Abstract
Wolbachia are maternally inherited endosymbiotic bacteria found within many insect species. Aedes mosquitoes experimentally infected with Wolbachia are being released into the field for Aedes‐borne disease control. These Wolbachia infections induce cytoplasmic incompatibility which is used to suppress populations through incompatible matings or replace populations through the reproductive advantage provided by this mechanism. However, the presence of naturally occurring Wolbachia in target populations could interfere with both population replacement and suppression programs depending on the compatibility patterns between strains. Aedes aegypti were thought to not harbor Wolbachia naturally but several recent studies have detected Wolbachia in natural populations of this mosquito. We therefore review the evidence for natural Wolbachia infections in A. aegypti to date and discuss limitations of these studies. We draw on research from other mosquito species to outline the potential implications of natural Wolbachia infections in A. aegypti for disease control. To validate previous reports, we obtained a laboratory population of A. aegypti from New Mexico, USA, that harbors a natural Wolbachia infection, and we conducted field surveys in Kuala Lumpur, Malaysia, where a natural Wolbachia infection has also been reported. However, we were unable to detect Wolbachia in both the laboratory and field populations. Because the presence of naturally occurring Wolbachia in A. aegypti could have profound implications for Wolbachia‐based disease control programs, it is important to continue to accurately assess the Wolbachia status of target Aedes populations.
Keywords: Aedes aegypti, cytoplasmic incompatibility, dengue, Wolbachia
We review the evidence for natural Wolbachia infections in Aedes aegypti mosquitoes and attempt to validate previous previous reports through screening of laboratory and field populations. We detected no Wolbachia infection and therefore discuss the limitations of current studies and describe the evidence needed by future studies.

1. Wolbachia INFECTIONS IN NATURAL POPULATIONS
Wolbachia are best known for their profound effects on host reproduction and more recently for their applied use in disease control programs. Wolbachia infect approximately half of all insect species but their prevalence varies widely between orders and genera (Weinert, Araujo‐Jnr, Ahmed, & Welch, 2015). Variation in infection also occurs within species, ranging from low frequencies to fixation (Charlesworth, Weinert, Araujo, & Welch, 2019; Hilgenboecker, Hammerstein, Schlattmann, Telschow, & Werren, 2008). The prevalence of Wolbachia infections may be underestimated because infections can occur at low densities that are undetectable by conventional PCR (Mee, Weeks, Walker, Hoffmann, & Duchemin, 2015). Multiple Wolbachia variants have been detected within the same species, such as in Drosophila simulans (Martinez et al., 2017) and Culex pipiens (Atyame, Delsuc, Pasteur, Weill, & Duron, 2011). Superinfections, where multiple Wolbachia strains infect the same insect (Arthofer et al., 2009; Sinkins, Braig, & O'Neill, 1995), also occur.
Although Wolbachia are maternally inherited, interspecific transfer may occur through parasitism (Ahmed et al., 2015; Heath, Butcher, Whitfield, & Hubbard, 1999), consumption of infected individuals (Le Clec'h et al., 2013, Brown & Lloyd, 2015), sharing a common environment (Huigens, Almeida, Boons, Luck, & Stouthamer, 2004; Li et al., 2017), or other mechanisms. Successful horizontal transmission is likely to be rare, but Wolbachia can spread rapidly throughout populations once introduced (Kriesner, Hoffmann, Lee, Turelli, & Weeks, 2013; Turelli & Hoffmann, 1991). For Wolbachia to spread, they must increase host fitness. Wolbachia infections may alter host reproduction to favor infected females over uninfected females, particularly through cytoplasmic incompatibility, which gives a frequency‐dependent advantage to infected females (O'Neill et al., 1997). Cytoplasmic incompatibility results in fewer viable offspring in crosses between Wolbachia‐infected males and uninfected females. Wolbachia may also provide fitness advantages through the protection of hosts against viruses (Hedges, Brownlie, O'Neill, & Johnson, 2008; Teixeira, Ferreira, & Ashburner, 2008), nutritional provisioning (Brownlie et al., 2009), increased fertility (Dobson, Marsland, & Rattanadechakul, 2002), or changes in life history (Cao, Jiang, & Hoffmann, 2019).
Insects that are not naturally infected with Wolbachia may be amenable to infection experimentally. Novel Wolbachia infections have been generated through microinjection, where cytoplasm or purified Wolbachia from an infected donor is transferred to an uninfected embryo (Hughes & Rasgon, 2014). Deliberate transfers of Wolbachia between species are challenging and can take thousands of attempts to generate a stable line (McMeniman et al., 2009; Walker et al., 2011). But once an infection is introduced, Wolbachia infections have applications for pest and disease vector control since they can alter host reproduction and block virus replication and transmission (Hoffmann, Ross, & Rašić, 2015).
2. RELEASES OF NOVEL Wolbachia INFECTIONS FOR VECTOR AND DISEASE CONTROL
There is increasing interest in deploying mosquitoes with experimentally generated Wolbachia infections into the field for disease control. Over 25 novel Wolbachia infection types have been generated in mosquitoes through embryonic microinjection, mainly in the principal dengue vectors Aedes aegypti and Aedes albopictus (Ross, Turelli, & Hoffmann, 2019). Most of these infections induce cytoplasmic incompatibility, and many also reduce the ability of their hosts to transmit viruses, making them desirable for field release. For mosquito species that are naturally Wolbachia‐infected such as A. albopictus, novel infections can be generated either by first removing the natural infections with antibiotics (Calvitti, Moretti, Lampazzi, Bellini, & Dobson, 2010; Suh, Mercer, Fu, & Dobson, 2009) or by introducing the novel infection into an infected mosquito, resulting in a superinfection (Suh, Fu, Mercer, & Dobson, 2016; Zhang, Zheng, Xi, Bourtzis, & Gilles, 2015). Different novel Wolbachia infections may be incompatible with each other (Ant, Herd, Geoghegan, Hoffmann, & Sinkins, 2018) and the addition of Wolbachia strains to create superinfections can lead to unidirectional incompatibility, where females of the superinfected strain produce viable offspring following matings with males with any infection type, but superinfected males induce cytoplasmic incompatibility when mated with singly infected and uninfected females (Joubert et al., 2016).
Mosquitoes with novel Wolbachia infections are being released into the field for two main purposes: population replacement and population suppression. The objective of the former approach is to replace natural populations with mosquitoes possessing Wolbachia infections that interfere with virus transmission. This is achieved through the release of males that induce cytoplasmic incompatibility and females that transmit the Wolbachia infection and have reduced vector competence (Walker et al., 2011). Successful population replacement of A. aegypti with novel Wolbachia infections has been achieved in several countries (Garcia et al., 2019; Hoffmann et al., 2011; Nazni et al., 2019). Following releases in Australia and Malaysia, Wolbachia infections have maintained a stable, high frequency in most locations, coinciding with reduced local dengue transmission (Nazni et al., 2019; O'Neill et al., 2018; Ryan et al., 2019). Population suppression can be achieved through male‐only releases of Wolbachia‐infected males, resulting in cytoplasmic incompatibility with wild females. This was first demonstrated in 1967 in Cx. pipiens (Laven, 1967) by exploiting the natural variation in Wolbachia infection types between mosquitoes from different locations (Atyame et al., 2014). Other releases have used Wolbachia from a closely related species through introgression (O'Connor et al., 2012) and novel Wolbachia transinfections generated through microinjection (Mains, Brelsfoard, Rose, & Dobson, 2016; Zheng et al., 2019).
Both population replacement and suppression approaches rely on the novel Wolbachia infection types inducing cytoplasmic incompatibility with the resident mosquito population. Thus, the presence of natural Wolbachia infections in mosquitoes may interfere with disease control programs, making population replacement or suppression challenging or even impossible.
3. DETECTIONS OF Wolbachia IN Aedes aegypti
A. aegypti is the principal vector of dengue virus and has been the focus of Wolbachia‐based population replacement efforts, with releases of mosquitoes with novel Wolbachia infections now underway in over 10 countries (e.g., Nazni et al. (2019), Garcia et al. (2019), Hoffmann et al. (2011)). Until recently, A. aegypti was not thought to harbor Wolbachia naturally (Kittayapong, Baisley, Baimai, & O'Neill, 2000), though it is clearly amenable to infection given the number of stable experimental infections generated in this species (Ross, Turelli, et al., 2019). Evidence for horizontal gene transfer between Wolbachia and A. aegypti may reflect a historical infection (Klasson, Kambris, Cook, Walker, & Sinkins, 2009). The most comprehensive survey to date found no evidence for Wolbachia infection in A. aegypti through PCR assays on pools of mosquitoes, except in a single location where the experimentally generated wMel strain of Wolbachia had been released deliberately (Gloria‐Soria, Chiodo, & Powell, 2018). The lack of natural infection is advantageous for both population replacement and suppression programs because any cytoplasmic incompatibility‐inducing Wolbachia infection should be unidirectionally incompatible with wild populations.
Coon, Brown, and Strand (2016) detected Wolbachia in A. aegypti collected from Florida, USA, using 16S rRNA sequencing and multilocus sequence typing. This discovery suggested that natural Wolbachia infections may occur in A. aegypti, with its occurrence perhaps being geographically restricted or at a low frequency in other populations. Since then, seven further studies have purported to detect Wolbachia in natural populations of A. aegypti (Table 1). These studies report variable infection frequencies in populations and identify infections from several Wolbachia supergroups. Most studies found that the infections detected were closely related to or identical to the wAlbB infection that occurs natively in Aedes albopictus (Balaji et al., 2019; Carvajal et al., 2019; Coon et al., 2016; Kulkarni et al., 2019), while other studies also detected Wolbachia from supergroups that do not normally occur within Diptera (Carvajal et al., 2019; Thongsripong et al., 2018). Most evidence is limited to molecular detection, and not all studies claim to have discovered an active infection. However, some studies have established laboratory colonies and reported maternal transmission of Wolbachia (Kulkarni et al., 2019) or the loss of infection through antibiotic treatment (Balaji et al., 2019).
Table 1.
Detections of Wolbachia in natural populations of Aedes aegypti
| Location | Collection date(s) | Evidence for infection | Infection frequency (n tested) | Supergroup | Reference |
|---|---|---|---|---|---|
| Jacksonville, Florida, USA | July 2014 | Molecular detection (16S rRNA sequencing, MLST) | Not specified | A and B | Coon et al. (2016) |
| Kuala Lumpur, Malaysia | Not specified | Molecular detection (wsp) | 25% (16) | Unknown | Teo, Lim, Voon, and Mak (2017) |
| Nakhon Nayok, Thailand | 2008 | Molecular detection (16S and 18S rRNA sequencing) | Not specified | C, others | Thongsripong et al. (2018) |
| Houston, Texas, USA | Not specified | Molecular detection (16S rRNA sequencing) | Not specified | Unknown | Hegde et al. (2018) |
| Tamil Nadu, India | August 2015 | Molecular detection (16S rRNA, wsp, ftsZ, MLST) | Not specified | B | Balaji, Jayachandran, and Prabagaran (2019) |
| Electron microscopy | |||||
| qPCR across developmental stages and tissues | |||||
| Removal through antibiotic treatment | |||||
| New Mexico and Florida, USA | 2016, 2017 | Molecular detection (PCR, LAMP) | 44.8% (194) | B | Kulkarni et al. (2019) |
| Maternal transmission | |||||
| Manila, Philippines | May 2014–January 2015 | Molecular detection (wsp, 16S rDNA) | 11.9% (672) | A, B, C, D and J | Carvajal, Hashimoto, Harnandika, Amalin, and Watanabe (2019) |
| Panama | Not specified | Molecular detection (16S rRNA sequencing) | 0.2% (490) | Unknown | Bennett et al. (2019) |
Similar to A. aegypti, Anopheles mosquitoes (which transmit Plasmodium parasites that cause malaria) were also thought to be uninfected by Wolbachia, though several recent studies have detected Wolbachia in this genus (Ayala et al., 2019; Baldini et al., 2014; Jeffries et al., 2018). In a critical analysis of studies in Anopheles gambiae, Chrostek and Gerth (2019) assert that the evidence is currently insufficient to diagnose natural infections in this species. We highlight similar issues with detections of Wolbachia in A. aegypti but also discuss the potential implications for disease control if Wolbachia do occur naturally in this species.
4. POTENTIAL IMPLICATIONS OF NATURAL Wolbachia INFECTIONS FOR RELEASES OF NOVEL INFECTIONS
The presence of natural Wolbachia infections may influence compatibility patterns between mosquitoes with the novel Wolbachia infection and the natural population. These patterns are summarized in Figure 1, although crossing patterns in nature are likely to be more complex. Natural Wolbachia infections can have heterogeneous densities and frequencies in populations (Calvitti, Marini, Desiderio, Puggioli, & Moretti, 2015), making compatibility patterns hard to predict. Crosses may differ in the strength of incompatibility in different directions (O'Neill and Paterson, 1992, Joubert et al., 2016; Sinkins et al., 1995), and there are also environment‐dependent effects on cytoplasmic incompatibility including adult age (Kittayapong, Mongkalangoon, Baimai, & O'Neill, 2002) and temperature (Ross, Ritchie, Axford, & Hoffmann, 2019). The presence of Wolbachia superinfections also increases the number of potential compatibility patterns (Dobson, Rattanadechakul, & Marsland, 2004).
Figure 1.
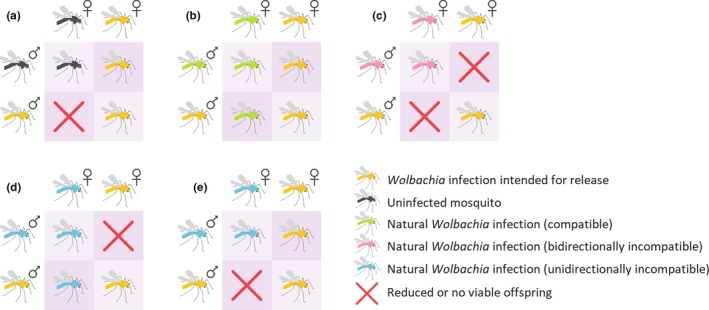
Potential crossing patterns between mosquitoes with novel Wolbachia infections that induce cytoplasmic incompatibility and mosquito populations with or without the presence of natural Wolbachia infections of different crossing types. (a) Crosses between mosquitoes with a novel Wolbachia infection and uninfected mosquitoes result in unidirectional cytoplasmic incompatibility. (b) When novel and natural Wolbachia infections exhibit the same crossing type, no cytoplasmic incompatibility occurs. (c) Bidirectional incompatibility occurs when novel and natural Wolbachia infections exhibit different crossing types. (d,e) Unidirectional cytoplasmic incompatibility may occur in favor of the natural (d) or (e) novel infection. These situations are most likely when the natural (d) or novel (e) infection is a superinfection, where one strain is compatible with the single infection but the other is not
With most novel infections generated in A. aegypti, the release of Wolbachia‐infected mosquitoes into an uninfected population will lead to cytoplasmic incompatibility (Figure 1a). Reduced egg hatch from crosses between infected males and uninfected females favors infected females. For a Wolbachia infection to invade an uninfected population, its frequency must exceed a threshold which depends on the fidelity of cytoplasmic incompatibility and maternal transmission and any fitness costs of the infection (O'Neill et al., 1997).
The presence of natural Wolbachia infections in a population may result in crossing patterns that make population replacement or suppression more challenging (Figure 1b‐e). The following scenarios assume that the natural infection is at fixation in the population, though infections may be at intermediate frequencies (Table 1) so any impacts on Wolbachia release programs will be weaker. When novel and natural infections are compatible with each other (no reduction in egg hatch in any combination), invasion will depend on the relative fitness of each infection type due to a lack of cytoplasmic incompatibility (Figure 1b). Since transinfections in mosquitoes typically impose fitness costs while natural infections tend to be beneficial (Ross, Turelli, et al., 2019), population replacement may be unachievable even if high frequencies are reached. In this situation, population suppression is impossible due to the lack of cytoplasmic incompatibility in any direction. Such patterns occur in Cx. pipiens, with multiple compatible strains coexisting within natural populations (Atyame et al., 2014; Duron, Raymond, & Weill, 2011).
Incompatibility between males of novel and natural infections and females of the opposite infection type in both directions, or bidirectional cytoplasmic incompatibility, may occur (Figure 1c). Bidirectional incompatibility is desirable for population suppression programs because it reduces the risk that inadvertently released females will replace natural populations (Moretti, Marzo, Lampazzi, & Calvitti, 2018). Novel Wolbachia infections that are bidirectionally incompatible with natural populations have been generated in A. albopictus (Calvitti et al., 2010; Xi, Khoo, & Dobson, 2006) by first removing the native superinfection which is at high frequency in most natural populations (Joanne et al., 2015; Kittayapong, Baisley, Sharpe, Baimai, & O'Neill, 2002). Such strains have been deployed successfully for population suppression (Mains et al., 2016). Bidirectional incompatibility can also occur between natural populations of Drosophila simulans (O'Neill and Karr, 1990, Montchamp‐Moreau, Ferveur, & Jacques, 1991), Nasonia wasps (Bordenstein & Werren, 2007), and Cx. pipiens (Yen & Barr, 1973).
When bidirectional incompatibility occurs, population replacement will be difficult to achieve unless high frequencies of the novel infection are reached. Where population replacement is successful, spread beyond the release area is unlikely since the frequency required for invasion is 50% when fitness is equal (O'Neill et al., 1997). Novel infections may instead persist with natural infections (Telschow, Yamamura, & Werren, 2005), particularly in fragmented populations (Keeling, Jiggins, & Read, 2003).
Unidirectional incompatibility may also occur between natural and novel infections (Figure 1d,e). If a natural population harbors a double infection and a novel infection with a single Wolbachia strain is released, this can result in unidirectional incompatibility favoring the natural infection if one strain of the superinfection is compatible and the other is not (Figure 1d). In this situation, population suppression is impossible and population replacement will be challenging; therefore, such infections are not being considered for release. Natural populations of A. albopictus are superinfected with the wAlbA and wAlbB strains at a high frequency although either strain may occasionally be lost (Joanne et al., 2015; Kittayapong, Baisley, et al., 2002), resulting in unidirectional cytoplasmic incompatibility (Dobson et al., 2004).
Aedes albopictus with novel Wolbachia infections have not been released for population replacement but triple infections may suitable for this purpose (Fu, Gavotte, Mercer, & Dobson, 2010; Zhang et al., 2015). Novel triple infections are unidirectionally incompatible with the natural double infection (Fu et al., 2010; Zheng et al., 2019) (Figure 1e), resulting in a similar pattern to crosses with uninfected mosquitoes (Figure 1a). In cases of unidirectional cytoplasmic incompatibility with the target population (Figure 1a,e), the accidental release of Wolbachia‐infected females during releases of males for population suppression could lead to population replacement (Dobson et al., 2002). This may be avoided by irradiating release stocks to sterilize any released females, as demonstrated in a recent A. albopictus population suppression program (Zheng et al., 2019).
Unidirectional cytoplasmic incompatibility can also occur in crosses between two single Wolbachia infections (Figure 1d,e) as demonstrated in Cx. pipiens (Atyame et al., 2014; Bonneau et al., 2018). In this situation, both strains induce cytoplasmic incompatibility, but one lacks the ability to restore compatibility with males of the other infection. Cytoplasmic incompatibility induction by males is governed by two genes while the ability to restore compatibility by females is governed by a single gene (Shropshire, On, Layton, Zhou, & Bordenstein, 2018); the two phenotypes are therefore not always linked.
Although natural infections may interfere with releases of novel infections, their presence may also provide opportunities for disease control. Wolbachia infections that cause cytoplasmic incompatibility can be released in other locations for population suppression without the need for novel infections (Laven, 1967). Natural infections may also be useful for population replacement if they can block virus transmission (Glaser & Meola, 2010; Mousson et al., 2012), but like releases of novel infections, it will be important to match the genetics of the release strain to the target population (Garcia et al., 2019).
5. TESTING A PUTATIVELY Wolbachia‐INFECTED LABORATORY POPULATION OF A. aegypti
Of the eight studies reporting natural Wolbachia infections in A. aegypti, only two established laboratory populations (Table 1). We obtained one of these populations with the intention of examining crossing patterns between natural infections and novel infections that are being deployed into the field (Ant et al., 2018; Walker et al., 2011). An A. aegypti population from Las Cruces, New Mexico, USA, was established in the laboratory in September 2018 (Kulkarni et al., 2019) and kindly provided to us by the authors. We received eggs from the third and fourth generations of this population (denoted LC) which were hatched and maintained in our laboratory according to methods described previously (Ross, Axford, Richardson, Endersby‐Harshman, & Hoffmann, 2017).
We performed a single cross to test whether A. aegypti males with the wAlbB strain (Axford, Ross, Yeap, Callahan, & Hoffmann, 2016; Xi, Khoo, & Dobson, 2005) induced cytoplasmic incompatibility with LC females. LC males do not induce detectable cytoplasmic incompatibility with uninfected (Rockefeller strain) females (Jiannong Xu, personal communication). Zero eggs hatched from a cross between wAlbB‐infected males and LC females (n = 1,027 eggs), indicating that the infection is absent, at a low density, or is not closely related to the wAlbB infection. Due to the absence of Wolbachia in the LC strain as detected through molecular analyses (see below), we did not proceed with further crosses.
We used molecular approaches to try and confirm Wolbachia infection in the A. aegypti LC strain. According to the authors, this population harbors a natural Wolbachia infection closely related to the wAlbB infection from A. albopictus (Kulkarni et al., 2019). Real‐time PCR/high‐resolution melt (RT/HRM) assays were performed as previously described (Axford et al., 2016; Lee, White, Weeks, Hoffmann, & Endersby, 2012) using primers specific to the wAlbB Wolbachia strain as well as Aedes and A. aegypti‐specific primers (Supporting Information Appendix S1). We also used a loop‐mediated isothermal amplification (LAMP) assay which can detect the wAlbB infection with high sensitivity (Jasper et al., 2019). Uninfected A. aegypti originating from Cairns, Australia, and wAlbB‐infected A. aegypti (Axford et al., 2016) were included as negative and positive controls, respectively, in each assay. Through these two approaches, we did not detect any wAlbB infection in 120 mosquitoes (including larvae and adults from both generations) from the LC population (Supporting Information Appendix S2), demonstrating that the LC laboratory population is not infected with wAlbB.
To test whether the LC population harbors any Wolbachia infection, we performed additional assays with general Wolbachia primers. TaqMan probe assays were performed as described previously (Mee et al., 2015), targeting the 16S rDNA (Supporting Information Appendix S1). We also performed conventional PCR with 16S rDNA and gatB primers following methods described by the authors of the original study (Kulkarni et al., 2019). Finally, LAMP assays were performed using our protocol (Jasper et al., 2019) but with primers used to diagnose Wolbachia infections by the original study (Kulkarni et al., 2019). From analyses of 72 individuals from both generations with the three molecular assays, zero were infected (Supporting Information Appendix S2). Negative and positive controls were confirmed in all assays. Through these analyses, we demonstrate conclusively that the LC population does not harbor Wolbachia. These results conflict with those from the original study (Kulkarni et al., 2019) and more recent tests by the authors where Wolbachia is at a high frequency (28/32, 87.5%) in the fourth laboratory generation (Jiannong Xu, personal communication). Although the reason for this conflicting result is unclear, our study emphasizes the need for independent evaluation of Wolbachia infections in A. aegypti.
6. FIELD SURVEY FOR NATURAL Wolbachia INFECTIONS IN A. aegypti
Teo et al. (2017) detected Wolbachia in A. aegypti from a site in Kuala Lumpur, Malaysia. To further test Wolbachia from Kuala Lumpur, we conducted our own sampling, undertaken as part of a release program with the wAlbB Wolbachia infection (Nazni et al., 2019). We sampled 693 A. aegypti from a site in 2013–2014 before Wolbachia releases commenced. We also sampled 382 A. aegypti from July 2017 to September 2018 from a control site where no Wolbachia releases were undertaken. Through conventional PCR and RT/HRM assays (described above), we did not detect Wolbachia infection in any individual (Supporting Information Appendix S3), in contrast to Teo et al. (2017). Our results are consistent with a global survey of A. aegypti where no evidence for natural Wolbachia infections was found (Gloria‐Soria et al., 2018). Below, we discuss the limitations of current studies and describe the evidence needed to confirm the presence of putative natural Wolbachia infections.
7. LIMITATIONS OF STUDIES TO DATE
Detections of Wolbachia in A. aegypti are accumulating (Table 1) but the evidence is largely molecular, which is insufficient to diagnose an active Wolbachia infection (Chrostek & Gerth, 2019). Coon et al. (2016) were the first to report the detection of Wolbachia in natural A. aegypti populations. In this study, Wolbachia were found at a low abundance and frequency in Florida, USA, through 16S rRNA sequencing and then characterized with multilocus sequence typing (MLST). Bennett et al. (2019) and Hegde et al. (2018) also detected Wolbachia at a low frequency and abundance through 16S rRNA sequencing but these results could not be validated with PCR amplification. These observations may reflect true infections although there are several potential sources of contamination that can cause false positives (discussed in Chrostek and Gerth (2019)).
Several species of filarial nematodes that infect A. aegypti harbor obligate Wolbachia infections from supergroups C and D (Bouchery, Lefoulon, Karadjian, Nieguitsila, & Martin, 2013). Both Thongsripong et al. (2018) and Carvajal et al. (2019) detected Wolbachia in A. aegypti that aligned to supergroup C. Carvajal et al. (2019) observed substantial diversity in 16S rDNA and wsp sequences, with alignments to supergroups A, B, C, D, and J. Given that Wolbachia from supergroups C, D, and J are not known to occur in Diptera, such diversity is likely explained by contamination from other sources. Species misidentification may also cause false positives if one species harbors Wolbachia and the other does not. Both Teo et al. (2017) and Carvajal et al. (2019) used identification keys but did not confirm that samples were A. aegypti with molecular approaches. Since A. aegypti and A. albopictus are sympatric in both locations, detections of Wolbachia in A. aegypti could result from species misidentification. Interspecific matings between infected males and uninfected females might also lead to Wolbachia being detected in females through the transfer of sperm carrying Wolbachia, given that this has been observed at the intraspecific level in A. aegypti (Ross, Axford, Callahan, Richardson, & Hoffmann, 2019). For molecular confirmation of Wolbachia infections, appropriate positive and negative controls are needed. Carvajal et al. (2019) used water as a negative control, but this is inadequate because positive detections may be due to amplification of mosquito nDNA. Mosquitoes or other insects of a known infection status, both Wolbachia‐infected and uninfected, are needed in each assay for confident diagnosis.
Two studies, Balaji et al. (2019) and Kulkarni et al. (2019), established laboratory colonies of A. aegypti with natural Wolbachia infections, allowing for more robust evidence to be collected on infection status. Kulkarni et al. (2019) demonstrate maternal transmission of the natural Wolbachia infection; ten offspring selected randomly from a cross between Wolbachia‐infected females and uninfected males were infected, while none from the reciprocal cross were infected. However, our inability to detect a Wolbachia infection in this laboratory population (as discussed above) suggests that this result may not reflect a true infection.
Balaji et al. (2019) provide several lines of evidence for a natural Wolbachia infection in A. aegypti (Table 1), although there are also limitations to this study. The infected laboratory population exhibited a stable infection frequency of ~80% across four generations, though reciprocal crosses between infected and uninfected populations are needed to confirm maternal transmission. Treatment of the infected population with tetracycline for four consecutive generations removed the Wolbachia infection, although the evidence for this provided in the Supporting Information lacks controls. Relative Wolbachia densities determined by RT/HRM are broadly consistent with natural infections in A. albopictus where densities can vary across life stages and between sexes (Calvitti et al., 2015; Tortosa et al., 2010). High Wolbachia densities in the ovaries are also consistent with a true infection, since maternal transmission requires infection of the germ line (Veneti, Clark, Karr, Savakis, & Bourtzis, 2004) but not somatic tissues, although Wolbachia often occupy somatic tissues (Dobson et al., 1999). Electron microscopy images show apparent localization of Wolbachia to the ovaries, but images are low resolution and there is no clear distinction between Wolbachia and organelles as in other recent studies (Leclercq et al., 2016; Li et al., 2017).
8. EVIDENCE REQUIRED TO CONFIRM NATURAL Wolbachia INFECTIONS
From the studies discussed above, we believe the evidence is currently insufficient to indicate that A. aegypti mosquitoes harbor a natural Wolbachia infection. We propose three lines of evidence as a minimum requirement for confirming a Wolbachia infection in this species: intracellular localization, maternal transmission, and removal of Wolbachia. Following molecular detection (traditional PCR, qPCR, or LAMP assays targeting the wsp gene should suffice), laboratory populations can be established from larvae, pupae, or adults from Wolbachia‐positive locations to enable further characterization. Figure 2 shows a suggested approach for confirming natural Wolbachia infections in insects, following an initial field survey.
Figure 2.
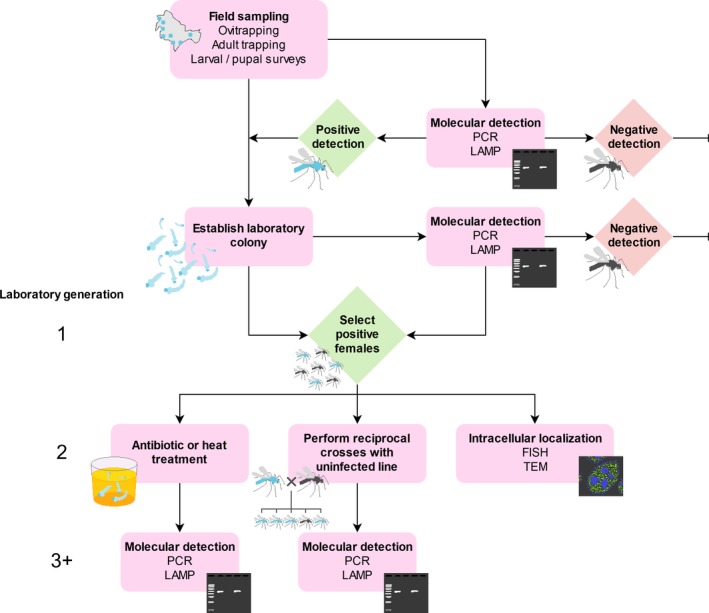
Suggested procedure for confirming natural Wolbachia infections
Intracellular localization is an important step in confirming a Wolbachia infection because it will help to distinguish between an active infection and merely the detection of Wolbachia sequences from horizontal gene transfer or the environment. It can be demonstrated by visualizing Wolbachia within host tissues, such as through fluorescence in situ hybridization (FISH) (Moreira et al., 2009). These observations require appropriate controls including separate probes for Wolbachia and host, and visualization of tissues with the Wolbachia infection removed (see below). If FISH is not available, transmission electron microscopy (TEM) can also be used (Binnington & Hoffmann, 1989; Li et al., 2017; Yen & Barr, 1973) though Wolbachia needs to be carefully distinguished from host organelles. Quantitative PCR of separate host tissues can also help to confirm Wolbachia infections because they often exhibit tissue‐specific localization.
Maternal inheritance of Wolbachia can be demonstrated through reciprocal crosses between Wolbachia‐infected and uninfected mosquitoes, ideally with all four combinations (see Figure 1). The uninfected mosquitoes may be from a laboratory colony or collected from the field. In a true natural infection, only offspring from infected mothers are expected to test positive for Wolbachia. Paternal transmission may occur very rarely (Hoffmann & Turelli, 1988), and maternal transmission may be imperfect, particularly if the infection has a low density in the ovaries (Narita, Nomura, & Kageyama, 2007), so we recommend testing at least ten offspring from ten individual females from each cross to account for this. Other patterns of inheritance point against a Wolbachia infection or may indicate horizontal transmission. Because filarial nematodes are only transmitted horizontally by mosquitoes, Wolbachia from filarial nematodes that infect mosquitoes would not be expected to persist across mosquito generations.
Wolbachia infections can be removed from insects through antibiotic or heat treatment (Li, Floate, Fields, & Pang, 2014). Novel Wolbachia infections can be cleared from A. aegypti with tetracycline added to larval rearing water or sugar solution fed to adults, through rearing larvae at high temperatures, or a combination of approaches (Endersby‐Harshman, Axford, & Hoffmann, 2019; Ross, Wiwatanaratanabutr, et al., 2017). The removal of natural Wolbachia infections from A. aegypti points to a true infection because these treatments should only affect microbes and not host DNA. Following removal, which may require multiple generations of treatment, the lack of infection can be confirmed through PCR or LAMP assays or by observing intracellular localization. This should be paired with a confirmation that the infection has persisted across generations in an untreated colony. Because Wolbachia infections may be heat (Ross, Wiwatanaratanabutr, et al., 2017) or antibiotic (Li et al., 2014) resistant, failure to eliminate Wolbachia does not necessarily confirm the lack of an active infection, so claims in this case should be supported by the other lines of evidence.
Together, these experiments should demonstrate conclusively whether the population harbors a Wolbachia infection. Following confirmation, additional experiments would likely be worthwhile, as we discuss below.
9. FUTURE DIRECTIONS
The confirmation of natural Wolbachia infections in A. aegypti would open avenues for further research, including applications for disease control programs. Laboratory crosses between natural infections and novel infections are needed to test the potential for natural infections to interfere with releases of novel infections. Surveys for natural infections prior to releases of novel infections may inform release strategies, including the choice of Wolbachia strain. Effects of natural infections on host fitness, reproduction, and vector competence should be evaluated since they may possess properties useful for reducing virus transmission and/or decreasing population size. Genome sequencing may provide insights into their origin. Finally, natural infections could be transferred to other species through microinjection to study their effects in novel hosts and provide further opportunities for disease control.
Although several studies have now claimed to detect Wolbachia in natural A. aegypti populations, the evidence is not compelling. Studies to date have relied mostly on molecular approaches that may be prone to contamination. These results conflict with a growing body of evidence for a lack of infection in this species which includes a comprehensive global survey (Gloria‐Soria et al., 2018), monitoring undertaken before releases of novel infections (Hoffmann et al., 2011) and the data presented here. Our inability to detect Wolbachia in a putatively infected laboratory population demonstrates the need for more robust evidence when reporting natural Wolbachia infections. Although natural Wolbachia infections in A. aegypti may not exist, releases of novel Wolbachia infections are continuing to expand, and new target populations should therefore continue to be monitored prior to releases taking place.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
PAR conceived the study, performed the live mosquito work, made the figures and tables, and drafted the manuscript; AGC, QY, and MJ performed the molecular diagnostics on the laboratory population; MAKA, ANA, and WAN conducted the field survey; AAH supervised and coordinated the project; and all authors contributed to writing and editing the manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors thank Kelly Richardson for assistance with molecular diagnostics, Jiannong Xu and Aditi Kulkarni for providing the Aedes aegypti LC strain, and Nancy Endersby‐Harshman for coordinating the import of the strain into our quarantine facility. We also thank the anonymous reviewers for their constructive feedback on the manuscript. AAH was supported by the National Health and Medical Research Council (1132412, 1118640, https://www.nhmrc.gov.au), the Australian Research Council (LE150100083, https://www.arc.gov.au), and the Wellcome Trust (108508, wellcome.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ross PA, Callahan AG, Yang Q, et al. An elusive endosymbiont: Does Wolbachia occur naturally in Aedes aegypti? Ecol Evol. 2020;10:1581–1591. 10.1002/ece3.6012
DATA AVAILABILITY STATEMENT
All data are contained within the manuscript and its appendices.
REFERENCES
- Ahmed, M. Z. , Li, S. J. , Xue, X. , Yin, X. J. , Ren, S. X. , Jiggins, F. M. , … Qiu, B. L. (2015). The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathogens, 10, e1004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ant, T. H. , Herd, C. S. , Geoghegan, V. , Hoffmann, A. A. , & Sinkins, S. P. (2018). The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti . PLoS Pathogens, 14, e1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthofer, W. , Riegler, M. , Schneider, D. , Krammer, M. , Miller, W. J. , & Stauffer, C. (2009). Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae). Molecular Ecology, 18, 3816–3830. [DOI] [PubMed] [Google Scholar]
- Atyame, C. M. , Delsuc, F. , Pasteur, N. , Weill, M. , & Duron, O. (2011). Diversification of Wolbachia endosymbiont in the Culex pipiens mosquito. Molecular Biology and Evolution, 28, 2761–2772. [DOI] [PubMed] [Google Scholar]
- Atyame, C. M. , Labbe, P. , Dumas, E. , Milesi, P. , Charlat, S. , Fort, P. , & Weill, M. (2014). Wolbachia divergence and the evolution of cytoplasmic incompatibility in Culex pipiens . PLoS ONE, 9, e87336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axford, J. K. , Ross, P. A. , Yeap, H. L. , Callahan, A. G. , & Hoffmann, A. A. (2016). Fitness of wAlbB Wolbachia infection in Aedes aegypti: Parameter estimates in an outcrossed background and potential for population invasion. The American Journal of Tropical Medicine and Hygiene, 94, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala, D. , Akone‐Ella, O. , Rahola, N. , Kengne, P. , Ngangue, M. F. , Mezeme, F. , … Simard, F. J. E. A. (2019). Natural Wolbachia infections are common in the major malaria vectors in Central Africa. Evolutionary Applications, 12, 1583–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji, S. , Jayachandran, S. , & Prabagaran, S. R. (2019). Evidence for the natural occurrence of Wolbachia in Aedes aegypti mosquitoes. FEMS Microbiology Letters, 366, fnz055. [DOI] [PubMed] [Google Scholar]
- Baldini, F. , Segata, N. , Pompon, J. , Marcenac, P. , Robert Shaw, W. , Dabiré, R. K. , … Catteruccia, F . (2014). Evidence of natural Wolbachia infections in field populations of Anopheles gambiae . Nature Communications, 5, 3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, K. L. , Gómez‐Martínez, C. , Chin, Y. , Saltonstall, K. , McMillan, W. O. , Rovira, J. R. , & Loaiza, J. R. (2019). Dynamics and diversity of bacteria associated with the disease vectors Aedes aegypti and Aedes albopictus . Scientific Reports, 9, 1–12. 10.1038/s41598-019-48414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnington, K. C. , & Hoffmann, A. A. (1989). Wolbachia—Like organisms and cytoplasmic incompatibility in Drosophila simulans . Journal of Invertebrate Pathology, 54, 344–352. [Google Scholar]
- Bonneau, M. , Atyame, C. , Beji, M. , Justy, F. , Cohen‐Gonsaud, M. , Sicard, M. , & Weill, M. (2018). Culex pipiens crossing type diversity is governed by an amplified and polymorphic operon of Wolbachia . Nature Communications, 9, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein, S. R. , & Werren, J. H. (2007). Bidirectional incompatibility among divergent Wolbachia and incompatibility level differences among closely related Wolbachia in Nasonia . Heredity, 99, 278–287. [DOI] [PubMed] [Google Scholar]
- Bouchery, T. , Lefoulon, E. , Karadjian, G. , Nieguitsila, A. , & Martin, C. (2013). The symbiotic role of Wolbachia in Onchocercidae and its impact on filariasis. Clinical Microbiology and Infection, 19, 131–140. [DOI] [PubMed] [Google Scholar]
- Brown, A. N. , & Lloyd, V. K. (2015). Evidence for horizontal transfer of Wolbachia by a Drosophila mite. Experimental and Applied Acarology, 66, 301–311. [DOI] [PubMed] [Google Scholar]
- Brownlie, J. C. , Cass, B. N. , Riegler, M. , Witsenburg, J. J. , Iturbe‐Ormaetxe, I. , McGraw, E. A. , & O'Neill, S. L. (2009). Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathogens, 5, e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvitti, M. , Marini, F. , Desiderio, A. , Puggioli, A. , & Moretti, R. (2015). Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: Concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One, 10, e0121813 10.1371/journal.pone.0121813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvitti, M. , Moretti, R. , Lampazzi, E. , Bellini, R. , & Dobson, S. L. (2010). Characterization of a new Aedes albopictus (Diptera: Culicidae) Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip Strain From Culex pipiens (Diptera: Culicidae). Journal of Medical Entomology, 47, 179–187. [DOI] [PubMed] [Google Scholar]
- Cao, L.‐J. , Jiang, W. , & Hoffmann, A. A. (2019). Life history effects linked to an advantage for wAu Wolbachia in Drosophila . Insects, 10, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal, T. M. , Hashimoto, K. , Harnandika, R. K. , Amalin, D. M. , & Watanabe, K. (2019). Detection of Wolbachia in field‐collected Aedes aegypti mosquitoes in metropolitan Manila, Philippines. Parasites & Vectors, 12, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, J. , Weinert, L. A. , Araujo, E., Jr. , & Welch, J. J . (2019). Wolbachia, Cardinium and climate: An analysis of global data. Biology Letters, 15, 20190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek, E. , & Gerth, M. (2019). Is Anopheles gambiae a natural host of Wolbachia? mBio, 10, e00784‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon, K. L. , Brown, M. R. , & Strand, M. R. (2016). Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Molecular Ecology, 25, 5806–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, S. L. , Bourtzis, K. , Braig, H. R. , Jones, B. F. , Zhou, W. , Rousset, F. , & O'Neill, S. L. (1999). Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochemistry and Molecular Biology, 29, 153–160. [DOI] [PubMed] [Google Scholar]
- Dobson, S. L. , Marsland, E. J. , & Rattanadechakul, W. (2002). Mutualistic Wolbachia infection in Aedes albopictus: Accelerating cytoplasmic drive. Genetics, 160, 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, S. L. , Rattanadechakul, W. , & Marsland, E. J. (2004). Fitness advantage and cytoplasmic incompatibility in Wolbachia single‐ and superinfected Aedes albopictus . Heredity, 93, 135–142. [DOI] [PubMed] [Google Scholar]
- Duron, O. , Raymond, M. , & Weill, M. (2011). Many compatible Wolbachia strains coexist within natural populations of Culex pipiens mosquito. Heredity, 106, 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endersby‐Harshman, N. M. , Axford, J. K. , & Hoffmann, A. A. (2019). Environmental concentrations of antibiotics may diminish Wolbachia infections in Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology, 56, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Fu, Y. , Gavotte, L. , Mercer, D. R. , & Dobson, S. L. (2010). Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Applied Environmental Microbiology, 76, 5887–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, G. D. A. , Sylvestre, G. , Aguiar, R. , da Costa, G. B. , Martins, A. J. , Lima, J. B. P. , … Maciel‐De‐freitas, R. (2019). Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Neglected Tropical Diseases, 13, e0007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, R. L. , & Meola, M. A. (2010). The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One, 5, e11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloria‐Soria, A. , Chiodo, T. G. , & Powell, J. R. (2018). Lack of evidence for natural Wolbachia infections in Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology, 55, 1354–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, B. D. , Butcher, R. D. , Whitfield, W. G. , & Hubbard, S. F. (1999). Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Current Biology, 9, 313–316. [DOI] [PubMed] [Google Scholar]
- Hedges, L. M. , Brownlie, J. C. , O'Neill, S. L. , & Johnson, K. N. (2008). Wolbachia and virus protection in insects. Science, 322, 702. [DOI] [PubMed] [Google Scholar]
- Hegde, S. , Khanipov, K. , Albayrak, L. , Golovko, G. , Pimenova, M. , Saldaña, M. A. , … Hughes, G. L. (2018). Microbiome interaction networks and community structure from laboratory‐reared and field‐collected Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquito vectors. Frontiers in Microbiology, 9, 2160 10.3389/fmicb.2018.02160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker, K. , Hammerstein, P. , Schlattmann, P. , Telschow, A. , & Werren, J. H. (2008). How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiology Letters, 281, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A. , Montgomery, B. L. , Popovici, J. , Iturbe‐Ormaetxe, I. , Johnson, P. H. , Muzzi, F. , … O'Neill, S. L. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature, 476, 454–457. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , Ross, P. A. , & Rašić, G. (2015). Wolbachia strains for disease control: Ecological and evolutionary considerations. Evolutionary Applications, 8, 751–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A. , & Turelli, M. (1988). Unidirectional incompatibility in Drosophila simulans: Inheritance, geographic variation and fitness effects. Genetics, 119, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , & Rasgon, J. L. (2014). Transinfection: A method to investigate Wolbachia–host interactions and control arthropod‐borne disease. Insect Molecular Biology, 23, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigens, M. , de Almeida, R. , Boons, P. , Luck, R. , & Stouthamer, R. (2004). Natural interspecific and intraspecific horizontal transfer of parthenogenesis–inducing Wolbachia in Trichogramma wasps. Proceedings of the Royal Society B: Biological Sciences, 271, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper, M. E. , Yang, Q. , Ross, P. A. , Endersby‐Harshman, N. , Bell, N. , & Hoffmann, A. A. J. B. (2019). A LAMP assay for the rapid and robust assessment of Wolbachia infection in Aedes aegypti under field and laboratory conditions. PLoS One, 730689 10.1371/journal.pone.0225321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries, C. L. , Lawrence, G. G. , Golovko, G. , Kristan, M. , Orsborne, J. , Spence, K. , … Walker, T . (2018). Novel Wolbachia strains in Anopheles malaria vectors from Sub‐Saharan Africa. Wellcome Open Research, 3, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanne, S. , Vythilingam, I. , Yugavathy, N. , Leong, C.‐S. , Wong, M.‐L. , & Abubakar, S. J. A. T. (2015). Distribution and dynamics of Wolbachia infection in Malaysian Aedes albopictus . Acta Tropica, 148, 38–45. [DOI] [PubMed] [Google Scholar]
- Joubert, D. A. , Walker, T. , Carrington, L. B. , de Bruyne, J. T. , Kien, D. H. T. , Hoang, N. L. T. , … O'Neill, S. L. (2016). Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathogens, 12, e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling, M. J. , Jiggins, F. M. , & Read, J. M. (2003). The invasion and coexistence of competing Wolbachia strains. Heredity, 91, 382–388. [DOI] [PubMed] [Google Scholar]
- Kittayapong, P. , Baisley, K. J. , Baimai, V. , & O'Neill, S. L. (2000). Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). Journal of Medical Entomology, 37, 340–345. [DOI] [PubMed] [Google Scholar]
- Kittayapong, P. , Baisley, K. J. , Sharpe, R. G. , Baimai, V. , & O'Neill, S. L. (2002). Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. The American Journal of Tropical Medicine and Hygiene, 66, 103–107. [DOI] [PubMed] [Google Scholar]
- Kittayapong, P. , Mongkalangoon, P. , Baimai, V. , & O'Neill, S. (2002). Host age effect and expression of cytoplasmic incompatibility in field populations of Wolbachia‐superinfected Aedes albopictus . Heredity, 88, 270–274. [DOI] [PubMed] [Google Scholar]
- Klasson, L. , Kambris, Z. , Cook, P. E. , Walker, T. , & Sinkins, S. P. (2009). Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti . BMC Genomics, 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner, P. , Hoffmann, A. A. , Lee, S. F. , Turelli, M. , & Weeks, A. R. (2013). Rapid sequential spread of two Wolbachia variants in Drosophila simulans . PLoS Pathogens, 9, e1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, A. , Yu, W. , Jiang, J. , Sanchez, C. , Karna, A. K. , Martinez, K. J. , … Xue, R. D. (2019). Wolbachia pipientis occurs in Aedes aegypti populations in New Mexico and Florida, USA. Ecology and Evolution, 9, 6148–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven, H. (1967). Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature, 216, 383–384. [DOI] [PubMed] [Google Scholar]
- Le Clec'h, W. , Chevalier, F. D. , Genty, L. , Bertaux, J. , Bouchon, D. , & Sicard, M . (2013). Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS One, 8, e60232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq, S. , Thézé, J. , Chebbi, M. A. , Giraud, I. , Moumen, B. , Ernenwein, L. , … Cordaux, R. (2016). Birth of a W sex chromosome by horizontal transfer of Wolbachia bacterial symbiont genome. Proceedings of the National Academy of Sciences U S A, 113, 15036–15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. F. , White, V. L. , Weeks, A. R. , Hoffmann, A. A. , & Endersby, N. M. (2012). High‐Throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans . Applied and Environmental Microbiology, 78, 4740–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. J. , Ahmed, M. Z. , Lv, N. , Shi, P. Q. , Wang, X. M. , Huang, J. L. , & Qiu, B. L. (2017). Plant‐mediated horizontal transmission of Wolbachia between whiteflies. ISME Journal, 11, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.‐Y. , Floate, K. , Fields, P. , & Pang, B.‐P. (2014). Review of treatment methods to remove Wolbachia bacteria from arthropods. Symbiosis, 62, 1–15. [Google Scholar]
- Mains, J. W. , Brelsfoard, C. L. , Rose, R. I. , & Dobson, S. L. (2016). Female adult Aedes albopictus suppression by Wolbachia‐infected male mosquitoes. Scientific Reports, 6, 33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, J. , Tolosana, I. , Ok, S. , Smith, S. , Snoeck, K. , Day, J. P. , & Jiggins, F. M. (2017). Symbiont strain is the main determinant of variation in Wolbachia‐mediated protection against viruses across Drosophila species. Molecular Ecology, 26, 4072–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman, C. J. , Lane, R. V. , Cass, B. N. , Fong, A. W. C. , Sidhu, M. , Wang, Y. F. , & O'Neill, S. L. (2009). Stable introduction of a life‐shortening Wolbachia infection into the mosquito Aedes aegypti . Science, 323, 141–144. [DOI] [PubMed] [Google Scholar]
- Mee, P. T. , Weeks, A. R. , Walker, P. J. , Hoffmann, A. A. , & Duchemin, J.‐B. (2015). Detection of low‐level Cardinium and Wolbachia infections in Culicoides. Applied and Environmental Microbiology, 81, 6177–6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montchamp‐Moreau, C. , Ferveur, J.‐F. , & Jacques, M. J. G. (1991). Geographic distribution and inheritance of three cytoplasmic incompatibility types in Drosophila Simulans . Genetics, 129, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L. A. , Iturbe‐Ormaetxe, I. , Jeffery, J. A. , Lu, G. J. , Pyke, A. T. , Hedges, L. M. , … O'Neill, S. L. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium . Cell, 139, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Moretti, R. , Marzo, G. A. , Lampazzi, E. , & Calvitti, M. (2018). Cytoplasmic incompatibility management to support Incompatible Insect Technique against Aedes albopictus . Parasites & Vectors, 11, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousson, L. , Zouache, K. , Arias‐Goeta, C. , Raquin, V. , Mavingui, P. , & Failloux, A. B. (2012). The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus . PLoS Neglected Tropical Diseases, 6, e1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita, S. , Nomura, M. , & Kageyama, D. (2007). Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: Transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiology Ecology, 61, 235–245. [DOI] [PubMed] [Google Scholar]
- Nazni, W. A. , Hoffmann, A. A. , Noor Afizah, A. , Cheong, Y. L. , Mancini, M. V. , Golding, N. , … Sinkins, S. P . (2019). Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Current Biology, 29, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, L. , Plichart, C. , Sang, A. C. , Brelsfoard, C. L. , Bossin, H. C. , & Dobson, S. L. (2012). Open release of male mosquitoes infected with a Wolbachia biopesticide: Field performance and infection containment. PLoS Neglected Tropical Diseases, 6, e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, S. , Hoffmann, A. A. , & Werren, J. H. (1997). Influential passengers: Inherited microorganisms and arthropod reproduction. Oxford, UK: Oxford University Press. [Google Scholar]
- O'Neill, S. L. , & Karr, T. L. (1990). Bidirectional incompatibility between conspecific populations of Drosophila simulans . Nature, 348, 178-180. [DOI] [PubMed] [Google Scholar]
- O'Neill, S. L. , & Paterson, H. E. H. (1992). Crossing type variability associated with cytoplasmic incompatibility in Australian populations of the mosquito Culex quinquefasciatus Say. Medical and Veterinary Entomology, 6, 209–216. [DOI] [PubMed] [Google Scholar]
- O'Neill, S. L. , Ryan, P. A. , Turley, A. P. , Wilson, G. , Retzki, K. , Iturbe‐Ormaetxe, I. , … Ritchie, S. A. (2018). Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Research, 2, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. A. , Axford, J. K. , Callahan, A. G. , Richardson, K. M. , & Hoffmann, A. A. (2019). Stable phenotypic effects of an unstable deleterious wolbachia infection. BioRxiv, 853473 10.1101/853473 [DOI] [Google Scholar]
- Ross, P. A. , Axford, J. K. , Richardson, K. M. , Endersby‐Harshman, N. M. , & Hoffmann, A. A. (2017). Maintaining Aedes aegypti mosquitoes infected with Wolbachia . Journal of Visualized Experiments, e56124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. A. , Ritchie, S. A. , Axford, J. K. , & Hoffmann, A. A. (2019). Loss of cytoplasmic incompatibility in Wolbachia‐infected Aedes aegypti under field conditions. PLoS Neglected Tropical Diseases, 13, e0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. A. , Turelli, M. , & Hoffmann, A. A. (2019). Evolutionary ecology of Wolbachia releases for disease control. Annual Review of Genetics, 53, 93-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. A. , Wiwatanaratanabutr, I. , Axford, J. K. , White, V. L. , Endersby‐Harshman, N. M. , & Hoffmann, A. A. (2017). Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathogens, 13, e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, P. A. , Turley, A. P. , Wilson, G. , Hurst, T. P. , Retzki, K. , Brown‐Kenyon, J. , … O'Neill, S. L. (2019). Establishment of Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Research, 3, 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire, J. D. , On, J. , Layton, E. M. , Zhou, H. , & Bordenstein, S. R . (2018). One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster . Proceedings of the National Academy of Sciences, 115, 4987–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkins, S. P. , Braig, H. R. , & O''Neill, S. L. (1995). Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proceedings of the Royal Society B: Biological Sciences, 261, 325–330. [DOI] [PubMed] [Google Scholar]
- Suh, E. , Fu, Y. , Mercer, D. R. , & Dobson, S. L. (2016). Interaction of Wolbachia and bloodmeal type in artificially infected Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology, 53, 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, E. , Mercer, D. R. , Fu, Y. , & Dobson, S. L. (2009). Pathogenicity of life‐shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster . Applied and Environmental Microbiology, 75, 7783–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, L. , Ferreira, A. , & Ashburner, M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biology, 6, 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telschow, A. , Yamamura, N. , & Werren, J. H. (2005). Bidirectional cytoplasmic incompatibility and the stable coexistence of two Wolbachia strains in parapatric host populations. Journal of Theoretical Biology, 235, 265–274. [DOI] [PubMed] [Google Scholar]
- Teo, C. , Lim, P. , Voon, K. , & Mak, J. J. T. B. (2017). Detection of dengue viruses and Wolbachia in Aedes aegypti and Aedes albopictus larvae from four urban localities in Kuala Lumpur, Malaysia. Tropical Biomedicine, 34, 583–597. [PubMed] [Google Scholar]
- Thongsripong, P. , Chandler, J. A. , Green, A. B. , Kittayapong, P. , Wilcox, B. A. , Kapan, D. D. , & Bennett, S. N. (2018). Mosquito vector‐associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod‐borne diseases. Ecology and Evolution, 8, 1352–1368. 10.1002/ece3.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa, P. , Charlat, S. , Labbe, P. , Dehecq, J.‐S. , Barre, H. , & Weill, M. (2010). Wolbachia age‐sex‐specific density in Aedes albopictus: A host evolutionary response to cytoplasmic incompatibility? PLoS ONE, 5, e9700 10.1371/journal.pone.0009700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M. , & Hoffmann, A. A. (1991). Rapid spread of an inherited incompatibility factor in California Drosophila . Nature, 353, 440–442. [DOI] [PubMed] [Google Scholar]
- Veneti, Z. , Clark, M. E. , Karr, T. L. , Savakis, C. , & Bourtzis, K. (2004). Heads or tails: Host‐parasite interactions in the Drosophila‐Wolbachia system. Applied and Environmental Microbiology, 70, 5366–5372. 10.1128/AEM.70.9.5366-5372.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, T. , Johnson, P. H. , Moreira, L. A. , Iturbe‐Ormaetxe, I. , Frentiu, F. D. , McMeniman, C. J. , … Hoffmann, A. A. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature, 476, 450–453. [DOI] [PubMed] [Google Scholar]
- Weinert, L. A. , Araujo‐Jnr, E. V. , Ahmed, M. Z. , & Welch, J. J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings of the Royal Society B: Biological Sciences, 282, 20150249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Z. Y. , Khoo, C. C. H. , & Dobson, S. L. (2005). Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science, 310, 326–328. [DOI] [PubMed] [Google Scholar]
- Xi, Z. , Khoo, C. C. , & Dobson, S. L. (2006). Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus . Proceedings of the Royal Society B: Biological Sciences, 273, 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, J. H. , & Barr, A. R. (1973). The etiological agent of cytoplasmic incompatibility in Culex pipiens . Journal of Invertebrate Pathology, 22, 242–250. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Zheng, X. , Xi, Z. , Bourtzis, K. , & Gilles, J. R. (2015). Combining the sterile insect technique with the incompatible insect technique: I‐impact of Wolbachia infection on the fitness of triple‐and double‐infected strains of Aedes albopictus . PLoS One, 10, e0121126 10.1371/journal.pone.0121126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Zhang, D. , Li, Y. , Yang, C. , Wu, Y. , Liang, X. , … Xi, Z. (2019). Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature, 572, 56–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript and its appendices.


