Abstract
The endoplasmic reticulum (ER) is the entry point to the secretory pathway and major site of protein biogenesis. Translocation of secretory and integral membrane proteins across or into the ER membrane occurs via the evolutionarily conserved Sec61 complex, a heterotrimeric channel that comprises the Sec61p/Sec61α, Sss1p/Sec61γ, and Sbh1p/Sec61β subunits. In addition to forming a protein-conducting channel, the Sec61 complex also functions to maintain the ER permeability barrier, preventing the mass free flow of essential ER-enriched molecules and ions. Loss in Sec61 integrity is detrimental and implicated in the progression of disease. The Sss1p/Sec61γ C terminus is juxtaposed to the key gating module of Sec61p/Sec61α, and we hypothesize it is important for gating the ER translocon. The ER stress response was found to be constitutively induced in two temperature-sensitive sss1 mutants (sss1ts) that are still proficient to conduct ER translocation. A screen to identify intergenic mutations that allow for sss1ts cells to grow at 37 °C suggests the ER permeability barrier to be compromised in these mutants. We propose the extreme C terminus of Sss1p/Sec61γ is an essential component of the gating module of the ER translocase and is required to maintain the ER permeability barrier.
Keywords: endoplasmic reticulum (ER), endoplasmic reticulum–associated protein degradation (ERAD), translocation, gating, endoplasmic reticulum stress (ER stress), Sec61 complex, Sss1p/Sec61γ, translocon gating, Sec61p/Sec61α
Introduction
The endoplasmic reticulum (ER)3 plays a major role in the biosynthesis of about a third of a cell's proteome (1). Proteins are targeted to and translocated across the ER membrane either cotranslationally or posttranslationally. Cotranslational translocation is initiated upon recognition of the hydrophobic signal sequence by the signal recognition particle (SRP) as it emerges from the ribosome (2–6). The resulting complex is targeted to the ER by association of the SRP with the SRP receptor (3, 5, 6). Interaction with the translocon results in SRP dissociation followed by looped insertion of the nascent chain into the translocation channel (3, 7, 8). Upon resumption of translation the nascent chain moves through the pore and into the lumen of the ER where cleavable signal sequences are processed by the signal peptidase complex (9–12).
Smaller proteins or those containing a moderately hydrophobic signal sequence are completely synthesized in the cytoplasm and translocated posttranslationally (3, 13). Cytosolic chaperones associate with these substrates to maintain them in a translocation-competent state (12, 14, 15). Posttranslational translocation proceeds via the SEC complex, an assembly of the translocon and the Sec62p/Sec63p/Sec71p/Sec72p subcomplex (3, 16–20). Upon association with the SEC complex, chaperones are released and the signal peptide of the secretory precursor is inserted into the translocation channel after which translocation is facilitated by Kar2p (BiP in mammals) (16, 21).
The translocon is formed by the conserved heterotrimeric Sec61 complex. It comprises two essential subunits, Sec61p and Sss1p, and the nonessential Sbh1p subunit (Sec61α, Sec61γ, and Sec61β in mammals, respectively) (3, 16, 22). This complex forms an hourglass-shaped structure with an aqueous pore. At the center lies the pore ring, formed by hydrophobic amino acids, the side chains of which form a gasket through which a polypeptide is threaded during translocation (12, 23, 24). In the closed conformation the pore is sealed by a short helix of TM2 in Sec61p, TM2a, which acts as a plug (23, 25). A Sec61 paralog exists that exclusively functions in the cotranslational pathway (26–28). This paralog is known as Ssh1 and Sec61A2 in yeast and mammals, respectively (29). In yeast the Ssh1 complex comprises Ssh1p and Sbh2p, related to Sec61p and Sbh1p, respectively, as well as Sss1p (26, 27).
The conformational changes that take place within the translocon upon the initiation of translocation are best understood for cotranslational translocation. Interaction of the ribosome-nascent chain complex with cytosolic loops 6 and 8 of Sec61p is critical for initiating the preliminary conformational changes that facilitate translocation (3, 30–33). This includes partial displacement of the plug and opening of the lateral gate, formed by TM2b and TM7 of Sec61p, to allow the intercalation of the signal sequence (23, 34). The latter promotes further conformational changes that include full displacement of the plug and further lateral gate separation to establish an open channel to allow protein translocation to proceed.
Previous studies have shown Sss1p/Sec61γ to stabilize the translocon; defects in the Sss1p/Sec61γ TMD lead to various outcomes such as inefficient ribosomal binding, breakdown of ER structure, defective cotranslational and posttranslational translocation, and loss of cell viability (22, 35). Additionally, structural analyses show the extreme C terminus of Sss1p is juxtaposed to the key gating module of Sec61p (23, 32). Therefore, we hypothesized that the highly conserved C terminus of Sss1p is critical in gating the ER translocon. Through phenotypical characterization of two mutants, sss1–6 and sss1–7, we show that the C terminus of Sss1p is important for ER homeostasis but does not influence the stability of the ER translocation machinery. Furthermore, we found that mutations in key gating modules of Sec61p suppress the temperature sensitivity of sss1–6 and sss1–7. Together, this provides insight into the role of Sss1p/Sec61γ plays in translocon function and in mammalian ER physiology and pathology associated with dysregulated diffusion of small molecules through the ER translocon.
Results
The C terminus of Sss1p is highly conserved
The Sec61 complex comprises Sec61/Sec61α, Sbh1p/Sec61β, and Sss1p/Sec61γ. Eukaryotes also encode for a second ER translocase which contains Ssh1p/Sec61α2, Sss1p/Sec61γ, and Sbh2p/Sec61β (Fig. 1A). Given that Sss1p/Sec61γ is the only essential protein to be a component of both ER translocases we considered the possibility that its activity may be tightly regulated. The Sss1p transmembrane domain is highly conserved, in particular the C-terminal portion where the K69LIHIPI75 heptapeptide is absolutely conserved. We hypothesized this region to be functionally important and performed double-alanine scanning mutagenesis throughout it, creating variants Sss1pI68A,K69A, Sss1pL70A,I71A, Sss1pH72A,I73A, and Sss1pP74A,I75A (Fig. 1B) to investigate the role of these residues in Sss1p function. As SSS1 is essential, we tested if expression of these variants could sustain cell viability. YCp SSS1 and each mutant were transformed into BWY530 (sss1Δ::KanMX4 FKp53) and tested for the ability of these strains to grow after loss of FKp53 on 5-FOA medium. Strains harboring YCp SSS1 or any of the mutants, unlike cells transformed with vector alone, produced viable colonies that expressed stable Sss1 protein (Fig. 1, C and D).
Figure 1.
The Sss1p C terminus is highly conserved. A, ribbon diagram of the Sec61 homologue complex crystal structure (PDB ID: 2WWA) (32) was composed using Chimera software. Ssh1p, Sbh2p, and Sss1p are colored gray, blue, and red, respectively. The highly conserved KLIHIPI heptapeptide is highlighted in gold. B, the sequence of the extreme C terminus of Sec61γ and Sss1p are aligned using Clustal Omega sequence alignment software and the position of each double alanine scanning mutation indicated. C, BWY530 yeast transformed with either YCp HIS3, YCp SSS1, YCp SSS11I68A,K69A, YCp SSS1L70A,I71A, YCp SSS1H72A,I73A, or YCp SSS1P74A,I75A were streaked onto His selective medium and medium containing FOA and incubated at 30 °C for 2 days. D, cell extracts derived from WT cells or cells expressing either SSS1I68A,K69A, SSS1L70A,I71A, SSS1H72A,I73A, or SSS1P74A,I75A were immunoblotted with anti-Sss1p or anti-Sec61p antibodies.
Mutations in the Sss1p C terminus disrupt ER homeostasis
The growth of cells expressing the sss1P74A,I75A double mutant, referred to as sss1–6 herein, is temperature sensitive as they grow poorly at 37 °C (Fig. 2A). We have isolated a second mutation within the C terminus that is temperature sensitive (Fig. 2A). The Sss1pH72K variant (referred to as sss1–7) was originally generated to enable us to identify potential lumenal interacting proteins by crosslinking. The resulting strain was even more temperature sensitive than sss1–6 cells (Fig. 2, A and B).
Figure 2.
sss1–6 and sss1–7 are inserted into the ER membrane. A, WT, sss1–6 or sss1–7 yeast were spotted on YPD agar in a 10-fold dilution series and incubated at 30 °C or 37 °C for 2 days. B, cell extracts derived from WT sss1–6 or sss1–7 yeast were immunoblotted with anti-Sss1p, anti-Sec61p, and anti-Sec63p antibodies. C, GFP-Sss1p, GFP-Sss1–6p, and GFP-Sss1–7p was visualized in cells grown at 30 °C and 37 °C and colocalized with Sec63p-RFP (5 μm bar).
The hydrophobicity of the extreme C terminus of tail-anchored proteins is a critical property that allows their stable integration into membranes by the GET complex (37). Hydropathy analysis shows that the mutations in the C terminus of Sss1–6p and Sss1–7p have little effect on the hydrophobicity of this region relative to Sss1p (Fig. S1). We used fluorescence microscopy to investigate if these mutations affect the integration of Sss1p into the ER membrane. GFP-Sss1p shows a perinuclear and peripheral locality and colocalizes with the ER resident protein Sec63p (Fig. 2C). As with GFP-Sss1p, we observe GFP-Sss1–6p and GFP-Sss1–7p to localize to the perinuclear and peripheral ER and both entirely colocalize with Sec63p (Fig. 2C).
Given that Sss1p is an essential translocon component, we investigated if the integrity of the translocation apparatus was affected in Sss1p variants by measuring the stability of other essential translocon-associated proteins. We find the stability of both Sec61p and Sec63p to be unaffected in sss1–6 and sss1–7 at 30 °C or 37 °C (Fig. 2B). Next, we investigated the integrity of the translocon. The interaction between Sss1p and Sec61p required to form the translocon can be stabilized by the crosslinking reagent disuccinimidyl suberate (DSS) (38, 39). We detected a DSS-dependent immunoreactive band of ∼46 kDa with both anti-Sss1p– and anti-Sec61p–specific antibodies in membranes isolated from WT cells (Fig. 3A). This adduct was also detected in membranes isolated from either sss1–6 or sss1–7 cells treated with DSS, regardless of whether cells were grown at 30 °C or 37 °C (Fig. 3A).
Figure 3.
ER translocation is not affected in sss1–6 and sss1–7. A, membranes derived from WT sss1–6 or sss1–7 yeast incubated with and without 1 mm DSS were immunoblotted with anti-Sss1p and anti-Sec61p antibodies. B, membranes prepared from WT sss1–6 or sss1–7 yeast were subject to ConA chromatography. An equal portion of each fraction was analyzed by immunoblotting with either anti-Sss1p, anti-Sec61p, or anti-Sec63p specific antibodies. The bound fraction is shown. C, cell extracts derived from WT sss1–6 or sss1–7 yeast were immunoblotted with anti-Sss1p, anti-Sec61p, anti-α factor, and anti-DPAP B antibodies. Secretory mutants, sec63–1 and sec65–1 (sects), were included as a negative control for α factor and DPAP B, respectively.
Binding of the Sec71p glycoprotein with the lectin concanavalin A (ConA) enables the affinity purification of the heptameric SEC complex (20, 40). Digitonin solubilized membranes isolated from WT, sss1–6, and sss1–7 cells were incubated with ConA-coupled Sepharose beads, the bound fraction retained, and Sec61p, Sss1p, and Sec63p were visualized by immunoblot analysis (Fig. 3B). As expected, Sec61p, Sss1p, and Sec63p were associated with the ConA-bound fraction of digitonin-solubilized membranes isolated from WT cells. Likewise, these proteins were associated with the ConA-bound fraction of digitonin-solubilized membranes isolated from sss1–6 and sss1–7 cells, grown at 30 °C or 37 °C. Therefore, neither the sss1H72K nor the sss1P74A I75A mutations disrupted the ability to form protein complexes required for ER translocation.
The biogenesis of DPAP B, which is translocated cotranslationally, as well as Lhs1p and α-factor, which are translocated by posttranslational translocation (41), were monitored to determine whether ER translocation is compromised in sss1–6 and sss1–7. There was no obvious translocation defect in sss1–6 and sss1–7 mutants, irrespective of growth conditions, as the levels of both pDPAP B and Lhs1p did not exceed that that accumulates in WT cells (Fig. 3C). This was also the case for a second posttranslational translocation substrate prepro-α factor (Fig. 3C). To further validate this we reasoned that the mating efficiency of MATα variants of both sss1–6 and sss1–7 would be comparable to WT cells at both permissive and semi-permissive temperatures. Indeed, we observed no difference in the ability of these sss1 mutants to mate as compared with WT cells (Fig. S2A).
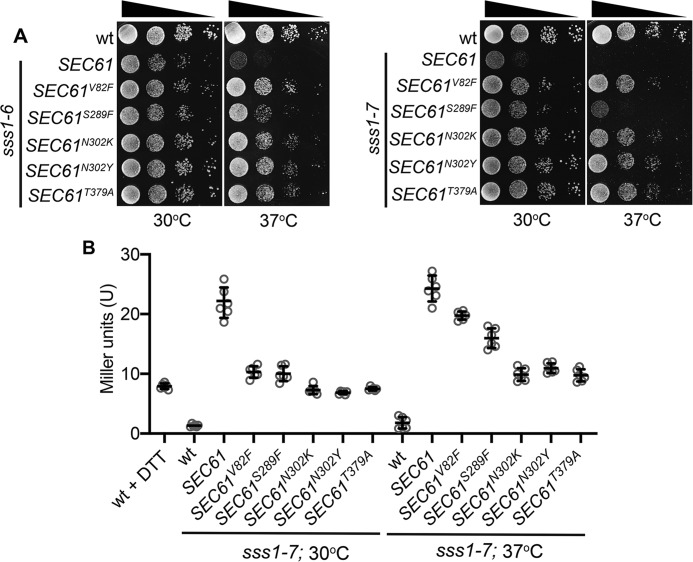
We observed significant ER distension and expansion in sss1–6, in particular, and sss1–7, indicating the ER to be stressed in these mutants (Fig. 2C). Given this, we tested if the unfolded protein response (UPR) was induced in sss1–6 and sss1–7 mutants. For this we used a lacZ reporter placed under transcriptional control of a yeast UPR enhancer (UPRE) (Wilkinson et al. 36). WT cells were treated with the reducing agent dithiothreitol (DTT) to gauge a typical UPR response. UPR-dependent LacZ activity was significantly elevated in DTT-treated cells compared with WT (Fig. 4A), ensuring the range of response expected from these controls. LacZ activity in both sss1 mutants at 30 °C and 37 °C was up to 11-fold greater than that of WT. This confirms that the UPR is constitutively induced in sss1–6 and sss1–7 cells.
Figure 4.

ER homeostasis is perturbed in sss1–6 and sss1–7. A, WT, sss1–6, and sss1–7 yeast transformed with pJT30 (UPRE-LACZ) were grown in Ura medium and β-galactosidase activity determined. WT cells treated with 5 mm DTT for 2 h were used as a positive control. B, WT, SEC61N302D, and sss1–6 yeast transformed with YEp HGT1 were grown in Ura selective medium with increasing concentrations of GSH. The relative growth of each strain determined and the GSH sensitivity (1/relative growth) presented. C, WT, sss1–6, and sss1–7 yeast were spotted on YPD agar or YPD agar containing 1 μg/ml terbinafine in a 10-fold dilution series and incubated at 30 °C or 34 °C for 2 days.
Together, these experiments confirmed that despite their large effects on cell viability at 37 °C and causing constitutive induction of the UPR, these Sss1p point mutations did not affect the abundance, overall integrity, or general translocational activity of the translocon. This suggested that the role of this conserved section of Sss1p is independent of protein translocation.
The ER is more permeable in sss1–6 and sss1–7
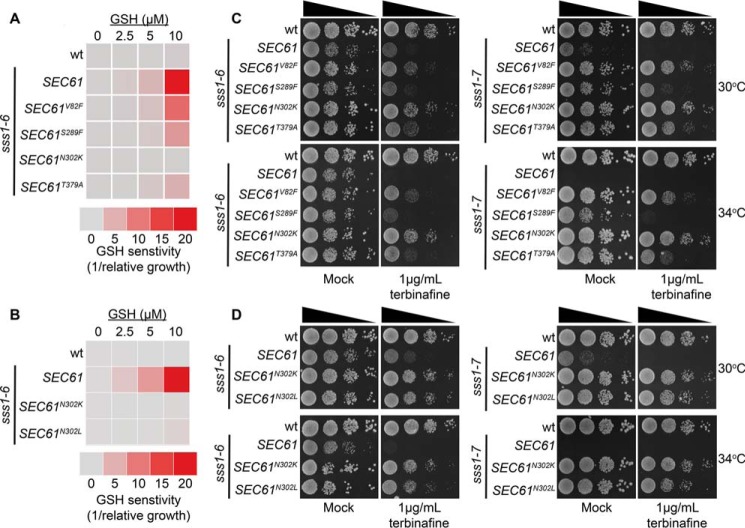
The Sec61 complex has been shown by Toledano and colleagues (42) to facilitate the diffusion of reduced GSH into the ER. Furthermore, it has also been suggested that the Sec61 complex may facilitate the diffusion of other small molecules across the ER membrane. We wanted to determine whether sss1–6 and sss1–7 cells possessed phenotypes consistent with altered ER permeability. WT cells that overexpress Hgt1p, the plasma membrane high-affinity GSH transporter (↑HGT1 cells hereafter), accumulate high levels of GSH when supplied with exogenous GSH, which becomes cytotoxic because of hyperoxidation of the ER (42, 43). HGT1 is overexpressed in ↑HGT1 cells by placing the HGT1 ORF under transcriptional control of the constitutive and robust PMA1 promoter on a multicopy plasmid (YEp). In our hands, WT ↑Hgt1p cells easily tolerate up to 10 μm GSH (Fig. 4B) whereas the growth of SEC61N302D ↑Hgt1p cells is hypersensitive (Fig. 4B). sss1–6 ↑Hgt1p growth is also hypersensitive to GSH as it was severely perturbed by 2.5 μm GSH and 5 μm GSH and completely arrested by 10 μm GSH (Fig. 4B).
Mn2+ is an essential cofactor for cytoplasmic farnesyl pyrophosphate (FPP) synthetase (Fpp1p) (44–46). Schuldiner and colleagues (44) have shown that Fpp1p activity is elevated upon deletion of the ER resident Mn2+ transporter Spf1p because of a failure to store Mn2+ in the ER which gives rise to elevated Mn2+ levels in the cytosol. Elevated Fpp1p activity leads to increased squalene synthesis, which inhibits cell proliferation if cells cannot remove it through metabolism, such as when cells are challenged with the squalene epoxidase inhibitor terbinafine (44). We used this system to test if Sss1p mutants were also defective in maintaining normal Mn2+ homeostasis and possessed increased Fpp1p activity. sss1–6 and sss1–7, in particular, cell growth is extremely sensitive to terbinafine as, unlike WT, 1 μg/ml terbinafine completely inhibited the growth of sss1–7 and sss1–6 at 30 °C and 34 °C, respectively (Fig. 4C). Importantly, neither sss1–6 nor sss1–7 cells are hypersensitive to the 14α-sterol demethylase inhibitor miconazole (Fig. S2B). Therefore, the hypersensitivity of both sss1–6 and sss1–7 cells to terbinafine is not simply because of these mutants being hypersensitive to small molecules that inhibit ergosterol biosynthesis.
Mutations in residues of Sec61p located in important gating modules suppress sss1–6 and sss1–7 temperature sensitivity
To understand how mutations in the C terminus of Sss1p disrupt ER homeostasis we sought to identify mutations in the major interacting partner of Sss1p, Sec61p, which could suppress sss1–7 temperature-sensitive growth. Missense mutations were incorporated into the SEC61 gene by error-prone PCR, and mutagenized SEC61 genes were then transfected into sss1–7 yeast and integrated into the SEC61 genetic locus by homologous recombination. In total, 203 Ts+ colonies were isolated and the complete nucleotide sequence of 50 was determined. On average EP-PCR yielded 3.5 mutations per 1 kb and 23 of the 50 mutants sequenced contained a single nucleotide substitution in the SEC61 ORF. In total, five different SEC61 mutant alleles, V82F, S289F, N302K, N302Y, and T379A were obtained from this screen. We were surprised to discover that each mutation was able to suppress the temperature-sensitive growth of sss1–6 and sss1–7 (Fig. 5A) and reduced UPR induction (Fig. 5B) in these sss1 mutants when expressed from a low-copy, centromeric plasmid, avoiding the need to integrate these mutations into the SEC61 locus.
Figure 5.
Mutations in residues of Sec61p located in important gating modules suppress sss1–6 and sss1–7 temperature sensitivity. A, WT, sss1–6, or sss1–7 yeast transformed with either YCp SEC61, YCp SEC61V82F, YCp SEC61S289F, YCp SEC61N302K, YCp SEC61N302Y, or YCp SEC61T379A were spotted on YPD agar in a 10-fold dilution series and incubated at 30 °C or 37 °C for 2 days. B, WT, sss1–6, or sss1–7 yeast transformed with either YCp SEC61, YCp SEC61V82F, YCp SEC61S289F, YCp SEC61N302K, YCp SEC61N302Y, or YCp SEC61T379A and with pJT30 (UPRE-LacZ) were grown in Ura selective medium and β-galactosidase activity determined. As a positive control WT cells were treated with 5 mm DTT for 2 h.
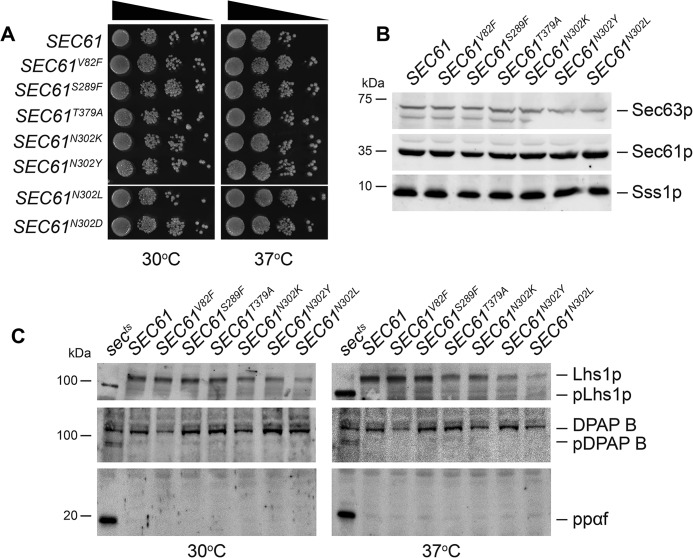
The functionality of these suppressive SEC61 mutants is not compromised as each mutant supports the robust growth of cells when expressed as the only copy of SEC61 (Fig. 6, A and B). Furthermore, neither mutant possessed temperature growth phenotype (Fig. 6A). We did not detect any major SRP-dependent or SRP-independent translocation defects in these mutants. The SRP-dependent precursor pDPAP B readily accumulates in the sec65–1ts control at both permissive and nonpermissive temperatures but not in our panel of SEC61 mutants (Fig. 6C). Also, neither SRP-independent translocation precursor pLhs1p nor prepro-α factor accumulated in these SEC61 mutants at 30 °C and 37 °C, unlike the sec62–1ts control (Fig. 6C).
Figure 6.
SEC61-dependent suppressors of sss1–6 and sss1–7 are functional. A, WT, SEC61V82F, SEC61S289F, SEC61N302K, SEC61N302Y, SEC61T379A, SEC61N302L, or SEC61N302D yeast were spotted on YPD agar in a 10-fold dilution series and incubated at 30 °C or 37 °C for 2 days. B, cell extracts derived from WT SEC61V82F, SEC61S289F, SEC61N302K, SEC61N302Y, SEC61T379A, or SEC61N302L yeast were immunoblotted with anti-Sss1p, anti-Sec61p, and anti-Sec63p antibodies. C, cell extracts derived from WT SEC61V82F, SEC61S289F, SEC61N302K, SEC61N302Y, SEC61T379A, or SEC61N302L yeast grown at 30 °C or 37 °C were immunoblotted with anti-Lhs1p, anti-DPAP B, and anti-α factor antibodies.
Mapping each mutation on the crystal structure of the Sec61 homologue Ssh1 revealed them to be positioned at critical sites for translocon gating including the pore ring (V82F) and the lateral and lumenal gate (S289F, N302K/Y, and T379A) (Fig. S3). Residue Asn-302 is part of a network that is responsible for the opening and closing of the lateral and lumenal gates of the translocon. That the SEC61N302K and SEC61N302Y mutations suppress both sss1–6 and sss1–7 suggests that dynamics of the lateral and lumenal gate of the translocon is altered somehow in these sss1 mutants. Substitution of Asn-302 to a polar (N302D) or hydrophobic residue (N302L) destabilizes the closed or open conformation of the Sec61 complex, respectively (47). To investigate if sss1H72K and sss1P74A, I75A mutations destabilize either the open or the closed conformation of the translocon, we tested if the SEC61N302L or the SEC61N302D mutation suppressed sss1–6 and sss1–7 temperature sensitivity. The SEC61N302L mutant suppressed sss1–6 and sss1–7 temperature-sensitive growth (Fig. 7A) and reduced UPR induction (Fig. 7B) like SEC61N302K and SEC61N302Y. In contrast, co-expression of SEC61N302D with either sss1–6 or sss1–7 exacerbated the growth defects of these mutants (Fig. 7, C and D). Furthermore, we were unable to isolate sss1–7 sec61N302D double mutants (Fig. 7C), suggesting the possibility that these mutations, when co-expressed, are synthetically lethal. Together, this suggests that the sss1–6 and sss1–7 mutations may destabilize the closed conformation of the translocon.
Figure 7.
Mutations in the lumenal lateral gate genetically interact with sss1–6 and sss1–7. A, WT, and sss1–6 or sss1–7 yeast transformed with either YCp SEC61, YCp SEC61N302K, or YCp SEC61N302L were spotted on YPD agar in a 10-fold dilution series and incubated at 30 °C or 37 °C for 2 days. B, WT or sss1–7 yeast transformed with either YCp SEC61, YCp SEC61N302K, or YCp SEC61N302L and with pJT30 (UPRE-LacZ) were grown in Ura selective medium and β-galactosidase activity was determined. As a positive control WT cells were treated with 5 mm DTT for 2 h. C, CMY5 yeast were cotransformed with either YCp SSS1, YCp sss1–6, or YCp sss1–7 and either YCp SEC61 or YCp SEC61N302D. Transformants were streaked out onto either Leu, Trp selective medium or medium containing FOA and incubated at 30 °C for 3 days. D, CMY5 yeast cotransformed with YCp sss1–6 and either YCp SEC61 or YCp SEC61N302D recovered from FOA containing medium were spotted on YPD agar in a 10-fold dilution series and incubated at 30 °C or 37 °C for 2 days.
Mutations in residues located in important Sec61p gating modules suppress the elevated ER permeability observed in sss1–6 and sss1–7
Given the impressive manner in which our SEC61 mutants were able to diminish the level of ER stress in both sss1–6 and sss1–7 we were keen to determine whether these mutants were also able to suppress phenotypes associated with altered ER permeability. Co-expression of each of the SEC61 suppressors of sss1–7 temperature sensitive growth were able to suppress the hypersensitivity of the sss1–6 mutant to GSH (Fig. 8, A and B). Furthermore, co-expression of the SEC61 mutants that suppressed sss1–6 and sss1–7 temperature-sensitive growth also suppressed their hypersensitivity to terbinafine, albeit with varying strength (Fig. 8, C and D).
Figure 8.
Mutations in residues of Sec61p located in important gating modules suppress ER permeability defects in sss1–6 and sss1–7. A, WT, or sss1–6 yeast transformed with either YCp SEC61, YCp SEC61V82F, YCp SEC61S289F, YCp SEC61N302K, or YCp SEC61T379A were transformed with YEp HGT1 and were grown in Ura selective medium with increasing concentrations of GSH. The relative growth of each strain determined and the GSH sensitivity (1/relative growth) presented. B, WT, sss1–6 or sss1–6 yeast transformed with either YCp SEC61N302K or YCp SEC61N302L and YEp HGT1 were grown in Ura medium with increasing concentrations of GSH, the relative growth of each strain determined and the GSH sensitivity (1/relative growth) presented. C, WT and sss1–6 or sss1–7 yeast transformed with either YCp SEC61, YCp SEC61V82F, YCp SEC61S289F, YCp SEC61N302K, or YCp SEC61T379A were spotted on YPD agar or YPD agar containing 1 μg/ml terbinafine in a 10-fold dilution series and incubated at 30 °C or 34 °C for 2 days. D, WT, sss1–6, sss1–7 or sss1–6 and sss1–7 yeast transformed with either YCp SEC61N302K or YCp SEC61N302L were spotted on YPD agar or YPD agar supplemented with 1 μg/ml terbinafine in a 10-fold dilution series and incubated at 30 °C or 34 °C for 2 days.
Discussion
Sss1p/Sec61γ is an essential and highly conserved subunit of the ER translocase yet its function is poorly understood. A heptapeptide, KLIHIPI, located toward the C terminus of the protein is absolutely conserved in all eukaryotes studied to date. Herein, we discover the KLIHIPI peptide to be an important factor that influences ER permeability.
The extreme Sss1p/Sec61γ C terminus influences ER permeability
Cells expressing sss1 mutants with defective TM domains have been shown to be defective in ER translocation (22, 35). We were surprised to find no ER translocation defect in both sss1–6 and sss1–7 cells even though the UPR was highly induced in both mutants. Sss1p, like Sec61p, is essential for both SRP-dependent and SRP-independent ER translocation. That we find no evidence of the accumulation of secretory precursor proteins at steady state suggests that mutation of the highly conserved C terminus of Sss1p does not result in the gross perturbation in the formation of protein complexes that are required to conduct ER translocation. Given that Sss1p/Sec61γ is a C-terminally anchored protein it is possible that both Sss1–6p and Sss1–7p may not be efficiently targeted to and incorporated into the ER membrane. Our data do not support this notion. We do not observe a decrease in the membrane-associated pool of Sss1–6p and Sss1–7p nor do we observe a decreased ability to crosslink both Sec61p and Sss1p with DSS. The latter point is crucial to this conclusion as the insertion of C-terminal–anchored proteins proceeds via the GET complex and not the ER translocase. Therefore, the crosslinking of Sec61p with both Sss1–6p and Sss1–7p reflects a functional interaction rather than a trivial interaction of Sec61p with a translocation intermediate. In further support, we observe no reduction in the ability to isolate components of the ER SEC complex by ConA pulldown.
Those secretory proteins that are N-glycosylated are modified by the oligosaccharyl transferase (OST) as they are translocated through the translocation channel. Sss1p is physically located at the interface between the translocon and OST. It is possible that mutation of the highly conserved Sss1p C terminus perturbs the association of OST with the translocon. However, we find no evidence of N-glycosylation being perturbed in sss1–6 and sss1–7, the N-glycosylation status of Lhs1p and DPAP B is indistinguishable from WT. Furthermore, MAT α derivatives of both sss1–6 and sss1–7 secrete sufficient quantity of α factor to enable their mating with MAT a strains. We therefore have to consider other possibilities to explain the severe ER stress in sss1–6 and sss1–7 mutants.
A directed evolution approach was therefore used to obtain SEC61 mutants that suppressed the temperature-sensitive growth defect of sss1–7 cells to understand what aspect of translocon function was disrupted in these sss1 mutants. We isolated two different classes of suppressor mutation: class one mutations (V82F) are located in the pore ring and class two mutations (S289F, N302K/Y, and T379A) are located in the lateral and lumenal gate that coordinates the opening and closing of the protein-conducting channel. A common feature of both classes of suppressor mutation is that they are situated in regions of the Sec61 protein that are required to gate the channel. The lateral gate, formed by TMs 2 and 7 of Sec61p, enables the incorporation of signal peptides or transmembrane helices into the lipid bilayer once they have emerged from the ribosome. Thus, the opening of the “lateral gate” is a critical process that allows protein translocation to proceed while maintaining the ER permeability barrier to ensure that ER lumenal equivalents do not leak into the cytosol and vice versa. Apolar residues in the lumenal gate and a cluster of polar residues within the lateral gate form a highly conserved gating motif that regulates the opening and closing of the Sec61 complex (47). One critical residue is Sec61p Asn-302. Importantly, mutations that reconfigure the hydrogen bonding network have been shown to elicit a Prl phenotype (47). In contrast, mutations that increase the hydrophobicity enhance the nonpolar interactions between the lateral and lumenal gates and stabilize the closed conformation. Enhancing the nonpolar interaction network suppressed both sss1–6 and sss1–7 temperature sensitivity.
Taken together this suggests the possibility that the ER is more permeable in sss1–6 and sss1–7. We consider that our panel of SEC61 mutations in the lumenal and lateral gate negate the ability of sss1–6 and sss1–7 to destabilize the closed conformation of the Sec61 complex. It is possible that these mutations suppress sss1–6 and sss1–7 by reducing the rate of ER translocation to such an extent that a translocating intermediate can seal the channel by clogging the translocon. However, we consider this to be highly unlikely as each mutant is fully functional with no detectable defect in either SRP-dependent or SRP-independent translocation.
The Sec61 complex has been shown by Toledano and colleagues (42) to facilitate the diffusion of reduced GSH into the ER. It is important to note that the SEC61N302L mutation in the lumenal-lateral gate was also utilized in this study to verify that the translocon forms the channel that facilitates the diffusion of GSH into the ER. Our panel of SEC61 suppressors as well as SEC61N302L suppresses sss1–6 hypersensitivity to GSH. These SEC61 mutations also rescue the hypersensitivity of both sss1–6 and sss1–7 to terbinafine, a phenotype that has been previously shown to arise in mutants defective in ER Mn2+ storage (44). We appreciate that the hypersensitivity of both sss1–6 and sss1–7 growth to terbinafine is itself an indirect measure of increased ER permeability in these mutants. However, both sss1–6 and sss1–7 are not hypersensitive to the 14α-sterol demethylase inhibitor, miconazole ruling out that this phenotype is because of these sss1 mutants being hypersensitive to reagents that inhibit ergosterol biosynthesis. Together we hypothesize that the C terminus of Sss1p/Sec61γ constitutes an important component of the gating module that coordinates the opening and closing of the translocon, both sss1–6 and sss1–7 destabilizing the closed state. Future work will provide a mechanistic insight to the role of this domain. The importance of the C terminus of Sss1p as a key component of the translocon gating module is reflected in the absolute conservation of the KLIHIPI heptapeptide throughout the eukaryota.
Materials and methods
Yeast strains and growth
Saccharomyces cerevisiae strains are listed in Table S1 and plasmids are listed in Table S2. Yeast strains were grown routinely at 30 °C in YP medium (2% peptone, 1% yeast extract) containing 2% glucose (YPD) or in minimal medium (0.67% yeast nitrogen base; YNB) with 2% glucose plus appropriate supplements for selective growth. All media were from Formedium (Hunstanton, UK). For growth assays, yeast were spotted in a 10-fold dilution series on YPD agar and grown at either 30 °C, 34 °C, or 37 °C for 2 to 3 days. 1 μg/ml terbinafine or DMSO was added to YPD agar where indicated.
Mutagenesis and selection for SEC61 suppressors of sss1–7 temperature sensitivity
EP-PCR was carried out with TaqDNA polymerase in the presence of 500 μm MnCl2 and run for 10 cycles. Reactions were also performed with inverse dNTP concentrations. Samples were pooled after EP-PCR and digested with HindIII for 1 h at 37 °C. sss1–7 yeast were transformed with restricted PCR product and transformants were plated on YPD agar and incubated at 37 °C for up to 5 days. The SEC61 locus of suppressor colonies was sequenced and individual mutations were introduced in pBW11 by site-directed mutagenesis using the Q5® Site-Directed Mutagenesis Protocol (New England Biolabs Inc.); oligonucleotides used are listed in Table S3.
Fluorescence microscopy
The GFP ORF was amplified with oligonucleotides flanked with 5′ PacI and 3′ SphI and ligated into pJKB2 in which nucleotides −8 to −1 and 1 to 6 were mutated to encode PacI and SphI restriction sites, respectively, giving pLB4 (YCp GFP-SSS1). The sss1–6 and sss1–7 mutations were incorporated into pLB4 using the Q5® Site-Directed Mutagenesis Protocol (New England Biolabs Inc.) giving pLB5 and pLB6 respectively. Oligonucleotides used are listed in Table S3. Static images were collected of live cells attached to a concanavalin A–coated slide using an Ultraview Spinning Disk Confocal Microscope (Perkin Elmer Life Sciences).
DSS crosslinking
Yeast microsomes were prepared according to Rothblatt and Meyer (48). Membranes were treated with DMSO or 1 mm DSS at 30 °C or 37 °C for 30 min and then quenched by the addition of 10 mm lysine and 100 mm Tris for 10 mins.
Immunoblotting
Antibodies used are listed in Table S4.
ConA dependent fractionation of SEC proteins
The fractionation of SEC proteins by ConA was performed according to Pilon et al. (40). Briefly, microsomes were isolated and resuspended in 100 μl of solubilization buffer (50 mm HEPES/KOH, pH 7.4, 400 mm KAc, 5 mm MgAc, 10% (w/v) glycerol, 0.05% (v/v) β-mercaptoethanol) on ice containing protease inhibitors (5 μg/ml leupeptin, 0.5 μg/ml pepstatin, 1 mm amino-benzamidine, 2.5 μg/ml chymostatin, and 0.1 mm PMSF). Membranes were solubilized by the addition of 400 μl solubilization buffer containing 3.75% (w/v) digitonin. Next, samples were centrifuged at 100,000 × g in a Beckman TLA100.3 rotor for 60 min at 4 °C to isolate the ribosome attached membrane proteins. The supernatant fraction was added to 100 μl of a suspension of ConA-Sepharose equilibrated in 50 mm HEPES/KOH (pH 7.4), 10% (w/v) glycerol, 0.05% (v/v) β-mercaptoethanol, 1% (w/v) digitonin, and protease inhibitors, and incubated for 60 min at 4 °C. The beads were recovered by centrifugation at 2500 × g and the supernatant fraction was cleared from any remaining beads at 12,000 × g (free fraction). The ConA beads were washed three times with 1 ml of equilibration buffer. Equal aliquots of both fractions were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies.
β-Galactosidase assays
β-Galactosidase assays were performed according to Tyson and Stirling (49). Briefly, yeast cells were grown at 30 °C in minimal medium containing 2% glucose and appropriate supplements. Cultures were diluted to A600 nm of 0.2 and grown for a further 4 h. Cells were isolated and resuspended in 2 ml of Z buffer (60 mm Na2HPO4, 40 mm NaH2PO4, 10 mm KCl, 10 mm MgSO4, 50 mm 2-mercaptoethanol, pH 7.0). Aliquots (0.8 ml) were collected, cells were permeabilized in 50 μl of 0.1% (w/v) SDS and 100 μl of CHCl3, and samples were equilibrated to 30 °C. Assays were initiated by addition of 160 μl of o-nitrophenyl-galactopyranoside (4 mg/ml stock solution in Z buffer) and incubated at 30 °C for 20 min. Reactions were terminated by addition of 400 μl of 1 m Na2CO3, pH 9.0, the A420 nm was measured, and LacZ activity (U) was calculated by multiplying A420 nm/A600 nm by 1000. Three independent biological replicates and at least two technical replicates were performed.
GSH sensitive growth assay
Cells harboring YEp HGT1 were grown to mid logarithmic-phase and then subcultured to 0.01 A600 nm in SC media without uracil containing 0–10 μm GSH and the growth rate recorded. The growth rate of cells cultured in the absence of GSH was set to 100% and relative growth rates plotted. Three independent biological replicates and at least two technical replicates were performed.
Author contributions
C. M. W., H. G. D., A. L. P., K. L. P. S., L. B., P. F. W., A. L. B., and C. J. M. investigation; K. L. P. S., R. F. L. S., C. J. S., and C. J. M. supervision; R. F. L. S., C. J. S., B. L. S., and C. J. M. conceptualization; R. F. L. S., B. L. S., and C. J. M. writing-original draft; C. J. M. project administration.
Supplementary Material
Acknowledgments
We thank Curtin University Schools of Pharmacy and Biomedical Science and Graduate Research School for providing funds for graduate research.
The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S3 and Table S1–S4.
- ER
- endoplasmic reticulum
- SRP
- signal recognition particle
- DSS
- disuccinimidyl suberate
- ConA
- concanavalin A
- UPR
- unfolded protein response
- UPRE
- UPR enhancer
- OST
- oligosaccharyl transferase.
References
- 1. Behnke J., Feige M. J., and Hendershot L. M. (2015) BiP and its nucleotide exchange factors Grp170 and Sil1: Mechanisms of action and biological functions. J. Mol. Biol. 427, 1589–1608 10.1016/j.jmb.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janda C. Y., Li J., Oubridge C., Hernández H., Robinson C. V., and Nagai K. (2010) Recognition of a signal peptide by the signal recognition particle. Nature 465, 507–510 10.1038/nature08870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapoport T. A., Li L., and Park E. (2017) Structural and mechanistic insights into protein translocation. Annu. Rev. Cell Dev. Biol. 33, 369–390 10.1146/annurev-cellbio-100616-060439 [DOI] [PubMed] [Google Scholar]
- 4. Voorhees R. M., and Hegde R. S. (2015) Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. eLife 4, e07975 10.7554/eLife.07975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walter P., Ibrahimi I., and Blobel G. (1981) Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J. Cell Biol. 91, 545–550 10.1083/jcb.91.2.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keenan R. J., Freymann D. M., Stroud R. M., and Walter P. (2001) The signal recognition particle. Annu. Rev. Biochem. 70, 755–775 10.1146/annurev.biochem.70.1.755 [DOI] [PubMed] [Google Scholar]
- 7. Luirink J., and Sinning I. (2004) SRP-mediated protein targeting: Structure and function revisited. Biochim. Biophys. Acta 1694, 17–35 10.1016/j.bbamcr.2004.03.013 [DOI] [PubMed] [Google Scholar]
- 8. Ulbrandt N. D., Newitt J. A., and Bernstein H. D. (1997) The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell 88, 187–196 10.1016/S0092-8674(00)81839-5 [DOI] [PubMed] [Google Scholar]
- 9. Böhni P. C., Deshaies R. J., and Schekman R. W. (1988) SEC11 is required for signal peptide processing and yeast cell growth. J. Cell Biol. 106, 1035–1042 10.1083/jcb.106.4.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans E. A., Gilmore R., and Blobel G. (1986) Purification of microsomal signal peptidase as a complex. Proc. Natl. Acad. Sci. U.S.A. 83, 581–585 10.1073/pnas.83.3.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braakman I., and Hebert D. N. (2013) Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5, a013201 10.1101/cshperspect.a013201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmermann R., Eyrisch S., Ahmad M., and Helms V. (2011) Protein translocation across the ER membrane. Biochim. Biophys. Acta Biomembr. 1808, 912–924 10.1016/j.bbamem.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 13. Lakkaraju A. K., Thankappan R., Mary C., Garrison J.L., Taunton J., and Strub K. (2012) Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol. Biol. Cell 23, 2712–2722 10.1091/mbc.e12-03-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen R. E., and Johnson A. E. (1999) Protein translocation: Is Hsp70 pulling my chain? Curr. Biol. 9, R779–R782 10.1016/S0960-9822(00)80012-3 [DOI] [PubMed] [Google Scholar]
- 15. Ngosuwan J., Wang N. M., Fung K. L., and Chirico W. J. (2003) Roles of cytosolic Hsp70 and Hsp40 molecular chaperones in post-translational translocation of presecretory proteins into the endoplasmic reticulum. J. Biol. Chem. 278, 7034–7042 10.1074/jbc.M210544200 [DOI] [PubMed] [Google Scholar]
- 16. Park E., and Rapoport T. A. (2012) Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 41, 21–40 10.1146/annurev-biophys-050511-102312 [DOI] [PubMed] [Google Scholar]
- 17. Plath K., and Rapoport T. A. (2000) Spontaneous release of cytosolic proteins from posttranslational substrates before their transport into the endoplasmic reticulum. J. Cell Biol. 151, 167–178 10.1083/jcb.151.1.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young B. P., Craven R. A., Reid P. J., Willer M., and Stirling C. J. (2001) Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J. 20, 262–271 10.1093/emboj/20.1.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshaies R. J., Sanders S. L., Feldheim D. A., and Schekman R. (1991) Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349, 806–808 10.1038/349806a0 [DOI] [PubMed] [Google Scholar]
- 20. Panzner S., Dreier L., Hartmann E., Kostka S., and Rapoport T. A. (1995) Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81, 561–570 10.1016/0092-8674(95)90077-2 [DOI] [PubMed] [Google Scholar]
- 21. Matlack K. E., Misselwitz B., Plath K., and Rapoport T. A. (1999) BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell 97, 553–564 10.1016/S0092-8674(00)80767-9 [DOI] [PubMed] [Google Scholar]
- 22. Falcone D., Henderson M. P., Nieuwland H., Coughlan C. M., Brodsky J. L., and Andrews D. W. (2011) Stability and function of the Sec61 translocation complex depends on the Sss1p tail-anchor sequence. Biochem. J. 436, 291–303 10.1042/BJ20101865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van den Berg B., Clemons W. M. Jr., Collinson I., Modis Y., Hartmann E., Harrison S. C., and Rapoport T. A. (2004) X-ray structure of a protein-conducting channel. Nature 427, 36–44 10.1038/nature02218 [DOI] [PubMed] [Google Scholar]
- 24. Mandon E. C., Trueman S. F., and Gilmore R. (2013) Protein translocation across the rough endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5, a013342 10.1101/cshperspect.a013342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li W., Schulman S., Boyd D., Erlandson K., Beckwith J., and Rapoport T. A. (2007) The plug domain of the SecY protein stabilizes the closed state of the translocation channel and maintains a membrane seal. Mol. Cell 26, 511–521 10.1016/j.molcel.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 26. Finke K., Plath K., Panzner S., Prehn S., Rapoport T. A., Hartmann E., and Sommer T. (1996) A second trimeric complex containing homologs of the Sec61p complex functions in protein transport across the ER membrane of S. cerevisiae. EMBO J. 15, 1482–1494 10.1002/j.1460-2075.1996.tb00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang Y., Cheng Z., Mandon E. C., and Gilmore R. (2008) An interaction between the SRP receptor and the translocon is critical during cotranslational protein translocation. J. Cell Biol. 180, 1149–1161 10.1083/jcb.200707196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prinz A., Hartmann E., and Kalies K. U. (2000) Sec61p is the main ribosome receptor in the endoplasmic reticulum of Saccharomyces cerevisiae. Biol. Chem. 381, 1025–1029 10.1515/BC.2000.126 [DOI] [PubMed] [Google Scholar]
- 29. Grupe A., Li Y., Rowland C., Nowotny P., Hinrichs A. L., Smemo S., Kauwe J. S., Maxwell T. J., Cherny S., Doil L., Tacey K., van Luchene R., Myers A., Wavrant-De Vrièze F., Kaleem M., et al. (2006) A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease. Am. J. Hum. Genet. 78, 78–88 10.1086/498851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gogala M., Becker T., Beatrix B., Armache J. P., Barrio-Garcia C., Berninghausen O., and Beckmann R. (2014) Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion. Nature 506, 107–110 10.1038/nature12950 [DOI] [PubMed] [Google Scholar]
- 31. Voorhees R. M., Fernandez I. S., Scheres S. H., and Hegde R. S. (2014) Structure of the mammalian ribosome-Sec61 complex to 3.4 A resolution. Cell 157, 1632–1643 10.1016/j.cell.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Becker T., Bhushan S., Jarasch A., Armache J.P., Funes S., Jossinet F., Gumbart J., Mielke T., Berninghausen O., Schulten K., Westhof E., Gilmore R., Mandon E. C., and Beckmann R. (2009) Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome. Science 326, 1369–1373 10.1126/science.1178535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng Z., Jiang Y., Mandon E. C., and Gilmore R. (2005) Identification of cytoplasmic residues of Sec61p involved in ribosome binding and cotranslational translocation. J. Cell Biol. 168, 67–77 10.1083/jcb.200408188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plath K., Mothes W., Wilkinson B. M., Stirling C. J., and Rapoport T. A. (1998) Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 94, 795–807 10.1016/S0092-8674(00)81738-9 [DOI] [PubMed] [Google Scholar]
- 35. Wilkinson B. M., Brownsword J. K., Mousley C. J., and Stirling C. J. (2010) Sss1p is required to complete protein translocon activation. J. Biol. Chem. 285, 32671–32677 10.1074/jbc.M110.128256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilkinson B. M., Tyson J. R., Reid P. J., and Stirling C. J. (2000) Distinct domains within yeast Sec61p involved in post-translational translocation and protein dislocation. J. Biol. Chem. 275, 521–529 10.1074/jbc.275.1.521 [DOI] [PubMed] [Google Scholar]
- 37. Schuldiner M., Metz J., Schmid V., Denic V., Rakwalska M., Schmitt H. D., Schwappach B., and Weissman J. S. (2008) The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645 10.1016/j.cell.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esnault Y., Feldheim D., Blondel M. O., Schekman R., and Képès F. (1994) SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J. Biol. Chem. 269, 27478–27485 [PubMed] [Google Scholar]
- 39. Wilkinson B. M., Esnault Y., Craven R.A., Skiba F., Fieschi J., K'epès F., and Stirling C. J. (1997) Molecular architecture of the ER translocase probed by chemical crosslinking of Sss1p to complementary fragments of Sec61p. EMBO J. 16, 4549–4559 10.1093/emboj/16.15.4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pilon M., Römisch K., Quach D., and Schekman R. (1998) Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane. Mol. Biol. Cell 9, 3455–3473 10.1091/mbc.9.12.3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ng D. T., Brown J. D., and Walter P. (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol. 134, 269–278 10.1083/jcb.134.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ponsero A. J., Igbaria A., Darch M. A., Miled S., Outten C. E., Winther J. R., Palais G., D'Autréaux B., Delaunay-Moisan A., and Toledano M. B. (2017) Endoplasmic reticulum transport of glutathione by Sec61 is regulated by Ero1 and Bip. Mol. Cell 67, 962–973.e965 10.1016/j.molcel.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar C., Igbaria A., D'Autreaux B., Planson A.G., Junot C., Godat E., Bachhawat A. K., Delaunay-Moisan A., and Toledano M. B. (2011) Glutathione revisited: A vital function in iron metabolism and ancillary role in thiol-redox control. EMBO J. 30, 2044–2056 10.1038/emboj.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen Y., Megyeri M., Chen O. C., Condomitti G., Riezman I., Loizides-Mangold U., Abdul-Sada A., Rimon N., Riezman H., Platt F. M., Futerman A. H., and Schuldiner M. (2013) The yeast p5 type ATPase, spf1, regulates manganese transport into the endoplasmic reticulum. PLoS One 8, e85519 10.1371/journal.pone.0085519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davis C. D., Ney D. M., and Greger J. L. (1990) Manganese, iron and lipid interactions in rats. J. Nutr. 120, 507–513 10.1093/jn/120.5.507 [DOI] [PubMed] [Google Scholar]
- 46. Hansen S. L., Spears J. W., Lloyd K. E., and Whisnant C. S. (2006) Growth, reproductive performance, and manganese status of heifers fed varying concentrations of manganese. J. Anim. Sci. 84, 3375–3380 10.2527/jas.2005-667 [DOI] [PubMed] [Google Scholar]
- 47. Trueman S. F., Mandon E. C., and Gilmore R. (2012) A gating motif in the translocation channel sets the hydrophobicity threshold for signal sequence function. J. Cell Biol. 199, 907–918 10.1083/jcb.201207163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rothblatt J. A., and Meyer D. I. (1986) Secretion in yeast: Translocation and glycosylation of prepro-α-factor in vitro can occur via an ATP-dependent post-translational mechanism. EMBO J. 5, 1031–1036 10.1002/j.1460-2075.1986.tb04318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tyson J. R., and Stirling C. J. (2000) LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO J. 19, 6440–6452 10.1093/emboj/19.23.6440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.