Melanins are macromolecules that are ubiquitous in nature and impart a large variety of biological functions, including structure, coloration, radiation resistance, free radical scavenging, and thermoregulation. Currently, in the majority of investigations, melanins are either chemically synthesized or extracted from animals, which presents significant challenges for large-scale production. Bacteria have been used as biocatalysts to synthesize a variety of biomaterials due to their fast growth and amenability to genetic engineering using synthetic biology tools. In this study, we engineered the extremely fast-growing bacterium V. natriegens to synthesize melanin nanoparticles by expressing a heterologous tyrosinase gene with inducible promoters. Characterization of the melanin produced from V. natriegens-produced tyrosinase revealed that it exhibited physical and chemical properties similar to those of natural and chemically synthesized melanins, including nanoparticle structure, protection against UV damage, and adsorption of toxic compounds. We anticipate that producing and controlling melanin structures at the nanoscale in this bacterial system with synthetic biology tools will enable the design and rapid production of novel biomaterials for multiple applications.
KEYWORDS: Vibrio natriegens, biomanufacturing, fast growing, melanin, melanin biosynthesis, nanoparticle, synthetic biology
ABSTRACT
Melanin is a pigment produced by organisms throughout all domains of life. Due to its unique physicochemical properties, biocompatibility, and biostability, there has been an increasing interest in the use of melanin for broad applications. In the vast majority of studies, melanin has been either chemically synthesized or isolated from animals, which has restricted its use to small-scale applications. Using bacteria as biocatalysts is a promising and economical alternative for the large-scale production of biomaterials. In this study, we engineered the marine bacterium Vibrio natriegens, one of the fastest-growing organisms, to synthesize melanin by expressing a heterologous tyrosinase gene and demonstrated that melanin production was much faster than in previously reported heterologous systems. The melanin of V. natriegens was characterized as a polymer derived from dihydroxyindole-2-carboxylic acid (DHICA) and, similarly to synthetic melanin, exhibited several characteristic and useful features. Electron microscopy analysis demonstrated that melanin produced from V. natriegens formed nanoparticles that were assembled as “melanin ghost” structures, and the photoprotective properties of these particles were validated by their protection of cells from UV irradiation. Using a novel electrochemical reverse engineering method, we observed that melanization conferred redox activity to V. natriegens. Moreover, melanized bacteria were able to quickly adsorb the organic compound trinitrotoluene (TNT). Overall, the genetic tractability, rapid division time, and ease of culture provide a set of attractive properties that compare favorably to current E. coli production strains and warrant the further development of this chassis as a microbial factory for natural product biosynthesis.
IMPORTANCE Melanins are macromolecules that are ubiquitous in nature and impart a large variety of biological functions, including structure, coloration, radiation resistance, free radical scavenging, and thermoregulation. Currently, in the majority of investigations, melanins are either chemically synthesized or extracted from animals, which presents significant challenges for large-scale production. Bacteria have been used as biocatalysts to synthesize a variety of biomaterials due to their fast growth and amenability to genetic engineering using synthetic biology tools. In this study, we engineered the extremely fast-growing bacterium V. natriegens to synthesize melanin nanoparticles by expressing a heterologous tyrosinase gene with inducible promoters. Characterization of the melanin produced from V. natriegens-produced tyrosinase revealed that it exhibited physical and chemical properties similar to those of natural and chemically synthesized melanins, including nanoparticle structure, protection against UV damage, and adsorption of toxic compounds. We anticipate that producing and controlling melanin structures at the nanoscale in this bacterial system with synthetic biology tools will enable the design and rapid production of novel biomaterials for multiple applications.
INTRODUCTION
Melanins are macromolecules formed by oxidative polymerization of phenolic and/or indolic compounds. These black or brown pigments are hydrophobic, negatively charged, and ubiquitous in nature and impart a large variety of biological functions to organisms, including structure, coloration, free radical scavenging, radiation resistance, and thermoregulation (1). Inspired by the physicochemical, optoelectronic, self-assembling, and adhesive properties of natural melanin, a number of research groups have synthesized melanin nanoparticles for a broad range of applications, including protective coatings, functional films, environmental sensors, and energy storage devices (2, 3) Currently, however, commercially available melanins are either chemically synthesized (4) or extracted from sepia (5), and both approaches contain significant challenges for the generation of yields amenable to large-scale applications. Since microorganisms are easily cultivated and economically sustainable, they show great potential to produce advanced biomaterials. Many bacteria isolated from nature, including species of Bacillus, Aeromonas, Rhizobium, and Streptomyces, were reported to produce melanin via tyrosinase (monophenol monooxygenase EC 1.14.18.1), a copper-containing enzyme (6–11). The enzyme catalyzes the oxidation of l-tyrosine to o-dihydroxyphenylalanine (DOPA) and dopaquinone, which undergoes cyclization to 5,6-dihydroxyindole (DHI) or 5,6-dihydroxyindole-2-carboxylic acid (DHICA) and further polymerizes spontaneously into melanin (see Fig. S1 in the supplemental material) (12). With recombinant DNA technology, the tyrosinase gene has previously been cloned and heterologously expressed in Escherichia coli, and the resulting melanin was tested in a variety of applications (13, 14).
The broad application of biomaterials like melanin on a large scale requires high productivity. However, yields of melanin production from various bacteria are determined by the quantities of substrates (l-tyrosine or l-DOPA) that are fed into the growth cultures and routed toward processing by tyrosinase, so improving melanin production efficiency has been achieved by natural selection of active enzymes or growth optimization (15, 16). Two of the most critical physiological features that impact a microbial production system are the growth rate and biomass-specific substrate uptake rate (17). Therefore, faster-growing organisms provide clear advantages over slower-growing organisms in industrial production. E. coli is often the gold-standard organism for genetic and metabolic engineering efforts, but it exhibits a ∼40-min doubling time in glucose minimal medium (17). Alternatively, the Gram-negative marine bacterium Vibrio natriegens is recognized as one of the fastest-growing organisms currently known, with a reported doubling time of less than 10 min in rich medium and of less than 25 min in glucose minimal medium (18–21). With nutritional versatility, a high growth rate, and lack of pathogenicity, V. natriegens has become an attractive alternative to E. coli for biotechnological applications (22, 23). Additionally, a number of genetic tools have been developed to engineer V. natriegens (20, 21, 24) which make it an attractive chassis for synthetic biology, metabolic engineering, and biomaterial production.
In this study, we engineered V. natriegens to synthesize melanin by expressing a tyrosinase gene from Bacillus megaterium under the control of inducible promoters and demonstrated that V. natriegens was able to produce melanin faster than were previously reported heterologous systems. We found that the melanin produced from V. natriegens-produced tyrosinase could be found in cell-free supernatants as nanoparticles as well as associated with the cell wall, and it exhibited physical and chemical characteristics similar to those of natural and chemically synthesized melanins. This study demonstrated that V. natriegens could be used as a biocatalyst for fast production of a biopolymer.
RESULTS
Expression of the tyrosinase gene and melanin production in V. natriegens.
The tyrosinase gene tyr1 from B. megaterium (25) was synthesized and placed under the control of the Ptac promoter and the lacIq repressor in vector pJV298 (26) using the method described by Tschirhart et al. (20) (Fig. S2). This plasmid was electroporated into V. natriegens, and after overnight incubation at 30°C, transformants (pJV-Tyr1) with black and diffusible pigments were observed on M9 agar plates supplemented with isopropyl-β-d-1-thiogalactopyranoside (IPTG) and l-tyrosine (data not shown). To verify the pigment identity, both pigmented and nonpigmented cells were incubated with anti-melanin antibodies. Fluorescence microscopy showed that immunofluorescence signals were localized on the black cells only (Fig. S3). This result confirmed that the pigments formed were melanins that were associated with the V. natriegens cells.
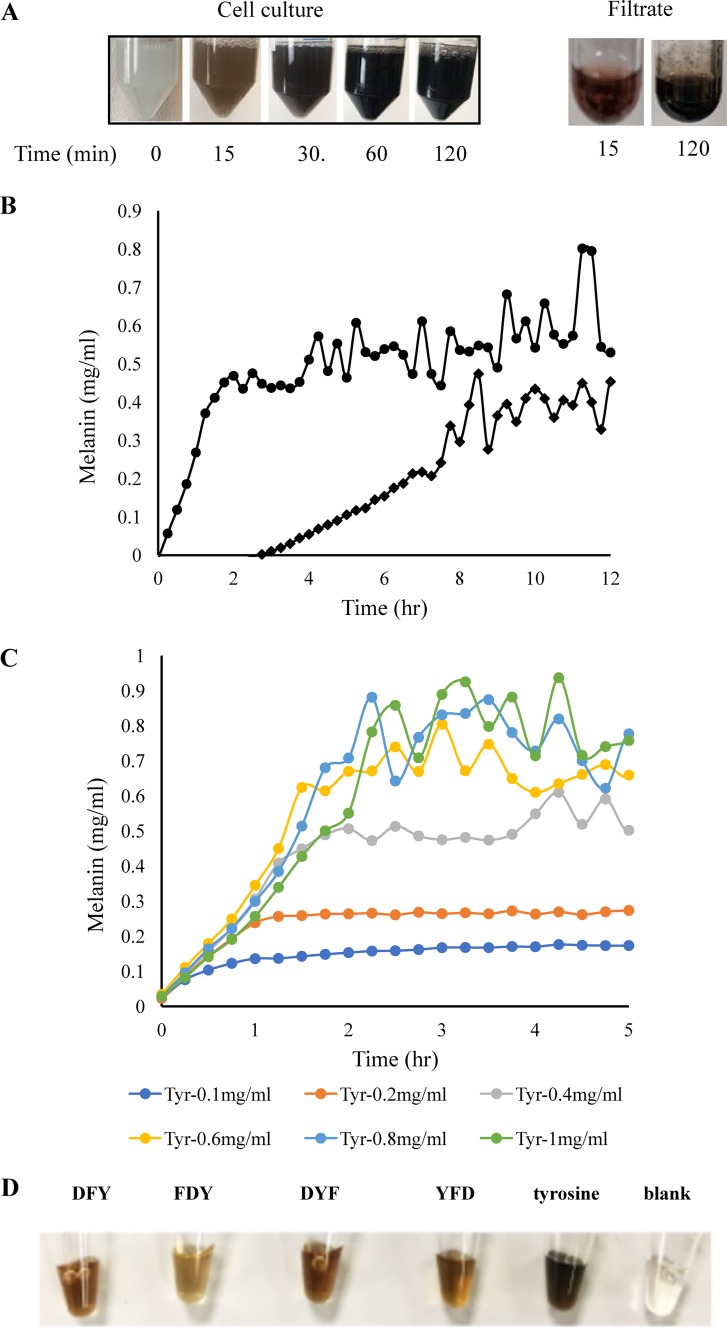
To measure tyrosinase expression and the efficacy of this construct, the recombinant strain was grown for 2 h to log phase and induced by IPTG in LBv2 liquid medium for 3 h. Cells were then collected for proteomic analysis. As accessed by shotgun proteomics, tyrosinase expression increased approximately 100-fold by induction in four biological replicates (Table 1), indicating that the Ptac promoter was efficiently regulated in V. natriegens. Induced cells were then resuspended in LBv2 and M9 liquid media with added l-tyrosine (0.4 mg/ml) and CuSO4, respectively. Within 15 minutes, a black pigment was observed in the M9 culture (Fig. 1A), and melanin yield reached the maximal level (∼0.45 mg/ml) within 2 h, with a rate of approximately 0.32 mg/ml per hour (Fig. 1B), which was equivalent to 420 mg melanin (mel)/g cell dry weight [CDW]/h and significantly faster than any other melanin-producing microorganism (Table 2). Theoretically, 1 g of l-tyrosine is expected to make 1.15 g of melanin by incorporating one atom of oxygen to the l-tyrosine molecule resulting from the tyrosine hydroxylase activity of tyrosinase (14). The melanin yield from this study suggested that l-tyrosine was almost completely converted into melanin by the recombinant bacteria. However, it was noted that the melanin production rate was significantly lower in the LBv2 culture, with a rate of ∼0.05 mg/ml per hour (equivalent to 66 mg mel/gCDW/h), and melanin was saturated after 8 h with less yield than in M9 culture (Fig. 2B). We also found that some tyrosinase was released from the cells, as some tyrosinase activity could be detected in the supernatant of induced cells in M9 culture that had been filtered prior to the addition of l-tyrosine (Fig. 1A), indicating that shifting bacterial cells into the minimal medium resulted in the release of the expressed tyrosinase into the medium, perhaps from increased permeability or cell lysis, so as to accelerate accessing the substrates. To determine the effect of l-tyrosine quantity on melanin yields, M9 cultures containing IPTG-induced cells were supplemented with a range of l-tyrosine concentrations, from 0.1 mg/ml to 1 mg/ml. Figure 1C shows that the melanin production rates were very similar and that concentrations of synthesized melanin were correlated with concentrations of l-tyrosine under 0.6 mg/ml. Starting with an l-tyrosine concentration over 0.6 mg/ml resulted in concentrations of melanin that were above the detection limit of the assay due to product aggregation, which was reflected by fluctuation in the measurements at the later stage.
TABLE 1.
Proteomic quantification of expressed tyrosinase from four independent V. natriegens/pJV-Tyr1 cultures induced by IPTG
| Protein | Avg area ratio induced/uninduced for culture: |
|||
|---|---|---|---|---|
| VN1 | VN2 | VN3 | VN4 | |
| Tyrosinase | 350 | 70 | 90 | 480 |
| DnaK | 2 | 4 | 4 | 2 |
FIG 1.
Expression of tyrosinase gene and melanin production in V. natriegens through IPTG induction. (A) Left, melanin production in M9 medium supplemented with 40 μg/ml CuSO4 and 0.4 mg/ml l-tyrosine after tyrosinase was induced in rich medium; right, melanin production from the filtered supernatant supplemented with CuSO4 and l-tyrosine after the induced cells were incubated in M9 medium for 15 min. (B) Kinetics of melanin production in M9 (•) and LBv2 (♦) media supplemented with 40 μg/ml CuSO4 and 0.4 mg/ml l-tyrosine after tyrosinase was induced in rich medium. (C) Effect of l-tyrosine concentration (in milligrams per milliliter) on melanin production in M9 medium. Graphs in panels B and C represent the averages of the results from five independent experiments. (D) Syntheses of melanin variants with tripeptide precursors. IPTG-induced V. natriegens/pJV-Tyr1 cells were transferred into M9 medium supplemented with CuSO4 and 5 mM tripeptides and incubated at 37°C for 1 h.
TABLE 2.
Comparison of melanin production levels in different microbial hosts
| Host | Gene | Tm (oC)a | Production time (h)b | Reference or source |
|---|---|---|---|---|
| Bacillus weihenstephanensis | Laccasec | 30 | 120 | 50 |
| Streptomyces kathirae | Tyrosinasec | 28 | 128 | 15 |
| Streptomyces glaucescens | Tyrosinasec | 30, 37 | >48 | 9 |
| E. coli | MelAd | 30 | 30 | 14 |
| E. coli | Tyr1d | 30 | 33 | 13 |
| V. natriegens | Tyr1d | 30, 37 | <10 | This study |
Tm, growth temperature.
Production time is counted as from the start of bacterial growth to the beginning of melanin saturation period.
Endogenous gene.
Heterologously expressed gene.
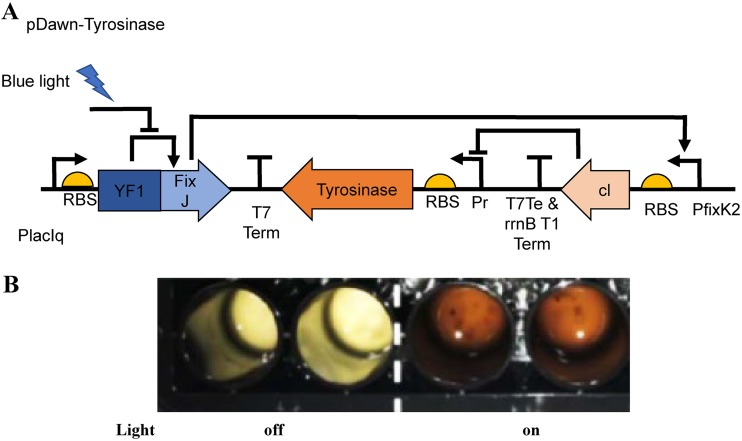
FIG 2.
Induction of tyrosinase gene expression and melanin production in V. natriegens in an optogenetic system. (A) pDawn plasmid for light-activated gene expression in V. natriegens. YF1/FixJ drives gene expression from the pfixK2 promoter and is repressed by blue light. Insertion of the λ phage repressor cI and the λ promoter pR makes expression of the tyrosinase gene light activated. (B) Melanin production in the light plate array. The engineered bacterial cells were incubated in each well for 48 h. Left, duplicate wells with light off; right, duplicate wells with light on. RBS, ribosome-binding site.
Tyrosinase has been demonstrated to catalyze a variety of substrates, including short peptides containing l-tyrosine, for the synthesis of melanin-like polymeric pigments with diverse physicochemical properties in vitro (27). To determine the ability of V. natriegens-produced tyrosinase to catalyze the formation of melanin from multiple substrates, we added four tripeptides (DFY, FDY, DYF, and YFD) to tyrosinase-producing cultures of V. natriegens at 5 mM. Orange pigments with a variety of color intensities were observed in cell cultures after 2 h (Fig. 1D). In agreement with the in vitro experiment (28), tripeptides that contain paired aromatics (DFY, DYF, and YFD) gave rise to a relatively darker orange color than that of the tripeptide that has the pair separated by a charged aspartic acid. This result indicates that the paired aromatics in tripeptides not only tend to aggregate during the self-assembly process but also enhance the polymerization of oxidized peptides. It also suggests that tyrosinase-expressing V. natriegens can be used to produce functionalized melanin-like biopolymers with tunable properties by using various l-tyrosine-containing short peptides as the substrates.
Induction of melanin production with optogenetics.
In an industrial setting, it may be desirable to have temporal control over the production of certain molecules in a chassis organism. Nonchemical induction using optogenetics offers many exciting opportunities and applications for controlling cell responses in programmable ways and is becoming a valuable part of synthetic biology circuits. Therefore, we also tested induction of tyrosinase in V. natriegens using the light-driven pDawn system (Fig. 2A) (29). In the absence of blue light, the histidine kinase YF1, which contains a photosensory domain, phosphorylates its cognate response regulator FixJ and drives robust gene expression of λ repressor cI from the FixK2 promoter. The cI repressor prevents tyrosinase expression. Upon light exposure, a significant reduction in kinase activity of YF1 and, consequently, cI expression results in derepression of the pR promoter and tyrosinase expression, which then catalyzes melanin production in the presence of l-tyrosine. To control light input, we built the 48-well-plate-fitted light plate array (LPA) designed by the Tabor lab (30) to test our constructs. As can be seen in Fig. 2B, with the tested parameters, we saw melanin production in V. natriegens upon induction with light and no melanin where no light was applied. This proof-of-concept experiment demonstrated induction of a bioproduct through optogenetics in V. natriegens.
Bacterial melanin nanoparticles and “melanin ghosts.”
Due to their high biocompatibility, melanin nanoparticles have been investigated in many biomedical and material applications and been made from chemical or enzymatic oxidation of the precursors l-DOPA or dopamine (27). In order to determine whether the recombinant bacterium was able to make melanin nanoparticles, the black supernatant separated from the V. natriegens culture 30 min after melanization and was subjected to transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses. TEM revealed uniform black particles of less than 20 nm, and DLS measurements showed a dominant cluster with particle sizes around 20 nm as well (Fig. 3A). However, these particles were not observed in the supernatant from the nonmelanized cell cultures. In the nonmelanized sample, two large peaks with small percentages of particle numbers were observed, and they might be attributed to by-products of cell culture extracts. Moreover, the sizes of melanin nanoparticles in the supernatant 12 h after melanization were around 70 nm (Fig. 3A), suggesting that longer incubation times increased the size of the nanoparticles. The addition of an extra 100 mM NaCl to the M9 medium resulted in larger particles (larger than 100 nm; Fig. 3A) and spontaneous precipitation of melanin aggregates, indicating that the sizes of melanin particles were also affected by salt concentration.
FIG 3.
Extracellular melanin particles and bacterial melanin ghosts. (A) a, TEM image of melanin nanoparticles in the supernatant of V. natriegens cell culture after l-tyrosine addition in the bacterial culture at 30 min. b, hydrodynamic sizes of melanin nanoparticles measured by DLS. Melanin nanoparticles were formed after the addition of l-tyrosine in the presence of 250 mM NaCl. The measured average sizes were 16 ± 0.6 nm with l-tyrosine addition (similar to melanin nanoparticle measurement by TEM) and 97 ± 39 nm for the control without l-tyrosine addition that were attributed to the by-products of cell culture extract. c, melanin nanoparticles formed after l-tyrosine addition at 12 h with different concentrations of salt. The peaks showed at 35 ± 3.4 nm (250 mM NaCl, blue), 73 ± 5.3 nm (350 mM NaCl, orange), and 86 ± 9.6 nm (550 mM NaCl, gray). (B) SEM and TEM images of melanin ghosts and melanized bacterial cells.
Melanin synthesized from other microbes such as fungi is typically located within the cell wall and plays a significant role in maintaining cell wall integrity (31). Melanin structures associated with the fungal cell walls, so-called “melanin ghosts,” are generated by exposure of melanized cells to 4 M guanidinium isothiocyanate followed by 6 M HCl at 100°C. This protocol was applied to treat melanized and nonmelanized V. natriegens cells. Under the same chemical treatment conditions, the nonmelanized bacterial cells were completely solubilized, but the melanized cells remained as black particulate materials. Scanning electron microscopy (SEM) images showed that these black particles exhibited morphologies similar to those of bacterial cells (Fig. 3B). It was also noted that there were nanosized granules on the surface, which might be melanin aggregates resulting from acid precipitation. In contrast to fungal melanin ghosts that had melanin walls without internal structures, TEM revealed that the bacterial black particles were filled with irregular electron-dense structures with no defined organelles or cell wall layers (Fig. 3B). This result showed that melanin ghost-like structures could be generated from bacteria as well even after other cellular components were depleted by acid hydrolysis.
Chemical characterization of melanin produced from V. natriegens.
To verify the chemical composition of melanin produced from V. natriegens, black powder was extracted from the supernatant of melanized bacteria and analyzed with Fourier transform infrared (FTIR) spectroscopy. A comparison of the bacterium-producing melanin and synthetic melanin revealed significant similarities between IR spectra (Fig. 4A) representing equivalent functional groups, such as the hydrogen bond of an -OH group, an alkyl -CH2 group, and an aromatic ring including a C=C group. Notably, a peak at 1,722 cm−1 suggested a C=O carbonyl group vibration, indicating the carboxylic acid from dihydroxyindole-2-carboxylic acid (DHICA).
FIG 4.
Characterization of melanin produced from V. natriegens. (A) FTIR spectra of bacterial (Bac) melanin and synthetic (Syn) melanin. Peaks in common and their assignments are 1,625 cm−1, C=C vibration in aromatic (indole) ring; 1,722 cm−1, C=O carbonyl group vibration from carboxylic acid in DHICA; triple peaks at ∼2,900 cm−1, CH2 alkyl vibration; and broad peak centered at 3,415 cm−1, O-H hydroxyl vibration. a.u., arbitrary units. (B) EPR spectra of melanin (Mel) powders, dried melanized and nonmelanized V. natriegens (Vnat) cells, and melanin ghosts. (C) EPR changes of melanin ghosts and dried melanized cells responding to illumination. arb., arbitrary.
Stable free radicals are characteristic of melanin and have a unique electron paramagnetic resonance (EPR) signal. To examine this signal in bacterial synthesized melanin, equal amounts of extracted melanin powders, melanized bacterial cells, bacterial melanin ghosts, and nonmelanized cells were examined using EPR (Fig. 4B). The first three samples exhibited distinct, stable free radical signals with Zeeman splitting g-values of 2.004 that are highly similar to synthetic eumelanin, fungal 1,8-dihydroxynaphthalene (DHN)-melanin, and bacterial pyomelanin (22). It was noted that melanin ghosts had the relatively highest signal intensity, and extracted melanin powders had the weakest signals. As expected, nonmelanized cells did not show an EPR signal. Illumination of melanin ghosts and melanized bacteria with UV-visible light from a xenon (Xe) lamp for 30 min increased the EPR signals (Fig. 4C).
Redox activity of melanized bacteria.
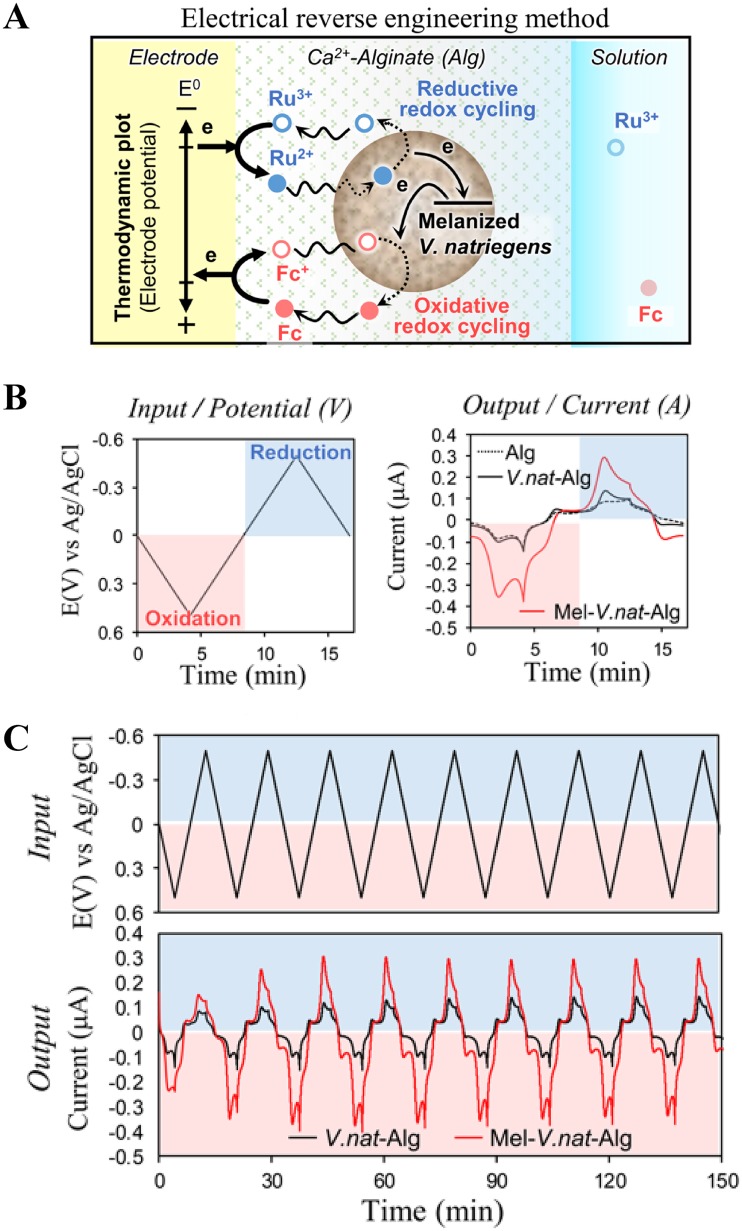
We employed an electrochemical reverse engineering method (40) to characterize the redox activity of melanized V. natriegens cells. Figure 5A shows the schematic of the electrochemical reverse engineering method. Here, the melanized V. natriegens cells were immobilized at an electrode surface by codepositing it within a permeable alginate hydrogel film. The film-entrapped cells were probed for redox activity by immersing the film-coated electrode into a solution containing two diffusible mediators that can freely diffuse through the matrix and access the electrode. When the underlying electrode is cycled to oxidative (more positive) voltages, one mediator (50 μM ferrocene dimethanol [Fc]) can undergo oxidative redox cycling, in which Fc donates an electron to the electrode, the oxidized form (Fc+) diffuses into the film and accepts an electron from melanin, and this rereduced Fc mediator can then diffuse back to the electrode where it can be reoxidized by donating the electron to the electrode. This oxidative redox cycling serves to extract electrons from the melanin. The other mediator [50 μM Ru(NH3)6Cl3 [Ru3+]) can undergo reductive redox cycling when the electrode potential is cycled to a reducing (more negative) voltage, in which Ru3+ accepts an electron from the electrode; the reduced form, Ru2+, diffuses into the film and donates its electron to melanin; and the reoxidized Ru3+ form diffuses back to the electrode where it can be rereduced. This reductive redox cycling serves to transfer electrons from the electrode to the melanin.
FIG 5.
Redox activity of melanized V. natriegens (V.nat) revealed by reverse electrical engineering method. (A) Schematic shows that melanin can donate/accept electrons to/from mediators by oxidative/reductive redox cycling process (details are described in the supplemental material). (B) The imposed input potential (i.e., voltage) and observed output current response associated with Ru3+ and Fc mediators. (C) Long-term cyclic experiments test for the reversibility of redox-activity; steady amplifications are signatures of reversible and repeated oxidation and reduction of melanin. E(V), potential in volts.
To control these redox cycling processes, an oscillating input voltage is imposed to the electrode (Fig. 5B). As the redox cycling processes occurs, the output currents associated with mediator oxidation/reduction reactions are measured. Two negative controls including the alginate-only film and the alginate film with nonmelanized V. natriegens showed small output peak currents. However, the melanized V. natriegens-alginate film-coated electrode showed large peak currents for both Ru3+ reduction and Fc oxidation (Fig. 5B). The high amplification of the redox currents provides evidence that the melanin produced by V. natriegens confers redox activity. To test the reversibility of this redox activity, the imposed input potential was repeatedly cycled over 150 min, as shown in Fig. 5C. The output current curve shows that when the melanized V. natriegens cells were embedded in the film, the mediator currents were amplified during both oxidation and reduction. Importantly, this amplification remained nearly steady over 2 h. This result indicates that the melanin produced by V. natriegens can be reversibly oxidized and reduced, and thus, the melanin can be repeatedly switched between redox states.
UV protection.
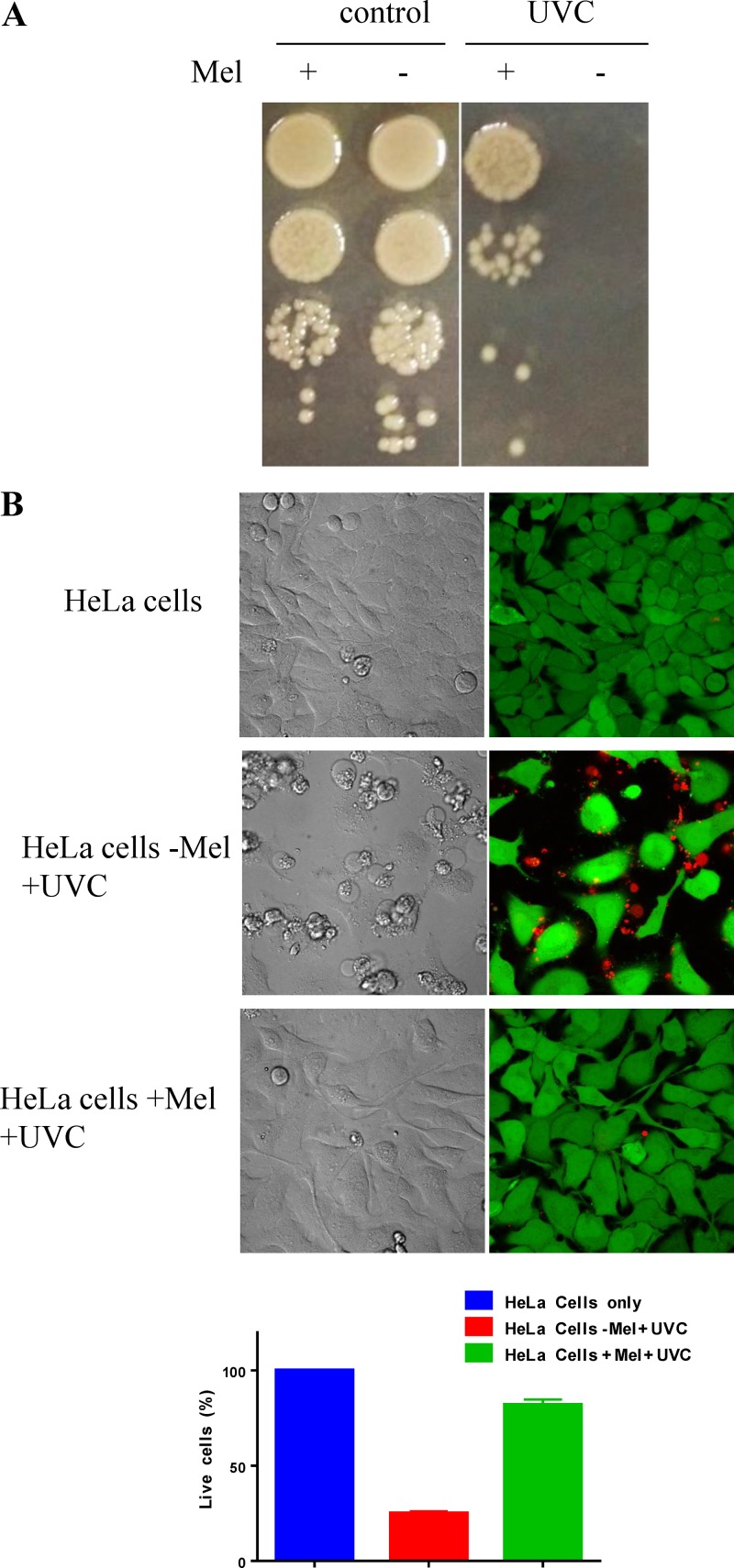
Melanin has been demonstrated to have a role in protecting against oxidative stresses such as UV irradiation (33). When washed melanized and nonmelanized V. natriegens cells suspended in phosphate-buffered saline (PBS) were irradiated with UVC, both types of cells were killed completely, indicating that the melanin in the cell walls was not sufficient to protect the cells. However, we did observe the protective property of melanin when melanized cell culture in the growth medium supplemented with l-tyrosine was exposed to UVC. UV at 450 mJ/cm2 completely killed bacterial cells in the nonmelanized culture (without adding l-tyrosine), but the cells in the melanized culture survived (Fig. 6A). Furthermore, supernatants filtered from the nonmelanized and melanized cell cultures were added into HeLa cell cultures, which were then irradiated with 4 mJ/cm2 UVC. Figure 6B shows that more than 90% of HeLa cells mixed with the melanized supernatant were still alive after UVC irradiation, but less than 20% of cells mixed with the nonmelanized supernatant survived under the same conditions. Therefore, the extracellular melanin nanoparticles produced here have the ability to protect mammalian cells from UVC irradiation.
FIG 6.
Photoprotective properties of biosynthesized melanin. (A) Survival of the nonmelanized and melanized V. natriegens cell cultures irradiated with 450 mJ/cm2 UVC. (B) Survival of HeLa cells suspended in the supernatants of the nonmelanized (−Mel) and the melanized (+Mel) V. natriegens and irradiated with 4 mJ/cm2 UVC. The control includes HeLa cells only without irradiation. Live/dead staining is shown as green for live cells and red for dead cells.
TNT adsorption.
Melanin also exhibits promiscuous binding capabilities as a result of its complex polymeric structure. This situates melanin-producing bacteria as candidates for bioremediation applications. We therefore explored the ability of melanin-producing V. natriegens to bind and sequester the common environmental contaminant 2,4,6-trinitrotoluene (TNT), an explosive material. We added melanin-producing V. natriegens to a solution of TNT with a final concentration of 125 ppb in PBS and observed that melanized cells could bind and remove most of the compound from solution. This process was time dependent, with only ∼25% of TNT removed after a 5-min incubation but nearly 100% removed after a 30-min incubation (Fig. 7A). Additionally, the binding of TNT was pH dependent, with the efficiency of TNT removal from solution going from nearly 100% at pH 6, to 80% at pH 5, and to only 10% at pH 4 and pH 3 (Fig. 7B).
FIG 7.
(A and B) Time-dependent (A) and pH-dependent (B) adsorption of TNT by ∼5 × 108 nonmelanized (light gray) and melanized (dark gray) V. natriegens cells.
DISCUSSION
Melanin production by tyrosinase has been described in several bacterial species (9, 25, 34–37). Due to their unique physiochemical properties and potential industrial applications, it is desirable to produce melanins at large scale and low cost. Heterologous expression of tyrosinase genes in E. coli has been reported to produce melanins when growth media are supplemented with l-tyrosine, but melanin synthesis was slow, and melanins were fully produced only after at least 30 h (Table 2). We previously demonstrated that genetic modules adopted from E. coli functioned similarly in V. natriegens (20). The successful regulation of tyrosinase gene expression and melanin production through engineering inducible promoters in V. natriegens supports that this fast-growing marine bacterium represents a new synthetic biology chassis able to host a broad range of the genetic circuits and modulate diverse biomaterial manufactures. The proteomics analysis also validated that the expression of tyrosinase was steadily regulated by the Ptac promoter in V. natriegens. With a growth rate that is two times higher than that of E. coli, V. natriegens has a clear advantage as a biomanufacturing host by significantly reducing fermentation time, which will result in an economical benefit for large-scale melanin production. It was noted that, with the substrate concentrations below 0.6 mg/ml, melanin production accumulated to a maximal level and then became steady probably due to complete consumption of l-tyrosine. However, adding the substrate l-tyrosine at a concentration of or higher than 0.6 mg/ml resulted in melanin aggregation in the media due to solubility limitations at higher concentrations of these final products (Fig. 1C). We also demonstrated induction of this biopigment through optogenetics in V. natriegens. This exciting avenue not only illustrates that this fast-growing bacterium is an excellent chassis to host synthetic biology circuits but also revealed its great potential to make novel sensing and protective biomaterials in response to easily manipulated external signals. Since optogenetic systems that respond to UV light are available (30), they could be used to trigger melanin production to protect the cells from further UV damage or create patterns based on UV exposure.
Previously, V. natriegens was demonstrated to speed up selenium nanoparticle production by its own reducing capability (38). Our study suggested that V. natriegens could be used as a fast biocatalyst for biopolymer production. In this study, the tyrosinase was not tagged with the adhesin involved in diffuse adherence (AIDA) autotransporter vehicle for surface expression like in the previous investigation in E. coli (13), but melanins were not only found to be distributed in the cytoplasm and the cell surface by electronic microscopy but also largely produced in the cell cultures as nanoparticles (Fig. 3). Moreover, V. natriegens might be more susceptible to changes in pH and osmolarity than is E. coli, which can result in increased cell permeability or cell lysis, especially where V. natriegens is transferred from rich medium to minimal medium (Fig. 1A). This susceptibility resulted in the release of tyrosinase into growth media that accelerated melanin production and could potentially result in lower downstream cost due to the reduced energy or time required to break open cells for protein harvest.
The formation of melanin nanoparticles has been well described in chemical reactions (39) and usually takes more than 12 h. In this study, we demonstrated that bacteria were able to produce melanin nanoparticles (MNPs) in as little as 30 min in cell culture (Fig. 4A). Correlations of particle sizes to incubation time and salt concentrations in the media (Fig. 3A) indicated that the sizes of bacteria producing MNPs could be tuned by controlling growth conditions. Melanin ghosts have been hallmarks for the study of melanin in fungi and are very stable after harsh hydrolysis treatment. Interestingly, we demonstrated that melanin ghosts could be formed in melanized bacteria as well, but melanin not only aggregated on the cell surface but also in the internal structure of ghosts (Fig. 4B), which was different from the fungal melanin ghosts that melanin assembled in the fungal cell walls. Heterogeneity due to cellular components and spherical morphologies, therefore, appears to provide melanin ghosts with novel properties not exhibited by pure melanins.
Both EPR and electrochemical analyses demonstrated that the melanized V. natriegens cells possess stable free radical scavenger and active redox properties. These properties will render additional and broader biological functions to this fast-growing bacterium. Along with mechanical resistance, the melanized bacteria may be a synthetic biology chassis well suited for functioning in extreme environments. Like fungal DHN-melanin and sepia melanin (40), the melanized bacteria in this study could be repeatedly engaged in redox-cycling reactions that yielded amplified output currents, which indicated that melanin can be reversely and stably switched between oxidized and reduced redox states. This property will allow bacterial cells to exchange electrons with diffusible redox-active species in the cellular environment and sense biological or environmental redox signals. Moreover, it has been reported that the biological melanin may perform energy-harvesting activities (41, 42) using an unknown mechanism. Thus, the melanized bacteria potentially can be developed as living materials to preserve charge storage capacity and power transient electrical devices.
To test the photoprotective property of bacterial melanin, we irradiated bacterial cells with short-wavelength UVC, which is the most damaging type of UV radiation. UVC is absorbed by DNA and results in the formation of pyrimidine adducts and strand breaks (43). Initially, we irradiated the PBS-washed melanized bacterial cells with UVC and did not see improved UV resistance compared with that with the nonmelanized cells. This finding suggested that the melanin granules deposited in the cell wall and cytoplasm were not sufficient to absorb UV radiation. When melanized cell cultures were irradiated with UVC, cells grown in the melanin-containing medium showed significantly higher resistance (Fig. 6A). This result was similar to findings from our previous report that pyomelanin-containing supernatant from Vibrio campbellii ΔhmgA culture demonstrated protective property against oxidative stress (44). Melanin is known to scavenge reactive oxygen species (ROS) generated by UV in solution, and its scavenging capability is proportional to its concentration (32). Therefore, our result indicated that the larger volume of melanin particles in the cell medium greatly improved melanin’s ability to attenuate UVC penetrating into cells and scavenge ROS generated from irradiation in the aqueous environment. The photoprotective property of MNPs was further confirmed by applying the filtered supernatant to HeLa cells (Fig. 6B). Therefore, large-scale production of MNPs from V. natriegens may have potential to make cost-effective photoprotection materials.
Melanin has been known for its affinity to adsorb various chemicals and drugs, including harmful substances, which results in protection of pigmented cells and tissues (45). Gustavsson et al. (13) successfully demonstrated that E. coli cells coated with melanin were able to adsorb up to 80% of the antimalarial drug chloroquine at concentrations typical for pharmaceutical pollution in wastewater. Here, we showed that the melanized marine bacterium was able to robustly sequester the explosive compound TNT from an aqueous solution. The discharge of explosive compounds such as TNT from explosive manufacturing and ammunition loading plants to wastewater has been of increasing concern. Currently, TNT-contaminated water is treated with granular activated carbon (GAC) adsorption (46). However, the regeneration of GAC may be an issue when a large amount of TNT is adsorbed. Our study demonstrates that melanin-producing V. natriegens is able to provide dynamic and economic TNT-adsorbing materials that may be regenerated through changes in pH.
In summary, we successfully engineered a V. natriegens strain to rapidly and economically produce melanin from tyrosinase, and analyses revealed that this melanin exhibited physical and chemical properties similar to those of natural and chemically synthesized melanins. We anticipate that producing and controlling melanin structures at the nanoscale in this fast-growing bacterial system with synthetic biology tools will lead to the generation of novel biomaterials for multiple applications, including protection of chemical and radiation threats, therapeutics, electronics, sensing, and bioremediation that could benefit both military and civilian populations.
MATERIALS AND METHODS
Strains and growth conditions.
V. natriegens strain ATCC 14048 was grown in LBv2 (Luria broth [Miller] supplemented with 204 mM NaCl, 4.2 mM KCl, and 23.14 mM MgCl2) or M9 medium (11.28 g M9 minimal salts 5×, 4 g glucose, 15 g NaCl, 0.5 g MgSO4, 10 mg CaCl2, and 40 mg CuSO4 per liter), unless otherwise stated. Tripeptides were purchased from Peptide 2.0, Inc. (Chantilly, VA). HeLa ATCC CCL-2 cells (ATCC, Manassas, VA) were cultured in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, IL).
Construction of melanin-producing V. natriegens.
The tyrosinase gene from Bacillus megaterium Tyr1 was synthesized by Eurofins Genomics (Louisville, KY), similar to a previous study (13). The PCR-amplified tyr1 sequence was cloned into the plasmid pJV298 under the control of the inducible promoter Ptac (26), replacing the green fluorescent protein (GFP) gene with the Gibson Assembly master kit (New England BioLabs, Ipswich, MA) with four primers, as follows: tyr1-1, ATGTATATCTCCTTAAGCTTACG; tyr-2, TGAGGATCCGGTGATTGATTG; vect-1, GCTTAAGGAGATATACATATGGTAACAAGTATAGAGTTAGAAAAAAC; and vect-2, AATCACCGGATCCTCATGAGGAACGTTTTGATTTTC). The pDawn-tyr1 plasmid was constructed as follows: pDawn (29) was cut with NdeI and BamHI and incubated with alkaline phosphatase and calf intestinal (CIP; New England BioLabs). The tyr1 gene was amplified using the primers tyr-F (cggagctcgaattcgTCATGAGGAACGTTTTGATTTTC) and tyr-R (ccgcgcggcagccaATGGGTAACAAGTATAGAGTTAG) (lowercase letters complement the vector sequence, and uppercase letters complement the tyr1 sequence), and a Gibson Assembly reaction was set up to insert the PCR product into the digested pDawn vector using the Gibson Assembly Master kit (New England BioLabs). Plasmid maps are shown in Fig. S2.
Melanin production.
V. natriegens/pJV-Tyr1 from a glycerol stock was inoculated into 3 ml LBv2 liquid medium supplemented with 6 μg/ml chloramphenicol (LBv2-Cm) and grown at 30°C overnight. A 0.5-ml aliquot of the overnight culture was transferred into 50 ml LBv2-Cm medium and incubated at 200 rpm at 37°C for 2 h. Tyrosinase production was induced by the addition of 200 μM isopropyl-β-d-1-thiogalactopyranoside (IPTG) for three more hours. Cells were pelleted from the culture via centrifugation, washed with M9 minimal medium once, and resuspended in 50 ml M9 medium supplemented with 50 μg/ml CuSO4 and 0.4 mg/ml l-tyrosine (nonmelanized bacterial cells were prepared by omitting l-tyrosine in the same medium). The melanized bacterial cells were harvested by centrifugation, and the black supernatant that contained melanin nanoparticles was passed through a Millipore 0.2-μm polyethersulfone membrane to remove cell debris. Melanin was precipitated from the supernatant by adding a 1/10 volume of 6 N HCl, washed with deionizing water until neutral pH, and lyophilized into black powder. Bacterial growth curves were measured at an optical density at 600 nm (OD600) using a Bioscreen C analyzer (Growth Curves USA, Piscataway, NJ). Pigment intensities from melanized bacterial cultures (adding l-tyrosine in the medium) were measured at an OD492 by subtracting readings from nonmelanized bacterial cultures (omitting l-tyrosine in the same medium) using the same instrument. OD492 values were converted to melanin yields using the standard curve made from the melanin powder precipitated from the supernatant. The rate of melanin production was calculated as the quantity of melanin produced and divided by the estimated cell dry weight in 1 ml of culture within a 1-h period before melanin saturation (14). The immunofluorescent analysis method for the melanized cells is described in the supplemental material.
Optogenetic induction using the light plate apparatus.
The light plate apparatus (LPA) for optogenetic induction was constructed based on the paper by Gerhardt et al. (30). Overnight cultures of V. natriegens were inoculated into a 24-well plate with 1 ml LBv2 plus 200 μg/ml kanamycin, 0.4 mg/ml l-tyrosine, and 10 μg/ml CuSO4 and 2 to 3% cell culture per well. The plate was covered with a BreathEasy membrane and assembled into the LPA. The LPA was loaded with a program in Iris from the Tabor lab (http://taborlab.github.io/Iris/). The program was set up with the following parameters: light intensities always on for each column in the plate, with three of 0 and three of 2,000. The LPA was incubated at 37°C in a foil-covered incubator for ∼48 h at 250 rpm to allow for tyrosinase production and the conversion of l-tyrosine to melanin.
Proteomic quantification of the expressed tyrosinase.
IPTG-induced and -uninduced V. natriegens/pJV-Tyr1 cultures were normalized to the same cell numbers after counting with the flow cytometry. Cell pellets were reconstituted in 100 μl of 10% n-propanol in 50 mM ammonium bicarbonate (NPABC). Thirty microliters of the cell suspension was lysed, digested with trypsin in a Barocycler (Pressure BioSciences, Inc.), dried in the speed vacuum, and further suspended in 90 μl of 0.1% formic acid. Three microliters of this suspension was injected into the liquid chromatography-tandem mass spectrometry (LC-MS/MS) system for analysis (Thermo U3000 nano-LC coupled to a Thermo Orbitrap Fusion Lumos mass spectrometer). The acquired data were converted into mgf files and searched with Mascot against a Vibrio natriegens database, custom database containing the tyrosinase sequence, and common contaminant database. Selected peptides assigned to the tyrosinase protein and DnaK were then manually extracted from the raw data, and the chromatographic peak area was calculated. Area ratios between induced and uninduced samples are reported in Table 1. The DnaK protein was used as a representative of endogenous protein not controlled by the inducer.
Generation and characterization of melanin ghosts.
The protocol for making bacterial melanin ghosts was modified from a method previously developed in fungi (31). Melanized bacteria were incubated with 4 M guanidinium thiocyanate at room temperature (RT) for 10 min. Cells were washed with deionized water once and hydrolyzed by boiling in 6 M HCl for 20 min. The resulting black particles (melanin ghosts) were washed with deionized water multiple times until a pH of 6 was obtained. Imaging of the melanin ghosts and melanized V. natriegens cells was conducted using a Philips CM200 TEM and a FEI Quanta 600 SEM, respectively.
Sample preparation for imaging.
Bacterial cells or melanin ghosts were prefixed with 2.5% glutaraldehyde, 100 mM HEPES buffer, 50 mM l-lysine, and 7.5% ruthenium red for 30 min, fixed with the same solution minus l-lysine for 2 h, and postfixed with a 4% osmium tetroxide (OsO4) solution for 2 h. Each sample was dehydrated in increasing amounts of acetone (25%, 50%, 70%, 95%, and 100%) for 30 min at each step. TEM samples were trimmed and ultramicrotomed using an RMC Ultracut microtome with a 35° DiaTome diamond knife. Sections were cut at a thickness of 70 nm and picked up onto 400-mesh Cu grids. Once the grids were dried, they were stained with UranyLess stain.
Measurement of melanin nanoparticles.
Dynamic light scattering (DLS) measurements were carried out using a ZetaSizer Nano series instrument equipped with a HeNe laser source (λ = 633 nm) (Malvern Instruments Ltd., Worcestershire, UK) and analyzed using dispersion technology software (DTS) (Malvern Instruments Ltd.). Structural characterization of melanin nanoparticles was carried out using a JEM-2100 TEM. Samples for TEM were prepared by spreading a drop (5 to ∼10 μl) of melanin nanoparticles onto ultrathin carbon/holey support film on a 300-mesh Au grid (Ted Pella, Inc.) and letting it dry. Individual particle sizes were measured using a Gatan digital micrograph (Pleasanton, CA); average sizes along with standard deviations were extracted from an analysis of ∼100 nanoparticles.
Fourier transform infrared spectroscopy.
One milligram of melanin powder was ground with 160 mg anhydrous potassium bromide (KBr; FTIR grade, ≥99% trace metals basis; Sigma-Aldrich) and compressed into a semitransparent pellet using a hydraulic press (Omega CN9000). The transmission FTIR measurements (iS50 FTIR; Nicolet) were taken with an air background.
Electron paramagnetic resonance.
Melanin powder, melanin ghosts, and dried, melanized bacteria were characterized by EPR at 300 K in a Bruker 9.5-GHz spectrometer. Typical microwave powers of 5 to 20 mW with 1-G modulation amplitude and 100 kHz field modulation were employed for these experiments. EPR spectra were also obtained for the melanin samples after illumination with a xenon (Xe) lamp (75 W, 350 to 1,000 nm wavelength) for 30 min at RT.
Electrochemical measurements.
Melanized and nonmelanized V. natriegens cells were probed for redox activity by first immobilizing them within a Ca2+-alginate hydrogel film at an electrode surface. These films were prepared by electrodeposition by suspending the cells in a mixed solution containing 1% alginate and 0.25% CaCO3 particles and electrodepositing the film onto a standard gold electrode by immersing this electrode into the mixed solution and biasing it to serve as the anode (4 A/m2, 60 s), using a Pt wire as the cathode (2400 SourceMeter; Keithley) (47, 48). Electrochemical probing for the redox activity of these films was performed using cyclic voltammetry (CV) in a three-electrode system (CHI Instruments 600C electrochemical analyzer), as follows: (i) the film-coated standard gold electrode was the working electrode, (ii) Ag/AgCl was the reference electrode, and (iii) Pt wire was the counterelectrode. Air was excluded by purging N2 during the experiment. All of the CV experiments were performed at the scan rate of 2 mV/s.
Cell survival assay.
Twenty microliters of the nonmelanized and the melanized V. natriegens cell cultures or washed cells suspended in PBS was irradiated with UVC (254 nm) at 450 mJ/cm2 for 20 min, diluted with PBS, and spotted on LBv2 agar plates. HeLa cells were seeded on 35-mm dishes with 14-mm glass-bottom inserts (no. 1.0 cover glass; MatTek Corp., MA, USA) at a density of ∼7 × 104 cells/ml (3 ml/well) and incubated under regular cell culturing condition (5% CO2 at 37°C) for 24 h to a confluence of 70 to 80%. Dishes were coated with fibronectin (10 to 20 μg/ml) in Dulbecco’s phosphate-buffered saline (DPBS) before adding the cell suspension. The cell monolayers on the dishes were washed with DPBS three times and submerged with the supernatant filtered from the melanized V. natriegens culture diluted (1:1) with live cell imaging solution (LCIS). Cells were then subjected to UV irradiation (254 nm, 6 mJ/cm2) for 10 s, followed by incubation for 24 h in under regular culturing medium and conditions. Next, cells were washed with LCIS, followed by staining with a fluorescence LIVE/DEAD viability kit (Thermo Fisher Scientific, Grand Island, NY) for live/dead quantification. The stained cells were imaged with confocal laser scanning microscopy (CLSM) using a Nikon A1Rsi confocal microscope. Fluorescence images of individual cells were counted and quantified for live (green) and dead (red) status for 50 to 70 cells for each sample from two independent experiments.
Chemical adsorption assays.
For the TNT absorption assays, melanized cell cultures were developed as described above, and 500 μl of cells (∼109/ml) was added to 500 μl of a 125 ppb solution of 2,4,6-trinitrotoluene (TNT) (Cerilliant, Inc., Round Rock, TX). These cell suspensions were then vortexed for 5, 10, 30, 60, or 180 min before pelleting. For determining the pH dependence of binding, the melanized V. natriegens cells were pelleted and resuspended in citrate-phosphate buffer (pH 3 to 8) (49). Five hundred microliters of cells in this buffer was then mixed with a solution containing 125 ppb TNT in the same buffer with the appropriate pH. This mixture was vortexed for 30 min before pelleting by centrifugation. The concentration of TNT in the supernatant was measured using an Agilent 1290 Infinity high-performance liquid chromatography (HPLC) system equipped with an Agilent reverse-phase C18 analytical column (Eclipse XDB-C18; 5 mm; 4.6 by 250 mm2; Santa Clara, CA, USA). UV-Vis detection at 254 nm was performed to monitor the elution of TNT.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julia van Kessel (Indiana University) for providing the pJV298 plasmid.
This work was supported by the Assistant Secretary of Defense for Research and Engineering [ASD(R&E)] through the Applied Research for Advancement of S&T Priorities Synthetic Biology for Military Environments program. We also gratefully acknowledge financial support from the U.S. National Science Foundation (grant DMREF-717 1435957), the Air Force Office of Scientific Research under the Multidisciplinary Research Program of the University Research Initiative (MURI) (grant FA9550-18-1-0142), and the Department of Defense (Defense Threat Reduction Agency, grants HDTRA1-13-1-0037 and HDTRA1-719 15-1-0058).
The views expressed here are those of the authors and do not represent those of the U.S. Navy, the U.S. Department of Defense, or the U.S. Government.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.d'Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S, Galván I, Ghanem G, Kenzo K, Meredith P, Pezzella A, Santato C, Sarna T, Simon JD, Zecca L, Zucca FA, Napolitano A, Ito S. 2015. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell Melanoma Res 28:520–544. doi: 10.1111/pcmr.12393. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Khetan A, Wu W, Chun SE, Viswanathan V, Whitacre JF, Bettinger CJ. 2016. Evidence of porphyrin-like structures in natural melanin pigments using electrochemical fingerprinting. Adv Mater 28:3173–3180. doi: 10.1002/adma.201504650. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer AD, Howell RC, Jiang Z, Bryan RA, Gerfen G, Chen CC, Mah D, Cahill S, Casadevall A, Dadachova E. 2009. Physico-chemical evaluation of rationally designed melanins as novel nature-inspired radioprotectors. PLoS One 4:e7229. doi: 10.1371/journal.pone.0007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel RP, Okun MR, Edelstein LM, Cariglia N. 1974. Peroxidatic conversion of tyrosine to dopachrome. J Invest Dermatol 63:374–377. doi: 10.1111/1523-1747.ep12680867. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Simon JD. 2003. Isolation and biophysical studies of natural eumelanins: applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res 16:606–618. doi: 10.1046/j.1600-0749.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 6.Aghajanyan AE, Hambardzumyan AA, Hovsepyan AS, Asaturian RA, Vardanyan AA, Saghiyan AA. 2005. Isolation, purification and physicochemical characterization of water-soluble Bacillus thuringiensis melanin. Pigment Cell Res 18:130–135. doi: 10.1111/j.1600-0749.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera-Valladares N, Martínez A, Piñero S, Lagunas-Muñoz VH, Tinoco R, de Anda R, Vázquez-Duhalt R, Bolívar F, Gosset G. 2006. Expression of the melA gene from Rhizobium etli CFN42 in Escherichia coli and characterization of the encoded tyrosinase. Enzyme Microb Technol 38:772–779. doi: 10.1016/j.enzmictec.2005.08.004. [DOI] [Google Scholar]
- 8.Cubo MT, Buendia-Claveria AM, Beringer JE, Ruiz-Sainz JE. 1988. Melanin production by Rhizobium strains. Appl Environ Microbiol 54:1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Naggar NEA, El-Ewasy SM. 2017. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci Rep 7:42129. doi: 10.1038/srep42129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Rivera J, Eisenman HC, Nosanchuk JD, Aisen P, Zaragoza O, Moadel T, Dadachova E, Casadevall A. 2005. Comparative analysis of Cryptococcus neoformans acid-resistant particles generated from pigmented cells grown in different laccase substrates. Fungal Genet Biol 42:989–998. doi: 10.1016/j.fgb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Kanteev M, Goldfeder M, Fishman A. 2015. Structure-function correlations in tyrosinases. Protein Sci 24:1360–1369. doi: 10.1002/pro.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plonka PM, Grabacka M. 2006. Melanin synthesis in microorganisms–biotechnological and medical aspects. Acta Biochim Pol 53:429–443. doi: 10.18388/abp.2006_3314. [DOI] [PubMed] [Google Scholar]
- 13.Gustavsson M, Hornstrom D, Lundh S, Belotserkovsky J, Larsson G. 2016. Biocatalysis on the surface of Escherichia coli: melanin pigmentation of the cell exterior. Sci Rep 6:36117. doi: 10.1038/srep36117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagunas-Muñoz VH, Cabrera-Valladares N, Bolívar F, Gosset G, Martínez A. 2006. Optimum melanin production using recombinant Escherichia coli. J Appl Microbiol 101:1002–1008. doi: 10.1111/j.1365-2672.2006.03013.x. [DOI] [PubMed] [Google Scholar]
- 15.Guo J, Rao Z, Yang T, Man Z, Xu M, Zhang X, Yang ST. 2015. Cloning and identification of a novel tyrosinase and its overexpression in Streptomyces kathirae SC-1 for enhancing melanin production. FEMS Microbiol Lett 362:fnv041. doi: 10.1093/femsle/fnv041. [DOI] [PubMed] [Google Scholar]
- 16.Tarangini K, Mishra S. 2014. Production of melanin by soil microbial isolate on fruit waste extract: two step optimization of key parameters. Biotechnol Rep (Amst) 4:139–146. doi: 10.1016/j.btre.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long CP, Gonzalez JE, Cipolla RM, Antoniewicz MR. 2017. Metabolism of the fast-growing bacterium Vibrio natriegens elucidated by 13C metabolic flux analysis. Metab Eng 44:191–197. doi: 10.1016/j.ymben.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eagon RG. 1962. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J Bacteriol 83:736–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HH, Ostrov N, Wong BG, Gold MA, Khalil AS, Church GM. 2019. Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat Microbiol 4:1105–1113. doi: 10.1038/s41564-019-0423-8. [DOI] [PubMed] [Google Scholar]
- 20.Tschirhart T, Shukla V, Kelly EE, Schultzhaus Z, NewRingeisen E, Erickson JS, Wang Z, Garcia W, Curl E, Egbert RG, Yeung E, Vora GJ. 2019. Synthetic biology tools for the fast-growing marine bacterium Vibrio natriegens. ACS Synth Biol 8:2069–2079. doi: 10.1021/acssynbio.9b00176. [DOI] [PubMed] [Google Scholar]
- 21.Weinstock MT, Hesek ED, Wilson CM, Gibson DG. 2016. Vibrio natriegens as a fast-growing host for molecular biology. Nat Methods 13:849–851. doi: 10.1038/nmeth.3970. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Lin B, Mostaghim A, Rubin RA, Glaser ER, Mittraparp-Arthorn P, Thompson JR, Vuddhakul V, Vora GJ. 2013. Vibrio campbellii hmgA-mediated pyomelanization impairs quorum sensing, virulence, and cellular fitness. Front Microbiol 4:379. doi: 10.3389/fmicb.2013.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffart E, Grenz S, Lange J, Nitschel R, Muller F, Schwentner A, Feith A, Lenfers-Lucker M, Takors R, Blombach B. 2017. High substrate uptake rates empower Vibrio natriegens as production host for industrial biotechnology. Appl Environ Microbiol 83:e01614-17. doi: 10.1128/AEM.01614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalia TN, Hayes CA, Stolyar S, Marx CJ, McKinlay JB, Dalia AB. 2017. Multiplex genome editing by natural transformation (MuGENT) for synthetic biology in Vibrio natriegens. ACS Synth Biol 6:1650–1655. doi: 10.1021/acssynbio.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuster V, Fishman A. 2009. Isolation, cloning and characterization of a tyrosinase with improved activity in organic solvents from Bacillus megaterium. J Mol Microbiol Biotechnol 17:188–200. doi: 10.1159/000233506. [DOI] [PubMed] [Google Scholar]
- 26.van Kessel JC, Rutherford ST, Cong JP, Quinodoz S, Healy J, Bassler BL. 2015. Quorum sensing regulates the osmotic stress response in Vibrio harveyi. J Bacteriol 197:73–80. doi: 10.1128/JB.02246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vij M, Grover R, Gotherwal V, Wani NA, Joshi P, Gautam H, Sharma K, Chandna S, Gokhale RS, Rai R, Ganguli M, Natarajan VT. 2016. Bioinspired functionalized melanin nanovariants with a range of properties provide effective color matched photoprotection in skin. Biomacromolecules 17:2912–2919. doi: 10.1021/acs.biomac.6b00740. [DOI] [PubMed] [Google Scholar]
- 28.Lampel A, McPhee SA, Park HA, Scott GG, Humagain S, Hekstra DR, Yoo B, Frederix P, Li TD, Abzalimov RR, Greenbaum SG, Tuttle T, Hu C, Bettinger CJ, Ulijn RV. 2017. Polymeric peptide pigments with sequence-encoded properties. Science 356:1064–1068. doi: 10.1126/science.aal5005. [DOI] [PubMed] [Google Scholar]
- 29.Ohlendorf R, Vidavski RR, Eldar A, Moffat K, Moglich A. 2012. From dusk till dawn: one-plasmid systems for light-regulated gene expression. J Mol Biol 416:534–542. doi: 10.1016/j.jmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Gerhardt KP, Olson EJ, Castillo-Hair SM, Hartsough LA, Landry BP, Ekness F, Yokoo R, Gomez EJ, Ramakrishnan P, Suh J, Savage DF, Tabor JJ. 2016. An open-hardware platform for optogenetics and photobiology. Sci Rep 6:35363. doi: 10.1038/srep35363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Aisen P, Casadevall A. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun 64:2420–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geng J, Yu SB, Wan X, Wang XJ, Shen P, Zhou P, Chen XD. 2008. Protective action of bacterial melanin against DNA damage in full UV spectrums by a sensitive plasmid-based noncellular system. J Biochem Biophys Methods 70:1151–1155. doi: 10.1016/j.jprot.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Garcia-Pichel F. 2011. Microbial ultraviolet sunscreens. Nat Rev Microbiol 9:791–802. doi: 10.1038/nrmicro2649. [DOI] [PubMed] [Google Scholar]
- 34.Fuqua WC, Weiner RM. 1993. The melA gene is essential for melanin biosynthesis in the marine bacterium Shewanella colwelliana. J Gen Microbiol 139:1105–1114. doi: 10.1099/00221287-139-5-1105. [DOI] [PubMed] [Google Scholar]
- 35.Mercado-Blanco J, García F, Fernández-López M, Olivares J. 1993. Melanin production by Rhizobium meliloti Gr4 is linked to nonsymbiotic plasmid pRmeGR4b: cloning, sequencing, and expression of the tyrosinase gene mepA. J Bacteriol 175:5403–5410. doi: 10.1128/jb.175.17.5403-5410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena D, Ben-Dov E, Manasherob R, Barak Z, Boussiba S, Zaritsky A. 2002. A UV tolerant mutant of Bacillus thuringiensis subsp. kurstaki producing melanin. Curr Microbiol 44:25–30. doi: 10.1007/s00284-001-0069-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang GL, Aazaz A, Peng ZR, Shen P. 2000. Cloning and overexpression of a tyrosinase gene mel from Pseudomonas maltophila. FEMS Microbiol Lett 185:23–27. doi: 10.1111/j.1574-6968.2000.tb09035.x. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Llamosas H, Castro L, Blazquez ML, Diaz E, Carmona M. 2017. Speeding up bioproduction of selenium nanoparticles by using Vibrio natriegens as microbial factory. Sci Rep 7:16046. doi: 10.1038/s41598-017-16252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YR, Li YW, Hu ZY, Yue XJ, Proetto MT, Jones Y, Gianneschi NC. 2017. Mimicking melanosomes: polydopamine nanoparticles as artificial microparasols. ACS Cent Sci 3:564–569. doi: 10.1021/acscentsci.6b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim E, Kang M, Tschirhart T, Malo M, Dadachova E, Cao G, Yin JJ, Bentley WE, Wang Z, Payne GF. 2017. Spectroelectrochemical reverse engineering demonstrates that melanin’s redox and radical scavenging activities are linked. Biomacromolecules 18:4084–4098. doi: 10.1021/acs.biomac.7b01166. [DOI] [PubMed] [Google Scholar]
- 41.Kim YJ, Wu W, Chun SE, Whitacre JF, Bettinger CJ. 2014. Catechol-mediated reversible binding of multivalent cations in eumelanin half-cells. Adv Mater 26:6572–6579. doi: 10.1002/adma.201402295. [DOI] [PubMed] [Google Scholar]
- 42.Kim YJ, Wu W, Chun SE, Whitacre JF, Bettinger CJ. 2013. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc Natl Acad Sci U S A 110:20912–20917. doi: 10.1073/pnas.1314345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sies H, Schulz WA, Steenken S. 1996. Adjacent guanines as preferred sites for strand breaks in plasmid DNA irradiated with 193 nm and 248 nm UV laser light. J Photochem Photobiol B 32:97–102. doi: 10.1016/1011-1344(95)07192-X. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Lin B, Hervey WJ, Vora GJ. 2013. Draft genome sequence of the fast-growing marine bacterium Vibrio natriegens strain ATCC 14048. Genome Announc 1:e00589-13. doi: 10.1128/genomeA.00589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsson BS. 1993. Interaction between chemicals and melanin. Pigment Cell Res 6:127–133. doi: 10.1111/j.1600-0749.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 46.Marinović V, Ristic M, Dostanic M. 2005. Dynamic adsorption of trinitrotoluene on granular activated carbon. J Hazard Mater 117:121–128. doi: 10.1016/j.jhazmat.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Tsao CY, Kim E, Tschirhart T, Terrell JL, Bentley WE, Payne GF. 2017. Using a redox modality to connect synthetic biology to electronics: hydrogel-based chemo-electro signal transduction for molecular communication. Adv Healthc Mater 6. doi: 10.1002/adhm.201600908. [DOI] [PubMed] [Google Scholar]
- 48.Shi XW, Tsao CY, Yang XH, Liu Y, Dykstra P, Rubloff GW, Ghodssi R, Bentley WE, Payne GF. 2009. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels. Adv Funct Mater 19:2074–2080. doi: 10.1002/adfm.200900026. [DOI] [Google Scholar]
- 49.McIlvaine TC. 1921. A buffer solution for colorimetric comparison. J Biol Chem 49:183–186. [Google Scholar]
- 50.Drewnowska JM, Zambrzycka M, Kalska-Szostko B, Fiedoruk K, Swiecicka I. 2015. Melanin-like pigment synthesis by soil Bacillus weihenstephanensis isolates from northeastern Poland. PLoS One 10:e0125428. doi: 10.1371/journal.pone.0125428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.