Abstract
Mitochondrial Ca2+ dynamics are involved in the regulation of multifarious cellular processes, including intracellular Ca2+ signalling, cell metabolism and cell death. Use of mitochondria-targeted genetically encoded Ca2+ indicators has revealed intercellular and subcellular heterogeneity of mitochondrial Ca2+ dynamics, which are assumed to be determined by distinct thresholds of Ca2+ increases at each subcellular mitochondrial domain. The balance between Ca2+ influx through the mitochondrial calcium uniporter and extrusion by cation exchangers across the inner mitochondrial membrane may define the threshold; however, the precise mechanisms remain to be further explored. We here report the new red fluorescent genetically encoded Ca2+ indicators, R-CEPIA3mt and R-CEPIA4mt, which are targeted to mitochondria and their Ca2+ affinities are engineered to match the intramitochondrial Ca2+ concentrations. They enable visualization of mitochondrial Ca2+ dynamics with high spatiotemporal resolution in parallel with the use of green fluorescent probes and optogenetic tools. Thus, R-CEPIA3mt and R-CEPIA4mt are expected to be a useful tool for elucidating the mechanisms of the complex mitochondrial Ca2+ dynamics and their functions.
Subject terms: Biological techniques, Biotechnology, Cell biology
Introduction
Mitochondrial Ca2+ dynamics contribute to the control of various cellular functions such as formation of spatiotemporal patterns of cytosolic Ca2+ dynamics, cellular metabolism and cell survival1. Ca2+ concentrations in the mitochondrial matrix are regulated by the balance between the influx of Ca2+ through the mitochondrial Ca2+ uniporter (MCU) and the efflux of Ca2+ by Na+/Ca2+ or H+/Ca2+ exchangers1–3. Recent studies have elucidated the molecular identity of the channel and regulatory components of MCU4–11 as well as Na+-Ca2+-Li+ exchanger in the mitochondrial inner membrane12,13. Furthermore, the advent of genetically encoded Ca2+ indicators (GECIs) that are targeted to the mitochondrial matrix has enabled monitoring mitochondrial Ca2+ dynamics with high spatiotemporal resolution, revealing both the subcellular and intercellular heterogeneity of mitochondrial Ca2+ responses upon agonist-induced increases in the cytosolic Ca2+ concentration14–16. These imaging results suggest that the threshold of the net Ca2+ flux into the mitochondrial matrix is differentially determined in individual cells or even in each subcellular mitochondrial domain. However, the mechanism of these heterogeneous mitochondrial Ca2+ dynamics and their functional significance remains to be clarified. Thus, further analyses combined with high-resolution mitochondrial Ca2+ imaging are required.
We have previously developed a Ca2+ indicator protein family of Calcium-measuring organelle-Entrapped Protein IndicAtors (CEPIA) to visualize Ca2+ signals in both the endoplasmic reticulum (ER) and mitochondria15. ER-targeted CEPIAs have Kd values for Ca2+ ranging between 558 and 672 µM, which are higher than those of other ER-targeted GECIs such as D1ER17, GCaMPer18, ER-GCaMPs19, and ER-LAR-GECOs20. Mitochondrial Ca2+ imaging analyses using green fluorescent protein (GFP)-based CEPIA variants with lower Ca2+ affinities (Kd = 14.5 or 90.2 µM) than cytosolic Ca2+ indicators suggested that mitochondrial Ca2+ concentrations can increase beyond 50 µM in a small fraction of HeLa cells. While a red fluorescent protein (RFP)-based low-affinity GECI, mito-LAR-GECO1.2 (Kd = 12 µM)20, has been developed, lower-affinity mitochondrial RFP-based CEPIAs have not been developed, yet. Moreover, GFP-based GECIs cannot be used simultaneously with other green fluorescent imaging tools, including synthetic Ca2+ indicators, nor are they simultaneously used with optogenetic tools that are activated by blue light, such as channelrhodopsin-2 and OptoXRs21,22. These limitations can be circumvented by GECIs with longer excitation and emission wave lengths. Therefore, CEPIA variants with red fluorescence may allow simultaneous use of other optical tools to increase the utility of mitochondria-targeted GECIs.
To this end, we generated the red-fluorescent CEPIAmt variants, R-CEPIA3mt and RCEPIA4mt, of which the Ca2+-affinity was optimized to measure mitochondrial Ca2+ concentrations. These variants allow visualization of mitochondrial Ca2+ signals with high spatiotemporal resolution that enables the detection of mitochondrial Ca2+ dynamics at subcellular local domains. Furthermore, simultaneous use of green fluorescent Ca2+ indicators and optogenetic tools is possible. Thus, R-CEPIA3mt and R-CEPIA4mt are expected to be a valuable tool for obtaining deeper insight into the cellular functions of mitochondrial Ca2+ dynamics.
Results
In vitro characterization of R-CEPIA3mt and R-CEPIA4mt
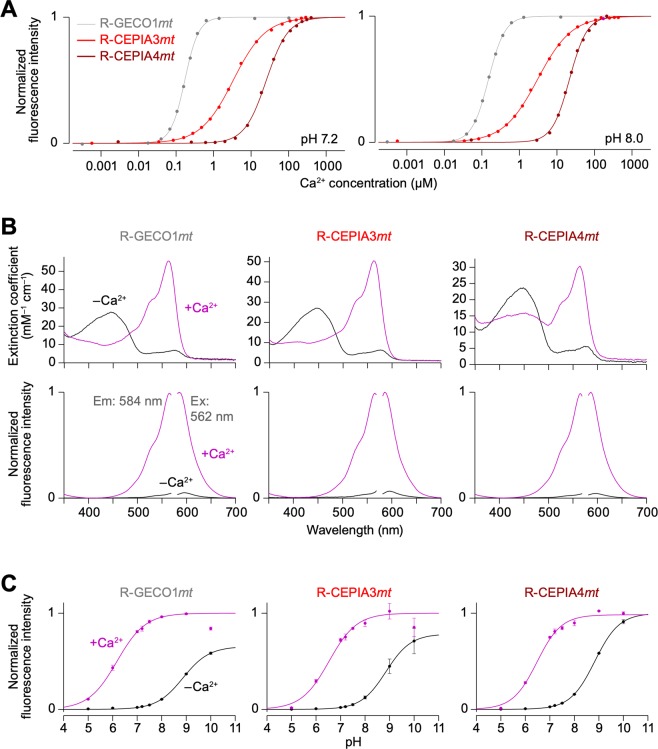
On the basis of an amino acid substitution strategy to produce low Ca2+ affinity variants of CEPIA15, we generated R-CEPIA3mt and R-CEPIA4mt by modifying one (E31D) and three (E31D, F92W and D133E) amino acids, respectively, in the calmodulin domain of R-GECO1mt (Supplementary Fig. 1A). As expected, these mutant indicators had reduced Ca2+ affinities (Kd = 3.7 µM for R-CEPIA3mt; Kd = 26.9 µM for R-CEPIA4mt) and high dynamic ranges (Fig. 1A and Table 1) without apparent alterations in the extinction coefficient, both excitation and emission spectra, and pH dependence of the original R-GECO1mt (Fig. 1B,C and Table 1). Notably, R-CEPIA3mt had a broader dynamic range with reduced cooperativity compared with those of R-GECO1mt and R-CEPIA4mt, indicating that R-CEPIA3mt may be useful for detecting dynamic changes in mitochondrial Ca2+ levels ranging from 0.5 to 100 µM.
Figure 1.
In vitro characterization of R-CEPIA3mt and R-CEPIA4mt. All the extracted parameters are summarized in Table 1. (A) In vitro Ca2+ titration curves of R-GECO1mt (gray), R-CEPIA3mt (red) and R-CEPIA4mt (brown) at pH 7.2 (left) or 8.0 (right) solution. (B) Absorption (upper), excitation and emission (lower) spectra of R-GECO1mt (left), R-CEPIA3mt (middle) and R-CEPIA4mt (right) in Ca2+-containing (1 mM, magenta) or Ca2+-free (1 mM EGTA, black) solution. (C) pH titration curves of R-GECO1mt (left), R-CEPIA3mt (middle) and R-CEPIA4mt (right) in Ca2+-containing (1 mM, magenta) or Ca2+-free (1 mM EGTA, black) solution. The plots were fitted by a single Hill equation. Mean ± SEM (n = 3).
Table 1.
Properties of R-CEPIA3mt and R-CEPIA4mt.
| Probe | Ca2+ | ε (mM−1 cm−1) (λABS*) | pKa† | λEx* | pH 7.2 | pH 8.0 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kd for Ca2+ (μM) | Dynamic range‡ | Hill coefficient | Kd for Ca2+ (μM) | Dynamic range‡ | Hill coefficient | |||||
| R-GECO1mt | − | 27 (445), 7 (576) | 8.9 | 565 | 0.19 ± 0.02§ | 22.2 ± 0.5§ | 2.20 ± 0.10§ | 0.14 ± 0.01§ | 8.8 ± 0.1§ | 2.12 ± 0.10§ |
| + | 54 (562) | 6.2 | ||||||||
| R-CEPIA3mt | − | 26 (445), 6 (576) | 8.9 | 565 | 3.7 ± 0.5 | 30.0 ± 1.5 | 0.96 ± 0.02 | 3.3 ± 0.2 | 8.9 ± 0.1 | 0.93 ± 0.01 |
| + | 49 (562) | 6.5 | ||||||||
| R-CEPIA4mt | − | 25 (445), 6 (576) | 8.8 | 565 | 26.9 ± 1.0 | 23.9 ± 0.4 | 1.51 ± 0.01 | 21.4 ± 0.3 | 4.7 ± 0.1 | 1.86 ± 0.02 |
| + | 17 (450), 30 (562) | 6.5 | ||||||||
*λABS and λEx are the maximum wavelength of absorption and fluorescence excitation spectra, respectively.
†pKa is determined as the pH at half-maximal fluorescence intensity calculated by fitting Hill equation to each plot.
‡Dynamic range indicates the ratio of the maximum to minimum fluorescence intensity (Fmax/Fmin).
§Mean ± s.e.m.
Mitochondrial Ca2+ signals visualized by R-CEPIA3mt and R-CEPIA4mt
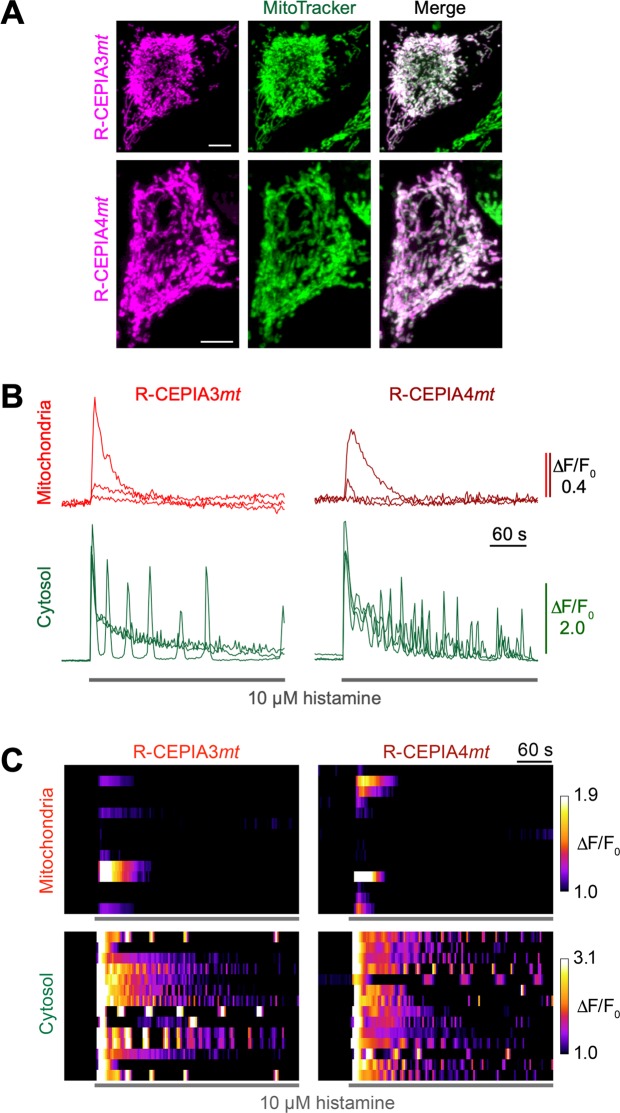
Using these CEPIA variants, we visualized mitochondrial Ca2+ signals in HeLa cells. The mitochondrial distribution of both variants was confirmed by colocalization with MitoTracker Green (Fig. 2A). Simultaneous Ca2+ imaging in mitochondria and the cytosol (the latter was visualized by the green fluorescent Ca2+ indicator, Cal-520) demonstrated that only a fraction of the cells (35.7%, n = 34 for R-CEPIA3mt; 28.6%, n = 38 for R-CEPIA4mt) showed a transient mitochondrial Ca2+ increase in response to cytosolic Ca2+ elevations induced by the inositol trisphosphate-producing agonist, histamine (Fig. 2B,C). Interestingly, mitochondrial Ca2+ transients were elicited only by the initial peak of the cytosolic Ca2+ oscillations, which reached the threshold of mitochondrial Ca2+ increases. Similar observations have previously been reported using GFP-based mitochondrial CEPIAs15.
Figure 2.
Global mitochondrial Ca2+ signals visualized by R-CEPIA3mt and R-CEPIA4mt. (A) Representative images of HeLa cells expressing R-CEPIA3mt (upper) or R-CEPIA4mt (lower). Fluorescence of R-CEPIAs (left), MitoTracker Green staining (middle) and the merged images (right) are shown. Scale bars, 5 µm. (B) Time course of agonist-induced Ca2+ response in the mitochondria (upper) and cytosol (lower) in three representative HeLa cells expressing R-CEPIA3mt (left) or R-CEPIA4mt (right). Cytosolic Ca2+ signals were visualized by a green fluorescent synthetic Ca2+ indicator, Cal-520. (C) Heat maps of cell population data (n = 14) of global Ca2+ signals in mitochondria (upper) and cytosol (lower). Each horizontal strip corresponds to the time course of the Ca2+ signal in each cell.
To examine the spatial resolution of R-CEPIA3mt and R-CEPIA4mt, we next focused on subcellular mitochondrial domains in single HeLa cells. Both R-CEPIAmt variants successfully detected heterogenous Ca2+ signals in mitochondrial subdomains within close proximity (Fig. 3A,B). We next performed simultaneous imaging of mitochondrial Ca2+ using two CEPIAmt variants with different colours and Ca2+ affinities to effectively expand the range of Ca2+ concentrations detectable with the indicators. HeLa cells were co-transfected with R-CEPIA3mt or R-CEPIA4mt and the GFP-based high Ca2+ affinity CEPIA2mt (Kd = 160 nM)15. Additionally, the cells were loaded with fura-2 for simultaneous detection of cytosolic Ca2+ dynamics. As shown in Fig. 3C,D, we found that individual HeLa cells had heterogenous mitochondrial domains, of which Ca2+ signals were detected either by both CEPIA2mt and one of the R-CEPIAmt variants (domain 1 in Fig. 3C,D) or only by CEPIA2mt (domain 2 in Fig. 3C,D). It is remarkable that agonist-induced mitochondrial Ca2+ dynamics were detected by indicators with varying affinities to Ca2+. On the basis of the calibrations of the indicators (Fig. 1A), these results suggest that each mitochondrial subdomain reaches distinct Ca2+ levels that range between 0.1 and 100 µM.
Figure 3.
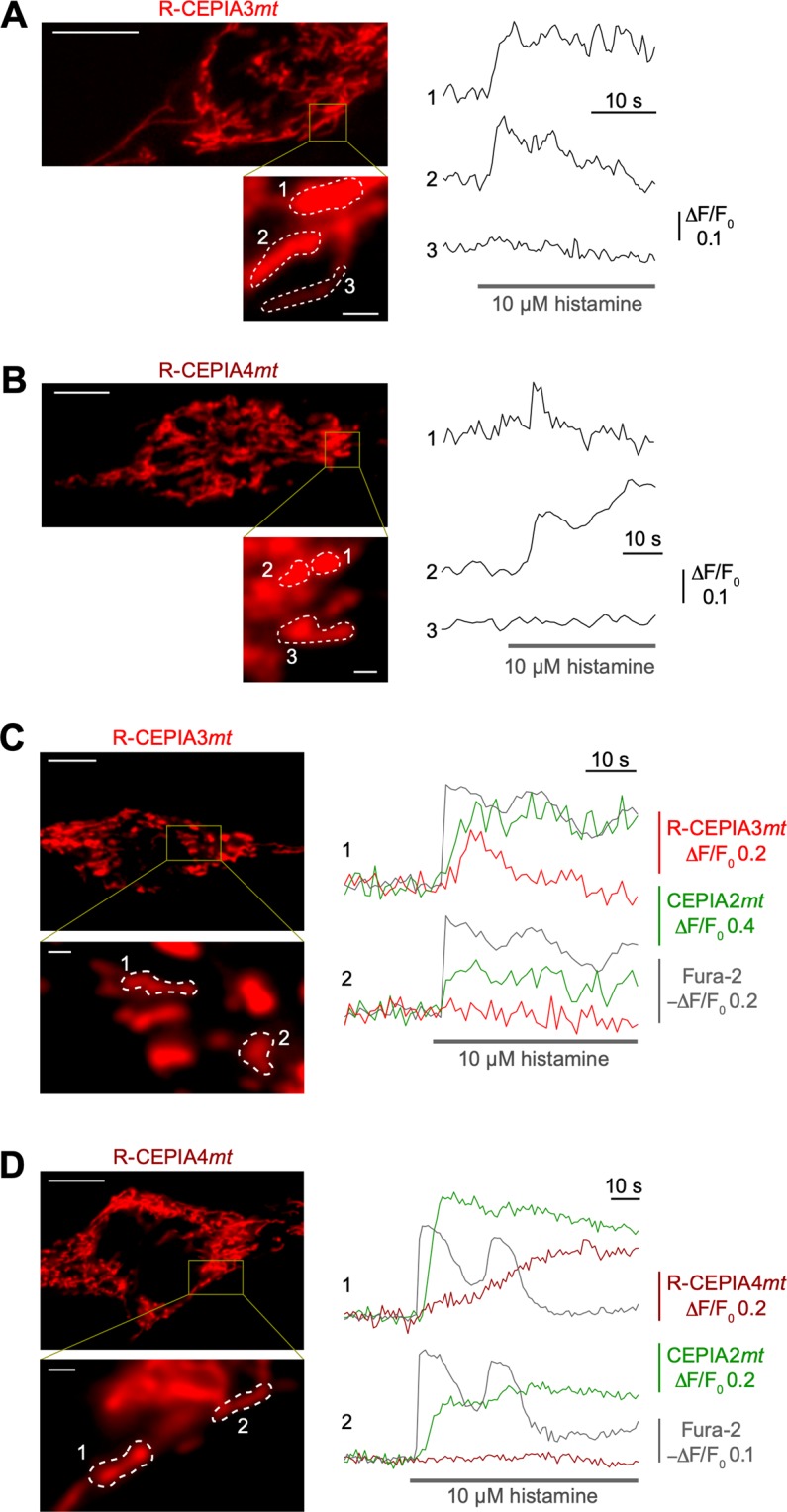
Local mitochondrial Ca2+ signals visualized by R-CEPIA3mt and R-CEPIA4mt. (A,B) Representative red fluorescence images and time courses of agonist-induced Ca2+ signals in subcellular mitochondrial domains in a HeLa cell expressing R-CEPIA3mt (A) or R-CEPIA4mt (B). Regions of interest (ROI) are indicated in high magnification images shown in the left lower panels. Scale bars, 10 µm (upper) and 1 µm (lower). (C,D) Simultaneous Ca2+ imaging of mitochondria with low Ca2+ affinity R-CEPIAs (red fluorescence), high Ca2+ affinity CEPIA2mt (green fluorescence) and the cytosolic Ca2+ indicator, fura-2 (405-nm excitation). Representative red fluorescence images and time courses of agonist-induced Ca2+ signals in subcellular mitochondrial domains in a HeLa cell are shown. ROI are indicated in high magnification images shown in the left lower panels. The cells expressing both R-CEPIA3mt and CEPIA2mt (C), and both R-CEPIA4mt and CEPIA2mt (D) were loaded with fura-2. Scale bars, 10 µm (upper) and 1 µm (lower).
pH dependence of R-CEPIA3mt and R-CEPIA4mt
Using HeLa cells co-expressing CEPIA2mt and one of the new R-CEPIAmt variants, we examined the pH dependence of R-CEPIA3mt and R-CEPIA4mt. Although alkalization by NH4Cl induced a sustained elevation in the fluorescence intensity of all mitochondrial CEPIAs, the amplitude of the change in R-CEPIA3mt and R-CEPIA4mt was significantly smaller than that of CEPIA2mt (Supplementary Fig. 2). This is attributable to the acidity shifted acid dissociation constant of R-CEPIA3mt and R-CEPIA4mt (Table 1; pKa = 6.5 in the presence of Ca2+ for both the R-CEPIAmt variants) compared with the GFP-based CEPIAs (pKa = 7.0 in the presence of Ca2+ for CEPIA2mt, Supplementary Fig. 1B)15. Thus, R-CEPIA3mt and R-CEPIA4mt enable a more stable detection of Ca2+ signals in mitochondria, which may undergo dynamic pH changes.
Simultaneous use of an optogenetic tool and mitochondrial Ca2+ indicators
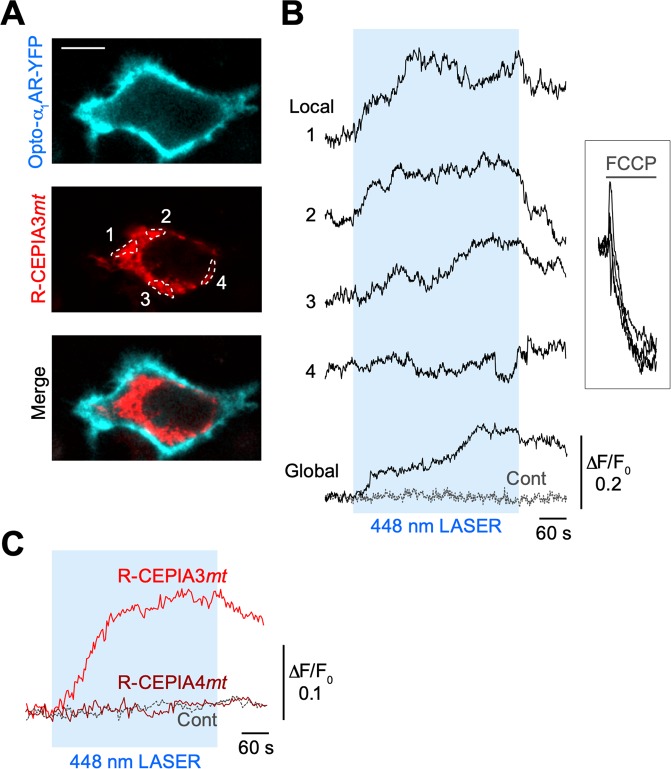
As the excitation and emission spectra of the R-CEPIAmt variants do not overlap with the excitation spectrum (peaking at ~450 nm) of the phospholipase C (PLC) activator, Opto-α1AR-YFP22, we performed mitochondrial Ca2+ imaging during optogenetic activation. In response to 448-nm laser irradiation, several mitochondrial domains (positions 1, 2 and 3 in Fig. 4A) in a HeLa cell expressing Opto-α1AR-YFP and R-CEPIA3mt showed Ca2+ increases (Fig. 4B), whereas another mitochondrial domain in the same cell (position 4 in Fig. 4A) did not show Ca2+ signals. The ensemble averaging of these local responses represents the global mitochondrial Ca2+ signal (bottom solid line in Fig. 4B). Another cell without Opto-α1AR-YFP expression in the same dish failed to show mitochondrial Ca2+ signals in response to light activation (“Cont” in Fig. 4B). Bath application of the mitochondrial oxidative phosphorylation uncoupler, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), rapidly decreased the fluorescence intensity of R-CEPIA3mt in all mitochondrial domains, indicating that R-CEPIA3mt reported Ca2+ increases in mitochondria induced by optogenetic activation of Ca2+ release from the ER. Averaged traces show that the global mitochondrial Ca2+ responses were detected by R-CEPIA3mt but not by R-CEPIA4mt (Fig. 4C), suggesting that optogenetic PLC activation may induce mitochondrial Ca2+ increases up to 1–2 µM at most in our experimental conditions.
Figure 4.
Simultaneous application of mitochondrial Ca2+ imaging and an optogenetic activator of the Ca2+ release machinery. (A) Representative confocal section images of Opto-α1AR-YFP and R-CEPIA3mt in a transfected HeLa cell. A merged image is shown in the lower panel. Scale bar, 10 µm. (B) Time courses of R-CEPIA3mt-reported Ca2+ signals in the subcellular mitochondrial domains indicated in (A) (Local; ROI 1–4). Laser irradiation (448 nm) during the period indicated by the blue box was used to activate Opto-α1AR-YFP, which is a chimera protein of rhodopsin, α1-adrenergic receptor and YFP. Traces in the right box show Ca2+ responses during bath application of the mitochondrial uncoupler, FCCP. Time courses of global mitochondrial Ca2+ signals in the same cell and in a negative control cell expressing R-CEPIA3mt (without expressing Opto-α1AR-YFP) in the same culture dish are shown at the bottom (Global and Cont). (C) Averaged time courses of optogenetics-induced global mitochondrial Ca2+ signals that were reported by R-CEPIA3mt (red) or R-CEPIA4mt (brown). As a control, the averaged response of HeLa cells transfected with only R-CEPIA3mt is shown (grey dashed line). n = 6, 3 and 3 cells for R-CEPIA3mt, R-CEPIA4mt and control, respectively, from 2 culture dishes each.
Discussion
We succeeded to produce two variants of RFP-based mitochondrial CEPIAs, R-CEPIA3mt and R-CEPIA4mt. These new indicators have lower Ca2+ sensitivity than that of the previously developed high Ca2+ affinity RFP-based mitochondrial GECI, R-GECO1mt (Table 1)15,23. The Kd values for Ca2+ of R-CEPIA3mt and R-CEPIA4mt are similar to those of CEPIA3mt and CEPIA4mt, respectively, which were developed in our previous study15. Owing to the large dynamic range and high brightness, R-CEPIA3mt and R-CEPIA4mt allow spatiotemporal resolution imaging that is high enough to visualize heterogenous Ca2+ dynamics in subcellular mitochondrial domains (Fig. 3). They can be used simultaneously with other tools, such as GFP-based CEPIAs, Cal-520 and fura-2 (Fig. 3) as well as optogenetic PLC activator (Fig. 4). Furthermore, they have a lower pH dependence than the GFP-based CEPIAmt variants (Supplementary Fig. 2).
We have previously shown that there is no significant pH change within the mitochondrial matrix in histamine-stimulated HeLa cells15. Thus, the mitochondrial Ca2+ changes reported by R-CEPIAmts in the present study are unlikely to be influenced by mitochondrial pH changes. It has been reported that under certain conditions mitochondria may undergo transient alkalizations called “mitochondrial flash” (estimated pH range: 7.5 to 8.0)24,25. The effect of such pH changes would be less in R-CEPIA3mt and R-CEPIA4mt than in GFP-based CEPIAmts.
The mechanism underlying the intercellular and subcellular heterogeneity in mitochondrial Ca2+ dynamics is of great interest. Recent studies have identified molecules involved in Ca2+ influx through the MCU complex, including MCU, MCUb, EMRE and MICU1–34–11. Furthermore, the Na+-Ca2+-Li+ transporter has been identified as a Ca2+ extrusion mechanism on the mitochondrial inner membrane12,13. Therefore, it is important to study whether there is intercellular and subcellular heterogeneity in the expression levels or activities of these Ca2+-handling proteins, and whether protein heterogeneity corresponds to the heterogeneity in mitochondrial Ca2+ dynamics. Moreover, it will be informative to study whether the heterogeneity in mitochondrial Ca2+ dynamics produces intercellular heterogeneity in cell death as well as subcellular heterogeneity in mitochondrial motility, ATP production, opening of permeability transition pores, and mitochondrial membrane potentials. Various optical tools for tagging, probing and controlling these cellular processes have been developed. Simultaneous use of these optical tools and the R-CEPIAmt variants is expected to make a great contribution to the field.
In summary, we added new members to the library of green and red emission mitochondrial Ca2+ indicators that cover a broad range of mitochondrial Ca2+ concentrations. Further imaging analyses using these potent and useful tools may facilitate uncovering the mode of action of mitochondrial Ca2+ dynamics and their functions in health and disease.
Methods
Gene manipulation and plasmid construction
For engineering R-CEPIA3 and R-CEPIA4, we introduced point mutations into pET19b R-GECO115 using primers 1–6 (Supplementary Table 1). For mammalian expression, the cDNAs of R-CEPIA3 and R-CEPIA4 were subcloned into pCMV vector containing the mitochondria-targeting sequence (pCMV R-GECO1mt15) by restriction enzyme digestion.
Bacterial expression and in vitro spectroscopy
BL21-CodonPlus(DE3)-RIL bacteria (Agilent, Santa Clara, CA, USA) were transformed with the pET19b plasmids having R-CEPIA3, R-CEPIA4, R-GECO1 or CEPIA2. The bacteria were incubated for 16–24 h at 37 °C in 2 × YT medium containing ampicillin and chloramphenicol (20 μg•ml–1). After harvesting the bacteria by centrifugation, the cells were resuspended in KCl/MOPS buffer (130 KCl, 50 MOPS in mM, pH 7.2) and lysed by French press (Thermo Fisher, Waltham, MA, USA) at 20,000 psi. The recombinant proteins were purified using TALON metal affinity resin (Takara Clontech, Shiga, Japan) and eluted with KCl/MOPS buffer containing 250 mM imidazole.
The absorbance spectra were measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher) in KCl/MOPS buffer containing 1 mM EGTA or 1 mM CaCl2. The protein concentration was calculated by measuring the absorbance following alkaline denaturation, assuming ε = 38,000 M–1•cm–1 at 455 nm for R-CEPIA3, R-CEPIA4, and R-GECO1, and ε = 44,000 M–1•cm–1 at 446 nm for CEPIA226. The molar extinction coefficient was calculated by dividing the peak absorbance value by the protein concentration.
Ca2+ titration curves were obtained by adding small aliquots of CaCl2 to the recombinant indicators in KCl/MOPS or KCl/HEPES (130 KCl, 50 HEPES in mM, pH 8.0) buffer. The indicators’ concentration was 25–75 nM. The Ca2+ concentrations were clamped with any of the following Ca2+ buffers: 1 mM EGTA, 1 mM BAPTA, 1 mM Br2BAPTA (5,5′-Dibromo BAPTA) and 1 mM Nitrilotriacetic acid (NTA). Free Ca2+ concentration was calculated by Maxchelator (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/). Fluorescence intensity was measured with F-4500 FL spectrofluorometer (Hitachi, Tokyo, Japan) at 562 ± 5/584 ± 5 nm (excitation/emission) wavelength for R-CEPIA3, R-CEPIA4, and R-GECO1, and 492 ± 10/514 ± 10 nm for CEPIA2. The obtained relationship between the Ca2+ concentration and the fluorescence intensity was fitted by the following single Hill plot equation with the KaleidaGraph software (Synergy Software, Reading, PA, USA).
K′d represents apparent dissociation constant or the Ca2+ concentration at which half of the indicator molecules bind to Ca2+. n represents Hill coefficient. The fluorescence intensity at various Ca2+ concentrations was normalized by (F − Fmin)/(Fmax − Fmin). The dynamic range of the indicator was calculated as the ratio of Fmax to Fmin.
pH titration curves for each indicator were obtained by measuring fluorescence intensity in the solutions containing 130 mM KCl and 50 mM pH buffer (MES for pH 5.0; MES/HEPES for pH 6.0; MOPS/HEPES for pH 7.0–7.5; HEPES for pH 8.0; HEPES/CHES for pH 9.0; CHES for pH 10.0) with 1 mM EGTA or 1 mM Ca2+. pKa was obtained by fitting the obtained fluorescence intensity with a single Hill equation.
Cell culture
HeLa cells were cultured on collagen-coated plastic dishes (IWAKI, Shizuoka, Japan) in DMEM supplemented with 10% fetal bovine serum, penicillin (100 U•ml–1) and streptomycin (100 U•ml–1). For Ca2+ imaging, the cells were plated on collagen type-I-coated glass-bottom dishes (MatTek, Ashland, MA, USA) and cultured for 16 h before imaging.
Imaging
Cultured HeLa cells were transfected using Lipofectamine 3000 (Thermo Fisher) 2 or 3 days before imaging. The plasmids used in the current study were: pCMV R-CEPIA3mt, pCMV R-CEPIA4mt, pCMV CEPIA2mt and pcDNA3 Opto-α1AR-YFP. For cytosolic Ca2+ imaging using Cal-520 or fura-2, cells were loaded with 5 µM Cal-520 AM (AAT Bioquest, USA) or 5 µM fura-2 AM (Dojindo, Japan) at room temperature (22–24 °C) for 30 min in culture medium. Before imaging, the loading solution was washed three times and replaced with physiological salt solution (PSS) containing (in mM) 150 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5.6 glucose and 25 HEPES (pH 7.4).
Time-lapse images of Cal-520, R-CEPIA3mt and R-CEPIA4mt (hereafter, R-CEPIA3/4mt) were captured using an inverted IX81 microscope (Olympus, Tokyo, Japan) equipped with a ×20 UPlanSApo oil immersion objective [numerical aperture (NA) = 0.75; Olympus], an electron-multiplying cooled-coupled device (EM-CCD) ImagEM camera (Hamamatsu Photonics, Japan), a Lambda 10–3 filter wheel (Sutter Instrument, Navato, CA, USA), an ebx75 xenon lamp (Leica, Wetzlar, Germany) and an EL6000 metal halide lamp (Leica) at a rate of one frame per 1 or 2 s with the following set of excitation and emission filters, respectively: 472 ± 15 nm and 520 ± 17.5 nm for Cal-520; 562 ± 20 nm and 641 ± 37.5 nm for R-CEPIA3/4mt.
For Ca2+ imaging in subcellular mitochondrial domains, time lapse or snapshot images of R-CEPIA3/4mt, CEPIA2mt, fura-2 and Opto-α1AR-YFP were captured using a TCS SP8 confocal microscope (Leica) equipped with a × 63 HC PL APO oil immersion objective (NA = 1.40; Leica) at a rate of one frame per 1–3 s with the following wavelengths [excitation laser (nm); emission spectra (nm)]: R-CEPIA3/4mt (552; 560–750), CEPIA2mt (488; 520–550), fura-2 (405; 430–515) and Opto-α1AR-YFP [for confirmation of transfection by YFP fluorescence, shown in Fig. 4A (488; 500–550), for light activation (448; 530–555)]. Photobleaching was corrected for by a linear or exponential fit to the fluorescence intensity change before agonist stimulation.
For imaging of subcellular localization of CEPIA, the mitochondria in R-CEPIA3/4mt-expressing cells were stained by a 30-min incubation in culture medium containing 500 nM MitoTracker Green (Thermo Fisher) at 37 °C. Images were captured with a TCS SP8 confocal microscope using a ×63 HC PL APO oil immersion objective at excitation: 488 nm and emission: 500–540 nm for MitoTracker Green and excitation: 552 nm and emission: 560–750 nm for R-CEPIA3/4mt.
Data analysis and statistics
Two-tailed Student’s t-tests were performed to determine the statistical significance using Origin 7 (OriginLab, Northampton, MA, USA). The calculations, processing and analysis of obtained images were performed with ImageJ and ImageJ Fiji (NIH, Bethesda, MD, USA). Graphs and time course traces were produced with Origin 7 and arraigned with Illustrator CC (Adobe, San Jose, CA, USA), respectively.
Supplementary information
Acknowledgements
We thank Y. Kawashima for technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan and Takeda Science Foundation, the Pharmacological Foundation Tokyo and The Naito Foundation, Japan Society for the Promotion of Science Overseas Research Fellowships, and American Heart Association Postdoctoral Fellowship.
Author contributions
J.S. engineered R-CEPIA3/4mt and carried out in vitro characterization; K.K. and I.T. carried out the cell biology experiments; J.S. and K.K. analysed the data and produced figures; J.S., K.K. and M.I. wrote the manuscript. All authors discussed the results and approved the submission of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kazunori Kanemaru and Junji Suzuki.
Supplementary information
is available for this paper at 10.1038/s41598-020-59707-8.
References
- 1.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nature Reviews Molecular Cell Biology. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.De Stefani D, Rizzuto R, Pozzan T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016;85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 4.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefani D, Raffaello A, Teardo E, Szabó I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri D, Sancak Y, Mootha VK, Clapham DE. MCU encodes the pore conducting mitochondrial calcium currents. Elife. 2013;2013:4–11. doi: 10.7554/eLife.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallilankaraman K, et al. MICU1 is an essential gatekeeper for mcu-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perocchi F, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26:179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamer KJ, Mootha VK. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 12.Palty R, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S, Dey K, Hershfinkel M, Ohana E, Sekler I. Identification of residues that control Li+ versus Na+ dependent Ca2+ exchange at the transport site of the mitochondrial NCLX. Biochim. Biophys. Acta - Mol. Cell Res. 2017;1864:997–1008. doi: 10.1016/j.bbamcr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki J, et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki J, Kanemaru K, Iino M. Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging. Biophys. J. 2016;111:1119–1131. doi: 10.1016/j.bpj.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson MJ, et al. A low affinity GCaMP3 variant (GCaMPer) for imaging the endoplasmic reticulum calcium store. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0139273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Juan-Sanz J, et al. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron. 2017;93:867–881.e6. doi: 10.1016/j.neuron.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, et al. Red fluorescent genetically encoded Ca2+ indicators for use in mitochondria and endoplasmic reticulum. Biochem. J. 2014;464:13–22. doi: 10.1042/BJ20140931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 22.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 23.Lynes EM, et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J. Cell Sci. 2013;126:3893–3903. doi: 10.1242/jcs.125856. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. Protons Trigger Mitochondrial Flashes. Biophys. J. 2016;111:386–394. doi: 10.1016/j.bpj.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santo-Domingo J, Giacomello M, Poburko D, Scorrano L, Demaurex N. OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J. 2013;32:1927–40. doi: 10.1038/emboj.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, et al. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.