ABSTRACT
Background
Mild parkinsonian signs are important clinical symptoms related to the decline of motor and cognitive functions. We aimed to identify predictors for the incidence of mild parkinsonian signs in older Japanese by conducting an 8-year longitudinal community-based cohort study.
Methods
Participants aged 65 years or older, living in Ama-cho, a rural island town in western Japan, underwent a baseline assessment of motor function, cognitive function, depression score, the Pittsburgh Sleep Quality Index (PSQI), the Tanner questionnaire, and cerebral white matter lesions on brain magnetic resonance imaging from 2008 to 2010, and then underwent a follow-up neurological examination from 2016 to 2017. Mild parkinsonian signs were defined according to a modified Unified Parkinson’s Disease Rating Scale score.
Results
Of the 316 participants without mild parkinsonian signs at baseline, 94 presented with incident mild parkinsonian signs at follow-up. In addition to an absence of exercise habits, a higher score on the Tanner questionnaire, PSQI, and deep white-matter hyperintensity Fazekas scores were significant independent predictors for incidence of mild parkinsonian signs.
Conclusion
We suggest multiple factors related to incidence of mild parkinsonian signs. Vascular lesions and sleep disorders are associated with a pathogenesis of mild parkinsonian signs, the Tanner questionnaire is useful for early detection of subclinical mild parkinsonian signs, and exercise has a possibility of being associated with preventing onset of mild parkinsonian signs.
Keywords: cerebral white matter lesions, exercise habits, sleeping disorder, subjective motor symptoms
Mild parkinsonian signs (MPS) are defined as features of bradykinesia, rigidity, and tremor, in addition to gait and postural instability occurring in isolation or combinations that do not meet clinical criteria for a diagnosis of parkinsonism. MPS are common in aging with their prevalence ranging from 15% to 52%.1,2,3 Subjects with MPS have more cerebral white matter lesions than those without.4,5,6 MPS is known to have the same risk factors as Parkinson’s disease, such as depression, hyposmia, REM sleep behavior disorder, and substantia nigra hyperechogenicity.3, 7, 8 Neuropathological changes of MPS have not yet been fully elucidated. Heterogeneous pathological changes, such as age related decline in nigrostriatal dopaminergic neurons, degenerative changes of Lewy body or Alzheimer’s disease, and vascular changes in the cerebral subcortical white matter, probably relate to MPS.9
MPS are associated with increased incidence of dementia10, 11 as well as functional disability such as parkinsonism12, 13 and mortality,14 although these can be reversible.15 We have previously reported that the presence of MPS is a risk factor for the development of parkinsonism and dementia in a prospective community-based cohort study.16 MPS are thought to be an important clinical sign in aging, and it is important to prevent the incidence of MPS at an early stage to extend healthy life expectancy. Here, we report an 8-year follow-up in our cohort to identify predictors for incident MPS in older community-dwelling Japanese.
SUBJECTS AND METHODS
Participants
We conducted this study in the municipality of Ama-cho, a rural island town with a large older population located 70 km from Yonago city, in the northwestern part of Japan.3 To be eligible for the study, participants were required to be physical and legal residents of the town on March 31, 2008. Inclusion criteria were as follows: (i) 65 years of age or older (n = 731), (ii) agreement to participate in the study, and (iii) completion of an examination survey at baseline and follow-up. Exclusion criteria were as follows: (i) participants who had died or moved out of town, (ii) participants who had a medical history of psychiatric disorders, and (iii) participants with severe organ dysfunction. Participants who did not undergo this survey despite our vigorous and repeated appeals prompting their participation were classified as nonresponders.
The study was approved by the Tottori University Committee for Medical Research Ethics (approval No. 1695) and followed the principles outlined in the Declaration of Helsinki and its contemporary amendments. All participants provided their written informed consent to participate.
Survey procedure and data collection
This study design was a prospective longitudinal study. The baseline study was conducted from 2008 to 2010 and the follow-up study was from 2016 to 2017. We conducted a uniform and standardized examination program at baseline and 8 years later. We first administered a questionnaire survey by mailing the questionnaires to the residents legally living in Ama-cho at the start of the baseline and follow-up study, and subsequently conducted an examination survey.
Questionnaire survey
The questionnaire was constructed to collect personal information and demographic information such as age, sex, formal education level, medical history including hypertension, dyslipidemia, diabetes mellitus, and current exercise habits. Medical history data were also obtained via a review of electronic healthcare system databases and patient administered questionnaires. For assessments of subjective motor and nonmotor symptoms of the participants, we also administered the Tanner questionnaire, which is validated for Parkinson’s disease screening.17 The Japanese version of the Geriatric Depression Scale (GDS) with 15 questions, which has been validated for the diagnosis of depression,18 was administered to evaluate symptoms of depression. We administered the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ) to assess REM sleep behavior disorder,19 and the Pittsburgh Sleep Quality Index (PSQI), which evaluates quality of sleep.20 GDS, RBDSQ, and PSQI were evaluated by the real number of scores, not by grouping by cut-off, in order to capture the effects of minor changes. The presence of constipation, hyposmia, and orthostatic hypotension was obtained by a self-administered questionnaire in which participants were asked if they experienced these symptoms or not.
Examination survey
Neurologists conducted a standardized neurological examination including an abbreviated (10-item) version of the motor portion of the Unified Parkinson’s Disease Rating Scale (modified UPDRS). The mUPDRS is able to diagnose parkinsonian signs in older community-dwelling people.3, 10, 21 The 10 item scale (each item rated from 0 to 4) assessed speech, facial expression, tremor at rest, rigidity (rated separately in the neck, right arm, left arm, right leg, and left leg), posture, and body (axial) bradykinesia. A Mini-Mental State Examination (MMSE) was administered to assess global cognitive function.22 We performed routine examinations in community centers and visited individual houses and nursing homes to raise participant rates in those who would otherwise have difficulty participating.
Assessment of cerebral white-matter hyperintensities
In the baseline study, brain magnetic resonance (MR) imaging was performed between March 2010 and May 2010, using a 1.5 T system (Philips Gyroscan Intera 1.5 T, Philips, Tokyo, Japan).23 Fazekas scores were used to assess periventricular hyperintensity (PVH) and deep white-matter hyperintensity (DWMH).24
Diagnostic Criteria
Those participants who had 2 or more cardinal signs (mUPDRS rating ≥ 2) on the standardized neurological examination were classified as having parkinsonism. Participants were classified as having MPS when they had 2 or more mUPDRS ratings = 1, one mUPDRS rating ≥ 2, or an mUPDRS resting tremor rating ≥ 1.3, 7, 21 Subtypes of MPS were classified as follows. Axial dysfunction: (1) UPDRS ratings = 1 in 2 or more of the 4 items of axial function (changes in speech, facial expression, posture, and axial bradykinesia), or (2) one UPDRS rating ≥ 2 on one of the 4 items. Rigidity: (1) UPDRS ratings = 1 in 2 or more of the 5 items of rigidity, or (2) one UPDRS rating ≥ 2 on one of the 5 items. Tremor: UPDRS resting tremor rating ≥ 1. Mixed: combination of the above 2 or 3 subtypes.
Statistical analysis
Descriptive statistics for each baseline clinical characteristic of normal individuals stratified by prognosis are given as the mean (standard deviation) for age and education, the median (quartile) for other noncategorical data, and the number (percentage) for categorical data. The variables age and education were analyzed using Student t test, other noncategorical variables using Mann–Whitney U test, and categorical variables using chi-squared test. Binary logistic regression analyses were conducted to determine predictors for incident MPS. Unadjusted univariate regressions were conducted first, then multivariate regressions with a likelihood ratio test were conducted for the variables that had been found to be associated with incidence of MPS in the univariate regressions. A 2-tailed P < 0.05 was considered significant. All analyses were performed with IBM SPSS Statistics for Windows (version 23.0; IBM Corp., Armonk, NY).
RESULTS
Study flow
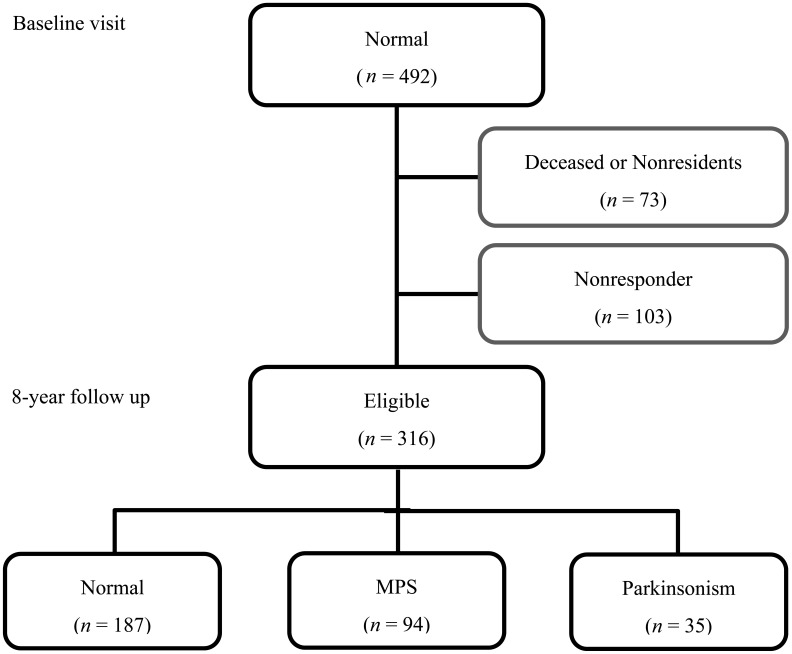
The flow of participants is presented in Fig. 1. Those participants having MPS (n = 168) or parkinsonism (n = 71) at baseline were excluded. Of the 492 remaining normal participants at baseline, 73 subjects were deceased or had moved outside of the town at the time of the 8-year follow up. We lost 103 subjects to follow up as a result of withdrawal from study. Of 316 participants who were eligible for the present study, 187 remained motor-normal (persistent normal), 94 had developed MPS (incident MPS), and 35 had developed parkinsonism (incident parkinsonism). Participants who dropped out of the study were significantly older than those who completed the 8-year follow-up evaluation (data not shown).
Fig. 1.
The flow of participants.
Related factor of incidence of MPS
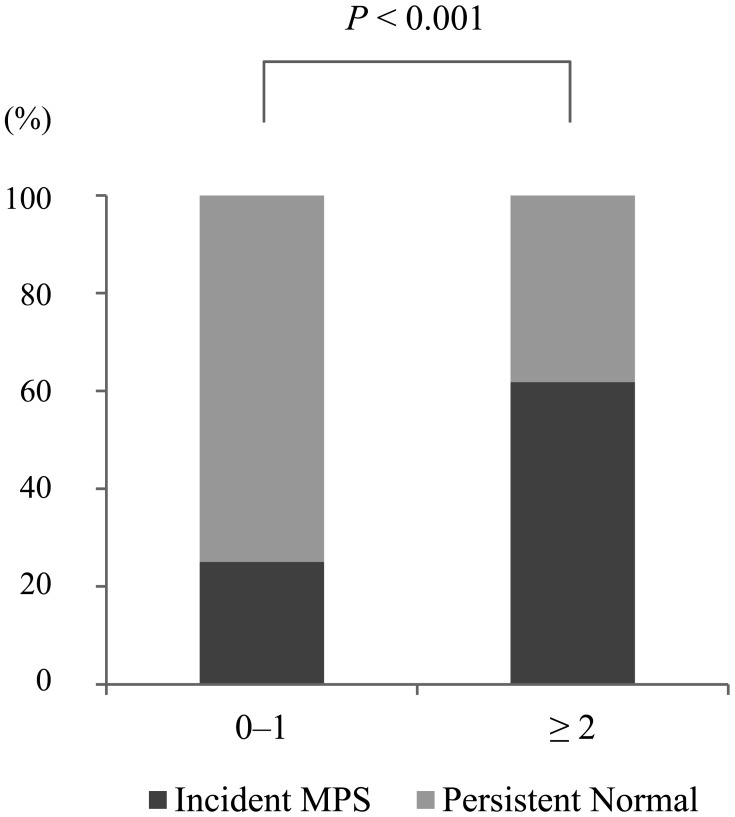
Baseline characteristics of the persistent normal group and the incident MPS group are presented in Table 1. Univariate analysis revealed that older age, not having an exercise habit, and a higher score for the Tanner questionnaire, GDS, RBDSQ, PSQI, or Fazekas (PVH and DWMH) assessments were associated with incidence of MPS. Binary logistic regression analysis adjusted for age and sex revealed that the absence of habitual exercise, the Tanner questionnaire score, PSQI score, and DWMH Fazekas score were significant independent predictors for incidence of MPS (Table 2). Participants who scored 2 points or more on the Tanner Questionnaire developed significantly more MPS than participants who scored 1 point or less (Fig. 2).
Table 1. Baseline characteristics of the normal individuals stratified by prognosis.
| Persistent normal n = 187 |
Incident MPS n = 94 |
||
| Age (year)* | mean (SD) | 72.1 (5.2) | 74.2 (5.5) |
| Sex (women) | n (%) | 106 (56.7) | 60 (63.8) |
| Education (year) | mean (SD) | 10.1 (2.2) | 9.9 (2.1) |
| Exercise habits* | n (%) | 68 (38.9) | 15 (17.2) |
| mUPDRS score | median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Tanner questionnaire score* | median (IQR) | 0.0 (0.0–1.0) | 1.0 (0.0–3.0) |
| MMSE score | median (IQR) | 28.0 (26.0–29.0) | 27.5 (26.0–29.0) |
| GDS score* | median (IQR) | 2.0 (0.0–3.0) | 3.0 (1.0–5.0) |
| RBDSQ score* | median (IQR) | 1.0 (0.0–3.0) | 2.0 (1.0–3.8) |
| Constipation | n (%) | 28 (16.4) | 18 (21.7) |
| Hyposmia | n (%) | 13 (7.3) | 9 (10.6) |
| Orthostatic hypotension | n (%) | 24 (13.5) | 19 (22.6) |
| PSQI score* | median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) |
| Hypertension | n (%) | 116 (62.0) | 68 (72.3) |
| Dyslipidemia | n (%) | 54 (28.9) | 30 (31.9) |
| Diabetes mellitus | n (%) | 25 (13.4) | 18 (19.1) |
| PVH score* | median (IQR) | 1.0 (0.0–1.0) | 1.0 (1.0–2.0) |
| DWMH score* | median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) |
*Significant (P < 0.05) differences between individuals with persistent normal and incident MPS.
Table 2. Predictors for incident MPS.
| Unit of increase | Univariate | Multivariate | |
| Age (year) | 1 year | 1.08 (1.03–1.13)* | – |
| Sex (women) | 0 = men 1 = women |
0.74 (0.45–1.24) | |
| MMSE score | 1 score | 0.95 (0.85–1.06) | |
| Exercise habits | 0 = absent 1 = present |
0.33 (0.17–0.62)* | 0.42 (0.21–0.86)* |
| Tanner questionnaire score | 1 score | 1.59 (1.33–1.92)* | 1.45 (1.19–1.78)* |
| GDS score | 1 score | 1.18 (1.07–1.30)* | – |
| RBDSQ score | 1 score | 1.21 (1.04–1.40)* | – |
| PSQI score | 1 score | 1.14 (1.03–1.26)* | 1.19 (1.06–1.35)* |
| PVH score | 1 score | 1.51 (1.11–2.05)* | – |
| DWMH score | 1 score | 1.80 (1.31–2.47)* | 1.62 (1.12–2.34)* |
Odds ratio (95% CI) is indicated. *P < 0.05
Fig. 2.
Development of mild parkinsonian signs (MPS) by Tanner score at baseline. Participants who scored 2 points or more on the Tanner Questionnaire developed significantly more MPS than participants who scored 1 point or less (chi-squared test).
DISCUSSION
In the present study, we investigated the predictors for incidence of MPS in a longitudinal 8-year study of an older adult community. Multivariate analysis revealed that a higher score on DWMH Fazekas, PSQI, or Tanner questionnaire assessments, and the absence of habitual exercise at baseline were independently associated with onset of MPS. To our knowledge, these predictors have not been reported to date and only 2 studies have investigated other predictors of MPS; a longitudinal 5-year study found that SN-hyperechogenicity and hyposmia were risk factors for predicting MPS,25 and a 1-year study found cardiovascular disease (any one of myocardial infarction, angina, arrhythmia, and chronic heart failure) predicted MPS.15 Several cross-sectional studies have found that cerebral white-matter lesions are associated with MPS.4,5,6 These previous studies proved that subjects with MPS have more cerebral small-vessel disease such as white-matter hyperintensities, lacunar infarctions, and cerebral microbleeds, suggesting that vascular mechanisms are a key pathophysiology of MPS. Previously, we reported that higher DWMH and PVH Fazekas scores were associated with the progression of parkinsonian signs of MPS subjects at 3 years.16 Our present study shows that a higher DWMH Fazekas score at baseline is associated with the onset of MPS from normal within 8 years. By contrast, there were no significant associations between vascular risk factors such as hypertension, dyslipidemia, and diabetes and the onset of MPS. Taken together with our results, vascular pathological changes in cerebral white matter are key contributors to incidence of MPS or prognosis of parkinsonism in the older adults.
Our present study also showed that a higher PSQI score is an independent predictor for incident MPS. It was unclear whether the presence of sleeping disorders meeting the criteria for PSQI score cutoff (≥ 6) significantly related to the onset of MPS, because few participants met the criteria. In the present study, participants with PSQI scores of 1 to 3 (mild sleeping disturbance) comprised the majority. Sleeping disorder and motor dysfunction are both common conditions in old age, and causal relationship between the 2 clinical symptoms is complex. There is a question of whether mild sleeping disorder is a risk factor for onset of MPS or is a prodromal symptom of MPS. Lysen et al. have shown that worse sleep quality and shorter sleep duration relate to a higher risk of parkinsonism including Parkinson’s disease in a prospective population-based study, but that this increased risk disappears with longer follow-up.26 They have argued that in the general population, sleep disturbances are markers of the prodromal phase of parkinsonism. In any case, conducting further interventional studies on sleep and the risk of developing MPS is warranted.
It is important to detect MPS at risk at an early stage, and follow them carefully. The Tanner questionnaire assesses the subjective motor symptoms of the participants and has been validated for screening for Parkinson’s disease. Our present study showed that a higher Tanner questionnaire score is associated with onset of MPS. In particular, participants who scored 2 or more developed significantly more MPS than participants who scored 1 or less. We suggest that the Tanner questionnaire is useful for screening to detect subclinical MPS in risk groups. Those who are aware of a decline in motor function subjectively could possibly develop a movement disorder within a few years, even if their parkinsonism cannot be identified objectively at baseline. Mitchell et al. have reported that subjective cognitive decline is a risk factor for objective cognitive decline.27 Just as there is a subjective cognitive impairment as a prodromal stage of mild cognitive impairment, there may be a similar stage before MPS. Subjective symptoms are therefore considered important for early detection of MPS, and additional investigation is needed that follows up or intervenes by targeting participants with subjective symptoms alone.
We also found that an absence of exercise habits at baseline was significantly associated with onset of MPS. Higher levels of physical activity may lower the risk of Parkinson’s disease, especially in men.28 There have been no reports of longitudinal studies of the association between exercise and MPS to date. This study showed that exercise is possibility associated with preventing onset of MPS. We propose some mechanistic hypotheses by which exercise indirectly prevents MPS, perhaps by preventing lifestyle disease and consequently preventing white matter lesions, or perhaps exercise prevents MPS by another mechanism such as maintenance of skeletal muscle, or prevents degenerative mechanisms, especially in those with a predisposition to MPS who tended to avoid exercise in the early years before onset. Further studies involving quantitation of exercise or exercise intervention will be needed to clarify the diverse relationships between exercise and MPS.
The present study has a few limitations. First, although we were able to investigate the entire population over the age of 65 on Ama-cho, there were 103 non-responders at the time of follow-up, and the non-responders were significantly older and could cause selection bias in the analysis. Second, we did not evaluate MPS as it developed over the 8 year period. Our present study should be replicated with higher response rates and an analysis including time of onset.
In conclusion, we found vascular lesions and sleep disorders were associated with the underlying pathogenesis of onset of MPS, the Tanner questionnaire was useful for early detection of subclinical symptoms of MPS, and exercise is possibly associated with preventing the onset of MPS. It is necessary to identify individuals with high risk of MPS and prevent onset of MPS by modifying their lifestyle. However, whether MPS is preventable or not requires further research.
Acknowledgments
Acknowledgments: We are particularly thankful for the efforts of the researchers who conducted the cognitive testing, the researchers who evaluated brain images, and the community health nurses in Ama-cho.
This work was supported, in part, by the Japan Agency for Medical Research and Development (dk0207025).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Bennett DA,Beckett LA,Murray AM,Shannon KM,Goetz CG,Pilgrim DM,et al. . Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71-6. 10.1056/NEJM199601113340202 [DOI] [PubMed] [Google Scholar]
- 2.Louis ED,Tang MX,Schupf N,Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62:297-302. 10.1001/archneur.62.2.297 [DOI] [PubMed] [Google Scholar]
- 3.Uemura Y,Wada-Isoe K,Nakashita S,Nakashima K. Mild parkinsonian signs in a community-dwelling elderly population sample in Japan. J Neurol Sci. 2011;304:61-6. 10.1016/j.jns.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Buchman AS,Leurgans SE,Nag S,Bennett DA,Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011;42:3183-9. 10.1161/STROKEAHA.111.623462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Laat KF,van Norden AGW,Gons RAR,van Uden IWM,Zwiers MP,Bloem BR,et al. . Cerebral white matter lesions and lacunar infarcts contribute to the presence of mild parkinsonian signs. Stroke. 2012;43:2574-9. 10.1161/STROKEAHA.112.657130 [DOI] [PubMed] [Google Scholar]
- 6.Hatate J,Miwa K,Matsumoto M,Sasaki T,Yagita Y,Sakaguchi M,et al. . Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinsonism Relat Disord. 2016;26:29-34. 10.1016/j.parkreldis.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 7.Lerche S,Brockmann K,Wurster I,Gaenslen A,Roeben B,Holz D,et al. . Reasons for mild parkinsonian signs – Which constellation may indicate neurodegeneration? Parkinsonism Relat Disord. 2015;21:126-30. 10.1016/j.parkreldis.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 8.Louis ED,Marder K,Tabert MH,Devanand DP. Mild Parkinsonian signs are associated with lower olfactory test scores in the community-dwelling elderly. Mov Disord. 2008;23:524-30. 10.1002/mds.21777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED,Bennett DA. Mild Parkinsonian signs: an overview of an emerging concept. Mov Disord. 2007;22:1681-8. 10.1002/mds.21433 [DOI] [PubMed] [Google Scholar]
- 10.Louis ED,Tang MX,Schupf N. Mild parkinsonian signs are associated with increased risk of dementia in a prospective, population-based study of elders. Mov Disord. 2010;25:172-8. 10.1002/mds.22943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS,Schneider JA,Bienias JL,Evans DA,Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60:539-44. 10.1001/archneur.60.4.539 [DOI] [PubMed] [Google Scholar]
- 12.Louis ED,Schupf N,Marder K,Tang MX. Functional correlates of mild parkinsonian signs in the community-dwelling elderly: poor balance and inability to ambulate independently. Mov Disord. 2006;21:411-6. 10.1002/mds.20735 [DOI] [PubMed] [Google Scholar]
- 13.Fleischman DA,Wilson RS,Schneider JA,Bienias JL,Bennett DA. Parkinsonian signs and functional disability in old age. Exp Aging Res. 2007;33:59-76. 10.1080/03610730601006370 [DOI] [PubMed] [Google Scholar]
- 14.Zhou G,Duan L,Sun F,Yan B,Ren S. Association between mild parkinsonian signs and mortality in an elderly male cohort in China. J Clin Neurosci. 2010;17:173-6. 10.1016/j.jocn.2009.07.102 [DOI] [PubMed] [Google Scholar]
- 15.Mahoney JR,Verghese J,Holtzer R,Allali G. The evolution of mild parkinsonian signs in aging. J Neurol. 2014;261:1922-8. 10.1007/s00415-014-7442-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada-Isoe K,Tanaka K,Uemura Y,Nakashita S,Tajiri Y,Tagashira S,et al. . Longitudinal course of mild parkinsonian signs in elderly people: A population-based study in Japan. J Neurol Sci. 2016;362:7-13. 10.1016/j.jns.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 17.Tanner CM,Gilley DW,Goetz CG. A brief screening questionnaire for parkinsonism. Ann Neurol. 1990;28:267-8. [Google Scholar]
- 18.Niino N. A Japanese translation of the Geriatric Depression Scale. Clin Gerontol. 1991;10:85-7. [Google Scholar]
- 19.Stiasny-Kolster K,Mayer G,Schäfer S,Möller JC,Heinzel-Gutenbrunner M,Oertel WH. The REM sleep behavior disorder screening questionnaire-A new diagnostic instrument. Mov Disord. 2007;22:2386-93. 10.1002/mds.21740 [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ,Reynolds CF III,Monk TH,Berman SR,Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 21.Louis ED,Tang MX,Mayeux R. Factor structure of parkinsonian signs in the community-dwelling elderly. Mov Disord. 2004;19:268-72. 10.1002/mds.20013 [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF,Folstein SE,McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23.Yamawaki M,Wada-Isoe K,Yamamoto M,Nakashita S,Uemura Y,Takahashi Y,et al. . Association of cerebral white matter lesions with cognitive function and mood in Japanese elderly people: a population-based study. Brain Behav. 2015;5:n/a. 10.1002/brb3.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazekas F,Chawluk JB,Alavi A,Hurtig HI,Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351-6. 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 25.Mahlknecht P,Kiechl S,Stockner H,Willeit J,Gasperi A,Poewe W,et al. . Predictors for mild parkinsonian signs: A prospective population-based study. Parkinsonism Relat Disord. 2015;21:321-4. 10.1016/j.parkreldis.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 26.Lysen TS,Darweesh SKL,Ikram MK,Luik AI,Ikram MA. Sleep and risk of parkinsonism and Parkinson’s disease: a population-based study. Brain. 2019;142:2013-22. 10.1093/brain/awz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell AJ,Beaumont H,Ferguson D,Yadegarfar M,Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439-51. 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 28.Chen H,Zhang SM,Schwarzschild MA,Hernán MA,Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664-9. 10.1212/01.WNL.0000151960.28687.93 [DOI] [PubMed] [Google Scholar]