Abstract
Colorectal cancer (CRC) is the most commonly diagnosed cancer among Saudi males and ranks third in females with up to 73% of cases diagnosed at late stage. This review provides an analysis of CRC situation in the Kingdom of Saudi Arabia (KSA) from healthcare perspective. A PUBMED (1986–2018) search was done to identify publications focusing on CRC in KSA. Due to reports of increased CRC incidence among young age group (< 50), and given the young population of KSA, the disease may burden the national healthcare system in the next decades. Environmental factors attributed to increasing incidence rates of CRC include red meat consumption, sedentary lifestyle, and increased calorie intake. Despite substantial investment in healthcare, attention to predictive diagnostics and targeted prevention is lacking. There is a need to develop national screening guidelines based on evidence that supports a reduction in incidence and mortality of CRC when screening is implemented. Future approaches are discussed based on multi-level diagnostics, risk assessment, and population screening programs focused on the needs of young populations that among others present the contents of the advanced approach by predictive, preventive, and personalized medicine. Recommendations are provided that could help to develop policies at regional and national levels. Countries with demographics and lifestyle similar to KSA may gain insights from this review to shape their policies and procedures.
Keywords: Predictive preventive personalized medicine, colorectal cancer; Screening; Personalized patient profiling; Young population; Risk factors; Treatment tailored to the person; Targeted prevention, healthcare strategy, biomarkers; Saudi Arabia
Introduction
According to 2015 estimate by the World Health Organization (WHO), cancer is the third leading cause of death before age 70 in the Kingdom of Saudi Arabia (KSA) among 22 countries, with one in eight cancer deaths due to colorectal cancer (CRC) [1, 2]. One third of CRC cases is diagnosed with distant metastases, a contributor of the premature death among Saudis [3]. CRC screening is associated with reduced mortality due to early detection of precancerous lesions or tumors; therefore, preventing the occurrence of CRC or detecting disease at an early stage where mortality rates are lower in comparison with late-stage CRC would be an effective strategy. In this review, we summarize the epidemiology of CRC in KSA, discuss latest development of molecular biology that would help in predictive diagnostics as well as personalized medicine for CRC patients, and make recommendations about avenues for CRC prevention in KSA.
Epidemiology of CRC in KSA
CRC is the most common type of cancer in KSA
Globally, the incidence of CRC is rising at an alarming rate especially in affluent nations that among other reflect multifarious dietary and lifestyle choices based on local traditions and known to be conducive to a variety of colonic diseases. A similar contextual epidemiological trend is observed in KSA, where 2047 newly diagnosed cases of CRC were reported in 2014 [2]. The age-standardized incidence rate of CRC in KSA doubled from 5.0 per 105 in 2001 to 10.5 per 105 in 2009. The increasing CRC incidence rate in KSA is among the highest in the Gulf Cooperation Council (GCC) countries [4]. The rate of new CRC cases is projected to double in the next decade mainly due to lack of predictive, preventive, and personalized medicine (PPPM) [5, 6]. The PPPM approach addresses both non-modifiable (e.g., genetic) and modifiable (preventable) risk factors, corresponding mitigating measures, patient stratification, population screening programs, and treatments tailored to the personalized patient profiles, among others, as the complex concept developed and promoted by the European Association for Predictive, Preventive and Personalized Medicine (EPMA, Brussels) [5, 6]. Frequent comorbid conditions specific for the country should be taken into consideration such as obesity and diabetes mellitus [7, 8].
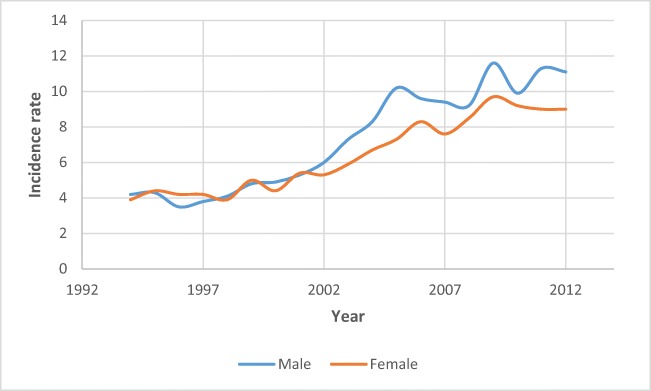
CRC is the most commonly diagnosed cancer among Saudi males and the third most diagnosed cancer in females [9, 10]. While the disease is more common among Saudi males with a median age at diagnosis of 60 years, females are diagnosed at a younger age (55 years). The 2017 data from Bazarbashi et al. (Fig. 1) shows an increasing pattern of the CRC incidence rate for both males and females, with a steeper increase since the year 2002, accompanied by a divergence in the rates between males and females [10]. Though females have the same lifetime risk of CRC as males, the incidence and mortality rates are 30% and 40% less among females due to longer life expectancy [11, 12].
Fig. 1.
Trends of CRC incidence rates across gender in Saudi Arabia (adapted from [9])
Comprehensive cancer mortality data is not available for KSA. However, the International Agency for Research on Cancer (IARC) estimated mortality rates in the Saudi population, using a modeled survival method (taking into account the incidence and survival rates of CRC in a given country to predict mortality rates). The estimated 2012 mortality rate among Saudi males was 10 per 105 and 7.0 per 105 for Saudi females [13].
Early onset of CRC could pose major healthcare problems in KSA
KSA is a country with prevalence of young population
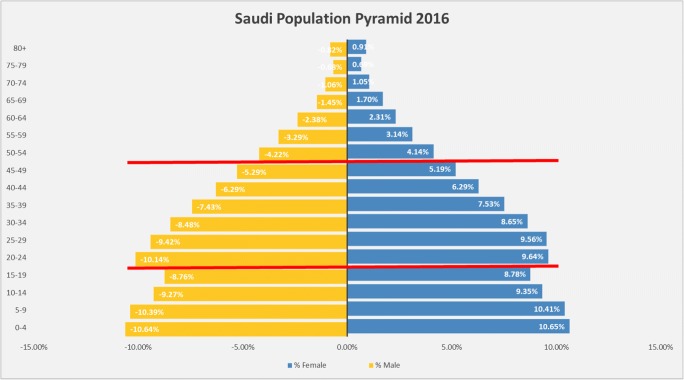
According to the General Authority for Statistics of Saudi Arabia, the total Saudi population in 2018 was 20,768,627 (49% female and 51% male). The majority live in the central (22.83%), western (22.13%), or eastern region (15.39%). The Saudi Arabian population is primarily young, with almost half of the population under the age of 25 years, and 35% between 20 and 39 years (Fig. 2). This trend is particularly alarming in the context of recent reports describing the early onset of CRC in the 20–49-year age group [12, 14–16], which could lead to increase in the disease burden in the next 15–20 years.
Fig. 2.
Saudi population pyramid (General Authority for Statistics of Saudi Arabia, 2016). There is a significant proportion of young population in the age group 20–50
CRC may burden KSA healthcare particularly affecting young generations
Notably, the CRC incidence has been increasing among individuals younger than 50 years old [12, 17]. A report estimated that, by 2030, the incidence of colon and rectal cancers among 20–34 years age group would increase by 90% and 124.2% and for the 35–49 year cohort by 27.7% and 46%, respectively [18]. Several studies provided evidence that early-onset CRC is associated with an advanced stage at diagnosis and aggressive tumor characteristics [19–22].
Alsanea and colleagues compared the CRC incidence rates between the USA and KSA and found that the rates were similar in the 20–24 years age group (0.6 in the USA vs. 0.5 in KSA); however, the rates were higher among US patients in the older age groups (25–29, 30–34, and 35–39 years) [9]. If combined, the rate of increase in Saudi patients in the age group 20–39 years during 2000–2006 was lower than what has been reported in the literature; however, the burden could increase in the next 15–20 years due to the high proportion of young Saudis in the population.
Along with age, geographical location is an important indicator of CRC incidence in KSA [10, 23]. The incidence rates in eastern and central regions were 17.0 per 105 and 16.8 per 105, respectively, but only 2.8 per 105 in the southern region. Possible reasons for the increased detection rates are the accessibility to advanced healthcare facilities and more patient referrals in the central and eastern regions [3]. Also, the presence of the petroleum industry in the eastern region could be a possible contributing factor to the increased incidence rate [24]. This is also corroborated by the low incidence rates reported for the southern region which has the least number of industries.
The current epidemiological situation of colorectal cancer in KSA therefore emphasizes the urgent need of understanding available options that can help in prevention at different levels.
Public health prevention
Primary prevention
The goal of the primary prevention is to prevent risks for CRC development. Contextually, the primary prevention of CRC optimally should utilize educational measures and occur at both individual and community levels as proposed by concepts of person-centered medicine (PCM) [5, 25]. The PCM proposes health promotion methods at both individual and community levels.
Lifestyle and family history have been important factors contributing to the incidence of CRC [26, 27]. Major lifestyle factors attributed to the occurrence of CRC include physical inactivity, tobacco smoking, increased calorie intake, and change in cuisine [26, 28].
The consumption of fruits and vegetables is associated with a lower risk of CRC while the increased consumption of red and processed meat is associated with a higher risk of CRC [29]. In KSA, there has been a 143.3% increase in the consumption of fat between 1970 and 1997 and a 48.3% increase in calorie intake [30]. While a study conducted among Saudi population found a reduction in CRC risk as a result of dairy intake, legume, black tea, coffee, leafy vegetables, and olive oil [28], only 10.8% of Saudis reported consuming fruits according to dietary recommendations, and only about 25% met the vegetable consumption recommendations [31]. Although all GCC states (except Kuwait and UAE) have operational policies in place to reduce unhealthy diet or promote healthy diet [32], executing and following up on those policies are unclear. Modifications of dietary lifestyle could potentially contribute to prevention of CRC among the Saudi population and the GCC countries.
Tobacco smoking is associated with 17 cancers including CRC and the association between smoking and rectal cancer is higher than smoking and colon cancer [33, 34]. According to the World Health Organization (WHO), the tobacco smoking rates in 2011 among Saudi men and women were 37.9% and 6.8%, respectively. Between the years of 1996 and 2012, the rate of change in tobacco consumption has increased by 12.5% in KSA, while in the same period, the rates have decreased in Kuwait by 8.3%, in UAE by 3%, and in Sultanate of Oman by 2.1% [35]. In 2015, a study estimated that 2141 cancer deaths in the GCC region (or 15% of all deaths) were attributed to smoking; the highest proportion of these premature deaths were in KSA [36]. Fortunately, intervention that contributes to reduction in tobacco smoking will result in an approximate 9% reduction in occurrence of CRC [37].
Furthermore, a recent review by the IARC showed that obesity (measured by body mass index (BMI); BMI ≥ 30) is associated with an increased risk of 13 cancers [38]. While Kuwait is the GCC state with the highest percentage of obesity (42%) and Sultanate of Oman with the lowest percentage (20.9%), KSA still has high percentage of obesity (33%) [32]. In 2014, two in five Saudi women and approximately one in three Saudi men were considered obese [2]. The prevalence of obesity is expected to increase due to the urbanization in KSA and the GCC countries at large.
Moreover, physical inactivity and sedentary behavior are common in the GCC region. For instance, in KSA, half of Saudi men and two thirds of Saudi women were found to be physically inactive [2]. There is a dose-response relationship between physical activity and colon cancer. Physical activity is known to reduce the risk of colon cancer by 20–25% [39] and precancerous lesions by about 15% [40]. The mechanism behind the reduction in risk is attributed to a stronger immune system and a reduction in inflammation in people who exercise regularly compared to no regular exercise [41, 42]. Physical activity is also associated with a reduction in mortality; a minimum of 2.5 h of moderate-to-vigorous weekly exercise is associated with a 13% reduction in CRC-related mortality [43]. Considering PPPM approach through focusing on promoting the health of healthy individuals, for instance, by collaborating with local municipalities to establish sidewalk, would help in creating physically active communities.
Moreover, patients with specific diseases also have an increased risk of CRC occurrence. Patients diagnosed with inflammatory bowel disease have a 1.7-fold increase, and a diagnosis of diabetes mellitus increases the risk of CRC development to 1.3-fold. The impact is clear as the prevalence of diabetes mellitus in GCC countries is among the highest in the world, with an estimated rate in KSA of up to 31.6% [44, 45]. Other GCC states have high rates of diabetes mellitus as well. For instance, while Sultanate of Oman has the least prevalence of diabetes mellitus (11.5%), Kuwait and Qatar are both with a prevalence of about 17% but the Kingdom of Bahrain has a prevalence of 30% [46]. Targeted prevention of CRC could thus be significantly improved by reducing the smoking and dietary habits and increasing the physical activity.
Secondary prevention
The goal of secondary prevention is to reduce the incidence of CRC in persons at risk. Accordingly, this section describes screening tests and programs of proven efficacy in preventing CRC.
CRC screening can prevent cancer with the use of polypectomy and can detect CRC at an early stage [47]. The US National Polyp Study found that polypectomy could decrease the incidence of CRC up to 76% [48]. Subsequent studies corroborated these findings but with a lesser reduction in CRC incidence [49, 50]. The US National Polyp study also estimated a reduction of 53% in CRC deaths due to polypectomy [51]. Many other studies have found decreased mortality with screening [52]. In combination, evidence suggests a reduction in both the incidence and mortality rate with CRC screening.
Nevertheless, the adoption of CRC screening at the population-level is challenging. There are many factors associated with the use of CRC screening including race, socioeconomic status (SES), health insurance coverage, availability of a usual source of care, communication with provider, level of knowledge about CRC screening, rural residence, and geographic access to screening facilities [53, 54]. Individuals in the minority groups, those with lower SES and limited healthcare coverage, are less likely to undergo screening compared to their counterparts [53]. Additionally, rural residents are 17% less likely to be up-to-date about overall CRC screening compared with urban residents [55]. Moreover, compared to urban and rural residents, remote rural residents are the least likely to receive CRC screening.
While designing an effective screening program is crucial, their successful implementation needs support from healthcare providers. Primary care physician (PCP) ensures the availability of a usual source of care, which is a well-established factor associated with the increased uptake of CRC screening [53]. Favorable CRC outcomes have been associated with PCP visits. Improved outcomes such as a lower incidence of late-stage CRC and a higher survival rate are proportional to the availability of PCPs [56–59]. For each 10% increase in PCPs measured by the number of PCPs per 105 people, the odds of a late-stage diagnosis of CRC are reduced by 5%. In contrast, each 10% increase in the supply of specialists such as gastroenterologists, general surgeons, or colorectal surgeons is associated with a 5% increase in late-stage CRC diagnosis. This could be because of the nature of the relationship between PCPs and patients, which tends to be longer and the provision of comprehensive healthcare, as opposed to the limited contact between specialists and patients [60].
PCPs, such as general practitioners or family medicine, internists, and general pediatricians, are the patient’s first contact with the healthcare system, and the preventive services are often initiated through primary care. Unlike North America and New Zealand, the GCC states, except United Arab Emirates (UAE), Qatar, and Bahrain, lack standards for the management of non-communicable diseases through a primary care approach [35]. The absence of PPPM resulted in late-stage diagnosis of many cancers except for countries that adopted the said approach. For instance, although not enough evidence has been accumulated about CRC screening in the GCC states, prior research conducted since the launch of breast cancer screening in the UAE showed a reduction in late-stage breast cancer diagnosis among UAE women compared to women from other GCC countries [35].
In case of CRC prevention and control, the roles of PCPs include discussion and recommendation regarding screening, performing non-invasive screening (e.g., FOBT), and referring patients to specialists (e.g., gastroenterologists, general surgeons, or colorectal surgeons) who can perform an endoscopic screening test [47].
Despite the aforementioned factors, screening programs of proven efficacy in preventing CRC have been established. For example, the USA and New Zealand are examples of countries with extensive primary prevention programs proven to be effective in reducing the incidence and mortality due to CRC. A review of guidelines/recommendations for CRC screening in North America and New Zealand is presented in Table 1.
Table 1.
Guidelines for CRC screening by different organizations in the USA and New Zealand
| Recommendation | Recommended by | Year | Reference |
|---|---|---|---|
| Colonoscopy every 10 years, annual FIT/FOBT, or flexible sigmoidoscopy every 5 years plus FOBT every 3 years | USPSTF | 2016 | [61] |
|
Tier 1: colonoscopy every 10 years and annual FIT Tier 2: CT-colonography every 5 years, FIT-DNA every 3 years, and FS every 5 years Tier 3: capsule colonoscopy every 5 years |
American Cancer Society, American College of Radiology, and the US Multi-Society Task Force | 2017 | [62] |
|
Test of choice: colonoscopy every 10 years Other recommendations: stool-based tests FS every 5 years or CT colonography |
NCCN | 2018 | [63] |
| Annual FIT/FOBT, stool DNA test every 3 years, colonoscopy every 10 years, CT-colonography and FS every 5 years | American Cancer Society | 2018 | [64] |
| Annual FIT or high-sensitivity gFOBT, FS every 5 years, FIT or high-sensitivity gFOBT every 3 years plus FS every 5 years or colonoscopy every 10 years | American College of Physicians | 2015 | [65] |
| Biennial FIT or gFOBT or FS every 10 years | Canadian Task Force on Preventive Health Care | 2016 | [66] |
| Biennial iFOBT for age group 60–74 years. Colonoscopy or CTC offered for those with positive iFOBT results | New Zealand | 2017 | [67] |
|
Still at the recommendation stage No established national guideline |
Saudi Society of Colon & Rectal Surgery | 2015 | [68] |
In the USA, CRC screening is recommended at age 50 years for the average-risk population defined as individuals with no history of CRC, polyps, or inflammatory bowel disease [69, 70]. The available CRC screening tests consist of three stool-based tests, four imaging tests, and two endoscopy tests (Table 2). The stool tests are the gFOBT, the FIT, and a fecal DNA test. The imaging tests are the double-contrast barium enema (DCBE), computed tomographic colonography (CTC), magnetic resonance colonography (MRC), and capsule endoscopy [52]. The endoscopy tests are flexible sigmoidoscopy (FS) and colonoscopy.
Table 2.
Screening tests for the detection of adenomatous polyps or cancer
| Screening test | Target population | Scope of detection | Reference |
|---|---|---|---|
| Colonoscopy every 10 years (less interval among increased or high-risk individuals) | Average, increased risk (personal or family history of polyps or cancer), or high-risk individuals (genetic disease; FAP or Lynch syndrome, or inflammatory disease) | Adenomatous polyps and cancer | [47] |
| FS every 5 years | Average, increased, or high-risk individuals | Adenomatous polyps and cancer | |
| DCBE every 5 years | Average or increased risk individuals | Adenomatous polyps and cancer | |
| CT-colonography every 5 years | Average or increased risk individuals | Adenomatous polyps and cancer | |
| Annual gFOBT with high sensitivity to cancer | Average or increased risk individuals | Mainly to detect cancer | |
| Annual FIT with high sensitivity to cancer | Average or increased risk individuals | Mainly to detect cancer | |
| Stool DNA test with high sensitivity to cancer | Average or increased risk individuals | Mainly to detect cancer |
Furthermore, the tests most commonly used in the USA are colonoscopy, FS, FIT, and high-sensitivity gFOBT [71, 72]. The current National Comprehensive Cancer Network (NCCN) guidelines recommend the following screening methods and frequency: annual high-sensitivity gFOBT or FIT; FS every 5 years with stool blood tests (FOBT or FIT); or colonoscopy every 10 years [47, 73]. The current use of screening tests in Saudi Arabia has not been published and necessitates the formulation of guidelines.
Moreover, because high-risk individuals (i.e., individuals with personal or family history of CRC or adenomatous polyps, personal or family history of inflammatory bowel disease, or hereditary CRC syndrome such as familial adenomatous polyps or Lynch syndrome) have increased risk of CRC, the screening recommendations are different. For those individuals, the Amsterdam criteria and Bethesda guideline were devised to establish the diagnosis [29]. Table 3 describes the main characteristics of the criteria. In KSA, a previous study advocated for screening for high-risk individuals based on genetic profiling [76].
Table 3.
Criteria for the diagnosis of hereditary non-polyposis CRC (HNPCC)
| Guidelines | Criteria | Reference |
|---|---|---|
| Bethesda Guideline (1997) | 1) Amsterdam criteria individuals | [74] |
| 2) Individuals with two HNPCC-related cancers: synchronous/metachronous colorectal cancers; endometrial, ovarian, gastric, hepatobiliary, small intestine, or renal tract transitional cell cancers | ||
|
3) Individuals with colorectal cancer and a first-degree relative with one or more of the following: (a) Colorectal cancer diagnosed under 45 years (b) HNPCC-related cancer diagnosed under 45 years (c) Adenoma diagnosed under 40 years | ||
| 4) Individuals under 45 years of age with colorectal or endometrial cancer | ||
| 5) Individuals with right-sided cancer of undifferentiated type | ||
| 6) Individuals under 45 years of age with signet ring cancer | ||
| 7) Individuals under 40 years of age with adenomas | ||
| Amsterdam Criteria-II (1999) | 1) Three familial cases with HNPCC-associated cancer (CRC, cancer of small bowel, endometrium, ureter, or renal pelvis) in which two of the affected individuals are first-degree relatives of the third | [75] |
| 2) Colorectal cancers occurring in two successive generations | ||
| 3) At least one colorectal cancer diagnosed under age 50 years | ||
| 4) Familial adenomatous polyposis should be excluded in CRC cases | ||
| 5) Tumors should be verified by a pathologist |
In KSA, several groups have recently advocated for the implementation of CRC screening guidelines, described the need for CRC screening, the age to start screening, and the suggested screening tests [68, 77]. They recommended screening for average-risk asymptomatic patients between 45 and 70 years old (70+ depending on patients’ health), although others advocated for screening to start at 40 years of age. Authors also advocated for the use of a colonoscopy every 10 years as the gold standard followed by sigmoidoscopy every 5 years with guaiac fecal occult blood test (gFOBT) or fecal immunochemical test (FIT). Initiatives for CRC screening among other GCC countries are lacking.
For the Saudi population and countries with similar demography, the required resources and the implementation of a CRC screening program will depend on the type of screening approach (organized vs. opportunistic). Whereas the organized approach provides unique advantages, a careful examination of the existing healthcare delivery system is warranted to determine if such an approach will yield the best results.
Tertiary prevention
The aim of tertiary prevention is to design therapeutic and rehabilitative strategies among patients diagnosed with CRC to prevent the disease progression such as in the case of metastatic CRC. Early diagnosis followed by appropriate treatment could help to increase survival rates. While a small proportion (9.4%) of Saudi patients is diagnosed with localized cancer, about 24–28.4% of patients are diagnosed with distant metastatic CRC that might necessitate non-surgical interventions [9, 20, 78, 79]. Prevention through lifestyle changes, early diagnosis, and molecular evidence-based personalized medicine (PM) could be the most promising strategies to manage CRC in KSA and elsewhere [80–84]. PM is central to all domains of CRC-related clinical care. Since the successful completion of the human genome project, through successive technological advances in genomic sequencing traditionally restricted to research, it is now becoming part of routine clinical care [85–87]. In the last 10 to 15 years, therapeutic approaches for CRC have gradually shifted from the application of non-specific and thus far more cytotoxic agents such as 5-fluorouracil, and oxaliplatin to the addition of selective agents aimed at targeting the function of specific oncoproteins [88]. The identification of specific CRC biomarkers enabling prognostic stratification of patients into subgroups with a high probability to respond to a specific therapy is the subject of intense investigation [89].
There is growing evidence suggesting that molecular alterations could be associated with a specific population. These alterations, presented as genetic predisposition, are one of the most significant risk factors in the development of CRC. For instance, a third of CRC patients (30%) have a family history of CRC, and 5% were linked to hereditary factors. This could be an important risk factor for societies with high rates of consanguinity. Previous studies reported that a high proportion of familial MSI cases are distinctive among Saudi patients [90–92]. Below is a brief review about the molecular evidence among Saudi CRC patients, which presents an avenue to analyze and develop treatment targets, and markers that will be relevant to the local population (Table 4).
Table 4.
Altered gene characteristics in Saudi CRC patients
| Gene | Molecular event reported in Saudi CRC patients | Comments | References |
|---|---|---|---|
| KRAS | Mutation on exon 4 | Incidence of KRAS and BRAF mutations is 50% compared to 30–40% globally. Exon 4 (codons 134–150) might be a novel mutational analysis | [93, 94] |
| PIK3CA | Mutation on exon 9 | Higher prevalence (12%) compared to CRC patients in Europe and North America | [91, 92] |
| MED12 | TGF-β signaling pathway dysregulation | Prevalence 2.82% and a rate of somatic mutation of 0.115. Might be resistant to alkalizing chemotherapeutic agents | [95] |
| PARP-1 | Alternation of exon 21 (Lys933Asn and Lys945Asn) | Associated with tumor progression and a poor prognosis | [96, 97] |
| hTERT | Correlated with telomere length of − 0.643. | Higher expression in Saudis over 75 years old | [98] |
| ATR | Correlated with telomere length of 0.207 | Associated in females more than males | [98] |
| TOP2A | Sensitive to anthracycline | May serve as a predictive biomarker for anthracycline-based chemotherapy | [99] |
| HER2 | Sensitive to anthracycline | May serve as a predictive biomarker for anthracycline-based chemotherapy | [99] |
| IL-17A | Increased heterozygous AG genotype and homozygous AA genotype | Observed in 70% of early-stage CRC, while observed only in 30% of late-stage CRC. G197A variant can be utilized as genetic screening for early detection of CRC | [100] |
| ATP8B1 | Region of loss, 18q21.31 | Aminophospholipid transporter | [101–103] |
| NARS | Region of loss, 18q21 | Asparaginyl-tRNA synthetase | [101–103] |
| ATP5A1 | Region of loss, 18q21.1 | Tumor suppressor, regulation of apoptosis | [101–103] |
| CTCFL | Region of gain, 20q13.31 | Gene regulation, oncogene in ovarian cancer | [101–103] |
| SPO11 | Region of gain, 20q13.31 | Gene binding, ATP binding | [101–103] |
| ZNF217 | Region of gain, 20q13.2 | Role in breast cancer | [101–103] |
| HOXA3 | Region of gain, 7p15.2 | Transcription factor | [101–103] |
| GPNMB | Region of gain, 7p15.3 | Role in metastasis | [101–103] |
| PLEKHA8I | Region of gain, 7p21 | Intracellular trafficking | [101–103] |
| GF2BP3 | Region of gain, 7p15.3 | Binding protein | [101–103] |
| PCAT1 | Region of gain, 8q24.21 | Non-protein coding prostate cancer transcript | [101–103] |
The RAS gene mutation is well established as one of the hallmark genetic alterations occurring during CRC pathogenesis in populations studied globally. In Saudi Arabia, the KRAS and BRAF mutations have a higher incidence, up to 50% among Saudi patients, compared to 30–40% in global studies [93, 94]. The BRAF mutation is higher in Saudi female patients compared to male patients. However, the KRAS exon 2–3 mutations have a lower incidence in Saudi patients with CRC, compared with international studies. In addition, seven novel somatic mutations on exon 4 of the KRAS gene were reported. These mutations are located in previously unreported loci [104]. It is suggested that exon 4 (codons 134–150) might be a novel hotspot for routine KRAS mutational analysis in Saudi CRC patients [105].
Dysregulation of the PI3K/Akt signaling pathway with a mutation in the TP53 gene is considered to be shared mechanisms in the tumorigenesis of CRC. Studies with Saudi CRC patients reported a higher prevalence of mutations in exon 9 of the PIK3CA gene, compared to CRC patients in Europe and North America. The incidence of the TP53 gene mutation is much lower in Saudi Arabia compared to reports globally [91, 92]. Mutation in the MED12 gene has an established role in uterine and breast neoplasms [106] and has been implicated in Saudi CRC patients as well [95].
Telomerase reverse transcriptase (hTERT) has a higher expression level in older CRC patients in KSA. Unlike previous studies done elsewhere, the expression of Rad3-related protein (ATR) was reported to be significantly higher in Saudi females compared to Saudi males [98]. Well-known amplifications that frequently occur in breast cancer were reported in Saudi CRC patients [91]. High-level amplifications of human epidermal growth factor receptor 2 (HER2) and DNA topoisomerase 2-alpha (TOP2A) are classically seen in patients with breast cancer [107]. Although reports of such amplifications in Saudi CRC patients were lower than in breast cancer, HER2 and TOP2A amplifications may serve as a predictive biomarker for anthracycline-based chemotherapy [99]. A novel genetic alternation of PARP-1 has been observed among Saudi CRC patients [96]. Since it has a significant association with tumor progression and a poor prognosis, the expression of PARP-1 exon 21 may present a novel potential role in the prediction of prognosis in Saudi CRC patients [96, 97]. In addition, MiRNA expression may also serve as a potential novel diagnostic biomarker for Saudi CRC patients due to the observed downregulation of miR-145 and miR-195, as well as the upregulation of miR-29 and miR-92 [108].
Interleukin-17 (IL-17) may play a role in human malignancy by inhibiting tumor angiogenesis [109]. The heterozygous AG genotype and homozygous AA genotype of IL-17A gene were increased 57% and 48%, respectively, in Saudi CRC patients compared to a control group [100]. These findings concurred with studies conducted in Iran and Tunisia, but not with a study conducted in Sweden, where no difference in IL-17 expression between CRC patients and healthy individuals was found [110]. Therefore, IL-17A may also serve as a diagnostic biomarker of CRC among Saudi patients.
Novel genes associated with CRC in Saudi patients were reported by employing patient-wise comparison of cytogenetic microarray data. Three genes were identified in regions of significant loss (ATP8B1, NARS, and ATP5A1) and eight genes were located in the region of gain (CTCFL, SPO11, ZNF217, PLEKHA8, HOXA3, GPNMB, IGF2BP3, and PCAT1) [101–103]. There is evidence of differential expression of genes and its isoforms in Saudi CRC patients [87, 111, 112]. These genes provide opportunities for developing biomarkers for CRC diagnosis and progression in Saudi patients and elsewhere.
Based on the current evidence from KSA, gene modifications provide pathways to develop a predictive diagnostic and personalized medicine approach for CRC patients. However, preventive strategies (i.e., primary and secondary preventions) could be more effective in reversing the increasing trend of CRC.
Study limitation
Although the current review study covered all published work since 1986, we might have missed some prior works about CRC in KSA. Furthermore, although the findings of this study can be generalized to populations other than KSA, e.g., the GCC counties, it is still limited to regional countries with similar environmental and genetic characteristics.
Expert recommendations and conclusion
Prediction by multi-level diagnostics
Multi-level diagnostics is an emerging concept that helps in achieving more precision and accurate results. Though it is biologically relevant, the cost of carrying multiple tests could be prohibitive. There should be an individual assessment for each case to determine the need for multi-level diagnostics.
Prevention focused on the needs of young populations
Governmental policymakers have a major role to play in population-level cancer prevention through legislation aimed at health promotion, early detection, and treatment of common cancers such as CRC [113]. Given the well-established risk factors and rising incidence, resource-intensive programs can be devised and implemented to positively modify risk factors and to facilitate the early detection of CRC, a major determinant in the successful eradication of this cancer. For example, by applying PCM, such initiatives may include strategies to encourage the consumption of a healthy diet, an increase in physical activities, a reduction in tobacco consumption, and the effective treatment of chronic diseases such as diabetes mellitus. The PCM is in alignment with the Saudi Vision 2030, which promotes a healthcare strategy through the adoption of preventive care among the young population.
Furthermore, the establishment of cancer registries in both rural and urban areas of KSA are essential to enable monitoring the progress in cancer control through primary prevention, early detection, and timely diagnosis and treatment of common cancers such as CRC [114]. Similarly, such initiatives will also assist in monitoring the impact of CRC screening, stage distribution, and to determine if disparities exist between rural and urban areas in terms of screening as well as treatment.
Personalization of medical services
There is an increasing level of acknowledgment about the heterogeneity and complexity of challenges faced in addressing the management of colorectal cancer. Due to the demographical features, KSA needs to address CRC problem at a priority level. There are identified risk factors embedded in the lifestyle as well as molecular landscape of the people. Screening and preventive strategies need to be devised and implemented to ensure best outcomes. Early diagnosis and molecular evidence-based PM could be the most promising ways to counter CRC in KSA and elsewhere [89, 115].
For CRC therapy, KRAS mutational status is now well recognized as the key to determining the likelihood of response to anti-EGFR agents cetuximab and panitumumab [116, 117]. There is an urgent need for comprehensive CRC genetic expression profiling and a systematic approach to the identification of biomarkers which can enable stratification of patients according to their potential to response to specific therapies.
Apart from the growing need of PM to capitalize on these emerging focused therapeutic options, PM has even more appealing role to reduce the healthcare costs and to promote cancer prevention by identifying tailored interventions to specific level of risk. For example, for a patient with low or average risk of CRC, morbidities associated with repeated colonoscopies or gastrointestinal bleeding due to long-term aspirin administration can be avoided. Conversely, in the setting of a high risk for CRC, such as a person with familial adenomatous polyposis, early colonoscopy examinations could decrease the morbidity and mortality by facilitating risk-based colectomy decision, thus preventing the occurrence of CRC all together. Several clinical parameters are currently utilized to guide and implement precision CRC prevention strategies such as race, age, family history, personal history of polyps or adenomas, germ line genetic risk, and the results of the screening tests. As the role of the genetic and the environmental variables and their interplay is further delineated, the catalog of clinical and laboratory variables potentially applicable for precision CRC prevention strategies may expand. This illustrates the significance of establishing well-orchestrated primary care networks to be able to expeditiously capitalize on the emerging evidence-based tools for prevention and treatment of CRC.
Though evidence about gene-level changes associated with CRC in Saudi Arabia has begun to emerge, the complexity involved in the host-environment interactions in the evolution of CRC needs more basic and clinical research. This will help to design and implement community-specific measures enabling prevention, early diagnosis, and successful therapy of CRC.
Population screening
The social-ecological framework is a valuable conceptual model for implementing strategies that help with CRC screening. The framework has been adopted and used by the Center of Disease Control and Prevention (CDC) for CRC screening [118]. At the interpersonal level, PCPs should be incentivized to provide screening services to the eligible population. While the approach is very useful because it will encourage PCPs to offer CRC screening to their patients, it relies on the initiative and discretion of PCPs on when and to whom the screening services should be offered. For instance, PCPs might provide screening services to educated individuals and therefore leaving those with lower health literacy without screening [119].
At the organizational level, successful examples of healthcare systems that have invested heavily in CRC screening efforts in the USA are Kaiser Permanente (KP) and Veterans Affairs (VA) [120]. These programs succeeded in changing the perspectives of screening from an individual-based screening to a population-based one. To do so, the programs advocated for the use of stool-based tests (FOBT/FIT) that can reach more people over the use of tests that are limited to few (e.g., colonoscopy). Such successful experience can be implemented in programs already established in KSA such as National Guard Health Affairs (NGHA), Security Forces Hospitals, and Armed Forces Hospitals. The said programs have the capabilities that make the implementation of CRC screening successful. For instance, at the NGHA, BESTCare is an established registration system that has access to member’s phone and address with the possibility of reaching out for screening, establishing reminder system, and monitoring screening uptakes, and follow up individuals with positive findings.
At the community level, Primary Health Care Centers (PHCCs), private healthcare clinics, and community pharmacies are potential contributors to CRC screening. PHCCs are governmentally funded centers that are non-profit and directed primarily to community. These centers provide essential treatment services but lack screening services such as colonoscopy. Accordingly, screening services that focus on stool-based tests (FIT or FOBT) are more practical even in the remotely rural areas. Private healthcare clinics and community pharmacies could bundle CRC screening with other preventive services such as vaccination programs [121].
In conclusion, the “road map” for advanced PPPM approach in CRC management as the proof-of-principle model in KSA healthcare suggests that promoting healthy diets, remaining physically active, refraining from smoking, maintaining healthy weight, and undergoing CRC screening when eligible could positively modify the risk of CRC among Saudi population. It also advocates for the utilization of PPPM approach since it considers the interest of both healthy and non-healthy individuals, specific population or the whole society, and also the healthcare system at large [5].
This review contains evidence and analyses from the Saudi population. However, the information and conclusions would be useful for GCC and other countries with similar demographics, lifestyle, and dietary patterns.
From reactive approach to PPPM in KSA: a paradigm shift
Nowadays, the healthcare system in KSA is focused on disease care and minimal efforts are directed toward healthcare. That is, satisfying current unmet needs of healthy population is lacking. For instance, infrastructure is not adequate to promote health and encourage physical activities among healthy individuals. Adopting PPPM, one would expect collaboration between healthcare sector and local municipalities and Ministry of Housing. Thus, a paradigm shift from reactive to PPPM is imperative.
Author contributions
All authors contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Consent of publication
Not applicable.
Ethical approval
Not applicable.
Core tips
The incidence of CRC is rising at an alarming rate especially in affluent nations, a reflection of the prevalent dietary and lifestyle choices known to be conducive to a variety of colonic diseases. No prior study reviewed current CRC epidemiology in Saudi Arabia. Our objective is to consolidate current research on the epidemiology and prevention of CRC in Saudi Arabia.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization, Cancer Country Profile - World Health Organization. 2014.
- 3.KFSHRC, O.C.R.U., TUMOR REGISTRY ANNUAL REPORT 2014. 2017.
- 4.Aziz MA, Allah-Bakhsh H. Colorectal cancer: a looming threat, opportunities, and challenges for the Saudi population and its healthcare system. Saudi J Gastroenterol. 2018;24(3):196–197. doi: 10.4103/sjg.SJG_164_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation-EPMA position paper 2016. EPMA Journal. 2016;7(1):23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golubnitschaja, O., V. Costigliola, and Epma, General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J, 2012. 3(1): p. 14. [DOI] [PMC free article] [PubMed]
- 7.Ng SW, et al. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12(1):1–13. doi: 10.1111/j.1467-789X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 8.The Lancet O Addressing the burden of cancer in the Gulf. Lancet Oncol. 2014;15(13):1407. doi: 10.1016/S1470-2045(14)71141-6. [DOI] [PubMed] [Google Scholar]
- 9.Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia: incidence, survival, demographics and implications for national policies. Ann Saudi Med. 2015;35(3):196–202. doi: 10.5144/0256-4947.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazarbashi S, Al Eid H, Minguet J. Cancer incidence in Saudi Arabia: 2012 data from the Saudi Cancer Registry. Asian Pac J Cancer Prev. 2017;18(9):2437–2444. doi: 10.22034/APJCP.2017.18.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668–1675. doi: 10.1002/ijc.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017.
- 13.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 14.Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216–224. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Imperiale TF. The rising prevalence of early-onset colorectal cancer: ready and FIT to tackle? Gastrointest Endosc. 2017;86(5):900–902. doi: 10.1016/j.gie.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 16.McKay A, Donaleshen J, Helewa RM, Park J, Wirtzfeld D, Hochman D, Singh H, Turner D. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. doi: 10.1186/1477-7819-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guraya SY, Eltinay OE. Higher prevalence in young population and rightward shift of colorectal carcinoma. Saudi Med J. 2006;27(9):1391–1393. [PubMed] [Google Scholar]
- 18.Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Chang GJ. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isbister WH. Colorectal cancer below age 40 in the Kingdom of Saudi Arabia. Aust N Z J Surg. 1992;62(6):468–472. doi: 10.1111/j.1445-2197.1992.tb07227.x. [DOI] [PubMed] [Google Scholar]
- 20.Mosli MH, Al-Ahwal MS. Colorectal cancer in the Kingdom of Saudi Arabia: need for screening. Asian Pac J Cancer Prev. 2012;13(8):3809–3813. doi: 10.7314/apjcp.2012.13.8.3809. [DOI] [PubMed] [Google Scholar]
- 21.O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69(10):866–872. [PubMed] [Google Scholar]
- 22.Zahir MN, et al. Clinical features and outcome of sporadic colorectal carcinoma in young patients: a cross-sectional analysis from a developing country. ISRN Oncol. 2014;2014:461570. doi: 10.1155/2014/461570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents. 1966. 1966. Berlin Springer CrossRef Google Scholar.
- 24.Al-Ahmadi K, Al-Zahrani A. NO(2) and cancer incidence in Saudi Arabia. Int J Environ Res Public Health. 2013;10(11):5844–5862. doi: 10.3390/ijerph10115844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Sarsina PR, Tassinari M. Person-centred healthcare and medicine paradigm: it’s time to clarify. EPMA Journal. 2015;6(1):11. doi: 10.1186/s13167-015-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alamri FA, Saeedi MY, Kassim KA. Dietary and other risk factors for colo-rectal cancer in Saudi Arabia. J Med Med Sci. 2014;5:222–229. [Google Scholar]
- 27.Arafa MA, Farhat K. Colorectal cancer in the Arab world—screening practices and future prospects. Asian Pac J Cancer Prev. 2015;16(17):7425–7430. doi: 10.7314/apjcp.2015.16.17.7425. [DOI] [PubMed] [Google Scholar]
- 28.Azzeh FS, Alshammari EM, Alazzeh AY, Jazar AS, Dabbour IR, el-Taani HA, Obeidat AA, Kattan FA, Tashtoush SH. Healthy dietary patterns decrease the risk of colorectal cancer in the Mecca Region, Saudi Arabia: a case-control study. BMC Public Health. 2017;17(1):607. doi: 10.1186/s12889-017-4520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91(11):916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 30.Musaiger AO. Diet and prevention of coronary heart disease in the Arab Middle East countries. Med Princ Pract. 2002;11(Suppl 2):9–16. doi: 10.1159/000066415. [DOI] [PubMed] [Google Scholar]
- 31.Moradi-Lakeh M, el Bcheraoui C, Afshin A, Daoud F, AlMazroa MA, al Saeedi M, Basulaiman M, Memish ZA, al Rabeeah AA, Mokdad AH. Diet in Saudi Arabia: findings from a nationally representative survey. Public Health Nutr. 2017;20(6):1075–1081. doi: 10.1017/S1368980016003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization, W.H . Noncommunicable diseases country profiles 2014. 2014. [Google Scholar]
- 33.Terry PD, Miller AB, Rohan TE. Prospective cohort study of cigarette smoking and colorectal cancer risk in women. Int J Cancer. 2002;99(3):480–3. doi: 10.1002/ijc.10364. [DOI] [PubMed] [Google Scholar]
- 34.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Othman S, et al. Tackling cancer control in the gulf cooperation council countries. The Lancet Oncology. 2015;16(5):e246–e257. doi: 10.1016/S1470-2045(15)70034-3. [DOI] [PubMed] [Google Scholar]
- 36.Abdulmalik M and Thavorncharoensap M.. Burden of cancer attributable to smoking in Gulf Cooperation Council (GCC) countries. in Proceedings of the International Conference on Applied Science and Health. 2018.
- 37.Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, Sinha R, Allison J. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104(18):1353–1362. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104(20):1548–1561. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 40.Song JH, Kim YS, Yang SY, Chung SJ, Park MJ, Lim SH, Yim JY, Kim JS, Jung HC. Physical activity and other lifestyle factors in relation to the prevalence of colorectal adenoma: a colonoscopy-based study in asymptomatic Koreans. Cancer Causes Control. 2013;24(9):1717–1726. doi: 10.1007/s10552-013-0247-4. [DOI] [PubMed] [Google Scholar]
- 41.Kruijsen-Jaarsma M, et al. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19. [PubMed]
- 42.Zheng Q, Cui G, Chen J, Gao H, Wei Y, Uede T, Chen Z, Diao H. Regular exercise enhances the immune response against microbial antigens through up-regulation of toll-like receptor signaling pathways. Cell Physiol Biochem. 2015;37(2):735–746. doi: 10.1159/000430391. [DOI] [PubMed] [Google Scholar]
- 43.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Wei S, Nie S, Liu L. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339–345. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 44.Al-Daghri NM, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9(1):76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alotaibi A, Perry L, Gholizadeh L, al-Ganmi A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: an overview. J Epidemiol Glob Health. 2017;7(4):211–218. doi: 10.1016/j.jegh.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alhyas L, McKay A, Majeed A. Prevalence of type 2 diabetes in the States of the co-operation council for the Arab States of the Gulf: a systematic review. PLoS One. 2012;7(8):e40948. doi: 10.1371/journal.pone.0040948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ, American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 48.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, Ackroyd F, Shike M, Kurtz RC, Hornsby-Lewis L, Gerdes H, Stewart ET. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 49.Alberts DS, Martínez ME, Roe DJ, Guillén-Rodríguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, Earnest DL, Parish D, Koonce K, Fales L, Sampliner RE. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342(16):1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 50.Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, Burke CA, Snover DC, Bresalier RS, McKeown-Eyssen G, Mandel JS, Bond JH, van Stolk RU, Summers RW, Rothstein R, Church TR, Cole BF, Byers T, Mott L, Baron JA. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129(1):34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin, J., et al., Screening for colorectal cancer: an updated systematic review for the U.S. Preventive Services Task Force. 2015.
- 53.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19(4):339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 54.McLachlan SA, Clements A, Austoker J. Patients’ experiences and reported barriers to colonoscopy in the screening context—a systematic review of the literature. Patient Educ Couns. 2012;86(2):137–146. doi: 10.1016/j.pec.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: cross-sectional analysis of 1998-2005 data from the Centers for Disease Control’s Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ananthakrishnan AN, Hoffmann RG, Saeian K. Higher physician density is associated with lower incidence of late-stage colorectal cancer. J Gen Intern Med. 2010;25(11):1164–1171. doi: 10.1007/s11606-010-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mainous AG, et al. The relationship between continuity of care and trust with stage of cancer at diagnosis. Fam Med. 2004;36(1):35–39. [PubMed] [Google Scholar]
- 58.Plascak JJ, Fisher JL, Paskett ED. Primary care physician supply, insurance type, and late-stage cancer diagnosis. Am J Prev Med. 2015;48(2):174–178. doi: 10.1016/j.amepre.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roetzheim RG, et al. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48(11):850–858. [PubMed] [Google Scholar]
- 60.Corkum M, Urquhart R, Kendell C, Burge F, Porter G, Johnston G. Impact of comorbidity and healthcare utilization on colorectal cancer stage at diagnosis: literature review. Cancer Causes Control. 2012;23(2):213–220. doi: 10.1007/s10552-011-9875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bibbins-Domingo K, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 62.Rex DK, Boland RC, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112(7):1016–1030. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- 63.Provenzale D, Gupta S, Ahnen DJ, Markowitz AJ, Chung DC, Mayer RJ, Regenbogen SE, Blanco AM, Bray T, Cooper G, Early DS, Ford JM, Giardiello FM, Grady W, Hall MJ, Halverson AL, Hamilton SR, Hampel H, Klapman JB, Larson DW, Lazenby AJ, Llor X, Lynch PM, Mikkelson J, Ness RM, Slavin TP, Jr, Sugandha S, Weiss JM, Dwyer MA, Ogba N. NCCN guidelines insights: colorectal cancer screening, version 1.2018. J Natl Compr Cancer Netw. 2018;16(8):939–949. doi: 10.6004/jnccn.2018.0067. [DOI] [PubMed] [Google Scholar]
- 64.Wolf AM, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 65.Wilt TJ, Harris RP, Qaseem A. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 66.Care, C.T.F.o.P.H., Recommendations on screening for colorectal cancer in primary care. Cmaj, 2016. 188(5): p. 340–348. [DOI] [PMC free article] [PubMed]
- 67.Benard F, et al. Systematic review of colorectal cancer screening guidelines for average-risk adults: summarizing the current global recommendations. World J Gastroenterol. 2018;24(1):124–138. doi: 10.3748/wjg.v24.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alsanea N, Almadi MA, Abduljabbar AS, Alhomoud S, Alshaban TA, Alsuhaibani A, Alzahrani A, Batwa F, Hassan AH, Hibbert D, Nooh R, Alothman M, Rochwerg B, Alhazzani W, Morgan RL. National guidelines for colorectal cancer screening in Saudi Arabia with strength of recommendations and quality of evidence. Ann Saudi Med. 2015;35(3):189–195. doi: 10.5144/0256-4947.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabatino SA, White MC, Thompson TD, Klabunde CN, Centers for Disease Control and Prevention (CDC) Cancer screening test use—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):464–468. [PMC free article] [PubMed] [Google Scholar]
- 70.USPSTF, Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med, 2008. 149(9): p. 627–637. [DOI] [PubMed]
- 71.Davis T, Arnold C, Rademaker A, Bennett C, Bailey S, Platt D, Reynolds C, Liu D, Carias E, Bass P, III, Wolf M. Improving colon cancer screening in community clinics. Cancer. 2013;119(21):3879–3886. doi: 10.1002/cncr.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fenton JJ, Elmore JG, Buist DSM, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med. 2010;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitlock EP, L.J. Liles E, Beil T, Fu R, O'Connor E, Thompson RN, Cardenas T. Screening for colorectal cancer: an updated systematic review [internet] 2008. [PubMed] [Google Scholar]
- 74.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Srivastava S, Jass JR, Khan PM, Lynch H, Smyrk T, Perucho M, Sobin L. A National Cancer Institute workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89(23):1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 75.Vasen HF, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 76.Alqahtani M, Grieu F, Carrello A, Amanuel B, Mashour M, Alattas R, al-Saleh K, Alsheikh A, Alqahtani S, Iacopetta B. Screening for lynch syndrome in young colorectal cancer patients from Saudi Arabia using microsatellite instability as the initial test. Asian Pac J Cancer Prev. 2016;17(4):1917–1923. doi: 10.7314/apjcp.2016.17.4.1917. [DOI] [PubMed] [Google Scholar]
- 77.Aljumah AA, Aljebreen AM. Policy of screening for colorectal cancer in Saudi Arabia: a prospective analysis. Saudi J Gastroenterol. 2017;23(3):161–168. doi: 10.4103/sjg.SJG_468_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alhumaid A, AlYousef Z, Bakhsh HA, AlGhamdi S, Aziz MA. Emerging paradigms in the treatment of liver metastases in colorectal cancer. Crit Rev Oncol Hematol. 2018;132:39–50. doi: 10.1016/j.critrevonc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Aziz MA., Chapter 6—colorectal cancer metastasis A2 - Ahmad, Aamir, in Introduction to cancer metastasis. 2017, Academic Press. p. 95–116.
- 80.Janssens JP, Schuster K, Voss A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018;9(2):113–123. doi: 10.1007/s13167-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9(1):77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng T, Zhan X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 2017;8(1):51–60. doi: 10.1007/s13167-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, Vittadini G, Desiderio DM. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):9. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aziz MA, Yousef Z, Saleh AM, Mohammad S, al Knawy B. Towards personalized medicine of colorectal cancer. Crit Rev Oncol Hematol. 2017;118:70–78. doi: 10.1016/j.critrevonc.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 85.Aziz M, et al. Abstract# 2436: microarray analysis of the expression levels of molecular components of relevant signaling pathways in response to FUra/IFN-gamma treatment protocols in human colon carcinoma cell lines. Cancer Res. 2009;69(9 Supplement):2436–6.
- 86.Aziz M, Hussein M, Gabere M. Filtered selection coupled with support vector machines generate a functionally relevant prediction model for colorectal cancer. OncoTargets and Therapy. 2016;9:3313. doi: 10.2147/OTT.S98910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aziz MA, Periyasamy S, Yousef Z, Deeb A, AlOtaibi M. Colorectal cancer driver genes identified by patient specific comparison of cytogenetic microarray. Genom Data. 2014;2:29–31. doi: 10.1016/j.gdata.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rashid, M., et al., Molecular classification of colorectal cancer using the gene expression profile of tumor samples. Exp Biol Med (Maywood), 2019: p. 1535370219850788. [DOI] [PMC free article] [PubMed]
- 90.Abubaker J, Bavi P, al-Harbi S, Ibrahim M, Siraj AK, al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, al-Dayel F, Uddin S, al-Kuraya KS. Clinicopathological analysis of colorectal cancers with PIK3CA mutations in Middle Eastern population. Oncogene. 2008;27(25):3539–3545. doi: 10.1038/sj.onc.1211013. [DOI] [PubMed] [Google Scholar]
- 91.Al-Kuraya KS, et al. Colorectal carcinoma from Saudi Arabia. Analysis of MLH-1, MSH-2 and p53 genes by immunohistochemistry and tissue microarray analysis. Saudi medical journal. 2006;27(3):323–328. [PubMed] [Google Scholar]
- 92.Al-Kuraya KS. KRAS and TP53 mutations in colorectal carcinoma. Saudi journal of gastroenterology: official journal of the Saudi Gastroenterology Association. 2009;15(4):217–219. doi: 10.4103/1319-3767.56087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aldiab A, al Khayal KA, al Obaid OA, Alsheikh A, Alsaleh K, Shahid M, Alkharji H. Clinicopathological features and predictive factors for colorectal cancer outcome in the Kingdom of Saudi Arabia. Oncology. 2017;92(2):75–86. doi: 10.1159/000450857. [DOI] [PubMed] [Google Scholar]
- 94.Bader T, Ismail A. Higher prevalence of KRAS mutations in colorectal cancer in Saudi Arabia: propensity for lung metastasis. Alexandria Journal of Medicine. 2014;50(3):203–209. [Google Scholar]
- 95.Siraj AK, et al. MED12 is recurrently mutated in Middle Eastern colorectal cancer. Gut. 2018;67(4):663–671. doi: 10.1136/gutjnl-2016-313334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alhadheq AM, et al. The effect of poly(ADP-ribose) polymerase-1 gene 3'untranslated region polymorphism in colorectal cancer risk among Saudi cohort. Dis Markers. 2016;2016:8289293. doi: 10.1155/2016/8289293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alshammari AH, Shalaby MA, Alanazi MS, Saeed HM. Novel mutations of the PARP-1 gene associated with colorectal cancer in the Saudi population. Asian Pac J Cancer Prev. 2014;15(8):3667–3673. doi: 10.7314/apjcp.2014.15.8.3667. [DOI] [PubMed] [Google Scholar]
- 98.Aljarbou F, Almousa N, Bazzi M, Aldaihan S, Alanazi M, Alharbi O, Almadi M, Aljebreen AM, Azzam NA, Arafa M, Aldbass A, Shaik J, Alasirri S, Warsy A, Alamri A, Parine NR, Alamro G. The expression of telomere-related proteins and DNA damage response and their association with telomere length in colorectal cancer in Saudi patients. PLoS One. 2018;13(6):e0197154. doi: 10.1371/journal.pone.0197154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Leo A, et al. HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. The lancet oncology. 2011;12(12):1134–1142. doi: 10.1016/S1470-2045(11)70231-5. [DOI] [PubMed] [Google Scholar]
- 100.Al Obeed Omar, Vaali-Mohamed Mansoor-Ali, Alkhayal Khayal, Traiki Thamer, Zubaidi Ahmad, Arafah Maha, Harris Robert, Khan Zahid, Abdulla Maha-Hamadien. IL-17 and colorectal cancer risk in the Middle East: gene polymorphisms and expression. Cancer Management and Research. 2018;Volume 10:2653–2661. doi: 10.2147/CMAR.S161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gonbad RA, et al. Influence of cytokinins in combination with GA(3) on shoot multiplication and elongation of tea clone Iran 100 (Camellia sinensis (L.) O. Kuntze) ScientificWorldJournal. 2014;2014:943054. doi: 10.1155/2014/943054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goerlitz D, et al. Genetic polymorphisms in NQO1 and SOD2: interactions with smoking, schistosoma infection, and bladder cancer risk in Egypt. Urol Oncol. 2014;32(1):47 e15–47 e20. doi: 10.1016/j.urolonc.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eldai H, Periyasamy S, al Qarni S, al Rodayyan M, Muhammed Mustafa S, Deeb A, al Sheikh E, Afzal Khan M, Johani M, Yousef Z, Aziz MA. Novel genes associated with colorectal cancer are revealed by high resolution cytogenetic analysis in a patient specific manner. PLoS One. 2013;8(10):e76251. doi: 10.1371/journal.pone.0076251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naser WM, Shawarby MA, al-Tamimi DM, Seth A, al-Quorain A, Nemer AMA, Albagha OME. Novel KRAS gene mutations in sporadic colorectal cancer. PLoS One. 2014;9(11):e113350. doi: 10.1371/journal.pone.0113350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dadduzio V, Basso M, Rossi S, Cenci T, Capodimonti S, Strippoli A, Orlandi A, Cerchiaro E, Schinzari G, Cassano A, Martini M, Barone C. KRAS exon 2 mutations as prognostic indicators in advanced colorectal cancer in clinical practice: a mono-institutional study. Molecular diagnosis & therapy. 2016;20(1):65–74. doi: 10.1007/s40291-015-0178-8. [DOI] [PubMed] [Google Scholar]
- 106.Laé M, Gardrat S, Rondeau S, Richardot C, Caly M, Chemlali W, Vacher S, Couturier J, Mariani O, Terrier P, Bièche I. MED12 mutations in breast phyllodes tumors: evidence of temporal tumoral heterogeneity and identification of associated critical signaling pathways. Oncotarget. 2016;7(51):84428–84438. doi: 10.18632/oncotarget.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arriola E, Marchio C, Tan DSP, Drury SC, Lambros MB, Natrajan R, Rodriguez-Pinilla SM, Mackay A, Tamber N, Fenwick K, Jones C, Dowsett M, Ashworth A, Reis-Filho JS. Genomic analysis of the HER2/TOP2A amplicon in breast cancer and breast cancer cell lines. Lab Investig. 2008;88(5):491–503. doi: 10.1038/labinvest.2008.19. [DOI] [PubMed] [Google Scholar]
- 108.Al-Sheikh YA, et al. Expression profiling of selected microRNA signatures in plasma and tissues of Saudi colorectal cancer patients by qPCR. Oncol Lett. 2016;11(2):1406–1412. doi: 10.3892/ol.2015.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi Y, Fang W, Xiong B, Wan Y, Dong C. A protective role by interleukin-17F in colon tumorigenesis. PLoS One. 2012;7(4):e34959. doi: 10.1371/journal.pone.0034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Omrane I, Marrakchi R, Baroudi O, Mezlini A, Ayari H, Medimegh I, Stambouli N, Kourda N, Bouzaienne H, Uhrhammer N, Bougatef K, Bignon YJJ, Benammar-Elgaaied A. Significant association between interleukin-17A polymorphism and colorectal cancer. Tumor Biol. 2014;35(7):6627–6632. doi: 10.1007/s13277-014-1890-4. [DOI] [PubMed] [Google Scholar]
- 111.Aziz MA, Periyasamy S, al Yousef Z, AlAbdulkarim I, al Otaibi M, Alfahed A, Alasiri G. Integrated exon level expression analysis of driver genes explain their role in colorectal cancer. PLoS One. 2014;9(10):e110134. doi: 10.1371/journal.pone.0110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deeb AM, Yousef Z, al-Johani M, Aziz MA. Effect of sampling procedure on the quality control metrics of cytoscan HD array for studying cytogenetic aspects of colorectal cancer. Int J Health Sci (Qassim) 2018;12(4):49–55. [PMC free article] [PubMed] [Google Scholar]
- 113.Brawley OW. The role of government and regulation in cancer prevention. The Lancet Oncology. 2017;18(8):e483–e493. doi: 10.1016/S1470-2045(17)30374-1. [DOI] [PubMed] [Google Scholar]
- 114.Lortet-Tieulent J, Siegel R. Expansion of cancer registration in China. Annals of translational medicine. 2014:2(7). [DOI] [PMC free article] [PubMed]
- 115.Aziz MA. Precision medicine in colorectal cancer. Saudi J Gastroenterol. 2019;25(2):139–140. doi: 10.4103/sjg.SJG_24_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lievre A, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 117.Wilson PM, Lenz HJ. Integrating biomarkers into clinical decision making for colorectal cancer. Clin Colorectal Cancer. 2010;9(Suppl 1):S16–S27. doi: 10.3816/CCC.2010.s.003. [DOI] [PubMed] [Google Scholar]
- 118.Joseph DA, DeGroff AS, Hayes NS, Wong FL, Plescia M. The colorectal cancer control program: partnering to increase population level screening. Gastrointest Endosc. 2011;73(3):429–434. doi: 10.1016/j.gie.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 119.Peterson NB, Dwyer KA, Mulvaney SA, Dietrich MS, Rothman RL. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007;99(10):1105–1112. [PMC free article] [PubMed] [Google Scholar]
- 120.Moiel D, Thompson J. Early detection of colon cancer-the kaiser permanente northwest 30-year history: how do we measure success? Is it the test, the number of tests, the stage, or the percentage of screen-detected patients? Perm J. 2011;15(4):30–38. doi: 10.7812/tpp/11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Potter MB. Strategies and resources to address colorectal cancer screening rates and disparities in the United States and globally. Annu Rev Public Health. 2013;34:413–429. doi: 10.1146/annurev-publhealth-031912-114436. [DOI] [PubMed] [Google Scholar]