Abstract
A recently discovered ornithine–ammonia cycle (OAC) serves as a conduit in the nitrogen storage and remobilization machinery in cyanobacteria. The OAC involves an arginine catabolic reaction catalyzed by the arginine dihydrolase ArgZ whose catalytic mechanism is unknown. Here we determined the crystal structures at 1.2–3.0 Å of unliganded ArgZ from the cyanobacterium Synechocystis sp. PCC6803 and of ArgZ complexed with its substrate arginine, a covalently linked reaction intermediate, or the reaction product ornithine. The structures reveal that a key residue, Asn71, in the ArgZ active center functions as the determinant distinguishing ArgZ from other members of the guanidino group–modifying enzyme superfamily. The structures, along with biochemical evidence from enzymatic assays coupled with electrospray ionization MS techniques, further suggest that ArgZ-catalyzed conversion of arginine to ornithine, ammonia, and carbon dioxide consists of two successive cycles of amine hydrolysis. Finally, we show that arginine dihydrolases are broadly distributed among bacteria and metazoans, suggesting that the OAC may be frequently used for redistribution of nitrogen from arginine catabolism or nitrogen fixation.
Keywords: enzyme catalysis, nitrogen metabolism, enzyme structure, cyanobacteria, hydrolase, enzyme mechanism, arginine, arginine dihydrolase, arginine metabolism, cyanobacteria, ornithine–ammonia cycle
Introduction
Arginine is a nitrogen-rich amino acid involved in multiple biological processes in living organisms (1, 2). Beyond its role as a precursor for biosynthesis of proteins and polyamines, arginine is a key intermediate of the ornithine–urea cycle, an essential pathway for detoxification of ammonium and disposal of excess nitrogen in terrestrial animals (3). In plants, arginine is a major storage form of organic nitrogen, and arginine metabolism through arginase and urease plays a key role in nitrogen distribution and recycling (4). In bacteria, arginine also serves as a reservoir of nitrogen, carbon, and energy, which could be catabolized through various pathways, including the arginase pathway, the arginine deiminase (ADI)4 pathway, and the arginine succinyltransferase (AST) pathway. These pathways usually have different functions. For example, arginine degradation by the ADI pathway provides energy for anaerobic growth of many bacteria such as Streptococcus faecalis (5). The AST pathway serves as the major pathway in Escherichia coli and related bacteria for arginine catabolism as a sole nitrogen source (6).
Recently, we have discovered an ornithine–ammonia cycle (OAC) in cyanobacteria that starts with carbamoyl phosphate synthesis by a bacterium- and plant-type glutamine-dependent enzyme and ends with conversion of arginine to ornithine and ammonia by a new arginine dihydrolase, ArgZ (7). The OAC allows rapid remobilization of nitrogen reserves under starvation and a high rate of nitrogen assimilation and storage after the nutrient becomes available. This pathway confers substantial adaptability and robustness on cyanobacterial metabolism under environmental nitrogen fluctuations. The OAC might be a more ancient pathway than the ornithine–urea cycle because cyanobacteria have been distributed on Earth since the Proterozoic era (∼2.3 billion years ago). The OAC is widely present among cyanobacteria. Particularly, it is found in most oceanic N2-fixing cyanobacteria, which are distributed in oligotrophic tropical and subtropical oceans and are key contributors to marine nitrogen fixation (8). The OAC may be used for storing and redistributing fixed nitrogen within cyanophycin; thus, integration of the OAC with N2 fixation may have contributed to the success of these diazotrophs in the open ocean.
A key step of OAC is a new arginine catabolic reaction catalyzed by the arginine dihydrolase ArgZ, which employs two water molecules to catalyze the conversion of arginine to ornithine with release of two molecules of ammonia and one molecule of carbon dioxide (Fig. 1A). Although an identical reaction can be achieved from a concerted action of arginase and urease, ArgZ does not share sequence homology with either of the enzymes (7). The ArgZ protein comprises a N-terminal domain belonging to the guanidino group–modifying enzyme family (residues 1–269, referred to as the GME domain) (9–11), a middle domain similar to the N-terminal conserved domain of the lysine-oxoglutarate reductase/saccharopine dehydrogenase bifunctional enzyme (Pfam PF04455, residues 286–356, referred to as the LOR/SDH-NC domain)(12), and a C-terminal uncharacterized region (residues 364–705) (Fig. 1B). The arginine dihydrolase activity is attributed to the N-terminal domain of ArgZ, which exhibits a distant homology with arginine deiminases that convert arginine to citrulline with release of one molecule of ammonium (13), and with N-succinylarginine dihydrolase, which catalyzes the conversion of N-succinylarginine to N-succinylornithine (6). The catalytic mechanism of arginine dihydrolase is unknown.
Figure 1.
Overall structure of full-length ArgZ. A, diagram of enzymatic reactions by ArgZ. B, schematic of ArgZ domains. C, overall structure of ArgZ. The four protomers are shown in different colors; the N-terminal domains (NTD) are shown as in ribbon; and the middle and C-terminal domains (CTD) are shown as in surface. D, the size-exclusion chromatography profile indicates that ArgZ is a tetramer in solution. The calculated mass of the ArgZ tetramer is 313 kDa, and the molecular mass markers used are thyroglobin (669 kDa), apoferritin (443 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), albumin (66 kDa), and carbonic anhydrolase (29 kDa). AU, absorption unit. E, the structure of one ArgZ protomer. The dashed line indicates 5-fold pseudosymmetry of the N-terminal AT domain. The colors are as in B.
In this study, we determined a crystal structure of the unliganded enzyme of the full-length cyanobacterium Synechocystis sp. PCC6803 ArgZ at 3.0 Å and three crystal structures of the N-terminal domain of ArgZ (ArgZ-N) complexed with its substrate arginine, a reaction intermediate, or its product ornithine at 1.1–1.2 Å. The structures show that the ArgZ N-terminal GME domain exhibits a classic α/β propeller fold responsible for dihydrolysis and that the ArgZ middle and C-terminal domains are responsible for tetramer organization. Together with biochemical data, the high-resolution crystal structures of ArgZ-N complexes suggest a “bond rotation” catalytic mechanism that involves two successive hydrolysis steps and a rotation of a covalently linked reaction intermediate in between. We demonstrate that an asparagine residue (Asn71) in the ArgZ active center functions as the determinant to distinguish arginine dihydrolase from the large GME superfamily. In the latter, we find that the homologs of ArgZ-N are broadly distributed in bacteria and metazoans.
Results
Overall structure of the full-length ArgZ
To understand the structural basis of the unique enzymatic reaction catalyzed by ArgZ, we determined a crystal structure of an unliganded enzyme of full-length Synechocystis sp. PCC6803 ArgZ at 3.0 Å (Table S1). ArgZ folds into a tetramer in one asymmetric unit (Fig. 1C), consistent with its elution profile of size-exclusion chromatography (Fig. 1D). The interprotomer interactions are mainly mediated by the middle LOR/SDH-NC and C-terminal domains of ArgZ, and the ArgZ-N GME domain of each protomer protrudes from the tetramer complex (Fig. 1C). The electron density map resolves most residues of the ArgZ-N catalytic domain (4–274), the middle LOR/SDH-NC domain (residues 286–360), and the C-terminal domain (residues 361–699), except some flexible loops.
The ArgZ protomers exhibit an extended conformation with minimal intradomain interactions. The ArgZ-N adopts the canonical fold of the GME superfamily, with 5-fold pseudosymmetric repeats of the α/β propeller consisting of a three-stranded β sheet and an α helix (Fig. 1E) (9–11). A structural similarity search using the Dali server suggests that the N-terminal catalytic domain shares high structural similarity with Arg:Gly amidinotransferase (14), ADI, dimethylarginine dimethylaminohydrolase (15), peptidyl-arginine deiminase (16), and the N-succinylarginine dihydrolase AstB (Fig. S1A) (6). The middle LOR/SDH-NC domain adopts a compact fold with an anti-parallel β-sheet packed on two α helices. The C-terminal region of ArgZ comprises a small domain (residues 364–442) of β strands (Fig. 1, B and E, blue) and a large Rossmann fold domain (residues 466–699; Fig. 1, B and E, and Fig. S1B, light blue) (17). Structures with folds similar to the C-terminal domain of ArgZ are dinucleotide-dependent enzymes with various activities (Fig. S1C) (18, 19).
The crystal structures of ArgZ-N complexed with substrate or product
Our previous study showed that ArgZ-N is capable of hydrolyzing arginine and releasing ammonium by a coupled enzymatic reaction (7). We confirmed that the ArgZ-N is sufficient to convert arginine to ornithine, carbon dioxide, and ammonium and even exhibits higher activity compared with the full-length ArgZ protein, suggesting that the ArgZ-N is the functional/catalytic domain of ArgZ dihydrolase (Fig. S2, A and B). To understand the structural basis of the dihydrolase activity of ArgZ, we determined a crystal structure at 1.20 Å of a substrate-bound complex by cocrystallization of substrate arginine with ArgZ-N (C264S) and a crystal structure at 1.14 Å of a product-bound complex by cocrystallization of the substrate arginine with WT ArgZ-N (Table S1). The ArgZ-N in the high-resolution structures folds exactly the same as the domain in the full-length ArgZ (root mean square deviation, 0.447 Å between Cα atoms; Fig. S2D), confirming that ArgZ-N is an independent catalytic domain (7). Superimposition of the two high-resolution binary structures of substrate or product-bound ArgZ-N reveals no conformational change of ArgZ-N (root mean square deviation, 0.077 Å between Cα atoms; Fig. 2A).
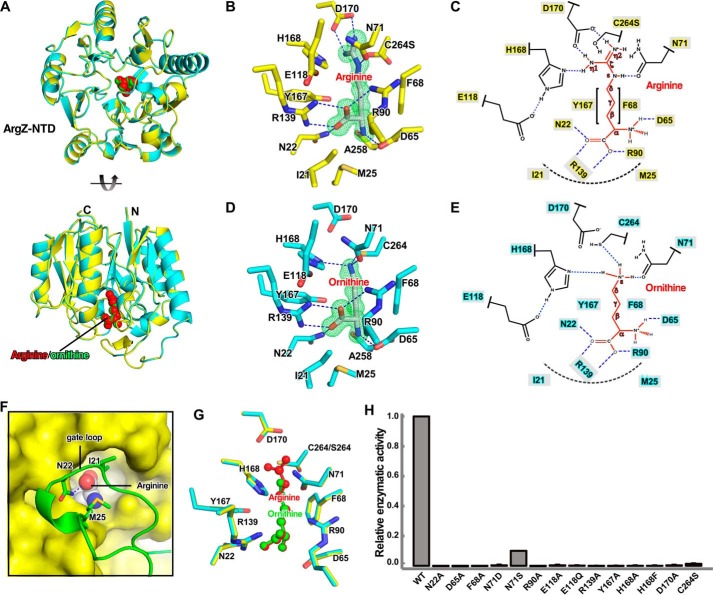
Figure 2.
Structure of ArgZ-N. A, side and top views of the superimposition of crystal structures of ArgZ(C264S)-N–arginine (yellow) and ArgZ-N–ornithine (cyan). Arginine (red) and ornithine (green) in the active site are shown as spheres. B, detailed interactions between the substrate arginine and the active center of ArgZ. The carbon atoms of the protein and the substrate arginine are shown in yellow and white, respectively. The N and O atoms are shown in blue and red, respectively. C, schematic of the interactions in B. D, detailed interactions between the product ornithine and the active center of ArgZ. The carbon atoms of the protein are shown in cyan and the rest of colors are as in B. E, schematic of the interactions in D. F, a gate loop (green) covers the active center (yellow surface) and stabilizes the substrate (spheres). G, comparison between substrate and product in the active center of ArgZ Atoms are colored as above. H, enzymatic activities of ArgZ derivatives, determined by GDH-coupled reactions. The experiments were repeated three times, and the results are presented as mean ± S.E. H-bonds and salt bridges are shown as blue dashes and van der Waals interactions are shown in dashed black arcs. Green mesh in B and D represents a simulated Fo-Fc omit polder map contoured at 6.0 σ.
In the structures, the catalytic cavity is located in a deep pocket at the middle of the 5-fold α/β propellers. The Fo-Fc simulated omit polder maps show clear electron density for the substrate arginine and the product ornithine in the structures of ArgZ-N(C264S)–arginine and ArgZ-N–ornithine, respectively (Fig. 2, B and D). The substrate arginine is buried in the catalytic pocket; a “gate loop” (residues Val17-His30, disordered in the ligand-free structure of the full-length ArgZ) seals the pocket and makes extensive interactions with the substrate arginine (Fig. 2F). Asn22 of the gate loop forms an H bond with the main-chain carboxyl moiety of the substrate arginine, whereas Ile21 and Met25 of the gate loop also contribute to substrate binding by making van der Waals interactions with the main-chain atoms of the substrate arginine (Fig. 2F).
ArgZ contacts every atom of the substrate arginine, explaining its high substrate specificity (7). The main-chain carboxyl moiety of the substrate arginine is stabilized by one H bond made by Asn22 and two salt bridges made by Arg139 and Arg90. The main-chain amine moiety of the substrate arginine is also stabilized by one H bond made by Ala258 and one salt bridge made by Asp65. The methylene moiety of the substrate arginine is embraced by hydrophobic side walls created by Phe68 and Tyr167. The guanidinium moiety of the substrate arginine resides in the center of the catalytic residues Cys264, His168, and Glu118 and are stabilized by two salt bridges with Asp170 and one H bond with Asn71. The less reactive C264S adopts two alternative conformations in the structure and makes no contact with the substrate (Fig. 2, B and C).
The product ornithine adopts the same conformation and makes exactly the same interactions with ArgZ as the substrate arginine, except that the guanidinium moiety has been converted into an amine, which makes three H-bonds with Cys264, His168, and Asn71 (Fig. 2, D, E, and G). Alanine substitution of residues contacting the substrate arginine or the product ornithine (N22A, D65A, F68A, R90A, R139A, and Y167A) all resulted in significant loss of catalytic activity (Fig. 2H).
A proposed catalytic mechanism of the dihydrolase ArgZ
Structure comparison of ArgZ with the PaADI (an arginine deiminase also in the GME superfamily; PDB code 2A9G) shows a very similar disposition of their catalytic residues around the guanidinium moiety of their substrate arginine (Fig. 3, A and B) (20). Mutating each residue of the catalytic Cys-His-Glu triad (C264S, H168F, or E118A) of ArgZ resulted in complete loss of enzymatic activity (Fig. 2H), suggesting that ArgZ utilizes the same Cys-His-Glu triad for catalysis as arginine deiminase.
Figure 3.
Proposed reaction mechanism of ArgZ. A, the active center of substrate-bound ArgZ(C264S). B, the active center of substrate-bound Pseudomonas aeruginosa ADI(C406A) (PDB code 2A9G). C, the active center of Mycoplasma arginini ADI (PDB code 1S9R) with a covalently bound intermediate II. D, the active center of substrate-bound E. coli AstB(C365S) (PDB code 1YNI). The carbon atoms of ArgZ, ADI, and AstB are shown in yellow, green, and light blue, respectively. The N and O atoms are shown in blue and red, respectively. E, the proposed reaction steps of the ArgZ-catalyzed two successive cycles of hydrolysis. The proposed side reaction producing L-citrulline is shown in a pink box. In the first cycle of hydrolysis, deprotonated Cys264 attacks and subsequently covalently links the Cζ of arginine, resulting in a tetrahedral intermediate I. Subsequently, the η1 amine group of the guanidium moiety attracts a proton back from imidazolinium of His168, resulting in collapse of the Nη1-Cζ bond to form intermediate II and release one molecule of ammonia. 180° rotation of the Nϵ-Cζ bond brings the η2 amine group close to His168, forming intermediated II*, and a water molecule deprotonated by the His-Glu pair attacks the Cζ of intermediate II* to form the tetrahedral intermediate III. In the second cycle of hydrolysis, His168 donates a proton to the η2 amine group and releases the second molecule of ammonia. A second water diffuses into the active center, where it is deprotonated by His168. The activated water molecule subsequently attacks Cζ of the covalently linked intermediate IV and forms a tetrahedral intermediate V. In the last step, the final products (CO2 and ornithine) are released, and Cys264 is freed through an unknown mechanism.
Arginine deiminase catalyzes one cycle of amine hydrolysis, converting one molecule of arginine and one molecule of water into one molecule of citrulline and one molecule of ammonia. The catalytic mechanism involves activation of the catalytic cysteine through His/Glu-aided deprotonation; nucleophilic attack of the substrate arginine by the catalytic cysteine, resulting in a covalently linked tetrahedral intermediate; collapse of the tetrahedral bond and release of one amine group; nucleophilic water attack, resulting in another covalently linked tetrahedral intermediate; and release of the product citrulline (13, 15, 20–22). Therefore, we propose that, like arginine deiminase, ArgZ employs Cys264 as a nucleophile for attacking the substrate arginine and His168/Glu118 as a general acid–base pair for proton abstraction and donation, but unlike arginine deiminase, ArgZ is able to catalyze two cycles of amine hydrolysis, and each cycle of hydrolysis resembles the deamination process catalyzed by arginine deiminase (13, 20) (Fig. 3E).
In the first cycle of hydrolysis by ArgZ, the thiol group of Cys264 attacks and subsequently covalently links to the atom Cζ of the substrate arginine, resulting in tetrahedral “intermediate I.” Subsequently, the η1 amine group of the guanidium moiety abstracts a proton back from imidazolinium of His168, resulting in collapse of the Nη1-Cζ bond, release of one molecule of ammonia, and formation of “intermediate II” (Fig. 3E).
To utilize the same catalytic triad for the second cycle of hydrolysis, ArgZ has to place the η2 amine group close to the His-Glu pair, which could be accomplished by a simple ∼180° rotation of the Nϵ-Cζ bond of intermediate II (Fig. 3E). A water molecule soon diffuses into the active center, becomes deprotonated/activated by the His-Glu pair, and attacks Cζ of “intermediate II*” to form a tetrahedral “intermediate III.” We were able to successfully detect a mass increase of ArgZ during the reaction with the ESI-MS technique, which exactly matches the molecular mass of covalently linked intermediate II (or intermediate II*), validating the first steps of the proposed reaction pathway (Fig. 4A). Subsequently, ArgZ catalyzes hydrolysis of the η2 amine group of intermediate III in a process similar to hydrolysis of the η1 amine group, releases CO2 and ornithine, and finally frees the thiol moiety of Cys264. We noticed that ArgZ also produces trace amounts of citrulline, probably through a side reaction branched from the pre-rotation intermediate II, consistent with the proposed catalytic mode of two successive amine hydrolyses (Figs. S2A and 3E).
Figure 4.
N71 is the determinant of the dihydrolase activity of ArgZ. A, the deconvoluted ESI-MS spectra of trapped reaction intermediates using WT ArgZ. Top panel, the spectra of ArgZ itself. Bottom panel, the spectra after a 5-min reaction. The calculated mass for unmodified ArgZ-N is 34,399 Da. The expected mass of ArgZ-N with covalently linked intermediate II is 34,557 (red peak with an expected excess mass of 158 Da). The peaks marked with an asterisk are attributable to a smaller fraction of nonenzymatically α-N-gluconoylated protein modified during expression with an expected excess mass of 178. B, the deconvoluted ESI-MS spectra of trapped reaction intermediates using ArgZ-N(N71S). The calculated mass for unmodified ArgZ-N(N71S) is 34,372 Da, and the expected mass of ArgZ-N(N71S) with covalently linked intermediate II is 34,530 (red peak with an expected excess mass of 158 Da). Representative ESI-MS plots are shown and typically have an error of 0.5 Da. C, the HPLC spectra show reaction products of WT or derivatives of ArgZ. The inset shows the m/z values of the products citrulline (Cit) and ornithine (Orn), detected by MS. D, the active center of the crystal structure of ArgZ-N(N71S)–intermediate II. The carbon atoms of protein and the substrate arginine are shown in light blue and white, respectively, and the N and O atoms are shown in blue and red, respectively. Green mesh represents a simulated Fo-Fc omit polder map contoured at 6.0. E, schematic of the active center in D.
N71 serves as the determinant for the dihydrolase activity of ArgZ
We propose that the ArgZ-specific residue Asn71 probably plays a key role in proceeding through the two successive amine hydrolyses process (Fig. 3, A and B). ArgZ and ADI share identical residues in the active center, except that dihydrolase contains an asparagine (Asn71 of ArgZ) that forms one H bond with the guanidinium moiety of their substrates, but deiminases contain an aspartate (Asp166 of PaADI and Asp161 of McADI) at the corresponding position that make two stronger salt bridge bonds with the guanidinium moiety of their substrates (Fig. 3, A–C). During the initial steps of the reaction, arginine deiminase, which has an Asp residue at the corresponding position of Asn71 of ArgZ, stabilizes the pre-rotation state of intermediate II by making two salt bridges with Nϵ and Nη2 amines, allowing a water molecule to quickly diffuse into the active center from the left side after release of the η1 amine and to trigger completion of one-round amine hydrolysis (Fig. 3, B and C). In contrast, Asn71 of ArgZ poses less restraint for the Nϵ-Cζ bond, allowing the η2 amine group to freely rotate to the left (post-rotation intermediate II*) after release of the η1 amine, where it could be stabilized by one additional H bond with His168 besides the H bond with Asp170 (Fig. 3E). Such rotation allows the second water to diffuse into the active center from the right for subsequent nucleophilic attack (Fig. 3, A and E), thus committing to the second cycle of amine hydrolysis. Moreover, Asn71 could also stabilize intermediates III, IV, and V during the process of the second cycle of amine hydrolysis by making two H bonds with Nϵ and Oη (Fig. 3E).
The hypothesis highlights that Asn71 serves as the determinant for the dihydrolase activity of ArgZ. To explore whether substitution of Asn71 would shift the equilibrium between prerotation (intermediate II) and post-rotation (intermediate II*) states and thus change the preference of ArgZ for catalyzing one or two cycles of amine hydrolysis, we prepared two ArgZ substitutions, N71D and N71S, and analyzed the products by using arginine as the substrate. N71D was designed to lock the Nϵ-Cζ bond in a pre-rotation conformation that only produces citrulline, as N71D would make two H bonds with and stabilize pre-rotation intermediate II. The results showed that the N71D derivative of ArgZ produces equal trace amounts of citrulline and ornithine, suggesting that N71D substantially reduced the ability of ArgZ to proceed through the second cycle of amine hydrolysis (Fig. 4C). N71S was predicted to allow Nϵ-Cζ bond rotation but be deficient in stabilizing the attacking water and subsequent intermediates (i.e. intermediates III, IV, and IV), thus inhibiting the second cycle of hydrolysis. The HPLC results show that N71S successfully transforms ArgZ from a dihydrolase to a deiminase (Fig. 4C). The results are consistent with our proposed “two successive hydrolyses” model and demonstrate that Asn71 is the key determinant for the dihydrolase activity of ArgZ.
Taking advantage of the reduced activity of the ArgZ substitutions, we sought to trap reaction intermediates with ArgZ mutant protein in crystallo. We cocrystallized the substrate arginine with ArgZ-N(N71S) and determined a crystal structure of ArgZ-N(N71S)–intermediate II at 1.21 Å (Table S1). In the structure of ArgZ(N71S)–intermediate II, the simulated Fo-Fc omit polder map clearly shows that an intermediate covalently bound to Cys264. The covalently linked intermediate II could be perfectly fitted into the electron density (Fig. 4D), consistent with the finding that intermediate II (or intermediate II*) was able to be trapped in the ArgZ(N71S)-catalyzed reaction and detected by ESI-MS (Fig. 4B). The structure clearly shows a pre-rotation state of the Nϵ-Cζ bond and a water molecule that makes H bonds with His168 and the covalently bound intermediate II and is presumably ready for nucleophilic attack of Cζ of the intermediate II (Fig. 4E). The crystal structure graphically demonstrates the presence of reaction intermediate II during the ArgZ-catalyzed reaction and further supports the decisive role of Asn71 in the catalytic property of ArgZ (i.e. to be an arginine dihydrolase rather than arginine deiminase).
Arginine dihydrolases are widely distributed in bacteria
We searched homologs of ArgZ-N from Synechocystis sp. PCC 6803 by PSI-BLAST in the nonredundant protein sequences database. A maximum likelihood phylogenetic tree was constructed for the representative ArgZ-N homologs, which are clearly divided into two groups (Fig. 5A). They can be distinguished by the residue corresponding to Asn71 of ArgZ, which in one group is an asparagine and in the other group is an aspartate (Fig. 5B). Based on the catalytic mechanism described above, we propose that the former group is arginine dihydrolase, whereas the latter group is arginine deiminase. Proteins WP_064813212.1 from Bacillus subtilis and WP_090011708.1 from Clostridium sp. DSM431 in the latter group have been shown to be arginine deiminases (1), consistent with our sequence-based classification.
Figure 5.
Broad distribution of arginine dihydrolase in three domains of life. A, the phylogenetic tree of ArgZ homologs in bacteria, eukaryotes, and archaea. Red pentagons label enzymes for subsequent experimental characterization. Blue pentagons label annotated enzymes in the literature. The protein codes are NCBI reference sequence IDs. B, the sequence alignment shows that the top group contains an Asn and the bottom group contains an Asp at the corresponding position of Asn71 of Synechosistis PCC 6803 ArgZ. C, the sequence alignment of proteins from the top group in A. Red stars label catalytic residues. Blue circles label residues making polar interactions with the substrates. Black circles label residues making hydrophobic interactions with the substrate. The gray box indicates the determinant residue Asn71.
To test whether the ArgZ-N homologs with a corresponding Asn71 are arginine dihydrolases, we selected several representatives from the tree and performed biochemical assays using purified recombinant proteins that include NP_001022509.1 from Caenorhabditis elegans, NP_625511.1 from Streptomyces coelicolor A3(2), ACI97994.1 from Rhodospirillum centenum SW, WP_011391214.1 from Rhodospirillum rubrum, and ATQ69541.1 from Methylosinus trichosporium OB3b. Incubation of individual proteins with arginine led to the formation of ornithine and ammonia (Fig. S3), confirming that these proteins are arginine dihydrolases. Thus, 2300 homologs of ArgZ-N were proposed as arginine dihydrolases (supporting information file). In addition to the conserved Asn71 residue, all proposed arginine dihydrolases contain the catalytic triad residues (Cys264, His168, and Glu118 in ArgZ) and the residues for substrate recognition (Asp65, Arg90, Arg139, Phe68, and Tyr167 in ArgZ) (Fig. 5C).
The arginine dihydrolases are widely present among bacteria and metazoa (File S1). They were found in 17 bacterial phyla, mostly in Actinobacteria, Cyanobacteria, Proteobacteria, Nitrospirae, and Planctomycetes. The Actinobacteria phylum, particularly the genera of Streptomyces, Mycobacterium, and Frankia, has the largest number of arginine dihydrolases. Notably, arginine dihydrolases were identified in many nitrogen-fixing bacteria, such as the free-living Rhodospirillum and cyanobacteria as well as the symbiotic Frankia and Herbaspirillum. In addition, a large number of arginine dihydrolases were also found in Metazoa, mostly in the Nematoda phylum.
Discussion
In this study, we have determined high-resolution crystal structures of ArgZ complexed with its substrate arginine, a covalently linked reaction intermediate, or its product ornithine. Our work provides structural basis for the substrate specificity and catalytic mechanism of ArgZ. We propose that ArgZ utilizes the conserved Glu118-His168-Cys264 catalytic triad to catalyze two successive steps of hydrolysis, accompanied by release of two molecules of ammonia (11), and a key spontaneous rotation of a reaction intermediate covalently linked to Cys264 allows the second cycle of amine hydrolysis. We demonstrate that Asn71 serves as the determinant for the dihydrolase activity of ArgZ.
Cygler and co-workers (6) reported crystal structures of another dihydrolase (Fig. 3D), E. coli AstB, which converts N-succinylarginine to N-succinylornithine, and predicted that a similar Asn residue (Asn110) in the active center might be critical for the dihydrolysis activity of AstB (6, 23). In agreement with the prediction, we demonstrate that, in this study, the active-center asparagine (Asn71) serves as the determinant to distinguish arginine dihydrolase from the rest of the GME family by showing that mutating Asn71 to Ser, Ala, or Asp greatly diminishes the dihydrolase activity of ArgZ, that ArgZ homologs with an Asn residue at the corresponding position of Asn71 exhibit activity of arginine dihydrolase, and that ArgZ homologs with an Asp residue at the corresponding position of Asn71 exhibit activity of arginine deiminase. Our study provides the catalytic mechanism for arginine dihydrolase, a subcategory of the GME family, and demonstrates experimentally that the identity of one residue in the active center is able to sufficiently distinguish dihydrolases from the rest of the GME superfamily.
Arginine dihydrolases are present in many nitrogen-fixing bacteria, including actinobacteria (Frankia) and cyanobacteria, which are widely distributed on land and in the sea and contribute substantially to global nitrogen cycling (24, 25). In cyanobacteria, arginine dihydrolase is a key enzyme in the OAC, which serves as a conduit in the nitrogen storage and remobilization machinery (7). Because the ability to synthesize arginine from ornithine is found in virtually all organisms, the OAC may also occur in Frankia, which could be used for redistributing the fixed nitrogen. Arginine dihydrolases were also identified in many other actinobacteria, such as Streptomyces spp. Previous reports have shown that arginine metabolism plays an important role in antibiotic production and differentiation in Streptomyces spp. (26, 27). However, the genes encoding arginine-degrading enzymes are not known. Our study indicates that arginine dihydrolase may be used for arginine catabolism in Streptomyces spp. In addition to bacteria, arginine dihydrolases have also been found in C. elegans and other nematode species. This finding raises the possibility that the OAC might occur in them. Further studies are required to investigate whether the OAC plays a role in arginine metabolism and stress resistance in C. elegans (28).
We have resolved the structure of a large C-terminal portion of ArgZ, which contains a LOR/SDH-NC domain, a small domain of β sheets, and a large Rossman fold domain. Unlike the wide distribution of the N-terminal dihydrolase domain, the homolog of the C-terminal region of ArgZ is only distributed in cyanobacteria and Archaea (mainly euryarchaeotes). A recent study reported that the cyanobacterium Anabaena AgrE, an enzyme closely related to ArgZ, is able to convert ornithine or arginine to proline and suggests that its C-terminal domain functions as an atypical ornithine cyclodeaminase, albeit with very low efficiency (29). However, we were not able to detect the activity of the ornithine cyclodeaminase of ArgZ in our enzymatic assays. Further structural and biochemical studies are needed to elucidate the potential function of the C terminus of ArgZ.
GME is a large enzyme superfamily distributed in all three domains of life. The two main reactions (hydrolase and amidinotransferase) catalyzed by enzymes in the superfamily have been well characterized (9, 11). Our results here demonstrate the unique catalytic mechanism (a bond rotation inducing two successive cycles of hydrolysis) of a third category of reaction and validate that a determinant asparagine in the active center could serve as a criterion for annotating dihydrolase from the GME superfamily.
Experimental procedures
Plasmids
The argZ of Synechocystis sp. PCC 6803 was cloned into the pProEX HTa vector using NcoI and XhoI restriction sites with a tobacco etch virus protease–cleavable His6 tag at the N terminus, resulting in pProEX-HTa-ArgZ. The N-terminal domain of ArgZ (residues 1–281) was cloned into pET28a, resulting in pET28a-ArgZ-N. The substitutions of ArgZ were made by site-directed mutagenesis (Transgene). The plasmids and primers are summarized in Table S2. The genes for proteins WP_064813212.1 from B. subtilis, WP_090011708.1 from Clostridium sp. DSM431, NP_001022509.1 from C. elegans, NP_625511.1 from S. coelicolor A3(2), ACI97994.1 from R. centenum SW, WP_011391214.1 from R. rubrum, and ATQ69541.1 from M. trichosporium OB3b were chemically synthesized (Genscript, Inc.) and then ligated into the expression vector pET28a.
Proteins
To obtain the full-length ArgZ, E. coli Transetta (DE3) cells carrying pProEX-HTa-ArgZ were grown at 37 °C to reach A600 of 0.8, and protein expression was induced at 18 °C for 12 h with 0.4 mm isopropyl 1-thio-β-d-galactopyranoside. Cells were harvested and resuspended in binding buffer containing 50 mm Tris (pH 8.0), 500 mm NaCl, 5% (v/v) glycerol, and 5 mm β-mercaptoethanol and disrupted using an EmulsiFlex-C5 cell disruptor (AVESTIN, Inc.). Cellular debris was removed by centrifugation at 13,000 × g for 30 min at 4 °C. The supernatant was filtered (0.45-μm syringe-driven filters, JET BIOFIL) and loaded onto an open column packed with 3 ml of nickel-nitrilotriacetic acid resin (SMART Inc.). The column was washed with binding buffer containing 30 mm imidazole, and the recombinant ArgZ was eluted by binding buffer containing 300 mm imidazole. The eluted fractions were pooled, incubated with tobacco etch virus protease, and dialyzed into buffer A (50 mm Tris-HCl (pH 8.0), 200 mm NaCl, 5% glycerol, and 5 mm β-mercaptoethanol) at 4 °C overnight and then loaded onto a HiPrep Q HP 16/10 column (GE Healthcare, 120-ml linear salt gradient from 200 mm to 1 m NaCl). The fractions containing ArgZ were pooled, concentrated, and loaded onto a HiLoad 16/600 Superdex 200 PG column (GE Healthcare) in 20 mm Tris (pH 8.0), 100 mm NaCl, 1% glycerol, and 1 mm DTT. The fraction containing ArgZ was collected, concentrated, and stored at −80 °C. The ArgZ-N and its homologous proteins were obtained using the same method as described above.
Crystallization, data collection, and structure determination
The crystal structure of ArgZ-N-ornithine was obtained by cocrystallization of ArgZ-N and the substrate arginine. Briefly, the crystallization drops contained 1 μl of 8 mg/ml ArgZ-N supplemented with 5 mm l-arginine and 1 μl of reservoir solution A (0.2 m KI, 0.1 m MES (pH 6.5), and 19% PEG 4000) was equilibrated with 400 μl of reservoir solution A in a hanging-drop vapor diffusion setup. Cubic crystals were transferred into the reservoir solution A containing 20% ethylene glycol and flash-frozen in liquid nitrogen. The diffraction data were collected at Shanghai Synchrotron Radiation Facility beamline BL19U1 and processed using HKL2000 (30). The molecular replacement was performed on the I-TASSER-MR online server, and the initial 3D models used for molecular replacement were generated by I-TASSER based on structural assembly simulations (31). Structure refinement was carried out using PHENIX (32) combined with several rounds of COOT manual fitting. The final model of ArgZ-N–ornithine was refined to Rwork and Rfree of 0.172 and 0.191, respectively, and deposited into the PDB with accession number 6JV0.
The crystals of ArgZ-N(C264S)–arginine were obtained by cocrystallization of ArgZ-N(C264S) and the substrate arginine through an analogous procedure as above. The structure was determined by molecular replacement using Phaser MR and ArgZ-N–ornithine as the search template. The final model of ArgZ-N(C264S)–arginine was refined to Rwork and Rfree of 0.175 and 0.188, respectively, and deposited into the PDB with accession number 6JV1.
The crystals of ArgZ-N (N71S)–intermediate II were obtained by cocrystallization of ArgZ-N(N71S) and the substrate arginine through an analogous procedure as above. The structure was determined by molecular replacement using Phaser MR and ArgZ-N–ornithine as the search template. The final model of ArgZ-N(N71S)–intermediate II was refined to Rwork and Rfree of 0.166 and 0.175, respectively, and deposited into the PDB with accession number 6JUZ.
The crystals of the full-length ArgZ were grown at 22 °C in a 2-μl drop containing 1 μl of 10 mg/ml ArgZ and 1 μl of reservoir solution (0.24 m ammonium formate and 17% (w/v) PEG 3350) by the hanging-drop vapor diffusion method. The crystals were transferred to reservoir solution containing 25% ethylene glycerol for flash-freezing in liquid nitrogen. The diffraction data were collected at Shanghai Synchrotron Radiation Facility beamline BL17U1 and processed using HKL2000 (30). The initial phase was obtained by a stepwise molecular replacement procedure. First, a partial solution of ArgZ (360–699) using PDB code 3C2Q as a template was obtained by the online MoRDa server; the high-resolution ArgZ-N and PDB code 3MGJ served as additional search templates for the second-step molecular replacement in PHASER-MR. Structure refinement and model building were carried out as above. The final model of ArgZ was refined to Rwork and Rfree of 0.246 and 0.279, respectively, and deposited into the PDB with accession number 6JUY.
GDH-coupled enzymatic assay
The dihydrolase activity of ArgZ was measured by a GDH-coupled enzymatic assay as in Ref. 7. GDH catalyzes the conversion of ammonium (released by ArgZ), 2-ketoglutarate, and NADH to glutamate, NAD+, and H2O. To compare the activity of the WT and ArgZ mutants, the reaction mixture (200 μl) contained 1 μg of ArgZ, ArgZ-N, or ArgZ-N derivatives; 50 mm triethanolamine buffer (pH 7.5); 5 units of glutamate dehydrogenase (GDH, Sigma); 5 mm α-ketoglutarate (Sigma); and 0.2 mm NADH. The reactions were initiated by addition of 5 mm l-arginine (working concentration). The change in NADH absorbance was monitored at 340 nm by a Beckman DU-800 spectrophotometer.
For determination of the apparent kcat and Km values of ArgZ and ArgZ-N, the reactions were performed essentially as above, except that the l-arginine concentration varied in the range of 0.1 to 10 mm (working concentration). Kinetic data were analyzed in GraphPad Prism 5.0.
HPLC-based enzymatic assay
The identities of reaction products were determined by a HPLC-based enzymatic assay as in Ref. 7. The reaction mixtures (200 μl) contained 1 μg of ArgZ, ArgZ-N or ArgZ-N derivatives and 50 mm triethanolamine buffer (pH 7.5) and were incubated for 2 h at 30 °C. Then they were derivatized with 200 μl of phenylisothiocyanate (Sigma) at room temperature for 45 min. The resulting phenylisothiocyanate products (10 μl) were separated by an Ultimate amino acid column (4.6 × 250 mm, Welch, Shanghai, China) on an Agilent model 1260 instrument with a custom gradient (0 min, 0%; 11 min, 7%; 13.9 min, 12%; 14 min, 15%; 29 min, 34%; 32 min, 70%; 35 min, 100%; 42 min, 100%; 45 min, 0%; 60 min, 0%) of solvent A (100 mm sodium acetate (pH 6.5) and 7% acetonitrile) and solvent B (80% acetonitrile) with a flow rate of 1 ml/min. The components were quantitated based on absorbance at 254 nm by a UV detector (Agilent).
ESI-MS
The reaction intermediates were trapped using the electrospray ionization (ESI)–MS technique. ArgZ-N (10 μm) and l-arginine (10 mm) were incubated in 50 mm triethanolamine at 30 °C, followed by quenching with formic acid at different time points to a final concentration of 5% (v/v). Protein buffer was exchanged into 1% formic acid solution by PD Minitrap G25 columns (GE Healthcare).
Samples (5 μl) were injected into an ACQUITY UPLC Protein BEH C4 column (Waters, Inc.) and separated by ultra-high-performance liquid chromatograph equipment (Acquity, Waters) with solvent A (0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid) using a custom program (0 min, 20%; 11 min, 90%; 12 min, 90%; 12.1 min, 20%; 15 min, 20%) at 65 °C; the molecular mass of the separated components was determined by a coupled Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher). Data processing was performed in Protein Deconvolution 3.0 (Thermo Fisher).
Phylogenetic tree
Protein sequences were retrieved based on homology to the N-terminal domain of ArgZ from Synechosistis PCC 6803 using BLAST with an E value threshold 1.0E−5. To filter out false positives, the presence of the characteristic catalytic triad was analyzed, which is essential for enzyme activity. The MUSCLE program was used for protein sequence alignments and the MEGA program for phylogenetic analysis. A phylogenetic tree of protein homologs was built using the maximum likelihood method, with calculation of bootstraps from 1000 replications.
Author contributions
N. Z., H. Z., L. L., and X. W. investigation; H. Z., C. Y., and Y. Z. supervision; H. Z. methodology; C. Y. and Y. Z. conceptualization; C. Y. resources; C. Y. and Y. Z. project administration; C. Y. and Y. Z. writing-review and editing; Y. Z. visualization; Y. Z. writing-original draft.
Supplementary Material
Acknowledgments
We thank the staff at beamline BL18U1/BL19U1 of the National Center for Protein Science Shanghai and at beamline BL17U1 of the Shanghai Synchrotron Radiation Facility for assistance during data collection.
This work was supported by National Key Research and Development Program of China Grant 2018YFA0900700, National Natural Science Foundation of China Grant 31822001, and Leading Science Key Research Program of the Chinese Academy of Sciences Grant QYZDB-SSW-SMC005. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S3, Tables S1 and S2, and supporting information file.
The atomic coordinates and structure factors (codes 6JUY, 6JV0, 6JV1, and 6JUZ) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- ADI
- arginine deiminase
- AST
- arginine succinyltransferase
- OAC
- ornithine–ammonia cycle
- ESI
- electrospray ionization
- GDH
- glutamate dehydrogenase
- GME
- guanidino group-modifying enzyme
- LOR/SDH-NC
- N-terminal conserved domain of the lysine-oxoglutarate reductase/saccharopine dehydrogenase bifunctional enzyme.
References
- 1. Cunin R., Glansdorff N., Piérard A., and Stalon V. (1986) Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50, 314–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morris S. M., Jr. (2007) Arginine metabolism: boundaries of our knowledge. J. Nutr. 137, 1602S–1609S 10.1093/jn/137.6.1602S [DOI] [PubMed] [Google Scholar]
- 3. Mommsen T. P., and Walsh P. J. (1989) Evolution of urea synthesis in vertebrates: the piscine connection. Science 243, 72–75 10.1126/science.2563172 [DOI] [PubMed] [Google Scholar]
- 4. Winter G., Todd C. D., Trovato M., Forlani G., and Funck D. (2015) Physiological implications of arginine metabolism in plants. Front. Plant Sci. 6, 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon J. P., Wargnies B., and Stalon V. (1982) Control of enzyme synthesis in the arginine deiminase pathway of Streptococcus faecalis. J. Bacteriol. 150, 1085–1090 10.1128/JB.150.3.1085-1090.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tocilj A., Schrag J. D., Li Y., Schneider B. L., Reitzer L., Matte A., and Cygler M. (2005) Crystal structure of N-succinylarginine dihydrolase AstB, bound to substrate and product, an enzyme from the arginine catabolic pathway of Escherichia coli. J. Biol. Chem. 280, 15800–15808 10.1074/jbc.M413833200 [DOI] [PubMed] [Google Scholar]
- 7. Zhang H., Liu Y., Nie X., Liu L., Hua Q., Zhao G. P., and Yang C. (2018) The cyanobacterial ornithine-ammonia cycle involves an arginine dihydrolase. Nat. Chem. Biol. 14, 575–581 10.1038/s41589-018-0038-z [DOI] [PubMed] [Google Scholar]
- 8. Sohm J. A., Webb E. A., and Capone D. G. (2011) Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 499–508 10.1038/nrmicro2594 [DOI] [PubMed] [Google Scholar]
- 9. Linsky T., and Fast W. (2010) Mechanistic similarity and diversity among the guanidine-modifying members of the pentein superfamily. Biochim. Biophys. Acta 1804, 1943–1953 10.1016/j.bbapap.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shirai H., Blundell T. L., and Mizuguchi K. (2001) A novel superfamily of enzymes that catalyze the modification of guanidino groups. Trends Biochem. Sci. 26, 465–468 10.1016/S0968-0004(01)01906-5 [DOI] [PubMed] [Google Scholar]
- 11. Shirai H., Mokrab Y., and Mizuguchi K. (2006) The guanidino-group modifying enzymes: structural basis for their diversity and commonality. Proteins 64, 1010–1023 10.1002/prot.20863 [DOI] [PubMed] [Google Scholar]
- 12. Kemper E. L., Cord-Neto G., Capella A. N., Gonçalves-Butruile M., Azevedo R. A., and Arruda P. (1998) Structure and regulation of the bifunctional enzyme lysine-oxoglutarate reductase-saccharopine dehydrogenase in maize. Eur. J. Biochem. 253, 720–729 10.1046/j.1432-1327.1998.2530720.x [DOI] [PubMed] [Google Scholar]
- 13. Das K., Butler G. H., Kwiatkowski V., Clark A. D. Jr, Yadav P., and Arnold E. (2004) Crystal structures of arginine deiminase with covalent reaction intermediates; implications for catalytic mechanism. Structure 12, 657–667 10.1016/j.str.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 14. Humm A., Fritsche E., Steinbacher S., and Huber R. (1997) Crystal structure and mechanism of human l-arginine:glycine amidinotransferase: a mitochondrial enzyme involved in creatine biosynthesis. EMBO J. 16, 3373–3385 10.1093/emboj/16.12.3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stone E. M., Costello A. L., Tierney D. L., and Fast W. (2006) Substrate-assisted cysteine deprotonation in the mechanism of dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry 45, 5618–5630 10.1021/bi052595m [DOI] [PubMed] [Google Scholar]
- 16. Wang S., and Wang Y. (2013) Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta 1829, 1126–1135 10.1016/j.bbagrm.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanukoglu I. (2015) Proteopedia: Rossmann fold: a β-α-β fold at dinucleotide binding sites. Biochem. Mol. Biol. Educ. 43, 206–209 10.1002/bmb.20849 [DOI] [PubMed] [Google Scholar]
- 18. Liao D. I., Wolff E. C., Park M. H., and Davies D. R. (1998) Crystal structure of the NAD complex of human deoxyhypusine synthase: an enzyme with a ball-and-chain mechanism for blocking the active site. Structure 6, 23–32 10.1016/S0969-2126(98)00004-5 [DOI] [PubMed] [Google Scholar]
- 19. Berthold C. L., Toyota C. G., Moussatche P., Wood M. D., Leeper F., Richards N. G., and Lindqvist Y. (2007) Crystallographic snapshots of oxalyl-CoA decarboxylase give insights into catalysis by nonoxidative ThDP-dependent decarboxylases. Structure 15, 853–861 10.1016/j.str.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 20. Galkin A., Lu X., Dunaway-Mariano D., and Herzberg O. (2005) Crystal structures representing the Michaelis complex and the thiouronium reaction intermediate of Pseudomonas aeruginosa arginine deiminase. J. Biol. Chem. 280, 34080–34087 10.1074/jbc.M505471200 [DOI] [PubMed] [Google Scholar]
- 21. Stone E. M., Person M. D., Costello N. J., and Fast W. (2005) Characterization of a transient covalent adduct formed during dimethylarginine dimethylaminohydrolase catalysis. Biochemistry 44, 7069–7078 10.1021/bi047407r [DOI] [PubMed] [Google Scholar]
- 22. Linsky T. W., Monzingo A. F., Stone E. M., Robertus J. D., and Fast W. (2008) Promiscuous partitioning of a covalent intermediate common in the pentein superfamily. Chem. Biol. 15, 467–475 10.1016/j.chembiol.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schneider B. L., Kiupakis A. K., and Reitzer L. J. (1998) Arginine catabolism and the arginine succinyltransferase pathway in Escherichia coli. J. Bacteriol. 180, 4278–4286 10.1128/JB.180.16.4278-4286.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wall L. G. (2000) The actinorhizal symbiosis. J. Plant Growth Regul. 19, 167–182 10.1007/s003440000027 [DOI] [PubMed] [Google Scholar]
- 25. Zehr J. P. (2011) Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 19, 162–173 10.1016/j.tim.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 26. Wang B., Liu J., Liu H., Huang D., and Wen J. (2015) Comparative metabolic profiling reveals the key role of amino acids metabolism in the rapamycin overproduction by Streptomyces hygroscopicus. J. Ind. Microbiol. Biotechnol. 42, 949–963 10.1007/s10295-015-1611-z [DOI] [PubMed] [Google Scholar]
- 27. Botas A., Pérez-Redondo R., Rodríguez-García A., Álvarez-Álvarez R., Yagüe P., Manteca A., and Liras P. (2018) ArgR of Streptomyces coelicolor is a pleiotropic transcriptional regulator: effect on the transcriptome, antibiotic production, and differentiation in liquid cultures. Front. Microbiol. 9, 361 10.3389/fmicb.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma H., Ma Y., Zhang Z., Zhao Z., Lin R., Zhu J., Guo Y., and Xu L. (2016) l-Arginine Enhances resistance against oxidative stress and heat stress in Caenorhabditis elegans. Int. J. Environ. Res. Public Health 13, 969 10.3390/ijerph13100969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burnat M., Picossi S., Valladares A., Herrero A., and Flores E. (2019) Catabolic pathway of arginine in Anabaena involves a novel bifunctional enzyme that produces proline from arginine. Mol. Microbiol. 111, 883–897 10.1111/mmi.14203 [DOI] [PubMed] [Google Scholar]
- 30. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 31. Wang Y., Virtanen J., Xue Z., and Zhang Y. (2017) I-TASSER-MR: automated molecular replacement for distant-homology proteins using iterative fragment assembly and progressive sequence truncation. Nucleic Acids Res. 45, W429–W434 10.1093/nar/gkx349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.