Abstract
BACKGROUND AND PURPOSE:
Simple-but-precise evaluation of cerebral perfusion is crucial for the treatment of Moyamoya disease. We aimed to develop a standardized scoring system for MR perfusion suitable for Moyamoya disease evaluation and investigate the postoperative serial changes and outcome predictors.
MATERIALS AND METHODS:
From January 2013 to December 2016, patients diagnosed with Moyamoya disease and receiving indirect revascularization were recruited prospectively. Clinical data and serial imaging studies were analyzed. The TTP maps were standardized using cerebellar reference values. We developed a scoring system of standardized TTP maps: 14 points for each hemisphere with higher points indicating better perfusion.
RESULTS:
In total, 24 children (4–17 years of age, 41 hemispheres) and 20 adults (18–51 years of age, 34 hemispheres) were included. The mean preoperative TTP scores were higher in children (7.34 ± 3.90) than in adults (4.88 ± 3.24). The standardized TTP maps revealed dynamic improvement with an increase in the corresponding scores at the 1-, 3-, and 6-month postoperative follow-ups; the scores stabilized after 6 months. The mean improvement in the 6-month scores of the pediatric and adult groups was 4.15 ± 3.55 and 6.03 ± 3.04, respectively. The 6-month TTP score improvements were associated with Matsushima grades. If we took score improvement as the outcome, the preoperative TTP score was the only significant predictor in multivariable analysis.
CONCLUSIONS:
The standardized TTP maps and scoring system facilitated the quantification of the sequential perfusion changes during Moyamoya disease treatment. The preoperative perfusion status was the only predictor of indirect revascularization outcome.
Moyamoya disease (MMD) is a progressive occlusive disease of the supraclinoid segment of the ICA associated with collateral vessel formation at the base of the brain. MMD has 2 age peaks: at approximately 10 years and at 30–40 years. The major clinical presentation is ischemic stroke in both children and adults.1 Surgical revascularization is the only effective therapy for patients with MMD to reduce their risk of subsequent strokes. Although the clinical response to surgical treatment appears to be favorable in most cases, there is considerable debate regarding the advantages and disadvantages of direct and indirect revascularizations, particularly for adult patients.2–4 The effectiveness of indirect revascularization in adult patients with MMD, however, has been supported by clinical and angiographic evaluations.5–7
Evaluation of MMD severity has largely depended on conventional angiography. Suzuki and Takaku8 proposed 6 stages of angiographic evolution in MMD. However, only some patients follow this stepwise progression, and the staging is not correlated with the clinical symptoms. Thus far, the evaluation of surgical outcome after revascularization for MMD depends on angiography using the Matsushima grading system.9 However, conventional angiography is invasive, and the Matsushima grading system focuses only on the outer aspect of cerebral hemispheres.
Brain perfusion studies provide cross-sectional imaging through SPECT, Xe-CT, 15O-H2O PET, MR perfusion (MRP), and CT perfusion in MMD.10–14 Given the adverse effect of radiation, MRP is an efficient, noninvasive tool for evaluation. A study comparing MTT measured through DSC MR imaging and the oxygen extraction fraction measured through PET revealed that an MTT delay of 2 seconds (compared with the cerebellum) suggested misery perfusion.15 Changes in TTP maps after revascularization surgery were reported to be correlated with clinical outcome in patients with MMD.16 In clinical practice, the evaluation of the TTP map is generally qualitative rather than quantitative. A scoring system serving as a simple method of communication among clinicians, such as ASPECTS17 for acute stroke treatment, would be helpful for the treatment of MMD.
In this study, our purpose was 2-fold: First, develop a scoring system for standardized TTP maps of MRP that is suitable for evaluation of longitudinal perfusion changes; and second, on the basis of the proposed scoring system, investigate the outcome predictors for indirect revascularization in patients with MMD.
Materials and Methods
The institutional review board of National Taiwan University Hospital approved this study, and written informed consent was obtained from all participants.
Participants
From January 2013 to December 2016, patients with MMD were prospectively recruited and operated on by a single neurosurgeon at our institution. The diagnosis of MMD was made according to the criteria stated by the Research Committee on Spontaneous Occlusion of the Circle of Willis in Japan.18 Preoperative MR imaging and MRP were part of our study. The patients who did not have a preoperative MRP result of sufficient quality or had undergone previous revascularization surgery were excluded.
All patients were scheduled for MR imaging and MRP follow-ups at 1, 3, 6, and 12 months after the operation. Conventional angiography was performed before each operation and 6–12 months after the last operation. The age, sex, presenting symptoms, associated diseases, operative methods, neuroimaging and clinical results, and surgical complications were recorded. Only the operated hemispheres were analyzed in this study.
MR Imaging and MRP
MR imaging and MRP were performed using a 1.5T MR imaging scanner, Signa Excite HDx (GE Healthcare, Milwaukee, Wisconsin). The scanning protocol involved axial FLAIR, T2WI, DWI of 5-mm thickness, TOF-MRA, and precontrast and postcontrast 3D-T1WI (inversion recovery spoiled gradient recalled). We used DSC MR imaging for MRP (TR/TE = 1000/40 ms, flip angle = 90°, FOV = 24 × 24 cm, in-plane resolution = 1.9 × 1.9 mm, thickness = 5 mm, gap = 3–5 mm depending on the head size, 12 slices covering half of the cerebellum to the top of the cerebrum, 50 phases). The MRP was aligned to the subcallosal line with a slice through the subcallosal line. Gadovist (gadobutrol; 0.1 mmol/kg; Bayer Schering Pharma, Berlin, Germany) was injected 5 seconds after the DSC MR imaging commenced (injection rate = 3 cm3/s) followed by a 20-cm3 saline flush. The follow-up MR imaging and MRP followed the slice location of the preoperative study.
Semiquantitative TTP Scoring System
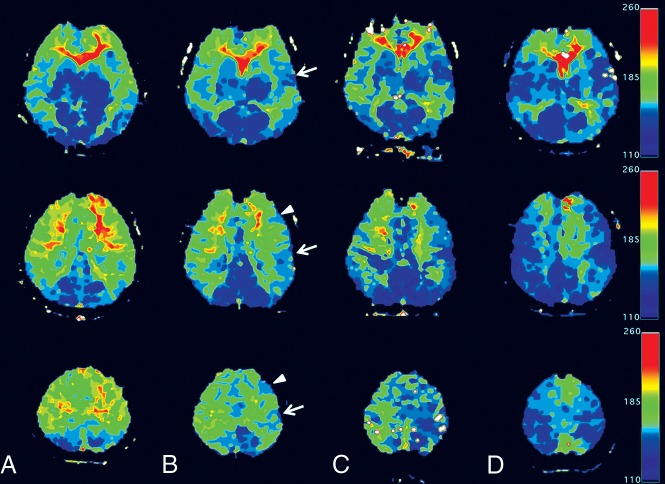
Postprocessing of DSC MR imaging data was performed using the Perfusion Mismatch Analyzer (PMA; Version 5.0.0.0; Advanced Medical Science Center, Iwate Medical University, Iwate, Japan). TTP maps were generated and input to OsiriX Imaging Software (http://www.osirix-viewer.com). We used the National Institutes of Health color bar for display and adjusted the window level and width for each brain according to the value of the TTP of the cerebellum. The numeric range of the color bar was fixed to 15 seconds, whereas the lower end was adjusted to 4 seconds lower than the cerebellar average TTP value so that the cerebellum would appear blue. The areas of prolonged TTP (≥2 seconds) would appear green to red on color maps. Figure 1 depicts the sequential change of a patient's standardized color maps before and after surgical revascularization.
Fig 1.
Serial changes in 3 levels in standardized TTP maps of an 18-year-old female patient with MMD. Preoperatively (A) and 1 (B) and 3 (C) months after left temporal EDAS and frontal EPS and a 6-month map after a right-sided operation (D). Preoperative maps show large areas of prolonged TTP in the anterior cerebral artery and MCA territories bilaterally (A). Areas of normalized TTP near the craniotomy site (arrows: temporal EDAS; arrowheads: frontal EPS) are shown in the 1-month postoperative map (B) and are enlarged in the 3-month map (C). The contralateral hemisphere shows simultaneous improvement. After a subsequent right-sided operation, the right cerebrum shows increased areas of TTP normalization. The final maps show only small areas of TTP prolongation at the bilateral medial cerebrum (D).
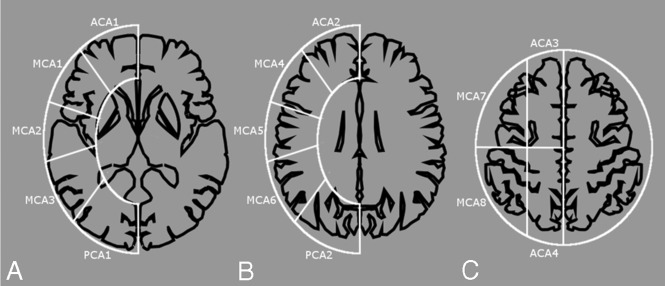
We developed a scoring system for semiquantification of the TTP maps, the TTP scoring system. MRP at 14 regions in 3 levels (ie, ganglionic, ventricular roof, and high convexity) was selected for scoring (Fig 2). For each region, if blue occupied more than half of the area, it was given 1 point. Each healthy cerebral hemisphere would score 14 points. The infarcted areas were not excluded from evaluation. Two neuroradiologists read the standardized TTP color maps independently and provided the scores according to the color in the 14 regions of each hemisphere.
Fig 2.
TTP scoring system. We counted and totaled 14 cortical regions in 3 levels of cerebral hemispheres: ganglionic level (A), ventricular roof level (B), and high convexity (C). Each level is approximately 2 cm apart. An area of blue larger than one-half of 1 region is counted as 1 point, otherwise, it is counted as 0 points. The total TTP score of 1 hemisphere ranges from 0 to 14 points: 4, 8, and 2 for the anterior cerebral artery, MCA, and posterior cerebral artery areas, respectively.
Conventional Angiography
A complete angiographic study of the common carotid, external carotid, and ICAs of each hemisphere, and vertebral angiography of the dominant side was performed. Preoperative and postoperative angiography was assessed using the Suzuki staging (I–VI) and Matsushima grading (A–C) systems, respectively, for each cerebral hemisphere.8,9
Surgical Procedure
All participants underwent encephaloduroarteriosynangiosis (EDAS) or encephalomyosynangiosis of the temporal region, with or without additional encephalopericraniosynangiosis (EPS) of the frontal or parietal regions. Patients requesting a 1-stage operation underwent multiple burr-hole operations for both cerebral hemispheres. During EPS and multiple burr-hole operations, craniotomy windows (diameter, 3–4 cm) were created to facilitate the exposure of the arteries located in the cerebral sulci and obtain effective contact with the donor vascular tissue. During EDAS, the horizontal width of the craniotomy window was >3 cm to expose at least 1 sulcus and the vertical length was >7 cm to acquire a long superficial temporal artery flap. An anchoring suture between the superficial temporal artery flap and dura was created at the 4 corners of the craniotomy window. In addition, duropexy was performed by infolding the dural flap into the cerebral sulci in every craniotomy window.
Statistical Analysis
Descriptive statistics for demographic data, clinical manifestation, radiologic evaluation, operative method, periprocedural complications, and follow-up data are provided. The TTP scores were treated as continuous data, without violating the assumption of normality. TTP score improvement was considered a radiologic outcome. The interrater reliability of the TTP score was assessed using the intraclass correlation coefficient. To investigate the predictor for this radiologic outcome, we used a linear mixed-regression model to control the correlation between hemispheres of the same subject. Categoric predictors were coded as dummy variables. If the predictor was a continuous variable, standardization of the variable was performed to facilitate direct comparison of coefficients. Univariable and multivariable analyses were conducted using the Wald test for the coefficients. The significance level was set at .05. All statistical analyses were performed on SAS (Version 9.4; SAS Institute, Cary, North Carolina).
Results
Patients' Clinical and Radiologic Data
During the study period, 31 pediatric and 23 adult patients with MMD were operated on at our institution; of them, 2 were excluded because of insufficient preoperative MRP quality and 8 due to previous revascularization. Finally, 44 patients (24 pediatric and 20 adult) were included. Among the 88 hemispheres, there were 75 operated hemispheres (41 pediatric and 34 adult) included in the analysis. Table 1 shows the patient characteristics.
Table 1:
Patient demographic and clinical data
| Pediatric Patients (n = 24) | Adult Patients (n = 20) | |
|---|---|---|
| Age (mean) (yr) | 9.21 ± 3.59 | 32.4 ± 10.94 |
| Sex (male) (No.) (%) | 16 (67) | 3 (15) |
| Clinical presentation (No.) (%) | ||
| Focal neurologic symptoms | 17 (71) | 13 (65) |
| Seizure | 3 (13) | 4 (20) |
| Headache | 5 (21) | 3 (15) |
| Infarction | 9 (38) | 14 (70) |
| Hemorrhage | 0 (0) | 1 (5) |
| Comorbidities (No.) (%)a | 3 (8) | 0 (0) |
There were 2 patients with neurofibromatosis type 1 and 1 patient with systemic lupus erythematosus.
The pre- and postoperative features of the operated hemispheres are shown in Table 2. Among the 75 hemispheres, an additional EPS was performed in the frontal or parietal area in 15 (36%) pediatric and 30 (88%) adult hemispheres, respectively. Three adults had immediate postoperative stroke related to relatively low blood pressure and diabetic ketoacidosis; however, they were stabilized thereafter. No periprocedural infarction occurred in the pediatric patients.
Table 2:
Pre- and postoperative radiologic characteristics
| Pediatric Hemispheres (n = 41) | Adult Hemispheres (n = 34) | |
|---|---|---|
| Preoperative Suzuki stage (No.) (%) | ||
| I | 1 (2) | 0 (0) |
| II | 7 (17) | 5 (15) |
| III | 18 (44) | 11 (32) |
| IV | 12 (29) | 10 (29) |
| V | 3 (7) | 3 (9) |
| VI | 0 (0) | 5 (15) |
| Preoperative TTP score (points) (mean) | 7.34 ± 3.90 | 4.88 ± 3.24 |
| Additional EPS (No.) (%) | 15 (36) | 30 (88) |
| Periprocedural infarction (No.) (%) | 0 (0) | 3 (9) |
| Duration of follow-up (mean) (mo) | 38.00 ± 16.57 | 29.85 ± 5.11 |
| Progressive PCA disease (No.) (%) | 3 (7) | 0 (0) |
| Recurrent stroke during follow-up (No.) (%)a | 3 (7) | 0 (0) |
Note:—PCA indicates posterior cerebral artery.
Between 2 operations: 2 on the nonoperated side, 1 in the PCA territory on the operated side.
The mean follow-up duration for pediatric and adult patients was 38 ± 16.57 and 29.85 ± 15.11 months, respectively. Three pediatric patients had new asymptomatic MR imaging–demonstrated infarctions after the operation: Two developed the infarction on the nonoperated side, whereas 1 had it in the posterior cerebral artery territory of the operated side. MRA showed enlargement of superficial temporal arteries and middle meningeal arteries on the operative sides with reciprocal change of the smaller anterior cerebral artery and MCA. MRA and MRP revealed disease progression in the posterior cerebral artery territories of 3 children during the follow-up period; 2 of them received adjuvant EPS to cover the posterior cerebral artery territory later. The patients presenting with transient ischemic attacks, cerebral infarction, hemorrhage, or syncope had no new attacks. The patients presenting with headaches had partial-to-complete symptom relief. One of the 7 patients with seizures was still taking 1 type of antiepileptic drug, whereas the other 6 were symptom-free.
Postoperative Sequential Changes of TTP Scores with Angiographic Correlation
The intraclass correlation coefficients of preoperative and postoperative TTP scores were 0.95 (95% CI, 0.925–0.967) and 0.86 (95% CI, 0.789–0.909), respectively. Only the results of scorer 1 were further analyzed.
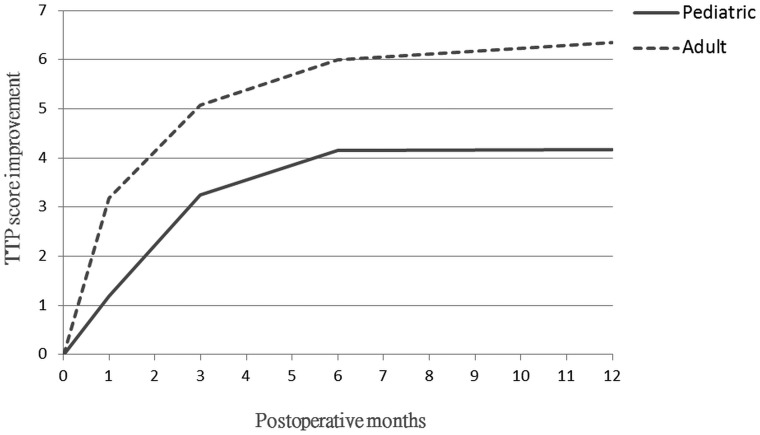
The mean preoperative TTP score for each hemisphere was 7.34 ± 3.90 and 4.88 ± 3.24 in pediatric and adult patients, respectively. The sequential changes in postoperative 1-, 3-, 6-, and 12-month TTP scores in all patients with available data were assessed. The mean pediatric hemisphere score improvement was 1.19 ± 1.64, 3.24 ± 2.36, 4.15 ± 3.55, and 4.17 ± 3.51 at the 1-, 3-, 6-, and 12-month follow-ups, respectively, compared with the preoperative score. Furthermore, the mean adult hemisphere score improvement was 3.18 ± 2.69, 5.08 ± 2.72, 6.03 ± 3.04, and 6.34 ± 2.93 at the 1-, 3-, 6-, and 12-month follow-ups, respectively (Fig 3). In both groups, the postoperative perfusion changes were more robust at 0–3 months than at 3–6 months of follow-up. The changes stabilized after 6 months. The difference between the 6- and 12-month scores was minor (mean difference, 0.08) and nonsignificant using the paired t test (P = .2). According to the color maps, the earliest perfusion changes occurred in the MCA territory around the craniotomy sites and grew larger on the follow-up maps (Fig 1). After unilateral revascularization, the contralateral cerebrum would also show perfusion improvement.
Fig 3.
Line chart revealing postoperative sequential changes of TTP score improvement. In both pediatric and adult groups, the score improvements are most robust at 0–3 months followed by 3–6 months and reach a plateau at the 6-month follow-up. Our adult group has more score improvement than the pediatric group, probably related to more severe preoperative disease status.
Most of the cerebral infarctions were small or in the white matter, which would not influence the TTP scores. Among the patients with cerebral infarction, only 7 of them (including 3 preoperatively, 3 immediately after operation, and 1 with disease progression) showed infarctions sizable enough to influence the TTP scores in the range of 1–3 points. These areas showed parenchymal defects on the follow-up studies, and the points were permanently lost.
Postoperative angiography was performed in 56 cerebral hemispheres (33 pediatric hemispheres and 23 adult hemispheres). We selected the 6-month TTP data after the final operation to evaluate the surgical outcome for both hemispheres. In these hemispheres, higher Matsushima grades (grade A being the highest) demonstrated higher TTP score improvement (Table 3). Matsushima grade A or B is usually considered adequate or satisfactory collateral formation; thus, the proportions of adult and pediatric patients with adequate results were similar (83% and 82%, respectively). The mean TTP score improvement in the 8 pediatric and 11 adult hemispheres without postoperative angiography was 4.13 ± 2.62 and 5.73 ± 2.69, respectively, which was close to that of Matsushima grade B in adult patients and between Matsushima grades A and B in pediatric patients, suggesting satisfactory results.
Table 3:
Postoperative Matsushima grading and TTP score improvement (6 months after the operation) indicating that TTP score improvement is correlated with Matsushima grading
| Pediatric |
Adult |
|||
|---|---|---|---|---|
| No. of Hemispheres (%) | Score Improvement (Mean) | No. of Hemispheres (%) | Score Improvement (Mean) | |
| Matsushima grade | ||||
| A | 17 (52) | 5.94 ± 3.72 | 10 (44) | 7.40 ± 2.37 |
| B | 10 (30) | 3.50 ± 2.83 | 9 (39) | 6.00 ± 2.96 |
| C | 6 (18) | 0.17 ± 1.17 | 4 (17) | 3.50 ± 4.73 |
| Unknowna | 8 | 4.13 ± 2.62 | 11 | 5.73 ± 2.69 |
The patients did not undergo postoperative catheter angiography.
Analyses of Postoperative TTP Score Improvement
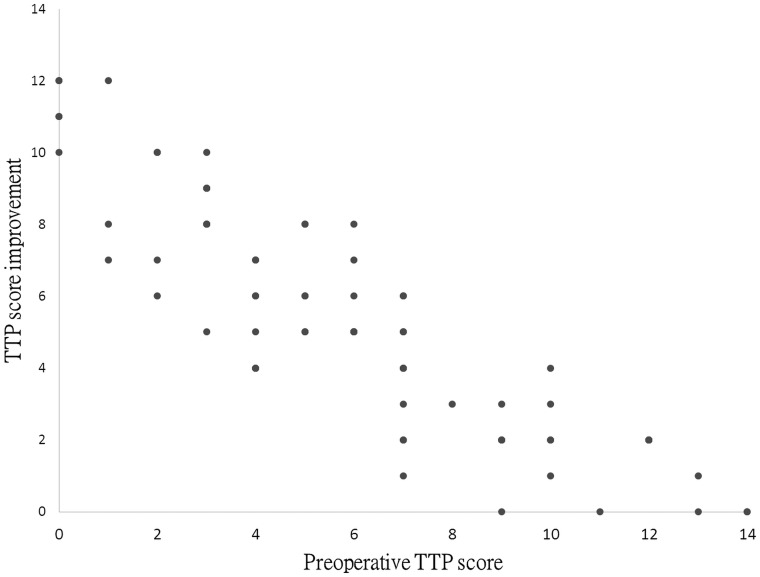
To compare the TTP score improvement across the hemispheres, linear mixed-model analysis was performed with random intercepts. To keep reasonable statistical power more than 0.8, we chose only 4 predictors in analyses. Age, male sex, preoperative TTP score, and additional EPS to the areas other than the temporal region were input as predictors. In univariable analyses, all 4 predictors were significant. After further controlling the correlation among these predictors, multivariable analyses were performed. The results indicated that only the preoperative TTP score was significant (coefficient = −2.93; P < .001). Regarding the point estimate of the coefficient, the absolute values of age, sex, and EPS decreased considerably, and that for age changed from a positive to a negative value. The detailed results are listed in Table 4. The results indicated that only the preoperative TTP score mattered when taking all predictors into account. Figure 4 illustrates the presumed linear relationship between the improvements in the preoperative and postoperative TTP scores.
Table 4:
Linear mixed regression model analyses of predictors for postoperative 6-month TTP score improvementa
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| Coefficient (SD) | P Valueb | Coefficient (SD) | P Valueb | |
| Age | 0.92 (0.40) | .03 | −0.28 (0.24) | .26 |
| Sex (male) | −2.47 (0.76) | .002 | −0.51 (0.44) | .26 |
| Preoperative TTP score | −3.03 (0.19) | <.001 | −2.93 (0.21) | <.001 |
| Additional EPSc | 3.03 (0.75) | <.001 | 0.58 (0.47) | .21 |
Linear mixed regression model with random intercepts and standardized age and preoperative TTP scores were used.
P values were obtained with the Wald test.
EPS to the hypoperfusion area at frontal or parietal regions in addition to temporal EDAS in the same procedure.
Fig 4.
Scatter diagram indicating that the preoperative TTP score is inversely correlated with the TTP score improvement after the operation.
Discussion
The standard evaluation before and after surgical revascularization for MMD has been dependent on conventional angiography, which is an invasive procedure. Although MRP studies have also been applied in the follow-up of MMD, no standardized scoring method has been widely accepted for long-term use. Similar to previous reports,19,20 our pediatric participants experienced more disease progression during the follow-up period, suggesting ongoing disease activity, while our adult patients presented with more severe disease and with more posterior circulation involvement, which also suggest a more advanced stage.21 The standardized TTP maps and scoring system, providing an understanding of the extent and severity of cerebral perfusion impairment, are suitable for long-term follow-up of subjects with MMD, particularly for the children in whom disease progression is expected and radiation risk is a particular concern.
Our study indicated that an improvement in cerebral perfusion after indirect revascularization can be seen as early as 1 month after the operation, and the postoperative 6-month TTP scores increased to approximately 11 points in each cerebral hemisphere. Multivariable analysis suggested that hemispheres with more severe perfusion impairment would benefit more from indirect revascularization, regardless of patient age. A higher level of vascular plasticity and the angiogenic potential are hallmarks of MMD compared with vascular narrowing of other etiologies.22,23 The ischemic brain may provoke revascularization if a vascularized graft is present nearby. Therefore, the hemispheres with poorer initial perfusion are supposed to improve more postoperatively if optimal revascularization coverage is provided. By contrast, the hemispheres with less perfusion impairment may not benefit much from revascularization surgery. Whether a preset EDAS or other revascularized flaps can provide collaterals if the cerebral perfusion deteriorates at a later time remains unknown. The optimal surgical timing should thus be carefully determined with the help of TTP maps and MRA.
Ishii et al24 reported that a preoperative MTT delay predicted the degree of postoperative revascularization and that no difference existed between adults and children in the degree of revascularization. The authors used ROIs to measure different cortical areas and obtain the MTT relative to the cerebellum. Ladner et al25 developed a complex scoring system to incorporate the prior infarcts, angiography, and cerebral reactivity to measure hemodynamic severity in MMD. We used the standardized TTP map, which is an easily obtainable method to evaluate perfusion independent of the deconvolution algorithms. This simple scoring system uses visual assessment rather than ROIs. The standardized TTP maps enable rapid detection of the abnormal perfusion areas. Although we used the PMA and OsiriX viewers, the generation of TTP maps and standardization of the color maps can be achieved in every MR imaging workstation for routine clinical use. By using standardized TTP maps and the scoring systems, semiquantifying perfusion changes during follow-up are feasible. Early detection of disease progression would be easier by comparing the interval changes on the standardized TTP maps and the MRA. Conventional angiography might be best reserved for preoperative evaluation and surgical planning.
Our study did not provide data comparing the effects of direct and indirect revascularization; however, the 6-month MRP and clinical outcomes indicated that indirect revascularization in the adult group seemed to be as safe and effective as in the pediatric group. For adult patients with MMD with sizable areas of cerebral perfusion impairment, indirect revascularization can serve as a safe and effective means to reconstitute cerebral perfusion. Whether direct revascularization or combined direct and indirect revascularization is a superior method for adult patients remains unclear. Our standardized TTP maps and scoring system may offer an effective imaging tool to evaluate revascularized areas and to quantitatively analyze the effects of different operation methods.
Our study had the following limitations: First, our sample size was small. Therefore, the number of predictors in regression analyses was limited because of concern of statistical power. However, it was a prospective study, and all procedures were performed by the same senior neurosurgeon. Our results provided an insight into how cerebral perfusion changes after indirect revascularization for MMD. Second, the mean follow-up duration was 2–3 years in our patients. A longer follow-up period is vital, particularly in growing children. Third, a stress test was not applied in our perfusion study because the intravenous form of acetazolamide was not available at our institution. Another consideration is the potential hazards of the stress test to the brain with impaired perfusion. Fourth, we had only 12 slices to cover the brain because of the limitation of our 1.5T scanner with TR = 1000 ms. However, we aligned our slices to the subcallosal line and placed a slice through the subcallosal line, and the gap and slice thickness of follow-up examinations also followed the preoperative ones; therefore, the differences in slice locations among examinations should have been minimized. Finally, the scoring system alone may not reflect the true perfusion condition. In this TTP scoring system, we could not further differentiate more pronounced perfusion impairment from modest perfusion impairment in a single region if the perfusion impairment area was larger than one-half of the region. However, we can simply compare the colors on the standardized maps to resolve the problem.
Conclusions
We developed a TTP scoring system that helped in quantitative evaluation of the preoperative severity and sequential changes in MRP of patients with MMD. The preoperative perfusion status was the only outcome predictor of indirect revascularization. The indirect revascularization has a favorable outcome in both pediatric and adult patients with MMD.
Acknowledgments
The authors acknowledge statistical assistance provided by the Department of Medical Research in the National Taiwan University Hospital.
ABBREVIATIONS:
- EDAS
encephaloduroarteriosynangiosis
- EPS
encephalopericraniosynangiosis
- MMD
Moyamoya disease
- MRP
MR perfusion
Footnotes
This work was supported by grants from Ministry of Science and Technology, Taiwan to Dr. Y.-F. Chen (MOST-103-2321-B-002-032) and Dr. M.-F. Kuo (MOST 105-2314-B-002-004-MY2 and 103-2321-B-002-033).
Paper previously presented in poster form at: XXI Symposium Neuroradiologicum, March 19–23, 2018; Taipei, Taiwan.
References
- 1. Chen PC, Yang SH, Chien KL, et al. Epidemiology of moyamoya disease in Taiwan: a nationwide population-based study. Stroke 2014;45:1258–63 10.1161/STROKEAHA.113.004160 [DOI] [PubMed] [Google Scholar]
- 2. Teo MK, Madhugiri VS, Steinberg GK. Editorial: direct versus indirect bypass for moyamoya disease—ongoing controversy. J Neurosurg 2017;126:1520–22 10.3171/2015.10.JNS152025 [DOI] [PubMed] [Google Scholar]
- 3. Macyszyn L, Attiah M, Ma TS, et al. Direct versus indirect revascularization procedures for moyamoya disease: a comparative effectiveness study. J Neurosurg 2017;126:1523–29 10.3171/2015.8.JNS15504 [DOI] [PubMed] [Google Scholar]
- 4. Uchino H, Kim JH, Fujima N, et al. Synergistic interactions between direct and indirect bypasses in combined procedures: the significance of indirect bypasses in moyamoya disease. Neurosurgery 2017;80:201–09 10.1227/NEU.0000000000001201 [DOI] [PubMed] [Google Scholar]
- 5. Dusick JR, Gonzalez NR, Martin NA. Clinical and angiographic outcomes from indirect revascularization surgery for moyamoya disease in adults and children: a review of 63 procedures. Neurosurgery 2011;68:34–43; discussion 43 10.1227/NEU.0b013e3181fc5ec2 [DOI] [PubMed] [Google Scholar]
- 6. Park SE, Kim JS, Park EK, et al. Direct versus indirect revascularization in the treatment of moyamoya disease. J Neurosurg 2018;129:480–89 10.3171/2017.5.JNS17353 [DOI] [PubMed] [Google Scholar]
- 7. Lin N, Aronson JP, Manjila S, et al. Treatment of moyamoya disease in the adult population with pial synangiosis. J Neurosurg 2014;120:612–17 10.3171/2013.11.JNS131027 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99 10.1001/archneur.1969.00480090076012 [DOI] [PubMed] [Google Scholar]
- 9. Matsushima Y, Inaba Y. Moyamoya disease in children and its surgical treatment: introduction of a new surgical procedure and its follow-up angiograms. Childs Brain 1984;11:155–70 [DOI] [PubMed] [Google Scholar]
- 10. Miller JH, Khonsary A, Raffel C. The scintigraphic appearance of childhood moyamoya disease on cerebral perfusion imaging. Pediatr Radiol 1996;26:833–38 10.1007/BF03178033 [DOI] [PubMed] [Google Scholar]
- 11. Lee SK, Kim DI, Jeong EK, et al. Postoperative evaluation of moyamoya disease with perfusion-weighted MR imaging: initial experience. AJNR Am J Neuroradiol 2003;24:741–47 [PMC free article] [PubMed] [Google Scholar]
- 12. Kang KH, Kim HS, Kim SY. Quantitative cerebrovascular reserve measured by acetazolamide-challenged dynamic CT perfusion in ischemic adult moyamoya disease: initial experience with angiographic correlation. AJNR Am J Neuroradiol 2008;29:1487–93 10.3174/ajnr.A1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Touho H, Karasawa J, Ohnishi H. Preoperative and postoperative evaluation of cerebral perfusion and vasodilatory capacity with 99mTc-HMPAO SPECT and acetazolamide in childhood moyamoya disease. Stroke 1996;27:282–89 10.1161/01.STR.27.2.282 [DOI] [PubMed] [Google Scholar]
- 14. Kuroda S, Kashiwazaki D, Hirata K, et al. Effects of surgical revascularization on cerebral oxygen metabolism in patients with moyamoya disease: an 15O-gas positron emission tomographic study. Stroke 2014;45:2717–21 10.1161/STROKEAHA.114.006009 [DOI] [PubMed] [Google Scholar]
- 15. Tanaka Y, Nariai T, Nagaoka T, et al. Quantitative evaluation of cerebral hemodynamics in patients with moyamoya disease by dynamic susceptibility contrast magnetic resonance imaging: comparison with positron emission tomography. J Cereb Blood Flow Metab 2006;26:291–300 10.1038/sj.jcbfm.9600187 [DOI] [PubMed] [Google Scholar]
- 16. Yun TJ, Cheon JE, Na DG, et al. Childhood moyamoya disease: quantitative evaluation of perfusion MR imaging—correlation with clinical outcome after revascularization surgery. Radiology 2009;251:216–23 10.1148/radiol.2511080654 [DOI] [PubMed] [Google Scholar]
- 17. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 18. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 2012;52:245–66 10.2176/nmc.52.245 [DOI] [PubMed] [Google Scholar]
- 19. Kim SK, Cho BK, Phi JH, et al. Pediatric moyamoya disease: an analysis of 410 consecutive cases. Ann Neurol 2010;68:92–101 10.1002/ana.21981 [DOI] [PubMed] [Google Scholar]
- 20. Ishii K, Isono M, Kobayashi H, et al. Temporal profile of angiographical stages of moyamoya disease: when does moyamoya disease progress? Neurol Res 2003;25:405–10 10.1179/016164103101201571 [DOI] [PubMed] [Google Scholar]
- 21. Lee JY, Kim SK, Phi JH, et al. Posterior cerebral artery insufficiency in pediatric moyamoya disease. J Korean Neurosurg Soc 2015;57:436–39 10.3340/jkns.2015.57.6.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acker G, Fekonja L, Vajkoczy P. Surgical management of moyamoya disease. Stroke 2018;49:476–82 10.1161/STROKEAHA.117.018563 [DOI] [PubMed] [Google Scholar]
- 23. Bedini G, Blecharz KG, Nava S, et al. Vasculogenic and angiogenic pathways in moyamoya disease. Curr Med Chem 2016;23:315–45 10.2174/092986732304160204181543 [DOI] [PubMed] [Google Scholar]
- 24. Ishii Y, Nariai T, Tanaka Y, et al. Practical clinical use of dynamic susceptibility contrast magnetic resonance imaging for the surgical treatment of moyamoya disease. Neurosurgery 2014;74:302–09 10.1227/NEU.0000000000000266 [DOI] [PubMed] [Google Scholar]
- 25. Ladner TR, Donahue MJ, Arteaga DF, et al. Prior Infarcts, Reactivity, and Angiography in Moyamoya Disease (PIRAMD): a scoring system for moyamoya severity based on multimodal hemodynamic imaging. J Neurosurg 2017;126:495–503 10.3171/2015.11.JNS15562 [DOI] [PMC free article] [PubMed] [Google Scholar]