Abstract
Acidic soils rapidly retain applied phosphorus fertilizers and consequently present low availability of this nutrient to plants. The use of phosphate-solubilizing microorganisms to help plant phosphorus (P) absorption is a promising sustainable strategy for managing P deficiencies in agricultural soils. Trichoderma strains have been one of the most studied filamentous fungi for improving the production and development of several crop species mainly due to their capability for symbiotic associations and their ability to control soil-borne plant diseases. Thus, this work sought to bioprospect Trichoderma strains from the Amazon rainforest capable of solubilizing/mineralizing soil phosphate and promoting soybean growth. Soybean plants inoculated with selected Trichoderma strains were cultivated in soil under greenhouse conditions and under a gradient of rock phosphate and triple superphosphate. As a result, 19.5% of the isolated Trichoderma strains were able to solubilize phosphate. In addition, those strains produced different organic acids during the solubilization process. Trichoderma spp. strains showed positive responses in the promotion of soybean growth—from 2.1% to 41.1%—as well as in the efficiency of P uptake-up to 141%. These results reveal the potential of Trichoderma spp. from the Amazon biome as promising biofertilizer agents.
Subject terms: Applied microbiology, Soil microbiology
Introduction
The high demand for fertilizers used in Brazilian agriculture is a result of the growing population, which necessitates an increase in food production1. Brazil is the second-largest supplier of food and agricultural products and expected to be the leading producer of food to meet global demand in the near future2. Thus, applications of fertilizer are a routine activity in agricultural production as an attempt to promote crop growth to increase productivity. The requirement of fertilizers in the field results in the accumulation of those inputs in soils and water and, therefore, environmental pollution, causing problems to human and animal health1,3. In the future, Brazilian agriculture has to identify alternatives to reduce its dependence on chemical fertilizers while at the same time functioning in a lucrative and more sustainable way4.
A range of nutrients is important for plant growth5, but the ones that limit agricultural production the most are nitrogen and phosphorus, which are important in the initial development of the plant6,7. Nitrogen fertilization in Brazil has decreased significantly with use of symbiotic associations with nitrogen-fixing bacteria8,9. However, Brazilian agriculture continues to depend on chemical phosphate fertilization4. The role of P in the plant is associated with three essential biochemical processes: energy production, respiration, and photosynthesis. P is also involved in enzymatic processes and is a component of nucleic acids and cell membranes10–13. Phosphorus is generally found in the lowest concentration in the soil, 0.01%, compared to 0.14% of nitrogen, mainly in tropical and subtropical regions14,15. Although there is a high amount of total phosphorus (P) in the soil, its low availability to plants is one of the main obstacles to agricultural productivity1. The amount of P absorbed by the crops varies from 10 to 40% of the total phosphate fertilizer applied to the soil16. This phenomenon is due to a high degree of reactivity that occurs between phosphorus and soil constituents, causing the fixation of phosphorus or its precipitation with soil particles, making it unavailable for plant absorption17.
In general, Brazilian soils present low phosphorus contents (0.03 mg available P.kg−1 of soil), requiring high applications of phosphate fertilizers to meet cultural demands18. The efficiency of the application of phosphate fertilizers to the soil varies from 10–25%, and the phosphorus accessible to microorganisms and plants provided by these fertilizers is very low19. Brazil is the fourth largest country in terms of fertilizer consumption. According to the National Association for the Diffusion of Fertilizers, imports of phosphate fertilizers and domestic production of phosphate inputs in 2017 increased by 56.2% and 33.7%, respectively, the largest increases in relation to nitrogen and potash fertilizers. The high import rate of these fertilizers strongly contributes to a negative deficit in the Brazilian trade balance.20. In the last twenty years, the application of phosphate fertilizers has surpassed the expansion of arable land because of the rapid fixation of phosphorus in the soil. Maize, soybean, and sugarcane are the crops that receive most of these phosphate fertilizers4. The fertility of tropical soils is generally dependent on a thin layer of organic matter associated with litter. Thus, soils are highly weathered and typically acidic, and the availability of nutrients depends primarily on nutrient cycling21,22. The Amazon rainforest is among the forests with the largest reserves of biodiversity23. The biodiversity of the Amazon rainforest is attributed to the high variability of niches within it, from dense forest to savannah, making Brazil among those countries with a large macro- and microbiological biodiversity24. A large number of micro-ecosystems, soil types and climate conditions are favorable for fungal soil communities, providing a constant degradation of forest biomass25,26, which makes the Amazon rainforest a substantial source of bioprospection from fungal-originating products.
The application of microorganisms as biofertilizers is a promising approach to assist in agricultural production; these applications have contributed to the growth of several crop species1,27,28, increase plant biomass and total P contents29 and participate in the cycling of P without affecting the environment. Agricultural and pasture soils are composed of larger communities of these microorganisms involved in the availability of P10,30. They are key to providing phosphorus that is retained in the soil to plants1 via the processes of mineralization and solubilization31. Sharma et al. (2013) reported the ability of fungi to occupy larger spaces and ranges within the soil than bacteria and to produce a range of organic acids that play a trivial role in the solubilization of inorganic phosphate. Within this context, we believe that the Amazon rainforest may be an excellent biome for the bioprospection of fungal strains capable of solubilizing/mineralizing insoluble phosphorus and making a portion of this nutrient pool available to plants. In this way, the Amazon rainforest can improve the growth and productivity of a wide variety of crop species.
Fungi of the genus Trichoderma pertain to the phylum Ascomycota, subdivision Pezizomycotina, class Sordariomycetes, order Hypocreales and family Hipocreaceae32,33. They are rapidly growing fungi in the culture medium, initially presenting colonies with the presence of white mycelium, which with development becomes cottony and compact with green tufts. They are cosmopolitan, found most in soils and have an important ecological function, because they participate in the decomposition and mineralization of plant waste, contributing to the availability of nutrients for the plants, interfering directly and indirectly in their growth34,35. Trichoderma is commonly studied for the control of soil phytopathogens and as a biofertilizer. Trichoderma have great prominence in studies28,36, due to their predominance in the rhizosphere of different plant species, role in the control of different phytopathogens, assistance in the promotion of plant growth via different mechanisms. Furthermore, easy isolation, rapid growth and ability to grow in different substrates, as well as their ability to produce an infinite amount of metabolites such as antibiotics and auxins37. Recent studies have reported the occurrence of Trichoderma in soils of the Amazon forest that have cellulolytic capabilities38,39, are endophytes of native tree species40 are producers of biosurfactants41 and are biological control agents42,43. Thus, our work sought to bioprospect Trichoderma strains from the Amazon rainforest with the ability to solubilize/mineralize phosphate and the potential to promote the growth of soybean plants, as the environment of the Amazon rainforest is highly dependent on microorganisms for fast nutrient cycling. With the application of Trichoderma strains in the soil, we sought to optimize the use of different sources of phosphates, as well as the quantity of these sources applied, to contribute to more sustainable agriculture and greater efficiency in the use of phosphorus sources.
Results
Selective isolation and selection of Trichoderma spp. capable of solubilizing phosphate in vitro
Phosphorus is the most important macronutrients in crop development and growth, and phosphate-solubilizing fungi play an important role in enhancing phosphorus availability for plants. A total of 251 isolates were obtained from Amazonian soils using the selective medium TSM for Trichoderma. The numbers of isolates per collection point are shown in Table 1. The isolates were preserved at the Collection of Microorganisms of Environmental and Agricultural Importance (CMAA) of EMBRAPA Environment, Jaguariúna, São Paulo, Brazil.
Table 1.
Collection data.
| Collection Point | Collection Time | Coordinates | Soil Temperature (°C) | Type of Soil | Number of Trichoderma isolates | |
|---|---|---|---|---|---|---|
| S | W | |||||
| 1 | April/2015 | 02°50′58,8″ | 59°24′52,2″ | 24,7 | clay | 39 |
| 2 | April/2015 | 02°54′41,7″ | 59°02′26,6″ | 28 | sandy | 7 |
| 3 | April/2015 | 03°00′45,4″ | 58°51′12,6″ | 25,3 | sandy | 10 |
| 4 | April/2015 | 03°07′33,2″ | 60°00′22,9″ | 24,1 | sandy | 18 |
| 5 | April/2015 | 03°12′35,8″ | 60°40′43,3″ | 25,9 | sandy | 17 |
| 6 | April/2015 | 02°59′21,6″ | 60°53′36,0″ | 24 | sandy | 29 |
| 7 | April/2015 | 02°51′36,5″ | 60°58′10,6″ | 26,2 | sandy | 18 |
| 8 | April/2015 | 02°17′49,9″ | 60°02′37,7″ | 24,3 | clay | 20 |
| 9 | April/2015 | 01°49′46,1″ | 60°07′55,0″ | 25,2 | clay | 24 |
| 10 | April/2015 | 01°28′39,2″ | 60°15′10,0″ | 27,5 | sandy | 20 |
| 11 | April/2015 | 01°28′52,4″ | 60°15′18,9″ | 25,1 | clay | 26 |
| 12 | April/2015 | 01°56′52,5″ | 60°02′31,8″ | 26 | clay | 23 |
| Total number of isolates | 251 | |||||
From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
To select the phosphate-solubilizing fungi, the clear zone was observed around the colonies of Trichoderma spp. isolates on solid NBRIP media. This effect occurs because, during their growth, the microorganisms use the phosphate present in the culture media. Of all Trichoderma spp. isolates screened, 49 showed potential for solubilizing phosphorus, with halos greater than 10 millimeters (Table 2). Of these isolates, eight with halos greater than 50 mm were selected for testing in NBRIP liquid media. The eight isolates presented a halo around the colony on PSM media (halos ranged from 5.3 to 10.7 millimeters in diameter), indicating the ability to mineralize organic phosphorus in the form of phytate.
Table 2.
Phosphate solubilization and mineralization in solid NBRIP and PSM culture medium and organic acid production by Trichoderma spp.
| Treatments | Solubilization/Mineralization | Average | Standard deviation | Organic Acid | ||
|---|---|---|---|---|---|---|
| Halo size (mm) | ||||||
| 1 | 2 | 3 | ||||
| AMS 34.39 | 53 | 53 | 54 | 53.3 | 0.58 | Latic Acid, Fumaric Acid |
| 10 | 10 | 12 | 10.7 | 1.15 | ||
| AMS 29.10 | 57 | 54 | 50 | 53.7 | 3.51 | Ascorbic Acid, Gluconic Acid |
| 11 | 9 | 10 | 10 | 1.0 | ||
| AMS 1.43 | 56 | 50 | 58 | 54.7 | 4.16 | Latic Acid |
| 6 | 5 | 5 | 5.3 | 0.58 | ||
| AMS 2.18b | 65 | 57 | 49 | 57 | 8.0 | D-Malic Acid |
| 7 | 15 | 9 | 10.3 | 4.2 | ||
| AMS 31.15 | 53 | 60 | 60 | 57.7 | 4.0 | D-Isocitric Acid, Phytic Acid, Citric Acid |
| 12 | 9 | 10 | 10.3 | 1.5 | ||
| AMS 26.10 | 53 | 60 | 60 | 57.7 | 4.0 | D-Malic Acid |
| 11 | 10 | 9 | 10 | 1 | ||
| AMS 2.18a | 55 | 62 | 64 | 60.3 | 4.7 | Ascorbic Acid, Gluconic Acid |
| 8 | 6 | 6 | 6.7 | 1.1 | ||
From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
The isolates AMS 31,15 (90,3%), AMS 1.43 (85.7%), AMS 2.18a (83.0%) and AMS 34.39 (82.6%) were also selected as fungi with potential for solubilization (Fig. 1). Of the four isolates of Trichoderma spp. with the best results, two (AMS 34.39 and AMS 31.15) did not inhibit the germination of soybeans, as determined via a culture used in an experiment in the greenhouse. For this reason, these two isolates were selected for bioassays in the greenhouse with soybean plants.
Figure 1.
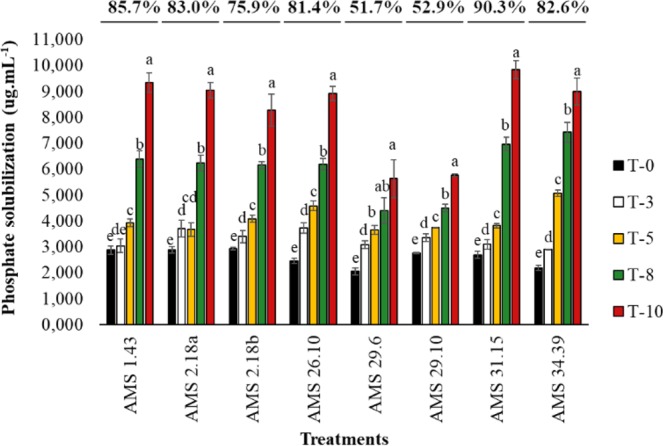
Values in percentage after the ten-day incubation period and µg.mL−1 of calcium phosphate solubilization (10 g.L−1) in liquid NBRIP medium by Trichoderma spp. during the ten-day incubation period. T-0 - first day of incubation, T-3 - third day of incubation, T-5 - fifth day of incubation, T-8 - eighth day of incubation, T-10 - tenth day of incubation. Different letters are significantly different according to Tukey test (P < 0.05). From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
The eight selected isolates produced organic acids during the solubilization process. They produced lactic acid, fumaric acid, ascorbic acid, gluconic acid, d-malic acid, d-isocitric acid, citric acid, and phytic acid, as shown in Table 2.
Impact of Trichoderma spp. and phosphate fertilizers on the development of soybean plant
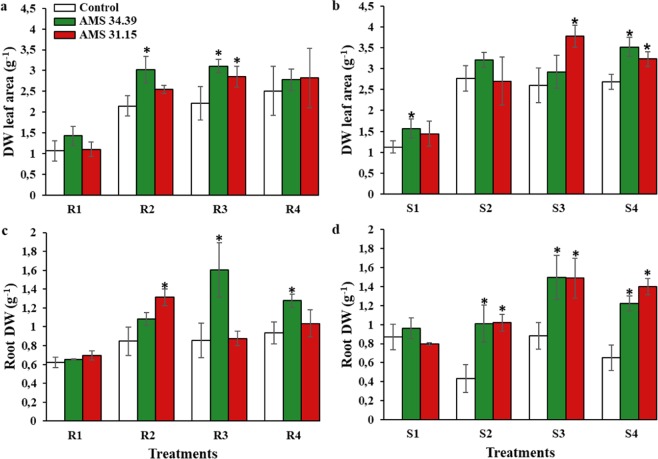
In the absence of a source of phosphorus applied to the soil (Level 1), that is, without the application of the sources of rock phosphate and super triple phosphate, the plants responded positively to the increase in aerial biomass in the three treatments applied (control, AMS 34.39 and AMS 31.15) (Fig. 2a,b). However, the combination of Trichoderma and phosphorus sources increased significantly at level 3 (P < 0.05) the biomass of soybean plants in rock phosphate (Fig. 2a). This difference was also observed in the super triple phosphate source at levels 3 and 4 about the control (Fig. 2b). In both sources of phosphate, the AMS 34.39 isolate showed a significant difference when compared to the control at the same level. The DW of the leaf area between the phosphorus application levels varied from 10.5–40.7% (AMS 34.39) and 2.1–41.1% (AMS 31.15) compared that of to the control treatment (without Trichoderma) for the Bayóvar rock phosphate and super triple phosphate sources. For roots, the DW varied 4.9–134.9% for AMS 34.39 isolate and from 0.9–137.2% for AMS 31.15. The efficiency in the absorption of phosphorus by the plants inoculated with the Trichoderma strains was also evaluated. Compared to the control (control, level 1) values, the treatment values ranged from 111.2–156.1% (AMS 34.39) and from 81.7–140.6% (AMS 31.15) (Table 3).
Figure 2.
Average dry matter mass of the leaf area and root of soybeans grown in soils with different levels of P (1, 2, 3 and 4) and sources of phosphorus (R- Bayóvar Rock Phosphate and S- Triple Super Phosphate) in the presence of Trichoderma (green and red) and Control (white). (a) DW leaf area - Bayóvar Rock Phosphate, (b) DW leaf area - Triple Super Phosphate, (c) Root DW - Bayóvar Rock Phosphate and (d) Root DW – Triple Super Phosphate. Different letters are significantly different according to Scott Knott test (P < 0.05). From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
Table 3.
Overview of the experimental design and percentage of P absorption efficiency and biomass improvement.
| Treatments | P source | Level P | Efficiency P (%) | Biomass improvement (%) | |
|---|---|---|---|---|---|
| Leaf | Root | ||||
| Control | Rock phosphate | R1 | ___ | ___ | ___ |
| AMS 34.39 | Rock phosphate | R1 | 120.5 | 33.8 | 4.9 |
| AMS 31.15 | Rock phosphate | R1 | 81.7 | 3.6 | 11.8 |
| Control | Rock phosphate | R2 | ___ | ___ | ___ |
| AMS 34.39 | Rock phosphate | R2 | 132.8 | 30.8 | 27.5 |
| AMS 31.15 | Rock phosphate | R2 | 111.5 | 10.5 | 3.5 |
| Control | Rock phosphate | R3 | ___ | ___ | ___ |
| AMS 34.39 | Rock phosphate | R3 | 156.1 | 40.7 | 87.5 |
| AMS 31.15 | Rock phosphate | R3 | 140.6 | 34.8 | 53.3 |
| Control | Rock phosphate | R4 | ___ | ___ | ___ |
| AMS 34.39 | Rock phosphate | R4 | 111.2 | 10.7 | 36.8 |
| AMS 31.15 | Rock phosphate | R4 | 90.7 | 12.5 | 11.1 |
| Control | Triple Super Phosphate | S1 | ___ | ___ | ___ |
| AMS 34.39 | Triple Super Phosphate | S1 | 140.9 | 39.6 | 10.7 |
| AMS 31.15 | Triple Super Phosphate | S1 | 121.8 | 28.7 | 0.9 |
| Control | Triple Super Phosphate | S2 | ___ | ___ | ___ |
| AMS 34.39 | Triple Super Phosphate | S2 | 121.5 | 10.5 | 134.9 |
| AMS 31.15 | Triple Super Phosphate | S2 | 87.5 | 2.1 | 137.2 |
| Control | Triple Super Phosphate | S3 | ___ | ___ | ___ |
| AMS 34.39 | Triple Super Phosphate | S3 | 135.1 | 23.1 | 130.2 |
| AMS 31.15 | Triple Super Phosphate | S3 | 127.0 | 41.1 | 129.2 |
| Control | Triple Super Phosphate | S4 | ___ | ___ | ___ |
| AMS 34.39 | Triple Super Phosphate | S4 | 127.8 | 22.9 | 39.0 |
| AMS 31.15 | Triple Super Phosphate | S4 | 119.4 | 18.9 | 59.1 |
From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
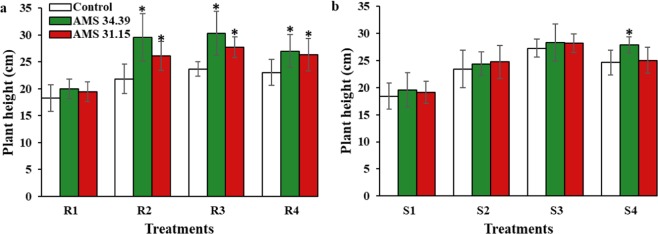
Significant differences (P < 0.05) were observed in the height of soybean plants when Trichoderma isolates were inoculated (Fig. 3a,b). This difference was most evident for the two isolates when P was applied (at levels 2, 3 and 4). It was also observed that the increase in plant height when inoculated with Trichoderma spp. compared with that of the controls was better with the source of Bayóvar rock phosphate.
Figure 3.
Average height of soybeans grown in soils with different levels of P (1, 2, 3 and 4) and sources of phosphorus (R- Phosphate of Bayóvar Rock and S- Triple Super Phosphate) in the presence of Trichoderma (green and red) and Control (white). (a) Bayóvar Rock Phosphate and (b) Triple Super Phosphate. Different letters are significantly different according to Scott Knott test (P < 0.05). From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
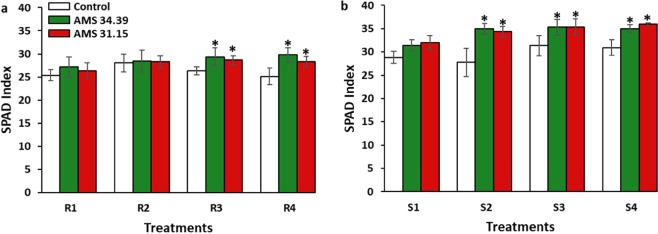
The chlorophyll indices presented significant differences (P < 0.05) in response to the level-3 and level-4 P for the two phosphate sources and the two Trichoderma isolates. For the super triple phosphate source, the effect of the application of the Trichoderma strains and the increase in the phosphorus level was more promising (Fig. 4a,b).
Figure 4.
Average chlorophyll index of soybeans grown in soils with different levels of P (1, 2, 3 and 4) and sources of phosphorus (R- Bayóvar Rock Phosphate and S- Triple Super Phosphate) in the presence of Trichoderma (green and red) and Control (white). (a) Bayóvar Rock Phosphate and (b) Triple Super Phosphate. Different letters are significantly different according to Scott Knott test (P < 0.05). From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
Enzymatic activities in the soil
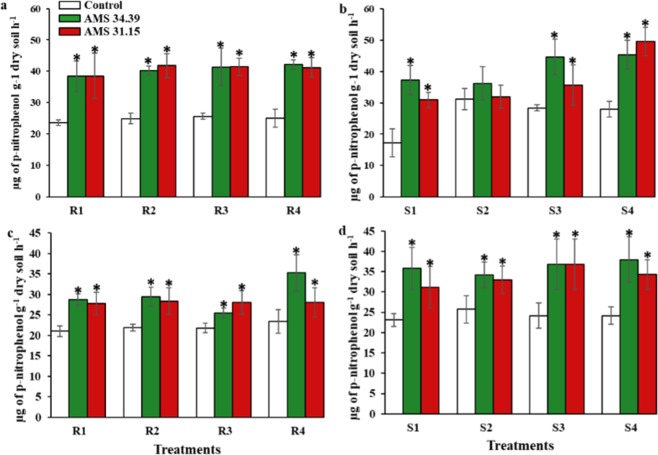
The activity of acid and alkaline phosphatases was similar between the isolates when the two sources of phosphorus were applied (Fig. 5a–d). In general, for the two phosphate sources studied, the AMS 34.39 and AMS 31.15 isolates showed significantly higher enzymatic activity (P < 0.05) than the controls at all levels of P applied to the soil.
Figure 5.
Average activity of acid and alkaline phosphatase in soils with different levels of P (1, 2, 3 and 4) and sources of phosphorus (R- Bayóvar Rock Phosphate and S- Triple Super Phosphate) in the presence of Trichoderma (green and red) and Control (white). (a) Acid Phosphatase - Bayóvar Rock Phosphate, (b) Acid Phosphatase – Triple Super Phosphate, (c) Alkaline Phosphatase - Bayóvar Rock Phosphate and (d) Alkaline Phosphatase – Triple Super Phosphate. Different letters are significantly different according to Scott Knott test (P < 0.05). From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
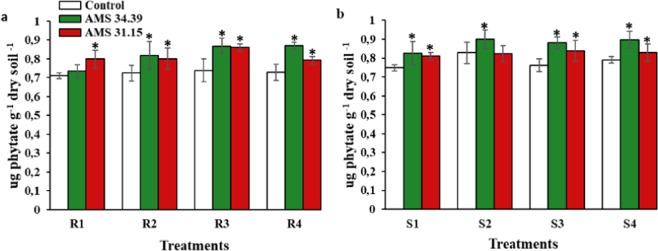
There was a significant difference between the treatments with the presence of Trichoderma and those without inoculation with the fungus for activity phytase. The AMS 34.39 and AMS 31.15 isolates showed increases of up to 17% and 16%, respectively, with the source of Bayóvar rock phosphate. On the other hand, the use of triple superphosphate showed increases of up to 15% and 10% for the isolates AMS 34.39 and AMS 31.15, respectively (Fig. 6a,b).
Figure 6.
Average enzyme activity of phytase in soils with different levels of P (1, 2, 3 and 4) and sources of phosphorus (R- Bayóvar Rock Phosphate and S- Triple Super Phosphate) in the presence of Trichoderma (green and red) and Control (white). (a) Bayóvar Rock Phosphate and (b) Triple Super Phosphate. Different letters are significantly different according to Scott Knott test (P < 0.05). From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
The combination of Trichoderma with a phosphate fertilizer can be much more advantageous for the plant compared with their separate use in the field, that is, only Trichoderma applied without a source of phosphorus or a source of phosphorus without applications of Trichoderma.
Discussion
In this study, Trichoderma isolated from soils of the Amazon rainforest demonstrated the potential for phosphate solubilization and increased soybean plant growth, highlighting the importance of the Amazon biome as a source of novel microbial stains with biotechnological importance. The solubilization process is caused by the release of organic acids and various enzymes, phosphatases, and compounds produced by microorganisms44. All this organic acid was already described as produced by Trichoderma strains45 (Table 2). Organic acids have great importance in the availability of phosphorus for the plant because they are capable of converting the phosphate present in the soil into di- or monobasic phosphates, which are readily available for absorption.
The fungus, applied in conjunction with a phosphorus source, promoted soybean plant growth (Fig. 3). The two phosphorus sources evaluated in this study showed higher positive effects when combined with the Trichoderma isolates than when applied alone. These effects were also P level dependent. Treatments involving different Trichoderma strains with beneficial attributes, including the promotion of plant growth and the biocontrol of phytopathogens, should be considered in the development of formulations. The positive effect of Trichoderma in the presence of a phosphorus source has also been reported by other authors45–47. Phosphorus that is readily available to the plant is a phosphate anion, a poorly mobile element in the soil and plants compared to other macronutrients. Thus, in addition to transforming organic phosphate into inorganic phosphate, Trichoderma helps to increase the root system, contributing to a greater region of nutrient absorption by the plant48. Few reported on the mechanisms of plant-Trichoderma interaction in promoting growth. Possibly one of the mechanisms involved in root development is due to acidification of the site with the presence of Trichoderma. This process results in the early development of the roots, which after the first days of development occurs an inhibition of the primary root and consequently the development of secondary roots37,49, as a mechanism to escape the acidification of the medium. According to Cornejo, Trichoderma enhances the lateral roots instead of the formation of new roots. Many authors have reported that during the process of P solubilization, the pH of the medium becomes acidified, probably due to the production of organic acids30,50–52. Thus, a correlation between the decrease in pH and the increase in P solubilization influences the biomass increase of the lateral roots12 and consequently increases the surface of P absorption by plants. Tandon (2019) demonstrated that by alkalinizing the medium in the phosphorus solubilization process, mycelial production and phosphatase activity by Trichoderma decreased significantly, which contributes to the importance of pH in the phosphorus solubilization process53. Combined with another mechanism that can be important in the formation of the root system is the production of metabolites, such as auxins and ethylene, produced by a range of Trichoderma species49,54.
The rock phosphate, being an insoluble phosphate, induces a higher secretion of phosphatases, for example, which facilitates the release of phosphorus to the plant promoting growth55. The mechanisms of P solubilization differ not only between fungal isolates but also between the phosphorus sources applied. Triple superphosphate has a higher content of soluble P available to the plant than does rock phosphate, considering that much of it is adsorbed to soil colloids. The microbial activity when rock phosphate is applied is higher because a greater amount of phosphate needs to be mineralized. The results obtained in this study showed the phosphate solubilization potential of two strains of Trichoderma spp. It is important to emphasize the use of rock phosphate, which has a relatively slow release of phosphorus in the soil, in addition to being a cheaper source because it requires a relatively low amount of manufacturing56. One of the major problems with the application of rock phosphate is that because it is slowly released, crops tend to have low yields in the initial few years. With the combined application of Trichoderma as presented in this paper, the response of the plants was positive (Fig. 2, Table 3). This joint application presents great importance for agriculture because there is relatively little expenditure with the use of rock phosphate and because the permanence of rock phosphate in the soil is greater than that of triple superphosphate, which is readily used; finally, with the Trichoderma, production can be relatively high.
In this work, The performance of Trichoderma spp. isolates were better presented in the application of phosphorus at level 3, especially with the AMS strain 34.39. Thus, the application of phosphorus could be in a smaller amount and with better efficiency when using together a Trichoderma strain (Fig. 2). For example, the phosphate level 3 applied represents the average productivity of the soybean crop. When applying AMS 34.39 isolate at level 3, we observed increases of 40.7% and 23.1% in response to the sources of Bayóvar and super triple rock phosphate, respectively (Table 3). When comparing the biomass values for the same strain of Trichoderma (AMS 34.39) combined with phosphorus at level 4, which is equivalent to the high productivity of the crop, it showed increases of 10.7% and 22.9% for the same sources of phosphorus applied. Thus, when applying 70 kg ha−1 of phosphate (level 3) the result was better for the biomass of soybean plants than the application equivalent to 90 kg ha-1 (level 4).
The low concentration of phosphorus in the soil reflects a decrease in ATP and NADPH production and the expression of genes related to photosynthesis57. Thus, these decreases are reflected in the chlorophyll index because it is an indication of photosynthetic pigments58. Therefore, the application of a phosphate near the Trichoderma may have reflected in the production of ATP in the plant, as well as in the expression of genes associated with photosynthesis, responding to the increase in chlorophyll in the results obtained (Fig. 4). Triple superphosphate, a readily available source in the soil, presented the most promising result because the analysis was performed twenty days after the planting of the crop, a result that was already expected. Some authors have demonstrated the increase on chlorophyll level due the presence of Trichoderma on different cultures as cucumber, wheat, soybean and lettuce plants59–62.
Some factors are involved in the process of phosphatase production by Trichoderma, such as the presence of an inorganic phosphate is essential for a better secretion of phosphatases, and it has been reported that the nature of the phosphate source linked to the solubilization process also interferes in the activity52,55,63,64. One of the mechanisms of action of Trichoderma for nutrient supplementation of plants is via the production of phosphatase enzymes. Some authors have already described the activity of this fungus in terms of its production of these enzymes47,48,52,65. The activity of phosphatases is reported mainly at sites where there is an absence of inorganic phosphorus52. In a study by Naik et al. (2013), acid phosphatase activity was higher for Trichoderma than for the other two fungi studied: Aspergillus and Penicillium12. The genus Trichoderma has been reported for its high phytase activity, which releases available phosphorus in the soil30,46,66. The results obtained in this study corroborate those found by those authors (Fig. 6a,b). The high association with the solid phase of the soil makes the phosphorus bound to phytate available in low quantities, limiting its absorption by plants67. Thus, phosphate fertilizers constitute the most soil-applied fertilizers to achieve good productivity. Many factors can interfere with the efficiency of phosphate-solubilizing microorganisms, such as the preparation of the inoculant, the form of application to the soil and the place where it is applied51. In addition, Garcia Lopes (2017) demonstrated that the type of soil may be related to the activity of microorganisms68. The concentration of P applied to the different soil was not affected, but its efficiency was affected by the physical and chemical properties of the soil69,70.
The efficiency of microorganisms that assist in the availability of P in the soil is correlated with their ability both to promote plant growth in other ways and to control phytopathogens that are present in the soil. Biological control agents with resources to make nutrients available to plants are increasingly being targeted by studies66,68. In this context, the genus Trichoderma comprises fungi of great importance in agriculture; these fungi are known as disease control agents for various pathogens and act as growth promoters of various crop species28,42,71,72.
Conclusions
In this study, Trichoderma isolated from soils of the Amazon rainforest demonstrated the potential for phosphate solubilization and increased soybean plant growth, highlighting the importance of the Amazon biome as a source of novel microbial stains with biotechnological importance. The fungus, applied in conjunction with a phosphorus source, promoted soybean plant growth. The two phosphorus sources evaluated in this study showed higher positive effects when combined with the Trichoderma isolates than when applied alone. These effects were also P level dependent. Treatments involving different Trichoderma strains with beneficial attributes, including the promotion of plant growth and the biocontrol of phytopathogens, should be considered in the development of formulations.
Materials and Methods
Collection sites and isolation of Trichoderma
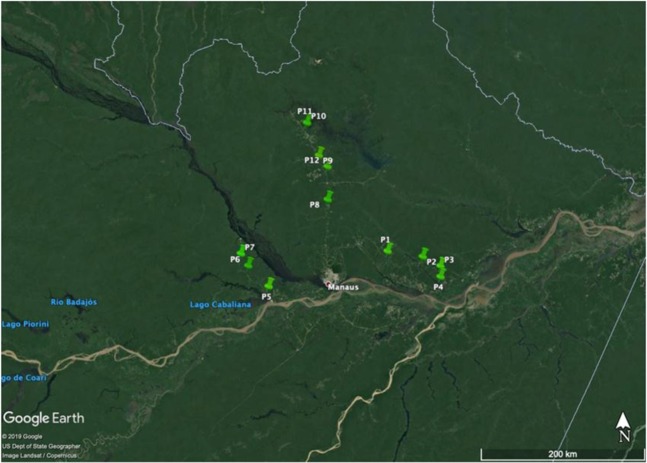
The soil collections were carried out in the State of Amazonas, Brazil, from the city of Manaus, extending to the cities of Itacoatiara, Novo Airão, and Presidente Figueiredo. In total, there were twelve collection points, with a distance between the points from 50 to 60 kilometers, containing three sub-samples per point, collected from 0–15 cm depth. The data of the characteristics of each point are shown in Fig. 7 and Table 1.
Figure 7.
Places for collecting soil samples from the 12 points in the State of Amazonas. From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
For the isolation of Trichoderma spp., the selective medium TSM for Trichoderma73 was used. The soil suspension (1 gr. of soil in 9 mL of sterile saline) was serially diluted and appropriated dilutions were spread plated on TSM medium. The cultures were incubated at 28 °C ± 2 °C for seven days, and after this period, typical Trichoderma colonies were purified and selected for further studies. The identification of isolates was performed according to morphological characteristics of the genus Trichoderma, by means of colony coloration, characteristics of spores and hyphae.
Screening of efficient phosphate-solubilizing Trichoderma spp
We evaluated the potential of 251 Trichoderma isolates capable of solubilize and mineralize P in vitro. The isolates were initially grown in potato dextrose agar (PDA) and, later, in solid NBRIP medium (National Botanical Research Institute’s Phosphate) containing 10 g of glucose; 5 g Ca5 (OH) (PO4)3; 5 g MgCl2 6H2O; 0.25 g MgSO47H2O; 0.2 g KCl; 0.1 g (NH4)2 SO4; 15 g agar and pH 7.0 in 1000 mL distilled water74. The plates were incubated at 28 °C ± 2 °C until the presence of a clear hydrolysis halo around the colonies, confirming the ability of the fungus to solubilize P75. Trichoderma spp. isolates with the highest solubilization halos were evaluated for the quantification of solubilized P in liquid NBRIP medium. The isolates were grown in PDA medium at 28 °C ± 2 °C for seven days. After this period, three 8.0 mm diameter discs were removed and transferred to a 50 mL Erlenmeyer, containing NBRIP medium (glucose, 10 grams (g); MgCl2. 6H2O, 5 g; MgSO4.7H2O, 0.25 g; KCl, 0.2 g; (NH4)2SO4, 0.1 g). In the media, 50 mL of K2HPO4 (10%) and 100 mL of CaCl2 (10%) were added to form an insoluble calcium phosphate precipitate (CaHPO4), incubated at 27 ± 2 °C in an orbital shaker at 150 rpm for ten days. The amount of phosphate in the medium before inoculation of the Trichoderma strains was approximately 2 µg. mL−1 calcium phosphate. Readings were taken at 0, 2, 4, 6, 8 and 10 days. Aliquots of 1 mL were removed and centrifuged at 10,000 g for 5 min to determine the concentration of soluble phosphorus according to the colorimetric method of Murphy & Riley76. To evaluate the mineralization potential of selected isolates for liquid NBRIP it was applied a Phytate Specific Media (PSM), containing 15 g C6H12O6; 5 g (NH4)2SO4, 0.1 g NaCl; 0.5 g KCl; 0.01 g FeSO4.7H2O; 0.01 g de MgSO47H2O; 0.01 g MnSO4; 5 g calcium phytate and 15 g agar77. The plates were incubated at 28 °C ± 2 °C until the presence of a clear hydrolysis halo around the colonies, confirming the ability of the fungus to mineralise P.
The potential for organic acid production was evaluated in high-performance liquid chromatography (HPLC). Aliquots of the samples with 10 days of incubation were collected and centrifuged at 10,000 g for 5 min and filtered in Millipore® 0.2 µm membrane. The extract was applied to a Bio-Rad aminex HPX-87H column, with 10.8% acetonitrile mobile phase at 0.0035 M H2SO4, and a constant outflow of 0.5 ml min−1, 35 °C, UV (210 nm) for 35 minutes.
Experimental design and bioassay
Soybean plants, variety NA 5909 RG, Brazil, were grown in three-liter pots using soil from the EMBRAPA Environment experimental area, Jaguariúna, São Paulo, Brazil. In the bioassay, a factorial model was applied, including the following factors: two sources of P (Bayóvar rock phosphate and Triple superphosphate), four levels of phosphates (0, 50, 70 and 90 kg ha−1), and the application of two Trichoderma sp. (AMS 34.39 and AMS 31.15). The control treatment consisted of all levels of phosphates in the two sources without the presence of Trichoderma. Twenty-four treatments were applied to the bioassay with five repetitions, counting 120 pots. The two Trichoderma isolates used in the bioassay were selected in the in vitro test in liquid medium and by a soybean germination bioassay, in order to evaluate if the isolates did not inhibit the germination of the culture used. The soil used in the experiment is characterized by being deficient in P and acid pH, as shown in Table 4.
Table 4.
Soil chemical analysis.
| pH (CaCl2) | M.O (g.dm−3) | Presina (mg.dm−3) | K ---------- | Ca ---------- | Mg mmolc.dm−3 | H + Al ---------- | SB ---------- | CTC ---------- | V % |
|---|---|---|---|---|---|---|---|---|---|
| 6.0 | 32 | 6 | <0.9 | 44 | 12 | <2 | 56.5 | 71.5 | 79 |
From: Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth.
Phosphorus levels were corrected according to Boletim 100 of the Agronomic Institute of Campinas, São Paulo, Brazil, by means of chemical analysis of the soil according to the crop evaluated. Four levels were assigned to the experiment: R1 or S1, only the phosphorus present in the soil, R2 or S2, R3 or S3 and R4 or S4 being 50, 70 and 90 kg ha−1, corresponding to low, medium and high productivity of soybean cultivation, respectively. The proportion of P2O5 from each of the two sources used was considered, Triple superphosphate (46% of P2O5) and Bayóvar Rock phosphate (31% of P2O5). The experiment was conducted for seven weeks until the R1 stage of the culture, under controlled conditions in the wandering house, temperature (25–35 °C), humidity (75–80%) and photoperiod of 10 h/14 h (light/dark). Soil moisture was determined once or two times a day. Bases saturation and pH were corrected with soil liming; and nitrogen and potassium were supplied by irrigating a solution containing 420 mg of urea and 300 mg of potassium chloride in each pot after planting.
Plant analysis
Twenty-one days after planting, the chlorophyll was measured with a portable SPAD-502Plus meter. At harvest, the height of the soy plants was analyzed. The roots were removed from the soil and washed. The roots were dried (60 °C) until they reached a constant weight for evaluation of the dry matter mass, as well as the leaf area of the plants. The leaves were collected and crushed for subsequent analysis of the P concentration, carried out at the Plant Tissue Laboratory of College of Agriculture “Luiz de Queiroz”, University of São Paulo, Piracicaba, São Paulo- Brazil. The rhizospheric soil was collected for enzymatic analysis of acid and alkaline phosphatases78 and phytase79.
Data analysis and statistics
All tests and treatments were performed with repetitions and the values were expressed as the mean between them. For the in vitro tests, a parametric variance test (ANOVA) was used to evaluate whether there was a significant difference in the solubilization of P, after considering the assumptions of normality tested by the Shapiro-Wilk and equality of variance by bartlett test. The significant data were compared using the Tukey and Scott Knott test (p < 0.05).
In the greenhouse experiment, a two-way ANOVA was applied to test the significance of each factor (levels of phosphorus and Trichoderma ssp.) and its interaction. As the interactions were always significant, Scott Knott mean comparation test was applied for the treatments considering the P levels, the Trichoderma isolates, and the control treatment.
To measure the effectiveness of the addition of Trichoderma in each level of P and the two sources of P, absolute values of dry weight (DW) (g/plant-1) were converted in the improvement of the biomass of the plants (in %) for each Trichoderma sp. calculated in relation to the control without Trichoderma. For that, we applied the following Eq. 1:
| 1 |
where each of the isolates of Trichoderma (x) - AMS 34.39 and AMS 31.15 - at each level of phosphorus (y) - 0, 50, 70 and 90 kg ha−1 - is compared with the control conditions at the same levels of P (y). The value different from 0% indicates that treatment with Trichoderma resulted in an increase or decrease in plant biomass (using the same source of P and the level applied). The amount of phosphorus in the aerial part of the soybean plants was evaluated between the Trichoderma and control isolates, in relation to the source of phosphorus and level of this applied. This value was obtained by multiplying the phosphorus content of the aerial part of the plant by its dry matter. In addition, the efficiency of P absorption (in %) between the phosphorus sources and the applied level was calculated by the Eq. 2:
| 2 |
where each of the isolates of Trichoderma (x) – AMS 34.39 and AMS 31.15 – at each level of phosphorus (y) - 0, 50, 70 and 90 kg ha-1 – and the control conditions at the same levels of P (y) were compared with the control without the addition of phosphorus- control Level 1.
Acknowledgements
The author’s acknowledge the support of EMBRAPA Environment for making the laboratories available to the research and also the Microbiology program for the opportunity to develop this work. This study was made possible thanks to the financial support provided by CAPES (Scholarship of the University of São Paulo) and FAPESP (2013/26659-9). The authors special thanks are due to Dra. Simone Raposo Cotta and Dr. Suikinai Nobre Santos for their support during the work development.
Author contributions
L.B., M.A.M., C.C.P. execution of experiments; L.B., M.A.M. data collection; L.B., J.B.C., I.S.M. tabulation, statistical analysis of data; L.B. creation of tables and figures; L.B., J.B.C., I.S.M. writing of the text and standardization of norms according to the journal. C.C.P. collection of samples.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017;8:1–8. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FAO & OECD. OECD-FAO Agricultural., 10.1787/19991142 (2015).
- 3.Rimando, A. M. & Duke, S. O. Natural Products for Pest Management. In ACS Symposium Series927, 2–21, 10.1021/bk-2006-0927.ch001 (2006).
- 4.Withers PJA, et al. Transitions to sustainable management of phosphorus in Brazilian agriculture. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 6.Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol Plant 10 p., 10.1007/s11738-013-1338-2 (2013).
- 7.Richardson AE, Barea JM, McNeill AM, Prigent C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. doi: 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- 8.Roper MM, Gupta VVSR. Enhancing Non-symbiotic N2 Fixation in Agriculture. Open Agric. J. 2016;10:7–27. doi: 10.2174/1874331501610010007. [DOI] [Google Scholar]
- 9.Martins JCR, de Freitas ADS, Menezes RSC, Sampaio EVdeSB. Nitrogen symbiotically fixed by cowpea and gliricidia in traditional and agroforestry systems under semiarid conditions. Pesqui. Agropecu. Bras. 2015;50:178–184. doi: 10.1590/S0100-204X2015000200010. [DOI] [Google Scholar]
- 10.Anand, K., Kumari, B. & Mallick, M. A. Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. Int. J. Pharm. Pharm. Sci. 8, 37–40, https://innovareacademics.in/journals/index.php/ijpps/article/view/9747 (2016).
- 11.Khan MS, Zaidi A, Wani PA. Role of phosphate-solubilizing microorganisms in sustainable agriculture – A review. Agron. Sustain. 2007;27:29–43. doi: 10.1051/agro:2006011. [DOI] [Google Scholar]
- 12.Naik SK, et al. Inorganic phosphate solubilization by phosphate solubilizing fungi isolated from acidic soils. African J. Microbiol. Res. 2013;7:4310–4316. doi: 10.5897/AJMR2013.5947. [DOI] [Google Scholar]
- 13.Sharma S, Kumar V, Tripathi RB. Isolation of Phosphate Solubilizing Microorganism (PSMs) From Soil. J. Microbiol. Biotechnol. Res. Sch. 2011;1:90–95. [Google Scholar]
- 14.Wang X, Pan Q, Chen F. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza. 2011;21:173–181. doi: 10.1007/s00572-010-0319-1. [DOI] [PubMed] [Google Scholar]
- 15.Fink JR, Inda AV, Tiecher T, Barrón V. Iron oxides and organic matter on soil phosphorus availability. Ciência e Agrotecnologia. 2016;40:369–379. doi: 10.1590/1413-70542016404023016. [DOI] [Google Scholar]
- 16.Adesemoye AO, Kloepper JW. Plant – microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol Biotechnol. 2009;85:1–12. doi: 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- 17.Thonar C, et al. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017;4:1–16. doi: 10.1186/s40538-017-0088-6. [DOI] [Google Scholar]
- 18.Coutinho FP, Felix WP, Yano-Melo AM. Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol. Eng. 2012;42:85–89. doi: 10.1016/j.ecoleng.2012.02.002. [DOI] [Google Scholar]
- 19.Richardson AE, Simpson RJ. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011;156:989–996. doi: 10.1104/pp.111.175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Costa Simões D, Caixeta-Filho JV, Palekar US. Fertilizer distribution flows and logistic costs in Brazil: Changes and benefits arising from investments in port terminals. Int. Food Agribus. Manag. Rev. 2018;21:407–422. doi: 10.22434/IFAMR2017.0037. [DOI] [Google Scholar]
- 21.Macrae, A., Coelho, R. E. E., Peixoto, R. & Rosado, A. S. Tropical Soil Microbial Communities. In The Prokaryotes, Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., T. F. 85–95, Springer International Publishing, 10.1007/978-3-642-30123-0_115 (2013).
- 22.Cenciani K, Lambais MR, Cerri CC, Azevedo LCBde, Feigl BJ. Bacteria diversity and microbial biomass in forest, pasture and fallow soils in the southwestern Amazon basin. Rev. Bras. Ciência do Solo. 2009;33:907–916. doi: 10.1590/S0100-06832009000400015. [DOI] [Google Scholar]
- 23.Hoorn C, et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- 24.Pereira, J. O., Souza, A. Q. L., Souza, A. D. L., França, S. C. & Oliveira, L. A. Overview on Biodiversity, Chemistry, and Biotechnological Potential of Microorganisms from the Brazilian Amazon Jose. Springer Int. Publ. 71–103, 10.1007/978-3-319-55804-2_5 (2017).
- 25.Stürmer SL, Siqueira JO. Species richness and spore abundance of arbuscular mycorrhizal fungi across distinct land uses in Western Brazilian Amazon. Mycorrhiza. 2011;21:255–267. doi: 10.1007/s00572-010-0330-6. [DOI] [PubMed] [Google Scholar]
- 26.Metcalfe DB, et al. Impacts of fire on sources of soil CO2 efflux in a dry Amazon rain forest. Glob. Chang. Biol. 2018;24:3629–3641. doi: 10.1111/gcb.14305. [DOI] [PubMed] [Google Scholar]
- 27.Xiao C, Zhang H, Fang Y, Chi R. Evaluation for rock phosphate solubilization in fermentation and soil-plant system using a stress-tolerant phosphate-solubilizing Aspergillus niger WHAK1. Appl. Biochem. Biotechnol. 2013;169:123–133. doi: 10.1007/s12010-012-9967-2. [DOI] [PubMed] [Google Scholar]
- 28.Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2013;2:1–14. doi: 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain R, Saxena J, Sharma V. Effect of phosphate-solubilizing fungi Aspergillus awamori S29 on mungbean (Vigna radiata cv. RMG 492) growth. Folia Microbiol. (Praha). 2012;57:533–541. doi: 10.1007/s12223-012-0167-9. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 31.Li, R. et al. Solubilisation of Phosphate and Micronutrients by Trichoderma harzianum and Its Relationship with the Promotion of Tomato Plant Growth. PLoS One 1–16, 10.5061/dryad.p460c (2015). [DOI] [PMC free article] [PubMed]
- 32.Waghunde RR, Shelake RM, Sabalpara AN. Trichoderma: A significant fungus for agriculture and environment. African J. Agric. Res. 2016;11:1952–1965. doi: 10.5897/AJAR2015.10584. [DOI] [Google Scholar]
- 33.Druzhinina IS, Shelest E, Kubicek CP. Novel traits of Trichoderma predicted through the analysis of its secretome. FEMS Microbiol. Ecol. 2012;337:1–9. doi: 10.1111/j.1574-6968.2012.02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brotman, Y. et al. Trichoderma-Plant Root Colonization: Escaping Early Plant Defense Responses and Activation of the Antioxidant Machinery for Saline Stress Tolerance. PLoS Pathog. 9, 10.1371/journal.ppat.1003221 (2013). [DOI] [PMC free article] [PubMed]
- 35.Shoresh M, Harman GE, Mastouri F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 36.Vinale F, et al. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett. Appl. Microbiol. 2009;48:705–711. doi: 10.1111/j.1472-765X.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- 37.Contreras-Cornejo HA, Macías-Rodriguez L, Cortés-Penagos C, López-Bucio J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent. Plant Physiol. 2009;149:1579–1592. doi: 10.1104/pp.108.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza MFde, Silva ASAda, Bon EPS. A novel Trichoderma harzianum strain from the Amazon Forest with high cellulolytic capacity. Biocatal. Agric. Biotechnol. 2018;14:183–188. doi: 10.1016/j.bcab.2018.03.008. [DOI] [Google Scholar]
- 39.Delabona P, et al. Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour. Technol. 2012;107:517–521. doi: 10.1016/j.biortech.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 40.Vaz ABM, et al. A multiscale study of fungal endophyte communities of the foliar endosphere of native rubber trees in Eastern Amazon. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-34619-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holanda Sena H, et al. Erratum to “Production of Biosurfactants by Soil Fungi Isolated from the Amazon Forest”. Int. J. Microbiol. 2018;2018:1–8. doi: 10.1155/2018/1586859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de França SKS, et al. Biocontrol of sheath blight by Trichoderma asperellum in tropical lowland rice. Agron. Sustain. Dev. 2015;35:317–324. doi: 10.1007/s13593-014-0244-3. [DOI] [Google Scholar]
- 43.Holmes KA, Schroers H-J, Thomas SE, Evans HC, Samuels GJ. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Prog. 2004;3:199–210. doi: 10.1007/s11557-006-0090-z. [DOI] [Google Scholar]
- 44.Ramesh A, Sharma SK, Joshi OP, Khan IR. Phytase, Phosphatase Activity and P-Nutrition of Soybean as Influenced by Inoculation of Bacillus. Indian J. Microbiol. 2011;51:94–99. doi: 10.1007/s12088-011-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart, A. & Hill, R. Applications of Trichoderma in Plant Growth Promotion. In:Biotechnology and Biology of Trichoderma 415–425 (2014).
- 46.Saravanakumar K, Shanmuga Arasu V, Kathiresan K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013;104:101–105. doi: 10.1016/j.aquabot.2012.09.001. [DOI] [Google Scholar]
- 47.García-López AM, Avilés M, Delgado A. Plant uptake of phosphorus from sparingly available P- sources as affected by Trichoderma asperellum T34. Agric. Food Sci. 2015;24:249–260. doi: 10.23986/afsci.49532. [DOI] [Google Scholar]
- 48.Zhao L, Liu Q, Zhang Y, Cui Q, Liang Y. Effect of acid phosphatase produced by Trichoderma asperellum Q1 on growth of Arabidopsis under salt stress. J. Integr. Agric. 2017;16:1341–1346. doi: 10.1016/S2095-3119(16)61490-9. [DOI] [Google Scholar]
- 49.Pelagio-Flores R, Esparza-Reynoso S, Garnica-Vergara A, López-Bucio J, Herrera-Estrella A. Trichoderma-induced acidification is an early trigger for changes in Arabidopsis root growth and determines fungal phytostimulation. Front. Plant Sci. 2017;8:1–13. doi: 10.3389/fpls.2017.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borges Chagas LF, Chagas Junior AF, Rodrigues de Carvalho M, de Oliveira Miller L, Orozco Colonia BS. Evaluation of the phosphate solubilization potential of Trichoderma strains (Trichoplus JCO) and effects on rice biomass. J. Soil Sci. Plant Nutr. 2015;15:794–804. doi: 10.4067/S0718-95162015005000054. [DOI] [Google Scholar]
- 51.García-López AM, Avilés M, Delgado A. Effect of various microorganisms on phosphorus uptake from insoluble Ca-phosphates by cucumber plants. J. Plant Nutr. Soil Sci. 2016;179:454–465. doi: 10.1002/jpln.201500024. [DOI] [Google Scholar]
- 52.Kapri A, Tewari L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Brazilian J. Microbiol. 2010;41:787–795. doi: 10.1590/S1517-83822010005000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tandon, A. et al. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. J. King Saud Univ. - Sci. 1–8, 10.1016/j.jksus.2019.02.001 (2019).
- 54.Garnica-Vergara A, et al. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive functioning. New Phytol. 2016;209:1496–1512. doi: 10.1111/nph.13725. [DOI] [PubMed] [Google Scholar]
- 55.Qualhato TF, et al. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: Evaluation of antagonism and hydrolytic enzyme production. Biotechnol. Lett. 2013;35:1461–1468. doi: 10.1007/s10529-013-1225-3. [DOI] [PubMed] [Google Scholar]
- 56.Ptáček, P. Phosphate Rocks. Intech13, 10.5772/57353 (2016).
- 57.Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant. Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- 58.Colla, G., Rouphael, Y., Di Mattia, E., El-Nakhel, C. & Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. 95, 1706–1715, 10.1002/jsfa.6875 (2015). [DOI] [PubMed]
- 59.Yedidia I, Benhamou N. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999;65:1061–1070. doi: 10.1128/AEM.65.3.1061-1070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang F, et al. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 2016;100:64–74. doi: 10.1016/j.plaphy.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, S., Gan, Y. & Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 7, 10.3389/fpls.2016.01405 (2016). [DOI] [PMC free article] [PubMed]
- 62.Zhang X, Li X, Xia L. Heterologous Expression of an Alkali and Thermotolerant Lipase from Talaromyces thermophilus in Trichoderma reesei. Appl Biochem Biotechnol. 2015;176:1722–1735. doi: 10.1007/s12010-015-1673-4. [DOI] [PubMed] [Google Scholar]
- 63.Zhao L, Zhang YQ. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015;14:1588–1597. doi: 10.1016/S2095-3119(14)60966-7. [DOI] [Google Scholar]
- 64.Peleg Y, Addison R, Aramayo R, Metzenberg RL. Translocation of Neurospora crassa transcription factor NUC-1 into the nucleus is induced by phosphorus limitation. Fungal Genet. Biol. 1996;20:185–191. doi: 10.1006/fgbi.1996.0034. [DOI] [PubMed] [Google Scholar]
- 65.Souza AA, et al. Trichoderma harzianum produces a new thermally stable acid phosphatase, with potential for biotechnological application. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0150455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Santiago A, García-López AM, Quintero JM, Avilés M, Delgado A. Effect of Trichoderma asperellum strain T34 and glucose addition on iron nutrition in cucumber grown on calcareous soils. Soil Biol. Biochem. 2013;57:598–605. doi: 10.1016/j.soilbio.2012.06.020. [DOI] [Google Scholar]
- 67.Gaind S, Nain L. Soil–Phosphorus Mobilization Potential of Phytate Mineralizing Fungi. J. Plant Nutr. 2015;38:2159–2175. doi: 10.1080/01904167.2015.1014561. [DOI] [Google Scholar]
- 68.García-López AM, Recena R, Avilés M, Delgado A. Effect of Bacillus subtilis QST713 and Trichoderma asperellum T34 on P uptake by wheat and how it is modulated by soil properties. J. Soils Sediments. 2018;18:727–738. doi: 10.1007/s11368-017-1829-7. [DOI] [Google Scholar]
- 69.Chuan-Qing Z, Guang-Xiang C, Wei-Yi H, Xing-She L, Yi-Fei Y. Dissolving mechanism of strain P17 on insoluble phosphorus of yellow-brown soil. Brazilian J. Microbiol. 2014;45:937–943. doi: 10.1590/S1517-83822014000300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin J, Tang C, Sale P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: A review. Ann. Bot. 2015;116:987–999. doi: 10.1093/aob/mcv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou D, Huang XF, Guo J, dos-Santos ML, Vivanco JM. Trichoderma gamsii affected herbivore feeding behaviour on Arabidopsis thaliana by modifying the leaf metabolome and phytohormones. Microb. Biotechnol. 2018;11:1195–1206. doi: 10.1111/1751-7915.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, F., Xu, X., Huo, Y. & Xiao, Y. Trichoderma-inoculation and mowing synergistically altered soil available nutrients, rhizosphere chemical compounds and soil microbial community, potentially driving alfalfa growth. Front. Microbiol. 10, 10.3389/fmicb.2018.03241 (2019). [DOI] [PMC free article] [PubMed]
- 73.Elad Y, Chet I. Degradation of plant pathogenic fungi by Trichoderma harzianum. Dep. plant Pathol. Microbiol. Fac. Agric. Hebr. Univ. Jerusalem. 1982;11:719–725. [Google Scholar]
- 74.Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Ecol. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 75.Lynn TM, Yu SS, Kyaw EP, Latt ZK. Enhancement of phosphate solubilizing activity of Enterobacter clocae by NTG mutagenesis. Sky J. Soil Sci. Environ. Manag. 2014;2:102–110. [Google Scholar]
- 76.Murphy J, RiIey JP. Determination Single Solution Method For The In Natural Waters. Anal. Chim. Act. 1962;27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- 77.Singh RK, Malik N, Singh S. Impact of rhizobial inoculation and nitrogen utilization in plant growth promotion of maize (Zea mays L.) Nusant. Biosci. 2014;5:8–14. doi: 10.13057/nusbiosci/n050102. [DOI] [Google Scholar]
- 78.Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Bd. Biochem. 1969;1:301–307. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- 79.Chen YP, et al. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006;34:33–41. doi: 10.1016/j.apsoil.2005.12.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.