Abstract
BACKGROUND AND PURPOSE:
Choroidal collateral anastomosis is associated with hemorrhage recurrence in patients with Moyamoya disease. However, the relationship between recurrent ipsilateral hemorrhage and choroidal collateral anastomosis subtypes (anterior choroidal artery anastomosis, lateral posterior choroidal artery anastomosis, and medial posterior choroidal artery anastomosis) is unclear. This study aimed to assess this potential association in adult patients with Moyamoya disease.
MATERIALS AND METHODS:
Patients angiographically diagnosed with Moyamoya disease who underwent conservative treatment between January 2008 and December 2018 were included in this retrospective study. Two readers assessed the angiographic images to identify choroidal collateral anastomosis subtypes, and Cox proportional hazard regression models were used to estimate the risk of recurrent hemorrhage associated with each subtype.
RESULTS:
Thirty-nine patients (mean age = 45.2 years) were included in this study. During 52.4 ± 37.0 months of follow-up, recurrent ipsilateral hemorrhage occurred in 48.7% (19/39) of patients. Patients with recurrent hemorrhage had a higher prevalence of choroidal collateral (94.8% versus 60.0%; P = .02) and lateral posterior choroidal artery (78.9% versus 25.0%; P < .01) anastomoses than those without recurrent hemorrhage. Lateral posterior choroidal artery anastomosis was associated with recurrent hemorrhage before (hazard ratio = 6.66; 95% CI, 2.18–20.39; P < .01) and after (hazard ratio = 5.78; 95% CI, 1.58–21.13; P < .01) adjustments were made for age, sex, and other confounding factors.
CONCLUSIONS:
Choroidal collateral anastomosis is responsible for most cases of recurrent hemorrhage in adult patients with Moyamoya disease; lateral posterior choroidal artery anastomosis is a significant risk factor for these recurrent events.
Moyamoya disease (MMD) is an uncommon but potentially catastrophic cerebrovascular disorder characterized by progressive occlusion in the terminal portion of the internal carotid artery and its main branches within the circle of Willis.1 Intracranial hemorrhage accounts for half of initial manifestations of MMD in adult patients,2,3 and the recurrence rate of hemorrhage can be as high as 17.1% per year during the natural course of the disease.4,5 Patients with hemorrhage recurrence generally have poor outcomes such as life-long disabilities or even death.4 Identifying risk factors for recurrent hemorrhage is therefore useful for clinical decision-making in terms of management and follow-up.
The occurrence of periventricular hemorrhage is a major clinical concern in adult patients with MMD who experience recurrent hemorrhage.6 In brain hemispheres with recurrent hemorrhage, the hemorrhage is more likely to occur around the posterior territory, particularly in the periventricular area around the atrium or the posterior portion of the body of the lateral ventricle.5,7 A previous postmortem study demonstrated that the subependymal collateral arteries supplying the above territories originate mainly from the lateral posterior choroidal arteries (LPChAs) but rarely from the anterior choroidal arteries (AChAs) or medial posterior choroidal arteries (MPChAs).8 Therefore, we suspect that the LPChAs may play a key role in the occurrence of recurrent hemorrhage. Choroidal collateral anastomosis (ChCA) has previously been shown to be associated with recurrent hemorrhage in this patient population.5,9,10 However, the relationship between the subtypes of ChCA and recurrent ipsilateral hemorrhage is still unclear.
In this retrospective study, we therefore sought to assess the association between the subtypes of ChCA and recurrent ipsilateral hemorrhage in adult patients with MMD.
MATERIALS AND METHODS
Study Population
This study was approved by The Affiliated Drum Tower Hospital of Nanjing University Medical School with a waiver of informed consent. Consecutive patients with MMD who had experienced initial intracranial hemorrhage and received conservative treatment (no surgical intervention) and clinical follow-up between January 2008 and December 2018 were recruited for this retrospective study. We enrolled as many patients as possible with >5 years of clinical follow-up for nonrecurrent hemorrhage controls. Considering that the main purpose of our study was to evaluate the association between the ChCA subtypes and recurrent ipsilateral hemorrhage, the factors associated with non-MMD-related hemorrhage and influencing the target vessel evaluation were excluded in the present study. All cases of MMD had bilateral involvement and were diagnosed via angiography according to the guidelines proposed by the Ministry of Health, Labor, and Welfare of Japan.11 Patients with autoimmune disease, meningitis, brain tumor, Down syndrome, neurofibromatosis type 1, or a history of head irradiation were excluded from the study. We also excluded patients who met any of the following 3 conditions: 1) age older than 65 years12; 2) the presence of factors influencing the evaluation of recurrent hemorrhage such as bleeding diathesis, uncontrolled diabetes mellitus (fasting blood glucose level of >300 mg/dL), or uncontrolled hypertension (systolic pressure of >180 mm Hg and/or diastolic pressure of >110 mm Hg); or 3) a history of intracranial hemorrhage. The occurrence of recurrent hemorrhage was recorded if there was an acute neurologic symptom during follow-up with a corresponding new intracranial hemorrhage on brain imaging. Demographic and clinical characteristics for all patients were collected from the medical records.
Imaging Techniques
For the initial hemorrhagic event, a cerebrovascular DSA examination was performed on an x-ray scanner (Allura Xper FD20/2D; Philips Healthcare, Best, the Netherlands); this examination included selective common, internal, and external carotid artery arteriography on both sides and vertebral arteriography on at least 1 side. The imaging parameters for this examination were as follows: 6 frames/s, injection pressure =300 psi/kg, and contrast medium administered at a rate of 3 mL/s. When a recurrent hemorrhage occurred, non-contrast-enhanced CT of the brain was performed to identify the intracranial hemorrhagic site. When diffuse ventricular hemorrhage occurred, SWI examinations were conducted within 2 months of the hemorrhagic event, with all scans obtained on a 3T MR imaging scanner (uMR 770; United Imaging Healthcare; Shanghai) with a 24-channel phased array head and neck coil. The MR images were acquired using the following parameters: TR/TE = 24.2/15 ms, flip angle = 15°, FOV = 190 × 190 mm2, section thickness = 1.2 mm, voxel size = 0.6 × 0.6 × 1.2 mm3, and acquisition time = 7 minutes 35 seconds.
Definitions and Analysis of Angiographic Variables
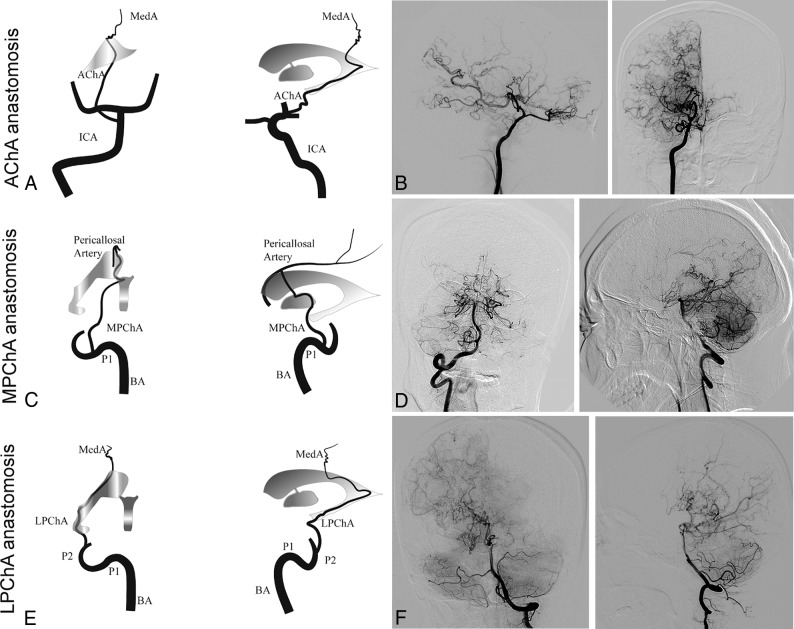
The definition of ChCA provided by Funaki et al9 was adapted to the present study. For this study, the subtypes of ChCA were categorized into AChA anastomosis, LPChA anastomosis, and MPChA anastomosis.9 AChA anastomosis is defined as anastomosis between the extreme dilation and extension of the AChA with sudden deviation from the shape of the lateral ventricle at its peripheral portion to connect the medial end of the medullary artery. LPChA anastomosis refers to the extreme extension of the LPChA beyond the atrium of the lateral ventricle to reach the body of the lateral ventricle. MPChA anastomosis is defined as the extreme extension of the MPChA beyond the level of the pericallosal artery to the corpus callosum. Schematic illustrations and representative examples of each subtype are shown in Fig 1. Other variables such as Suzuki stages1 (stages I–VI), involvement of the posterior cerebral arteries (Mugikura stage II–IV),13 thalamic collateral anastomosis,9 the presence of an intracranial aneurysm, and the presence of a fetal posterior communicating artery at the time of initial hemorrhage were also evaluated. The initial angiographic images were reviewed in consensus by 2 neuroradiologists, both with >5 years' experience in neurovascular imaging and both blinded to the brain images. A third investigator with >10 years' experience in neurovascular imaging resolved any discrepancies.
Fig 1.
Schematic illustrations and angiographic findings from representative cases of each subtype of choroidal collateral anastomosis. A and B, Anterior-posterior and lateral right carotid artery angiograms show a dilated anterior choroidal artery extending beyond the lateral ventricle to the cortex (arrows). C and D, Anterior-posterior and lateral right vertebral artery angiograms show a medial posterior choroidal artery extending beyond the level of the pericallosal artery (arrows) to the corpus callosum. E and F, Anterior-posterior and lateral left vertebral artery angiograms show a lateral posterior choroidal artery extending beyond the body of the lateral ventricle to the cortex (arrows). MedA indicates the medullary artery; P1 and P2, the proximal portion of posterior cerebral artery; BA, the basilar artery.
Follow-Up
After the initial hemorrhagic event was investigated and conservative treatment was initiated, all patients underwent follow-up in the outpatient clinic every 3–6 months so that cases of hemorrhage recurrence could be identified. When hemorrhage reoccurred, the site of the hemorrhage was determined on CT within 1 week after the onset of the event. When diffuse ventricular hemorrhage occurred, the presumed origin of the recurrent hemorrhage was identified by SWI within 2 months of the event. The interval between the initial event and hemorrhage recurrence was also recorded.
Reproducibility
All angiographic data were interpreted by the 2 raters twice, with a 1-month time interval between sessions to minimize memory bias. Interrater and intrarater agreement values for the subtypes of ChCA were calculated.
Statistical Analysis
Continuous variables were described as mean ± SD, and categoric variables were presented as number and percentage. An independent t test, a Mann-Whitney U test, and a χ2 or Fisher exact test were used to compare patients with and without recurrent hemorrhage and hemispheres with and without recurrent hemorrhage in terms of baseline characteristics. Univariate and multivariate Cox proportional hazard regression models were used to estimate the risk of recurrent ipsilateral events for each ChCA subtype. Interrater and intrarater agreement values for ChCA subtype evaluation were calculated using the unweighted Cohen κ. A P value < .05 was considered significant. All statistical analyses were performed using SPSS 22.0 (IBM, Armonk, New York).
RESULTS
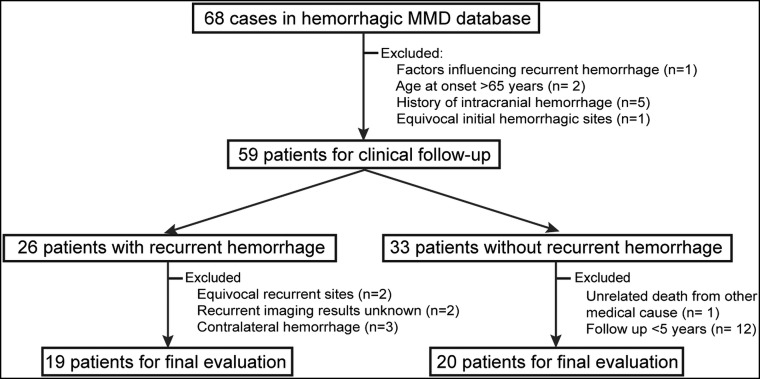
From January 2008 to December 2018, a total of 68 patients presented with hemorrhagic MMD and underwent conservative treatment. Of these 68 patients, 9 (13.2%) were excluded for the following reasons: uncontrolled hypertension and diabetes mellitus (n = 1), age older than 65 years (n = 2), history of intracranial hemorrhage (n = 5), and equivocal initial hemorrhagic sites (n = 1). During the follow-up period, another 20 (29.4%) patients were excluded for the following reasons: equivocal recurrent hemorrhagic sites (n = 2), follow-up images of recurrent hemorrhage unknown (n = 2), presenting with recurrent hemorrhage in the contralateral hemispheres (n = 3), unrelated death from other medical causes (liver tumor, n = 1), and follow-up period of <5 years (patients without recurrent hemorrhage, n = 12). The remaining 39 (57.4%) patients (29 women; mean age at diagnosis = 45.2 ± 9.1 years) met the eligibility criteria for the final analysis (Fig 2). Of these 39 patients, 2 (5.1%) were smokers, 13 (33.3%) had hypertension, 8 (20.5%) had dyslipidemia, 3 (7.7%) had diabetes mellitus, and 3 (7.7%) had a history of ischemic stroke (defined as symptomatic infarctions confirmed on DWI). None of the study patients were treated with antiplatelets or anticoagulants. During 52.4 ± 37.0 months (range, 1–114 months) of follow-up, 48.7% (19/39) of patients experienced recurrent ipsilateral hemorrhage. Of the baseline clinical risk factors assessed, only the number of subarachnoid hemorrhages in patients with recurrent events was significantly higher than in those without recurrent events (21.1% versus 0.0%; P = .05; Table 1).
Fig 2.
Flowchart of patient recruitment.
Table 1:
Baseline characteristics in patients with MMD with and without recurrent ipsilateral hemorrhage
| Characteristic | Patients without Recurrent Hemorrhage (n = 20) | Patients with Recurrent Hemorrhage (n = 19) | P Value |
|---|---|---|---|
| Women (No.) (%) | 15 (75.0) | 14 (73.7) | .93 |
| Age (mean) (yr) | 43.7 ± 10.6 | 46.9 ± 7.0 | .27 |
| Smokers (No.) (%) | 1 (5.0) | 1 (5.3) | >.99 |
| Concurrent disease (No.) (%) | |||
| Hypertension | 8 (40.0) | 5 (26.3) | .37 |
| Dyslipidemia | 4 (20.0) | 4 (21.1) | >.99 |
| Diabetes mellitus | 2 (10.0) | 1 (5.3) | >.99 |
| History of ischemia (No.) (%) | 1 (5.0) | 2 (10.5) | .61 |
| Hemorrhagic type (No.) (%) | |||
| IVH | 13 (65.0) | 13 (68.4) | .82 |
| Only IVH | 6 (30.0) | 10 (52.6) | .20 |
| ICH + IVH | 7 (35.0) | 3 (15.8) | .46 |
| SAH | 0 (0.0) | 4 (21.1) | .05 |
| Only SAH | 0 (0.0) | 1 (5.3) | .49 |
| SAH + IVH | 0 (0.0) | 3 (15.8) | .27 |
| ICH | 7 (35.0) | 2 (10.5) | .13 |
Note:—IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage.
Intracranial Hemorrhage
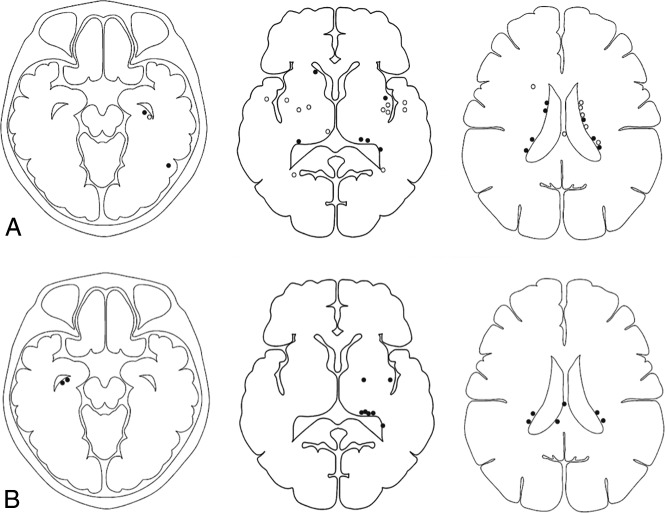
In 39 hemispheres, the initial hemorrhagic sites were in the subependymal area of the lateral ventricle in 21 hemispheres (53.8%), the insular lobe in 5 hemispheres (12.8%), the temporal lobe in 3 hemispheres (7.7%), the basal ganglia in 2 hemispheres (5.1%), the occipital lobe in 2 hemispheres (5.1%), the corpus callosum in 1 hemisphere (2.6%), the frontal lobe in 1 hemisphere (2.6%), and the subarachnoid in 4 hemispheres (10.2%). Of the 39 hemispheres with intracranial hemorrhage, 11 (28.2%) demonstrated recurrent hemorrhage at the initial hemorrhage site and 8 (20.5%) demonstrated recurrent hemorrhage at a different site in the same hemisphere. Comparisons of imaging features in hemispheres with and without recurrent hemorrhage are shown in Fig 3.
Fig 3.
Topographic analysis showing the distribution of initial and recurrent hemorrhagic sites. A, Topographic analysis of initial hemorrhagic sites for those with (black dots) and those without (white dots) recurrent hemorrhage. B, Another topographic analysis shows the distribution of recurrent hemorrhagic sites (black dots). Four patients with recurrent hemorrhage attributable to intracranial aneurysm rupture are not shown in this analysis.
Analysis of Angiographic Variables
Of the 39 cases in the study, none demonstrated intracranial lesions at Suzuki stage I, one (2.6%) demonstrated lesions at stage II, nineteen (48.7%) demonstrated lesions at stage III, five (12.8%) demonstrated lesions at stage IV, twelve (30.8%) demonstrated lesions at stage V, and 2 (5.1%) demonstrated lesions at stage VI. In addition, 11 (28.2%) intracranial arteries were found to have stenotic-occlusive lesions involving the posterior cerebral arteries. The fetal posterior communicating artery and thalamic anastomosis were identified in 33.3% (13/39) and 15.4% (6/39) of cases, respectively. An intracranial aneurysm was identified in those 4 patients presenting with subarachnoid hemorrhage, including the posterior cerebral artery in 2 patients, the lenticulostriate artery in 1 patient, and the middle meningeal artery in 1 patient.
A ChCA was identified in 76.9% (30/39) of patients, including AChA in 13 patients, LPChA in 20 patients, and MPChA in 14 patients. Compared with hemispheres without hemorrhage recurrence, hemispheres with hemorrhage recurrence demonstrated a higher prevalence of ChCA (94.8% versus 60.0%; P = .02), LPChA anastomosis (78.9% versus 25.0%; P < .01), and intracranial aneurysms (21.1% versus 0.0%; P = .03). A representative case demonstrating LPChA anastomosis and recurrent ipsilateral hemorrhage is shown in Fig 4. No other significant differences were noted in baseline angiographic characteristics between hemispheres with and those without recurrent hemorrhage (Table 2).
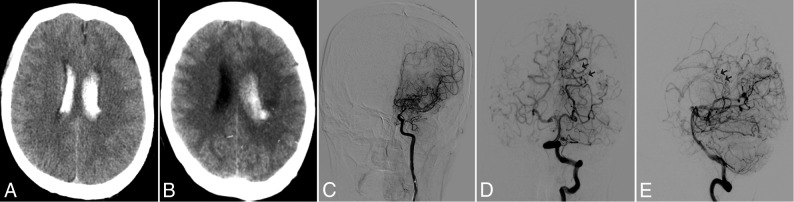
Fig 4.
A 50-year-old man experienced a recurrent hemorrhage in the ipsilateral hemisphere. A, CT image indicates the initial hemorrhage in the posterior portion of the body of the left lateral ventricle. B, CT image obtained 49 months later reveals a recurrent hemorrhage in the initial hemorrhagic site. C. Left anterior-posterior carotid artery angiogram obtained at baseline demonstrates no obvious anterior choroidal anastomosis from the internal carotid artery. Anterior-posterior (D) and lateral (E) views of the left vertebral angiogram obtained at baseline reveal the typical finding of lateral posterior choroidal anastomosis responsible for recurrent hemorrhage (black arrows).
Table 2:
Baseline variables in hemispheres with and without recurrent hemorrhage in adult patients with MMD
| Variables | Hemispheres without Recurrent Hemorrhage (n = 20) | Hemispheres with Recurrent Hemorrhage (n = 19) | P Value |
|---|---|---|---|
| Suzuki stage (No.) (%) | |||
| I | 0 (0.0) | 0 (0.0) | |
| II | 1 (5.0) | 0 (0.0) | |
| III | 10 (50.0) | 9 (47.4) | |
| IV | 3 (15.0) | 2 (10.5) | |
| V | 5 (25.0) | 7 (36.8) | |
| VI | 1 (5.0) | 1 (5.3) | |
| Suzuki stage >III (No.) (%) | 9 (45.0) | 10 (52.6) | .63 |
| Involved PCA (No.) (%) | 4 (20.0) | 7 (36.8) | .30 |
| Fetal PcomA (No.) (%) | 7 (35.0) | 6 (31.6) | .82 |
| IA (No.) (%) | 0 (0.0) | 4 (21.1) | .03 |
| TC anastomosis (No.) (%) | 2 (10.0) | 4 (21.1) | .41 |
| ChCA subtype (No.) (%) | 12 (60.0) | 18 (94.8) | .02 |
| AChA anastomosis | 5 (25.0) | 8 (42.1) | .26 |
| LPChA anastomosis | 5 (25.0) | 15 (78.9) | <.01 |
| MPChA anastomosis | 5 (25.0) | 9 (47.4) | .15 |
Note:—PCA indicates posterior cerebral artery; PcomA, posterior communicating artery; IA, intracranial aneurysm; TC, thalamic collateral.
Association between Subtypes of ChCA and Recurrent Ipsilateral Hemorrhage
Table 3 summarizes the radiographic characteristics of the 19 hemispheres in which recurrent hemorrhage was seen. In 14 hemispheres, ChCA was considered responsible for the recurrence because the recurrent hemorrhage occurred in the hemisphere containing a ChCA and corresponded to the distribution of the choroidal arteries: Five stemmed from the AChA, 7 stemmed from the LPChA, and 2 stemmed from the MPChA.
Table 3:
Clinical features of adult patients with MMD who experienced recurrent ipsilateral hemorrhage
| Case | Age (yr)/Sex | Hemorrhagic Site |
Interval (mo) | Target ChCA |
|||
|---|---|---|---|---|---|---|---|
| Site of Initial Hemorrhage | Site of Recurrent Hemorrhage | AChA | LPChA | MPChA | |||
| 1 | 46/M | Right body of lateral ventricle | Right body of lateral ventricle | 5 | + | ||
| 2 | 43/M | Left atrium | Left atrium | 10 | + | ||
| 3 | 44/F | Right anterior body of lateral ventricle | Right temporal horn of lateral ventricle | 2 | + | ||
| 4 | 42/F | Right posterior body of lateral ventricle | Right posterior body of lateral ventricle | 10 | + | ||
| 5 | 50/M | Left posterior body of lateral ventricle | Left posterior body of lateral ventricle | 49 | + | ||
| 6 | 63/F | Left occipital horn of lateral ventricle | Left occipital horn of lateral ventricle | 1 | + | ||
| 7 | 46/M | Right frontal horn of lateral ventricle | Right splenium of corpus callosum | 4 | + | ||
| 8 | 51/F | Left posterior horn of lateral ventricle | Left atrium | 12 | + | ||
| 9 | 51/F | Left atrium | Left atrium | 5 | + | ||
| 10 | 45/F | Left temporal horn of lateral ventricle | Left posterior body of lateral ventricle | 58 | + | ||
| 11 | 50/F | Left insular lobe | Left insular lobe | 47 | + | ||
| 12 | 58/F | Right anterior body of lateral ventricle | Right body of corpus callosum | 14 | + | ||
| 13 | 36/F | Left body of lateral ventricle | Left atrium | 59 | + | ||
| 14 | 32/F | Right posterior horn of lateral ventricle | Right temporal horn of lateral ventricle | 1 | + | ||
| 15a | 42/F | Left temporal-occipital lobe | Left putamen | 25 | |||
| 16b | 56/M | Left perimesencephalic subarachnoid | Left perimesencephalic subarachnoid | 39 | |||
| 17b | 45/F | Right insular lobe and subarachnoid | Right insular lobe and subarachnoid | 1 | |||
| 18b | 57/F | Left quadrigeminal cistern | Left quadrigeminal cistern | 57 | |||
| 19b | 42/F | Left temporal lobe and subarachnoid | Left temporal lobe and subarachnoid | 2 | |||
Note:—+ indicates present.
Recurrent hemorrhage not attributable to choroidal anastomosis.
Recurrent hemorrhage attributable to intracranial aneurysm rupture.
In univariate Cox regression analysis, a significant association was demonstrated between LPChA anastomosis and hemorrhage recurrence (hazard ratio [HR] = 6.66; 95% CI, 2.18–20.39; P < .01). In multivariate Cox regression analysis, after adjustments were made for age, sex, a history of ischemia, Suzuki stage >III, involvement of the posterior cerebral arteries, thalamic anastomosis, the fetal posterior communicating artery, and intracranial aneurysm, the association between LPChA anastomosis and hemorrhage recurrence remained significant (HR = 5.78; 95% CI, 1.58–21.13; P < .01) (Table 4).
Table 4:
Univariate and multivariate adjusteda analyses of the association between ChCA subtypes and recurrent ipsilateral hemorrhage in adult patients with MMD
| ChCA Subtype | Presence of Recurrent Hemorrhage |
|||||
|---|---|---|---|---|---|---|
| Univariate Analysis |
Multivariate Analysis |
|||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AChA anastomosis | 1.77 | 0.71–4.41 | .22 | 8.23 | 1.41–48.13 | .02 |
| MPChA anastomosis | 2.14 | 0.86–5.29 | .10 | 3.43 | 0.80–14.80 | .10 |
| LPChA anastomosis | 6.66 | 2.18–20.39 | <.01 | 5.78 | 1.58–21.13 | <.01 |
Multivariate analysis was adjusted for confounding factors including age, sex, a history of ischemia, Suzuki stage >III, involvement of posterior cerebral arteries, thalamic anastomosis, fetal posterior communicating artery, and intracranial aneurysm.
Reproducibility
The κ values for intrarater agreement in the identification of AChA, MPChA, and LPChA were 0.84, 0.71, and 0.69, respectively. The κ values for interrater agreement in the identification of AChA, MPChA, and LPChA were 0.76, 0.66, and 0.61, respectively.
DISCUSSION
In this study, we found that recurrent ipsilateral hemorrhage was common among adult patients with MMD, and ChCA was responsible for most cases of hemorrhage recurrence in these patients. In addition, LPChA anastomosis was found to be an independent predictor of recurrent ipsilateral hemorrhage. Our findings suggest that serial imaging follow-up of LPChA anastomosis may provide additional information for risk stratification in patients with MMD.
Previous research has suggested that MMD-related intracranial hemorrhage may differ from primary intracranial hemorrhage in terms of location: MMD-related hemorrhage is more likely to present as intraventricular hemorrhage with or without intracerebral hemorrhage.6 One study showed that the presence of intraventricular hemorrhage was significantly correlated with the occurrence of recurrent hemorrhage in patients with MMD.14 Similarly, our study demonstrated that >50% of hemorrhage recurrence events presented as intraventricular hemorrhages; however, this result did not reach statistical significance. These differences in results between studies may be partially due to the different exclusion criteria used in different studies.
In the current study, ChCA was found to be more prevalent in hemispheres with recurrent hemorrhage among patients with MMD, findings consistent with previous results.5,9 We also found that the subtypes of ChCA were more frequently observed in the hemispheres with recurrent hemorrhage, though this difference was not significant for AChA or MPChA anastomosis. The recurrent hemorrhagic sites were distributed mainly in the periventricular area around the atrium or the posterior portion of the body of the lateral ventricle, suggesting that fragile choroidal collateral vessels around the periventricular area are mainly derived from the posterior circulation, particularly for LPChA.
LPChA anastomosis was shown to be significantly correlated with recurrent ipsilateral hemorrhage in this study, suggesting that LPChA anastomosis plays a key role in the occurrence of recurrent hemorrhage in patients with MMD. However, the mechanism of this correlation is not fully understood. We propose 2 possible mechanisms to explain this relationship.
The first potential mechanism involves persistent hemodynamic stress of the collateral vessels in different periventricular regions. Yamamoto et al15 demonstrated that the Moyamoya collateral vessels longitudinally shift from the anterior to posterior circulation during disease progression. In the current study, we found that recurrent hemorrhage was rarely observed in the insular lobe and basal ganglia but was more prevalent in the atrium and the posterior portion of the body of the lateral ventricle. The collateral anastomoses around the insular lobe and basal ganglia are mainly derived from the single blood supply of the anterior circulation, whereas the collateral anastomoses around the atrium and the posterior portion of the body of the lateral ventricle derive from the multiple blood supplies of both the anterior and posterior circulations. Persistent hemodynamic stress is thus added from both the anterior and posterior circulations, a potential trigger of recurrent hemorrhage.
The second potential mechanism is related to the development of ventricular microaneurysms in the LPChA. A previous study using 7T TOF MRA found that a high number of patients with MMD had ventricular microaneurysms in the periventricular region.16 In the current study, excluding cases of intracranial aneurysm rupture, recurrent hemorrhage occurred in the initial hemorrhagic sites in 46.7% (7/15) of hemispheres. The coexisting anterior and posterior choroidal anastomoses in MMD indicate that ventricular microaneurysms may be present in choroidal collateral vessels, particularly in the LPChA. Future studies using high-resolution MR imaging to characterize the arteriopathy of the LPChA in patients with MMD are warranted.
This study is limited by its retrospective nature. In addition, because of the rarity of nonsurgical intervention in patients with hemorrhagic MMD and the large number of patients excluded, this study is limited by its small sample size. Furthermore, interrater and intrarater agreement values for the identification of LPChA were low due to technical flaws in angiographic imaging of some unilateral vertebral arteries and the use of a weak contrast agent for angiography in some cases. The relationship between subtypes of ChCA and recurrent hemorrhage was determined only via analysis of the initial angiographic images, with no follow-up angiographic validation. Therefore, the development of ChCA subtypes was unclear in our study. Nevertheless, this study remains one of the first to analyze the association between ChCA subtypes and recurrent ipsilateral hemorrhage in patients with MMD. Considering the unique collateral anastomosis seen in patients with MMD, multicenter prospective studies are needed to determine the natural course of these collateral vessels. MR imaging may be able to be used for serial imaging follow-up of LPChA anastomosis in patients with MMD. In a recent study, sliding thin-slab maximum-intensity-projection coronal MRA images were found to provide reliable follow-up of periventricular anastomosis.17 Future studies are warranted to assess surgical revascularization in the area of the LPChA in nonsurgical patients and serial imaging follow-up of LPChA changes in surgical patients with MMD.
CONCLUSIONS
Recurrent ipsilateral hemorrhage is common in the natural course of hemorrhagic MMD, and LPChA anastomosis is associated with recurrent hemorrhage in this patient population. In light of these findings, LPChA anastomosis may serve as a marker of the risk of recurrent ipsilateral hemorrhage in patients with MMD.
Acknowledgments
We thank Zhongzhi Jia, MD, The Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University, Changzhou, China, and Megan Griffiths, scientific writer, Cleveland, Ohio, for help with revising the manuscript.
ABBREVIATIONS:
- AChA
anterior choroidal artery
- ChCA
choroidal collateral anastomosis
- HR
hazard ratio
- LPChA
lateral posterior choroidal artery
- MMD
Moyamoya disease
- MPChA
medial posterior choroidal artery
Footnotes
This work was supported by the Natural Science Foundation of China (81720108022, B.Z.) and the Social Development Project of Science and Technology in Jiangsu Province (BE2016605, BE201707, B.Z.).
References
- 1. Suzuki J, Takaku A. Cerebrovascular “Moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99 10.1001/archneur.1969.00480090076012 [DOI] [PubMed] [Google Scholar]
- 2. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol 2008;7:1056–56 10.1016/S1474-4422(08)70240-0 [DOI] [PubMed] [Google Scholar]
- 3. Takahashi JC, Miyamoto S. Moyamoya disease: recent progress and outlook. Neurol Med Chir (Tokyo) 2010;50:824–32 10.2176/nmc.50.824 [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi E, Saeki N, Oishi H, et al. Long-term natural history of hemorrhagic Moyamoya disease in 42 patients. J Neurosurg 2000;93:976–80 10.3171/jns.2000.93.6.0976 [DOI] [PubMed] [Google Scholar]
- 5. Funaki T, Takahashi JC, Houkin K, et al. High rebleeding risk associated with choroidal collateral vessels in hemorrhagic Moyamoya disease: analysis of a nonsurgical cohort in the Japan Adult Moyamoya trial. J Neurosurg 2018. February 1:1–8 [Epub ahead of print] 10.3171/2017.9.JNS17576 [DOI] [PubMed] [Google Scholar]
- 6. Nah HW, Kwon SU, Kang DW, et al. Moyamoya disease-related versus primary intracerebral hemorrhage: [corrected] location and outcomes are different [erratum in Stroke 2013;44:e119]. Stroke 2012;43:1947–50 10.1161/STROKEAHA.112.654004 [DOI] [PubMed] [Google Scholar]
- 7. Takahashi J, Funaki T, Houkin K, et al. ; JAM Trial Investigators. Significance of the hemorrhagic site for recurrent bleeding: prespecified analysis in the Japan Adult Moyamoya Trial. Stroke 2016;47:37–43 10.1161/STROKEAHA.115.010819 [DOI] [PubMed] [Google Scholar]
- 8. Marinković S, Gibo H, Filipović B, et al. Microanatomy of the subependymal arteries of the lateral ventricle. Surg Neurol 2005;63:451–58; discussion 458 [DOI] [PubMed] [Google Scholar]
- 9. Funaki T, Takahashi JC, Houkin K, et al. Angiographic features of hemorrhagic moyamoya disease with high recurrence risk: a supplementary analysis of the Japan Adult Moyamoya Trial. J Neurosurg 2018;128:777–84 10.3171/2016.11.JNS161650 [DOI] [PubMed] [Google Scholar]
- 10. Funaki T, Takahashi JC, Houkin K, et al. Effect of choroidal collateral vessels on de novo hemorrhage in Moyamoya disease: analysis of nonhemorrhagic hemispheres in the Japan Adult Moyamoya Trial. J Neurosurg 2019. February 8:1–7 [Epub ahead of print] 10.3171/2018.10.JNS181139 [DOI] [PubMed] [Google Scholar]
- 11. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of Moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 2012;52:245–66 10.2176/nmc.52.245 [DOI] [PubMed] [Google Scholar]
- 12. Miyamoto S, Yoshimoto T, Hashimoto N, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic Moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke 2014;45:1415–21 10.1161/STROKEAHA.113.004386 [DOI] [PubMed] [Google Scholar]
- 13. Mugikura S, Takahashi S, Higano S, et al. Predominant involvement of ipsilateral anterior and posterior circulations in Moyamoya disease. Stroke 2002;33:1497–500 10.1161/01.STR.0000016828.62708.21 [DOI] [PubMed] [Google Scholar]
- 14. Kim KM, Kim JE, Cho WS, et al. Natural history and risk factor of recurrent hemorrhage in hemorrhagic adult Moyamoya disease. Neurosurgery 2017;81:289–96 10.1093/neuros/nyw179 [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto S, Hori S, Kashiwazaki D, et al. Longitudinal anterior-to-posterior shift of collateral channels in patients with Moyamoya disease: an implication for its hemorrhagic onset. J Neurosurg 2019;130:884–90 10.3171/2017.9.JNS172231 [DOI] [PubMed] [Google Scholar]
- 16. Matsushige T, Kraemer M, Schlamann M, et al. Ventricular microaneurysms in Moyamoya angiopathy visualized with 7T MR angiography. AJNR Am J Neuroradiol 2016;37:1669–72 10.3174/ajnr.A4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyakoshi A, Funaki T, Takahashi JC, et al. Restoration of periventricular vasculature after direct bypass for Moyamoya disease: intra-individual comparison. Acta Neurochir (Wien) 2019;161:947–54 10.1007/s00701-019-03866-9 [DOI] [PubMed] [Google Scholar]