Abstract
The evolution of increased competitive ability (EICA) hypothesis states that, when introduced in a novel habitat, invasive species may reallocate resources from costly quantitative defense mechanisms against enemies to dispersal and reproduction; meanwhile, the refinement of EICA suggests that concentrations of toxins used for qualitative defense against generalist herbivores may increase. Previous studies considered that only few genotypes were introduced to the new range, whereas most studies to test the EICA (or the refinement of EICA) hypotheses did not consider founder effects.
In this study, genetic and phenotypic data of Chromolaena odorata populations sampled across native and introduced ranges were combined to investigate the role of postintroduction evolution in the successful invasion of C. odorata.
Compared with native populations, the introduced populations exhibited lower levels of genetic diversity. Moreover, different founder effects events were interpreted as the main cause of the genetic structure observed in introduced ranges. Three Florida, two Trinidad, and two Puerto Rico populations may have been the sources of the invasive C. odorata in Asia.
When in free of competition conditions, C. odorata plants from introduced ranges perform better than those from native ranges at high nutrient supply but not at low nutrient level. The differences in performance due to competition were significantly greater for C. odorata plants from the native range than those from the introduced range at both nutrient levels. Moreover, the differences in performance by competition were significantly greater for putative source populations than for invasive populations.
Quantities of three types of secondary compounds in leaves of invasive C. odorata populations were significantly higher than those in putative source populations. These results provide more accurate evidence that the competitive ability of the introduced C. odorata is increased with postintroduction evolution.
Keywords: Chromolaena odorata, common garden experiment, EICA, founder effects, invasion
Three Florid, two Trinidad and two Puerto Rico populations may have been the sources of the invasive C. odorata in Asia. C. odorata increased resource investment into growth through post‐introduction evolution. Post‐introduction evolution enhances the competitive ability of C. odorata, and is essential for this species’ establishment and development in introduced ranges.

1. INTRODUCTION
The evolution of increased competitive ability (EICA) hypothesis suggests that invasive plants may reallocate resources from defense mechanisms into growth as a response to release from an enemy in their new range (Blossey & Notzold, 1995). Abela‐Hofbauerová and Münzbergová (2011) found that invasive Cirsium arvense in North America are larger in most size parameters than the native populations of the same species in Europe. However, most studies that evaluated this hypothesis have not differentiated specialist from generalist enemies, and, in introduced ranges, invasive plants may encounter generalist herbivores rather than total enemy release (Cano, Escarre, Vrieling, & Sans, 2009; Muller‐Scharer, Schaffner, & Steinger, 2004). Muller‐Scharer et al. (2004) refined the EICA hypothesis and proposed that, in introduced ranges, exotic plant species may adjust the allocation of resources from high‐cost quantitative defenses (i.e., resisting specialist herbivores) to growth and low‐cost qualitative defenses (i.e., resisting generalist herbivores). Nylund, Pereyra, Wood, Johannesson, and Pavia (2012) found that introduced Fucus vesiculosus increased the dosage of phlorotannins as a defense mechanism against generalist herbivores. The leaf total terpene contents in exotic plant species in Hawaii were 135% higher than that in native species, which facilitate them to resist the generalist herbivores from the introduced range, where specialist herbivores were scarce (Penuelas et al., 2010). Several studies have indicated that plant genotypes from introduced ranges had a more effective types of defenses than genotypes from native ranges (Blair & Wolfe, 2004; Joshi & Vrieling, 2005; Oduor, Kleunen, & Stift, 2017; Puritty, Mayfield, Azcarate, & Cleland, 2018; Turner, Hufbauer, & Rieseberg, 2014). Furthermore, Lin et al. (2015), based on features such as low root–shoot ratio, thin leaves, low leaf cell wall protein contents, and low leaf mass area, proposed that invasive Jacobaea vulgaris had poorer structural defense mechanisms than native genotypes.
Many plant secondary metabolites may act as defense mechanisms against herbivores and have allelopathic effects; if evolutionary mechanisms generate an increase in qualitative defenses against generalist herbivores, the allelopathic effect on indigenous plants may also be strengthened, eventually leading to the increase of the competitive ability of invasive species. Leaf extracts from the invasive Chromolaena odorata in China exerted stronger inhibitory effects on the germination of indigenous plants than the native populations of the same species from Mexico (Qin et al., 2013). Introduced C. odorata had higher resistance to three generalist herbivore species and higher tolerance to simulated herbivory (by shoot removal) than plants from native populations (Liao, Zheng, Lei, & Feng, 2014). Zheng et al. (2015) found that the concentration of odoratin (Eupatorium), a unique compound found in C. odorata with both allelopathic and defensive activities, in the introduced Chromolaena odorata was 2.4 times higher than that from the native range. The introduced population of Taraxacum officinale in the Chilean Andes produced more phenols and anthocyanins as a defensive response to herbivory than the native population in the French Alps (Gonzalez‐Teuber, Quiroz, Concha‐Bloomfield, & Cavieres, 2017).
Most common garden experiments that tested the EICA hypothesis did not include founder effects among the considered factors, which may have led to misleading conclusions, as source populations are only a fraction of the genotypes among native populations (Dlugosch & Parker, 2008). If the invasive populations are introduced from only one or a few native populations with stronger competitiveness, evidence supporting the EICA hypothesis would be found. However, source populations are weakly competitive, evidence contrary to the EICA hypothesis would be found. Both aforementioned situations could result from founder effects rather than postintroduction evolution. To exclude confounding founder effects, the difference between plants from invasive populations and the ones from their source populations should be compared (Williams & Fishman, 2014). For example, Sakata, Yamasaki, Isagi, and Ohgushi (2014) proposed that the features higher resistance, sexual reproduction, and asexual rhizome reproduction present in introduced populations of Solidago altissima resulted from a long history of pressure by Corythucha marmorata rather than from stochastic events such as genetic drift and founder effects.
Chromolaena odorata is a plant species native to North, Central, and South America, but is a noxious invasive perennial herb or subshrub throughout much of Asia, Oceania, and Africa. It was first introduced into India as an ornamental plant in the middle of the 19th century and has now become one of the most invasive species in southern China (Xie, Li, Gregg, & Dianmo, 2001). There are more than 200 arthropod enemies attacking C. odorata in its native range, and a quarter are specialists; however, some generalist herbivores are documented for Chromolaena odorata in invasive ranges, where specialists are absent (Zhang & Feng, 2007).
Although C. odorata has been introduced into Asia for nearly 100 years, the route of the spread of C. odorata throughout Asia is not well known. A study applying three DNA fragments and six pairs of microsatellite markers (SSRs) revealed that C. odorata in Asia originated from Trinidad and Tobago and adjacent areas in the West Indies (Yu, He, Zhao, & Li, 2014). However, Paterson and Zachariades (2013) indicated that the samples from Asia showed an affinity with samples from Trinidad, Florida, and Venezuela. Therefore, the sources of C. odorata in Asia could not be confirmed based on the existing researches (i.e., Paterson & Zachariades, 2013; Yu et al., 2014). Yu et al. (2014) indicated that the genotypes in Asia (introduced range) have strong competitive ability, which may facilitate the successful invasion of C. odorata. Therefore, it is reasonable to test the degree to which adaptation contributed to the higher competitive ability of C. odorata plants by comparing the invasive populations with the putative source populations.
During the invasion process, C. odorata strengthens its defense mechanisms against generalist herbivores (Liao et al., 2014) and enhances allelopathic effects (Qin et al., 2013). However, Liao et al. (2014) and Qin et al. (2013) did not consider the source of C. odorata and thus did not exclude founder effects. It is therefore necessary to confirm the source of C. odorata in native ranges and then compare invasive populations and source populations.
The hypotheses in this study are 1) the genetic diversity of C. odorata in native ranges is higher than that in introduced ranges; and 2) selective pressures in the introduced range cause an increase in the plant's growth rate and secondary compound production, which in turn increase its competitive ability. Evolution is predicted to increase the production of the secondary compounds active in defense and allelopathy, and to enhance growth traits.
2. MATERIALS AND METHODS
2.1. Plant materials
Chromolaena odorata weeds were collected in the species’ native regions in North America and the Caribbean and in the invasive ranges in Asia (Table S1). From each place (defined as a population), 10 plant seeds at least 20‐m intervals between any two plants were randomly selected and collected. A total of 10 and 12 geographical populations were collected from the invasive and native range, respectively. The seeds of various groups (populations) were seeded in the nursery bed.
2.2. Genetic analyses
Total genomic DNA was extracted from leaf tissues of C. odorata following the modified cetyltrimethylammonium bromide (CTAB) method described in Yu and Li (2011). In this study, 11 pairs of SSR primers were used to investigate the genetic diversity of 218 individuals from 10 introduced populations and 12 native populations (Table 1). The 11 PCR primers used are described in Table S1. The 20 μL PCR volume contained 1.5 μL of 10 × Buffer I, 1.0 µl of each primer (5 µmol/L), 0.3 µl of dNTP (10 mmol/L), 2.0 mmol/L of MgCl2, 0.1 µl of Taq polymerase (5 U/µL), and 15 ng of genomic DNA. PCR amplification was conducted using a Gene Amp 9,600 PCR system (ABI, USA). Amplification conditions were 10 min of denaturation at 95°C, followed by 35 to 40 cycles of 40 s at 94°C, 45 s at locus specific annealing temperature (Table S2), and 45 s at 72°C; and then a final extension step of 10 min at 72°C. Each forward primer was labeled with one of three fluorescent dyes (FAM, HEX, and TAMRA) for polymorphism analysis on a 3730xl DNA analyzer (ABI, USA) with internal lane Rox‐500 standards (Beijing Microread Genetics Co., Ltd, Beijing, China). PCR products with different sizes were multiplexed for detecting, and each mixture contained products of two to three primers labeled with different fluorescent dyes. The hierarchical partitioning of genetic variation within and among populations and regions was assessed by analysis of molecular variance (AMOVA; Armstrong & De Lange, 2005) based on the pairwise squared Euclidean distance among molecular loci using GenAlEx version 6.2 (Peakall and Smouse, Australian National University). Genetic relationships among native and invasive individuals were determined by constructing an UPGMA dendrogram using unweighted pairwise genetic distance matrices in POPGENE version 1.31 (Yeh & Boyle, 1997).
Table 1.
Information on sample populations of Chromolaena odorata
| Sample code | Country/region | G.P.S. coordinates | Elevation |
|---|---|---|---|
| Invasive populations | |||
| JD | JingDong, Yunnan, China | 24 º17'N 100 º50'E | 1263 |
| SM | SiMao, Yunnan, China | 22º46'N 100º56'E | 1380 |
| ML | MengLun, Yunnan, China | 21º56'N 101º15'E | 544 |
| SY | SanYa, Hainan, China | 18º19'N 109 º12'E | 23 |
| WX | Vientiane, Laos | 17º58'N102º37'E | 170 |
| BK | Central Thailand | 14º25'N101º23'E | 739 |
| YNS | Southern Vietnam | 11º20'N107º24'E | 125 |
| PH | Philippines | 8 º 10'N124 º10'E | 107 |
| SL | Sri Lanka | 7º11'N80º25'E | 451 |
| MY | Malaysia | 2º22'N102º21'E | 50 |
| Native populations | |||
| MAR | Florida, USA | 27º06'N80º15'W | 1 ~ 5 |
| BRO | Florida, USA | 26º08'N80º06'W | 1 ~ 5 |
| FAK | Florida, USA | 25º52'N80º29'W | 1 ~ 5 |
| MD | Florida, USA | 25º38'N80º20'W | 1 ~ 5 |
| CDV | Mexico | 23º40'N99º11'W | 600 |
| CUB | Cuba | 22º45'N82º50'W | 565 |
| MIC | Mexico | 18º51'N103º37'W | 950 |
| PM | Puerto Rico | 18º12'N67º06'W | 103 |
| PP | Puerto Rico | 18º12'N67º06'W | 103 |
| COY | Mexico | 16º44'N93º09'W | 640 |
| T2 | Felicity, Trinidad & Tobago | 10º31'N61º25'W | 10 |
| T1 | Mamoral, Trinidad & Tobago | 10º27'N61º17'W | 63 |
2.3. Common garden pot experiment in China
A common garden experiment was conducted in China (introduced range) to explore the biogeographical differences in performance (biomass and height) of C. odorata between invasive and native populations (considering their source populations), and to test whether adaptive evolution contributes to the competitive ability of the invasive C. odorata by comparing the invasive and putative source populations. The common garden experiment located at the Xishuangbanna Tropical Botanical Garden (21°560′N, 101°150′E; 570 m elevation) of the Chinese Academy of Sciences, located in Mengla County, Yunnan Province, China. Annual average temperature is 21.7°C; mean temperatures of the hottest (July) and coolest (January) months are 25.3°C and 15.6°C, respectively. Average annual precipitation is 1557 mm with a dry period from November to April.
In December 2012, the above‐ground parts of C. odorata were cut to allow vegetative propagation by sprouting. In March 2013, sprouts of same sizes from 218 individual plants were selected and placed in sand beds. When the seedlings were 7 cm tall, similar‐sized vigorous seedlings were transplanted into 15 dm3 pots; ten individuals from each C. odorata population were planted one per pot. Twelve individuals of each of the ten invasive populations of C. odorata were transplanted into pots with each of the twelve native species, totaling 120 pots. A few EICA studies have performed studies of intraspecific competition (Felker‐Quinn, Schweitzer, & Bailey, 2013; Feng et al., 2009, 2011; Parker et al., 2013; Qin et al., 2013), which is important because intraspecific competition eliminates the potential confounding effects of using a heterospecific as a “phytometer.” Pots contained a mixture of 60% forest topsoil and 40% river sand. Topsoil was used as a natural supply of macro‐ and micronutrients, while river sand provided adequate drainage and facilitated the harvesting of fine roots (Liao, Zhang, Barclay, & Feng, 2013). All seedlings were initially grown in shade with 50% irradiance for 4 weeks to facilitate initial survival; after this period, they were grown in full sun.
Two types of nutrient treatments were set: low nutrient with one‐time fertilizer (in August 2013; fertilizer with 0.1 g N + 0.1 g P + 0.1 g K/Kg) and high nutrient with three‐time fertilizer (in June, July, and August 2013; fertilizer with 0.1 g N + 0.1 g P + 0.1 g K/Kg). The high and low nutrient treatments were harvested in September and December 2013, respectively. The entire plants (including roots) were oven‐dried at 60°C for 72 hr and weighed.
To evaluate relative competition intensity, the competitive response of each population was measured as the percentage change in performance (i.e., biomass) when grown with competition, and the formula was described by Weigelt and Jolliffe (2003):
(P comp –P single)/P single × 100,where P single is plant performance when grown without competition and P comp is plant performance when grown with competition. The competitive effect of each population was measured as the percentage change in the performance of its competitor. In this study, P single was the average of all replicates per population per treatment and P comp was the value of the individual replicate.
2.4. Secondary metabolite extraction and isolation
To detect the increase in production of qualitative defense factors, three types of secondary compounds were extracted and verified: high in defense capacity and allelopathy (4`,5,6,7‐tetramethoxyflavone and Acutellerin‐4`,6,7‐trimethy ether); high in defense capacity but low in allelopathy (Isosakuranetin and 3,5‐dihydroxy‐7,4`‐dimethoxyflavone); and low in defense capacity but high in allelopathy (dihydrokaempferol‐3‐methoxy ether and Kaempferide‐4`‐methoxy ether).
In April 2013, newly mature leaves of C. odorata were collected from five plants of each of the 12 native and 10 introduced populations (Table S1) grown in the common garden at the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences. All leaves were individually dried under room temperature and ground; then, 500 mg of powder was extracted using 50 ml of methanol for 24 hr. Above six chemicals were measured following the assay method described in Zheng et al. (2015), using ACQUITY ultra‐performance liquid chromatography (Waters Corp., Miller, MA, USA) equipped with a BECH C18 column (2.1 mm × 50 mm, 1.7 μm; Waters Corporation). The mobile phase included (A) pure water and (B) acetonitrile. The concentration of eluent B was changed from 10% to 60% by a linear gradient in 10 min. The flow rate of the eluent was 0.6 ml/min, the injection volume of the extract was 5 μL, and the column oven was set at 25°C. Conditions for mass spectrometric detection were as follows: Electrospray ionization (ESI) was performed in positive ion mode at 1.8 kV, ion source temperature was 350°C, solvent temperature was 550°C, sheath gas flow rate was 800 L/h, and auxiliary gas flow rate was 150 L/h. All data for 6 chemicals were collected using multiple reaction monitoring (Table S3). All measurements were obtained in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
2.5. Statistical analysis
Two‐way nested ANOVAs, with range and population nested within range as fixed factors, were used to estimate the significance of differences between plants from invasive and native populations of C. odorata at each nutrient concentration. The variations between plants from invasive and putative source populations of C. odorata at each nutrient concentration were determined using two‐way nested ANOVAs, with range and population nested within range as fixed factors. All analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Genetic diversity and structure
Genetic diversity in the invasive populations was significantly lower than in the native ones. Number of alleles, expected heterozygosity, and Shannon's information index (I) in native populations were, respectively, 1.8 (F = 13.15, p < .01), 1.1 (F = 7.66, p < .05), and 1.9 (F = 12.65, p < .01) times higher than those in invasive populations (Table 2).
Table 2.
Diversity measures for polymorphic loci in 218 individuals of Chromolaena odorata
| Locus |
Native populations (n = 118) |
I | Invasive populations (n = 100) | |||||
|---|---|---|---|---|---|---|---|---|
| A | Ho | He | A | Ho | He | I | ||
| co77 | 5 | 0.03390 | 0.03368 | 0.1094 | 2 | 0.07000 | 0.06789 | 0.1517 |
| co227 | 11 | 0.76316 | 0.77394 | 1.8159 | 4 | 1.00000 | 0.52705 | 0.8060 |
| co250 | 11 | 0.61864 | 0.61576 | 1.2749 | 5 | 0.57000 | 0.43201 | 0.7186 |
| co26 | 14 | 0.24138 | 0.53986 | 1.2702 | 5 | 0.04000 | 0.03970 | 0.1258 |
| co115 | 12 | 0.30508 | 0.54064 | 1.1823 | 3 | 0.03000 | 0.02980 | 0.0874 |
| co195 | 4 | 0.55932 | 0.43747 | 0.7771 | 2 | 0.97938 | 0.50238 | 0.6929 |
| co65 | 19 | 0.44860 | 0.74560 | 2.0171 | 2 | 0.01000 | 0.01000 | 0.0315 |
| co15 | 4 | 0.63559 | 0.45283 | 0.7359 | 2 | 0.94000 | 0.50070 | 0.6913 |
| co56 | 4 | 0.31356 | 0.29005 | 0.5771 | 2 | 0.02041 | 0.02030 | 0.0569 |
| co189 | 19 | 0.61864 | 0.86812 | 2.3040 | 8 | 0.33000 | 0.31688 | 0.7041 |
| co50 | 10 | 0.73585 | 0.84400 | 1.9811 | 6 | 0.96939 | 0.53213 | 0.8490 |
| Mean | 10.273 | 0.47943 | 0.55836 | 1.2768 | 3.727 | 0.45083 | 0.27080 | 0.4468 |
| St. Dev | 5.623 | 0.23018 | 0.25220 | 0.6923 | 2.054 | 0.44592 | 0.23494 | 0.3457 |
A, number of alleles; He, expected heterozygosity; Ho, observed heterozygosity; and I, Shannon's information index.
These 22 populations consist of two separate groups. In one group, all invasive populations and three Florida populations (MD, MAR, and BRO) clustered together; in addition, two Trinidad populations (T1 and T2) and two Puerto Rico (PP and PM) populations were also close to them (Figure 1). The second group was formed by one Florida population (FAK), three Mexico populations (COY, CDV, and MIC), and the Cuba population (CUB).
Figure 1.
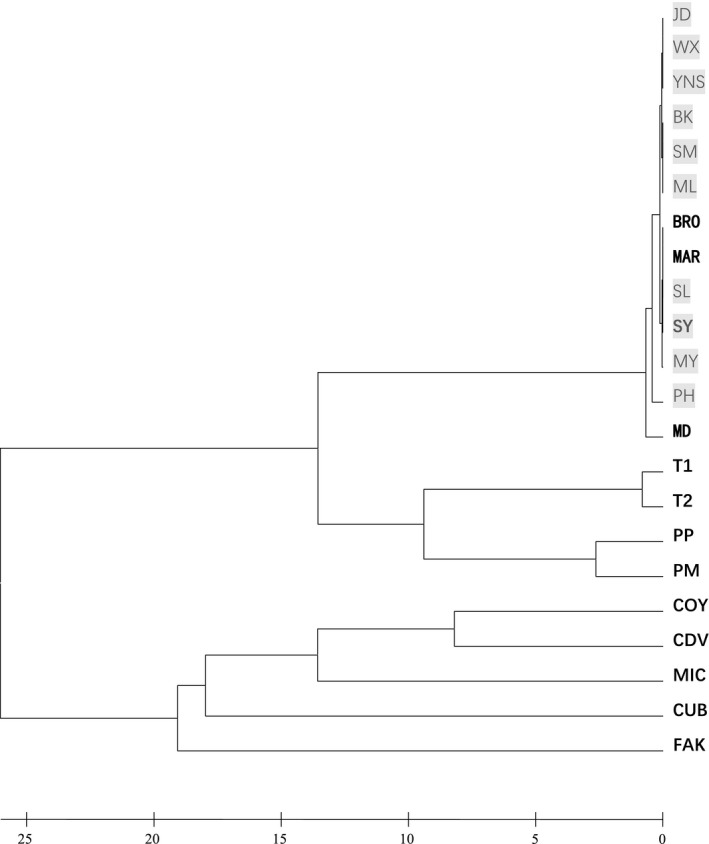
Cluster UPGMA of Chromolaena odorata plants from native and invasive ranges based on the data of microsatellite markers (SSR). JD: JingDong, Yunnan, China; WX: Vientiane, Laos; YNS: Southern Vietnam; BK: Central Thailand; SM: SiMao, Yunnan, China; ML: MengLun, Yunnan, China; BRO: Florida, USA; MAR: Florida, USA; SL: Sri Lanka; SY: SanYa, Hainan, China; MY: Malaysia; PH: Philippines; MD: Florida, USA; T1: Mamoral, Trinidad & Tobago; T2: Felicity, Trinidad & Tobago; PP: Puerto Rico; PM: Puerto Rico; COY: Mexico; CDV: Mexico; MIC: Mexico; CUB: Cuba; FAK: Florida, USA
3.2. Common garden pot experiment
When grown without competition, total biomass and height (Figures 2a and 3a) of plants from the invasive range were larger than those of plants from the native range at high nutrient supply, but not at low nutrient level (Figures 2b and 3b). The relative competition intensity in total biomass and height of plants from the native range were significantly lower than those from the nonnative range (Figures 2c,d and 3c,d).
Figure 2.

Comparisons between biomass of Chromolaena odorata plants from native and invasive populations, and invasive and putative source populations under high and low nutrient levels Populations grown in monoculture (a, b, e, f) and the respective changes influenced by competition (c, d, g, h). Panels a, c, e, and g represent plants grown at high nutrient level; panels b, d, f, and h represent plants grown at low nutrient level. Panels a, b, c, and d represent comparisons between invasive (n = 10) and native (n = 12) regions; panels e, f, g, and h represent comparisons between invasive (n = 10) and putative source (n = 7) regions. Striped columns represent Florida species. Narrow bars indicate mean + SE for each population (n = 10); central thick bars indicate mean + SE for each region (n = 10 for invasive; n = 12 for native). Significant differences between ranges according to one‐way nested ANOVAs: * = p < .05; ** = p < .01; *** = p < .001
Figure 3.

Comparisons between plant heights of Chromolaena odorata plants from native and invasive populations, and invasive and putative source populations. Populations grown in monoculture (a, b, e, f) and the respective changes caused by competition (c, d, g, h). Panels a, c, e, and g represent plants grown at high nutrient level, and panels b, d, f, and h represent plants grown at low nutrient level. Panels a, b, c, and d represent comparisons between invasive (n = 10) and native (n = 12) regions; panels e, f, g, and h represent comparisons between invasive (n = 10) and putative source (n = 7 for putative source) regions. Striped columns represent Florida species. Narrow bars indicate mean + SE for each population (n = 10); central thick bars indicate mean + SE for each region (n = 10 for invasive; n = 12 for native). Significant differences between ranges according to one‐way nested ANOVAs: * = p < .05
Regarding the comparisons conducted between C. odorata populations from invasive ranges and their putative source populations, when grown without competition, no significant differences in total biomass and plant height were observed between the two ranges at both nutrient levels (Figures 2e,f and 3e,f). Competition‐driven decreases in total biomass and plant height were significantly greater for C. odorata plants from the putative source populations than for those from the invasive ranges (Figures 2g,h and 3g,h).
3.3. Secondary metabolites differentiation
Concentrations of the three extracted and verified types of secondary compounds (4`,5,6,7‐tetramethoxyflavone and Acutellerin‐4`,6,7‐trimethy ether; Isosakuranetin and 3,5‐dihydroxy‐7,4`‐dimethoxyflavone; and dihydrokaempferol‐3‐methoxy ether and Kaempferide‐4`‐methoxy ether) in leaves of C. odorata plants in nonnative ranges were consistently and significantly higher than that in putative source populations (Figure 4).
Figure 4.

Comparison between secondary metabolite productions of Chromolaena odorata plants from invasive and putative source populations. Panels a, b, c, d, e, and f represent comparisons between introduced (n = 10) and putative source (n = 7) ranges. Striped columns represent Florida species. Narrow bars indicate mean + SE for each population (n = 10); central thick bars indicate mean + SE for each region (n = 10 for invasive; n = 7 for putative source). Significant differences between ranges according to one‐way nested ANOVAs: *** = p < .001
4. DISCUSSION
The level of genetic diversity of C. odorata plants throughout Asia is significantly lower than that in native populations; similar results were reported by Ye, Mu, Cao, and Ge (2004) and Yu et al. (2014). In introduced ranges, populations of an invader often originate from only few individuals from the native range, and the invasion into a new territory is associated with frequent founder effects, which potentially lead to a decrease in population‐level genetic diversity (Sakai et al., 2001; Tsutsui, Suarez, Holway, & Case, 2000; Ye et al., 2004). Williams and Fishman (2014) proposed that the phenotypic divergence between introduced and native‐range populations of Cynoglossum officinale was mainly caused by founder effects. It was believed that small founding sizes reduced genetic variation and fitness but did not prevent adaptation if the founders originated from genetically diverse populations (Szucs, Melbourne, Tuff, Weiss‐Lehman, & Hufbauer, 2017).
In this study, we found that total biomass and height of plants from the invasive range were larger than that from the native range at high nutrient supply, but not at low nutrient level. A similar trend was proposed for the invasive plant Poa annua, which exerted a competitive effect on the native plant Deschampsia, but only at high N availability (Cavieres, Sanhueza, Torres‐Mellado, & Casanova‐Katny, 2018). Liu, Zhang, van Kleunen (2018) and Witkowski (1991) found that the increase in biomass in response to nutrient addition for invasive species is higher than for noninvasive species. At low nutrient levels, soil nutrient is a limiting factor for plant growth and may offset the competitive advantage of invasive species. Competition‐driven decreases in total biomass and plant height were significantly greater for C. odorata plants from the putative source populations than for those from the invasive ranges, indicating that evolution actually occurred during the invasion process of C. odorata.
Invasive populations of C. odorata are not completely released from enemies. There are more than 200 herbivores in native ranges of C. odorata, and 25% of them are specialists (Zhang & Feng, 2007), whereas in the species’ invasive range in China, few generalists and no specialists on C. odorata have been found (Xu, Xiang, Chen, & Peng, 2011). Evolution occurred in C. odorata plants by increasing biomass, while it also increased the secondary chemical production in response to generalists in introduced ranges. Previous studies also reported the production of higher amounts of odoratin (Eupatorium; considered as qualitative defensive compounds) in introduced C. odorata than in native C. odorata (Zheng et al., 2015). Moreover, in the plant species Triadica sebifera, trading off chemical defenses production occurred as a response to a coevolution with novel natural enemies in introduced ranges; this contributed to its successful invasion by enhancing competitive ability (Wang et al., 2012). Consistent with our result, Ridenour, Vivanco, Feng, Horiuchi, and Callaway (2008) proposed that “evolution occurs at increasing competitive ability and defense traits of Centaurea maculosa in introduced range North American.”
The secondary chemical productions quantified in this study were all flavonoids. Flavonoids are beneficial for the plant itself as physiologically active compounds, stress protecting agents, attractants, or feeding deterrents and, in general, they play a significant role in plant resistance. Acutellerin‐4`,6,7‐trimethy ether and 4`,5,6,7‐tetramethoxyflavone have defense capacity and allelopathic effect, and the concentration of these compounds in introduced C. odorata was significantly higher than that in putative source populations. These results suggested that evolution indeed occurred in increasing production of qualitative defensive compounds in the process of invasion of C. odorata. Zheng et al. (2015) considered odoratin (Eupatorium) as an important qualitative defensive compound that contributes to the successful invasion of C. odorata. Invasive species could enhance their competitive ability through generating many types of compounds which may only have defending herbivores ability or allelopathic effect. In our study, the other two types of extracted compounds—with high defending capacity but low allelopathy (Isosakuranetin and 3,5‐dihydroxy‐7,4`‐dimethoxyflavone) and low defending capacity but high allelopathy (dihydrokaempferol‐3‐methoxy ether and Kaempferide‐4`‐methoxy ether)—also contributed to the plant's strong competition ability.
Although some studies attribute the invasion success of exotic species to founder effects or biased introduction (Williams & Fishman, 2014; Yu et al., 2014), evolution in competitive plant traits indeed exists in the process of invasion (Felker‐Quinn et al., 2013). Our results indicated that C. odorata increased resource investment into growth through postintroduction evolution, providing more convincing evidence for the EICA hypothesis than for the use of biogeographical comparisons of invasive plants without considering their source populations. Innate competitive advantages may have contributed to the successful introduction of C. odorata in Asia (Qin et al., 2013), but postintroduction evolution is also essential for the species’ establishment and expanding in introduced ranges.
AUTHORS’ CONTRIBUTIONS
This project was conceived by WTL under the supervision of YLF. YLZ, YBL, YPL, and ZYL collected the plant seeds; WTL and LKZ completed the experiments and analyzed the data. All of the authors contributed to the writing of the manuscript and approved this manuscript for submission.
Supporting information
ACKNOWLEDGMENTS
This study was funded by projects of the National Key Research and Development Program (2017YFC1200101, 2016YFC1200601), the National Natural Science Foundation of China (31670546), and the CAS 135 program (2017XTBG‐F01).
Li W, Zheng Y, Zhang L, et al. Postintroduction evolution contributes to the successful invasion of Chromolaena odorata . Ecol Evol. 2020;10:1252–1263. 10.1002/ece3.5979
Contributor Information
Yulong Zheng, Email: zhengyl@xtbg.org.cn.
Yulong Feng, Email: yl_feng@tom.com.
DATA AVAILABILITY STATEMENT
The data generated during this study are available from Weitao Li on reasonable request.
REFERENCES
- Abela‐Hofbauerová, I. , & Münzbergová, Z. (2011). Increased performance of Cirsium arvense from the invasive range. Flora ‐ Morphology, Distribution, Functional Ecology of Plants, 206, 1012–1019. 10.1016/j.flora.2011.07.007 [DOI] [Google Scholar]
- Armstrong, T. T. J. , & De Lange, P. J. (2005). Conservation genetics of Hebe speciosa (Plantaginaceae) an endangered New Zealand shrub. Botanical Journal of the Linnean Society, 149, 229–239. 10.1111/j.1095-8339.2005.00437.x [DOI] [Google Scholar]
- Blair, A. C. , & Wolfe, L. M. (2004). The evolution of an invasive plant: An experimental study with Silene latifolia. Ecology, 85, 3035–3042. 10.1890/04-0341 [DOI] [Google Scholar]
- Blossey, B. , & Notzold, R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants – A hypothesis. Journal of Ecology, 83, 887–889. 10.2307/2261425. [DOI] [Google Scholar]
- Cano, L. , Escarre, J. , Vrieling, K. , & Sans, F. X. (2009). Palatability to a generalist herbivore, defence and growth of invasive and native Senecio species: Testing the evolution of increased competitive ability hypothesis. Oecologia, 159, 95–106. 10.1007/s00442-008-1182-z [DOI] [PubMed] [Google Scholar]
- Cavieres, L. A. , Sanhueza, A. K. , Torres‐Mellado, G. , & Casanova‐Katny, A. (2018). Competition between native Antarctic vascular plants and invasive Poa annua changes with temperature and soil nitrogen availability. Biological Invasions, 20, 1597–1610. 10.1007/s10530-017-1650-7 [DOI] [Google Scholar]
- Dlugosch, K. M. , & Parker, I. M. (2008). Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology, 17, 431–449. 10.1111/j.1365-294X.2007.03538.x [DOI] [PubMed] [Google Scholar]
- Felker‐Quinn, E. , Schweitzer, J. A. , & Bailey, J. K. (2013). Meta‐analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecology and Evolution, 3, 739–751. 10.1002/ece3.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y.‐L. , Lei, Y.‐B. , Wang, R.‐F. , Callaway, R. M. , Valiente‐Banuet, A. , Inderjit, L. Y. P. , … Zheng, Y.‐L. (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106, 1853–1856. 10.1073/pnas.0808434106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. L. , Li, Y. P. , Wang, R. F. , Callaway, R. M. , Valiente‐Banuet, A. , & Inderjit, L. Y. P. (2011). A quicker return energy‐use strategy by populations of a subtropical invader in the non‐native range: A potential mechanism for the evolution of increased competitive ability. Journal of Ecology, 99, 1116–1123. 10.1111/j.1365-2745.2011.01843.x [DOI] [Google Scholar]
- Gonzalez‐Teuber, M. , Quiroz, C. , Concha‐Bloomfield, I. , & Cavieres, L. (2017). Enhanced fitness and greater herbivore resistance: Implications for dandelion invasion in an alpine habitat. Biological Invasions, 19, 647–653. 10.1007/s10530-016-1309-9 [DOI] [Google Scholar]
- Joshi, J. , & Vrieling, K. (2005). The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecology Letters, 8, 704–714. 10.1111/j.1461-0248.2005.00769.x [DOI] [Google Scholar]
- Liao, Z. Y. , Zhang, R. , Barclay, G. F. , & Feng, Y. L. (2013). Differences in competitive ability between plants from nonnative and native populations of a tropical invader relates to adaptive responses in abiotic and biotic environments. PLoS ONE, 8, e71767 10.1371/journal.pone.0071767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, Z. Y. , Zheng, Y. L. , Lei, Y. B. , & Feng, Y. L. (2014). Evolutionary increases in defense during a biological invasion. Oecologia, 174, 1205–1214. 10.1007/s00442-013-2852-z [DOI] [PubMed] [Google Scholar]
- Lin, T. T. , Doorduin, L. , Temme, A. , Pons, T. L. , Lamers, G. E. M. , Anten, N. P. R. , & Vrieling, K. (2015). Enemies lost: Parallel evolution in structural defense and tolerance to herbivory of invasive Jacobaea vulgaris . Biological Invasions, 17, 2339–2355. 10.1007/s10530-015-0879-2 [DOI] [Google Scholar]
- Liu, Y. J. , Zhang, X. Q. , & van Kleunen, M. (2018). Increases and fluctuations in nutrient availability do not promote dominance of alien plants in synthetic communities of common natives. Functional Ecology, 32, 2594–2604. [Google Scholar]
- Muller‐Scharer, H. , Schaffner, U. , & Steinger, T. (2004). Evolution in invasive plants: Implications for biological control. Trends in Ecology & Evolution, 19, 417–422. 10.1016/j.tree.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Nylund, G. M. , Pereyra, R. T. , Wood, H. L. , Johannesson, K. , & Pavia, H. (2012). Increased resistance towards generalist herbivory in the new range of a habitat‐forming seaweed. Ecosphere, 3(12), e125 10.1890/Es12-00203.1 [DOI] [Google Scholar]
- Oduor, A. M. O. , van Kleunen, M. , & Stift, M. (2017). In the presence of specialist root and shoot herbivory, invasive‐range Brassica nigra populations have stronger competitive effects than native‐range populations. Journal of Ecology, 105, 1679–1686. 10.1111/1365-2745.12779 [DOI] [Google Scholar]
- Parker, J. D. , Torchin, M. E. , Hufbauer, R. A. , Lemoine, N. P. , Alba, C. , Blumenthal, D. M. , … Wolfe, L. M. (2013). Do invasive species perform better in their new ranges? Ecology, 94, 985–994. 10.1890/12-1810.1 [DOI] [PubMed] [Google Scholar]
- Paterson, I. D. , & Zachariades, C. (2013). ISSRs indicate that Chromolaena odorata invading southern Africa originates in Jamaica or Cuba. Biological Control, 66, 132–139. 10.1016/j.biocontrol.2013.04.005 [DOI] [Google Scholar]
- Penuelas, J. , Sardans, J. , Llusia, J. , Owen, S. M. , Silva, J. , & Niinemets, U. (2010). Higher allocation to low cost chemical defenses in invasive species of Hawaii. Journal of Chemical Ecology, 36, 1255–1270. 10.1007/s10886-010-9862-7 [DOI] [PubMed] [Google Scholar]
- Puritty, C. E. , Mayfield, M. M. , Azcarate, F. M. , & Cleland, E. E. (2018). Different traits predict competitive effect versus response by Bromus madritensis in its native and invaded ranges. Biological Invasions, 20, 2553–2565. 10.1007/s10530-018-1719-y [DOI] [Google Scholar]
- Qin, R. M. , Zheng, Y. L. , Valiente‐Banuet, A. , Callaway, R. M. , Barclay, G. F. , Pereyra, C. S. , & Feng, Y. L. (2013). The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytologist, 197, 979–988. 10.1111/Nph.12071 [DOI] [PubMed] [Google Scholar]
- Ridenour, W. M. , Vivanco, J. M. , Feng, Y. L. , Horiuchi, J. , & Callaway, R. M. (2008). No evidence for trade‐offs: Centaurea plants from America are better competitors and defenders. Ecological Monographs, 78, 369–386. 10.1890/06-1926.1 [DOI] [Google Scholar]
- Sakai, A. K. , Allendorf, F. W. , Holt, J. S. , Lodge, D. M. , Molofsky, J. , With, K. A. , … Weller, S. G. (2001). The population biology of invasive species. Annual Review of Ecology and Systematics, 32, 305–332. 10.1146/annurev.ecolsys.32.081501.114037 [DOI] [Google Scholar]
- Sakata, Y. , Yamasaki, M. , Isagi, Y. , & Ohgushi, T. (2014). An exotic herbivorous insect drives the evolution of resistance in the exotic perennial herb Solidago altissima . Ecology, 95, 2569–2578. 10.1890/13-1455.1 [DOI] [Google Scholar]
- Szucs, M. , Melbourne, B. A. , Tuff, T. , Weiss‐Lehman, C. , & Hufbauer, R. A. (2017). Genetic and demographic founder effects have long‐term fitness consequences for colonising populations. Ecology Letters, 20, 436–444. 10.1111/ele.12743 [DOI] [PubMed] [Google Scholar]
- Tsutsui, N. D. , Suarez, A. V. , Holway, D. A. , & Case, T. J. (2000). Reduced genetic variation and the success of an invasive species. Proceedings of the National Academy of Sciences of the United States of America, 97, 5948–5953. 10.1073/pnas.100110397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, K. G. , Hufbauer, R. A. , & Rieseberg, L. H. (2014). Rapid evolution of an invasive weed. New Phytologist, 202, 309–321. 10.1111/nph.12634 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Siemann, E. , Wheeler, G. S. , Zhu, L. , Gu, X. , & Ding, J. Q. (2012). Genetic variation in anti‐herbivore chemical defences in an invasive plant. Journal of Ecology, 100, 894–904. 10.1111/j.1365-2745.2012.01980.x [DOI] [Google Scholar]
- Weigelt, A. , & Jolliffe, P. (2003). Indices of plant competition. Journal of Ecology, 91, 707–720. 10.1046/j.1365-2745.2003.00805.x [DOI] [Google Scholar]
- Williams, J. L. , & Fishman, L. (2014). Genetic evidence for founder effects in the introduced range of houndstongue (Cynoglossum officinale). Biological Invasions, 16, 205–216. 10.1007/s10530-013-0514-z [DOI] [Google Scholar]
- Witkowski, E. T. F. (1991). Growth and competition between seedlings of Protea Repens (L) L and the alien invasive, Acacia Saligna (Labill) Wendl in relation to nutrient availability. Functional Ecology, 5, 101–110. 10.2307/2389560 [DOI] [Google Scholar]
- Xie, Y. , Li, Z. Y. , Gregg, W. P. , & Dianmo, L. (2001). Invasive species in China – An overview. Biodiversity and Conservation, 10, 1317–1341. 10.1023/A:1016695609745 [DOI] [Google Scholar]
- Xu, J. C. , Xiang, C. L. , Chen, L. , & Peng, H. (2011). Orthezia quadrua (Homoptera: Ortheziidae): A natural enemy of Ageratina adenophora and Chromolaena odorata . Journal of Yunnan Agricultural University, 26, 577–579. [Google Scholar]
- Ye, W. H. , Mu, H. P. , Cao, H. L. , & Ge, X. J. (2004). Genetic structure of the invasive Chromolaena odorata in China. Weed Research, 44, 129–135. 10.1111/j.1365-3180.2004.00381.x [DOI] [Google Scholar]
- Yeh, F. C. , & Boyle, T. J. B. (1997). Population genetic analysis of co‐dominant and dominant marker and quantitative traits. Belgian Journal of Botany, 130, 129–157. [Google Scholar]
- Yu, X. Q. , He, T. H. , Zhao, J. L. , & Li, Q. M. (2014). Invasion genetics of Chromolaena odorata (Asteraceae): Extremely low diversity across Asia. Biological Invasions, 16, 2351–2366. 10.1007/s10530-014-0669-2 [DOI] [Google Scholar]
- Yu, X. Q. , & Li, Q. M. (2011). Isolation and characterization of microsatellite markers for a worldwide invasive weed, Chromolaena Odorata (Asteraceae). American Journal of Botany, 98, E259–E261. 10.3732/Ajb.1100169 [DOI] [PubMed] [Google Scholar]
- Zhang, L. H. , & Feng, Y. L. (2007). Potential biological control agents of Chromolaena odorata . Chinese Journal of Biological Control, 23, 83–88. [Google Scholar]
- Zheng, Y. L. , Feng, Y. L. , Zhang, L. K. , Callaway, R. M. , Valiente‐Banuet, A. , Luo, D. Q. , … Silva‐Pereyra, C. (2015). Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytologist, 205, 1350–1359. 10.1111/nph.13135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during this study are available from Weitao Li on reasonable request.


