Abstract
The RNA helicase bad response to refrigeration 2 homolog (BRR2) is required for the activation of the spliceosome before the first catalytic step of RNA splicing. BRR2 represents a distinct subgroup of Ski2-like nucleic acid helicases whose members comprise tandem helicase cassettes. Only the N-terminal cassette of BRR2 is an active ATPase and can unwind substrate RNAs. The C-terminal cassette represents a pseudoenzyme that can stimulate RNA-related activities of the N-terminal cassette. However, the molecular mechanisms by which the C-terminal cassette modulates the activities of the N-terminal unit remain elusive. Here, we show that N- and C-terminal cassettes adopt vastly different relative orientations in a crystal structure of BRR2 in complex with an activating domain of the spliceosomal Prp8 protein at 2.4 Å resolution compared with the crystal structure of BRR2 alone. Likewise, inspection of BRR2 structures within spliceosomal complexes revealed that the cassettes occupy different relative positions and engage in different intercassette contacts during different splicing stages. Engineered disulfide bridges that locked the cassettes in two different relative orientations had opposite effects on the RNA-unwinding activity of the N-terminal cassette, with one configuration enhancing and the other configuration inhibiting RNA unwinding compared with the unconstrained protein. Moreover, we found that differences in relative positioning of the cassettes strongly influence RNA-stimulated ATP hydrolysis by the N-terminal cassette. Our results indicate that the inactive C-terminal cassette of BRR2 can both positively and negatively affect the activity of the N-terminal helicase unit from a distance.
Keywords: allosteric regulation, protein conformation, ATPase, RNA helicase, RNA splicing, dual-cassette Ski2-like helicase, intramolecular regulation, nucleic acid-dependent nucleotide triphosphatase (NTPase), small nuclear ribonucleoprotein U5 subunit 200 (SNRNP200), superfamily 2 helicase, pre-mRNA splicing
Introduction
Nucleic acid–dependent nucleotide triphosphatases (NTPases)3 are present in all domains of life, as these enzymes are involved in virtually all aspects of gene expression and regulation (1). A subset of these NTPases acts on RNAs. Many RNA-dependent NTPases can function as RNA helicases in vitro (i.e. they unwind RNA duplexes in an NTP-dependent manner) (2, 3). In vivo, they might also perform other functions, such as RNA annealing (4, 5), RNA clamping (6), displacement of RNA-bound proteins (7), or displacement of RNA-bound RNA-protein complexes (RNPs) (8, 9).
To carry out these functions, RNA-dependent NTPases rely on a set of about 12 conserved sequence motifs that are involved in NTP binding, NTP hydrolysis, RNA binding, and coupling of NTP binding/hydrolysis/product release to RNA/RNP transactions (10). Based on the presence and the degree of conservation of particular motifs, RNA-dependent NTPases have been assigned to several superfamilies (SFs) of nucleic acid–dependent NTPases, each of which contains several distinct families and subfamilies of enzymes (11). The vast majority of RNA-dependent NTPases belong to SF1 and SF2, whose members typically act as monomers (11). SF1/2 RNA-dependent NTPases all comprise a core of two RecA-like motor domains, which embody the basic NTP and RNA/RNP-related activities. Depending on the specific family, the proteins can contain additional domains, which are appended to or inserted into the RecA-like domains, as well as intrinsically unstructured terminal extensions (12). These helicase-associated domains and regions can modulate the activities of the enzymes in diverse ways (12).
Several families of RNA-dependent NTPases also contain a conserved set of helicase-associated domains, which, together with the RecA-like core domains, form functional NTPase/helicase units or cassettes. For example, members of the Ski2-like subfamily of SF2 harbor helicase cassettes, in which the two RecA-like domains are followed by a winged-helix (WH), a helical bundle (HB), and a helix-loop-helix (HLH) domain, and some members comprise and additional C-terminal immunoglobulin-like (IG) domain. A small subgroup of Ski2-like enzymes contains duplicated helicase cassettes arranged in tandem. The genomes of yeast and humans each encode two such dual-cassette enzymes (i.e. Slh1/Rqt2 and BRR2 in yeast and ASCC3 and BRR2 (SNRNP200) in humans). ASCC3 is a subunit of the activating signal cointegrator complex, which was originally identified as a coactivator of nuclear receptors (75) and later found to be involved in down-tuning of cellular antiviral responses (14), regulation of myogenic differentiation (15, 16), and DNA dealkylation repair (17, 18). Slh1/Rqt2 may constitute the yeast ortholog of human ASCC3 (19).
BRR2 is an RNA helicase involved in precursor mRNA (pre-mRNA) splicing and is presently the best-investigated representative of the dual-cassette Ski2-like helicases. Pre-mRNA splicing is carried out by a large and dynamic RNP molecular machine, the spliceosome (20). The spliceosome assembles de novo on each pre-mRNA substrate in a stepwise manner, is then catalytically activated, carries out two transesterification reactions that lead to intron excision and exon ligation, and is finally disassembled in an ordered manner (20). The transitions between the assembly, activation, catalysis, and disassembly stages are accompanied by profound compositional and conformational remodeling of the spliceosome, which is promoted by at least eight conserved, spliceosome-associated RNA-dependent NTPases/RNA helicases. BRR2 is involved in the conversion of a precatalytic spliceosomal B complex to an activated Bact complex. In that process, BRR2 unwinds the initially base-paired spliceosomal U4 and U6 small nuclear RNAs (snRNAs) (21), enabling U6 to adopt a new structure, engage in new interactions, and form part of the spliceosome's active site (22). Regulation of the spliceosomal RNA helicases has been implicated in splicing fidelity (23) and in the regulation of alternative splicing (24) (i.e. the inclusion of different combinations of coding regions in mature mRNAs) by kinetic proofreading mechanisms and by funneling spliceosomal assembly intermediates into discard pathways (25). Thus, regulation of the activities of the spliceosomal helicases may have a major impact on gene expression and regulation, in particular in higher eukaryotes, where alternative splicing is pervasive (26).
A number of specific regulatory principles have been delineated for BRR2 (27). For example, besides the two helicase cassettes, BRR2 comprises a large N-terminal region of about 400 residues that can fold back onto the helicase cassettes and autoinhibit the enzyme by substrate competition and conformational clamping (28). In addition, a C-terminal Jab1 domain of the spliceosomal master regulator, Prp8, can either inhibit or activate BRR2, depending on whether or not a C-terminal tail of the domain is inserted into the helicase's RNA-binding tunnel (29–31). Other proteins binding to BRR2 can also modulate its activities in vitro (32, 33).
Only the N-terminal cassette (NC) of BRR2 is an active NTPase/RNA helicase, whereas the C-terminal cassette (CC) is considered a pseudoenzyme (13, 34). Nevertheless, the CC is essential for yeast viability (35), can still bind but does not hydrolyze ATP (34), and serves as an interaction platform for a number of other splicing factors (36, 37), some of which have been shown to modulate BRR2 activity by presently unknown mechanisms (32, 33). Indeed, in human BRR2 (hBRR2), the CC itself can activate the NC helicase (34). Presently, the regulatory potential of the BRR2 CC has not been thoroughly explored, and the molecular mechanisms underlying BRR2 regulation via its CC are poorly understood.
Here, we present direct evidence for the ability of the two hBRR2 cassettes to occupy vastly different relative positions and engage in different intercassette interactions in isolation and during different stages of splicing. Upon fixing two such relative arrangements by disulfide bridges, we observed opposite effects on RNA/ATP-related activities of the NC. Our results show that the CC can function as an intramolecular cofactor of the NC helicase, which, depending on its relative position and intercassette interactions, can exert positive or negative regulatory effects.
Results
The hBRR2 helicase cassettes can adopt diverse relative positions and orientations
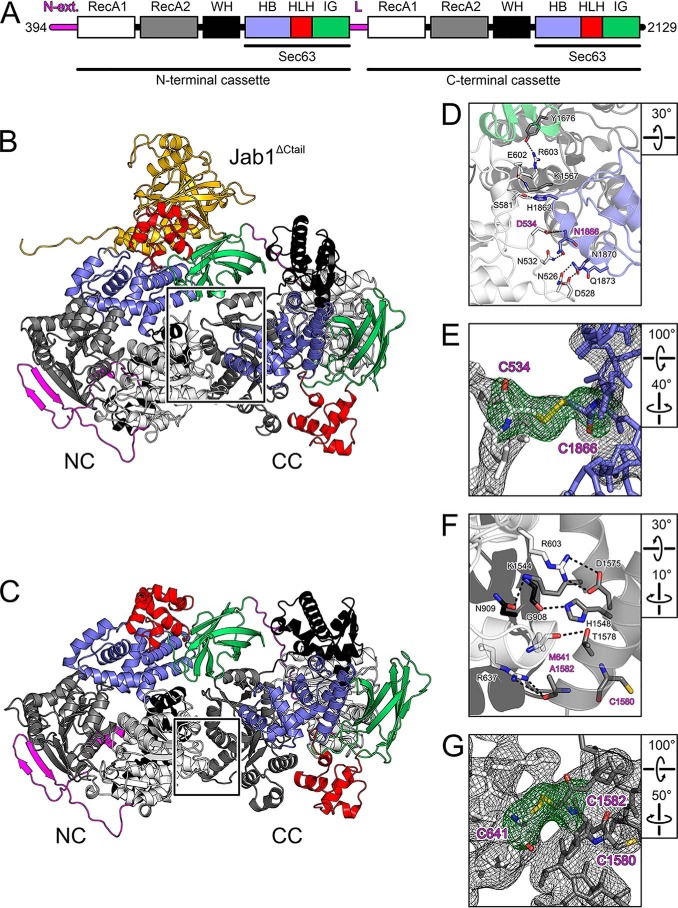
We had previously observed slight changes in relative cassette orientations in the crystal structures of a dual-cassette fragment of hBRR2 (residues 394–2129; here referred to as hBRR2 truncation 1 (hBRR2T1); Fig. 1A), in isolation (34) and in complex with the hPrp8 Jab1 domain, bearing a C-terminal tail in an hBRR2-inhibitory configuration (29). To further explore the intercassette flexibility in hBRR2, we determined the crystal structure of hBRR2T1 in complex with a hPrp8 Jab1 domain lacking the inhibitory tail (residues 2064–2320; hJab1ΔCtail), which we had previously shown to be a strong activator of the hBRR2 helicase (29). Crystals of the hBRR2T1-hJab1ΔCtail complex diffracted to 2.4 Å resolution, and the structure was solved by molecular replacement using the structure coordinates of isolated hBRR2T1 (PDB entry 4F91) (34) and of the tail-deleted hPrp8 Jab1 domain (PDB entry 4KIT) (29) (Table 1). In the hBRR2T1-hJab1ΔCtail structure, hBRR2T1 adopted a vastly different relative cassette orientation compared with hBRR2T1 alone or compared with hBRR2T1 within a hBRR2T1-hJab1 complex (Figs. 1 (B and C) and 2). Relative to isolated hBRR2T1, the apparent movement of the CC relative to the NC in the hBRR2T1-hJab1ΔCtail structure is described by a rotation of about 22° around the linker between NC and CC (apparent rotation from the hBRR2T1 structure to the hBRR2T1-hJab1ΔCtail structure defined as positive; Fig. 2, first panel, rot+). These observations show that the relative positions of the two cassettes in hBRR2 can cover a much larger spectrum than previously realized.
Figure 1.
Structures of hBRR2T1 and introduction of disulfide bridges. A, domain structure of hBRR2T1. N-ext., N-terminal extension; RecA, RecA-like domains; WH, winged-helix; HB, helical bundle; HLH, helix-loop-helix; IG, immunoglobulin-like domains; L, intercassette linker; Sec63, Sec63 homology units. B, structure of the hBRR2T1-hJab1ΔCtail complex. hBRR2T1 domains are colored as in A; hJab1ΔCtail is shown as gold. C, structure of the hBRR2T1 (34). hBRR2T1 domains are colored as in A. D, zoom into the intercassette interface in the hBRR2T1-hJab1ΔCtail complex (region boxed in B). In this and the following figure panels, interacting residues are shown as sticks. Carbon is shown as the respective protein region. Blue, nitrogen; red, oxygen. Dashed lines, hydrogen bonds or salt bridges. Residues mutated to cysteines to introduce a disulfide bridge are labeled in magenta. Rotation symbols represent the view relative to B and C. E, 2Fo − Fc electron density (gray mesh; 1σ level) and Fo − Fc “omit” electron density (green mesh; 3σ level) around the engineered disulfide bridge in hBRR2T1-D534C/N1866C (hBRR2T1-SS-rot+). hBRR2T1-D534C/N1866C was crystallized in complex with hJab1ΔCtail. Mutated residues are labeled in magenta. F, zoom into the intercassette interface in isolated hBRR2T1 (region boxed in C). G, 2Fo − Fc electron density (gray mesh; 1σ level) and Fo − Fc “omit” electron density (green mesh; 3σ level) around the engineered disulfide bridge in hBRR2T1-M641C/A1582C (hBRR2T1-SS-linear). hBRR2T1-M641C/A1582C was crystallized in isolation. Mutated residues and a neighboring cysteine are labeled in magenta.
Table 1.
Crystallographic data
Values in parentheses are for the highest-resolution shells.
| Data set | hBRR2T1-hJab1ΔCtail | hBRR2T1-M641C/A1582C | hBRR2T1-D534C/N1866C-hJab1ΔCtail |
|---|---|---|---|
| Data collection | |||
| Wave length (Å) | 0.91842 | 0.91840 | 0.91677 |
| Temperature (K) | 100 | 100 | 100 |
| Space group | P212121 | C2 | P212121 |
| Unit cell parameters | |||
| Axes (Å) | 99.5, 118.7, 187.8 | 145.6, 152.5, 142.5 | 99.2, 119.1, 187.9 |
| Angles (degrees) | 90.0, 90.0, 90.0 | 90.0, 120.7, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 50.0–2.4 (2.53–2.39) | 50.0–3.2 (3.36–3.17) | 50.0–2.6 (2.76–2.60) |
| Reflections | |||
| Unique | 88,725 (14,026) | 44,087 (6492) | 68,538 (10,962) |
| Completeness (%) | 99.8 (99.0) | 97.0 (89.3) | 98.9 (99.2) |
| Redundancy/multiplicity | 8.9 (8.4) | 3.4 (3.4) | 5.7 (5.6) |
| I/σ(I) | 11.3 (1.7) | 8.7 (1.0) | 9.6 (1.3) |
| Rmeas(I) (%)a | 14.4 (144.6) | 12.1 (131.6) | 15.9 (138.6) |
| CC½ (%)b | 99.7 (59.4) | 99.8 (64.1) | 99.7 (47.4) |
| Refinement | |||
| Resolution (Å) | 48.1–2.4 (2.48–2.39) | 48.4–3.2 (3.33–3.17) | 47.9–2.6 (2.69–2.59) |
| Reflections | |||
| Number | 88,212 (8,654) | 44,032 (5,199) | 68,531 (6,757) |
| Completeness (%) | 99.73 (98.84) | 97.2 (88.7) | 98.85 (98.77) |
| Test set | 2,100 (206) | 2,094 (247) | 2,099 (207) |
| Rworkc | 17.9 (25.8) | 26.4 (44.7) | 19.6 (31.4) |
| Rfreed | 25.3 (33.9) | 31.8 (52.3) | 27.9 (39.8) |
| Contents of A.U.e | |||
| Protein atoms | 16,866 | 13,858 | 15,823 |
| Ligand atoms | 10 | ||
| Water oxygens | 656 | 20 | 102 |
| Mean B factors (Å2) | |||
| Wilson | 56.6 | 94.0 | 58.2 |
| Protein | 59.6 | 118.9 | 69.2 |
| Ligands | 121.1 | ||
| Water oxygens | 49.2 | 107.2 | 49.7 |
| Ramachandran plotf | |||
| Favored (%) | 95.00 | 98.90 | 93.04 |
| Outliers (%) | 0.76 | 1.10 | 1.07 |
| r.m.s.d.g | |||
| Bond lengths (Å) | 0.009 | 0.002 | 0.006 |
| Bond angles (degrees) | 1.10 | 0.54 | 0.94 |
| PDB code | 6S8Q | 6S8O | 6S9I |
aRmeas(I) = ∑h (N/(N − 1))½ ∑i |Iih − 〈Ih〉|/∑h∑i Iih, in which 〈Ih〉 is the mean intensity of symmetry-equivalent reflections h, Iih is the intensity of a particular observation of h, and N is the number of redundant observations of reflection h.
b CC½ = (〈I2〉 − 〈I〉2)/(〈I2〉 − 〈I〉2) + σ2ϵ, in which σ2ϵ is the mean error within a half-data set (74).
c Rwork = ∑h|Fo − Fc|/∑Fo (working set, no σ cut-off applied).
d Rfree is the same as Rwork, but calculated on the test set of reflections excluded from refinement.
e A.U., asymmetric unit.
f Calculated with Phenix.
g r.m.s.d., root mean square deviation from target geometry.
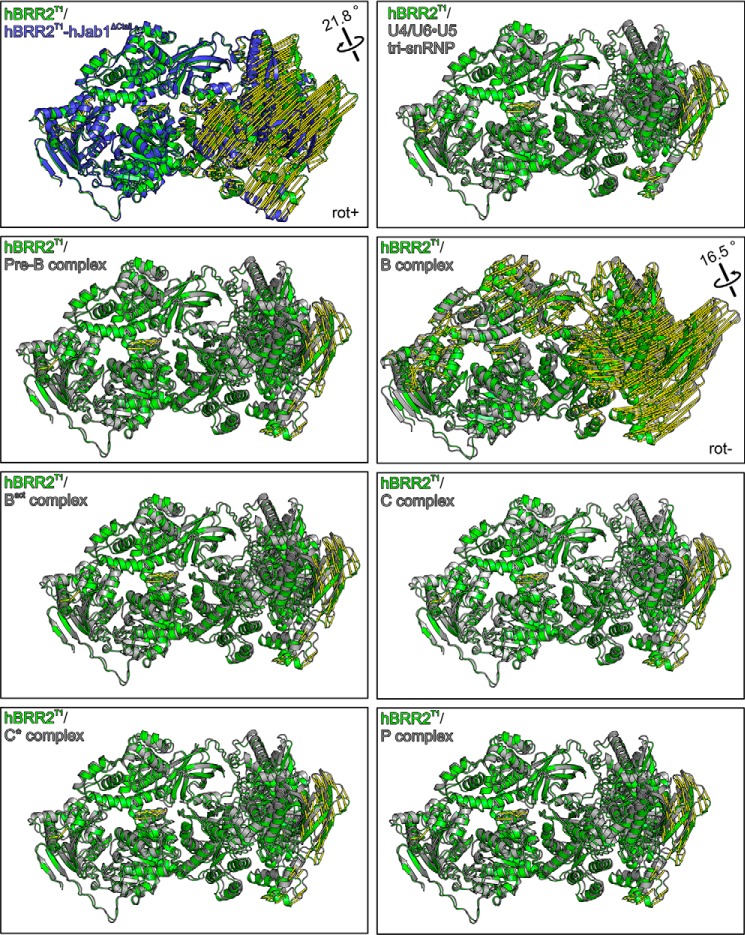
Figure 2.
Structural comparisons. Crystal structure of hBRR2T1 (green) (34) superimposed on the hBRR2T1-hJab1ΔCtail structure (blue) and on the hBRR2 subunits (gray) in cryo-EM structures of the U4/U6·U5 tri-snRNP (39), spliceosomal pre-B (40), B (38), Bact (42), C (43), C* (41), and P complexes (44). Alignments were based on NC residues 1–1288. Yellow “mode” vectors indicate structural differences as distances after alignment (displacements of common Cα positions of the compared structures to the isolated hBRR2T1 reference structure). rot+, apparent rotation of CC in the hBRR2T1 structure to the CC in the hBRR2T1-hJab1ΔCtail structure defined as positive; rot−, opposite apparent rotation sense for the CC seen in all structures of spliceosomal complexes, most prominently in the B complex.
The different relative positioning of the cassettes in the hBRR2T1-hJab1ΔCtail complex crystals may reflect conformational changes induced by the activatory hJab1ΔCtail or may be the consequence of crystal packing effects, as the hBRR2T1-hJab1ΔCtail complex crystallized in a different space group than hBRR2T1 or hBRR2T1-hJab1 (Table 1). To investigate whether hBRR2 also can undergo intercassette conformational changes in the framework of spliceosomal complexes, we compared hBRR2T1 crystal structures with the conformations of hBRR2 subunits extracted from recently published cryo-EM structures of human spliceosomes (38–44). Structural alignments via the NCs revealed that the CCs can adopt different relative orientations in different functional contexts (Fig. 2 and Table 2). A similar picture emerged when we compared the conformations of BRR2 subunits extracted from cryo-EM structures of yeast spliceosomal complexes (45–50) (Table 2). hBRR2 conformations of all cryo-EM structures aligned more closely to the conformation of isolated hBRR2T1 than to the conformation of hBRR2T1 in the crystal structure of the hBRR2T1-hJab1ΔCtail complex (Table 2). Moreover, compared with isolated hBRR2T1, all hBRR2 conformations in spliceosomal cryo-EM structures exhibited rotations of the CC relative to the NC in the opposite sense compared with hBRR2T1 in the hBRR2T1-hJab1ΔCtail complex, with BRR2 in the B complex exhibiting the largest rotation of 16.5° (Fig. 2, rot−). BRR2 resides in a peripheral, less well-resolved region in most spliceosomal cryo-EM structures, which may obscure the full extent of BRR2 conformational changes in the imaged contexts, and not all assembly, activation, and catalysis intermediates of a splicing cycle have so far been structurally analyzed. Thus, the conformational spectrum explored by BRR2 during a splicing cycle might exceed the conformational flexibility portrayed by the presently available spliceosome structures. Irrespectively, these analyses reveal that BRR2 apparently undergoes intercassette conformational changes during a splicing cycle.
Table 2.
Structural comparisons
| Structure | PDB code | r.m.s.d.a hBRR2T1 | r.m.s.d. hBRR2T1-Jab1ΔCtail |
|---|---|---|---|
| Å | Å | ||
| Human U4/U6·U5 tri-snRNP | 3JCR | 1.5 | 5.6 |
| Human B complex | 5O9Z | 2.7 | 9.6 |
| Human Bact complex | 5Z57 | 1.5 | 5.7 |
| Human C complex | 5YZG | 1.5 | 5.7 |
| Human C* complex | 5XJC | 1.5 | 5.7 |
| Yeast U4/U6·U5 tri-snRNP | 3JCM | 5.6 | 9.3 |
| Yeast U4/U6·U5 tri-snRNP | 5GAO | 5.9 | 9.6 |
| Yeast B complex | 5NRL | 5.9 | 9.7 |
| Yeast Bact complex | 5GM6 | 4.4 | 6.6 |
| Yeast Bact complex | 5LQW | 4.1 | 5.7 |
| Yeast C complex | 5LJ5 | 3.0 | 6.5 |
a r.m.s.d., root mean square deviation after superposition according to all Cα atoms of the BRR2 subunits.
Alternative hBRR2 conformations can be stabilized via engineered disulfide bridges
The hBRR2T1 conformations in the isolated and hBRR2T1-hJab1ΔCtail crystal structures exhibit major differences in the contacts of the CC to the NC at the interface between the cassettes (Fig. 1, D and F). Whereas the conformation of isolated hBRR2T1 encompasses several salt bridges and hydrogen bonds between the N-terminal RecA1/winged-helix domains and the C-terminal RecA2 domain (Fig. 1F), the cassette interface in the hBRR2T1-hJab1ΔCtail structure comprises fewer and longer-distance polar and hydrophobic interactions between the N-terminal RecA1 and the C-terminal RecA2/HB domains (Fig. 1D). In the following, we refer to the relative cassette arrangement in isolated hBRR2T1 and hBRR2T1-hJab1ΔCtail complex as the linear and positively rotated (rot+) conformations, respectively.
Previously, we had shown that exchanges of residues, which foster intercassette contacts in the linear conformation of isolated hBRR2T1 or in the extended linker region connecting the two cassettes, led to alterations in the NC helicase activity (34). We therefore set out to test whether the two conformations represented by isolated hBRR2T1 and hBRR2T1-hJab1ΔCtail crystal structures would correlate with differences in the helicase activity of hBRR2. To this end, we selected pairs of NC/CC residues that resided in suitable relative positions in the two structures to lead to the formation of disulfide bridges upon joint exchange for cysteine residues, thereby stabilizing the linear or the rot+ conformation (Fig. 1, D and F). Cysteine residues were introduced at the selected positions (M641C/A1582C for the linear conformation of isolated hBRR2T1; D534C/N1866C for the rot+ conformation of hBRR2T1-hJab1ΔCtail) by site-directed mutagenesis, and the corresponding hBRR2T1 variants were purified (omitting the addition of DTT in the final gel filtration step; Fig. S1) and crystallized alone (hBRR2T1-M641C/A1582C; in the following referred to as hBRR2T1-SS-linear) or in complex with hJab1ΔCtail (hBRR2T1-D534C/N1866C; in the following referred to as hBRR2T1-SS-rot+).
Crystal structure analyses unequivocally revealed the presence of the intended disulfide bridges (Fig. 1, E and G). Moreover, structural alignments showed that the disulfide-bridged hBRR2T1 variants adopted essentially the same conformations as the WT variant in the two different structural contexts (isolated hBRR2T1/hBRR2T1-SS-linear, root mean square deviation (r.m.s.d.) of 0.519 Å for 1,485 matching pairs of hBRR2T1 Cα atoms; hBRR2T1/hBRR2T1-SS-rot+-Jab1ΔCtail, r.m.s.d. of 0.278 Å for 1,523 matching pairs of hBRR2T1 Cα atoms).
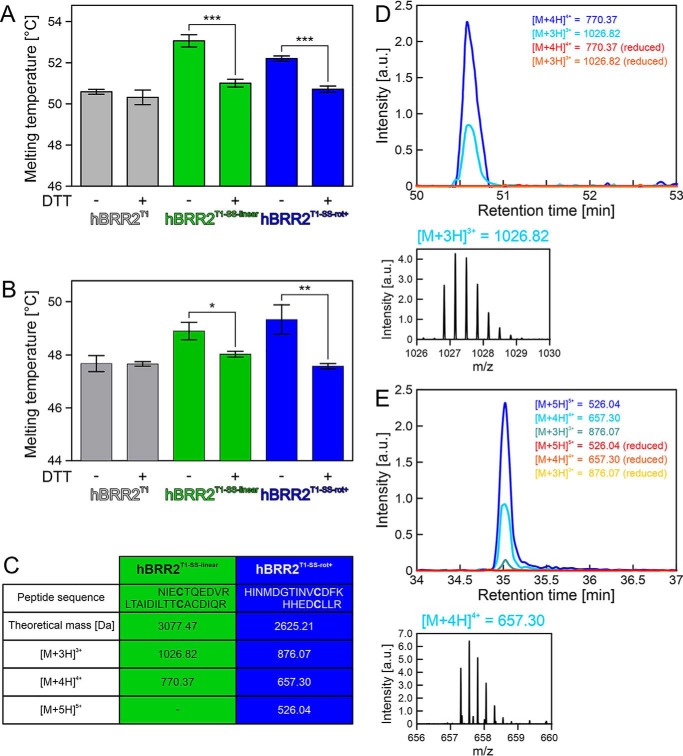
To test whether the intended disulfide bridges also formed in solution prior to crystallization, we compared the fold stabilities of WT and cysteine-derivatized hBRR2T1 using differential scanning fluorimetry (DSF) in two different buffers and in the presence or absence of the reducing agent DTT (Fig. 3, A and B). Not surprisingly, the buffer composition per se did have an influence on the melting temperatures of the proteins. However, in both buffer systems, the presence or absence of DTT influenced the melting temperatures of the cysteine variants, but not those of the WT protein, in the same way and in complete agreement with the formation of the intended disulfide bridges under nonreducing conditions (i.e. in the absence of DTT, melting temperatures of both cysteine variants were increased by 2–3 °C in the two buffers compared with those of the WT protein (Fig. 3 (A and B), columns 3 and 5)). In contrast, in the presence of DTT, the melting temperatures of both cysteine variants were indistinguishable and essentially identical to those of the WT protein in DTT-containing buffers (Fig. 3 (A and B), columns 4 and 6).
Figure 3.
Analysis of the hBRR2T1 bearing engineered disulfide bridges. A, DSF-derived melting temperatures for hBRR2T1 (gray), hBRR2T1-SS-linear (green), and hBRR2T1-SS-rot+ (blue) with and without the addition of the reducing agent, DTT, in size-exclusion chromatography buffer. Values represent means ± S.D. (error bars) of at least three independent experiments. ***, p ≤ 0.001. B, DSF-derived melting temperatures for hBRR2T1 (gray), hBRR2T1-SS-linear (green), and hBRR2T1-SS-rot+ (blue) with and without the addition of the reducing agent, DTT, in 40 mm TRIS-HCl, pH 7.5, 50 mm NaCl, 0.5 mm MgCl2. Values represent means ± S.D. of at least three independent experiments. *, p ≤ 0.05; **, p ≤ 0.01. C, mass-to-charge ratios (m/z) of disulfide-bridged peptides identified in LC-MS analyses. D, extracted ion chromatograms for disulfide bridge–containing peptides of hBRR2T1-SS-linear present in the nonreduced/oxidized samples and missing in the reduced samples. One exemplary MS1 spectrum of the triply charged bridged peptide is shown. E, extracted ion chromatograms for disulfide bridge–containing peptides of hBRR2T1-SS-rot+ present in the nonreduced/oxidized samples and missing in the reduced samples. One exemplary MS1 spectrum of the quadruply charged bridged peptide is shown. a.u., arbitrary units.
To further test the formation of the engineered disulfide bridges in solution, we conducted MS analyses (Fig. 3, C–E). Free thiol groups in hBRR2T1-SS-linear and hBRR2T1-SS-rot+ were modified with N-ethylmaleimide (NEM) before or after reduction with DTT, and subsequently proteins were digested with LysC and trypsin. MS1 signals corresponding to the expected disulfide-bridged peptides (638NIECTQEDVR647/1571LTAIDILTTCACDIQR1586 for hBRR2T1-SS-linear; 524HINMDGTINVCDFK537/1862HHEDCLLR1869 for hBRR2T1-SS-rot+) were identified in the nonreduced samples (Fig. 3C). For hBRR2T1-SS-linear, two different charge states of the bridged peptide were seen, whereas three different charge states were detected for the bridged peptide of hBRR2T1-SS-rot+ (Fig. 3C; examples for MS1 peaks are shown in Fig. 3 (D and E)). Extracted-ion chromatograms of the reduced samples lacked signals of the disulfide-bridged peptides (Fig. 3, D and E), confirming that the identified peaks in the nonreduced samples originated from disulfide-bridged peptides. The identities of the bridged peptides were further confirmed by MS2 spectra of material in peaks m/z 770.4 (hBRR2T1-SS-linear) and m/z 657.3 (hBRR2T1-SS-rot+; Fig. S2). The latter analysis revealed that the m/z 770.4 peak that originated from hBRR2T1-SS-linear corresponded to 638NIECTQEDVR647/1571LTAIDILTTCACDIQR1586 with a disulfide bridge between Cys-641 and Cys-1580 rather than the engineered Cys-1582. Therefore, either a Cys-641/Cys-1580 disulfide bridge formed in hBRR2T1-SS-linear in solution whereas a Cys-641/Cys-1582 disulfide bridge was favored in the crystal (Fig. 1G), or disulfide shuffling between the neighboring Cys-1580 and Cys-1582 took place during sample preparation for MS. Irrespectively, both disulfide-bridged configurations would stabilize hBRR2T1-SS-linear in the linear conformation seen in isolated WT hBRR2T1.
To estimate the fraction of bridged and unbridged protein molecules, the intensities of the corresponding unbridged peptides were compared between the nonreduced and reduced samples. This analysis indicated a high degree of disulfide bridge formation in the hBRR2T1-SS-linear variant, but a lower degree of disulfide bridge formation in the hBRR2T1-SS-rot+ variant.
Together, the above analyses suggest that the engineered variants indeed form the intended disulfide bridges in solution, albeit not at 100%, and that the replacements of the selected residues in the variants with cysteine or the presence of DTT per se have no effect on the fold stabilities. They therefore also indicate that the linear and rot+ conformations observed in hBRR2T1 and hBRR2T1-hJab1ΔCtail crystals, respectively, are also adopted by hBRR2T1 in solution. hBRR2T1 in solution apparently populates the two conformations to different extents, with a higher fraction of hBRR2T1 molecules in the linear conformation and a lower fraction in the rot+ conformation.
Restraining the cassettes in different conformations influences BRR2 helicase activity
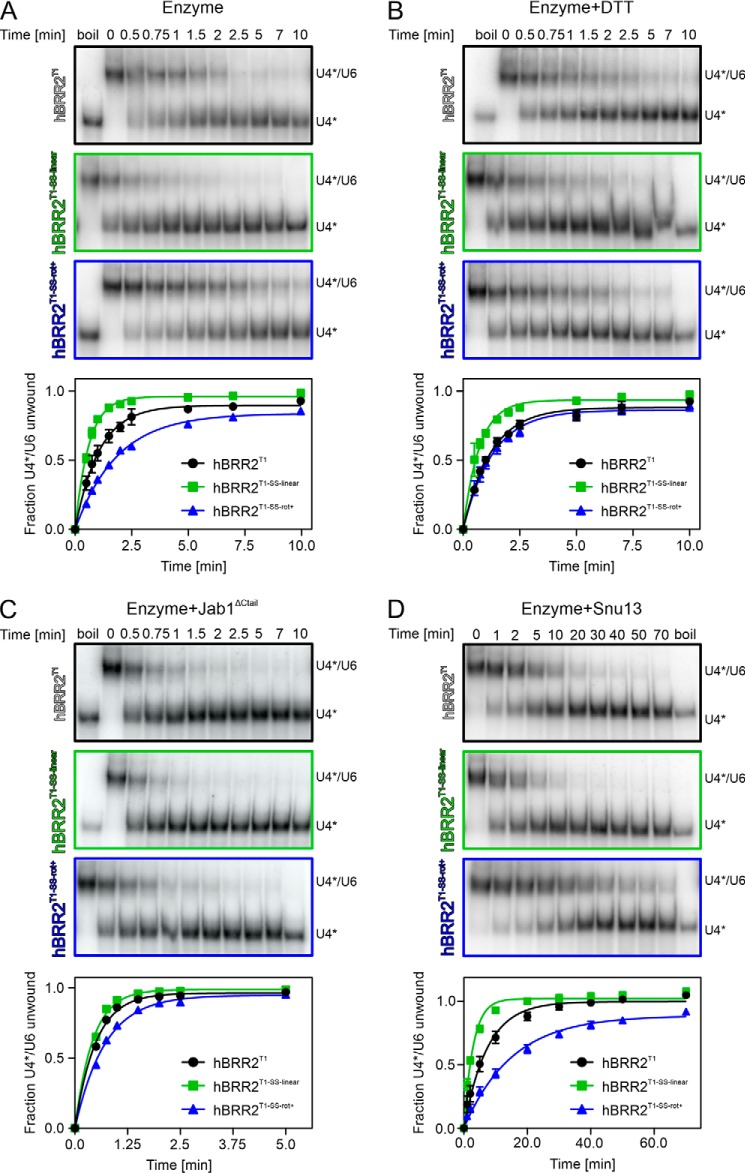
To test the consequences of arresting hBRR2T1 in two different intercassette conformations, we monitored the effects of hBRR2T1-SS-linear and hBRR2T1-SS-rot+ in gel-based, radioactive U4/U6 unwinding assays. Compared with WT hBRR2T1, hBRR2T1-SS-linear exhibited a 1.7-fold higher unwinding rate (1.645 ± 0.049 min−1 versus 0.941 ± 0.040 min−1), whereas hBRR2T1-SS-rot+ showed a 1.8-fold lower activity (0.536 ± 0.012 min−1; Fig. 4A and Table 3). Notably, upon inclusion of DTT in the unwinding buffer, the activity of hBRR2T1-SS-linear decreased whereas the activity of hBRR2T1-SS-rot+ increased, both approaching the unwinding rates of the WT variant under the same conditions (Fig. 4B and Table 3). These trends were significant, as indicated by nonoverlapping 95% confidence intervals compared with the values without reducing agent (Table 3).
Figure 4.
Helicase activities. A–D, unwinding of U4*/U6 duplexes by the hBRR2T1 variants indicated on the left of the gels under the conditions listed on top of the gels. A, enzyme alone, untreated. B, enzyme alone, treated with reducing agent. C, enzyme in complex with hJab1ΔCtail. D, enzyme with the addition of ySnu13 to the RNA. Top, exemplary gels monitoring the unwinding reactions. U4*, radioactively labeled U4 snRNA. Bottom, quantification of the data at the top. Black, hBRR2T1; green, hBRR2T1-SS-linear; blue, hBRR2T1-SS-rot+. Data points represent means ± S.D. (error bars) of at least three independent experiments.
Table 3.
Amplitudes and rate constants of U4/U6 unwinding
Results from gel-based unwinding assays were quantified by densitometry and fit to a first-order reaction (fraction unwound = A(1 − exp(−kut)), in which A is the amplitude of the reaction, ku is the apparent first-order rate constant of unwinding, and t is time). Values represent means ± S.E.M. of at least three independent experiments.
| A | ku | 95% confidence intervals of ku | |
|---|---|---|---|
| min−1 | min−1 | ||
| −DTT | |||
| WT | 0.899 ± 0.012 | 0.941 ± 0.040 | 0.859–1.023 |
| SS-linear | 0.964 ± 0.007 | 1.645 ± 0.049 | 1.546–1.744 |
| SS-rot+ | 0.841 ± 0.007 | 0.536 ± 0.012 | 0.511–0.561 |
| +DTT | |||
| WT | 0.881 ± 0.011 | 0.815 ± 0.030 | 0.753–0.877 |
| SS-linear | 0.935 ± 0.013 | 1.394 ± 0.070 | 1.250–1.538 |
| SS-rot+ | 0.862 ± 0.013 | 0.772 ± 0.035 | 0.701–0.844 |
| +Jab1ΔCtail, −DTT | |||
| WT | 0.969 ± 0.010 | 1.987 ± 0.098 | 1.787–2.187 |
| SS-linear | 0.992 ± 0.007 | 2.337 ± 0.086 | 2.161–2.512 |
| SS-rot+ | 0.953 ± 0.010 | 1.385 ± 0.055 | 1.272–1.498 |
| +Snu13, −DTT | |||
| WT | 0.998 ± 0.014 | 0.136 ± 0.008 | 0.119–0.152 |
| SS-linear | 1.021 ± 0.011 | 0.340 ± 0.018 | 0.304–0.376 |
| SS-rot+ | 0.888 ± 0.016 | 0.066 ± 0.004 | 0.058–0.074 |
Although the effects of both mutants compared with the WT protein are mild, direct comparison between both mutants indicates that stabilizing BRR2 in two different intercassette conformations can lead to a >3-fold change in its unwinding rate. Moreover, given that the engineered disulfide bridges are not formed at 100% in solution (see above), the unwinding assays underestimate the effects of completely locking hBRR2T1 in either linear or rot+ conformation, in particular for the latter situation.
We reasoned that the effects of the disulfide-bridged variants should be dampened under conditions where BRR2 helicase is activated and may be augmented under conditions where BRR2 helicase activity is hindered. To test this idea, we first repeated the unwinding assays in the presence of the hJab1ΔCtail activator. hJab1ΔCtail increased the unwinding rates of all hBRR2T1 constructs, consistent with previous studies (29) (Fig. 4C and Table 3). These results show that hJab1ΔCtail can still bind to hBRR2T1 when restrained in the linear conformation seen in the isolated hBRR2T1 crystal structure (hBRR2T1-SS-linear), consistent with only the NC being involved in Jab1 binding (29) (Fig. 1B). As the relative position of the CC is, thus, most likely not directly influenced by this interaction partner, the altered conformation of hBRR2T1 in the hBRR2T1-hJab1ΔCtail co-crystal structure is probably a consequence of crystal packing forces. Irrespectively, the same trends in unwinding rates were observed when the experiments were conducted in the presence of hJab1ΔCtail, although the effects were significantly dampened, as expected (Fig. 4C and Table 3) (i.e. in the presence of the activator, hBRR2T1-SS-linear showed an ∼1.2-fold higher unwinding rate constant (2.337 ± 0.086 min−1), whereas hBRR2T1-SS-rot+ exhibited an ∼1.4-fold reduced unwinding (1.385 ± 0.055 min−1) compared with WT hBRR2T1 (1.987 ± 0.098 min−1; Fig. 4C and Table 3)).
To test the effects of disulfide cross-linking under conditions where BRR2-mediated U4/U6 unwinding is slowed down, we included the ySnu13 protein in the assay. ySnu13 binds to and stabilizes the U4/U6 duplex and drastically decreases BRR2-mediated unwinding (9). Consistently, the addition of ySnu13 resulted in an ∼7-fold reduced unwinding rate for WT BRR2T1 (Fig. 4D and Table 3). Under these conditions, the unwinding rate of hBRR2T1-SS-linear (0.340 ± 0.018 min−1) was 2.5-fold higher than the rate of the WT (0.136 ± 0.008 min−1), whereas the unwinding rate of hBRR2T1-SS-rot+ (0.066 ± 0.004 min−1) was about 2-fold lower (Fig. 4D and Table 3). Thus, the unwinding rates of hBRR2T1-SS-linear and hBRR2T1-SS-rot+ differed more than 5-fold in the hindered system.
Together, the above findings indicate that hBRR2 is more active in unwinding a biologically relevant RNA substrate or in disrupting a biologically relevant RNP when it is restrained in the conformation represented by the isolated hBRR2T1 crystal structure, whereas restraining hBRR2T1 in the conformation seen in the hBRR2T1-hJab1ΔCtail complex structure leads to decreased RNA-unwinding and RNP disruption activities.
Altered ATP hydrolysis may contribute to altered helicase activities
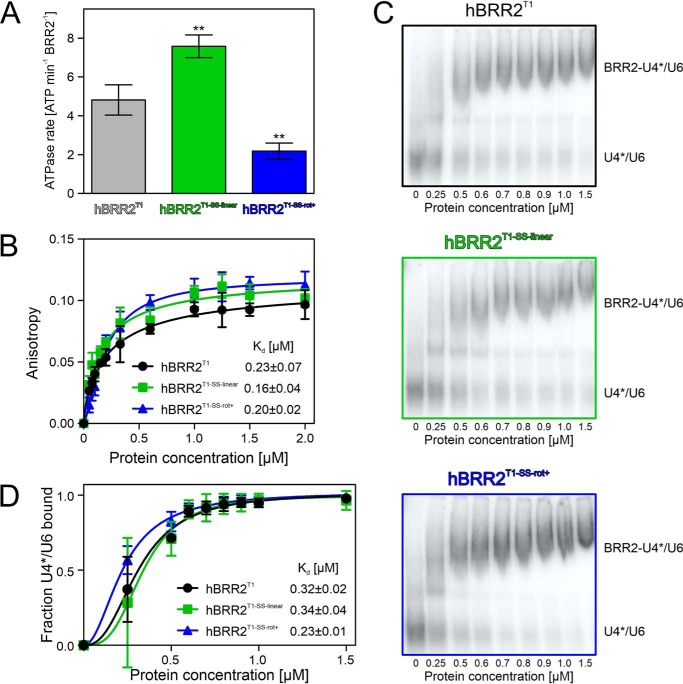
To further track down the mechanism by which the CC exerts influence on the NC, depending on its relative position and orientation, we tested the consequence of stabilizing hBRR2T1 in different cassette configurations on ATP hydrolysis. We determined the RNA-stimulated ATPase rates of the disulfide-bridged hBRR2T1 variants via TLC, which again revealed significant differences compared with the WT protein, fully consistent with the trends in the unwinding activities (Fig. 5A). Thus, hBRR2T1-SS-linear exhibited higher stimulated ATPase activity (7.58 ± 0.59 ATP/hBRR2T1-SS-linear/min), whereas the stimulated ATPase activity of hBRR2T1-SS-rot+ was decreased (2.18 ± 0.42 ATP/hBRR2T1-SS-rot+/min) compared with the WT protein (4.82 ± 0.78 ATP/hBRR2T1/min).
Figure 5.
ATPase- and RNA-binding activities. A, RNA-stimulated ATPase rates of the indicated hBRR2T1 variants (hBRR2T1 (black), hBRR2T1-SS-linear (green), and hBRR2T1-SS-rot+ (blue)), analyzed via a radioactive, TLC-based ATPase assay. Data points represent means ± S.D. (error bars) of at least three independent experiments. **, p ≤ 0.01. B, fluorescence polarization–based analysis of RNA-binding activities of the indicated hBRR2T1 variants (hBRR2T1 (black), hBRR2T1-SS-linear (green), and hBRR2T1-SS-rot+ (blue)). The indicated Kd values were determined by fitting the data to a Hill equation, fraction bound = Acprotn/(cprotn + Kdn), in which A is the maximum of bound RNA, cprot is the protein concentration, Kd is the dissociation constant, and n is the Hill coefficient. Values represent means ± S.E.M. (error bars) of at least three independent experiments. C, representative electrophoretic gel mobility shift assays monitoring binding of the indicated hBRR2T1 variants (hBRR2T1 (black), hBRR2T1-SS-linear (green), and hBRR2T1-SS-rot+ (blue)) to U4*/U6 di-snRNAs. U4*, radioactively labeled U4 snRNA. D, quantification of the data shown in C. The indicated Kd values were determined by fitting the quantified data to a Hill equation, fraction bound = Acprotn/(cprotn + Kdn), in which A is the maximum of bound RNA, cprot is the protein concentration, Kd is the dissociation constant, and n is the Hill coefficient. Data points represent means ± S.E.M. of at least three independent experiments.
Finally, we tested for differences in the RNA affinities of the different hBRR2T1 variants. Fluorescence polarization assays using a fluorescence-labeled RNA duplex with a 3′-single-stranded overhang, which we had employed previously in hBRR2-RNA interaction studies (51), suggested that RNA binding was unaffected by the engineered disulfide bridges (Fig. 5B). Likewise, all proteins exhibited similar affinities to U4/U6 di-snRNAs based on electrophoretic gel mobility shift assays (Fig. 5, C and D).
Discussion
Intramolecular modulation in a dual-cassette RNA helicase
Peripheral domains of SF2 helicases can influence the enzymes' subcellular localization (52), oligomerization state (53), substrate specificities (54), molecular mechanisms (55), and activities (56). The CC of BRR2 can be considered a large (about 100 kDa) and unique helicase-associated region of the N-terminal helicase unit, as it is catalytically inactive (13, 34) yet influences the NC helicase from a distance (34). The functional importance of the CC is underscored by the observation that its deletion is lethal in yeast (35). However, the molecular mechanisms by which it modulates the NC helicase are presently poorly understood.
By comparing BRR2 subunits from several crystal and cryo-EM structures of isolated BRR2, small BRR2 complexes, and spliceosomal complexes, we showed that the CC can adopt multiple positions and orientations relative to the NC, stabilized by different intercassette contacts. Locking hBRR2T1 in two different conformations that exhibit different relative positioning of NC and CC via engineered disulfide bridges affected the helicase activity of the NC in opposite ways. We previously reported that the exchange of residues that mediate intercassette contacts in an isolated hBRR2T1 crystal structure, mutations in the intercassette linker, and mutations in the CC nucleotide-binding pocket led to reduced BRR2 helicase activity, suggesting that the CC can stimulate the activity of the NC (34). Our present observation that an engineered disulfide bridge, which stabilizes the linear cassette conformation of isolated hBRR2T1, leads to an increase in BRR2 helicase activity is consistent with this notion. On the other hand, a disulfide-bridged hBRR2T1 variant that stabilizes at least part of the enzymes in a sample in the alternative rot+ conformation, seen in the hBRR2T1-hJab1ΔCtail co-crystal structure, led to a decreased unwinding activity. Together, these findings show that, depending on its position, orientation, and contacts to the NC, the CC can either activate or inhibit the NC.
We further characterized the molecular mechanism underlying this flexible regulatory influence. Our data indicate that the different NC-CC conformations do not influence RNA binding by the NC. In contrast, we observed a modulation of stimulated ATPase rates. The trends observed in ATP hydrolysis matched the trends seen in RNA helicase activities.
It remains to be seen whether the principles uncovered here for the BRR2 RNA helicase also exist in other dual-cassette Ski2-like enzymes, such as ASCC3 and Slh1/Rqt2. In this respect, it is interesting that the CC of ASCC3 is an active DNA helicase (17), but whether the ASCC3 NC can also unwind nucleic acid duplexes and/or whether intercassette modulation of activity is also possible in the other direction (i.e. the NC influencing the activity of the CC) are open questions.
Implications for BRR2 regulation by protein partners
In none of the presently structurally characterized splicing stages does hBRR2 adopt a conformation that corresponds to the rot+ conformation observed in the hBRR2T1-hJabΔCtail complex. However, not all stages of a canonical splicing reaction have so far been structurally characterized, and additional stages may be populated in certain alternative splicing events. Irrespectively, also in some of the presently structurally characterized splicing stages, hBRR2 exhibits a cassette configuration distinct from the linear conformation seen in isolated hBRR2T1, the most extreme case represented by the rot− conformation in the B complex. As the linear conformation has a strong stimulatory effect on the NC helicase, all deviations from this conformation may lead to more or less pronounced BRR2 helicase inhibition.
Many protein interaction partners of BRR2 bind to the CC (32, 33, 36, 37). However, the CC most likely does not simply represent a passive landing pad for other proteins, as some of the interacting proteins also influence the activity of BRR2. For example, the intrinsically disordered proteins Ntr2 (in yeast) and FBP21 (in humans) bind to the CC of BRR2 and tune down its unwinding activity in vitro (32, 33). In the respective studies, it was speculated that their binding to the CC might influence the communication between the cassettes. Based on these previous observations and results presented here, we propose a more general mechanism, wherein the CC can be used by interacting proteins to modulate the NC helicase activity from a distance, by stabilizing the CC in a helicase-promoting or in a helicase-attenuating conformation. Based on our results and our comparisons of hBRR2 structures in diverse molecular contexts, BRR2 might adopt a near continuum of different cassette conformations. The equilibrium between the conformations could be shifted by protein interaction partners binding to the CC, which might consequently gradually stimulate or inhibit the enzyme. Such protein-mediated communication between the cassettes may contribute to a retinitis pigmentosa–linked BRR2 mutation found in the CC (57).
Magnitude of the putative regulatory influence
The effects of both mutants on BRR2-mediated U4/U6 unwinding relative to the WT protein were rather mild. However, we would like to point out that the observed effects are of a magnitude comparable with the effects previously observed due to BRR2-binding or U4/U6-binding proteins, which were suggested to be relevant (e.g. the yeast BRR2-binding protein, Ntr2, induces an up to 50% reduction of BRR2's unwinding amplitudes without an effect on the unwinding rate, and human FBP21 reduces the unwinding rate of BRR2 about 2-fold upon binding to the helicase (32, 33)). Similarly, the yeast U4/U6-binding proteins Prp31 and Prp3 decrease BRR2's rates about 2-fold and about 1.5-fold, respectively (9).
One reason for the rather moderate effects is obviously that disulfide bridge formation in solution was incomplete for both double-cysteine variants, and in particular for hBRR2T1-SS-rot+. Another reason may be the reductionist conditions under which we can presently test BRR2 helicase activity. In the context of the spliceosome, BRR2 does not simply unwind a naked U4/U6 snRNA duplex, but has to overcome the stabilizing effect of U4/U6-bound proteins. In this context, differences in the activities of different BRR2 conformers may be augmented. In agreement with this notion, we observed a relatively greater effect of the two disulfide-bridged variants relative to the WT, when we monitored BRR2-mediated disruption of a Snu13-bound U4/U6 duplex. Binding of Snu13 has previously been shown to reduce BRR2-mediated U4/U6 unwinding (9).
Implications for splicing regulation
Apart from the CC and proteins binding the CC, BRR2 activity is also regulated via an autoinhibitory N-terminal region (28), via the Prp8 Jab1 domain that can either inhibit or activate the helicase (29–31), via the Prp8 RNase H–like domain that can sequester the U4/U6 substrate (58), and via other proteins that alter the stability of the U4/U6 substrate (9). The question emerges of why BRR2 is regulated in an intricate manner on many levels and not simply shut on and off by one mechanism. We suggest that the fine-tuning of BRR2 activity could be part of proofreading events or contribute to the selection of alternative splice sites.
Proofreading describes a mechanism for discriminating between suboptimal/aberrant and optimal splice substrates to maintain splicing fidelity. At least five of the spliceosome-associated NTPases/helicases, Prp5, Prp28, Prp16, Prp22, and Prp43, have been implicated in proofreading mechanisms in yeast (23), including proofreading of 5′-splice sites (59, 60), of branch sites (61), and of 3′-splice sites (62). It is thought that these helicases can alternatively remodel suboptimal splicing intermediates in an off-pathway manner. After such off-pathway remodeling, often the helicase Prp43 is thought to be responsible for discarding aberrant intermediates (63). The decision or distribution between on-pathway and off-pathway remodeling may be determined by the speed at which a given helicase can remodel an intermediate along the on-pathway direction. In principle, similar mechanisms might underlie alternative splicing decisions in higher eukaryotes. Weak splice sites (analogous to suboptimal substrates) would exhibit slow splicing kinetics and thus be discarded more frequently than strong splice sites (analogous to optimal substrates) that would splice faster and thus be less prone to discard. Interestingly, a recent study directly linked spliceosomal helicases to alternative splicing by showing that Prp16 and Prp22 enable the selection of alternative branch sites and 3′-splice sites, respectively (24).
Some evidence suggests that also BRR2 might be involved in proofreading and alternative splicing. For example, changes in alternative splicing patterns were detected upon knockdown of core splicing factors, including BRR2 (64, 65). Furthermore, two retinitis pigmentosa–linked mutations that give rise to BRR2 variants enhance the use of a cryptic splice site (66). Regulatory factors or regions, including the CC and CC-binding proteins, may modulate the kinetics of U4/U6 disruption by BRR2, which could tune the velocity of Bact formation. In addition, BRR2 lacking the N-terminal region can lead to noncanonical disruption of the U4/U6·U5 tri-snRNP (into U4/U6 di-snRNP and U5 snRNP) (28), and B complex spliceosomes have been shown to undertake repeated attempts at activation with intermittent release of U4 (67). Thus, BRR2 might be able to remodel the B complex in diverse ways and thereby differentially funnel B complexes based on different pre-mRNA substrates along or off the splicing pathway. Fine-tuning of BRR2 activity might then influence to what extent a given B complex is discarded or further productively processed. Finally, unlike the other spliceosomal helicases, after formation of the B complex, BRR2 remains stably associated with the spliceosome during the subsequent stages of splicing. Its helicase activity is thus in principle available to serve also as a discard factor. Although direct evidence is presently missing, tuning of the activity of BRR2 might thus even be used directly for proofreading.
Experimental procedures
Site-directed mutagenesis, cell culture, and gene expression
A codon-optimized gene, encoding an N-terminal deletion construct of hBRR2 (residues 395–2129, here referred to as hBRR2T1), in a pFL vector, which directs the production of a fusion protein bearing an N-terminal, cleavable His10 tag (34), was used as a PCR template. Mutagenesis was performed with the QuikChange site-directed mutagenesis kit II (Stratagene), and successful mutagenesis was confirmed by sequencing. For production of hJab1ΔCtail, we employed a DNA construct in a pFL vector that directs the production of a fusion protein with an N-terminal, cleavable GST tag (29).
The pFL constructs were transformed into Escherichia coli DH10 MultiBac cells, and blue-white screening was used to select for colonies with successful Tn7 transposition into the baculovirus genome. The Bacmid DNA was purified and transfected into SF9 insect cells with Xtreme gene 9 DNA transfection reagent (Roche Applied Science). The first virus generation, V0, was harvested and used to infect H5 insect cells to produce the second virus generation, V1. The V1 virus was used to infect H5 cells for protein production. Cells were harvested before the cell viability decreased below 90%.
Protein purification
All purification steps were carried out at 4 °C. For hBRR2T1 variants, cell pellets were resuspended in lysis buffer (50 mm HEPES-NaOH, pH 7.5, 600 mm NaCl, 10% (w/v) glycerol, 0.05% (v/v) Nonidet P-40, 20 μg/ml DNase 1, 2 mm β-mercaptoethanol, supplemented with Complete EDTA-free protease inhibitors) and sonicated for 30 min using a Sonoplus Ultrasonic Homogenizer HD 3100 (Bandelin). The lysate was centrifuged for 1 h at 21,500 rpm. The supernatant was loaded on a Histrap FF column (GE Healthcare), washed, and eluted in a gradient to 250 mm imidazole. The His tag was cleaved, and the protein sample was dialyzed overnight in 40 mm HEPES-NaOH, pH 7.5, 500 mm NaCl, 10% (w/v) glycerol, 15 mm imidazole, 2 mm β-mercaptoethanol. The cleaved protein was again loaded on a Histrap column and collected in the flow-through. The sample was then treated with RNase A (Sigma) and loaded on a HiPrepTM Heparin 16/60 column (GE Healthcare) in 25 mm TRIS-HCl, pH 8.0, 50 mm NaCl, 5% (v/v) glycerol, 2 mm DTT, washed, and eluted with a linear gradient to 750 mm NaCl. Fractions containing the protein of interest were pooled, concentrated, and loaded on a HiLoad Superdex 200 16/60 column (GE Healthcare) in gel filtration buffer (10 mm TRIS-HCl, pH 7.5, 200 mm NaCl). Fractions containing the protein of interest were pooled, concentrated to 10 mg/ml, flash-frozen in liquid nitrogen and stored at −80 °C until use. For activity assays, the protein tag was not cleaved, and 20% (v/v) glycerol was included in all buffers.
hJab1 variants were purified as described before (29). Briefly, insect cell pellets were resuspended in 50 mm TRIS-HCl, pH 8.0, 300 mm NaCl, 5% (v/v) glycerol, 0.05% (v/v) Nonidet P-40, 2 mm DTT, supplemented with Complete EDTA-free protease inhibitors. After sonication and centrifugation, the protein was captured on GSH beads. After washing, the protein was eluted with 10 mm reduced GSH. The buffer was exchanged with a HiLoad Superdex 75 26/60 column to 50 mm TRIS-HCl, pH 8.0, 300 mm NaCl, 5% (v/v) glycerol, 2 mm DTT. The GST tag was cleaved overnight with PreScission protease, and the protein was collected in the unbound fraction after adding fresh GSH beads. The protein was further purified by gel filtration in 10 mm TRIS-HCl, pH 8.0, 150 mm NaCl. Fractions containing the protein of interest were pooled, concentrated to 10 mg/ml, flash-frozen in liquid nitrogen, and stored at −80 °C until use.
For complex formation, hBRR2T1 variants were combined with a 1.5-fold molar excess of hJab1 variants and loaded on a Superdex 200 10/300 global increase column (GE Healthcare) in 20 mm TRIS-HCl, pH 8.0, 150 mm NaCl. Fractions containing the complex of interest were pooled, concentrated to 6 mg/ml, flash-frozen in liquid nitrogen, and stored at −80 °C until use.
The ySnu13 protein was purified as described before (9). The buffer was exchanged to 20 mm HEPES-NaOH, pH 7.0, 300 mm NaCl, and the protein was concentrated to 17.5 mg/ml.
Crystallographic analyses
hBRR2T1-hJab1 complexes were crystallized in sitting drops on 24-well plates with drops containing 1 μl of protein complex solution and 1 μl of reservoir solution (0.1 m HEPES-NaOH, pH 8.0, 0.1 m MgCl2, 8% PEG 3350). Crystals were cryoprotected by transfer into reservoir solution supplemented with 25% (v/v) ethylene glycol.
Isolated hBRR2T1 variants were crystallized in sitting drops on 24-well plates with drops containing 1 μl of protein solution and 1 μl of reservoir solution (0.1 m sodium citrate, 1.5 m sodium malonate, pH 7.0). Crystals were cryoprotected in 0.1 m sodium citrate, 3.0 m sodium malonate, pH 7.0, 0.1 m NaCl. All crystals were flash-frozen in liquid nitrogen.
Data sets were collected on beamlines 14.1 and 14.2 of the BESSY II storage (Berlin, Germany). The data were processed with XDS (68). Structures were solved by molecular replacement with Phenix (69), using structure coordinates of isolated hBRR2T1 (PDB entry 4F91) and hBRR2T1-hJab1 complex (PDB entry 4KIT). Models were refined by automated refinement in Phenix or REFMAC5 (70) alternating with manual model building in Coot (71). Structure figures were prepared using PyMOL (version 1.8; Schrödinger, LLC).
Structural comparisons
For visualization of the different domain orientations (Fig. 2), hBRR2 models from crystal and cryo-EM structures were aligned to the crystal structure of isolated hBRR2T1 (PDB entry 4F91) according to Cα atoms of residues 1–1288 (NC), using the “Align” routine in PyMOL. For calculation of overall r.m.s.d. values (Table 2), hBRR2 models from cryo-EM structures were aligned to the crystal structures of isolated hBRR2T1 (PDB entry 4F91) or of hBRR2T1 in the hBRR2T1-hJab1ΔCtail complex (this work) according to all Cα atoms, using the “Align” routine in PyMOL.
For the calculation of the rotation angles, hBRR2 structures were aligned to hBRR2T1 (PDB entry 4F91) using the “LSQ Superpose” routine in Coot. Cα atoms of residues 1291–2125 (CC) of isolated hBRR2T1 were then aligned to the repositioned hBRR2 models from crystal and cryo-EM structures. The angle α was calculated from the trace from the resulting rotation matrix (Scheme 1),
where trace = a11 + a22 + a33 = n − 2 + 2 × cosα, in which n was set to 3 for three-dimensional space.
Differential scanning fluorimetry
For thermal shift analyses, hBRR2T1 variants were diluted to 0.2 mg/ml in 40 mm TRIS-HCl, pH 7.5, 50 mm NaCl, 0.5 mm MgCl2 with or without 2 mm DTT, and supplemented with SyproOrange Protein Gel Stain 1:500 (Sigma). After incubation on ice for 1.5 h, the protein solutions were 1:1 diluted in either 40 mm TRIS-HCl, pH 7.5, 50 mm NaCl, 0.5 mm MgCl2 with or without 2 mm DTT or in size exclusion buffer with or without 2 mm DTT. Melting curves were recorded by monitoring the fluorescence at 569 nm while heating the samples from 25 to 95 °C at 1 °C/min.
Mass spectrometry
Protein digestion for LC-MS analysis was conducted according to Ref. 72. Briefly, 25 μg of protein were denatured in 8 m urea, 100 mm TRIS-HCl, pH 6.5. One half of the sample was directly alkylated by the addition of 2 mm NEM for 4 h at 37 °C (nonreduced sample). The other half was first reduced by the addition of 4 mm DTT for 2 h at 37 °C, and subsequently free thiols were alkylated by the addition of 2 mm NEM for 2 h at 37 °C (reduced sample). Proteins were digested by the addition of LysC protease for 4 h at 37 °C. After a 4-fold dilution in 100 mm TRIS-HCl, pH 6.5, the digestion was completed by the addition of trypsin and overnight incubation at 37 °C. The reaction was stopped by the addition of 3% (v/v) TFA, 5% (v/v) acetonitrile, and samples were desalted by STAGE tips as described before (73).
Peptides were reconstituted in 20 μl of 0.1% (v/v) TFA, 5% (v/v) acetonitrile in water, and 2 μl were analyzed by a reverse-phase capillary nano-LC system (Ultimate 3000, Thermo Scientific) connected to an Orbitrap Velos mass spectrometer (Thermo Scientific). Samples were desalted on a trap column (PepMap100 C18, 3 μm, 100 Å, 75-μm inner diameter × 2 cm; Thermo Scientific) using a mobile phase of 0.05% (v/v) TFA, 2% (v/v) acetonitrile in water. LC separations were performed on a capillary column (Acclaim PepMap100 C18, 2 μm, 100 Å, 75-μm inner diameter × 25 cm; Thermo Scientific) at an eluent flow rate of 300 nl/min. Mobile phase A contained 0.1% (v/v) formic acid in water, and mobile phase B contained 0.1% (v/v) formic acid in acetonitrile. The column was pre-equilibrated with 3% mobile phase B followed by a linear increase to 50% mobile phase B over 50 min. Mass spectra were acquired in a data-dependent mode, utilizing a single MS survey scan (r = 30,000) in the Orbitrap, followed by up to 20 higher-energy collisional dissociation scans (r = 7,500), using a normalized collision energy of 35 excluding +1 precursor ions. Extracted ion chromatograms and spectra were analyzed by Thermo Scientific Xcalibur 2.2.
U4*/U6 unwinding assays
U4*/U6 snRNA duplex (where U4* is U4 radioactively labeled) was prepared as described previously (9). Briefly, full-length yeast U4* snRNA was annealed with full-length U6 snRNA in 20 mm TRIS-HCl, pH 7.5, 50 mm NaCl, 18 mm MgCl2. Annealed duplex was separated from single strands by 6% native PAGE at 200 V for 1 h. The duplex regions were extracted from the gel in 20 mm TRIS-HCl, pH 8.0, 300 mm NaCl, 10 mm EDTA, 0.5% (v/v) SDS, further purified by phenol-chloroform extraction, and precipitated with LiCl and isopropyl alcohol. The RNA was washed with 70% ethanol and dissolved in 40 mm TRIS-HCl, pH 7.5, 100 mm NaCl.
For unwinding assays, 200 nm hBRR2T1 variants with or without 500 nm hJab1ΔCtail, or with or without 500 nm Snu13, were incubated for 3 min at 30 °C with 0.65 nm U4*/U6 duplex in unwinding buffer, supplemented with 8% (v/v) glycerol, 15 ng/μl acetylated BSA, 1 unit/μl RNasin (MoloX), with or without 2 mm DTT. The unwinding reactions were started by the addition of 1.7 mm ATP/MgCl2. 10-μl samples were taken at the indicated time points, and the reactions were stopped by mixing with 40 mm TRIS-HCl, pH 7.4, 50 mm NaCl, 25 mm EDTA, 1% (w/v) SDS, 10% (v/v) glycerol, 0.05% (w/v) xylene cyanol, 0.05% (w/v) bromphenol blue. The samples were loaded on a 6% native PAGE and separated at 200 V, 4 °C for 1 h. The gels were transferred to a filter paper and visualized by autoradiography, and bands were quantified with the ImageQuant software. The data of three independent replicates were fitted to the equation, y = A(1 − exp(−kut)), in which y is the fraction U4*/U6 unwound, A is the amplitude of the reaction, ku is the apparent rate constant of unwinding, and t is time, by using GraphPad Prism.
ATPase assays
ATP hydrolysis reactions were started by the addition of 1 mm ATP/MgCl2, supplemented with a small amount of [α-32P]ATP, to 100 nm hBRR2T1 variants and 1 μm U4/U6 duplex in unwinding buffer. Samples were incubated for 30 min at 30 °C, reactions were stopped by the addition of 50 mm EDTA, and samples were transferred to a TLC polyethyleneimine cellulose F plate (Merck). TLC was run with 20% (v/v) EtOH, 6% (v/v) acetic acid, 0.5 m LiCl. The plate was dried, and ATP/ADP signals were visualized by autoradiography. Radioactive spots were quantified with the ImageQuant software, and the number of hydrolyzed ATP molecules per hBRR2T1 variant molecule per minute was calculated based on three independent replicates.
Fluorescence polarization assays
10 nm FAM-labeled RNA duplex bearing a 3′-single-stranded overhang (5′-CGCGUGCUGGUCGAAAUUUAAUUAUAAACCAGACCGUC-3′/5′-GACCAGCACGCG-3′-[5-FAM]); regions forming the duplex in boldface type; IBA) were incubated with increasing concentrations of hBRR2T1 variants (0, 50, 75, 100, 150, 200, 500, 1,000, 1,250, 1,500, and 2,000 nm) in 40 mm HEPES-NaOH, pH 7.5, 50 mm NaCl, 0.5 mm MgCl2, 8% (v/v) glycerol, 10 nm acetylated BSA at room temperature. Samples were measured in a Victor V3 1420 plate reader. The fluorescence polarization values for three independent replicates were plotted with GraphPad Prism. Kd values were determined by fitting the data to a Hill equation, fraction bound = Acprotn/(cprotn + Kdn), in which A is the maximum of bound RNA, cprot is the protein concentration, Kd is the dissociation constant, and n is the Hill coefficient.
Electrophoretic mobility shift assays
Increasing concentrations of hBRR2T1 variants (0, 0.25, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.5, 2.0, and 3.0 μm) were incubated with 1 nm radioactive U4*/U6 duplex RNA for 3 min at 30 °C in unwinding buffer, supplemented with 0.25 mg/ml yeast tRNA. The samples were mixed with 40 mm TRIS-HCl, pH 7.4, 50 mm NaCl, 25 mm EDTA, 10% (v/v) glycerol, 0.05% (w/v) xylene cyanol, 0.05% (w/v) bromphenol blue and run on a 4% native PAGE at 170 V, 4 °C for 2–3 h. The gels were transferred to filter papers and visualized by autoradiography. Quantification of three independent replicates was done with ImageQuant software and plotted with GraphPad Prism. Kd values were determined as described above.
Author contributions
K. V., K. F. S., B. K., C. W., and M. C. W. formal analysis; K. V., K. F. S., B. K., C. W., and M. C. W. validation; K. V., K. F. S., B. K., and C. W. investigation; K. V., K. F. S., B. K., C. W., and M. C. W. visualization; K. V., K. F. S., B. K., C. W., and M. C. W. methodology; K. V. writing-original draft; K. F. S., C. W., and M. C. W. supervision; K. F. S., B. K., C. W., and M. C. W. writing-review and editing; C. W. and M. C. W. resources; M. C. W. conceptualization; M. C. W. data curation; M. C. W. funding acquisition; M. C. W. project administration.
Supplementary Material
Acknowledgments
We thank Eva Absmeier (Freie Universität Berlin) for helpful discussions. We accessed beamlines of the BESSY II storage ring (Berlin, Germany) via the Joint Berlin MX-Laboratory sponsored by Helmholtz Zentrum Berlin für Materialien und Energie, Freie Universität Berlin, Humboldt-Universität zu Berlin, Max-Delbrück Centrum for Molecular Medicine, Leibniz-Institut für Molekulare Pharmakologie, and Charité-Universitätsmedizin Berlin. For MS (B. K. and C. W.), we acknowledge the assistance of the Core Facility BioSupraMol supported by the Deutsche Forschungsgemeinschaft (DFG).
This work was supported by Deutsche Forschungsgemeinschaft Grant TRR186/A15-1 (to M. C. W.) and by a Dahlem International Network PostDoc Fellowship from Freie Universität Berlin (to K. F. S.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1 and S2.
The atomic coordinates and structure factors (codes 6S8Q, 6S8O, and 6S9I) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- NTPase
- nucleic acid–dependent nucleotide triphosphatase
- BESSY II
- Berliner Elektronenspeicherring-Gesellschaft für Synchrotronstrahlung II
- CC
- C-terminal cassette
- DSF
- differential scanning fluorimetry
- h
- human
- hBRR2T1-SS-linear
- hBRR2T1-M641C/A1582C
- hBRR2T1-SS-rot+
- hBRR2T1-D534C/N1866C
- NC
- N-terminal cassette
- NEM
- N-ethylmaleimide
- pre-mRNA
- precursor messenger RNA
- r.m.s.d.
- root mean square deviation
- RNP
- ribonucleoprotein complex
- snRNP
- small nuclear RNP
- snRNA
- small nuclear RNA
- T1
- truncation 1
- TFA
- trifluoroacetic acid
- SF
- superfamily
- PDB
- Protein Data Bank
- TLC
- thin layer chromatography
- FAM
- fluorescein amidite.
References
- 1. Byrd A. K., and Raney K. D. (2012) Superfamily 2 helicases. Front Biosci. (Landmark Ed.) 17, 2070–2088 10.2741/4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanner N. K., and Linder P. (2001) DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol. Cell 8, 251–262 10.1016/S1097-2765(01)00329-X [DOI] [PubMed] [Google Scholar]
- 3. Jankowsky A., Guenther U. P., and Jankowsky E. (2011) The RNA helicase database. Nucleic Acids Res. 39, D338–D341 10.1093/nar/gkq1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Q., and Jankowsky E. (2005) ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry 44, 13591–13601 10.1021/bi0508946 [DOI] [PubMed] [Google Scholar]
- 5. Halls C., Mohr S., Del Campo M., Yang Q., Jankowsky E., and Lambowitz A. M. (2007) Involvement of DEAD-box proteins in group I and group II intron splicing: biochemical characterization of Mss116p, ATP hydrolysis-dependent and -independent mechanisms, and general RNA chaperone activity. J. Mol. Biol. 365, 835–855 10.1016/j.jmb.2006.09.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballut L., Marchadier B., Baguet A., Tomasetto C., Séraphin B., and Le Hir H. (2005) The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat. Struct. Mol. Biol. 12, 861–869 10.1038/nsmb990 [DOI] [PubMed] [Google Scholar]
- 7. Jankowsky E., Gross C. H., Shuman S., and Pyle A. M. (2001) Active disruption of an RNA-protein interaction by a DExH/D RNA helicase. Science 291, 121–125 10.1126/science.291.5501.121 [DOI] [PubMed] [Google Scholar]
- 8. Bowers H. A., Maroney P. A., Fairman M. E., Kastner B., Lührmann R., Nilsen T. W., and Jankowsky E. (2006) Discriminatory RNP remodeling by the DEAD-box protein DED1. RNA 12, 903–912 10.1261/rna.2323406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Theuser M., Höbartner C., Wahl M. C., and Santos K. F. (2016) Substrate-assisted mechanism of RNP disruption by the spliceosomal Brr2 RNA helicase. Proc. Natl. Acad. Sci. U.S.A. 113, 7798–7803 10.1073/pnas.1524616113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caruthers J. M., and McKay D. B. (2002) Helicase structure and mechanism. Curr. Opin. Struct. Biol. 12, 123–133 10.1016/S0959-440X(02)00298-1 [DOI] [PubMed] [Google Scholar]
- 11. Singleton M. R., Dillingham M. S., and Wigley D. B. (2007) Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 76, 23–50 10.1146/annurev.biochem.76.052305.115300 [DOI] [PubMed] [Google Scholar]
- 12. Fairman-Williams M. E., Guenther U. P., and Jankowsky E. (2010) SF1 and SF2 helicases: family matters. Curr. Opin. Struct. Biol. 20, 313–324 10.1016/j.sbi.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim D. H., and Rossi J. J. (1999) The first ATPase domain of the yeast 246-kDa protein is required for in vivo unwinding of the U4/U6 duplex. RNA 5, 959–971 10.1017/S135583829999012X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J., Ding S. C., Cho H., Chung B. C., Gale M. Jr., Chanda S. K., and Diamond M. S. (2013) A short hairpin RNA screen of interferon-stimulated genes identifies a novel negative regulator of the cellular antiviral response. mBio 4, e00385–13 10.1128/mBio.00385-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knierim E., Hirata H., Wolf N. I., Morales-Gonzalez S., Schottmann G., Tanaka Y., Rudnik-Schöneborn S., Orgeur M., Zerres K., Vogt S., van Riesen A., Gill E., Seifert F., Zwirner A., Kirschner J., et al. (2016) Mutations in subunits of the activating signal cointegrator 1 complex are associated with prenatal spinal muscular atrophy and congenital bone fractures. Am. J. Hum. Genet. 98, 473–489 10.1016/j.ajhg.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davignon L., Chauveau C., Julien C., Dill C., Duband-Goulet I., Cabet E., Buendia B., Lilienbaum A., Rendu J., Minot M. C., Guichet A., Allamand V., Vadrot N., Fauré J., Odent S., et al. (2016) The transcription coactivator ASC-1 is a regulator of skeletal myogenesis, and its deficiency causes a novel form of congenital muscle disease. Hum. Mol. Genet. 25, 1559–1573 10.1093/hmg/ddw033 [DOI] [PubMed] [Google Scholar]
- 17. Dango S., Mosammaparast N., Sowa M. E., Xiong L. J., Wu F., Park K., Rubin M., Gygi S., Harper J. W., and Shi Y. (2011) DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol. Cell 44, 373–384 10.1016/j.molcel.2011.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brickner J. R., Soll J. M., Lombardi P. M., Vågbø C. B., Mudge M. C., Oyeniran C., Rabe R., Jackson J., Sullender M. E., Blazosky E., Byrum A. K., Zhao Y., Corbett M. A., Gécz J., Field M., et al. (2017) A ubiquitin-dependent signalling axis specific for ALKBH-mediated DNA dealkylation repair. Nature 551, 389–393 10.1038/nature24484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuo Y., Ikeuchi K., Saeki Y., Iwasaki S., Schmidt C., Udagawa T., Sato F., Tsuchiya H., Becker T., Tanaka K., Ingolia N. T., Beckmann R., and Inada T. (2017) Ubiquitination of stalled ribosome triggers ribosome-associated quality control. Nat. Commun. 8, 159 10.1038/s41467-017-00188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wahl M. C., Will C. L., and Lührmann R. (2009) The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 10.1016/j.cell.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 21. Raghunathan P. L., and Guthrie C. (1998) RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr. Biol. 8, 847–855 10.1016/S0960-9822(07)00345-4 [DOI] [PubMed] [Google Scholar]
- 22. Wolff T., and Bindereif A. (1993) Conformational changes of U6 RNA during the spliceosome cycle: an intramolecular helix is essential both for initiating the U4-U6 interaction and for the first step of slicing. Genes Dev. 7, 1377–1389 10.1101/gad.7.7b.1377 [DOI] [PubMed] [Google Scholar]
- 23. Koodathingal P., and Staley J. P. (2013) Splicing fidelity: DEAD/H-box ATPases as molecular clocks. RNA Biol. 10, 1073–1079 10.4161/rna.25245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Semlow D. R., Blanco M. R., Walter N. G., and Staley J. P. (2016) Spliceosomal DEAH-box ATPases remodel pre-mRNA to activate alternative splice sites. Cell 164, 985–998 10.1016/j.cell.2016.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Semlow D. R., and Staley J. P. (2012) Staying on message: ensuring fidelity in pre-mRNA splicing. Trends Biochem. Sci. 37, 263–273 10.1016/j.tibs.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graveley B. R. (2001) Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 17, 100–107 10.1016/S0168-9525(00)02176-4 [DOI] [PubMed] [Google Scholar]
- 27. Absmeier E., Santos K. F., and Wahl M. C. (2016) Functions and regulation of the Brr2 RNA helicase during splicing. Cell Cycle 15, 3362–3377 10.1080/15384101.2016.1249549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Absmeier E., Wollenhaupt J., Mozaffari-Jovin S., Becke C., Lee C. T., Preussner M., Heyd F., Urlaub H., Lührmann R., Santos K. F., and Wahl M. C. (2015) The large N-terminal region of the Brr2 RNA helicase guides productive spliceosome activation. Genes Dev. 29, 2576–2587 10.1101/gad.271528.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mozaffari-Jovin S., Wandersleben T., Santos K. F., Will C. L., Lührmann R., and Wahl M. C. (2013) Inhibition of RNA helicase Brr2 by the C-terminal tail of the spliceosomal protein Prp8. Science 341, 80–84 10.1126/science.1237515 [DOI] [PubMed] [Google Scholar]
- 30. Mozaffari-Jovin S., Wandersleben T., Santos K. F., Will C. L., Lührmann R., and Wahl M. C. (2014) Novel regulatory principles of the spliceosomal Brr2 RNA helicase and links to retinal disease in humans. RNA Biol. 11, 298–312 10.4161/rna.28353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maeder C., Kutach A. K., and Guthrie C. (2009) ATP-dependent unwinding of U4/U6 snRNAs by the Brr2 helicase requires the C terminus of Prp8. Nat. Struct. Mol. Biol. 16, 42–48 10.1038/nsmb.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wollenhaupt J., Henning L. M., Sticht J., Becke C., Freund C., Santos K. F., and Wahl M. C. (2018) Intrinsically disordered protein Ntr2 modulates the spliceosomal RNA helicase Brr2. Biophys. J. 114, 788–799 10.1016/j.bpj.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henning L. M., Santos K. F., Sticht J., Jehle S., Lee C. T., Wittwer M., Urlaub H., Stelzl U., Wahl M. C., and Freund C. (2017) A new role for FBP21 as regulator of Brr2 helicase activity. Nucleic Acids Res. 45, 7922–7937 10.1093/nar/gkx535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santos K. F., Jovin S. M., Weber G., Pena V., Lührmann R., and Wahl M. C. (2012) Structural basis for functional cooperation between tandem helicase cassettes in Brr2-mediated remodeling of the spliceosome. Proc. Natl. Acad. Sci. U.S.A. 109, 17418–17423 10.1073/pnas.1208098109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L., Xu T., Maeder C., Bud L. O., Shanks J., Nix J., Guthrie C., Pleiss J. A., and Zhao R. (2009) Structural evidence for consecutive Hel308-like modules in the spliceosomal ATPase Brr2. Nat. Struct. Mol. Biol. 16, 731–739 10.1038/nsmb.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Nues R. W., and Beggs J. D. (2001) Functional contacts with a range of splicing proteins suggest a central role for Brr2p in the dynamic control of the order of events in spliceosomes of Saccharomyces cerevisiae. Genetics 157, 1451–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cordin O., Hahn D., Alexander R., Gautam A., Saveanu C., Barrass J. D., and Beggs J. D. (2014) Brr2p carboxy-terminal Sec63 domain modulates Prp16 splicing RNA helicase. Nucleic Acids Res. 42, 13897–13910 10.1093/nar/gku1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bertram K., Agafonov D. E., Dybkov O., Haselbach D., Leelaram M. N., Will C. L., Urlaub H., Kastner B., Lührmann R., and Stark H. (2017) Cryo-EM structure of a pre-catalytic human spliceosome primed for activation. Cell 170, 701–713.e11 10.1016/j.cell.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 39. Agafonov D. E., Kastner B., Dybkov O., Hofele R. V., Liu W. T., Urlaub H., Lührmann R., and Stark H. (2016) Molecular architecture of the human U4/U6.U5 tri-snRNP. Science 351, 1416–1420 10.1126/science.aad2085 [DOI] [PubMed] [Google Scholar]
- 40. Zhan X., Yan C., Zhang X., Lei J., and Shi Y. (2018) Structures of the human pre-catalytic spliceosome and its precursor spliceosome. Cell Res. 28, 1129–1140 10.1038/s41422-018-0094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X., Yan C., Hang J., Finci L. I., Lei J., and Shi Y. (2017) An atomic structure of the human spliceosome. Cell 169, 918–929.e14 10.1016/j.cell.2017.04.033 [DOI] [PubMed] [Google Scholar]
- 42. Zhang X., Yan C., Zhan X., Li L., Lei J., and Shi Y. (2018) Structure of the human activated spliceosome in three conformational states. Cell Res. 28, 307–322 10.1038/cr.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhan X., Yan C., Zhang X., Lei J., and Shi Y. (2018) Structure of a human catalytic step I spliceosome. Science 359, 537–545 10.1126/science.aar6401 [DOI] [PubMed] [Google Scholar]
- 44. Zhang X., Zhan X., Yan C., Zhang W., Liu D., Lei J., and Shi Y. (2019) Structures of the human spliceosomes before and after release of the ligated exon. Cell Res. 29, 274–285 10.1038/s41422-019-0143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yan C., Wan R., Bai R., Huang G., and Shi Y. (2016) Structure of a yeast activated spliceosome at 3.5 Å resolution. Science 353, 904–911 10.1126/science.aag0291 [DOI] [PubMed] [Google Scholar]
- 46. Nguyen T. H. D., Galej W. P., Bai X. C., Oubridge C., Newman A. J., Scheres S. H. W., and Nagai K. (2016) Cryo-EM structure of the yeast U4/U6.U5 tri-snRNP at 3.7 Å resolution. Nature 530, 298–302 10.1038/nature16940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rauhut R., Fabrizio P., Dybkov O., Hartmuth K., Pena V., Chari A., Kumar V., Lee C. T., Urlaub H., Kastner B., Stark H., and Lührmann R. (2016) Molecular architecture of the Saccharomyces cerevisiae activated spliceosome. Science 353, 1399–1405 10.1126/science.aag1906 [DOI] [PubMed] [Google Scholar]
- 48. Wan R., Yan C., Bai R., Huang G., and Shi Y. (2016) Structure of a yeast catalytic step I spliceosome at 3.4 Å resolution. Science 353, 895–904 10.1126/science.aag2235 [DOI] [PubMed] [Google Scholar]
- 49. Plaschka C., Lin P. C., and Nagai K. (2017) Structure of a pre-catalytic spliceosome. Nature 546, 617–621 10.1038/nature22799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galej W. P., Wilkinson M. E., Fica S. M., Oubridge C., Newman A. J., and Nagai K. (2016) Cryo-EM structure of the spliceosome immediately after branching. Nature 537, 197–201 10.1038/nature19316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Absmeier E., Becke C., Wollenhaupt J., Santos K. F., and Wahl M. C. (2017) Interplay of cis- and trans-regulatory mechanisms in the spliceosomal RNA helicase Brr2. Cell Cycle 16, 100–112 10.1080/15384101.2016.1255384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yankiwski V., Noonan J. P., and Neff N. F. (2001) The C-terminal domain of the Bloom syndrome DNA helicase is essential for genomic stability. BMC Cell Biol. 2, 11 10.1186/1471-2121-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Putnam A. A., Gao Z., Liu F., Jia H., Yang Q., and Jankowsky E. (2015) Division of labor in an oligomer of the DEAD-box RNA helicase Ded1p. Mol. Cell 59, 541–552 10.1016/j.molcel.2015.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Singleton M. R., Scaife S., Raven N. D., and Wigley D. B. (2001) Crystallization and preliminary X-ray analysis of RecG, a replication-fork reversal helicase from Thermotoga maritima complexed with a three-way DNA junction. Acta Crystallogr. D Biol. Crystallogr. 57, 1695–1696 10.1107/S0907444901013105 [DOI] [PubMed] [Google Scholar]
- 55. Walbott H., Mouffok S., Capeyrou R., Lebaron S., Humbert O., van Tilbeurgh H., Henry Y., and Leulliot N. (2010) Prp43p contains a processive helicase structural architecture with a specific regulatory domain. EMBO J. 29, 2194–2204 10.1038/emboj.2010.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mojumdar A., De March M., Marino F., and Onesti S. (2017) The human RecQ4 helicase contains a functional RecQ C-terminal region (RQC) that is essential for activity. J. Biol. Chem. 292, 4176–4184 10.1074/jbc.M116.767954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X., Lai T. Y., Chiang S. W., Tam P. O., Liu D. T., Chan C. K., Pang C. P., Zhao C., and Chen L. J. (2013) Contribution of SNRNP200 sequence variations to retinitis pigmentosa. Eye (Lond.) 27, 1204–1213 10.1038/eye.2013.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mozaffari-Jovin S., Santos K. F., Hsiao H. H., Will C. L., Urlaub H., Wahl M. C., and Lührmann R. (2012) The Prp8 RNase H-like domain inhibits Brr2-mediated U4/U6 snRNA unwinding by blocking Brr2 loading onto the U4 snRNA. Genes Dev. 26, 2422–2434 10.1101/gad.200949.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koodathingal P., Novak T., Piccirilli J. A., and Staley J. P. (2010) The DEAH box ATPases Prp16 and Prp43 cooperate to proofread 5′ splice site cleavage during pre-mRNA splicing. Mol. Cell 39, 385–395 10.1016/j.molcel.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang F., Wang X. Y., Zhang Z. M., Pu J., Fan Y. J., Zhou J., Query C. C., and Xu Y. Z. (2013) Splicing proofreading at 5′ splice sites by ATPase Prp28p. Nucleic Acids Res. 41, 4660–4670 10.1093/nar/gkt149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liang W. W., and Cheng S. C. (2015) A novel mechanism for Prp5 function in prespliceosome formation and proofreading the branch site sequence. Genes Dev. 29, 81–93 10.1101/gad.253708.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mayas R. M., Maita H., and Staley J. P. (2006) Exon ligation is proofread by the DExD/H-box ATPase Prp22p. Nat. Struct. Mol. Biol. 13, 482–490 10.1038/nsmb1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mayas R. M., Maita H., Semlow D. R., and Staley J. P. (2010) Spliceosome discards intermediates via the DEAH box ATPase Prp43p. Proc. Natl. Acad. Sci. U.S.A. 107, 10020–10025 10.1073/pnas.0906022107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papasaikas P., Tejedor J. R., Vigevani L., and Valcárcel J. (2015) Functional splicing network reveals extensive regulatory potential of the core spliceosomal machinery. Mol. Cell 57, 7–22 10.1016/j.molcel.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 65. Park J. W., Parisky K., Celotto A. M., Reenan R. A., and Graveley B. R. (2004) Identification of alternative splicing regulators by RNA interference in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 101, 15974–15979 10.1073/pnas.0407004101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cvačková Z., Matějů D., and Staněk D. (2014) Retinitis pigmentosa mutations of SNRNP200 enhance cryptic splice-site recognition. Hum. Mutat. 35, 308–317 10.1002/humu.22481 [DOI] [PubMed] [Google Scholar]
- 67. Hoskins A. A., Rodgers M. L., Friedman L. J., Gelles J., and Moore M. J. (2016) Single molecule analysis reveals reversible and irreversible steps during spliceosome activation. eLife 5, e14166 10.7554/eLife.14166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., and Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 10.1107/S0907444902016657 [DOI] [PubMed] [Google Scholar]
- 70. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu S., Fan S. B., Yang B., Li Y. X., Meng J. M., Wu L., Li P., Zhang K., Zhang M. J., Fu Y., Luo J., Sun R. X., He S. M., and Dong M. Q. (2015) Mapping native disulfide bonds at a proteome scale. Nat. Methods 12, 329–331 10.1038/nmeth.3283 [DOI] [PubMed] [Google Scholar]
- 73. Rappsilber J., Ishihama Y., and Mann M. (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 10.1021/ac026117i [DOI] [PubMed] [Google Scholar]
- 74. Karplus P. A., and Diederichs K. (2015) Assessing and maximizing data quality in macromolecular crystallography. Curr. Opin. Struct. Biol. 34, 60–68 10.1016/j.sbi.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim H. J., Yi J. Y., Sung H. S., Moore D. D., Jhun B. H., Lee Y. C., and Lee J. W. (1999) Activating signal cointegrator 1, a novel transcription coactivator of nuclear receptors, and its cytosolic localization under conditions of serum deprivation. Mol Cell Biol. 19, 6323–6332 10.1128/mcb.19.9.6323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.