Abstract
The Qinghai–Tibet Plateau (QTP) plays an important role in avian diversification. To reveal the relationship between the QTP uplift and avian diversification since the Late Cenozoic, here, we analyzed the phylogenetic relationship and biogeographical pattern of the genus Tetraogallus (Galliformes, Phasianidae) and the probable factors of speciation in the period of the QTP uplift inferred from concatenated data of four nuclear and five mitochondrial genes using the method of the Bayesian inference. Phylogenetic analysis indicated that T. himalayensis had a close relationship with T. altaicus and conflicted with the previous taxonomy of dark‐bellied and white‐bellied groups. The molecular clock showed that the speciation of Tetraogallus was profoundly affected by the uplift of the QTP and glacial oscillations. Biogeographic analysis suggested that the extant snowcocks originated from the QTP, and the QTP uplift and glacial oscillations triggered the diversification of Tetraogallus ancestor. Specifically, the uplift of the mountain provided a prerequisite for the colonization of snowcocks Tetraogallus as a result of the collision between the Indian and the Arab plates and the Eurasian plate, in which ecological isolation (the glacial and interglacial periods alternate) and geographical barrier had accelerated the Tetraogallus diversification process. Interestingly, we discovered hybrids between T. tibetanus and T. himalayensis for the first time and suggested that T. tibetanus and T. himalayensis hybridized after a second contact during the glacial period. Here, we proposed that the hybrid offspring was the ancestor of the T. altaicus. In conclusion, the uplift of QTP and glacial oscillations triggered the snowcocks colonization, and then, isolation and introgression hybridization promoted diversification.
Keywords: diversification, introgression hybridization, Qinghai‐Tibet Plateau, speciation, Tetraogallus
A phylochronogy of the genus Tetraogallus species based on concatenated nucleotide data. Phylogenetic analysis indicated that T. himalayensis had a close relationship with T. altaicus and conflicted with the previous taxonomy of dark‐bellied and white‐bellied groups. The molecular clock showed that the speciation of Tetraogallus was profoundly affected by the uplift of the QTP and glacial oscillations.

1. INTRODUCTION
The uplift of Qinghai–Tibet Plateau (QTP) profoundly impacts speciation and diversification in the plateau birds (Lei, Qu, & Song, 2014). The uplift of the QTP results from the collision of the Indian Plate with Eurasia during the Eocene (55–50 million years ago [Mya]) and then has experienced different stages of growth to reach the current altitude, which has created the unique topography, complex climate, and diversified habitats of the QTP, and makes the QTP an area of worldwide importance for biodiversity (An et al., 2006; Favre et al., 2015; Li & Fang, 1999). Moreover, the unique geomorphological configuration provides multitudinous refugia for animals to survive in harsh environments during the series of major ice ages of the Quaternary (2.4 Mya to the present) (Hewitt, 2000; Lei et al., 2014). Most of the currently endemic birds of the QTP have went through speciation since the Late Cenozoic in response to the QTP uplift, such as partridge Perdix (Bao et al., 2010), monal‐partridge Tetraophasis (Wen & Liu, 2010), and ground tit Pseudopodoces humilis (James et al., 2003). Therefore, the study on Tetraogallus diversification in the QTP can help reveal the geographic history of the QTP and the speciation mechanism. Snowcocks inhabit and evolve in the high‐altitude mountain land (Liu, 1998; Shen & Wang, 1963), and make it an ideal animal to investigate the relationship between geological history and speciation. However, the diversification of Tetraogallus has long been ignored and its biogeographical history is poorly understood so far.
The genus Tetraogallus is composed of five species, including T. tibetanus, T. himalayensis, T. altaicus, T. caspius, and T. caucasicus, and distributed in the major mountains system of the Center Asia (Figure 1) (Liu, 1998; Zheng, 2002). T. tibetanus, an endemic to the QTP, live across almost mountain systems on the whole QTP, including Pamir Plateau (Liu, 1998; Zheng, Tan, Lu, & Tang, 1978). T. himalayensis inhabit the Tien Shan, Pamir Plateau, and parts of the QTP, and the distribution area overlaps with T. tibetanus on the QTP (Liu, 1998; Zheng et al., 1978). T. altaicus are distributed in the Altai–Sayan–Hangay Mountains (Liu, 1998; Potapov, 1987; Zabelin, 2007), including the Altai Mountains in China (Huang, Mi, & Shao, 1992). T. caspius are distributed in the southern part of the Caspian Sea from the eastern Anatolian Plateau to the Iranian Plateau, and T. caucasicus only survive in the Caucasus Mountains (Dementiev & Gladkov, 1967; Liu, 1998). Bianki (1898) once thought that extant five snowcocks can classify into dark‐bellied (T. himalayensis, T. caspius, and T. caucasicus) and white‐bellied (T. tibetanus and T. altaicus) groups based mainly on adult abdomen plumage color. Liu (1998) suggested that T. tibetanus and T. himalayensis first split from the Tetraogallus ancestor based on their morphological characteristics and represent white‐bellied and white‐bellied groups. T. altaicus split from T. tibetanus; both T. caspius and T. caucasicus split from T. himalayensis and speculated that T. altaicus is likely to hybridize with T. himalayensis (Liu, 1998). Liu (1998) systematically elaborated the phylogenetic relationship of Tetraogallus, but the aforementioned conclusion has not been validated by molecular phylogenetic approach up to now. Stein, Brown, and Mooers (2015) reconstructed the phylogenetic relationship of Tetraogallus except for T. caucasicus using molecular data and the result indicated that T. altaicus has a close relationship with T. caspius; however, this result did not explain the morphological characteristics and the distribution pattern of extant snowcocks. Thus, a further phylogenetic study on the genus Tetraogallus remains critical to understand the speciation in relation to the geographic history.
Figure 1.
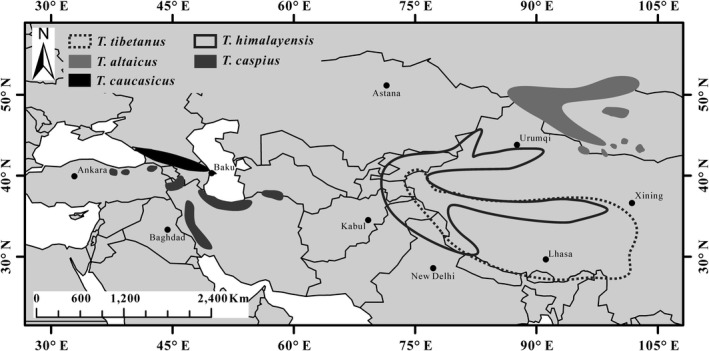
Geographical distribution of Tetraogallus species. Species distribution information is cited from Liu (1998)
The origin of Tetraogallus has been controversial over the past few decades (Liu, 1998). In the aspect of origin time of the Tetraogallus ancestor, Koslova (1952) and Baziev (1978) suggested that snowcocks originate in the Early Pleistocene (about 2.5 Mya) based on the orogeneses of the QTP. Potapov (1992) disagreed with the aforementioned point and proposed that the ancestor of Tetraogallus occurs in the first glacier of the Pleistocene (Minde glacial period) because the climate of the Pliocene/Pleistocene boundary is warm and does not have the conditions for the origin of the Tetraogallus, but that is too late for snowcocks to have evolved obviously. As such, Liu (1998) pointed out that the snowcock first occurs in the Quaternary Early glaciation (Hongya glaciation, about 3.5–2.6 Mya). However, recent molecular phylogenetic studies have greatly advanced the origin of snowcocks. Stein et al. (2015) suggested that the ancestor of Tetraogallus can be dated back to the Middle Oligocene (about 29.32 Mya) and diversification begins in the Late Miocene (about 7.31 Mya) inferred from the concatenation dataset of nuclear and mitochondrial genes. In the aspect of origin area of the Tetraogallus ancestor, Koslova (1952) pointed out that the origin of the snowcock‐like ancestors is related to the uplift of the QTP, and they may be originated from the jungles of the Eastern Kunlun Mountains and the Hengduan Mountains (Western Sichuan Province, China). Baziev (1978) believed that the snowcock‐like ancestor is not alpine birds, and they inhabit the hilly areas from the Caucasus to Central China; the snowcock‐like ancestor will rise with the process of mountain uplift, so each mountain system has a kind of snowcock. Consequently, the distribution area of extant snowcocks is the origin of ancestor. However, Potapov (1992) found a paradox that snowcocks are unlikely to inhabit the Hengduan Mountains during the Pliocene/Pleistocene boundary because of lower altitude and suggest that snowcocks originated in the Pamir Plateau, Tien Shan, and Kunlun mountains. Fossil data can be used to estimate the origin of snowcocks, in addition, however snowcocks have few fossils available, and precious fossil data are only found in the Altai and Caucasus Mountains, which can date back to the Middle and Late Pleistocene, and these are not sufficient to infer the origin of Tetraogallus because time is too short (Panteleev, 2002; Potapov, 1992). In summary, the origin of the Tetraogallus ancestor has not yet been determined in terms of spatial and temporal.
To gain knowledge on the speciation mechanisms in snowcocks, here, we investigated the phylogeny, biogeographical history, and diversification rate using a concatenated DNA dataset. As a consequence, the present investigation aimed at solving three questions: 1. When and where did snowcocks originate? 2. Was the diversification of snowcocks affected by the QTP uplift and glacial oscillations? 3. Was hybridization promoting the speciation of T. altaicus?
2. MATERIALS AND METHODS
2.1. Taxon set and DNA data
The genus Tetraogallus is composed of five species (T. himalayensis, T. tibetanus, T. altaicus, T. caspius, and T. caucasicus) according to taxonomic data, which belong to Galliformes, Phasianidae (Zheng, 2002). Here, T. caucasicus was not taken into account in the ingroup taxon set because its DNA data were not available up to now. All DNA sequence data were downloaded from the GenBank database (downloaded on or before December 30, 2018; Table A1) in the present study. Two outgroup taxa were selected from the genus Alectoris based on the previous study (Stein et al., 2015), including A. chukar and A. rufa. We assembled a DNA data matrix which was composed of 3 protein‐coding genes (COX1, CYTB, and ND2) and 2 nonprotein‐coding genes (12S and D‐loop) mitochondrial loci combined with 4 nonprotein‐coding genes (CLTC, CLTCL1, RHO, and EEF2) nuclear loci (Table A1). The DNA matrix was 6,975 base pairs (bp) long at its maximum extent (including gaps), and the alignment data were sparse and average coverage was approximately 85% across DNA markers (mitochondrial locus: 67%–100%; nuclear locus: 83%). In order to improve the resolution ability of the phylogenetic inference, here, we chose to mark as high coverage as possible rather than adopted all publicly available nucleotide sequences. In addition, D‐loop sequences were downloaded from the GenBank database and were used to construct phylogenetic analysis of populations between T. tibetanus and T. himalayensis. Sequence information please see Table A2.
2.2. Phylogenetic analysis, genetic distance, nucleotide mutation rate, and estimation of divergence time
Multiple‐sequence alignments of mitochondrial and nuclear genes were performed using BioEdit v. 7.1.3.0 program (Hall, 1999) with default parameters. Each gene was aligned separately and manually concatenated these sequences using Sequence Matrix v. 1.7.8 (Vaidya, Lohman, & Meier, 2011). All concatenated sequences were format converted for further analyses using Geneious v. 9.1.4 program. The genetic distance was calculated by MEGA 6.0 (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013) with the Kimura 2‐parameter (K2P) model of nucleotide substitution (Kimura, 1980) using concatenated data. Phylogenetic analysis and divergence date on the genus Tetraogallus species were estimated by BEAST version 1.7.4 program (Drummond & Rambaut, 2007) using the Markov Chain Monte Carlo (MCMC) to estimate the divergence times of the genus Tetraogallus. The best‐fit model of substitution and best partition schemes for the dataset were identified with the corrected Akaike information criterion (AICc) (Akaike, 1974), implemented in Modeltest 3.7 program (Posada & Crandall, 1998).
To acquire as accurate divergence time as possible, we estimated the number of nucleotide substitutions per site (d) from comparisons of the focal species and an outgroup species using the formula: d = (tv + tvR)/m, where tv is the number of transversions between the genus Tetraogallus and outgroup taxa, R is the transition/transversion ratio within the genus Tetraogallus, and m is the sequence length (Nei, 1992; Rooney, Honeycutt, & Derr, 2001). Transition and transversion values were calculated in the program MEGA 6.0. The rate of nucleotide substitutions per site per lineage per year is λ = d/2T when an estimate of d was obtained, where T is the divergence time between the ingroup and outgroup species (Rooney et al., 2001). The mutation rate per nucleotide site per generation is μ = λg, where g is the generation time (g = 3 years of snowcock; Huang, Ma, Shao, & Jiang, 1990). Here, d was 0.084 (tv = 84, R = 5.96, m = 6,975) for combined genes. The rate of nucleotide substitution per site per lineage per year (λ) was about 0.14 × 10–8 (T = 29.32 Mya, quoted from Stein et al. (2015)), and the mutation rate of per generation (μ) was about 0.40% Mya for concatenation DNA sequence of snowcocks. Similarly, we used the same method to calculate the rate of nucleotide substitute of D‐loop and the result showed that μ was about 0.20% Mya.
The relaxed molecular clock was performed with the uncorrelated lognormal clock model for species divergence of Tetraogallus to infer branch lengths and nodal ages under the GTR + I substitution model in BEAST. The phylogenetic relationship was reconstructed under the Yule speciation process (Steel & McKenzie, 2001), with enforced monophyly of the ingroup. Chain lengths were 50 million, sampled every 1,000 generations, with the first 10% discarded as burn‐in. The posterior distribution of the estimated divergence times was obtained by specifying one calibration point that was used as prior for the time for the most recent common ancestor of A. chukar and A. rufa. According to the previous studies, the age of the A. chukar and A. rufa divergence was as time calibration point (about 2.84 Mya) (Stein et al., 2015). The prior for the age of the tree root was corresponding to the basal split, and a normal distribution was as the prior model for the calibration with mean 2.84 Mya and standard deviation 0.01. As such, the similar setting in BEAST was used to estimate the population divergence date between T. tibetanus (35 haplotypes; An, Zhang, Liu, & Wang, 2015) and T. himalayensis (37 haplotypes; Wang, Qu, Liu, Bao, & Song, 2011) with the Bayesian strict clock under the HKY + I + G substitution model. The final molecular clock tree was generated in the program TreeAnnotator 1.7.4 (a subprogram of BEAST) using the mean as node heights. The program Tracer v1.5 was used to test the results validity of sampling (effective sample size (ESS) > 200). Here, Bayesian posterior probability (PP) equal or above 0.95 was considered as strong relationships (Leaché & Reeder, 2002). Finally, we used the FigTree v. 1.3.1 program to visualize all tree files.
2.3. Biogeographical analysis
To infer the possible origin area of the genus Tetraogallus, we used the program with the method of BBM (Bayesian binary MCMC) in RASP (Reconstruct Ancestral State in Phylogenies) (Yu, Harris, Blair, & He, 2015) to reconstruct the possible ancestral region of snowcocks on the phylogenetic tree. Here, the biogeographic distribution range of extant species of Tetraogallus was divided into four sections according to the available literature (Liu, 1998; Potapov, 1987; Zheng et al., 1978) and the International Union for Conservation of Nature online source (IUCN, https://www.iucnredlist.org). These areas were A (Qinghai–Tibet Plateau [including Pamir Plateau]), B (Tien Shan Mountains), C (Altai–Sayan–Hangay Mountains), and D (Iranian–Anatolia Plateau) (Figure 3a, Top‐left). We used a phylogenetic tree of Tetraogallus that derived from BEAST analysis output and ran BBM on all of them, and the number of maximum areas was set to 1 area. The MCMC chains were run five millions generation and sampled every 100 generations. Fixed JC + G (Jukes–Cantor + gamma) was used for BBM analysis with null root distribution. The possible ancestral ranges were determined at each node on a selected tree.
Figure 3.
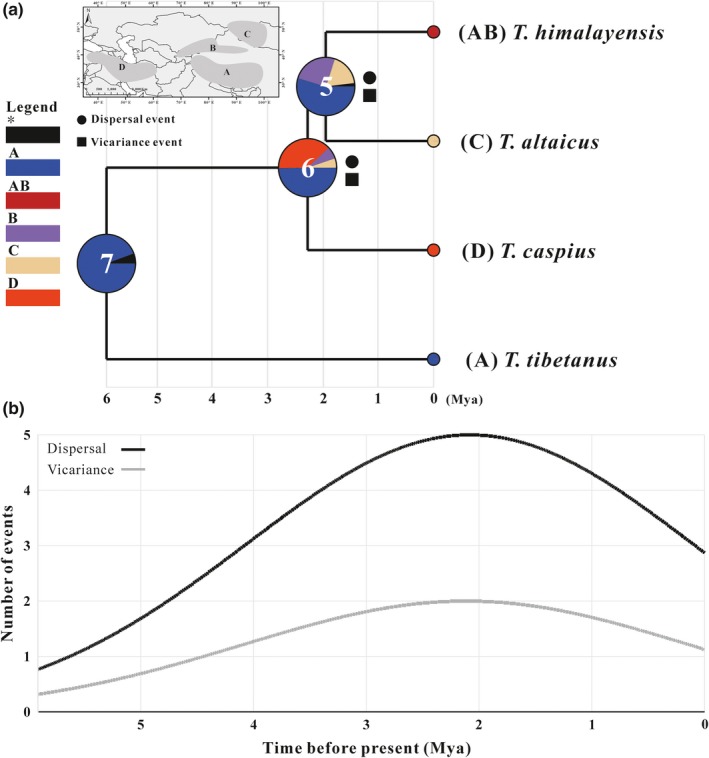
Diagram of ancestral distribution ranges is estimated using BBM analysis (output from RASP). (a) Biogeographical areas used in RASP analysis are divided into four regions (Top‐left). The possible ancestral range is estimated by BBM based on each node of the phylogeny of the genus Tetraogallus, and Pie charts at each node show probabilities of alternative ancestral ranges. (b) The frequency of biogeographic events of snowcocks changes over time
2.4. Diversification analysis
To reconstruct the macroevolutionary dynamics of Tetraogallus over time, we implemented a diversification rate analysis. BAMM software used a Bayesian framework to account for rate variation through time and among lineages in phylogenetic trees, and we employed it to simulate posterior distributions of rate‐shift configurations (Rabosky, Donnellan, Grundler, & Lovette, 2014a). We then used BAMMtools package in R to conduct rate‐through‐time analysis and to identify and visualize diversification rate shift along branches (the BEAST output result) (R Core Team, 2012; Rabosky, Grundler, et al., 2014b). BAMM was run by setting four independent MCMC running for 10 million generations and sampled every 1,000 generations. After removing 25% of trees as burn‐in, the BAMM output was analyzed with employing BAMMtools and the 95% credible rate‐shift configurations were estimated using Bayes factors. The best shift configuration with the highest maximum a posteriori probability was estimated in this analysis. In addition, we demonstrated a relationship between diversification rate shifts and ecological opportunity processes, which if ecological opportunity occurred in new regions could trigger rapid radiation and cause speciation rate increase and colonization of a new region (Schenk, Rowe, & Steppan, 2013; Yoder et al., 2010). The phytools package in R was used to estimate a gamma statistic (γ) with a phylogenetic tree to test region‐specific patterns in the diversification rates and to visualize lineage‐through‐time (LTT) plot (Revell, 2012). Herein, if γ value was positive (γ > 0), it suggested a late outbreak of speciation rate, and conversely, a negative value for γ (γ < 0) indicated that this branch has a process of decreasing the speciation rate (Pybus & Harvey, 2000).
3. RESULTS
3.1. Phylogenetic analysis, divergence time, and genetic distance
Phylogenetic relationships of the genus Tetraogallus were reconstructed using the concatenated DNA data with the method of Bayesian inference. The results indicated that T. tibetanus and T. himalayensis were first split from the Tetraogallus ancestor (PP = 1) and dated back to the Late Miocene (about 5.91 Mya) with 95% confidence intervals (95% CI) of 3.09–8.87 Mya (Figure 2). T. caspius split from T. himalayensis (PP = 1) that occurred in the Early Pleistocene (about 2.28 Mya, 95% CI: 1.10–3.66 Mya). T. altaicus was closely related to T. himalayensis by comparison with T. tibetanus (PP = 0.51) and split from T. himalayensis that first occurred in the Early‐Middle Pleistocene (about 1.95 Mya, 95% CI: 0.98–2.92 Mya). The K2P distance between snowcocks was calculated using concatenated data (Table 1). The T. tibetanus showed the high divergence (between 0.060 and 0.064), with the most divergent lineages differing by K2P distance inferred from the concatenated data. However, the K2P distance between T. himalayensis and T. altaicus was quite low (K2P = 0.007) by comparisons with other snowcocks, implicating the lower differentiation between them in line with the phylogeny tree (Table 1, Figure 2).
Figure 2.
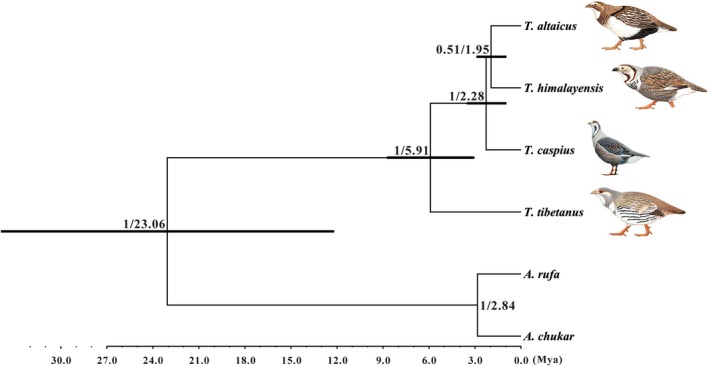
A phylochronogy of the genus Tetraogallus species based on concatenated nucleotide data. The divergence time is estimated using the BEAST with the calibration method under the relaxed molecular clock model (using the estimated mutation rate). Branch lengths represent the mean values of the posterior distribution. The posterior probability and divergence time are indicated at each inner node. The node bars indicate the posterior probability distribution of the node age under the 95% CI. Snowcock portraits are quoted from MacKinnon, Phillipps, He, and Lu (2000) and Svensson, Mullarney, Zetterstrom, and Grant (2009)
Table 1.
Pairwise distances divergence (K2P distances) for the concatenated DNA data between the genus Tetraogallus species
| T. altaicus | T. caspius | T. himalayensis | T. tibetanus | |
|---|---|---|---|---|
| T. altaicus | ||||
| T. caspius | 0.017 | |||
| T. himalayensis | 0.007 | 0.013 | ||
| T. tibetanus | 0.061 | 0.064 | 0.060 |
3.2. Biogeographical analysis
BBM analysis indicated that the ancestor of the genus Tetraogallus originated from the area A at node 7 with high marginal probability (94.4%) (Figure 3a). Similarly, the ancestral reconstruction of BBM suggested that A was the ancestral area of nodes 6 and 5, with 50.1% and 55.1% marginal probability, respectively. BBM results suggested that the speciation between T. tibetanus and T. himalayensis occurred in the QTP during the Late Miocene (node 7), and then they underwent dispersal and isolation that may be driven by the geographic and climatic events, as evident from ancestral ranges at nodes 6 and 5. BBM detected two vicariance events and three dispersal events at nodes 6 and 5, while node 7 without any biogeographic event occurred. Moreover, BBM estimated the possible colonization route of snowcocks (nodes 7, 6, and 5) as the result that the origin and spread of snowcocks were related to the QTP uplift. In addition, the time curve of dispersal and vicariance events illustrated that a fastigium of biogeographical events occurred in the Early Pleistocene (about 2.28 Mya) (Figure 3b). Herein, it was clear that this discontinuous distribution pattern was related to geological and climatic changes.
3.3. Diversification rate shift
The distinct shift configuration for diversification was shown in Figure 4a, along with the corresponding consensus phylorate plot and rate acceleration events within the genus Tetraogallus taxa which fell into the Early Pleistocene (about 2.28 Mya). Here, the speciation rate of snowcocks has undergone a rapid increase and then subsequent decrease, and we detected the best rate‐shift point which was noted in the phylorate plot (Figure 4a). The LTT plot showed an increase of diversification rate after colonized the QTP area (Figure 4b) and suggested a potential early initial burst at approximately 6 Mya, and after that the speciation rate has undergone a subsequent reduction (γ = −0.082, p > .05) in line with phylorate (Figure 4a).
Figure 4.
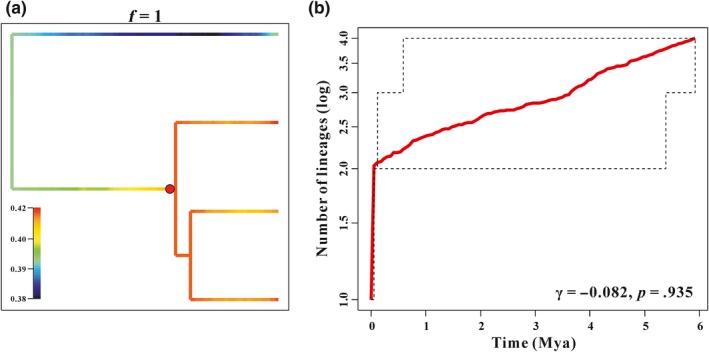
Diagram of macroevolutionary dynamics of Tetraogallus. (a) A phylorate plot shows speciation rates (cool colors = slow and warm = fast) along each branch of the Tetraogallus phylogeny. The red dot denotes the position of the diversification rate shift detected by BAMM analysis. (b) LTT plot of Tetraogallus. The red solid line shows the mean number of lineages and the black dashed lines along with it give the 95% confidence interval
3.4. Phylogeographic analysis between T. tibetanus and T. himalayensis
Phylochronology showed two distinct lineages (PP = 1) and the population divergence time between T. tibetanus and T. himalayensis occurred in the Middle Pliocene (about 3.54 Mya) with 95% confidence intervals of 2.19–5.20 Mya. There, no phylogeographic structure was observed with T. tibetanus and all haplotypes were intermixed with poor support and low divergence (Figure 5) and suggested that there were frequent genetic exchanges between populations. All haplotypes of T. himalayensis grouped into two clades (Clade A and Clade B) with high support value (PP = 1); Clade A represented the populations from the Northern Qinghai–Tibet Plateau and Clade B represented the populations from the Kunlun–Tien Shan mountains. The divergence time between two major clades dated back to the Early Pleistocene (about 1.66 Mya, 95% CI: 0.99–2.52 Mya). More importantly, we found three hybrids between T. tibetanus and T. himalayensis from the SB and HT populations (Figure 5, dark gray clade). Three hybrids should be T. himalayensis but have a close relationship with T. tibetanus (PP = 1), indicating that the male T. himalayensis hybridized with the female T. tibetanus as the result that hybrids embed into T. tibetanus clade. The molecular clock indicated that hybridization time between T. himalayensis and T. tibetanus occurred in the Early Pleistocene (about 1.83 Mya, 95% CI: 1.04–2.81 Mya). Here, we speculated that the hybridization area was likely to be located on the sympatric zone of T. tibetanus and T. himalayensis (Figure 5; Top‐left: dim gray shade).
Figure 5.
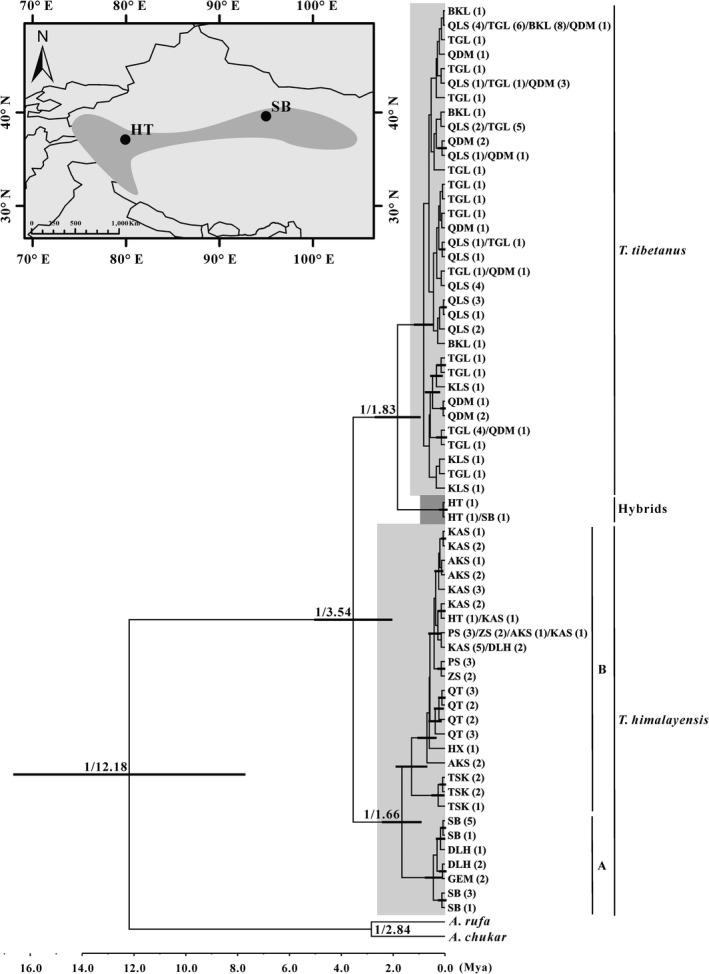
A chronogram of population divergence between T. tibetanus and T. himalayensis based on D‐loop haplotypes. The divergence time is estimated using the BEAST with the calibration method under the strict clock model (using the estimated mutation rate). Branch lengths represent the mean values of the posterior distribution. The posterior probability and divergence time are indicated at the major inner nodes, and population names are provided in Table A2. The node bars indicate the posterior probability distribution of the node age under the 95% CI. Proposed hybridization zone of T. tibetanus and T. himalayensis is marked in dim gray shade (Top‐left)
4. DISCUSSION
A multi‐locus phylogeny indicated that T. tibetanus and T. himalayensis first differentiated from the Tetraogallus ancestor, and then T. altaicus and T. caspius split from T. himalayensis (Figure 2), and our results supported the conclusions proposed by Liu (1998). Phylogenetic analysis suggested that T. himalayensis has a close relationship with T. altaicus by comparison with T. caspius which vary from previous phylogenetic result (Stein et al., 2015), and we believed the current results were rational because of the large number of phylogenetic information sites provided. As such, the K2P distance also supported this result (Table 1). Stein et al. (2015) suggested that T. altaicus has close relationship with T. caspius, but their work cannot explain the speciation mechanism because of the distribution pattern of extant snowcocks and morphological variations. Both T. tibetanus and T. altaicus are classified into white‐bellied group (Bianki, 1898), at this point, but T. altaicus was closely related to T. himalayensis in terms of morphological and genetic characteristics in fact rather than T. tibetanus. Phylogenetic results on the genus Tetraogallus broke the knowledge of white‐bellied and dark‐bellied groups by taxonomists for a century, making this conclusion be challenged. We would discuss how extant snowcocks evolved below. The divergence time of calibration point indicated that our time was basically consistent with Stein et al. (2015)' results and suggested that the ingroup divergence time was rational. The speciation event between snowcocks can be dated back to the Late Miocene to Early Pleistocene (5.91–1.95 Mya) which predated previous studies (Baziev, 1978; Koslova, 1952; Liu, 1998; Potapov, 1992) and can adequately reveal the speciation mechanism of snowcocks because for this timeframe takes about 2 million years for it a vertebrate to evolve into a new species (Avise, Walker, & Johns, 1998). More importantly, the speciation of snowcocks coincided with the uplift of the QTP and the Quaternary glacial oscillations. For instance, Bao et al. (2010) suggested that the speciation of the genus Perdix is triggered by the Late Pliocene/Early Pleistocene (3.63–2.00 Mya) intensive uplift of the QTP and the Pleistocene glaciations (2.05–1.28 Mya). Naturally, we proposed that the Tetraogallus diversification was affected by the uplift of the QTP and the Quaternary glacial period at that time.
BBM analysis indicated that the Tetraogallus ancestor originated from the QTP (including Pamir Plateau) (Figure 3). Previous studies on the origin area of snowcocks supported our result (Koslova, 1952; Liu, 1998; Potapov, 1992) and suggested that the uplift of the QTP triggered the Tetraogallus diversification. The uplift of the QTP is a complicated geological development process and has underwent several uplifts which is the progressive and heterogeneous uplift of the QTP from south to north (Favre et al., 2015; Mulch & Chamberlain, 2006; Wang et al., 2008). Subsequently, a series of rapid uplift events give rise to approach its present elevation during the Late Cenozoic (Fang, 2017; Li & Fang, 1999; Shi, Li, & Li, 1998), including Qing–Zang (between 3.6 and 1.7 Mya), Kunlun–Huanghe (between 1.1 and 0.6 Mya), and Gonghe (about 0.15 Mya) tectonic movements. Specifically, the Qing–Zang tectonic movement occurred in the Middle Pliocene to Early Pleistocene (between 3.6 and 1.7 Mya), which contained three phases A (begin in 3.6 Mya), B (2.5 Mya), and C (1.7 Mya) (Li & Fang, 1999). The progressive extension of the uplift of the QTP is associated with the major mountains uplift and has blocked the northward of the India Ocean warm air, such as the higher Himalayas and the rise of the Tien Shan, which causes the progressive aridification of Central Asia during the Miocene (Miao, Herrmann, Wu, Yan, & Yang, 2012; Miao et al., 2011; Sun, Gong, Tian, Jia, & Windley, 2015). Moreover, the development of the Pleistocene glaciers of the QTP is closely related to the progressive uplift of the plateau and the surrounding mountains (Zheng & Rutter, 1998). Consequently, the QTP after the uplift results in drastic shifts in the distribution of plant communities and major faunal turnover (Deng & Ding, 2015; Li, Fang, Pan, Zhao, & Song, 2001; Sun & Wang, 2005). All these factors can be beneficial snowcocks to acquire suitable ecological resource and promoted adaptive radiation, of which the geographic colonization is an important way to survive (Stroud & Losos, 2016). The colonization of novel territory (e.g., mountains uplift or glacier recession) can provide a release from competition and predation pressures, abundant food resources, or suitable climatic conditions, allowing them to differentiate a variety of species to colonize multiple unexploited ecological resources, such as ground tit (James et al., 2003) and voles (Lv, Xia, Ge, Wu, & Yang, 2016). In summary, this evolutionary model can be characterized by ecological opportunity. Ecological opportunity can affect speciation rate, and animals will soon fill unoccupied niche if rate increase and then decrease tend to be stable finally (Rabosky & Lovette, 2008; Schenk et al., 2013). These theories were in favor of the diversification mechanism of snowcocks. Here, the diversification analysis of snowcocks indicated that the increase of speciation rate can be dated back to the Late Miocene and peaked in the Early Pleistocene (about 2.28 Mya, Figure 4), which coincided with the active period of the Late Cenozoic of the QTP. Obviously, the phases of A and B profoundly influenced on the snowcocks speciation and supported the snowcocks speciation was subjected to ecological opportunity. As such, the LTT analysis showed that the snowcocks speciation was subjected to ecological opportunity even though the gamma value was not significant because of the small number of species. In conclusion, the uplift of the QTP provided ecological opportunity for Tetraogallus diversification.
The T. tibetanus, an endemic to the QTP, has the long evolutionary history among the extant snowcocks. T. tibetanus split from T. himalayensis that can date back to the Late Miocene to coincide with the geological active period of QTP and Asian interior aridification (Sun et al., 2015), namely that the uplift of the QTP promoted speciation of T. tibetanus. Here, similar geological event occurred on the Phrynocephalus theobaldi differentiation (about 5.65 Mya), and the plateau uplift promoted the differentiation of toad‐headed lizards that inhabited the QTP during the Late Miocene (Jin, Liu, & Brown, 2017), and maybe, similar geographic events induced the divergent possibility between T. tibetanus and T. himalayensis at that time. Theoretically, the Gloger's rule believes that melanin is abundant in hot and humid areas, while maroon pigment or tawny pigment is abundant in dry areas (Edward, Burtt, & Jann, 2004; Zheng, 1952). We speculated that the origin area of T. tibetanus was likely to occur in the Gangdise Mountains and the Himalayas with wetter air and warm climate before the intense uplift of the QTP during the Late Cenozoic (since 3.6 Mya, Li and Fang (1999)) based on black stripes of belly feathers. Moreover, the origin area of T. himalayensis was likely to take place in the Pamir Plateau or the area east of the Pamir Plateau (Western Kunlun Mountains) with drier air and cold climate. A rational explain was that the Gangdise and Himalayas are the first to uplift as the result that has caused ecological shift in different regions and can provide ecological opportunity for T. tibetanus and T. himalayensis to colonize because the geographical development of the QTP is progressive uplift from south to north (Li & Fang, 1999; Mulch & Chamberlain, 2006). Competitive exclusion and ecological isolation caused the differentiation between T. tibetanus and T. himalayensis (Liu, 1998, 1999). Ecological opportunity had created a novel habitat for T. tibetanus and T. himalayensis, and then adaptive radiation accelerated them to divergence. Consequently, ecological isolation drove two snowcocks (T. tibetanus and T. himalayensis) to occupy different niches to evolve in their respective directions (Liu, 1999), including altitude, diet, breeding strategy, and breeding time, and finally evolved into two separate species. Geographical or ecological isolation leads to a reduction or disruption of genetic exchange between populations, and alleles change under the influence of genetic drift, which ultimately give rise to reproductive isolation (Hoskin, Higgie, McDonald, & Moritz, 2005; Via, 2012). For example, the uplift of the QTP gives rise to the speciation of the genus Perdix (Bao et al., 2010), and ecological divergent is an important factor for eared pheasants Crossoptilon speciation (Wang et al., 2017). In summary, the uplift of the QTP and niche divergence promoted the speciation of T. tibetanus and T. himalayensis.
Liu (1998) suggested that snowcocks colonize a novel habitat during the glaciation period, and then, interglacial period isolation promotes speciation. T. caspius split from T. himalayensis can be dated to the Early Pleistocene (about 2.28 Mya) corresponding to the uplift of the QTP and the Quaternary glacial oscillations and suggested that T. himalayensis colonized to the Iranian Plateau and Anatolia Plateau during the ice age and interglacial period isolation forced the independent evolution of the dispersed populations to eventually evolve into T. caspius. It was worth noting that the dispersal of T. himalayensis reached a climax during this period (about 2.28 Mya) inferred from BBM analysis. The Iranian Plateau is a juvenile landmass, which begins to uplift as a result of the Arabian plate impinging on Eurasia during the Late Eocene to the Early Miocene (between 35 and 20 Mya) (Berberian, 2014; Mouthereau, 2011). After that, the tectonic movements of Iranian Plateau give rise to the climatic change and the formation extensions of steppe‐like landscape during the Middle and Late Miocene (Storch, 2004), in which provides several suitable habitats for snowcock survival and also provides a prerequisite for T. himalayensis colonization. Furthermore, the Northern hemisphere great glacial period has led to the widespread development of ice sheets or glaciers in low‐latitude areas (An et al., 1998; Curry, 1966; Wu & Li, 1990) and promoted the T. himalayensis to step out the Pamir Plateau to colonize the Iranian Plateau, Anatolia Plateau, and Caucasus Mountains. Interglacial period isolation caused the speciation of T. caspius. BBM analyses detected vicariance and dispersal events in the speciation proceed of T. caspius (Figure 3) and supported this interpretation.
Phylogeographic analysis results indicated that three hybrids were reproduced by the hybridization between female T. tibetanus and male T. himalayensis based on D‐loop haplotypes (Figure 5), and they embed into the T. tibetanus clade because of the maternal inheritance of mitochondrial DNA (Sato & Sato, 2012). Obviously, there was no rigorous reproductive isolation between T. tibetanus and T. himalayensis, and the hybridization can give birth to F1 generation in the sympatric zone (Figure 6). Here, hybridization time can be dated back to the Early Pleistocene (about 1.83 Mya, 95% CI: 1.04–2.81 Mya). When allopatric populations meet in the contact zone, theoretically, hybridization may occur if reproductive isolation is incomplete (Dowling & Secor, 1997). When allopatric taxa became sympatric again, moreover, speciation can accelerate because of secondary contact such as the genus Drosophila (Coyne & Orr, 1997). For instance, the Darwin's Heath (Coenonympha darwiniana) originate through hybridization between the Pearly Heath (C. arcania) and the Alpine Heath (C. gardetta) with different parental contributions (Capblancq, Després, Rioux, & Mavárez, 2015), and hybridization promotes speciation in Coenonympha butterflies. A possible explanation is that the Quaternary Early ice age (e.g., Danube‐Gonzi ice age (about 2.6–1.5 Mya), Hongya ice age (about 2.5 Mya) (Liu, 1998), and Xixiabangma ice age (about 2.5–0.78 Mya) (Zheng, Xu, & Shen, 2002)) caused T. tibetanus and T. himalayensis to secondary contact and hybridize in sympatric zone, and then, the introgressive hybridization between F1 and T. himalayensis occurred in the interglacial period (Figure 6). Here, we called F2 the ghost of introgression past to be an ancestor for T. altaicus. Consequently, F2 repeatedly backcrosses to T. himalayensis as the result that a large number of alleles form T. himalayensis introgress into the ancestor of T. altaicus (Fn) (Figure 6). A further study would be to investigate introgression of T. altaicus from T. tibetanus and T. himalayensis at the whole genome scale. Liu (1998) pointed out T. altaicus have introgression from T. himalayensis based on morphological comparison between them, and our results have validated his speculation. Introgression hybridization (the one‐way flow of genes) between F2 and T. himalayensis allows T. altaicus to obtain parental characteristics, including morphology, behavior, and life history. The T. altaicus inherited many of the characteristics of the T. himalayensis, such as similar body size, yellow skin around the eyes, the same life habits, and tail‐hanging behavior (snowcocks portrait in Figure 2). The hybrids between A. chukar and A. magna repeatedly backcross to A. magna, for instance, making hybrids closely related to A. magna rather than that of A. chukar in terms of morphological and genetic characteristics (Chen, An, & Liu, 2016; Liu, Wen, Huang, & Hou, 2006), and supported our inference. The niche overlap forced introgression zone shift to colonize new suitable habitats and finally arrived in the Altai–Sayan–Hangay Mountains along the Tien Shan Mountains (Figure 6), and the ecological opportunity made them occupy new ecological resources.
Figure 6.
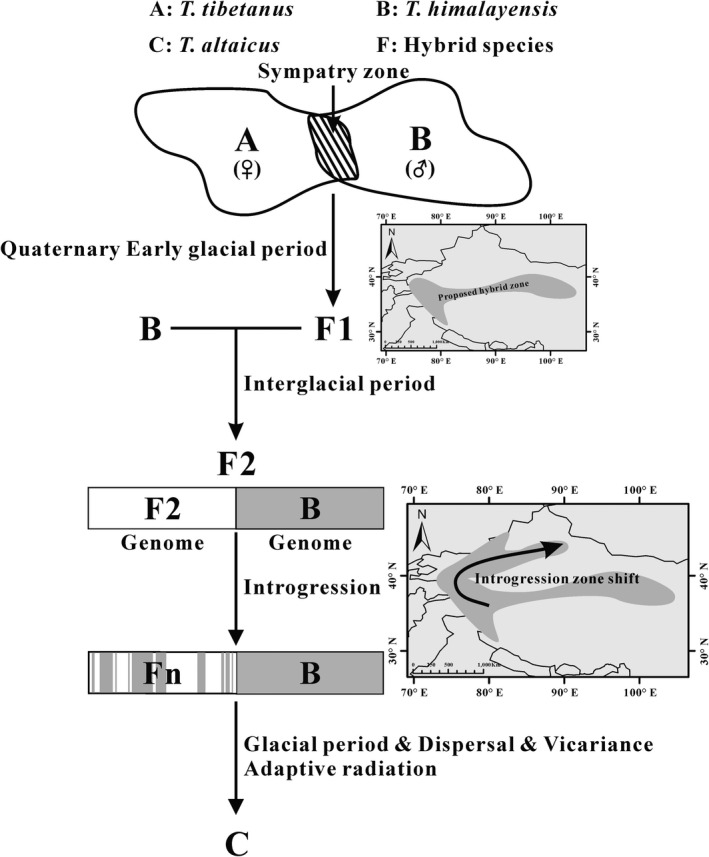
Proposed speciation model of T. altaicus. The hybridization between T. tibetanus and T. himalayensis occurs in the sympatric zone during the Quaternary Early glacial period, and then reproduces F1 generation. The F1 hybridizes with T. himalayensis and reproduces F2 during the interglacial period, and then several generations of introgression allowed hybrids to inherit numerous loci from T. himalayensis, which can be combined via gene flow through hybridization. Competitive exclusion between them gives rise to the introgression zone shift along with the Tien Shan and makes Fn (hybrids) to be an ancestor for T. altaicus. Hybrids (Fn generations) can potentially be adaptive and favored in a new habitat via adaptive introgression and can lead to a separate hybrid taxon (hybrid speciation). Finally, competitive exclusion and ecological opportunity can explain that hybrid species (the ancestor of T. altaicus) colonize the Altai–Sayan–Hangay Mountains during the glacial period, and then, geographical isolation and adaptive radiation have driven hybrids to be a novel species—T. altaicus
Introgression hybridization usually does not generate a new species, theoretically, but if hybrids (Fn) no longer backcross with the parental population and occupy new habitats, and then introgression of a few loci may promote adaptive divergence and so facilitate speciation (Abbott et al., 2013). The differentiation time between T. himalayensis and T. altaicus occurs in the Early Pleistocene corresponding to the uplift of Pamir–Tien Shan and the Asian aridification development (Fu, Ninomiya, & Guo, 2010; Wang et al., 2014). The uplift of the Pamir–Tien Shan provided an ecological opportunity for the ancestor of T. altaicus to colonize a novel territory and ecological resources, and provided a prerequisite for the introgression zone shift. In addition, the uplift of the Altai–Sayan–Hangay region occurred from the Oligocene to the Pliocene and approximately reached the present landscape the end of the Early Pliocene (3.6–2.4 Mya), blocking moisture from Siberian reached rain shadow, which caused aridization in the Central Asia (Caves, Sjostrom, Mix, Winnick, & Chamberlain, 2014; Xu, Ji, Sun, & Zhao, 2015; Zabelin, 2007). The uplift of the Altai and Hangay link to India–Asia convergence corresponds to the uplift of QTP (Caves et al., 2014; Xu et al., 2015) as a result that can provide suitable habits for hybrids (Fn) survival before the speciation of T. altaicus, and then, they can reach the Altai–Sayan–Hangay region through the Tien Shan during the Pleistocene glacial period. The mountain land of the Tien Shan and the Altai mountains is covered by the cover‐type glacier during the glacial maxima of the Middle Pleistocene, here, make the lower limit of glacier to spread low land with the above sea level of 1000 m (Chih‐chiu, 1968). These factors provide a prerequisite for the hybridization zone shift and the dispersal of the ancestor of T. altaicus. Since then, the aridification of central Asia has promoted Gobi Desert development, such as Gurbantunggut Desert and Badain Jaran Desert. Geographical barriers blocked genetic exchange with T. himalayensis, and BBM analysis detected vicariance and dispersal events in speciation of T. altaicus (Figure 3a). Here, hybridization and geographical isolation accelerated the process of T. altaicus speciation. A few studies suggested that some birds evolved a novel species by hybridizing in natural environments. For instance, Brelsford, Mila, and Irwin (2011) suggested that the speciation of Audubon's warbler (Dendroica auduboni) is hybridized by myrtle warbler (D. coronata) and black‐fronted warbler (D. nigrifrons) inferred from amplified fragment length polymorphism and molecular markers data. Joseph et al. (2008) considered that the intermediate plumage phenotypes of Western Slopes Rosella result from hybridization between Crimson Rosella and Yellow Rosella in a species complex of Australian parrots. Grant, Grant, and Deutsch (1996) supported that hybridization can contribute to the speciation process by enhancing genetic variation and relaxing genetic constrains on particular directions of evolutionary change, such as some island birds (Darwin's Finches). In conclusion, the genetic exchange between the backcross offspring and T. himalayensis was weakened as a result that the mountains uplift promoted the ancestor of T. altaicus to colonize and Middle‐Asia aridification caused geographical barriers, and the interaction of these factors promoted a new species differentiate—T. altaicus (Figure 6).
5. CONCLUSIONS
The extant snowcocks originated from the QTP, and the uplift of the QTP and the Quaternary glacial oscillations accelerated the genus Tetraogallus diversification. Specifically, mountains uplift and competitive exclusion promoted the differentiation between T. tibetanus and T. himalayensis. The T. himalayensis colonized other suitable habitats during the glacial period, and the interglacial isolation promoted the speciation of the T. caspius. The hybridization between T. tibetanus and T. himalayensis reproduced fertile hybrids during the Quaternary glacial period and repeatedly backcrosses with T. himalayensis during the interglacial period as a result of inheriting many characteristics from T. himalayensis, and glacial dispersal and isolation finally promoted the speciation of T. altaicus.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
AUTHOR CONTRIBUTIONS
NF L conceived the study; L D was responsible for obtaining and analyzing data and wrote the manuscript with the help of JC L.
ACKNOWLEDGMENTS
The work was supported by the National Natural Science Foundation of China (Nos. 30530130). Li Ding and Jicheng Liao dedicate this paper to the late Mr. Naifa Liu.
APPENDIX A.
Table A1.
GenBank accession number for mitochondrial and nuclear DNA sequences used in phylogenetic reconstructions for the Tetraogallus (4 ingroup and 2 outgroup taxa)
| Taxa | Species | Mitochondrial (Coverage (%)) | Nuclear (Coverage (%)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12S (100) | COX1 (83) | CYTB (100) | ND2 (83) | D‐loop (67) | CLTC (83) | CLTCL1 (83) | RHO (83) | EEF2 (83) | ||
| Ingroup | T. tibetanus | NC_023939.1 | NC_023939.1 | NC_023939.1 | NC_023939.1 | NC_023939.1 | KC778959.1 | KC778851.1 | KC778916.1 | KC778873.1 |
| T. himalayensis | NC_027279.1 | NC_027279.1 | NC_027279.1 | NC_027279.1 | NC_027279.1 | KC785632.1 | KC785646.1 | KC785713.1 | KC785663.1 | |
| T. caspius | KJ001802.1 | – | EU106676.1 | – | – | – | – | – | – | |
| T. altaicus | KC785617.1 | GQ482760.1 | AY563127.1 | KC785695.1 | – | KC785631.1 | KC785645.1 | KC785712.1 | KC785662.1 | |
| Outgroup | A. chukar | FJ752426.1 | FJ752426.1 | FJ752426.1 | FJ752426.1 | FJ752426.1 | KC749574.1 | KC749619.1 | EF569435.1 | KC749686.1 |
| A. rufa | KC749448.1 | GU951807.1 | HG940463.1 | DQ307002.1 | AJ586226.1 | KC749576.1 | KC749621.1 | EF569436.1 | KC749688.1 | |
12S, 12S ribosomal RNA; COX1, cytochrome c oxidase subunits 1; CYTB, cytochrome b; ND2, NADH dehydrogenase subunits 2; D‐loop, control region; CLTC, clathrin heavy polypeptide gene, intron 7; CLTCL1, clathrin heavy chain‐like 1 gene, intron 7; RHO, rhodopsin gene, intron 1; EEF2, eukaryotic elongation factor 2 (EEF2) gene, introns 5, 6 and partial coding sequence.
Table A2.
Sequences information (D‐loop) of T. tibetanus and T. himalayensis
| T. tibetanus | T. himalayensis | ||||
|---|---|---|---|---|---|
| Sampling site (size) | Haplotype | GenBank accession number | Sampling site (size) | Haplotype | GenBank accession number |
| QLS (1), TGL (5) | H1 | JX136799.1 | DLH (2) | H1 | GQ343513.1 |
| QLS (4) | H2 | JX136800.1 | GEM (2) | H2 | GQ343514.1 |
| QLS (2) | H3 | JX136801.1 | DLH (1) | H3 | GQ343515.1 |
| TGL (1) | H4 | JX136802.1 | SB (1) | H4 | GQ343516.1 |
| TGL (1) | H5 | JX136803.1 | SB (5) | H5 | GQ343517.1 |
| TGL (1) | H6 | JX136804.1 | SB (1) | H6 | GQ343518.1 |
| BKL (1) | H7 | JX136805.1 | SB (3) | H7 | GQ343519.1 |
| QLS (4), TGL (6), BKL (8), QDM (1) | H8 | JX136806.1 | DLH (2), KAS (3) | H8 | GQ343520.1 |
| TGL (1) | H9 | JX136807.1 | PS (3), ZS (2), AKS (1) | H9 | GQ343521.1 |
| QLS (1), TGL (1) | H10 | JX136808.1 | KAS (1) | H10 | GQ343522.1 |
| QLS (1), TGL (1), QDM (3) | H11 | JX136809.1 | PS (3) | H11 | GQ343523.1 |
| TGL (1) | H12 | JX136810.1 | KAS (1) | H12 | GQ343524.1 |
| QLS (1) | H13 | JX136811.1 | ZS (2) | H13 | GQ343525.1 |
| TGL (1) | H14 | JX136812.1 | AKS (1) | H14 | GQ343526.1 |
| TGL (1) | H15 | JX136813.1 | QT (3) | H15 | GQ343527.1 |
| TGL (1) | H16 | JX136814.1 | AKS (2) | H16 | GQ343528.1 |
| TGL (1), QDM (1) | H17 | JX136815.1 | HT (1) | H17 | GQ343529.1 |
| TGL (1) | H18 | JX136816.1 | KAS (1) | H18 | GQ343530.1 |
| TGL (1) | H19 | JX136817.1 | KAS (1) | H19 | GQ343531.1 |
| QLS (1) | H20 | JX136818.1 | KAS (2) | H20 | GQ343532.1 |
| TGL (4), QDM (1) | H21 | JX136819.1 | KAS (1) | H21 | GQ343533.1 |
| TGL (1) | H22 | JX136820.1 | QT (2) | H22 | GQ343534.1 |
| BKL (1) | H23 | JX136821.1 | KAS (3) | H23 | GQ343535.1 |
| BKL (1) | H24 | JX136822.1 | QT (1) | H24 | GQ343536.1 |
| KLS (1) | H25 | JX136823.1 | QT (1) | H25 | GQ343537.1 |
| KLS (1) | H26 | JX136824.1 | QT (1) | H26 | GQ343538.1 |
| KLS (1) | H27 | JX136825.1 | QT (1) | H27 | GQ343539.1 |
| QLS (1), QDM (1) | H28 | JX136826.1 | QT (1) | H28 | GQ343540.1 |
| QDM (2) | H29 | JX136827.1 | KAS (1) | H29 | GQ343541.1 |
| QDM (2) | H30 | JX136828.1 | KAS (1) | H30 | GQ343542.1 |
| QDM (1) | H31 | JX136829.1 | AKS (2) | H31 | GQ343543.1 |
| QDM (1) | H32 | JX136830.1 | TSK (2) | H32 | GQ343544.1 |
| QDM (1) | H33 | JX136831.1 | TSK (2) | H33 | GQ343545.1 |
| QLS (3) | H34 | JX136832.1 | TSK (1) | H34 | GQ343546.1 |
| QLS (1) | H35 | JX136833.1 | HT (1), SB (1) | H35 | GQ343547.1 |
| HT (1) | H36 | GQ343548.1 | |||
| HX (1) | H37 | GQ343549.1 | |||
Sampling sites of T. tibetanus: Qilian Mountains (QLS), Qaidam Basin (QDM), Baryan Har Mountains (BKL), Tanggula Mountains (TGL) and West Kunlun Mountains (WKL); Sampling sites of T. himalayensis: Delingha (DLH), Geermu (GEM), Subei (SB), Taxkorgan (TSK), Pishan (PS), Hotan (HT), Kashitashi (KAS), Aksu (AKS), Zhaosu (ZS), Qitai (QT) and Houxia (HX) (Please refer to the original for detailed sampling information; An et al., 2015; Wang et al., 2011).
Ding L, Liao J, Liu N. The uplift of the Qinghai–Tibet Plateau and glacial oscillations triggered the diversification of Tetraogallus (Galliformes, Phasianidae). Ecol Evol. 2020;10:1722–1736. 10.1002/ece3.6008
Contributor Information
Jicheng Liao, Email: liaojch@lzu.edu.cn.
Naifa Liu, Email: liu_naifa@163.com.
DATA AVAILABILITY STATEMENT
All data used in this review paper have been published elsewhere. Genbank accession number please see the Tables A1 and A2.
REFERENCES
- Abbott, R. , Albach, D. , Ansell, S. , Arntzen, J. W. , Baird, S. J. E. , Bierne, N. , … Zinner, D. (2013). Hybridization and speciation. Journal of Evolutionary Biology, 26, 229–246. 10.1111/j.1420-9101.2012.02599.x [DOI] [PubMed] [Google Scholar]
- Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723. 10.1007/978-1-4612-1694-0_16 [DOI] [Google Scholar]
- An, B. , Zhang, L. X. , Liu, N. F. , & Wang, Y. (2015). Refugia persistence of Qinghai‐Tibetan plateau by the cold‐tolerant bird Tetraogallus tibetanus (Galliformes: Phasianidae). PLoS ONE, 10, e0121118 10.1371/journal.pone.0121118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Z. , Wang, S. , Wu, X. , Chen, M. , Sun, D. , & Liu, X. (1998). Evidences of wind accumulation on the Loess Plateau of China: The beginning of the Northern hemisphere great glacial period during the Late Cenozoic and the uplifting of the Qinghai‐Tibet Plateau. Science in China (Series D), 28, 481–490 (Chinese). [Google Scholar]
- An, Z. S. , Zhang, P. Z. , Wang, E. , Wang, S. M. , Qiang, X. K. , Li, L. , … Zhou, W. J. . (2006). Changes of the monsoon‐arid environment in China and growth of the Tibetan Plateau since the Miocene. Quaternary Sciences, 26, 678–693 (English abstract). [Google Scholar]
- Avise, J. C. , Walker, D. , & Johns, G. C. (1998). Speciation durations and Pleistocene effects on vertebrate phylogeography. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265, 1707–1712. 10.1098/rspb.1998.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, X. K. , Liu, N. F. , Qu, J. Y. , Wang, X. L. , An, B. , Wen, L. Y. , & Song, S. (2010). The phylogenetic position and speciation dynamics of the genus Perdix (Phasianidae, Galliformes). Molecular Phylogenetics and Evolution, 56, 840–847. 10.1016/j.ympev.2010.03.038 [DOI] [PubMed] [Google Scholar]
- Baziev, D. K. (1978). The snowcocks of Caucasus. Ecology, Morphology, Evolution. Leningrad, Russia: Nauka. [Google Scholar]
- Berberian, M. (2014). Chapter 9 – Active tectonics and geologic setting of the Iranian plateau. Developments in Earth Surface Processes, 17, 151–171. 10.1016/B978-0-444-63292-0.00009-0 [DOI] [Google Scholar]
- Bianki, V. L. (1898). Review of the species of genus Tetraogallus Gray. St. Petersburg, Russia: Museum Emperor's Academy of Sciences. [Google Scholar]
- Brelsford, A. , Mila, B. , & Irwin, D. E. (2011). Hybrid origin of Audubon's warbler. Molecular Ecology, 20, 2380–2389. 10.1111/j.1365-294X.2011.05055.x [DOI] [PubMed] [Google Scholar]
- Capblancq, T. , Després, L. , Rioux, D. , & Mavárez, J. (2015). Hybridization promotes speciation in Coenonympha butterflies. Molecular Ecology, 24, 6209–6222. 10.1111/mec.13479 [DOI] [PubMed] [Google Scholar]
- Caves, J. K. , Sjostrom, D. J. , Mix, H. T. , Winnick, M. J. , & Chamberlain, C. P. (2014). Aridification of central Asia and uplift of the Altai and Hangay Mountains, Mongolia: Stable isotope evidence. American Journal of Science, 314, 1171–1201. 10.2475/08.2014.01 [DOI] [Google Scholar]
- Chen, Y. , An, B. , & Liu, N. (2016). Asymmetrical introgression patterns between rusty‐necklaced partridge (Alectoris magna) and chukar partridge (Alectoris chukar) in China. Integrative Zoology, 11, 403–412. 10.1111/1749-4877.12195 [DOI] [PubMed] [Google Scholar]
- Chih‐chiu, T., (1968). Problem of Quaternary ice‐cover patterns in western China. International Geology Review, 10, 1212–1228. 10.1080/00206816809474988 [DOI] [Google Scholar]
- Coyne, J. A. , & Orr, H. A. (1997). “Patterns of speciation in Drosophila” revisited. Evolution, 51, 295–303. 10.1111/j.1558-5646.1997.tb02412.x [DOI] [PubMed] [Google Scholar]
- Curry, R. R. (1966). Glaciation about 3,000,000 years ago in the Sierra Nevada. Science, 154, 770 10.1126/science.154.3750.770 [DOI] [PubMed] [Google Scholar]
- Dementiev, G. T. , & Gladkov, N. A. (1967). Birds of the Soviet Union (Vol. 4). Jerusalem, Israel: Israel Program for Scientific Translations. [Google Scholar]
- Deng, T. , & Ding, L. (2015). Paleoaltimetry reconstructions of the Tibetan Plateau: Progress and contradictions. National Science Review, 2, 417–437. 10.1093/nsr/nwv062 [DOI] [Google Scholar]
- Dowling, T. E. , & Secor, C. L. (1997). The role of hybridization and introgression in the diversification of animals. Annual Review of Ecology and Systematics, 28, 593–619. 10.1146/annurev.ecolsys.28.1.593 [DOI] [Google Scholar]
- Drummond, A. J. , & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7, 214–214. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward, H. , Burtt, J. R. , & Jann, M. I. (2004). Gloger's rule, feather‐degrading bacteria, and color variation among song sparrows. The Condor, 106, 681–686. 10.1650/7383 [DOI] [Google Scholar]
- Fang, X. (2017). Phased uplift of the Tibetan Plateau. Science and Technology Review, 35, 42–50 (English abstract). [Google Scholar]
- Favre, A. , Päckert, M. , Pauls, S. U. , Jähnig, S. C. , Uhl, D. , Michalak, I. , & Muellnerriehl, A. N. (2015). The role of the uplift of the Qinghai‐Tibetan Plateau for the evolution of Tibetan biotas. Biological Reviews of the Cambridge Philosophical Society, 90, 236–253. 10.1111/brv.12107 [DOI] [PubMed] [Google Scholar]
- Fu, B. , Ninomiya, Y. , & Guo, J. (2010). Slip partitioning in the northeast Pamir‐Tian Shan convergence zone. Tectonophysics, 483, 344–364. 10.1016/j.tecto.2009.11.003 [DOI] [Google Scholar]
- Grant, P. R. , Grant, B. R. , & Deutsch, J. C. (1996). Speciation and hybridization in island birds. Philosophical Transactions of the Royal Society B: Biological Sciences, 351, 765–772. 10.1098/rstb.1996.0071 [DOI] [Google Scholar]
- Hall, T. A. (1999). BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Hewitt, G. (2000). The genetic legacy of the Quaternary ice ages. Nature, 405, 907–913. 10.1038/35016000 [DOI] [PubMed] [Google Scholar]
- Hoskin, C. J. , Higgie, M. , McDonald, K. R. , & Moritz, C. (2005). Reinforcement drives rapid allopatric speciation. Nature, 437, 1353–1356. 10.1038/nature04004 [DOI] [PubMed] [Google Scholar]
- Huang, R. X. , Ma, L. , Shao, H. G. , & Jiang, T. (1990). Preliminary studies on the ecology and biology of the Himalayan snowcock in MT. Tian, Xinjiang. Journal of Xinjiang University, 7, 71–76 (English abstract). [Google Scholar]
- Huang, R. , Mi, E. , & Shao, H. (1992). A new record of birds from China ‐ Altai snowcock. Acta Zootaxonomica Sinica, 17, 501–502 (English abstract). [Google Scholar]
- James, H. F. , Ericson, P. G. P. , Slikas, B. , Lei, F.‐M. , Gill, F. B. , & Olson, S. L. (2003). Pseudopodoces humilis, a misclassified terrestrial tit (Paridae) of the Tibetan Plateau: Evolutionary consequences of shifting adaptive zones. Ibis, 145, 185–202. 10.1046/j.1474-919X.2003.00170.x [DOI] [Google Scholar]
- Jin, Y. , Liu, N. , & Brown, R. P. (2017). The geography and timing of genetic divergence in the lizard Phrynocephalus theobaldi on the Qinghai‐Tibetan plateau. Scientific Reports, 7, 2281 10.1038/s41598-017-02674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, L. , Dolman, G. , Donnellan, S. , Saint, K. M. , Berg, M. L. , & Bennett, A. T. (2008). Where and when does a ring start and end? Testing the ring‐species hypothesis in a species complex of Australian parrots. Proceedings of the Royal Society B: Biological Sciences, 275, 2431–2440. 10.1098/rspb.2008.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- Koslova, E. V. (1952). Avifauna of Tibetan Plateau: Its family ties and history. Proceedings of the Zoology Institute of the Academic Science of USSR, 9, 964–1028. [Google Scholar]
- Leaché, A. D. , & Reeder, T. W. (2002). Molecular Systematics of the Eastern Fence Lizard (Sceloporus undulatus): A comparison of parsimony, likelihood, and bayesian approaches. Systematic Biology, 51, 44–68. 10.1080/106351502753475871 [DOI] [PubMed] [Google Scholar]
- Lei, F. , Qu, Y. , & Song, G. (2014). Species diversification and phylogeographical patterns of birds in response to the uplift of the Qinghai‐Tibet Plateau and Quaternary glaciations. Current Zoology, 60, 149161 10.1093/czoolo/60.2.149 [DOI] [Google Scholar]
- Li, J. , & Fang, X. (1999). Uplift of the Tibetan Plateau and environmental changes. Chinese Science Bulletin, 44, 2117–2124 (English abstract). [Google Scholar]
- Li, J. J. , Fang, X. M. , Pan, B. T. , Zhao, Z. J. , & Song, Y. G. . (2001). Late Cenozoic in tensive uplift Qinghai‐Xizang plateau and its impacts on environments in surrounding area. Quaternary Sciences, 21, 381–391 (English abstract). [Google Scholar]
- Liu, N. F. (1998). Phylogenesis of snowcock genus (Tetraogallus) In Proceedings of the 3rd Cross-Strait Bird Symposium. Beijing, China: China Forestry Publishing House; (English abstract). [Google Scholar]
- Liu, N. (1999). Isolating mechanism between Tibetan snowcock (Tetraogallus tibetanus) and Himalay snowcock (Tetraogallus himalayensis) Conference proceedings of China zoological society (English abstract). [Google Scholar]
- Liu, N. F. , Wen, L. Y. , Huang, Z. H. , & Hou, P. (2006). Introgression hybridization between Alectoris magna and A. chukar in the Liupan Mountain region. Acta Zoologica Sinica, 52, 153–159. [Google Scholar]
- Lv, X. , Xia, L. , Ge, D. , Wu, Y. , & Yang, Q. (2016). Climatic niche conservatism and ecological opportunity in the explosive radiation of arvicoline rodents (Arvicolinae, Cricetidae). Evolution, 70, 1094–1104. 10.1111/evo.12919 [DOI] [PubMed] [Google Scholar]
- MacKinnon, J. , Phillipps, K. , He, F. Q. , & Lu, H. F. (2000). A field guide to the birds of China. Changsha, China: Hunan Education Press; (Chinese). [Google Scholar]
- Miao, Y. , Herrmann, M. , Wu, F. , Yan, X. , & Yang, S. (2012). What controlled Mid‐Late Miocene long‐term aridification in Central Asia? — Global cooling or Tibetan Plateau uplift: A review. Earth‐science Reviews, 112, 155–172. 10.1016/j.earscirev.2012.02.003 [DOI] [Google Scholar]
- Miao, Y. , Meng, Q. , Fang, X. , Yan, X. , Wu, F. , & Song, C. (2011). Origin and development of Artemisia (Asteraceae) in Asia and its implications for the uplift history of the Tibetan Plateau: A review (CPCI‐S). Quaternary International, 236, 3–12. 10.1016/j.quaint.2010.08.014 [DOI] [Google Scholar]
- Mouthereau, F. (2011). Timing of uplift in the Zagros belt/Iranian plateau and accommodation of late Cenozoic Arabia‐Eurasia convergence. Geological Magazine, 148, 726–738. 10.1017/S0016756811000306 [DOI] [Google Scholar]
- Mulch, A. , & Chamberlain, C. P. (2006). Earth science: The rise and growth of Tibet. Nature, 439, 670–671. 10.1038/439670a [DOI] [PubMed] [Google Scholar]
- Nei, M. (1992). Age of the common ancestor of human mitochondrial DNA. Molecular Biology and Evolution, 9, 1176–1178. 10.1093/oxfordjournals.molbev.a040785 [DOI] [PubMed] [Google Scholar]
- Panteleev, A. V. (2002). Specific features of the distribution of some bird species in Northern, Central, and Eastern Asia in the Quaternary. Russ Ornitol Zh, 11, 477–479. [Google Scholar]
- Posada, D. , & Crandall, K. A. (1998). MODELTEST: Testing the model of DNA substitution. Bioinformatics, 14, 817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Potapov, P. L. (1987). Order Galliformes, Birde of USSR: Galliformes‐Gruiformes. Leningrad, Russia: Nauka. [Google Scholar]
- Potapov, P. L. (1992). Adaptation to mountain conditions and evolution in snowcocks (Tetraogallus sp.). Gibier Fauna Snuvage, 9, 647–667. [Google Scholar]
- Pybus, O. G. , & Harvey, P. H. (2000). Testing macro‐evolutionary models using incomplete molecular phylogenies. Proceedings of the Royal Society B: Biological Sciences, 267, 2267–2272. 10.1098/rspb.2000.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2012). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved fromhttps://www.R-project.org/ [Google Scholar]
- Rabosky, D. L. , Donnellan, S. C. , Grundler, M. , & Lovette, I. J. (2014a). Analysis and visualization of complex macroevolutionary dynamics: An example from Australian scincid lizards. Systematic Biology, 63, 610–627. 10.1093/sysbio/syu025 [DOI] [PubMed] [Google Scholar]
- Rabosky, D. L. , Grundler, M. , Anderson, C. , Title, P. , Shi, J. J. , Brown, J. W. , … Larson, J. G. (2014b). BAMMtools: An R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods in Ecology and Evolution, 5, 701–707. 10.1111/2041-210X.12199 [DOI] [Google Scholar]
- Rabosky, D. L. , & Lovette, I. J. (2008). Density‐dependent diversification in North American wood warblers. Proceedings of the Royal Society B: Biological Sciences, 275, 2363–2371. 10.1098/rspb.2008.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. 10.1111/j.2041-210X.2011.00169.x [DOI] [Google Scholar]
- Rooney, A. P. , Honeycutt, R. L. , & Derr, J. N. (2001). Historical population size change of bowhead whales inferred from DNA sequence polymorphism data. Evolution, 55, 1678–1685. 10.1111/j.0014-3820.2001.tb00687.x [DOI] [PubMed] [Google Scholar]
- Sato, M. , & Sato, K. (2012). Maternal inheritance of mitochondrial DNA. Autophagy, 8, 424–425. 10.4161/auto.19243 [DOI] [PubMed] [Google Scholar]
- Schenk, J. J. , Rowe, K. C. , & Steppan, S. J. (2013). Ecological opportunity and incumbency in the diversification of repeated continental colonizations by muroid rodents. Systematic Biology, 62, 837–864. 10.1093/sysbio/syt050 [DOI] [PubMed] [Google Scholar]
- Shen, X. Y. , & Wang, J. J. (1963). Classification, geographical distribution, and ecology of Tetraogallus in China. Journal of Zoology, 5, 67–68 (Chinese). [Google Scholar]
- Shi, Y. F. , Li, J. J. , & Li, B. Y. (1998). Uplift and environmental changes of Qinghai‐Tibetan plateau in the late Cenozoic. Guangzhou, China: Guangdong science and Technology Press; (Chinese). [Google Scholar]
- Steel, M. , & McKenzie, A. (2001). Properties of phylogenetic trees generated by Yule‐type speciation models. Mathematical Biosciences, 170, 91–112. 10.1016/S0025-5564(00)00061-4 [DOI] [PubMed] [Google Scholar]
- Stein, R. W. , Brown, J. W. , & Mooers, A. O. (2015). A molecular genetic time scale demonstrates Cretaceous origins and multiple diversification rate shifts within the order Galliformes (Aves). Molecular Phylogenetics and Evolution, 92, 155–164. 10.1016/j.ympev.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Storch, G. (2004). Late Pleistocene rodent dispersal in the Balkans. Dordrecht, The Netherland: Kluwer Academic Publishers. [Google Scholar]
- Stroud, J. T. , & Losos, J. B. (2016). Ecological opportunity and adaptive radiation. Annual Review of Ecology Evolution & Systematics, 47, 507–532. 10.1146/annurev-ecolsys-121415-032254 [DOI] [Google Scholar]
- Sun, J. , Gong, Z. , Tian, Z. , Jia, Y. , & Windley, B. (2015). Late Miocene stepwise aridification in the Asian interior and the interplay between tectonics and climate. Palaeogeography, Palaeoclimatology, Palaeoecology, 421, 48–59. 10.1016/j.palaeo.2015.01.001 [DOI] [Google Scholar]
- Sun, X. , & Wang, P. (2005). How old is the Asian monsoon system?—Palaeobotanical records from China. Palaeogeography, Palaeoclimatology, Palaeoecology, 222, 181–222. 10.1016/j.palaeo.2005.03.005 [DOI] [Google Scholar]
- Svensson, L. , Mullarney, K. , Zetterstrom, D. , & Grant, P. J. (2009). Collins Bird Guide (2nd ed.). London, UK: HarperCollins Publishers Ltd. [Google Scholar]
- Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. , & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya, G. , Lohman, D. J. , & Meier, R. (2011). SequenceMatrix: Concatenation software for the fast assembly of multi‐gene datasets with character set and codon information. Cladistics, 27, 171–180. 10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Via, S. (2012). Divergence hitchhiking and the spread of genomic isolation during ecological speciation‐with‐gene‐flow. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 451–460. 10.1098/rstb.2011.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Zhao, X. , Liu, Z. , Lippert, P. C. , Graham, S. A. , Coe, R. S. , … Li, Y. (2008). Constraints on the early uplift history of the Tibetan Plateau. Proceedings of the National Academy of Sciences of the United States of America, 105, 4987–4992. 10.1073/pnas.0703595105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Liu, Y. , Liu, Y. , Chang, Y. , Wang, N. , & Zhang, Z. (2017). The role of niche divergence and geographic arrangement in the speciation of Eared Pheasants (Crossoptilon, Hodgson 1938). Molecular Phylogenetics and Evolution, 113, 1–8. 10.1016/j.ympev.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Wang, X. L. , Qu, J. Y. , Liu, N. F. , Bao, X. K. , & Song, S. (2011). Limited gene flow and partial isolation phylogeography of Himalayan Snowcock Tetraogallus himalayensis based on part mitochondrial D‐loop sequences. Current Zoology, 57, 58–767. 10.1093/czoolo/57.6.758 [DOI] [Google Scholar]
- Wang, X. , Sun, D. , Chen, F. , Wang, F. , Li, B. , Popov, S. V. , … Li, Z. (2014). Cenozoic paleo‐environmental evolution of the Pamir‐Tien Shan convergence zone. Journal of Asian Earth Sciences, 80, 84–100. 10.1016/j.jseaes.2013.10.027 [DOI] [Google Scholar]
- Wen, L. , & Liu, N. (2010). Cytochrome b gene based phylogeny and genetic divergence of Tetraophasis of China. Animal Biology, 60, 133–144. 10.1163/157075610X491699 [DOI] [Google Scholar]
- Wu, X. , & Li, Y. (1990). Moraines and environments in Qinghai‐Xizang Plateau. Quaternary Sciences, 10, 146–158 (English abstract). [Google Scholar]
- Xu, Q. , Ji, J. , Sun, D. , & Zhao, L. (2015). Late Cenozoic uplift‐exhumation history of Qinghe‐Fuyun region, Altay, Xinjiang‐Evidence from apatite fission track. Geological Bulletin of China, 34, 834–844 (English abstract). [Google Scholar]
- Yoder, J. B. , Clancey, E. , Des Roches, S. , Eastman, J. M. , Gentry, L. , Godsoe, W. , … Harmon, L. J. (2010). Ecological opportunity and the origin of adaptive radiations. Journal of Evolutionary Biology, 23, 1581–1596. 10.1111/j.1420-9101.2010.02029.x [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Harris, A. J. , Blair, C. , & He, X. (2015). RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Molecular Phylogenetics and Evolution, 87, 46–49. 10.1016/j.ympev.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Zabelin, V. I. (2007). Ecological features of species range formation in the Altai snowcock. Russian Journal of Ecology, 38, 347–352. 10.1134/S1067413607050098 [DOI] [Google Scholar]
- Zheng, B. , & Rutter, N. (1998). On the problem of Quaternary glaciations, and the extent and patterns of Pleistocene ice cover in the Qinghai‐Xizang (Tibet) Plateau. Quaternary International, 45–46, 109–122. 10.1016/S1040-6182(97)00009-8 [DOI] [Google Scholar]
- Zheng, B. , Xu, Q. , & Shen, Y. (2002). The relationship between climate change and Quaternary glacial cycles on the Qinghai‐Tibetan Plateau: Review and speculation. Quaternary International, 97–98, 93–101. 10.1016/S1040-6182(02)00054-X [DOI] [Google Scholar]
- Zheng, G. M. (2002). A checklist on the Classification and distribution of the birds of the world. Beijing, China: Science Press; (Chinese). [Google Scholar]
- Zheng, Z. X. (1952). Vertebrate taxonomy. Beijing, China: China Agriculture Press; (Chinese). [Google Scholar]
- Zheng, Z. X. , Tan, Y. K. , Lu, T. C. , & Tang, C. Z. (1978). Fauna sinica, Aves Galliformes. Beijing, China: Science Press; (Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this review paper have been published elsewhere. Genbank accession number please see the Tables A1 and A2.


