Plant-associated microbiomes are critical for plant health and other important agroecosystem processes. We assessed the bacterial and fungal microbiomes of wheat grown in soils from across a dryland wheat cropping systems in eastern Washington to identify the core microbiome on wheat roots that is consistent across soils from different locations and land use histories. Moreover, cross-domain co-occurrence network analysis identified core and hub taxa that may play important roles in microbial community assembly. Candidate core and hub taxa provide a starting point for targeting microbiome components likely to be critical to plant health and for constructing synthetic microbial communities for further experimentation. This work is one of the first examples of identifying a core microbiome on a major field crop grown across hundreds of square kilometers over a wide range of biogeographical zones.
KEYWORDS: soil, rhizosphere, fungi, bacteria, wheat, Triticum aestivum, Conservation Reserve Program, no-till, core microbiome, dryland wheat
ABSTRACT
The Inland Pacific Northwest is one of the most productive dryland wheat production areas in the United States. We explored the bacterial and fungal communities associated with wheat in a controlled greenhouse experiment using soils from multiple locations to identify core taxa consistently associated with wheat roots and how land use history influences wheat-associated communities. Further, we examined microbial co-occurrence networks from wheat rhizospheres to identify candidate hub taxa. Location of origin and land use history (long-term no-till versus noncropped Conservation Reserve Program [CRP]) of soils were the strongest drivers of bacterial and fungal communities. Wheat rhizospheres were especially enriched in many bacterial families, while only a few fungal taxa were enriched in the rhizosphere. There was a core set of bacteria and fungi that was found in >95% of rhizosphere or bulk soil samples, including members of Bradyrhizobium, Sphingomonadaceae, Massilia, Variovorax, Oxalobacteraceae, and Caulobacteraceae. Core fungal taxa in the rhizosphere included Nectriaceae, Ulocladium, Alternaria, Mortierella, and Microdochium. Overall, there were fewer core fungal taxa, and the rhizosphere effect was not as pronounced as with bacteria. Cross-domain co-occurrence networks were used to identify hub taxa in the wheat rhizosphere, which included bacterial and fungal taxa (e.g., Sphingomonas, Massilia, Knufia, and Microdochium). Our results suggest that there is a relatively small group of core rhizosphere bacteria that were highly abundant on wheat roots regardless of soil origin and land use history. These core communities may play important roles in nutrient uptake, suppressing fungal pathogens, and other plant health functions.
IMPORTANCE Plant-associated microbiomes are critical for plant health and other important agroecosystem processes. We assessed the bacterial and fungal microbiomes of wheat grown in soils from across a dryland wheat cropping systems in eastern Washington to identify the core microbiome on wheat roots that is consistent across soils from different locations and land use histories. Moreover, cross-domain co-occurrence network analysis identified core and hub taxa that may play important roles in microbial community assembly. Candidate core and hub taxa provide a starting point for targeting microbiome components likely to be critical to plant health and for constructing synthetic microbial communities for further experimentation. This work is one of the first examples of identifying a core microbiome on a major field crop grown across hundreds of square kilometers over a wide range of biogeographical zones.
INTRODUCTION
Plant-associated microbiomes are increasingly viewed as critical mediators of plant health and their manipulation as an important mechanism by which we can attain more sustainable agricultural production systems (1). Microbiomes in the rhizosphere can aid plants via enhanced nutrient and water uptake, pathogen suppression, phosphorus solubilization, and degradation of agrochemicals. However, with a few exceptions (e.g., Rhizobium and plant pathogens), we still lack an understanding of the microbial taxa or consortia that play key roles in specific cropping systems. The assembly of microbial communities in the rhizosphere is believed to be a function of interactions between the plant, soil characteristics, environmental factors, and microbial interactions (2) and thus can be highly variable among locations (3). However, to develop management practices that have consistent effects on soil communities, we need a broad understanding of the microbial components of soil communities that associate with plants across distinct locations.
Plants are thought to play an active role in “selecting” for microbial consortia in the rhizosphere via the provision of specific root exudates (4). As a result, despite wide variation in soil microbial communities across locations with different soil and environmental characteristics, plants can recruit a “core” microbiome of root symbionts that have resource use preferences adapted to the rhizosphere (5). Moreover, these core taxa are hypothesized to be coadapted with their plant hosts to successfully inhabit the rhizosphere of specific plant species or genotypes (6). Thus, core taxa or consortia are thought to be especially important for plant health and are particularly attractive targets for efforts to manipulate the plant microbiome or to design synthetic consortia (6). For example, the phenomenon of take-all decline occurs in cereal cropping systems globally (7). In this case, naturally occurring crop- or genotype-specific populations of fluorescent Pseudomonas species build up on roots over multiple seasons of monoculture and protect roots from infection by the take-all pathogen (Gaumannomyces graminis var. tritici) after an initial disease outbreak (7, 8). This phenomenon demonstrates the critical role that core taxa can play in plant health across a broad geographic range and variable environments.
In addition to variation in microbial communities among locations, land use history can have significant and long-lasting effects on soil- and plant-associated microbial communities. For example, a history of disturbance, nutrient inputs (e.g., fertilizers), pesticide or herbicide applications (9–12), and plant or crop species can alter soil community composition and subsequent plant colonists, so it takes decades for biotic communities to return to their previous state (13, 14). Moreover, cropping history, such as long-term monoculture, can result in plant-soil feedbacks that influence plant health. In eastern Washington State, many growers enroll in the Conservation Reserve Program (CRP) to provide natural habitat for wildlife and perform beneficial ecosystem services to control erosion and improve water quality (15). CRP land is often dominated by a diverse community of native or naturalized grasses, forbs, and shrubs, which is hypothesized to support a more diverse soil microbial community that provides more beneficial ecosystem services than species-poor microbial communities (16). However, the area of land in the CRP is declining as more growers convert it back to agricultural production (USDA Farm Services Agency, 2018 [fsa.usda.gov/programs-and-services/conservation-programs/reports-and-statistics/conservation-reserve-program-statistics/index]). Yet no work to date has explored how a legacy of CRP versus wheat cropping influences the microbiomes that associate with subsequent wheat plantings.
Microbial interactions are critical to the assembly of microbial communities in the rhizosphere (17, 18). Specifically, because of the high density, short generation times, and long coassociations with plants (suggesting shared niche preferences among microbes), it is hypothesized that microbe-microbe interactions are important selective drivers of plant-associated microbial assemblages (19). Although it is well recognized that microbial taxa in the rhizosphere engage in complex interspecific interactions, including nutrient exchange, signaling, antagonism, and cooperation (20), we know relatively little about the intricate network structure of microbial interactions (18). Recently, methods for construction of microbial coassociation networks have been used to infer these species interaction networks from amplicon sequencing data (21, 22). Although these methods do not directly identify positive or negative interactions among taxa, due to their correlative nature, they have proven to be useful in identifying “hub” taxa that play especially important roles in the assembly of plant-associated microbial communities (23). However, we still have little understanding of the structure of microbial co-occurrence networks, which microbial taxa constitute network hubs, and how network components relate to core microbial taxa (18, 19, 24).
In this work, we explored bacterial and fungal communities of wheat grown in soils from multiple locations across the wheat-growing region of eastern Washington. Specifically, we grew wheat in the greenhouse in soils from distinct locations and cropping histories in order to control for confounding local factors (weather, genotype, etc.). Our goals were to (i) determine how land use history of soils (no-till [NT] wheat versus CRP) influences community composition and diversity, (ii) identify the core microbial taxa that consistently associate with wheat roots from different soils and land use histories across this region, and (iii) explore the structure of the cross-domain microbial co-occurrence network and identify key taxa within networks. This work sheds important light on the key components and drivers of wheat-associated microbiomes and the complex structure of microbial interactions within wheat-associated microbial consortia.
RESULTS
For bacteria, 21,934,639 sequences were obtained that passed quality filtering for 224 samples. After rarefaction to 13,200 sequences/sample, sequences mapped to 21,183 distinct operational taxonomic units (OTUs) belonging to 22 identifiable phyla. The relative abundance of bacterial phyla varied significantly among soils from different locations, years, land use histories, and root proximities (Table 1), indicating substantial variation in soil and rhizosphere bacterial communities of wheat. Most notably, with respect to land use history, soils from no-till fields harbored greater proportions of Proteobacteria than those from CRP, and CRP soils had greater proportions of Firmicutes than no-till soils (Table 1). With respect to root proximity, the wheat rhizosphere was significantly enriched in Bacteroidetes and Proteobacteria versus bulk soil, whereas bulk soil harbored more Acidobacteria, Actinobacteria, and Planctomycetes.
TABLE 1.
Relative abundances of bacterial phyla among locations, years, cropping systems, and root proximitiesa
| Parameter | % abundance (mean ± SD) |
||||||
|---|---|---|---|---|---|---|---|
| Acidobacteria | Actinobacteria | Bacteroidetes | Firmicutes | Planctomycetes | Proteobacteria | Verrucomicrobia | |
| Location | |||||||
| Colfax | 13 ± 0.04 a | 30.3 ± 0.11 a | 12.6 ± 0.07 a | 0.69 ± 0.01 a | 3.5 ± 0.03 a | 31.8 ± 0.09 a | 3 ± 0.01 a |
| Lamont | 13 ± 0.05 ab | 27 ± 0.13 b | 13.2 ± 0.12 a | 1.1 ± 0.02 a | 3.8 ± 0.05 a | 33 ± 0.12 a | 3.4 ± 0.01 a |
| Ritzville | 11.7 ± 0.09 b | 27.7 ± 0.13 a | 13.7 ± 0.14 a | 0.46 ± 0.004 b | 2.6 ± 0.02 a | 38.7 ± 0.16 b | 2.1 ± 0.02 b |
| Genesee | 11.2 ± 0.05 b | 34.4 ± 0.12 c | 9.9 ± 0.11 b | 5.4 ± 0.13 c | 2.2 ± 0.01 a | 31.9 ± 0.13 a | 1.6 ± 0.02 b |
| Year | |||||||
| 2015 | 13.4 ± 0.04 | 26 ± 0.07 | 15.4 ± 0.07 | 3 ± 0.06 | 4.5 ± 0.02 | 30.9 ± 0.07 | 2.2 ± 0.02 |
| 2016 | 11.5 ± 0.02 | 32.9 ± 0.06 | 10.1 ± 0.03 | 0.9 ± 0.01 | 2 ± 0.01 | 35.7 ± 0.05 | 2.8 ± 0.01 |
| Land use history | |||||||
| No-till | 11 ± 0.03 a | 29.1 ± 0.07 a | 12.1 ± 0.06 a | 1 ± 0.01 a | 2.8 ± 0.02 a | 36.8 ± 0.08 a | 2.2 ± 0.01 a |
| CRP | 13.6 ± 0.03 b | 28.6 ± 0.07 a | 12.7 ± 0.06 a | 2.8 ± 0.05 b | 3.2 ± 0.02 a | 32.4 ± 0.06 b | 2.7 ± 0.01 b |
| Root proximity | |||||||
| Bulk | 14.2 ± 0.03 a | 33.4 ± 0.07 a | 9.1 ± 0.03 a | 1.9 ± 0.03 a | 3.5 ± 0.01 a | 30.5 ± 0.05 a | 2.6 ± 0.01 a |
| Rhizosphere | 10.5 ± 0.03 b | 26.6 ± 0.06 b | 15.5 ± 0.05 b | 1.7 ± 0.03 a | 2.6 ± 0.02 b | 36.7 ± 0.07 b | 2.5 ± 0.01 a |
Significant differences, indicated by different letters, were determined using Kruskal-Wallis tests. Differences were considered significant if FDR-adjusted P values were <0.05. Multiple comparisons were performed using Dunn’s test.
For fungi, we obtained 10,781,279 sequences that passed quality filtering. After rarefaction to 12,000 sequences/sample, 2,352,000 sequences mapped to 2,239 OTUs belonging to 7 identified fungal phyla. With respect to land use history, soil with a history of no-till harbored the largest portion of Ascomycota, whereas those with a history of CRP participation had significantly greater portions of Chytridiomycota and Glomeromycota (Table 2). Contrasting bulk and rhizosphere soil, the rhizosphere supported greater proportions of Basidiomycota and Zygomycota, whereas bulk soils were more enriched in Ascomycota (Table 2).
TABLE 2.
Relative abundances of fungal phyla among locations, years, cropping systems, and root proximitiesa
| Parameter | % abundance (mean ± SD) |
||||
|---|---|---|---|---|---|
| Ascomycota | Basidiomycota | Chytridiomycota | Glomeromycota | Zygomycota | |
| Location | |||||
| Colfax | 74.4 ± 0.20 a | 11.2 ± 0.20 a | 2.4 ± 0.04 ac | 0.05 ± 0.002 a | 5.5 ± 0.06 a |
| Lamont | 78.3 ± 0.22 a | 8.2 ± 0.20 a | 4.6 ± 0.11 b | 0.06 ± 0.004 a | 7.2 ± 0.10 b |
| Ritzville | 76.5 ± 0.41 a | 11.9 ± 0.37 a | 4.1 ± 0.14 bc | 0.08 ± 0.007 a | 5.3 ± 0.10 a |
| Genesee | 77.9 ± 0.27 a | 7.7 ± 0.17 a | 2.5 ± 0.09 c | 0.21 ± 0.02 b | 8.5 ± 0.13 b |
| Year (bulk soil only) | |||||
| 2015 | 81.1 ± 0.15 a | 6.9 ± 0.11 a | 1.6 ± 0.03 a | 0.05 ± 0.002 a | 6.5 ± 0.08 a |
| 2016 | 79.9 ± 0.17 b | 8.1 ± 0.13 a | 3.9 ± 0.07 b | 0.14 ± 0.01 b | 4.9 ± 0.06 b |
| Land use history | |||||
| No-till | 80.6 ± 0.12 a | 7.4 ± 0.09 a | 1.9 ± 0.03 a | 0.006 ± 0.003 a | 6.1 ± 0.06 a |
| CRP | 74.9 ± 0.16 b | 10.5 ± 0.14 a | 4.9 ± 0.07 b | 0.13 ± 0.007 b | 6.2 ± 0.05 a |
| Root proximity (2016 only) | |||||
| Bulk | 79.9 ± 0.17 a | 8.1 ± 0.13 a | 3.9 ± 0.07 a | 0.14 ± 0.01 a | 4.9 ± 0.06 a |
| Rhizosphere | 69.7 ± 0.21 b | 14.0 ± 0.22 b | 4.0 ± 0.06 a | 0.08 ± 0.003 a | 8.1 ± 0.07 b |
Significant differences, indicated by different letters, were determined using Kruskal-Wallis tests. Differences were considered significant if FDR-adjusted P values were <0.05. Multiple comparisons were performed using Dunn’s test.
Land use history and root proximity as drivers of wheat microbiota.
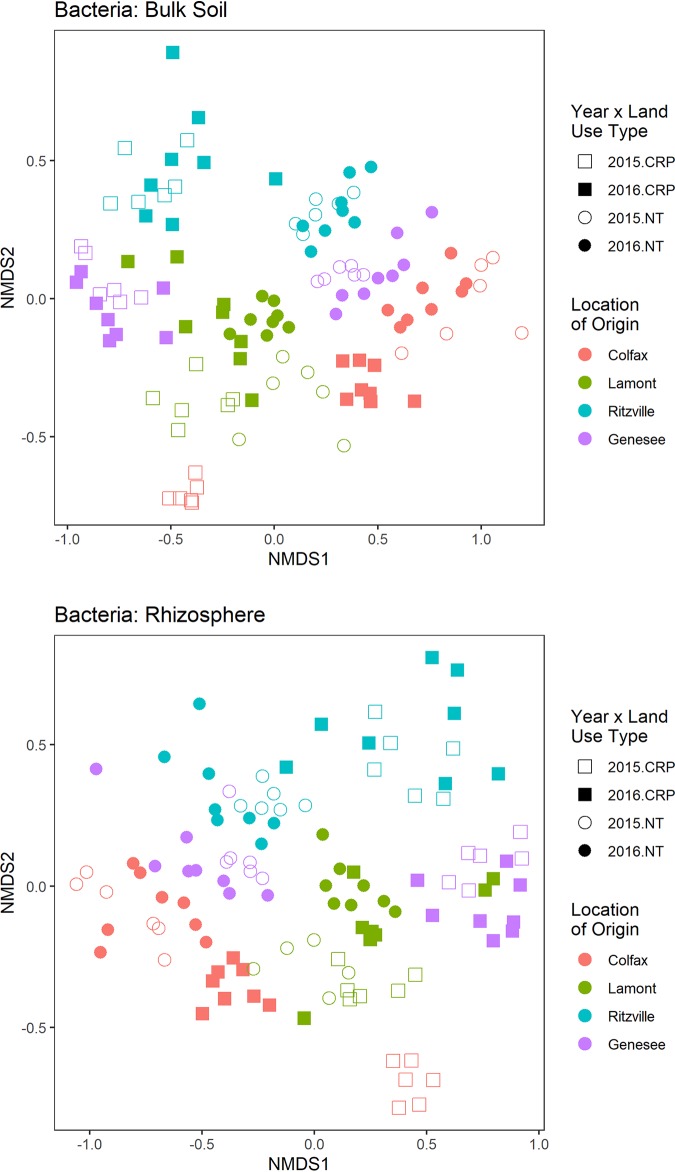
Despite consistent growth conditions in the greenhouse, location of soil origin and land use history were the strongest drivers of bacterial and fungal communities in both bulk soil and wheat rhizospheres (Fig. 1 and 2; Tables 3 and 4). Moreover, there were significant interactions between location of soil origin and land use history in determining bacterial and fungal communities in bulk soil and the rhizosphere (Tables 3 and 4), indicating that despite similar land use histories, soils from distinct locations support unique microbial communities. However, considering bacterial families, there were 32 abundant families that differed significantly in relative abundance between soils with different land use histories (Fig. 3). Most notably, members of Burkholderiaceae, Oxalobacteraceae, Sphingomonadaceae, Sphingobacteriaceae, Xanthomonadaceae, Flavobacteriaceae, Micrococcaceae, and Streptomycetaceae tended to be more prevalent in soils cultivated to wheat with a history of no-till. In soils historically in the CRP, members of Solirobrobacteraceae, Rubrobacteraceae, Propionibacteriaceae, Opitutaceae, and Cytophagaceae tended to be relatively more abundant. Contrasting bulk and rhizosphere soil, rhizospheres were especially enriched in Burkholderiaceae, Caulobacteraceae, Comamonadaceae, Cytophagaceae, Flavobacteriaceae, Microbacteriaceae, Mycobacteriaceae, Oxalobacteraceae, Phyllobacteriaceae, Pseudomonadaceae, Rhizobiaceae, and Sphingobacteriaceae. Bulk soil, in contrast, was enriched in only a few families, such as Gaiellaceae, Kineosporiaceae, Micromonospora, and Pseudonocardiaceae. Moreover, there were significant interactions between land use history and root proximity for Oxalobacteraceae, Pseudomonadaceae, and Phyllobacteriaceae (false-discovery rate [FDR] P values = 0.008, 0.0007, and 0.028, respectively), suggesting that despite similar relative abundances in bulk soils, members of these groups are better able to colonize wheat rhizospheres in soils with a long history of no-till.
FIG 1.
NMDS of bacterial communities from bulk (top) and rhizosphere (bottom) soil.
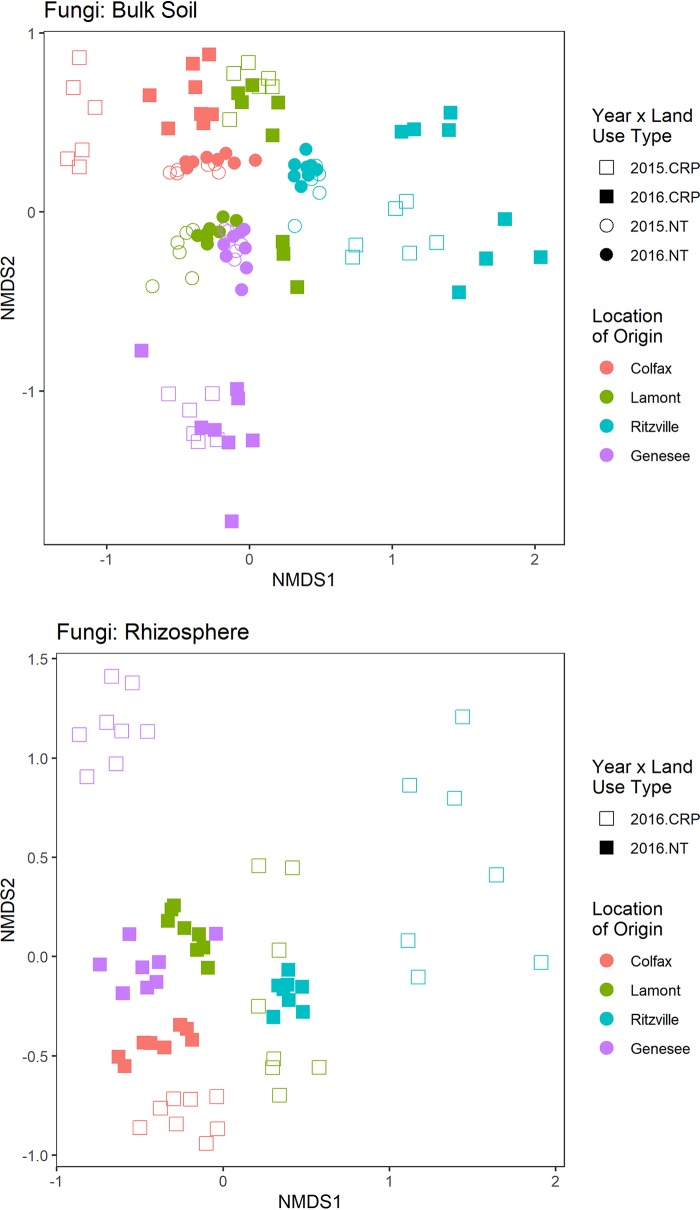
FIG 2.
NMDS of fungal communities from bulk (top; 2015 and 2016) and rhizosphere (bottom; 2016 only) soils.
TABLE 3.
PERMANOVA results for bacterial communities divided by year and root proximity
| Year | Parametera | r2 | P value | Root proximity | Parametera | r2 | P value |
|---|---|---|---|---|---|---|---|
| 2015 | L | 0.23 | 0.001 | Bulk | L | 0.15 | 0.001 |
| LUH | 0.21 | 0.001 | LUH | 0.21 | 0.001 | ||
| RP | 0.04 | 0.001 | Y | 0.04 | 0.001 | ||
| L × LUH | 0.14 | 0.001 | L × LUH | 0.09 | 0.001 | ||
| L × RP | 0.02 | 0.008 | L × Y | 0.06 | 0.001 | ||
| LUH × RP | 0.01 | 0.006 | LUH × Y | 0.03 | 0.001 | ||
| L × LUH × RP | 0.02 | 0.076 | L × LUH × Y | 0.04 | 0.001 | ||
| 2016 | L | 0.14 | 0.001 | Rhizo | L | 0.13 | 0.001 |
| LUH | 0.21 | 0.001 | LUH | 0.17 | 0.001 | ||
| RP | 0.03 | 0.001 | Y | 0.04 | 0.001 | ||
| L × LUH | 0.1 | 0.001 | L × LUH | 0.09 | 0.001 | ||
| L × RP | 0.015 | 0.064 | L × Y | 0.05 | 0.001 | ||
| LUH × RP | 0.011 | 0.04 | LUH × Y | 0.03 | 0.001 | ||
| L × LUH × RP | 0.012 | 0.33 | L × LUH × Y | 0.04 | 0.001 |
L, location; LUH, land use history; RP, root proximity; Y, year.
TABLE 4.
PERMANOVA results for fungal communities divided by year and root proximity
| Year | Parameter | r2 | P value | Root proximity | Parameter | r2 | P value |
|---|---|---|---|---|---|---|---|
| 2015 | L | 0.26 | 0.001 | Rhizo | L | 0.17 | 0.001 |
| LUH | 0.18 | 0.001 | LUH | 0.14 | 0.001 | ||
| L × LUH | 0.15 | 0.001 | L × LUH | 0.11 | 0.001 | ||
| 2016 | L | 0.15 | 0.001 | Bulk | L | 0.15 | 0.001 |
| LUH | 0.14 | 0.001 | LUH | 0.12 | 0.001 | ||
| RP | 0.02 | 0.001 | Y | 0.13 | 0.001 | ||
| L × LUH | 0.09 | 0.001 | L × LUH | 0.07 | 0.001 | ||
| L × RP | 0.02 | 0.058 | L × Y | 0.02 | 0.001 | ||
| LUH × RP | 0.01 | 0.062 | LUH × Y | 0.02 | 0.001 | ||
| L × LUH × RP | 0.01 | 0.5 | L × LUH × Y | 0.03 | 0.001 |
FIG 3.
Bacterial families that differ significantly between different cropping histories (squares) or root proximities (triangles). The color scale of the heatmap represents log2(1 + x)-transformed sequence counts. Hierarchical clustering was used to order families by similarity in abundances among samples (y axis).
Among fungal families, 14 families differed significantly in relative abundance between soils with different land use histories. Most notably, members of Chaetomiaceae, Hyaloscyphaceae, Nectriaceae, Coniochaetaceae, Pleosporaceae, and Tremellales were significantly more abundant in soils with a history of wheat production in no-till (Fig. 4). In contrast, Helotiales, Ceratobasidiaceae, Sebacinales, and Sporomiacae were enriched in soils with a history of CRP participation (Fig. 4). In contrast to bacteria, only a few fungal families differed in relative abundance between bulk soil and rhizosphere environments, where members of Nectriaceae, Pleosporaceae, and Mortierellaceae were enriched in the wheat rhizosphere (Fig. 4).
FIG 4.
Fungal families that differ significantly between different cropping histories (squares) or root proximities (triangles). The color scale of the heatmap represents log2(1 + x)-transformed sequence counts. Hierarchical clustering was used to order families by similarity in abundances among samples (y axis).
Land use history and location-dependent rhizosphere enrichment of microbial OTUs.
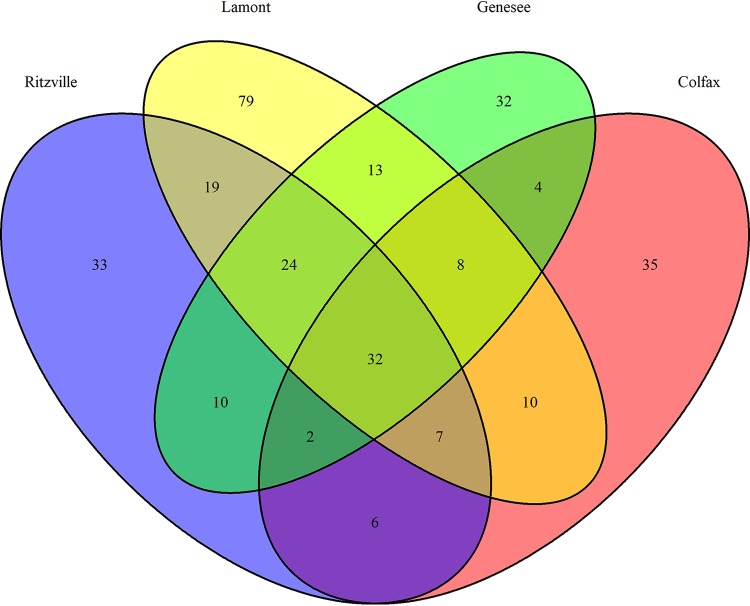
In soils with both a history of no-till wheat and CRP participation, bacterial OTUs with relative abundances enriched in wheat rhizospheres belonged primarily to Proteobacteria and Bacteroidetes (Fig. 5). Specifically, the rhizosphere was enriched in OTUs classified as Chitinophaga, Chryseobacterium, Acidovorax, Flavobacterium, Duganella, Mucilaginibacter, Massilia, Sphingomonas, Burkholderia, and Rhizobium. Interestingly, although there was substantial overlap in the taxa consistently enriched in the rhizospheres from soils with different land use histories (79% of OTUs [23 of 29, comprising an average of 12.8% ± 6.0% of sequences/sample] enriched in rhizosphere from NT soil were also enriched in those from CRP; 92% of OTUs [23 of 25, comprising an average of 9.2% ± 6.0% of sequences/sample] enriched in rhizosphere in CRP soil were also enriched in those from NT soil). When rhizosphere enrichment was evaluated for each location independently, an average of 24% of rhizosphere-enriched OTUs were significantly enriched in all locations (range of 16.7% to 30.7% from Lamont and Colfax soils, respectively, comprising an average of 9.6% ± 5.4% of sequences/sample) (Fig. 6), suggesting both a common core rhizosphere microbiome and location-dependent components. The enriched taxa common to plants grown in soils from each location included many of the same taxa as enriched in rhizospheres of plants grown in soils from each cropping history (Fig. 5).
FIG 5.
OTUs of bacteria that statistically differ in abundance between rhizosphere and bulk soils with a history of no-till or CRP. The x axis indicates DESeq2-estimated log2 fold differences between rhizosphere and bulk soils. The y axis indicates the lowest taxonomic rank to which OTUs could be confidently assigned and the OTU identifier. Points are colored by the phylum to which OTUs belong and the size of points is scaled by OTU abundance (base mean among all samples). OTUs more abundant in the rhizosphere are to the right of the red line (positive values), and those more abundant in bulk soil are to the left of the red line (negative values).
FIG 6.
Venn diagram of rhizosphere-enriched bacterial OTUs in soils from distinct locations.
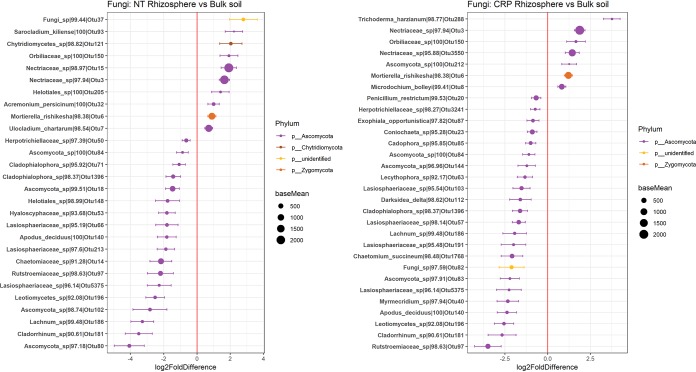
Despite a weak effect of the rhizosphere environment on fungal communities, there was a small number of fungal OTUs that were enriched in the rhizosphere in NT and CRP soils (Fig. 7). Most notably, OTUs within the family Nectriaceae and an OTU related to Mortierella rishikesha tended to be more abundant in the rhizosphere versus bulk soil under both land use histories. In contrast, a larger number of OTUs were significantly depleted in the rhizospheres, including those related to Cladophialophora, Lasiosphaeriaceae, and Chaetomiaceae. An OTU related to Ulocladium was enriched in the rhizosphere only in NT soils, whereas an OTU classified as Microdochium bolleyi was enriched only in CRP soils. In general, there tended to be less overlap in the fungal taxa that differed between bulk and rhizosphere soil in soils from different land use histories, where 59% (18 of 37 OTUs) of taxa that differed in CRP soils also differed in NT soils and 44% (18 of 41 OTUs) of OTUs that differed in NT soils also differed in CRP soils. When rhizosphere enrichment was evaluated for each location independently for fungi, only a single OTU (OTU3) was found to be enriched in the wheat rhizosphere in soils from each location (Fig. 8). This OTU was identified as a Nectriaceae species and BLAST matched to numerous Fusarium accessions with 100% identity in the UNITE ITS database (accessed 15 August 2019).
FIG 7.
OTUs of fungi in 2016 that statistically differ in abundance between rhizosphere and bulk soils with a history of no-till or CRP participation. The x axis indicates DESeq2-estimated log2 fold differences between rhizosphere and bulk soils. The y axis indicates the lowest taxonomic rank to which OTUs could be assigned, the BLAST percent identity, and OTU identifier. Points are colored by the phylum to which OTUs belong and the size of points is scaled by OTU abundance (base mean among all samples). OTUs more abundant in the rhizosphere are to the right of the red line (positive values), and those more abundant in bulk soil are to the left of the red line (negative values).
FIG 8.
Venn diagram of rhizosphere-enriched fungal OTUs in soils from distinct locations.
Core rhizosphere microbial taxa of wheat.
A core set of bacterial taxa was found in >95% of wheat rhizosphere samples when plants were grown in NT or CRP soils. Sixty-eight OTUs were found in 95% of rhizosphere samples irrespective of soil origin or land use history. Together these taxa comprised, on average, 20.1% ± 4.5% of rhizosphere communities. The core bacterial taxa of wheat primarily belonged to the classes Actinobacteria (n = 27 OTUs), Alphaproteobacteria (n = 25 OTUs), Betaproteobacteria (n = 10 OTUs), and Sphingobacteria (n = 3 OTUs) (Fig. 9).
FIG 9.
Neighbor joining tree of core bacterial OTUs. The size of the circles on tree tips are scaled by OTU mean abundance in rhizosphere samples, whereas tip colors represent the relative enrichment of an OTU in rhizosphere versus bulk soil samples.
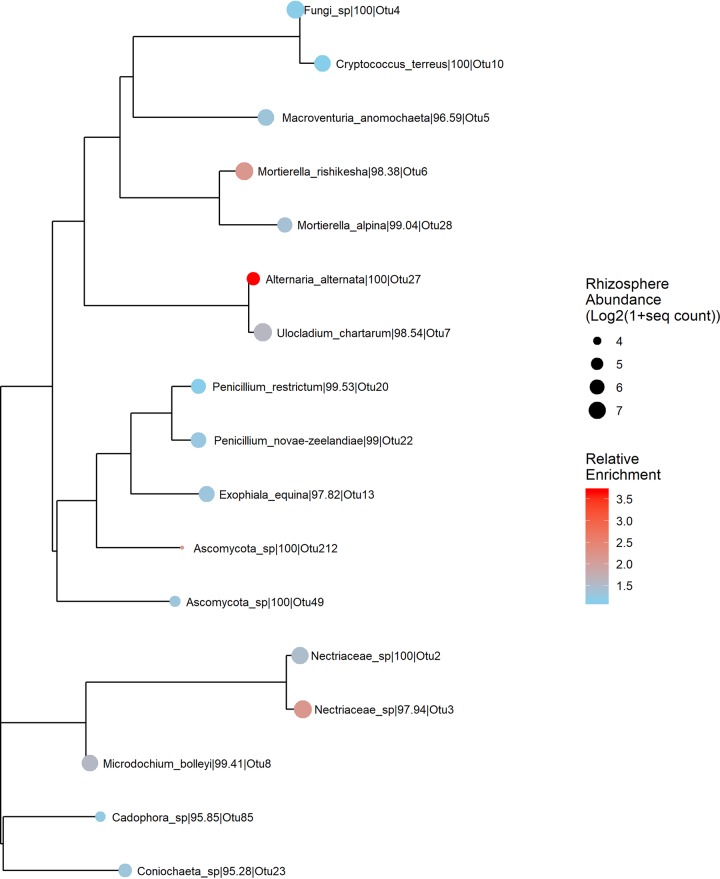
For fungi, the core rhizosphere taxa found in at least 95% of samples were primarily Ascomycota (13/15 taxa), with smaller numbers of Basidiomycota, Zygomycota, and unidentified phyla (Fig. 10). The core fungi with the greatest rhizosphere enrichment versus bulk soil included Alternaria, Ulocladium, Nectriacaeae (Fusarium), Mortierella, and Microdochium (Fig. 10). Thus, similar to the case with bacteria, there was a large overlap between fungal taxa that were widespread in bulk soil and those that were consistently found in the rhizosphere.
FIG 10.
Neighbor joining tree of core fungal OTUs. The size of the circles on tree tips are scaled by OTU mean abundance in rhizosphere samples, whereas tip colors represent the relative enrichment of an OTU in rhizosphere versus bulk soil samples.
Microbial diversity.
Bacterial richness and diversity varied significantly with year, location of soil origin, land use history, and root proximity (Fig. 11; see also Table S1 in the supplemental material). The richness and diversity of bacterial communities were significantly lower in no-till soils versus CRP soils and in rhizosphere soils versus bulk soil (Tukey’s honest significant difference [HSD] < 0.05). Moreover, bacterial richness and diversity (Shannon and Simpson) were significantly greater at Lamont than at other locations (HSD < 0.05). Fungal community richness, but not diversity, also varied significantly among soils from different locations and cropping histories in 2015 and 2016 (Fig. 11; Table S2). However, fungal richness was only significantly lower at the Ritzville location (HSD < 0.05), and no contrasts were significant for Shannon or Simpson’s diversity (HSD > 0.05). Moreover, although fungal community richness tended to be lower in the rhizosphere versus bulk soil, contrasts within soils from each land use history treatment were not significant (HSD > 0.05).
FIG 11.
Bacterial (left) and fungal (right) richness and diversity among locations, cropping histories, and root proximities.
Microbial cross-domain networks in the wheat rhizosphere.
After edge filtering, a cross-domain co-occurrence network among microbial taxa in the wheat rhizosphere consisted of 463 taxa (302 bacteria and 161 fungi) and 829 edges (553 positive and 276 negative co-occurrences) (Fig. 12). Hub taxa, or those with both high node degrees and betweenness centrality, included both bacterial and fungal taxa (Fig. S1). Bacterial hubs included Ilumatobacter, Ganulicella, Acidobacteria Gp1 and Gp17, Sphingomonas, and Massilia, whereas fungal hubs included Lecythophora, Cladophialophora, Knufia, Lophiostomataceae, Microdochium, and Devriesia. When mapped onto networks of positive and negative edges, hub taxa tended to be distributed throughout positive co-occurrence networks versus negative co-occurrence networks, suggesting that synergistic or commensal interactions with these taxa are important for rhizosphere community organization of consortia, whereas competitive or antagonistic interactions occur among specific taxa. When mapped to co-occurrence networks, core taxa tended to cluster together in the positive edge network, while being dispersed on the negative edge network (Fig. 12). This pattern suggests that plant selection for the presence of core taxa generates positive co-occurrences while minimizing negative co-occurrences among core taxa.
FIG 12.
Cross-domain co-occurrence network generated using the SpiecEasi method. Nodes represent individual genera, or the lowest rank to which OTUs could be classified with confidence. Edges represent significant positive (upper graphs) or negative (lower graphs) coassociations. Graphs on the left are colored by domain (black, bacterial taxa; red, fungal taxa). Graphs in the middle highlight hub nodes in green. Graphs on the right highlight core taxa in blue.
DISCUSSION
The composition of plant-associated microbial communities is often dependent on the environment in which crops are grown, such as soil characteristics, local weather conditions, growth stage, and land use history (9, 18, 25, 26). Despite variation in these factors, plants consistently recruit some taxa, considered to be the core microbiome, that play critical roles in plant nutrition and health (6). Moreover, central to the efforts to manipulate plant-associated microbiomes is identifying those taxa that perform beneficial functions for plants across distinct environmental conditions and cropping histories. We found that although soils from different locations and land use histories had strong impacts on the composition of microbial communities in the wheat rhizosphere, rhizosphere communities were consistently enriched in a relatively small number of microbial taxa within the phyla Proteobacteria, Bacteroidetes, Actinobacteria, and Ascomycota, paralleling results in other studies of grass crops (27–31).
Many of the core taxa commonly found in the wheat rhizosphere when grown in soils from different locations and land use histories in this study belonged to the family Oxalobacteraceae, including those identified to the genera Duganella and Massilia. These taxa may play important roles in root health. For example, Chapelle et al. (32) hypothesized that oxalic acid is released by Rhizoctonia solani AG-2 during invasion of the sugar beet rhizosphere, thereby increasing the motility, biofilm formation, and secondary metabolite production of bacterial taxa, including those within the family Oxalobacteraceae. Moreover, Duganella species, which were commonly enriched in the wheat rhizosphere, are producers of the antifungal antibiotic violacein (33) and have been found to be positively associated with plant productivity (34). Investigations of Massilia, another common rhizosphere member within Oxalobacteraceae that can attach to plant tissues and fungal mycelia and produce siderophores and lytic enzymes, revealed that this genus is coptiotrophic and reaches high abundances on roots in early succession stages of microbiome assembly before being displaced by more competitive taxa (35). Efforts to manipulate the plant microbiome must take into account microbial community assembly from the earliest stages of plant growth and microbial interactions during community succession (5). Thus, members of Massilia may play important roles in early stages of community assembly on wheat roots and set the stage for subsequent succession via priority effects.
Other rhizosphere-enriched taxa, especially Chitinophaga (Sphingobacteriaceae), Flavobacteria (Flavobacteriaceae), Variovorax (Comomonadaceae), Mucilaginibacter (Sphingobacteriaceae), and Chryseobacterium (Flavobacteriaceae), have also been implicated in the health and productivity of wheat, especially in response to Rhizoctonia infection, and tend to build up higher populations in the rhizosphere over multiple cycles of wheat (27). Interestingly, one OTU classified as Chryseobacterium (OTU13) was 99% identical in sequence identity to isolate CY31 described by Yin et al. (27) as an antagonist of Rhizoctonia solani AG8. In total, the prevalence of these taxa in the rhizosphere of wheat growing in soils from different locations and land use histories suggests that they are key components of a healthy wheat microbiome.
Although there were fewer core fungal versus bacterial taxa in the wheat rhizosphere, likely due to the weaker selective effect of the rhizosphere on fungi, especially enriched were members of the families Nectriaceae and Mortierellaceae. Notably, Nectriaceae includes the genus Fusarium, species of which can be important root pathogens of wheat (e.g., Fusarium culmorum and F. pseudograminearum) (36, 37). However, in other cases, members of Fusarium may be plant-beneficial saprophytes and compete with pathogens for resources (38). Unfortunately, the internal transcribed spacer (ITS) region is not adequate for species-level differentiation of Fusarium, and further research is needed on the role that Fusarium species play in rhizosphere community dynamics. Members of Mortierellaceae (phylum Zygomycota) are rapidly growing organisms that can degrade chitin and hemicellulose and are important decomposers in soil (39). Moreover, Mortierella has recently been described as a plant-beneficial rhizosphere colonist that mediates disease development in roots of Arabidopsis thaliana (40). Thus, Mortierella species may be beneficial colonizers of wheat roots and proliferate on senescing root material after harvest. However, future work employing culturing methods is needed to verify the functions of core wheat rhizosphere taxa and their interactions with each other and with root pathogens.
Microbial diversity is often suggested to be critical for crop productivity (41), where more diverse microbial communities are more resilient to disturbance and are better able to provide ecosystem services such as C and N cycling and disease protection (42, 43). Moreover, more diverse cropping histories can significantly enhance soil microbial diversity and plant-beneficial functions (44, 45). The greater diversity of bacteria and fungi in both wheat rhizospheres and bulk soils when the soils had a history of CRP participation versus no-till cropping likely reflects the greater diversity of plant communities in the CRP that support more diverse soil communities (46). In contrast, consistent selection for microbial taxa by wheat-based cropping practices in no-till soils has likely generated less diverse communities of taxa well-adapted to growth in the wheat rhizosphere or on residue (47). Interestingly, although microbial communities in the rhizosphere are thought to be buffered against changes in bulk soil communities due to management practices (30), the higher diversity in CRP soils translated to more diverse rhizosphere communities, suggesting that the dynamics of microbes recruited to the rhizosphere depend on the microbial diversity found in bulk soil.
Microbial co-occurrence networks in the wheat rhizosphere revealed rich patterns of bacterial and fungal interrelationships. Interestingly, microbial hub taxa, or those with many edges and high betweenness centrality, tended to be distinct from the core taxa (only sharing 3 taxa). This may be due to the consistent recruitment of core taxa in the rhizosphere, which may reduce the likelihood of many significant co-occurrence patterns across samples. Rather, hubs may reflect taxa that play a role in structuring more variable rhizosphere inhabitants. In particular, network hubs are hypothesized to serve as critical control points of networks that may be useful targets for manipulating microbiome composition and function (17, 23). For example, Agler et al. (23) found that factors that impacted hub taxa in a phyllosphere community had cascading effects throughout microbial communities with significant outcomes for microbial alpha- and beta-diversities. Hub taxa with roles as pathogens (e.g., Microdochium) or those that provide key metabolites (e.g., cellulose-degrading Cellulomonas) may be especially important in supporting cross-feeding or competitive interactions by other microbes and drive rhizosphere processes. In contrast to hub taxa, the core taxa were clustered in rhizosphere networks, suggesting that they form a more stable group of rhizosphere taxa. However, further work employing synthetic communities and time series data are needed to elucidate the roles of specific microbial taxa or network structures in rhizosphere community dynamics and function.
Although we identified components of the core wheat microbiome grown in soils from different locations and land use histories, growth under controlled greenhouse conditions may have influenced our observations. For example, destructive sampling and mixing of soils may have modified the composition of soil communities used in the greenhouse experiment. Moreover, field-grown wheat and soil communities are likely to experience greater variability in key environmental controls, such as moisture and temperature, that could impact microbiome assembly. However, controlled greenhouse experiments allow us to explicitly contrast location of soil origin and land use history as drivers of wheat-associated microbiomes without confounding variables.
Defining the core microbial associates of wheat across eastern Washington offers important insight into potential targets for phytobiome manipulation and the need to take account of different cropping histories in identifying keystone taxa. However, future work should expand on these results and experimentally examine the effects of different weather patterns and specific cropping rotations on wheat-associated microbiota in field settings. Moreover, detailed investigations of the associations between microbial community composition and soil health will shed light on the specific taxa that are correlated with plant health, productivity, and ecosystem processes. Finally, the role of specific core and keystone taxa in structuring wheat-associated microbiome composition and function should be explored experimentally in vitro in soils with different cropping histories to confirm their impacts on microbial interaction networks and plant health.
MATERIALS AND METHODS
Field sampling and experimental design.
Soils were collected from four farms representing different rainfall zones across eastern Washington State (described previously [11]). Fields were chosen that had a long history (>30 years) of no-till cropping practices and had adjacent fields managed under the Conservation Reserve Program (CRP). Soil was collected from the top 30 cm of each field using a clean spade and transported to the greenhouse (soil characteristics provided in Table S3). Greenhouse trials were performed to control for factors (e.g., weather, plant growth stage, and plant genotype) that may introduce variation in microbiome composition in field settings. Soil was potted in 13.0-cm by 13.0-cm plastic pots and wheat (Triticum aestivum L. cv. Louise) was planted (5 plants/pot). Pots were arranged in a randomized block design, and wheat was grown in the greenhouse with regular watering and a single round of fertilization (Miracle Gro; Scotts, Inc., Maryville, OH). The greenhouse temperature was maintained at 23°C during the day and 18°C at night with 16-h/day supplemental lighting with sodium vapor lamps. After 6 weeks of growth (Zadoks growth stage 30), bulk and rhizosphere samples were taken from each pot. Bulk soil (∼1 g) was taken between wheat plants using a sterile cork borer (∼0.5-cm diameter). For rhizosphere samples, a single plant was removed from each pot and shaken vigorously to remove loose soil from the root system, and root systems were placed in a sterile 50-ml tube. Root systems were then submerged in 10 ml of sterile distilled H2O, vortexed at maximum speed for 1 min, and sonicated for 1 min to remove rhizosphere soil. Experiments were repeated in 2 years (2015 and 2016) starting with fresh soil collected from the same fields in each year.
DNA extraction and Illumina sequencing.
DNA was extracted from bulk and rhizosphere samples using the MoBio/Qiagen PowerSoil kit (MoBio/Qiagen, Carlsbad, CA) according to the manufacturer’s instructions. For rhizosphere samples, 1 ml of solution was centrifuged (16,000 × g, 1 min) to pellet rhizosphere soil, H2O was pipetted off, and remaining soil was suspended in PowerSoil buffer. Samples were then transferred to PowerSoil bead tubes and processed via standard protocols. Homogenization of samples was performed on a FastPrep bead beater (MP Biomedical, Santa Ana, CA) using the “soil” program. DNA was quantified on a NanoDrop spectrophotometer and submitted for amplification of the V1 to V3 region of the bacterial 16S rRNA gene and paired-end sequencing using an Illumina MiSeq platform (V3 chemistry, 2 × 300). Samples for two locations in 2015 (Genesee and Ritzville) were sequenced by MR DNA (Shallowater, TX), whereas all other samples were submitted to the University of Minnesota Genomics Center (UMGC). Although the same region of the bacterial 16S rRNA gene was targeted, there were small differences in the amplification protocols and primer design between sequence providers, confounding location effects in 2015. PCR amplification at MR DNA used HotStartTaq Plus master mix (Qiagen, USA) and primers 27F (5′-AGRGTTTGATCMTGGCTCAG-3′) and 519R (5′-GWATTACCGCGGCKGCTG-3′). The thermocycling conditions consisted of 94°C for 3 min, followed by 28 cycles of 94°C for 30 s, 53°C for 40 s, and 72°C for 1 min, with a final elongation step at 72°C for 5 min. Samples were checked for successful amplification on a 2% agarose gel, pooled in equal proportions, purified with AMPure XP beads, and prepared for sequencing with the Illumina TruSeq DNA library preparation protocol. PCR amplification at the UMGC used a dual-indexing approach, as described previously (48). Briefly, this consisted of a first round of PCR using template-specific primers MN_27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and MN_534R (5′-ATTACCGCGGCTGCTGG-3′) with Kapa HiFi HotStart polymerase. The PCR conditions consisted of an initial denaturing at 95°C for 5 min, followed by 25 cycles of 98°C for 20 s, 55°C for 15 s, and 72°C for 1 min, with a final extension at 72°C for 1 min. The products from the first PCR were diluted 1:100, and 5 μl was included in a second PCR using indexing primers. The second PCR consisted of an initial denaturation at 95°C for 5 min and then 10 cycles of 98°C for 20 s, 55°C for 15 s, and 72°C for 1 min, with a final extension at 72°C for 5 min. The products were pooled, size selected, and spiked with 20% PhiX prior to sequencing with an Illumina MiSeq 600 cycle version 3 kit.
The fungal ITS1 region was amplified with ITS1-F and ITS2 primers (49) adapted to include linkers, barcoding tags, and additional bases to introduce a phase offset for Illumina sequencing (Table 5). PCR (20-μl total volume) consisted of 10 μl of FastStart master mix (Roche), 0.7 μl of primers (10 μM), 8.3 μl of PCR-grade H2O, and 1 μl of template DNA (1/10 diluted). Reactions were performed in triplicate with three separate annealing temperatures (50, 53, and 55°C) to minimize bias introduced by annealing temperature (50). Thermocycling conditions consisted of an initial denaturation at 95°C for 4 min, 30 cycles of 95°C for 30 s, annealing at 50, 53, or 55°C for 30 s, and extension at 72°C for 45 s, with a final extension at 72°C for 7 min. PCR products were pooled and checked for amplification on a 1.5% agarose gel. Illumina adapters and barcodes (provided by the University of Idaho) were added in an additional PCR step consisting of 10 μl 2× FastStart master mix, 0.75 μl of barcoding primers, 8.25 μl of H2O, and 1 μl of product from initial PCRs. Thermocycling for the barcoding PCR consisted of an initial denaturation at 95°C for 4 min, 10 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, and a final extension at 72°C for 7 min. Successful addition of barcodes was confirmed by visualization of an expected size shift on a 1.5% agarose gel. Barcoded amplicons were quantified with a PicoGreen fluorometry kit, pooled, and sequenced at the University of Idaho Core Genomics Center (2 × 300 MiSeq, version 3 chemistry). Sample-free DNA extractions were used as negative controls and were subjected to PCR, barcoding, Illumina sequencing, and subsequent data processing steps to evaluate potential contaminant sequences. Notably, rhizosphere samples were not sequenced for fungi in 2015.
TABLE 5.
Design of Illumina-tagged, phase-offset ITS1F and ITS2 primers for fungal amplification
| Primer direction and name | Illumina tag | Phase offset | Linker | Gene primer | Full primer sequence (5′→3′) |
|---|---|---|---|---|---|
| Forward | |||||
| ITS1F-A | ACACTGACGACATGGTTCTACA | GG | CTTGGTCATTTAGAGGAAGTAA | ACACTGACGACATGGTTCTACAGGCTTGGTCATTTAGAGGAAGTAA | |
| ITS1F-B | ACACTGACGACATGGTTCTACA | C | GG | CTTGGTCATTTAGAGGAAGTAA | ACACTGACGACATGGTTCTACACGGCTTGGTCATTTAGAGGAAGTAA |
| ITS1F-C | ACACTGACGACATGGTTCTACA | AC | GG | CTTGGTCATTTAGAGGAAGTAA | ACACTGACGACATGGTTCTACAACGGCTTGGTCATTTAGAGGAAGTAA |
| ITS1F-D | ACACTGACGACATGGTTCTACA | TAC | GG | CTTGGTCATTTAGAGGAAGTAA | ACACTGACGACATGGTTCTACATACGGCTTGGTCATTTAGAGGAAGTAA |
| Reverse | |||||
| ITS2-A | TACGGTAGCAGAGACTTGGTCT | CG | GCTGCGTTCTTCATCGATGC | TACGGTAGCAGAGACTTGGTCTCGGCTGCGTTCTTCATCGATGC | |
| ITS2-B | TACGGTAGCAGAGACTTGGTCT | G | CG | GCTGCGTTCTTCATCGATGC | TACGGTAGCAGAGACTTGGTCTGCGGCTGCGTTCTTCATCGATGC |
| ITS2-C | TACGGTAGCAGAGACTTGGTCT | TG | CG | GCTGCGTTCTTCATCGATGC | TACGGTAGCAGAGACTTGGTCTTGCGGCTGCGTTCTTCATCGATGC |
| ITS2-D | TACGGTAGCAGAGACTTGGTCT | ATG | CG | GCTGCGTTCTTCATCGATGC | TACGGTAGCAGAGACTTGGTCTATGCGGCTGCGTTCTTCATCGATGC |
Data processing.
Although there were primer and amplification differences for some samples in 2015, bacterial reads spanning the same 16S rRNA gene region were trimmed to an identical region, leaving no terminal gaps, and were processed together. For both bacteria and fungi, forward and reverse reads were paired using PEAR (version 0.9.6 [51]). Barcodes and primers were removed with cutadapt (version 1.9.1 [52]), and sequences with ambiguous bases and those shorter than 350 bp (bacteria) or 200 bp (fungi) were discarded. Processed sequences were clustered using the UPARSE pipeline (53), using vsearch (54) for all steps with the exception of OTU clustering, which used usearch (version 8.1). Briefly, reads were quality filtered (maximum expected error of 1) and dereplicated, and singletons were discarded prior to OTU clustering at 97% similarity using the “cluster_otus” command. Processed reads were then mapped to OTU clusters to generate an OTU abundance table. For bacteria, taxonomy was assigned to OTUs using the SINTAX algorithm (55) (80% confidence cutoff) and the Ribosomal Database Project 16S database (version 16). For fungi, taxonomy was assigned to OTU representative sequences (centroids) using BLAST+ to the UNITE database (31 January 2016 general release). For bacteria, OTUs with a total sequence count of <20 were discarded to reduce poor-quality OTUs. For fungi, OTUs were filtered to include only those with a match length of >75%, a percent identity of >80%, an E value of <10e−40 to UNITE representatives, and a total read count of ≥5. OTU tables were rarefied to 13,200 and 12,000 sequences/sample for bacteria and fungi, respectively, prior to analysis. Unrarefied OTU tables were retained for differential abundance and network construction algorithms. Material-free extraction controls indicated minimal cross-contamination prior to rarefaction, so no removal of contaminant OTUs was deemed necessary.
Statistical analyses.
Kruskal-Wallis tests were used to test for significant differences in relative abundances of phyla among treatments, followed by a Benjamini-Hochberg correction for the false-discovery rate (FDR) (56). Beta-diversity of bacterial and fungal communities was explored by nonmetric multidimensional scaling (NMDS) of Bray-Curtis dissimilarity among samples using the metaMDS function of the vegan package in R (57). The significance of differences in beta-diversity was assessed using permutational multivariate analysis of variance (PERMANOVA) with the “Adonis” function in the vegan package with 1,000 permutations to determine significance. Analysis of variance (ANOVA) on log10(1 + x)-transformed sequence counts of abundant (>1% of total rarefied sequences) was used to examine significant differences in bacterial or fungal families among treatments with the model ∼year + location + land use × root proximity, followed by a Benjamini-Hochberg correction for false discovery. We identified the core taxa of wheat rhizosphere communities using the criteria that taxa were found in at least 95% of rhizosphere samples and were significantly enriched in the wheat rhizosphere versus bulk soil from each location (see DESeq2 analysis below) from plants grown in soils from each land use history. Bacterial and fungal richness and diversity (Shannon’s H and inverse Simpson’s indices) were evaluated and compared among treatments with ANOVA followed by Tukey’s honest significant difference (HSD) post hoc tests for individual comparisons. Differences in the relative abundances of individual OTUs between bulk and rhizosphere soils with different cropping histories were assessed using DESeq2 (58) on unrarefied OTU tables filtered to include taxa with normalized counts of >5 and found in at least 3 samples. The land use history and root proximity factors were combined to facilitate contrasts between root proximities in soils from each history, and the DESeq model used was ∼location + year + (land use:root proximity). OTUs were considered differentially abundant (DAotus) if they had a base mean of >50, an FDR-adjusted P value of <0.1, and an estimated log2 fold change of >1. For determining enrichment for identification of the core taxa in the rhizosphere, DESeq2 was run on samples from each location of origin using the model ∼year + land use history + root proximity.
A co-occurrence network was constructed for rhizosphere samples using the “spiec-easi” method implemented in the SpiecEasi package in R (22). Sequence counts were combined at the genus-level taxonomic rank to reduce the number of features, and count tables were filtered prior to running SpiecEasi to include only taxa found in at least 25% of samples and sequence counts of >10. After filtering, 64 rhizosphere samples (those from 2016 only, since fungal communities were not sequenced from rhizosphere samples in 2015) including 168 bacterial and 106 fungal genera were included in the cross-domain network construction using the “multi.spiec.easi” function. Network inference used the Meinshausen-Buhlmann (MB) method for neighborhood selection and the bounded StARS approach with a lambda minimum ratio of 1e−5 and nlambda of 50. Hub and connector taxa were explored using node degrees (number of edges from a given node) and betweenness centrality (number of shortest paths through a node) calculated using the “igraph” package in R (59).
Data availability.
Bacterial and fungal sequencing data are available under NCBI SRA accession number PRJNA589625.
Supplementary Material
ACKNOWLEDGMENTS
D.C.S. was funded by an administrator-funded USDA-ARS Postdoctoral Research Associate Award. This research was funded by USDA-ARS and Regional Approaches to Climate Change-Pacific Northwest Agriculture (REACCH) award 2011-68002-30191 from the USDA National Institute for Food and Agriculture.
We thank the following growers for their collaboration: John Aeschliman, Ron Jirava, Tracy Eriksen, and Russ Zenner.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Finkel OM, Castrillo G, Herrera Paredes S, Salas González I, Dangl JL. 2017. Understanding and exploiting plant beneficial microbes. Curr Opin Plant Biol 38:155–163. doi: 10.1016/j.pbi.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sergaki C, Lagunas B, Lidbury I, Gifford ML, Schäfer P. 2018. Challenges and approaches in microbiome research: from fundamental to applied. Front Plant Sci 9:1205. doi: 10.3389/fpls.2018.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns JH, Anacker BL, Strauss SY, Burke DJ. 2015. Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AoB Plants 7:plv030. doi: 10.1093/aobpla/plv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loqué D, Bowen BP, Firestone MK, Northen TR, Brodie EL. 2018. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480. doi: 10.1038/s41564-018-0129-3. [DOI] [PubMed] [Google Scholar]
- 5.Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, Fukuda S, Ushio M, Nakaoka S, Onoda Y, Yoshida K, Schlaeppi K, Bai Y, Sugiura R, Ichihashi Y, Minamisawa K, Kiers ET. 2018. Core microbiomes for sustainable agroecosystems. Nat Plants 4:247–257. doi: 10.1038/s41477-018-0139-4. [DOI] [PubMed] [Google Scholar]
- 6.Busby PE, Soman C, Wagner MR, Friesen ML, Kremer J, Bennett A, Morsy M, Eisen JA, Leach JE, Dangl JL. 2017. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol 15:e2001793. doi: 10.1371/journal.pbio.2001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- 8.Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T. 2017. Disease suppressive soils: new insights from the soil microbiome. Phytopathology 107:1284–1297. doi: 10.1094/PHYTO-03-17-0111-RVW. [DOI] [PubMed] [Google Scholar]
- 9.Tardy V, Chabbi A, Charrier X, de Berranger C, Reignier T, Dequiedt S, Faivre-Primot C, Terrat S, Ranjard L, Maron P-A. 2015. Land use history shifts in situ fungal and bacterial successions following wheat straw input into the soil. PLoS One 10:e0130672. doi: 10.1371/journal.pone.0130672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlatter DC, Schillinger WF, Bary AI, Sharratt B, Paulitz TC. 2017. Biosolids and conservation tillage: impacts on soil fungal communities in dryland wheat-fallow cropping systems. Soil Biol Biochem 115:556–567. doi: 10.1016/j.soilbio.2017.09.021. [DOI] [Google Scholar]
- 11.Schlatter DC, Yin C, Hulbert S, Burke I, Paulitz T. 2017. Impacts of repeated glyphosate use on wheat-associated bacteria are small and depend on glyphosate-use history. Appl Environ Microbiol 83:e01354-17. doi: 10.1128/AEM.01354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somenahally A, DuPont JI, Brady J, McLawrence J, Northup B, Gowda P. 2018. Microbial communities in soil profile are more responsive to legacy effects of wheat-cover crop rotations than tillage systems. Soil Biol Biochem 123:126–135. doi: 10.1016/j.soilbio.2018.04.025. [DOI] [Google Scholar]
- 13.Barber NA, Chantos-Davidson KM, Amel Peralta R, Sherwood JP, Swingley WD. 2017. Soil microbial community composition in tallgrass prairie restorations converge with remnants across a 27-year chronosequence: microbial communities in restored prairies. Environ Microbiol 19:3118–3131. doi: 10.1111/1462-2920.13785. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons SM, Lekberg Y, Mummey DL, Sangwan N, Ramsey PW, Gilbert JA. 2017. Invasive plants rapidly reshape soil properties in a grassland ecosystem. mSystems 2:e00178-16. doi: 10.1128/mSystems.00178-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gewin VL, Kennedy AC, Veseth R, Miller BC. 1999. Soil quality changes in eastern Washington with Conservation Reserve Program (CRP) take-out. J Soil Water Conserv 54:432–438. [Google Scholar]
- 16.Purakayastha TJ, Smith JL, Huggins DR. 2009. Microbial biomass and N cycling under native prairie, conservation reserve and no-tillage in Palouse soils. Geoderma 152:283–289. doi: 10.1016/j.geoderma.2009.06.013. [DOI] [Google Scholar]
- 17.Poudel R, Jumpponen A, Schlatter DC, Paulitz TC, Gardener BBM, Kinkel LL, Garrett KA. 2016. Microbiome networks: a systems framework for identifying candidate microbial assemblages for disease management. Phytopathology 106:1083–1096. doi: 10.1094/PHYTO-02-16-0058-FI. [DOI] [PubMed] [Google Scholar]
- 18.Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK. 2016. The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol Lett 19:926–936. doi: 10.1111/ele.12630. [DOI] [PubMed] [Google Scholar]
- 19.Hassani MA, Durán P, Hacquard S. 2018. Microbial interactions within the plant holobiont. Microbiome 6:58. doi: 10.1186/s40168-018-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spraker JE, Wiemann P, Baccile JA, Venkatesh N, Schumacher J, Schroeder FC, Sanchez LM, Keller NP. 2018. Conserved responses in a war of small molecules between a plant-pathogenic bacterium and fungi. mBio 9:e00820-18. doi: 10.1128/mBio.00820-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barberán A, Bates ST, Casamayor EO, Fierer N. 2012. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J 6:343–351. doi: 10.1038/ismej.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz ZD, Müller CL, Miraldi ER, Littman DR, Blaser MJ, Bonneau RA. 2015. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput Biol 11:e1004226. doi: 10.1371/journal.pcbi.1004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agler MT, Ruhe J, Kroll S, Morhenn C, Kim S-T, Weigel D, Kemen EM. 2016. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol 14:e1002352. doi: 10.1371/journal.pbio.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker MG, Schlatter DC, Otto-Hanson L, Kinkel LL. 2014. Diffuse symbioses: roles of plant-plant, plant-microbe and microbe-microbe interactions in structuring the soil microbiome. Mol Ecol 23:1571–1583. doi: 10.1111/mec.12571. [DOI] [PubMed] [Google Scholar]
- 25.Berg G, Smalla K. 2009. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 26.Walters WA, Jin Z, Youngblut N, Wallace JG, Sutter J, Zhang W, González-Peña A, Peiffer J, Koren O, Shi Q, Knight R, Glavina del Rio T, Tringe SG, Buckler ES, Dangl JL, Ley RE. 2018. Large-scale replicated field study of maize rhizosphere identifies heritable microbes. Proc Natl Acad Sci U S A 115:7368–7373. doi: 10.1073/pnas.1800918115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin C, Hulbert SH, Schroeder KL, Mavrodi O, Mavrodi D, Dhingra A, Schillinger WF, Paulitz TC. 2013. Role of bacterial communities in the natural suppression of Rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.). Appl Environ Microbiol 79:7428–7438. doi: 10.1128/AEM.01610-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin C, Mueth N, Hulbert S, Schlatter D, Paulitz TC, Schroeder K, Prescott A, Dhingra A. 2017. Bacterial communities on wheat grown under long-term conventional tillage and no-till in the Pacific Northwest of the United States. Phytobiomes 1:83–90. doi: 10.1094/PBIOMES-09-16-0008-R. [DOI] [Google Scholar]
- 29.Bulgarelli D, Garrido-Oter R, Münch PC, Weiman A, Dröge J, Pan Y, McHardy AC, Schulze-Lefert P. 2015. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403. doi: 10.1016/j.chom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M. 2015. Evolution of bacterial communities in the wheat crop rhizosphere: rhizosphere bacteria in field-grown intensive wheat crops. Environ Microbiol 17:610–621. doi: 10.1111/1462-2920.12452. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney AK, Yin C, Hulbert SH. 2017. Community structure, species variation, and potential functions of rhizosphere-associated bacteria of different winter wheat (Triticum aestivum) cultivars. Front Plant Sci 8:132. doi: 10.3389/fpls.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapelle E, Mendes R, Bakker PAH, Raaijmakers JM. 2016. Fungal invasion of the rhizosphere microbiome. ISME J 10:265–268. doi: 10.1038/ismej.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi SY, Kim S, Lyuck S, Kim SB, Mitchell RJ. 2015. High-level production of violacein by the newly isolated Duganella violaceinigra str. NI28 and its impact on Staphylococcus aureus. Sci Rep 5:15598. doi: 10.1038/srep15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson M, Habiger J. 2012. Characterization and identification of productivity-associated rhizobacteria in wheat. Appl Environ Microbiol 78:4434–4446. doi: 10.1128/AEM.07466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ofek M, Hadar Y, Minz D. 2012. Ecology of root colonizing Massilia (Oxalobacteraceae). PLoS One 7:e40117. doi: 10.1371/journal.pone.0040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M, Migheli Q. 2013. Fusarium culmorum: causal agent of foot and root rot and head blight on wheat: the wheat pathogen Fusarium culmorum. Mol Plant Pathol 14:323–341. doi: 10.1111/mpp.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell JJ, Carere J, Fitzgerald TL, Stiller J, Covarelli L, Xu Q, Gubler F, Colgrave ML, Gardiner DM, Manners JM, Henry RJ, Kazan K. 2016. The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.). Ann Bot 119:853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alabouvette C. 1999. Fusarium wilt suppressive soils: an example of disease-suppressive soils. Austral Plant Pathol 28:57–64. doi: 10.1071/AP99008. [DOI] [Google Scholar]
- 39.Li F, Chen L, Redmile-Gordon M, Zhang J, Zhang C, Ning Q, Li W. 2018. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad Dev 29:1642–1651. doi: 10.1002/ldr.2965. [DOI] [Google Scholar]
- 40.Johnson JM, Ludwig A, Furch A, Mithöfer A, Scholz SS, Reichelt M, Oelmüller R. 2019. The beneficial root-colonizing fungus Mortierella hyalina promotes the aerial growth of Arabidopsis and activates calcium-dependent responses which restrict Alternaria brassicae-induced disease development in roots. Mol Plant Microbe Interact 32:351–363. doi: 10.1094/MPMI-05-18-0115-R. [DOI] [PubMed] [Google Scholar]
- 41.Tautges NE, Sullivan TS, Reardon CL, Burke IC. 2016. Soil microbial diversity and activity linked to crop yield and quality in a dryland organic wheat production system. Appl Soil Ecol 108:258–268. doi: 10.1016/j.apsoil.2016.09.003. [DOI] [Google Scholar]
- 42.Wagg C, Bender SF, Widmer F, van der Heijden M. 2014. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci U S A 111:5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu J, Wei Z, Friman V-P, Gu S-H, Wang X-F, Eisenhauer N, Yang T-J, Ma J, Shen Q-R, Xu Y-C, Jousset A. 2016. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. mBio 7:e01790-16. doi: 10.1128/mBio.01790-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jauri PV, Altier N, Pérez CA, Kinkel L. 2018. Cropping history effects on pathogen suppressive and signaling dynamics in Streptomyces communities. Phytobiomes 2:14–23. doi: 10.1094/PBIOMES-05-17-0024-R. [DOI] [Google Scholar]
- 45.Peralta AL, Sun Y, McDaniel MD, Lennon JT. 2018. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 9:e02235. doi: 10.1002/ecs2.2235. [DOI] [Google Scholar]
- 46.Zak DR, Holmes WE, White DC, Peacock AD, Tilman D. 2003. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84:2042–2050. doi: 10.1890/02-0433. [DOI] [Google Scholar]
- 47.Gómez P, Paterson S, De Meester L, Liu X, Lenzi L, Sharma MD, McElroy K, Buckling A. 2016. Local adaptation of a bacterium is as important as its presence in structuring a natural microbial community. Nat Commun 7:12453. doi: 10.1038/ncomms12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gohl DM, Vangay P, Garbe J, MacLean A, Hauge A, Becker A, Gould TJ, Clayton JB, Johnson TJ, Hunter R, Knights D, Beckman KB. 2016. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat Biotechnol 34:942–949. doi: 10.1038/nbt.3601. [DOI] [PubMed] [Google Scholar]
- 49.White TJ, Bruns TD, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, New York, NY. [Google Scholar]
- 50.Song Z, Schlatter D, Kennedy P, Kinkel LL, Kistler HC, Nguyen N, Bates ST. 2015. Effort versus reward: preparing samples for fungal community characterization in high-throughput sequencing surveys of soils. PLoS One 10:e0127234. doi: 10.1371/journal.pone.0127234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Kobert K, Flouri T, Stamatakis A. 2014. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 53.Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 54.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgar RC. 2016. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv 10.1101/074161. [DOI]
- 56.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 57.Oksanen J, Blanchette FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin D, O’Hara B, Simpson G, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2016. vegan: community ecology package. https://cran.r-project.org/web/packages/vegan/index.html.
- 58.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Csardi G, Nepusz T. 2006. The Igraph software package for complex network research InterJournal Complex Systems 2006:1695. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bacterial and fungal sequencing data are available under NCBI SRA accession number PRJNA589625.