 Population-based CRC screening programs enable detection of CRC at an earlier stage. Screening with fecal occult blood tests (FOBTs) has been shown to reduce CRC-related mortality.5,6,7 According to the Guidelines for Colon Cancer Screening (2005) based on the efficacy assessment in Japan,8 screening with the guaiac-based fecal occult blood test (gFOBT) has been shown to reduce CRC-related mortality in three randomized, controlled trials (Funen research, Nottingham study, and Minnesota study), with a 13% to 21% decrease of CRC deaths in a biennial screening and 33% in an annual screening.5,6,7
In Japan, CRC screening programs are based on the fecal immunochemical test (FIT), which does not require dietary restrictions. This strategy has consistently demonstrated both high sensitivity for detecting advanced adenoma and invasive CRC and good adherence by the target population,9,10,11 since FITs have a better diagnostic accuracy than gFOBTs.12 Despite a lack of randomized trials, the available evidence suggests that FIT-based CRC screening may reduce cancer mortality.13,14,15,16 The effectiveness of the FIT has been confirmed by case-control studies by Hiwatashi, Saito, and Zappa et al.17,18,19
Although FOBT screening is effective, not all CRCs will be detected within a screening program. These cancer cases partially consist of interval cancers: cancer cases detected after a negative screening examination and before the date of the next recommended screening.20 So far, most studies have reported interval cancers in programs using gFOBT, showing high proportions of interval cancers. In such programs, the proportion of interval cancer cases ranged from 48% to 55%.6, 7, 21, 22 Recently, van der Vlugt et al.23 reported low proportions of interval cancers (23%) using the FIT. These studies have shown better survival rates for screen-detected cancers than for interval cancers.24
Since not all CRCs are detected at an early stage in FOBT screening, we see cases that are found at an advanced CRC stage after a negative screening result the previous year (Negative advanced CRC). In this study, the characteristics of Negative advanced CRC in a FIT screening program are reported.
MATERIALS AND METHODS
Population and Design
As part of a community health checkup, Yonago City, Tottori Prefecture, conducts FIT screening every year for residents over 40 years of age. Fecal samples were collected for FIT, using a dedicated stool collection container distributed by the municipality. In addition, on the screening day, participants were asked about abdominal symptoms (constipation, diarrhea, bloody stool). For persons whose results were positive, considered subjects who required a detailed examination at a medical institution, colonoscopy was recommended.
A total of 109,639 participants underwent CRC screening by the two-day FIT between fiscal 2009 and 2017. Cases in which the test submitted only on the first day was negative were excluded. There was no designation of the stool test brands, and Yonago City does not know the stool test brands used in each medical institution. Thus, the positive threshold for hemoglobin was unclear. For all screening rounds combined, the FIT positivity rate was 10.0%, and adherence to post-FIT colonoscopy was 76.9%.
Definitions
Advanced CRC detected by a FIT screening program in participants with a negative screening result the previous year was defined as Negative advanced CRC, and advanced CRC found in a first-visit participant who had not had a FIT screening history for more than 3 years was defined as First advanced CRC. In Negative advanced CRC cases, those who had a false-positive result on a previous FIT but negative colonoscopy results were not included. The effects of sex, age at diagnosis, tumor location, and presence or absence of symptoms were also examined in both groups.
The rectum, sigmoid colon, and descending colon were included as the left-sided colon, and the transverse colon, ascending colon, cecum, and appendix were included as the right-sided colon in the analysis. Tumor staging was based on the 9th edition of the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma.25
Statistical analysis
Continuous variables are expressed as means ± standard deviation. The χ2 test or was used to compare categorical variables. Student’s t-test was used to compare continuous variables. All calculations were performed using Stat Flex (ver. 6.0; Artech Co., Ltd, Osaka, Japan), and P values of < 0.05 were considered significant.
Ethics approval
Approval was obtained from the Institutional Review Board of Tottori University (No.1511A079), and informed consent requirements were waived for this study.
RESULTS
Characteristics of screening-detected CRC
The clinicopathological characteristics of screening-detected CRC are shown in Table 1. A total 109,639 participants underwent CRC screening by the FIT between fiscal 2009 and 2017. During the study period, 339 cases of CRC, in 175 males and 164 females, were detected: 127 in the right-sided colon and 212 in the left-sided colon. The average age at diagnosis was 71.5 ± 8.97 years; 164 cases (48.4%) were female, and 166 cases (49.0%) were advanced stage; 138 cases (40.7%) were first-time screening, and 112 cases (33.0%) had symptoms.
Table 1. Characteristics of screening-detected colorectal cancer.
| Total (n = 339) | Tumor location | P-value | ||
| Right-sided (n = 127) | Left-sided (n = 212) | |||
| Age at diagnosis (years) | 71.5 ± 8.97 | 72.5 ± 9.27 | 70.9 ± 8.77 | 0.063 |
| Sex, n (%) | ||||
| Male | 175 | 44 (34.6) | 131 (61.8) | < 0.01 |
| Female | 164 | 83 (65.4) | 81 (38.2) | |
| Stage, n (%) | ||||
| Early | 173 (51.0) | 53 (41.7) | 120 (56.6) | < 0.01 |
| Advanced | 166 (49.0) | 74 (58.3) | 92 (43.4) | |
| Screening history, n (%) | ||||
| First time | 138 (40.7) | 39 (30.7) | 99 (46.7) | < 0.01 |
| Negative result previous year | 102 (30.1) | 54 (42.5) | 48 (22.6) | |
| Other | 99 (29.2) | 34 (26.8) | 65 (30.7) | |
| Symptoms, n (%) | ||||
| (+) | 112 (33.0) | 30 (23.6) | 82 (38.7) | < 0.01 |
| (–) | 227 (67.0) | 97 (76.4) | 130 (61.3) | |
The frequency of advanced CRC was 43.4% in the left-sided colon and 58.3% in the right-sided colon. On the other hand, the frequency of early stage CRC was 56.6% in the left-sided colon and 41.7% in the right-sided colon. The difference between the left-sided colon and the right-sided colon was significant. Moreover, the rate of right-sided CRCs was significantly higher in female (P < 0.01), negative result the previous year (P < 0.01), and symptom-negative (P < 0.01) participants than in each counterpart, respectively. No other correlations were found for age, sex, tumor location, stage and symptoms.
Screening history and stage of CRC
Screening history by cancer stage is shown in Table 2. Of 166 screening-detected advanced cancers, 83 (50.0%) were First advanced cancers and 35 (21.1%) were Negative advanced cancers. The rate (50.0%) of advanced cancer detected on first-time screening was significantly higher than that of early stage cancer (P < 0.01).
Table 2. Screening history and stage of colorectal cancer.
| Total | Stage | P -value | ||
| Early | Advanced | |||
| Screening-detected CRC, n | 339 | 173 | 166 | |
| Screening history, n (%) | ||||
| First time | 138 (40.7) | 55 (31.8) | 83 (50.0) | < 0.01 |
| Negative result previous year | 102 (30.1) | 67 (38.7) | 35 (21.1) | |
Characteristics of Negative advanced CRC (Table 3)
Table 3. Characteristics of Negative and First advanced colorectal cancers.
| Screening history of advanced CRC | P-value | ||
| Negative advanced CRC (n = 35) |
First advanced CRC (n = 83) |
||
| Age at diagnosis (years) | 71.6 ± 8.03 | 70.7 ± 8.72 | 0.289 |
| Sex, n (%) | |||
| Male | 13 (37.1) | 43 (51.8) | 0.145 |
| Female | 22 (62.9) | 40 (48.2) | |
| Tumor location, n (%) | |||
| Right-sided | 22 (62.9) | 28 (33.7) | < 0.01 |
| Left-sided | 13 (37.1) | 55 (66.3) | |
| Symptoms, n (%) | |||
| (+) | 6 (17.1) | 41 (49.4) | < 0.01 |
| (–) | 29 (82.9) | 42 (50.6) | |
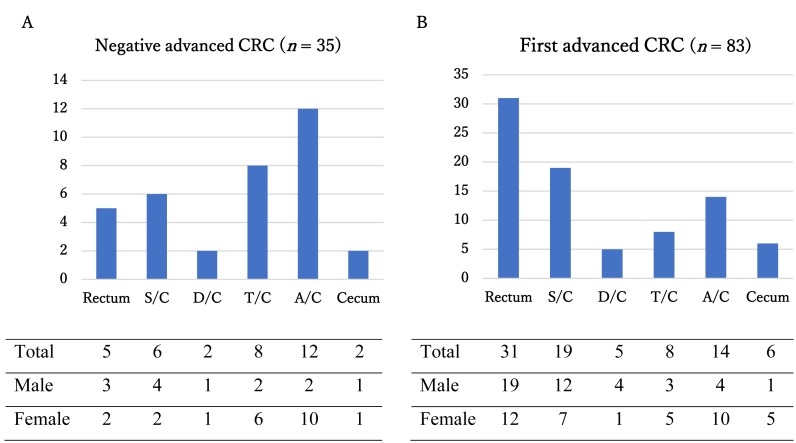
Of the detected CRCs, 35 (10.3%) were Negative advanced CRCs, and 83 (24.5%) were First advanced CRCs. The average age of Negative advanced CRC and First advanced CRC participants was 71.6 ± 8.03 and 70.7 ± 8.72 years, respectively. The ratio of female (22/35; 62.9%) participants in Negative advanced CRCs was higher than that (40/83; 48.2%) in First advanced CRCs, but it was not significantly different (P = 0.145). The frequency of right-sided location was significantly higher in Negative advanced CRC than in First advanced CRC (P < 0.01). In addition, the frequency of female participants was significantly higher in right-sided Negative advanced CRC than in right-sided First advanced CRC (P = 0.03) (Fig. 1). Symptoms were observed in 17.1% (6/35) of Negative advanced CRC and 49.4% (41/83) of First advanced CRC cases, and the rate was significantly lower in Negative advanced CRC cases (P < 0.01). Three-year sequential screening had been performed in 65.7% (23/35) of Negative advanced CRC cases (data not shown).
Fig. 1.
Anatomical locations of Negative advanced CRC (A) and First advanced CRC (B).
The frequency of female participants was significantly higher in right-sided Negative advanced CRC than in right-sided First advanced CRC (P = 0.03). A/C, Ascending Colon; D/C, Descending Colon; S/C, Sigmoid Colon; T/C, Transverse Colon.
DISCUSSION
The effectiveness of CRC screening by gFOBT has been demonstrated by several RCTs.5,6,7 However, we see not only interval cancers,20 but also advanced CRCs (Negative advanced CRCs) detected by FIT screening programs in participants with a negative screening result the previous year. In the present study, FIT screen-detected CRCs were frequently located at the right-sided colon in female, advanced stage, and symptom-negative participants. Negative advanced CRC was related to right-sided location and female sex, similar to interval cancer.
FIT is one of the most commonly used CRC screening methods worldwide; it is moderately sensitive, noninvasive for the detection of neoplasia, and has higher adherence rates compared to gFOBT. The greater adherence to screening with FIT may be the result of fewer dietary restrictions, easier sample collection, and the requirement for fewer samples compared to gFOBT.9,10,11,12 In the present study, most FIT-detected CRCs were located in the left-sided colon, and FIT-detected CRCs in the right-sided colon were frequent in female and advanced stage participants, in agreement with previous reports.23, 26 In the screen-detected CRCs, cases with symptoms were located less frequently in the right-sided colon than in the left-sided colon, where symptoms easily appear.27, 28
According to a 2015 nationwide summary of gastrointestinal cancer screening in the Japanese Society of Gastroenterological Cancer Screening,29 the percentage of CRCs found in first-time CRC screening was 36.7%.29 In this survey, 40.7% of CRCs found by CRC screening were first-time cases. The percentage of first-time cases was almost the same as in the national survey. The rate of advanced CRC detected by FIT screening was higher in first-time cases than in cases with a negative screening result the previous year. Thus, the CRC cases found on first-time CRC screening tend to be more advanced than the CRC cases found in second and subsequent screenings.
Several studies have reported that the FIT is less accurate in detecting right-sided than left-sided colorectal neoplasia.26, 30, 31 With regard to cancer location, lower FIT efficacy in the right colon may result from lesions that grow more rapidly and that bleed less due to phenotypic characteristics or weaker mechanical triggers. A longer transit time also may lead to degradation of hemoglobin, resulting in a negative result on the FIT. Therefore, the cancer location (right-sided vs. left-sided) might affect FIT sensitivity.26, 30,31,32,33 In the present study, the frequency of right-sided location was significantly higher in Negative advanced CRC than in First advanced CRC, indicating that FIT is not accurate enough to detect advanced CRC in the right-sided colon. Overall, these data are in agreement with previous studies that found a lower FIT accuracy for right-sided versus left-side advanced neoplasia.23, 26, 30,31,32,33
Previous studies have shown that interval cancer has a high incidence in female participants,21, 34,35,36 but recently it has been reported that there is no difference.22, 35, 37 In the present study, Negative advanced CRC had a higher incidence in women than men (62.9% vs 37.1%), yet this difference was not significant. However, in the right-sided colon, the rate of Negative advanced CRC was significantly higher in female participants.
Interval cancer on colonoscopy is detected in the right-sided colon as well as on FOBT, though colonoscopy has become better at detection through quality and technological improvements.38 Thus, quality control of colonoscopy, such as reducing inadequate bowel preparation and the operator’s adenoma detection rate, is considered important. The number of deaths from CRC continues to grow in Japan and decrease in the USA. One of the reasons for the decrease in colon cancer deaths in the United States may be that about 60% of people undergo colonoscopy once every 10 years.39 Moreover, a recent study revealed that net survival for right-sided colon cancer was significantly lower than that for left-sided disease in a Japanese population.40 Since a CRC right-sided shift is seen in persons older than 60 years,41, 42 colonoscopy might supplement FIT to enhance detection of right-sided lesions. A randomized, controlled trial to verify the effect of colon cancer screening by total colonoscopy has been carried out in Spain43 and the Nordic countries.44
The limitations of the present study were as follows. First, rectal neoplastic lesions were grouped with left-sided colon lesions. However, the natural history of rectal neoplastic lesions differs from that of cancers restricted to the colon. Therefore, different clinicopathological situations might have been combined within the same category.45 Second, the screening history of detected CRC cases, but not all participants, was confirmed.
The results of the present study showed that the characteristics of Negative advanced CRC were similar to those of interval cancer and were related to tumor location and sex, suggesting the value of integrating FIT programs with colonoscopy. Future studies may address a screening method that is highly sensitive to right-sided CRC.
Acknowledgments
Acknowledgments: The authors are grateful to Michiko Shabana and Kunihiko Miura for their valuable comments and supports on this study.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Siegel RL,Miller KD,Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Warschkow R,Sulz MC,Marti L,Tarantino I,Schmied BM,Cerny T,et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer. 2016;16:554. 10.1186/s12885-016-2412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnet E,Faivre J,Raymond L,Garau I. Influence of stage at diagnosis on survival differences for rectal cancer in three European populations. Br J Cancer. 1999;81:463-8. 10.1038/sj.bjc.6690716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Registry and Statistics [Internet]. Tokyo: Cancer Information Service, National Cancer Center; 2018. [cited 2019 Nov 1]. Available from: http://ganjoho.jp/reg_stat/statistics/stat/summary.html. Japanese.
- 5.Mandel JS,Church TR,Ederer F,Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. JNCI Journal of the National Cancer Institute. 1999;91:434-7. 10.1093/jnci/91.5.434 [DOI] [PubMed] [Google Scholar]
- 6.Scholefield JH,Moss S,Sufi F,Mangham CM,Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002;50:840-4. 10.1136/gut.50.6.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jørgensen OD,Kronborg O,Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50:29-32. 10.1136/gut.50.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Research Group for the Development of Appropriate Cancer Screening Methods and Its Assessment. [Evidence-based Guidelines for Colorectal Cancer Screening] [Internet]. 2005. [cited 2019 Mar 4]. Available from: http://canscreen.ncc.go.jp/pdf/guideline/colon_full080319.pdf. Japanese.
- 9.Allison JE,Sakoda LC,Levin TR,Tucker JP,Tekawa IS,Cuff T,et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99:1462-70. 10.1093/jnci/djm150 [DOI] [PubMed] [Google Scholar]
- 10.Brenner H,Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer. 2013;49:3049-54. 10.1016/j.ejca.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 11.van Rossum LGM,van Rijn AF,Verbeek ALM,van Oijen MGH,Laheij RJF,Fockens P,et al. Colorectal cancer screening comparing no screening, immunochemical and guaiac fecal occult blood tests: A cost-effectiveness analysis. Int J Cancer. 2011;128:1908-17. 10.1002/ijc.25530 [DOI] [PubMed] [Google Scholar]
- 12.Tinmouth J,Lansdorp-Vogelaar I,Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64:1327-37. 10.1136/gutjnl-2014-308074 [DOI] [PubMed] [Google Scholar]
- 13.Ventura L,Mantellini P,Grazzini G,Castiglione G,Buzzoni C,Rubeca T,et al. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig Liver Dis. 2014;46:82-6. 10.1016/j.dld.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 14.Zorzi M,Fedeli U,Schievano E,Bovo E,Guzzinati S,Baracco S,et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64:784-90. 10.1136/gutjnl-2014-307508 [DOI] [PubMed] [Google Scholar]
- 15.Libby G,Brewster DH,McClements PL,Carey FA,Black RJ,Birrell J,et al. The impact of population-based faecal occult blood test screening on colorectal cancer mortality: a matched cohort study. Br J Cancer. 2012;107:255-9. 10.1038/bjc.2012.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SR,Tucker GR,Osborne JM,Byrne SE,Bampton PA,Fraser RJL,et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust. 2013;198:327-30. 10.5694/mja12.11357 [DOI] [PubMed] [Google Scholar]
- 17.Hiwatashi N,Morimoto T,Fukao A,Sato H,Sugahara N,Hisamichi S,et al. An evaluation of mass screening using fecal occult blood test for colorectal cancer in Japan: a case-control study. Jpn J Cancer Res. 1993;84:1110-2. 10.1111/j.1349-7006.1993.tb02809.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H,Soma Y,Koeda J,Wada T,Kawaguchi H,Sobue T,et al. Reduction in risk of mortality from colorectal cancer by fecal occult blood screening with immunochemical hemagglutination test. A case-control study. Int J Cancer. 1995;61:465-9. 10.1002/ijc.2910610406 [DOI] [PubMed] [Google Scholar]
- 19.Zappa M,Castiglione G,Grazzini G,Falini P,Giorgi D,Paci E,et al. Effect of faecal occult blood testing on colorectal mortality: results of a population-based case-control study in the district of Florence, Italy. Int J Cancer. 1997;73:208-10. [DOI] [PubMed] [Google Scholar]
- 20.Sanduleanu S,le Clercq CMC,Dekker E,Meijer GA,Rabeneck L,Rutter MD,et al. ; Expert Working Group on ‘Right-sided lesions and interval cancers’, Colorectal Cancer Screening Committee, World Endoscopy Organization. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257-67. 10.1136/gutjnl-2014-307992 [DOI] [PubMed] [Google Scholar]
- 21.Gill MD,Bramble MG,Rees CJ,Lee TJW,Bradburn DM,Mills SJ. Comparison of screen-detected and interval colorectal cancers in the Bowel Cancer Screening Programme. Br J Cancer. 2012;107:417-21. 10.1038/bjc.2012.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia M,Domènech X,Vidal C,Torné E,Milà N,Binefa G,et al. Interval cancers in a population-based screening program for colorectal cancer in catalonia, Spain. Gastroenterol Res Pract. 2015;2015:672410. 10.1155/2015/672410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Vlugt M,Grobbee EJ,Bossuyt PMM,Bos A,Bongers E,Spijker W,et al. Interval Colorectal Cancer Incidence Among Subjects Undergoing Multiple Rounds of Fecal Immunochemical Testing. Gastroenterology. 2017;153:439-447.e2. 10.1053/j.gastro.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 24.Gill MD,Bramble MG,Hull MA,Mills SJ,Morris E,Bradburn DM,et al. Screen-detected colorectal cancers are associated with an improved outcome compared with stage-matched interval cancers. Br J Cancer. 2014;111:2076-81. 10.1038/bjc.2014.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The 9th edition of the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma, Japanese Society for Cancer of the Colon and Rectum; 2018. [DOI] [PMC free article] [PubMed]
- 26.Zorzi M,Hassan C,Capodaglio G,Narne E,Turrin A,Baracco M,et al. Divergent Long-Term Detection Rates of Proximal and Distal Advanced Neoplasia in Fecal Immunochemical Test Screening Programs: A Retrospective Cohort Study. Ann Intern Med. 2018;169:602-9. 10.7326/M18-0855 [DOI] [PubMed] [Google Scholar]
- 27.Hara N,Mastumoto T,Ikeda A,Harada S,Yasutake K,Kido M,et al. Clinicopathological feature of colorectal cancer detected by fecal occult blood test-comparison of early cancer rate by tumor location. Japanese Association for Cancer Detection and Diagnosis. 2013;21:184-90. [Google Scholar]
- 28.Nakae S,Ishikawa Y,Kuniyasu T,Konishi M,Kaneda K,Kawamura T,et al. Clinicopathological Feature of Colorectal Cancer Detected by Fecal Occult Blood Test. The Japanese Journal of Gastroenterological Surgery. 1999;32:1184-91. 10.5833/jjgs.32.1184 [DOI] [Google Scholar]
- 29.Mizuguchi M. Journal of Gastrointestinal Cancer Screening. 2018;56:1009-66. [Google Scholar]
- 30.Brenner H,Niedermaier T,Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int J Cancer. 2017;140:2015-22. 10.1002/ijc.30629 [DOI] [PubMed] [Google Scholar]
- 31.Wong MCS,Ching JYL,Chan VCW,Lam TYT,Shum JP,Luk AKC,et al. Diagnostic accuracy of a qualitative fecal immunochemical test varies with location of neoplasia but number of specimens. Clin Gastroenterol Hepatol. 2015;13:1472-9. 10.1016/j.cgh.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 32.de Wijkerslooth TR,Stoop EM,Bossuyt PM,Meijer GA,van Ballegooijen M,van Roon AHC,et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107:1570-8. 10.1038/ajg.2012.249 [DOI] [PubMed] [Google Scholar]
- 33.Hirai HW,Tsoi KKF,Chan JYC,Wong SH,Ching JYL,Wong MCS,et al. Systematic review with meta-analysis: faecal occult blood tests show lower colorectal cancer detection rates in the proximal colon in colonoscopy-verified diagnostic studies. Aliment Pharmacol Ther. 2016;43:755-64. 10.1111/apt.13556 [DOI] [PubMed] [Google Scholar]
- 34.Zorzi M,Fedato C,Grazzini G,Stocco FC,Banovich F,Bortoli A,et al. High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut. 2011;60:944-9. 10.1136/gut.2010.223982 [DOI] [PubMed] [Google Scholar]
- 35.Steele RJC,McClements P,Watling C,Libby G,Weller D,Brewster DH,et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61:576-81. 10.1136/gutjnl-2011-300535 [DOI] [PubMed] [Google Scholar]
- 36.Brenner H,Chang-Claude J,Seiler CM,Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012;61:1576-82. 10.1136/gutjnl-2011-301531 [DOI] [PubMed] [Google Scholar]
- 37.Digby J,Fraser CG,Carey FA,Lang J,Stanners G,Steele RJC. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J Med Screen. 2016;23:130-4. 10.1177/0969141315609634 [DOI] [PubMed] [Google Scholar]
- 38.Doubeni CA,Corley DA,Quinn VP,Jensen CD,Zauber AG,Goodman M,et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291-8. 10.1136/gutjnl-2016-312712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC). Vital signs: colorectal cancer screening test use--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881-8. [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa-Senda H,Hori M,Matsuda T,Ito H. Prognostic impact of tumor location in colon cancer: the Monitoring of Cancer Incidence in Japan (MCIJ) project. BMC Cancer. 2019;19:431. 10.1186/s12885-019-5644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cress RD,Morris C,Ellison GL,Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992–2001. Cancer. 2006;107(suppl):1142-52. 10.1002/cncr.22011 [DOI] [PubMed] [Google Scholar]
- 42.Cucino C,Buchner AM,Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035-40. 10.1007/s10350-004-6356-0 [DOI] [PubMed] [Google Scholar]
- 43.Quintero E,Castells A,Bujanda L,Cubiella J,Salas D,Lanas Á,et al. ; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697-706. 10.1056/NEJMoa1108895 [DOI] [PubMed] [Google Scholar]
- 44.Kaminski M,Bretthauer M,Zauber A,Kuipers E,Adami HO,van Ballegooijen M,et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44:695-702. 10.1055/s-0032-1306895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberman DA,Weiss DG,Bond JH,Ahnen DJ,Garewal H,Harford WV,et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-8. 10.1056/NEJM200007203430301 [DOI] [PubMed] [Google Scholar]