Twenty-one consecutive patients with ruptured brain AVMs who underwent transvenous embolization were prospectively followed between November 2016 and November 2018. Complete AVM nidus obliteration was shown in 16 (84%) of 19 patients. One (5%) patient with a small residual nidus after treatment showed complete obliteration at 13-month follow-up. There were 5 hemorrhages and 1 infarction; 4 patients' symptoms improved gradually. Transvenous embolization can be performed only in highly selected hemorrhagic brain AVMs with high complete obliteration rates, but it should not be considered as a first-line treatment.
Abstract
BACKGROUND AND PURPOSE:
The efficacy and safety of transvenous embolization for brain arteriovenous malformations remains unclear, given the very limited number of cases reported. This prospective study was performed to assess this technique in ruptured AVMs.
MATERIALS AND METHODS:
Twenty-one consecutive patients with ruptured brain AVMs who underwent transvenous embolization were prospectively followed between November 2016 and November 2018. The Spetzler-Martin grade was I in 3 AVMs (14.3%), II in four (19.0%), III in eleven (52.4%), and IV in three (14.3%). The complete AVM occlusion rate was calculated from 6-month follow-up DSA images. Occurrence of hemorrhage and infarction after embolization was evaluated using CT and MR imaging within 1 month after the operation. The mRS was used to assess the functional outcomes.
RESULTS:
Complete AVM nidus obliteration was shown in 16 (84%) of 19 patients with technically feasible AVMs immediately after embolization. One (5%) patient with a small residual nidus after treatment showed complete obliteration at 13-month follow-up. There were 5 hemorrhages and 1 infarction; 4 patients' symptoms improved gradually. The percentage of cases with mRS ≤ 2 rose from 57.1% (12/21) before embolization to 66.7% (14/21) at 1-month follow-up. Both the morbidity and mortality rates were 4.8% (1/21).
CONCLUSIONS:
Transvenous embolization can be performed only in highly selected hemorrhagic brain AVMs with high complete obliteration rates, improved functional outcomes, and acceptable morbidity and mortality rates, but it should not be considered as a first-line treatment.
Brain arteriovenous malformations are characterized by an intervening plexus of abnormal vessels (nidus) between feeding arteries and draining veins. The most common presentations of AVMs include hemorrhages, seizures, headaches, and progressive neurologic deficits due to chronic vascular steal.1 Hemorrhage can occur because of an inherent lack of normal smooth-muscle properties in the vascular architecture of AVMs. A first hemorrhagic event is associated with an increased risk of a new bleeding. Morbidity of AVM hemorrhage is estimated to be between 13% and 50%, and the mortality rate after intracranial hemorrhage from AVM rupture ranges from 12% to 67%.2
Treatment strategies (microsurgery, radiosurgery, and endovascular embolization) are chosen on the basis of angioarchitecture, location, and presentation of AVMs.3,4 Elimination of hemorrhage risk by extirpation or endoluminal closure of the nidus remains the primary goal of AVM treatment. Surgical resection of an AVM can be challenging in deep, inaccessible locations and eloquent areas. Radiosurgery may not be ideal in hemorrhagic AVMs as the first choice because of the long latency between treatment and AVM involution. Endovascular embolization through arterial routes may not be curative for AVMs in many situations, especially in cases with indirect feeders and extreme vessel tortuosity.5 Transvenous embolization can overcome these disadvantages.6–14 Current indications for transvenous embolization of AVMs include deep location, unfavorable arterial access, a small nidus, and a single draining vein.6–14 Because there are only a limited number of cases reported using this approach, the effectiveness and safety remain unclear. In this study, we tried to further validate this method.
Materials and Methods
The Medical Ethics Committee of Henan Provincial People's Hospital approved this study. Key inclusion criteria were as follows: 1) patients with a ruptured brain AVM; 2) patients not suitable for intra-arterial embolization due to the absence of arterial access, narrow arterial feeders, extremely tortuous course, too many feeders, and so forth; and 3) patients in whom lesions were not amenable to surgery or radiosurgery or patients who refused to undergo surgery or radiosurgery (AVMs not amendable to surgery or radiosurgery are defined as cases with modified Spetzler-Martin grades of III+, IV, and V based on modification of the Spetzler-Martin scale15 and those with scores of >1.5 based on Pollock-Flickinger grading scale,16 which are proved have high rates of iatrogenic complications); and 4) patients with favorable venous angioarchitecture and a single main draining vein.
Key exclusion criteria were as follows: 1) multiple AVMs, 2) patients with ≥2 main draining veins, 3) a history of severe allergies to contrast or nonadhesive embolic agents, and 4) uncontrolled active bleeding.
Twenty-one consecutive patients with ruptured brain AVMs underwent transvenous embolization between November 2016 and November 2018. Thirteen patients experienced brain AVM ruptures with intracranial hematoma and intraventricular hemorrhage, 6 patients had intracranial hematoma without intraventricular involvement, 1 patient had subarachnoid hemorrhage, and another one had intraventricular hemorrhage. The mean AVM size was 2.76 ± 1.24 cm, ranging from 1.2 to 5.5 cm. A summary of patient characteristics is shown in Table 1.
Table 1:
Baseline characteristics of the 21 patients in this study
| Variable | Value |
|---|---|
| Age (yr) | |
| Mean | 29.9 |
| Median | 29 |
| Range | 8–59 |
| SD | 17.0 |
| Sex (No.) (%) | |
| Male | 14 (66.7) |
| Female | 7 (33.3) |
| mRS before embolization (No.) (%) | |
| 0–2 | 12 (57.1) |
| 3–5 | 9 (42.9) |
| Location (No.) (%) | |
| Deep | 18 (85.7) |
| Superficial | 3 (14.3) |
| Size (No.) (%) | |
| ≤3 cm | 12 (57.1) |
| >3 cm | 9 (42.9) |
| Eloquent (No.) (%) | |
| Yes | 15 (71.4) |
| No | 6 (28.6) |
| Venous pattern (No.) (%) | |
| Superficial | 11 (52.4) |
| Deep | 9 (42.9) |
| Deep (main) + superficial | 1 (4.8) |
| No. of veins (%) | |
| Single | 20 (95.2) |
| Multiple | 1 (4.8) |
| Angioarchitecture (No.) (%) | |
| Aneurysms in the feeding artery or intranidus | 9 (42.9) |
| Venous stenosis | 4 (19.0) |
| Localized venous ectasia | 2 (9.5) |
| Spetzler-Martin grade (No.) (%) | |
| I | 3 (14.3) |
| II | 4 (19.0) |
| III | 11 (52.4) |
| IV | 3 (14.3) |
| V | 0 (0) |
All the embolization procedures were performed with the patient under general anesthesia. A 6F sheath was placed in the internal jugular vein followed by a 6F guiding catheter, which was advanced to the main draining vein of the brain AVMs. One or 2 microcatheters (Marathon, Covidien, Irvine, California; Apollo, Covidien; Echelon, Covidien; or Headway DUO, MicroVention, Tustin, California) were placed as close as possible to the nidus of the AVMs. A vascular sheath was placed in the right femoral artery followed by guide catheter placement though which a microcatheter was advanced into the feeding artery of the AVMs. Arterial inflow of the feeding artery was reduced by transarterial coil or liquid embolization or balloon inflation. Transvenous embolization was initiated by injecting ethylene-vinyl alcohol copolymer (Onyx; Covidien) into the nidus through the venous access route. Transvenous partial coiling in the draining vein through 1 microcatheter (known as the transvenous pressure cooker technique) was used to prevent reflux of Onyx.17–19 At the completion of the procedure, the microcatheter injecting Onyx was cut at the level of the jugular sheath.14
Preoperative baseline functional status was determined using the mRS score. The same evaluation was performed on postoperative days 2, 7, and 30 and at 3, 6, and 12 months. Occlusion of the AVM nidus was categorized as complete (no residual nidus) and near-complete obliteration (residual nidus of <3 mm in diameter). Comparison of the degree of occlusion was classified as progressive, stable, or recanalized.
Procedural safety was evaluated by assessing the periprocedural complications occurring within 1 month after embolization.20 Any deficit that resolved within the first 30 days was characterized as transient. Any deficit that persisted beyond 30 days was considered permanent. An mRS score of ≤2 indicated a nondisabling deficit. An mRS score of ≥3 indicated a disabling deficit. Periprocedural-related death was defined as any death occurring within 30 days after the procedure.
Statistical Analysis
Categoric variables are presented as numbers and percentages, and continuous variables are presented as mean and SD. A 2-sided P value < .05 was considered significant.
Results
Procedural/Technical Specifications
The median time between hemorrhage and transvenous treatment was 47 days (range, 9–164 days). The procedure was technically feasible in 19 (90.5%) cases (Table 2). There was failure of microcatheter placement into the draining vein via the nidus in 2 cases.
Table 2:
Safety and efficacy outcomes
| Variable | Value |
|---|---|
| Procedure | |
| Patients (No.) | 21 |
| Patients with technically feasible AVMs (No.) (%) | 19 (90.5%) |
| Procedure-related complications (No.) (%) | 6 (28.6) |
| Transient | 4 (19.0) |
| Permanent, nondisabling | 0 (0) |
| Permanent, disabling | 1 (4.8) |
| Death | 1 (4.8) |
| Non-neurologic | 0 (0) |
| Follow-up | |
| Immediate obliteration after procedure (No.) (%) | |
| In 19 patients with technically feasible AVMs | 16 (84.2) |
| In all 21 patients | 16 (76.2) |
| Imaging follow-up of patients (No.) | 14 |
| Follow-up time (median) (range) | 5.5 (3–15) |
| Obliteration at follow-up (No.) (%) | 13 (92.9) |
| Stable | 1 (7.1) |
| Recanalization | 0 (0) |
| Clinical follow-up of patients within 1 mo (No.) | 21 |
| Events | 6 |
| Stroke | 6 |
| Others | 0 |
| Clinical follow-up of patients beyond 1 mo (No.) | 20 |
| The latest follow-up time (median) (range) | 15 (2–26) |
| Events (No.) | 1 |
| Epilepsy | 1 |
| Others | 0 |
| The latest mRS | |
| 0–2 | 19 |
| 3–5 | 1 |
| 6 | 0 |
For the 19 embolization procedures, access included the straight sinus in 9 cases, the cortical veins via the superior sagittal sinus in 6 cases, and the cortical veins via the transverse sinus in 4 cases.
The transvenous pressure cooker technique and Onyx injection were accomplished using 1 microcatheter in 3 patients and 2 microcatheters in 16 patients. The microcatheters for Onyx injection were retained in all patients except one.
The mean procedural time of all 21 patients from puncture onset to puncture closing was 251.6 ± 73.1 minutes. The mean volume of Onyx used for embolization was 3.79 ± 3.30 mL (range, 0 –13 mL).
AVM Nidus Obliteration
Immediately after the procedure, complete obliteration was achieved in 16 cases (Table 2), with an obliteration rate of 84.2% in the 19 technically feasible cases and 76.2% in all 21 cases. A postoperative small residual nidus was present in 3 patients after transvenous embolization. Follow-up angiography was performed from 1 to 15 months after embolization. Stable obliteration was confirmed in 18 patients. No recurrence was noted (Fig 1). One AVM located in the parietal lobe and basal ganglia showed progressive occlusion (Fig 2).
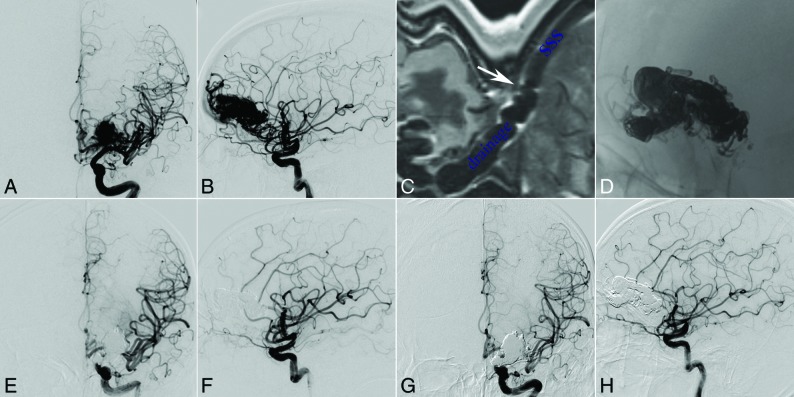
Fig 1.
A 31-year-old man with intraparenchymal hemorrhage. Selective DSA of the left ICA (anteroposterior [A] and lateral [B] views) demonstrates that the AVM located at the frontal lobe is fed by the branches and perforators of anterior cerebral artery, MCA, and ICA and drains a single venous outlet via the cortical vein to the superior sagittal sinus (SSS). The high-resolution MR imaging shows that there is no severe stenosis or valvelike chordae in the connection part of the draining vein and superior sagittal sinus (C, white arrow). The nidus cast is through the transvenous embolization (D, unsubtracted image of the DSA), and the AVM is completely angiographically obliterated at the end of the operation (anteroposterior [E] and lateral [F] views) and at the 5-month follow-up (anteroposterior [G] and lateral [H] views).
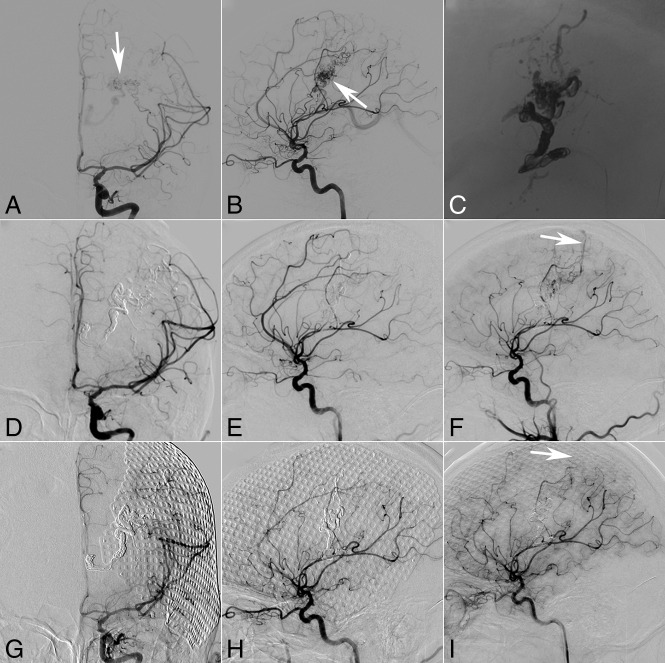
Fig 2.
A 28-year-old man with intraparenchymal and intraventricular hemorrhage. Ventriculostomy, decompressive craniectomy, and transarterial embolization were performed at the local hospital. Three months later, the selective DSA of the left ICA (anteroposterior [A] and lateral [B] views, both white arrows referring to the nidus) demonstrates that the parietal lobe and basal ganglia arteriovenous malformation are fed by the branches of the MCA and drain a single venous outlet via the deep vein to the straight sinus. The nidus cast was through transvenous embolization (C, unsubtracted image of the DSA), but there is a small residual AVM (anteroposterior [D] and lateral [E] views at the median arterial phase) with drainage via a cortical vein (F, white arrow) to the superior sagittal sinus, which appeared at the late arterial phase of DSA. Thirteen-month angiography follow-up confirms the complete occlusion of the residual AVM (anteroposterior [G] and lateral [H] views at the median arterial phase and lateral view [I] at the late arterial phase).
Procedural Safety
Procedure-related complications occurred in 6 patients, including 4 intraventricular hemorrhages, 1 intraparenchymal hemorrhage with combined intraventricular hemorrhage (Fig 3), and 1 cerebral infarction (Fig 4). Three of these patients underwent ventriculostomy, 1 of whom also underwent lumbar drainage; the other patients just had conservative management. Good outcomes (mRS ≤ 2) at the 1-month evaluation were achieved in 4 patients following the above therapies. The clinical outcome was poor (mRS = 5) in 1 patient (Fig 4) at the 1-month evaluation, and another patient died.
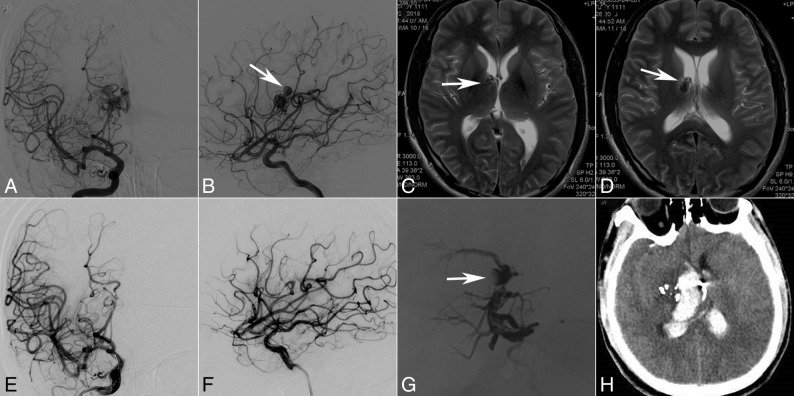
Fig 3.
A 28-year-old man with intraventricular hemorrhage. Selective DSA of the right ICA (anteroposterior [A] and lateral [B] views) demonstrates that the AVM with an intranidal aneurysm (B, white arrow) is fed by perforators of the MCA and ICA and drains a single venous outlet via the deep vein to the straight sinus. Axial MR image indicates a basal ganglia arteriovenous malformation (C, white arrow) with the intranidus aneurysm next to the ventricle (D, white arrow). At the end of the operation, the AVM does not appear at the last angiography (anteroposterior [E] and lateral [F] views), but the cast image shows that the aneurysm does not have complete penetration by the embolic agent (G, white arrow) after transarterial and transvenous embolization. Two days later, intraventricular hemorrhage occurred (H, CT).
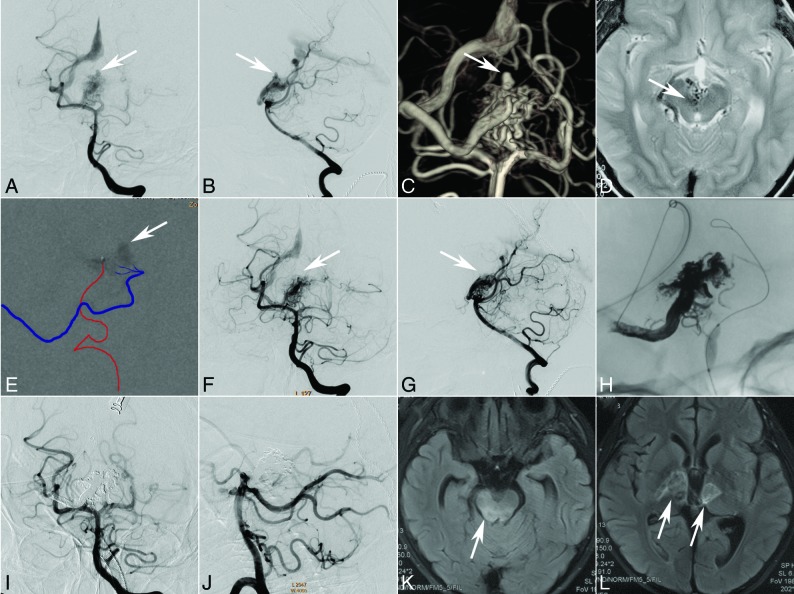
Fig 4.
An 8-year-old boy who presented with sudden headache and vomiting. CT shows intraventricular hemorrhage. Selective DSA of the left vertebral artery (anteroposterior [A] and lateral [B] views, white arrow) demonstrates that the AVM with an intranidus aneurysm (C, 3D reconstruction, white arrow) is fed by the perforators of the posterior cerebral artery and drains a single venous outlet via the deep vein to the straight sinus. Axial MR image indicates a diencephalon arteriovenous malformation (D, white arrow). Transarterial ethanol sclerotherapy (80% ethanol in iohexol, Omnipaque 300 [GE Healthcare, Piscataway, New Jersey]) was performed to occlude the aneurysm (E, white arrow, the injection course can be seen in the On-line Video). Both the immediate angiography after sclerotherapy and the 2-month follow-up angiography (anteroposterior [F] and lateral [G] views, white arrow) demonstrate occlusion of the aneurysm. At 2-month follow-up, transvenous embolization was performed under transarterial balloon blocking (H). The last angiography (anteroposterior [I] and lateral [J] views) shows complete occlusion of the AVM. The intraprocedure electroencephalography monitoring did not show an abnormality, but the patient presented with light coma or lethargy. The MR imaging performed 12 days after the operation shows multiple infarctions in the mesencephalon (K, white arrow) and thalamus (L, white arrows).
Among the above complications, 5 occurred in small-sized AVMs, 5 in eloquent areas, 5 with deep venous drainage, 9 in deep locations, 4 with Spetzler-Martin III, 4 with aneurysms in the feeding artery or AVM nidus, and 1 with venous outflow stenosis. The Fisher exact test indicated no significant difference among these factors (Table 3).
Table 3:
Relative analysis for the complications in 19 patients with technically feasible AVMs
| Variable | Complication |
P Value | |
|---|---|---|---|
| + | − | ||
| Spetzler-Martin grade | .801 | ||
| I–II | 1 | 5 | |
| III | 4 | 6 | |
| IV–V | 1 | 2 | |
| Size | .141 | ||
| ≤3 cm | 5 | 5 | |
| >3 cm | 1 | 8 | |
| Eloquent | .605 | ||
| + | 5 | 8 | |
| − | 1 | 5 | |
| Deep venous drainage | .057 | ||
| + | 5 | 4 | |
| − | 1 | 9 | |
Note:—+ indicates yes; −, no.
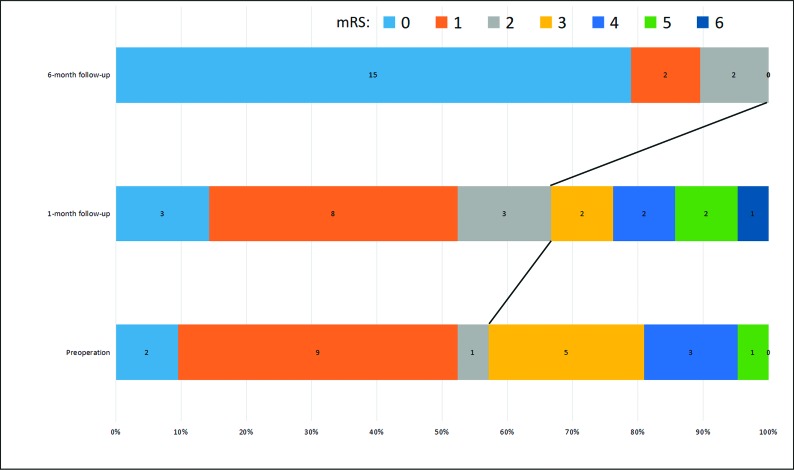
The mRS scores at the latest follow-up for all surviving patients were as follows: 0 for 15 patients, 1 for 2 patients, 2 for 2 patients, and 5 for 1 patient. The percentage of good outcome (mRS ≤ 2) increased from 57.1% (12/21) before embolization to 66.7% (14/21) at 1-month follow-up and 100% (19/19) at 6-month follow-up, respectively (Fig 5).
Fig 5.
The good functional outcome (mRS ≤ 2) ratios improved from 57.1% (12/21) before the operation to 66.7% (14/21) at 1-month follow-up and 100% (19/19) at 6-month follow-up, respectively.
Discussion
Transvenous Obliteration
Total occlusion of AVMs with detachable or nondetachable microcatheters in transarterial procedures is difficult to achieve, mostly due to a variety of challenges, including microcatheter navigation, multiplicity of arterial feeders, and achieving deep and complete Onyx penetration of the AVM nidus.5 In these challenging situations, transvenous embolization may be a viable alternative with total occlusion rates as high as 92.6%,21 which is comparable with ours in this study. Transvenous microcatheter navigation, which occurred in 2 of our cases, might be difficult. However, this issue might be resolved by balloon-assisted microcatheter navigation techniques22 or hybrid surgical techniques.23,24
The high occlusion rates of AVMs may be due to the small, compact architecture, which is more easily penetrated. Additionally, the single, embolized draining vein may, in turn, induce the occlusion of many shunts in the nidus. Because arteriovenous fistulas compose most brain AVMs with plexiform nidi and upstream shunts, arterial occlusion without obliteration of the venous drainage of brain AVMs can lead to recurrence.25 In the report of Viana et al,26 1 patient in whom immediate angiographic occlusion was not achieved showed spontaneous occlusion at the 6-month follow-up,26 which was also observed in this study.
Brain AVM–related events can be controlled once the AVM nidus has been obliterated. A case series demonstrated no events occurring in the 6-month follow-up in 11 patients.26 Another report indicated that only 1 (2.5%) patient had significant disability at the 6-month follow-up, but unfortunately, no detailed information was provided.21 In this study, 1 seizure occurred 3 months after the operation.
Procedural Safety
Previous reports on the transvenous embolization of AVMs showed low rates of periprocedural complications, disability, and morbility.21,27 In contrast, the complication rate was relatively high in our study. There are several possible reasons: First, the present study enrolled only patients with ruptured AVMs (100%), which had a higher risk for rebleeding.1 In the report of Mendes et al,21 the rate of ruptured AVMs was only 67.5%. Second, we enrolled more patients with higher Spetzler-Martin grades, and 66.7% of our patients were classified as Spetzler-Martin grade III or even higher. The percentage of higher Spetzler-Martin grades was 58.5% in the report of Mendes et al,21 and only 25% in the report of Trivelato et al,11 which are architecturally more complex.
Infarctions caused during transvenous embolization are supposed to be related to either penetration of Onyx into the feeding artery of the normal brain tissue or hyperemia and edema caused by draining vein occlusion. The outcome may depend on the local collateral circulation.2,28 In this study, 1 cerebral infarct that caused disability was thought to be due to the occlusion of the feeding artery with Onyx. Trivelato et al11 and Mendes et al21 reported 2 cases each of postprocedural parenchymal edema thought to be related to venous outflow obstruction, one of which caused a disability.
Hemorrhagic complications might occur in different locations. Among the 5 hemorrhagic complications, 4 occurred in the eloquent area and 4 with deep venous drainage, which are also the risk factors for transarterial embolization.29 All 5 AVMs with hemorrhagic complications were located in deep brain structures and around ventricles and bled into the ventricles, which is not a usual complication of transarterial embolization. Periventricular location has been cited as a risk factor for hemorrhage.30 These hemorrhages may be related to the following reasons:18 high arterial input pressure and venous outflow restriction in the deep AVMs; not enough brain tissue around the ventricle; and a large pressure gradient between the nidus and ventricle. Premature occlusion of draining veins during transvenous embolization before complete occlusion of the nidus can increase the pressure in the AVM nidus,31 which has been reported as one of the main causes of hemorrhagic complications.32 Therefore, transvenous embolization is considered an “all-or-nothing” technique. In this study, 3 AVMs had residual nidi postembolization, with hemorrhages occurring in 2. The 1 AVM with a residual that did not bleed had continued drainage through a cortical vein after the deep drainage vein had been occluded, which may explain the lack of hemorrhage postprocedure.
Most of the patients in this study with complications (4/6) had good outcomes after receiving appropriate treatment. For the ischemic complications, the outcome depends on the location and size of the infarction, especially whether it is in an eloquent area. In this study, the patient with infarction involving the midbrain and thalamus did not recover well. For the other 5 hemorrhagic complications, 4 patients had good outcomes and 1 died due to severe intracranial infection after hemorrhage. This might be related to several factors: First, intraparenchymal hemorrhage often occurs in the same area as prior hemorrhage and is less likely to immediately damage healthy brain; second, the subarachnoid hemorrhage can be treated by drugs or ventriculostomy; and finally, the patients were relatively young in this study.
Limitations
Although the number of cases in this study was the second largest in all the publications currently available,21 the sample size was still small. The patients were carefully selected and were selected for salvage procedures due to lack of good microsurgical, transarterial, and radiosurgical options, which may imply greater propensity for procedural risk than in other studies. Treatment strategy, patient factors, and doctors' experience are highly individualized and variable worldwide; thus, the findings of our study may not be consistent with other centers.
Conclusions
Transvenous embolization with high complete obliteration rates, improved functional outcomes, and acceptable morbidity and mortality rates can be performed in only carefully selected brain AVMs. However, more experience is necessary to discern the role of this technique in the management of ruptured AVMs, and it should not be considered a first-line treatment.
Acknowledgments
The authors thank Yanyan He, Bin Xu, Xiaoyu Kang at Henan Provincial People's Hospital for data collection; Jingge Zhao at Henan Provincial People's Hospital for statistical analyses; Ajmal Zemmar and Juha Hernesniemi at Henan Provincial People's Hospital for their English editing assistance; and David F. Kallmes, Ramanathan Kadirvel, Daying Dai, and Kimberly Collins at the Mayo Clinic for their help in providing learning office and reference material.
Footnotes
The study was funded by the National Natural Science Foundation of China (No. 81601583), the Scientific and Technological Project (No. 2018020424), and Aboard Research Project (2016054) of Henan Provincial Health Commission.
References
- 1. Solomon RA, Connolly EJ. Arteriovenous malformations of the brain. N Engl J Med 2017;376:1859–66 10.1056/NEJMra1607407 [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Choi EJ, McDougall CM, et al. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl Stroke Res 2014;5:316–29 10.1007/s12975-014-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. ; American Heart Association Stroke Council. Management of Brain Arteriovenous Malformations: a Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e200–24 10.1161/STR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 4. Cenzato M, Boccardi E, Beghi E, et al. European consensus con-ference on unruptured brain AVMs treatment (supported by EANS, ESMINT, EGKS, and SINCH). Acta Neurochir (Wien) 2017;159:1059–64 10.1007/s00701-017-3154-8 [DOI] [PubMed] [Google Scholar]
- 5. Elsenousi A, Aletich VA, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or Onyx: a meta-analysis. J Neurointerv Surg 2016;8:265–72 10.1136/neurintsurg-2014-011427 [DOI] [PubMed] [Google Scholar]
- 6. Nguyen TN, Chin LS, Souza R, et al. Transvenous embolization of a ruptured cerebral arteriovenous malformation with en-passage arterial supply: initial case report. J Neurointerv Surg 2010;2:150–52 10.1136/jnis.2009.001289 [DOI] [PubMed] [Google Scholar]
- 7. Kessler I, Riva R, Ruggiero M, et al. Successful transvenous embolization of brain arteriovenous malformations using Onyx in five consecutive patients. Neurosurgery 2011;69:184–93; discussion 193 10.1227/NEU.0b013e318212bb34 [DOI] [PubMed] [Google Scholar]
- 8. Pereira VM, Marcos-Gonzalez A, Radovanovic I, et al. Transvenous embolization of a ruptured deep cerebral arteriovenous malformation: a technical note. Interv Neuroradiol 2013;19:27–34 10.1177/159101991301900104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Consoli A, Renieri L, Nappini S, et al. Endovascular treatment of deep hemorrhagic brain arteriovenous malformations with transvenous Onyx embolization. AJNR Am J Neuroradiol 2013;34:1805–11 10.3174/ajnr.A3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed AZ. Endovascular venous approach in the treatment of ruptured intra-cerebral arterio-venous malformation. The Egyptian Journal of Radiology and Nuclear Medicine 2014;45:439–41 10.1016/j.ejrnm.2013.12.010 [DOI] [Google Scholar]
- 11. Trivelato FP, Manzato LB, Rezende MT, et al. Transitory brain stem edema following successfully transvenous embolization of a posterior fossa arteriovenous malformation. Clin Neuroradiol 2014;24:151–53 10.1007/s00062-013-0209-y [DOI] [PubMed] [Google Scholar]
- 12. Choudhri O, Ivan ME, Lawton MT. Transvenous approach to intracranial arteriovenous malformations: challenging the axioms of arteriovenous malformation therapy? Neurosurgery 2015;77:644–52 10.1227/NEU.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 13. Renieri L, Limbucci N, Consoli A, et al. Transvenous embolization: a report of 4 pediatric cases. J Neurosurg Pediatr 2015;15:445–50 10.3171/2014.11.PEDS13437 [DOI] [PubMed] [Google Scholar]
- 14. Iosif C, Mendes GA, Saleme S, et al. Endovascular transvenous cure for ruptured brain arteriovenous malformations in complex cases with high Spetzler-Martin grades. J Neurosurg 2015;122:1229–38 10.3171/2014.9.JNS141714 [DOI] [PubMed] [Google Scholar]
- 15. Lawton MT; UCSF Brain Arteriovenous Malformation Study Project. Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery 2003;52:740–49 10.1227/01.NEU.0000053220.02268.9C [DOI] [PubMed] [Google Scholar]
- 16. Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg 2002;96:79–85 10.3171/jns.2002.96.1.0079 [DOI] [PubMed] [Google Scholar]
- 17. Zhang G, Zhu S, Wu P, et al. The transvenous pressure cooker technique: a treatment for brain arteriovenous malformations. Interv Neuroradiol 2017;23:194–99 10.1177/1591019916682357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He Y, Bai W, Li T, et al. Curative transvenous embolization for ruptured brain arteriovenous malformations: a single-center experience from China. World Neurosurg 2018;116:e421–28 10.1016/j.wneu.2018.04.223 [DOI] [PubMed] [Google Scholar]
- 19. Chapot R, Stracke P, Velasco A, et al. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol 2014;41:87–91 10.1016/j.neurad.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 20. Jayaraman MV, Meyers PM, Derdeyn CP, et al. Reporting standards for angiographic evaluation and endovascular treatment of cerebral arteriovenous malformations. J NeuroIntervent Surg 2012;4:325–30 10.1136/neurintsurg-2011-010173 [DOI] [PubMed] [Google Scholar]
- 21. Mendes G, Kalani M, Iosif C, et al. Transvenous curative embolization of cerebral arteriovenous malformations: a prospective cohort study. Neurosurgery 2018;83:957–64 10.1093/neuros/nyx581 [DOI] [PubMed] [Google Scholar]
- 22. Mendes GA, Silveira EP, Saleme S, et al. Balloon-assisted microcatheter navigation for AVM embolization: technical note. J Neurosurg 2015;123:1120–24 10.3171/2014.10.JNS141359 [DOI] [PubMed] [Google Scholar]
- 23. Wang MZ, Qiu HC, Wang S, et al. A new technique for transvenous embolization of brain arteriovenous malformations in hybrid operation. Chin Med J (Engl) 2018;131:2993–96 10.4103/0366-6999.247199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kulcsar Z, Machi P, Schaller K, et al. Trans-venous embolization of a basal ganglia ruptured arteriovenous malformation with open surgical arterial control: a hybrid technique. J Neuroradiol 2018;45:202–05 10.1016/j.neurad.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 25. Houdart E, Gobin YP, Casasco A, et al. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology 1993;35:381–85 10.1007/BF00588376 [DOI] [PubMed] [Google Scholar]
- 26. Viana DC, de Castro-Afonso LH, Nakiri GS, et al. Extending the indications for transvenous approach embolization for superficial brain arteriovenous malformations. J NeuroIntervent Surg 2017;9:1053–59 10.1136/neurintsurg-2017-013113 [DOI] [PubMed] [Google Scholar]
- 27. Lv X, Song C, He H, et al. Transvenous retrograde AVM embolization: indications, techniques, complications and outcomes. Interv Neuroradiol 2017;23:504–09 10.1177/1591019917716817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stüer C, Ikeda T, Stoffel M, et al. Evidence for a predominant intrinsic sympathetic control of cerebral blood flow alterations in an animal model of cerebral arteriovenous malformation. Transl Stroke Res 2010;1:210–19 10.1007/s12975-010-0021-9 [DOI] [PubMed] [Google Scholar]
- 29. Pan J, He H, Feng L, et al. Angioarchitectural characteristics associated with complications of embolization in supratentorial brain arteriovenous malformation. AJNR Am J Neuroradiol 2014;35:354–59 10.3174/ajnr.A3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma L, Huang Z, Chen XL, et al. Periventricular location as a risk factor for hemorrhage and severe clinical presentation in pediatric patients with untreated brain arteriovenous malformations. AJNR Am J Neuroradiol 2015;36:1550–57 10.3174/ajnr.A4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D'Aliberti G, Talamonti G, Piparo M, et al. Venous flow rearrangement after treatment of cerebral arteriovenous malformations: a novel approach to evaluate the risks of treatment. World Neurosurg 2014;82:160–69 10.1016/j.wneu.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 32. Baharvahdat H, Blanc R, Termechi R, et al. Hemorrhagic complications after endovascular treatment of cerebral arteriovenous malformations. AJNR Am J Neuroradiol 2014;35:978–83 10.3174/ajnr.A3906 [DOI] [PMC free article] [PubMed] [Google Scholar]