Abstract
Prolonged cold exposure stimulates the formation of brownlike adipocytes expressing UCP1 (uncoupling-protein-1) in subcutaneous white adipose tissue which, together with classical brown adipose tissue, contributes to maintaining body temperature in mammals through nonshivering thermogenesis. The mechanisms that regulate the formation of these cells, alternatively called beige or brite adipocytes, are incompletely understood. Here we report that mice lacking CD137, a cell surface protein used in several studies as a marker for beige adipocytes, showed elevated levels of thermogenic markers, including UCP1, increased numbers of beige adipocyte precursors, and expanded UCP1-expressing cell clusters in inguinal white adipose tissue after chronic cold exposure. CD137 knockout mice also showed enhanced cold resistance. These results indicate that CD137 functions as a negative regulator of “browning” in white adipose tissue and call into question the use of this protein as a functional marker for beige adipocytes.
Keywords: adipose tissue metabolism, adipocyte, adipogenesis, adipose tissue, adipokine, beige adipocyte, brite adipocyte, thermogenesis, UCP1
Introduction
Prolonged exposure to cold temperatures induces the appearance of multilocular cells with thermogenic capacity in subcutaneous depots of white adipose tissue (WAT).4 These cells, referred to as either brite (1) or beige (19) adipocytes (the latter will be used here), express functional UCP1 (2) and, together with classic brown adipocytes, contribute to resistance to cold exposure in mammals. There is great interest in understanding the mechanisms that give rise to beige adipocytes, a process generally referred to as browning, and regulate their function as a means to develop strategies to increase energy expenditure in obesity and other metabolic conditions (3).
The mechanisms that regulate the formation of beige adipocytes, whether de novo from dedicated beige cell precursors or through transdifferentiation of existing white adipocytes, have been highly debated. There is compelling evidence supporting either mechanism, suggesting that both can contribute to beige fat biogenesis under different circumstances (4, 5). Cell sorting and in vitro experiments support the existence of beige adipocyte precursors in the stromal vascular fraction (SVF) of inguinal WAT (iWAT) (6, 7). On the other hand, it has been observed that in mice that have been exposed to cold, beige adipocytes adopt a white-like unilocular phenotype upon return to warm temperatures (8). Intriguingly, these adipocytes appear to be primed to adopt a thermogenic, beigelike phenotype when the mice are again exposed to cold (8), suggesting a possible mechanism of white to beige adipocyte conversion. Thus, mechanisms of browning could depend on whether mice have been exposed previously to cold temperatures, which would favor formation of beige adipocytes from memory cells of white appearance, or not, in which case beige adipocytes may be generated de novo (4).
Much of recent research on the generation and physiology of beige adipocytes has been based on the identification or utilization of markers specifically expressed by these cells. The transmembrane protein CD137, also referred to as Tnfrsf9 and 4–1BB, is one such molecule. CD137 is a member of the TNF receptor superfamily, initially characterized as an immunoregulatory molecule on the surface of activated leukocytes, particularly T cells and natural killer cells (9, 10). Chondrocytes and endothelial cells have also been shown to express CD137 upon stimulation by inflammatory cytokines. The CD137 ligand (CD137L) is a type II transmembrane protein expressed on antigen-presenting cells and is capable of reverse signaling upon interaction with CD137 presented on the surface of nearby cells (9, 10). CD137 was identified as a marker of beige adipocyte precursors and mature beige adipocytes in microarray screens of immortalized cell lines derived from iWAT of a strain of obesity-resistant Sv129 mice (6). However, subsequent studies have had mixed success in validating CD137 as a specific marker for beige adipocytes (8, 11–13). Moreover, the vast majority of studies have used CD137 mRNA, not the protein, as a marker for WAT browning or beige adipocytes and their precursors. Importantly, the function of CD137 in beige adipose tissue, if any, is totally unknown.
In this study, we have used knockout mice lacking CD137 to investigate possible functions of CD137 in the generation of beige adipocytes and the emergence of thermogenic properties in iWAT upon cold exposure. Contrary to what would have been expected for a beige adipocyte marker, we found that loss of CD137 increased the number of beige cell precursors and enhanced the expression of thermogenic markers and the size of UCP1-expressing cell clusters in iWAT of CD137 knockout mice exposed to cold.
Results
Enhanced expression of thermogenic proteins in iWAT of CD137 knockout mice after cold exposure
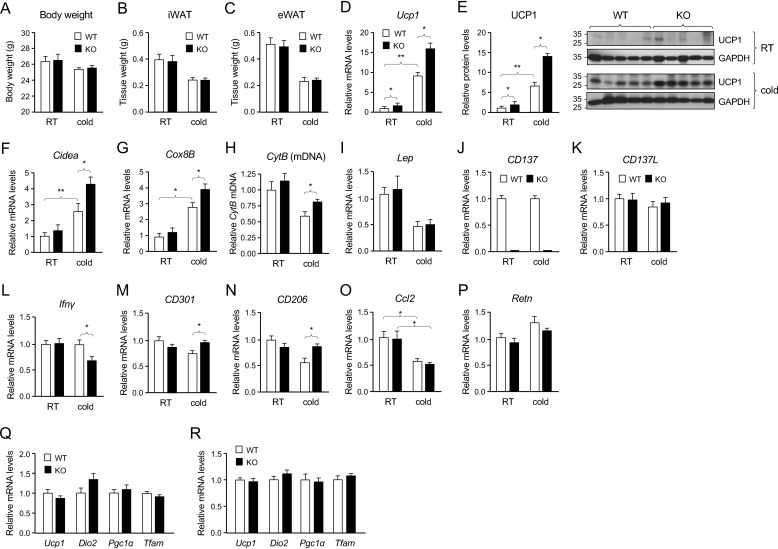
To investigate possible functions of CD137 in cold adaptation and iWAT browning, cohorts of young adult (6- to 7-week-old) WT and CD137 knockout male mice (C57/Bl6J background) were maintained on chow diet at room temperature (22–23 °C) or exposed to cold (8 °C for 4 weeks). No differences between genotypes could be detected in body weight or tissue weight of iWAT or epididymal fat depots in mice kept at room temperature or exposed to cold (Fig. 1, A–C). At room temperature, iWAT of CD137 knockout mice showed a small but detectable increase in the levels of expression of UCP1 mRNA and protein compared with WT (Fig. 1, D and E). As expected, cold exposure increased the expression of Ucp1 mRNA and UCP1 protein in iWAT of WT mice, but did significantly more so in CD137 knockout mice (Fig. 1, D and E), suggesting enhanced cold response in iWAT of mice lacking CD137. Similarly, the induction of thermogenic markers Cidea (cell death-inducing DNA fragmentation factor α-like effector A) and Cox8B (cytochrome c oxidase subunit 8–1) mRNAs was more pronounced in iWAT of CD137 knockout mice after cold exposure (Fig. 1, F and G). iWAT of cold-exposed CD137 knockout mice also showed increased mitochondria content as assessed by Q-PCR for the mitochondrial gene cytochrome B (Fig. 1H). As expected, cold exposure reduced the levels of mRNA encoding leptin, but there were no differences between genotypes (Fig. 1I). Interestingly, cold exposure had no effect on the levels of expression of mRNAs encoding CD137 or its ligand CD137L (Fig. 1, J and K). CD137 mRNA could not be detected in iWAT of the knockout mice (Fig. 1E). Given the established role of CD137 as an immunomodulatory molecule, we investigated the expression of a host of cell surface markers and cytokines associated with different inflammatory and immune responses. Although no significant changes between WT and knockout mice were detected in iWAT of mice kept at room temperature, the level of mRNA encoding IFN-γ was reduced in iWAT of CD137 knockout mice compared with WT after cold exposure (Fig. 1L). In line with an altered balance of type 1/2 immune responses, expression of mRNAs encoding markers of type 2 macrophages CD301 and CD206 was higher in iWAT of CD137 knockout mice compared with WT after cold exposure (Fig. 1, M and N). No differences were detected between genotypes in the levels of mRNAs encoding monocyte chemoattractant protein-1, encoded by the Ccl2 gene (Fig. 1O) or resistin, also known as adipose tissue-specific secretory factor (Fig. 1P). No differences between genotypes could be detected in the levels of thermogenic markers in brown adipose tissue, either at room temperature or after cold exposure (Fig. 1, Q and R).
Figure 1.
Enhanced expression of thermogenic proteins in iWAT of CD137 knockout mice after cold exposure. A, body weights of WT (open bars) and knockout (solid bars) mouse cohorts kept at room temperature (RT) and after chronic (4-week) cold exposure. B and C, weights of inguinal (B) and epididymal (C) fat depots in WT (open bars) and knockout (solid bars) mouse cohorts kept at RT and after chronic (4-week) cold exposure. D, expression of Ucp1 mRNA assessed by Q-PCR in iWAT of WT and CD137 knockout mice housed at RT or at 8 °C for 4 weeks (cold). Results are expressed as average ± S.E. of -fold change over WT at room temperature, n = 8 mice per group. *, p < 0.05; **, p < 0.01; 2-way ANOVA. E, expression of UCP1 protein assessed by Western blotting in iWAT of WT and CD137 knockout mice housed at RT or at 8 °C for 4 weeks (cold). Results are expressed as average ± S.E. of -fold change over WT at room temperature, n = 8 mice per group. *, p < 0.05; **, p < 0.01; 2-way ANOVA. Representative blots probed for UCP1 and GAPDH are show on the right-hand side. F and G, expression of Cidea (F) and Cox8B (G) mRNAs assessed by Q-PCR in iWAT of WT (open bars) and CD137 knockout (solid bars) mice housed at RT or at 8 °C for 4 weeks (cold). Results are expressed as average ± S.E. of -fold change over WT at RT, n = 8 mice per group. *, p < 0.05; **, p < 0.01; 2-way ANOVA. H, mitochondrial content assessed by Q-PCR for the mitochondrial gene cytochrome B (CytB) normalized to nuclear DNA in iWAT of WT (open bars) and CD137 knockout (solid bars) mice housed at RT or at 8 °C for 4 weeks (cold). Results are expressed as average ± S.E. of -fold change over WT at RT, n = 6 mice per group. *, p < 0.05. I to P, expression of Leptin (I), CD137 (J), CD137L (K), Ifnγ (L), CD301 (M), CD206 (N), Ccl2 (O), and Resistin (P) mRNAs assessed by Q-PCR in iWAT of WT (open bars) and CD137 knockout (solid bars) mice housed at RT or at 8 °C for 4 weeks (cold). Results are expressed as average ± S.E. of -fold change over WT at RT, n = 8 mice per group. *, p < 0.05; 2-way ANOVA. Q and R, expression of Ucp1, Dio2, Pgc1α, and Tfam mRNAs assessed by Q-PCR in brown adipose tissue of WT and CD137 knockout mice housed at room temperature (A) or at 5 °C for 4 weeks (B). Results are expressed as average ± S.E. of -fold change over WT, n = 8 mice per group.
CD137 knockout mice have expanded UCP1-expressing cell clusters in iWAT after cold exposure and show enhanced resistance to cold temperatures
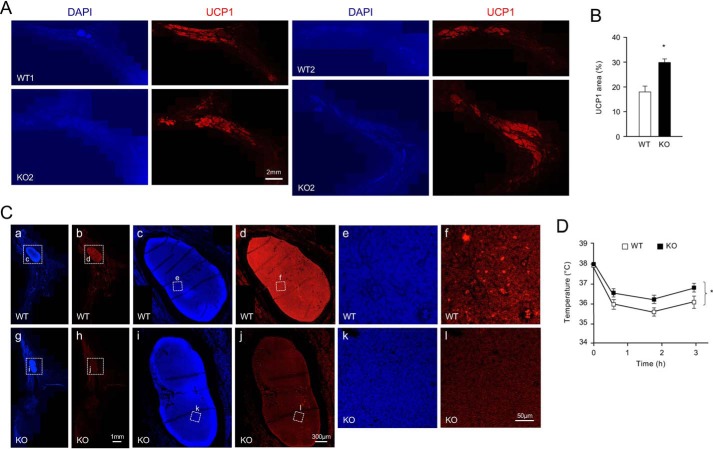
Enhanced expression of UCP1 in iWAT of CD137 knockout mice exposed to cold could be because of increased per cell levels of UCP1 mRNA, and protein, or expanded numbers of UCP1-expressing cells in the tissue. We investigated this by performing immunohistochemistry for UCP1 in sections of iWAT of WT and CD137 knockout mice after cold exposure. We determined the compound area of UCP1-expressing cell clusters relative to the total area of the tissue, assessed by DAPI staining, in 10 sections throughout the whole iWAT depot of 8 mice per genotype (Fig. 2A). We found a significant (p = 0.023) increase of 65% in the area occupied by UCP1-expressing cell clusters in iWAT from CD137 knockout compared with WT mice after cold exposure (Fig. 2B). No significant difference could be detected in the mean pixel intensity of UCP1 labeling between the two genotypes, suggesting that cold exposure induced a larger expansion of UCP1-expressing cell clusters in CD137 knockout mice compared with WT. Intriguingly, immunofluorescence signal for CD137 expression was below detection levels iWAT cells, except in the lymph node, where expression was clearly visible (Fig. 2C, panels a–f). Sections through iWAT of CD137 knockout animals confirmed that the lymph node signal was specific (Fig. 2C, panels g–l). Given that CD137 knockout mice showed elevated levels of UCP1 expression even when kept at room temperature, we investigated their ability to maintain body temperature during acute cold exposure compared with WT mice. We found a small but significant difference (∼1 °C) in body temperature between genotypes (Fig. 2D), suggesting that CD137 knockout mice are better adapted to acute cold exposure than WT mice.
Figure 2.
CD137 knockout mice have expanded UCP1-expressing cell clusters in iWAT after cold exposure and show enhanced resistance to cold temperatures. A, immunohistochemistry analysis of UCP1 expression in sections of iWAT from WT and CD137 knockout mice after 4 weeks at 8 °C. Examples from two different mice of each genotype are shown as composites of 15–20 images through the whole iWAT fat pad. Counterstaining with DAPI is shown on the left. Scale bar, 2 mm. B, percentage of tissue area occupied by UCP1-positive cell clusters in iWAT from WT and CD137 knockout mice after 4 weeks at 8 °C. Results are expressed as average ± S.E., n = 8 mice per group. *, p = 0.023; Student's t test. C, immunohistochemistry analysis of CD137 expression (red) in sections of iWAT from 6-week-old cold-exposed WT and CD137 KO mice. DAPI counterstaining is shown in blue. Expression of CD137 could be detected in the iWAT lymph node (high magnification insets) but remained at the background levels similar to KO iWAT, in the rest of the tissue, where adipocytes are located. Size bars are indicated. D, body temperature of WT and CD137 knockout mice measured during 3-h exposure to 5 °C. Results are expressed as average ± S.E., n = 8 mice per group. *, p < 0.05 KO versus WT; 2-way ANOVA.
Enhanced response to β-adrenergic stimulation in cultured beige adipocytes from CD137 knockout mice exposed to cold
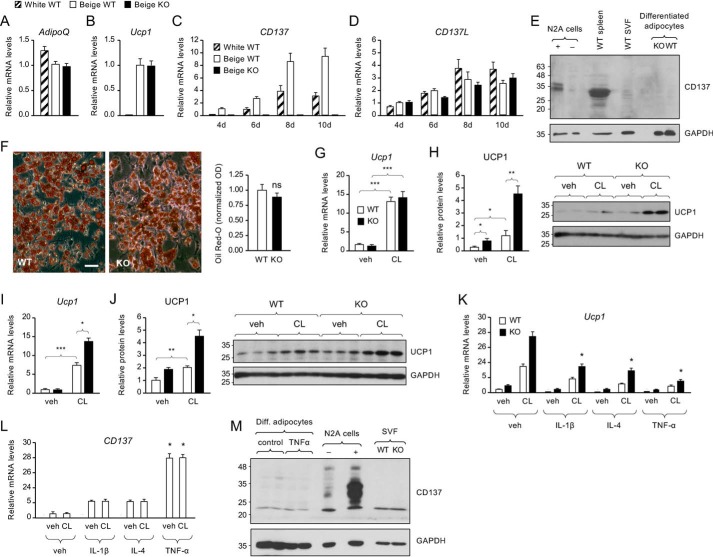
The enhanced expansion of UCP1-expressing cell clusters in iWAT of CD137 knockout mice after cold exposure prompted us to investigate the differentiation properties of cells in the SVF of iWAT derived from these mice compared with their WT counterparts. First, we established cultures of SVF cells from iWAT of WT mice kept at room temperature and tested conditions known to favor either white or beige adipocyte differentiation, following previously described protocols (e.g. (6, 13)). Although both protocols induced the expression of adipocyte markers such as mRNA encoding adiponectin (AdipoQ, Fig. 3A), only conditions known to favor development of beige adipocytes resulted in significant expression of Ucp1 mRNA (Fig. 3B). The levels of AdipoQ and Ucp1 mRNAs detected in cultures derived from iWAT lacking CD137 were similar to those observed in WT cultures (Fig. 3, A and B). Expression of CD137 mRNA increased during SVF cell differentiation using either protocol, although more so with conditions that favor the beige phenotype (Fig. 3C). No detectable levels of CD137 mRNA were seen in cultures derived from the SVF of CD137 knockout mice (Fig. 3C). Expression of mRNA encoding CD137L also increased during differentiation, although to a lesser extent (Fig. 3D). In this case, we saw no apparent differences between differentiation protocols or genotypes (Fig. 3D). Intriguingly, and despite mRNA expression, the levels of CD137 protein were below detection levels in cultured beige adipocytes or iWAT SVF cells (Fig. 3E). On the other hand, CD137 could be readily detected in spleen extracts or N2A cells stably transfected with the CD137 expression plasmid (Fig. 3E). No significant difference was detected between genotypes in the structure or size of iWAT adipocytes, nor lipid content, as assessed by Oil Red O staining (Fig. 3F).
Figure 3.
Enhanced response to β-adrenergic stimulation in cultured beige adipocytes from CD137 knockout mice after cold exposure. A and B, expression of Adiponectin (A) and Ucp1 (B) mRNAs assessed by Q-PCR in cultures of WT iWAT SVF cells differentiated for 8 days into white (hatched bars) or beige (open bars) adipocytes and CD137 knockout SVF cells differentiated to beige adipocytes (solid bars). Results are expressed in -fold change over beige WT as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. C and D, expression of CD137 (C) and CD137L (D) mRNAs assessed by Q-PCR in cultures of WT iWAT SVF cells differentiated for 4, 6, 8, or 10 days into white (hatched bars) or beige (open bars) adipocytes and CD137 knockout SVF cells differentiated to beige adipocytes (solid bars). Results are expressed in -fold change over beige WT as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. E, Western blot analysis of CD137 (molecular mass = 27kDa) expression in lysates of Neuro2A cells (N2A) stably transfected with CD137 expression vector (+) or control vector (−), extracts from WT spleen, iWAT SVF, and mature adipocytes differentiated in vitro from iWAT SVF derived from cold-exposed KO and WT mice. Loading control with GAPDH is shown in the lower panel. Expression of CD137 was detected in transfected N2A cells and spleen, but not in iWAT samples. F, representative phase contrast micrograph of Oil Red O staining in cultures of iWAT beige adipocytes isolated from WT or knockout mice after cold exposure. Scale bar, 20 μm. Histogram to the right shows quantification of Oil Red O staining in iWAT beige adipocytes isolated from WT or knockout mice after cold exposure. Shown are averages ± S.D. of triplicate measurements, normalized to the WT values. ns, not significantly different. G and I, expression of Ucp1 mRNA assessed by Q-PCR in cultures of iWAT beige adipocytes treated for 6 h with vehicle (veh) or the β-3 adrenergic agonist CL isolated from WT and knockout mice housed at room temperature (G) or for 4 weeks at 8 °C (I). Results are expressed in -fold change over WT (vehicle) as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001; 2-way ANOVA. H and J, expression of UCP1 protein assessed by Western blotting in cultures of iWAT beige adipocytes treated for 6 h with vehicle (veh) or the β-3 adrenergic agonist CL isolated from WT and knockout mice housed at room temperature (H) or for 4 weeks at 8 °C (J). Results are expressed in -fold change over WT (vehicle) as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001; 2-way ANOVA. Representative blots probed for UCP1 and GAPDH are show in the right-hand side. K, expression of Ucp1 mRNA assessed by Q-PCR in cultures of iWAT beige adipocytes, isolated from WT or knockout mice, treated for 6 h with vehicle (veh) or the β-3 adrenergic agonist CL after overnight treatment with the indicated cytokines. Results are expressed in -fold change over WT (vehicle) as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. L, expression of CD137 mRNA assessed by Q-PCR in cultures of iWAT beige adipocytes, isolated from WT mice, treated for 6 h with vehicle (veh) or the β-3 adrenergic agonist CL after overnight treatment with the indicated cytokines. Results are expressed in -fold change over WT (vehicle) as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. M, Western blot analysis of CD137 expression in lysates of in vitro differentiated adipocytes treated with TNFα, N2A cells transfected with CD137 plasmid, and iWAT SVF from cold-exposed KO and WT mice. Loading control with GAPDH is shown in the lower panel.
Adrenergic stimulation induces UCP1 expression in cultured adipocytes derived from iWAT (12). We examined this response by treating cultures of mature (day 10) beige adipocytes derived from either WT or knockout iWAT with the adrenergic receptor β-3 agonist CL316243 (CL). Fig. 3, G and H shows the levels of UCP1 mRNA and protein in cultures derived from iWAT of mice that were kept at room temperature. CL treatment increased Ucp1 mRNA levels by ∼13-fold in both WT and knockout cultures (Fig. 3E). UCP1 protein levels were also increased by CL, but in this case the response was significantly higher in the knockout cultures (Fig. 3F). Fig. 3, I and J show responses to CL treatment in cultures derived from mice that were exposed to cold, which stimulates expansion of beige adipocytes and browning in iWAT. In this case, CL induction of both UCP1 mRNA and protein was significantly stronger in cultures derived from knockout iWAT compared with WT. Interestingly, the levels of UCP1 protein were also higher in knockout cultures in the absence of CL stimulation (Fig. 3, H and J). Treatment of beige adipocytes with cytokines associated with inflammation, such as IL-1β, IL-4, and TNFα, significantly reduced Ucp1 mRNA levels (Fig. 3K), but dramatically increased CD137 mRNA expression (Fig. 3L), suggesting converse regulation of Ucp1 and CD137 expression during inflammation. In contrast to its effects on UCP1 expression, CL had no effect on the levels of CD137 mRNA in these cells (Fig. 3L). Despite the strong induction of CD137 mRNA expression observed pot stimulation with TNFα, protein levels of CD137 remained below detection levels (Fig. 3M).
Increased number of beige adipocyte precursors in iWAT isolated from CD137 knockout mice after cold exposure
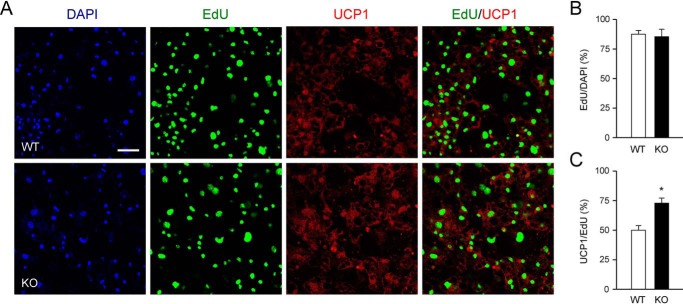
As indicated above, cold exposure induced a larger expansion of UCP1-expressing cell clusters and enhanced responsiveness to adrenergic induction of UCP1 expression in WAT from CD137 knockout mice compared with WT animals. Based on these observations, we considered the possibility that cold exposure produced a larger increase in the number of beige adipocyte precursors in iWAT from knockout compared with WT mice. To investigate this, we labeled proliferating precursors in SVF derived from iWAT of mice that had been exposed to cold with a pulse of ethynyl-deoxyuridine (EdU) and assessed the extent to which these cells differentiated to UCP1-expressing beige adipocytes following 10 days incubation in differentiation medium (Fig. 4A). We found no difference in the level of EdU incorporation between the two genotypes (Fig. 4B), indicating similar total numbers of precursor cells. However, the proportion of EdU-positive cells that expressed UCP1 was higher in the cultures derived from knockout iWAT compared with WT (Fig. 4C). This result suggested that cold exposure induced a larger expansion of beige adipocyte precursors in iWAT of CD137 knockout mice compared with WT iWAT.
Figure 4.
Increased number of beige adipocyte precursors in iWAT isolated from CD137 knockout mice after cold exposure. A, immunocytochemistry analysis of UCP1 expression in cultures of iWAT beige adipocytes labeled with a pulse of EdU prior to differentiation isolated from WT and knockout mice housed for 4 weeks at 8 °C. The panels show confocal images of DAPI, EdU, UCP1, and merged EdU/UCP1 labeling. Scale bar, 25 μm. B, percentage of EdU-positive cells among all cells labeled with DAPI in cultured iWAT beige adipocytes from WT and knockout mice housed for 4 weeks at 8 °C. Results are expressed as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. C, percentage of UCP1-positive cells among all cells labeled with EdU in cultured iWAT beige adipocytes from WT and knockout mice housed for 4 weeks at 8 °C. Results are expressed as average ± S.E. of three independent experiments (n = 3) each performed in duplicate. *, p < 0.05; Student's t test.
Discussion
This study represents the first investigation into the functional importance of CD137, widely used as a marker for beige adipocytes, for the browning of WAT and thermogenic responses to cold exposure. Unexpectedly, we found that mice lacking CD137 showed enhanced browning of iWAT when exposed to cold compared with WT animals, as manifested by increased expression of thermogenic markers, including UCP1, expanded clusters of UCP1-expressing cells in iWAT, and increased number of beige adipocyte precursors in iWAT SVF. CD137 knockout mice were also more resistant to acute cold exposure, perhaps because our room temperature conditions, which are 7–8 °C below the thermoneutral zone for mice, are experienced as a mild cold stress by these animals. Together, these data suggest that CD137 is a negative regulator of WAT browning.
As CD137 knockout mice lack this receptor in all tissues from birth, our present studies do not establish whether the effects of CD137 on beige adipocyte generation and function are cell-autonomous. As mentioned above, several earlier studies have been unable to validate enrichment of CD137 in beige adipocytes, or increased CD137 expression after cold exposure or adrenergic stimulation, as it would be expected for a functional marker of these cells (8, 11–13). In agreement with those studies, we did not observe increased expression of either mRNA encoding CD137 or its ligand upon cold exposure or treatment of beige adipocyte cultures with the adrenergic receptor β-3 agonist CL316243. Although our in vitro studies indicated increased expression of CD137 mRNA during beige adipocyte differentiation, CD137 protein levels remained below detection in these cultures, even after strong induction following TNFα treatment. Similar observations have been made by other groups (13). We also note that immunoreactivity for CD137 in iWAT sections from cold-exposed WT mice remained at background levels, similar to those observed in KO iWAT, although CD137 could be detected in the iWAT lymph node of WT mice. In this regard, it should also be mentioned that it is not uncommon for CD137 mRNA to be expressed in the absence of its corresponding protein (20). Intriguingly, inflammatory cytokines regulated the expression of Ucp1 and CD137 mRNAs in opposite directions in cultures of beige adipocytes, which also challenges the notion that CD137 mRNA expression correlates with the beige adipocyte phenotype. Additional studies using tissue-specific knockout of CD137 will be required to clarify whether CD137 affects beige adipocytes cell autonomously, or indirectly through other cellular components, such as immune cells.
Cold exposure induced a larger expansion of beige adipocyte clusters in iWAT of CD137 knockout mice, suggesting that CD137 exerts a negative effect on the formation of beige adipocytes induced by cold temperatures in vivo. These studies did not address whether this expansion was because of enhanced conversion of existing white adipocytes or de novo generation from an increased pool of beige adipocyte precursors. On the other hand, our studies in cultured SVF cells derived from iWAT of cold exposed mice would appear to support the latter possibility. The results suggested that CD137 exerts a negative effect on cold-induced expansion of proliferative adipocyte precursors that can be differentiated in vitro to beige adipocytes, as assessed by UCP1 expression. We consider the possibility that CD137 negatively affects in vitro differentiation of precursors to mature beige adipocytes as less likely, given that expression of CD137 mRNA in these cultures only became prominent in mature adipocytes, as well as our inability to detect significant expression of CD137 protein in these cells (as discussed above). It remains to be established whether this is indeed the predominant mechanism mediating cold-induced expansion of beige adipocytes in CD137 knockout mice in vivo.
The role of immune and inflammatory responses in browning of WAT is under intense investigation, with some studies reporting positive effects of type 2 lymphoid cells on browning (e.g. Ref. 14), and others describing negative effects of M2 type macrophages (15). Contradicting reports have also left the role of CD137 in adipose tissue inflammation unclear (16, 17). In our studies, we found that iWAT of mice lacking CD137 exposed to cold showed reduced levels of mRNA encoding IFN-γ, a potent inducer of type 1 immune responses, including M1 macrophages. In line with this, expression of mRNAs encoding markers of M2 macrophages, such as CD301 and CD206, were increased in iWAT of CD137 knockout compared with WT mice. Together, these data suggest reduced inflammation, and a bias toward type 2 immune responses in iWAT of cold-exposed mice lacking CD137, which is in agreement with the established role of this receptor as a driver of type I, cell-mediated immune responses (9). Additional studies will be required to address whether these changes contribute to enhanced browning of iWAT in the knockout mice.
In summary, the results of the present study reveal an unexpected role of the immunoregulatory receptor CD137 as negative modulator of WAT browning during cold exposure. Although the specific molecular mechanisms by which CD137 exerts these effects remain to be elucidated, our results favor an indirect, non–cell-autonomous role of CD137 in browning of white adipose tissue, possibly through regulation of pro-inflammatory cytokines and other molecules in immune cells, which in turn exert a negative effect on the expansion of beige adipocyte precursors during cold exposure. Our observations challenge the utility of CD137 as a functional marker for beige adipocytes and warrant future studies on the mechanisms by which CD137 affects the formation and function of beige adipocytes.
Experimental procedures
Animals
Mice were housed in a 12-h light–dark cycle and fed ad libitum on a standard chow diet. WT and CD137KO (21) male age-matched mice in C57/BL6J background were used for all the experiments. All animal procedures were approved by the National University of Singapore Institutional Animal Care and Use Committee.
Cold exposure
For cold exposure, animals were housed in a Memmert HPP750 climate chamber (Memmert GmbH, Germany) at the indicated temperatures. For chronic cold exposure, mice were first acclimatized to 16 °C in individual cages for 1 week. The chamber was then brought to 8 °C for additional 4 weeks. For acute cold exposure, mice were kept in individual cages for a period of 5 h at 5 °C. Rectal temperature was measured every hour using rectal temperature probe and animal temperature controller (World Precision Instruments, Sarasota, FL).
Tissue preparation and fluorescence immunohistochemistry
Mice were sacrificed under isoflurane anesthesia and adipose tissues were collected for further analysis. Immunohistochemistry was performed on paraffin-embedded sections of subcutaneous tissue. The tissue was collected and fixed in 4% paraformaldehyde for 48 h at 4 °C, washed with 70% ethanol, dehydrated in a series of graded ethanol and cleared in xylene. Tissues were then infiltrated with three changes of paraffin wax and finally embedded. The paraffin blocks were cut to obtain 5-μm thin sections in a microtome (Leica Microsystems, Buffalo Grove, IL) and placed over glass slides. For immunostaining, sections mounted on glass slides were deparaffinized in xylene and ethanol and rehydrated in water. Antigen retrieval was performed in sodium citrate buffer (10 mm sodium citrate, 0.05% Tween 20, pH = 6.0). The sections were washed in TNT buffer (100 mm Tris-HCl, 0.15 m NaCl, and 0.3% Triton X) and quenched for endogenous peroxidase in 3% H2O2 in water for 15 min. The sections were then washed three times with TNT buffer and blocked in goat serum as per instruction provided in the kit (Vectastain Elite ABC HRP kit, cat. no. PK-6101). Sections were then incubated overnight at 4 °C with primary antibody diluted in blocking buffer. A list of specific primary antibodies is provided in Table S2. The sections were further incubated with secondary antibody and ABC reagent as per instruction provided in the kit (Vectastain Elite ABC HRP kit, cat no. PK-6101). The immunostaining was developed with Alexa Fluor 594 Tyramide Superboost kit (Invitrogen, cat no. B40944) as per instructions provided by manufacturer, and images were captured in a confocal microscope (Leica Microsystems, Buffalo Grove, IL) using constant conditions for all sections and genotypes.
Primary culture of beige adipocytes
Inguinal adipose tissue was collected in Hanks' Balanced Salt Solution and digested with collagenase type I (Sigma) for 45 min at 37 °C with constant shaking. At the end of the digestion, Ham's F-12 Medium (Gibco) containing 5% FBS was added to stop the digestion. The digested tissue was passed through a 100-μm nylon filter to remove debris and clumps, then washed with complete medium (10% FBS, DMEM, glutamate, and penicillin-streptomycin mix) and collected by gentle centrifugation at RT. Cell pellets were resuspended in erythrocyte lysis buffer (155 mm NH4Cl, 10 mm KHCO3, 0.1 mm EDTA) and incubated for 3 min at 37 °C, then filtered through a 20-μm nylon filter. The cells (SVF) were further washed two times with complete medium, plated onto laminin (Sigma) coated glass coverslips, and kept in a humidified 5% CO2 incubator at 37 °C. On the next day, the cells were supplied with fresh medium and allowed to expand in complete medium for another 3 days. For differentiation into beige adipocytes, equal numbers of cells were plated in 24-well culture plates and incubated in differentiation medium (Ham's F12 with 10% FBS, 1 μm rosiglitazone, 1 nm T3, 10 μg/ml insulin, 1 μm dexamethasone, and 0.5 mm IBMX). After 2 days, the cells were maintained in a Ham's F-12 medium containing 10% FBS, rosiglitazone, T3, and insulin (no dexamethasone or IBMX) for additional 4–8 days as indicated. CL316243 was used at 1 μm for 6 h for studies of mRNA and for 24 h for analysis of UCP1 protein by immunoblotting.
Immunocytochemistry and EdU incorporation
Adipocytes cultured on coverslips were briefly washed in PBS, fixed for 15 min in 4% formaldehyde solution (Sigma-Aldrich) and blocked in PBS containing 0.2% gelatin and 0.25% Triton X-100. Fixed cells were then incubated overnight at 4 °C with the appropriate antibodies as listed in Table S2, followed by incubation in fluorophore-conjugated secondary antibodies. Coverslips were mounted onto microscopy slides using Fluoromount-G (SouthernBiotech). Confocal laser scanning microscopy was performed on a Leica SP8 microscope. For EdU labeling, plated SVF cells were incubated with 2.5 μm EdU (Sigma-Aldrich) for 1 h on the last day of expansion, then switched to differentiation medium and maintenance medium as indicated above. EdU was visualized by Click-chemistry following manufacturer's instructions (Invitrogen).
Image analysis
For each mouse, 10 sections through the iWAT depot 200 μm apart were quantified. ImageJ software was used to quantify positive signal area for UCP1 using a constant threshold for all the sections, determined from sections in which the primary antibody had been omitted. The area occupied by the tissue was delineated and measured on the DAPI images. Area occupied by UCP1 cell clusters relative to total tissue area (from DAPI staining) was calculated for each section. The procedure was repeated for all eight mice in each genotype and the mean value ± S.E. was then calculated. ImageJ software was also used to quantify Edu/DAPI and EdU/UCP1 double-labeled cells in cultures of beige adipocytes, using constant conditions and threshold for all the coverslips. Three different fields per coverslip were quantified in two independent wells. The same procedure was applied to two additional experiments and the mean values ± S.E. of Edu/DAPI and UCP1/EdU percentages were calculated.
Quantitative real-time PCR (Q-PCR)
Total RNA was extracted from tissues using TRIzol (Invitrogen, cat. no. 15596026) and further purified by using RNeasy kit (Qiagen cat. no. 74106). cDNA was synthesized from total RNA using high-capacity cDNA reverse transcription kit (Applied Biosystems, cat. no. 4390778) as per instruction provided in the kit. Q-PCR was run as a 20-μl reaction mixture using SYBR Select Master Mix (Thermo Fisher Scientific cat. no. 4472919) and primers as described in Table S1. The mRNA level was calculated by comparative Ct method normalized to TATA-binding protein (Tbp) mRNA. For quantification of mitochondria DNA, total tissue DNA was extracted with a DNeasy kit and used for real-time quantitative PCR using primers for the mitochondrial gene CytB, encoding cytochrome B (18).
Western blotting
Tissues were homogenized in 1 ml TRIzol (Invitrogen cat. no. 15596026) and centrifuged at 21,000 × g for 15 min at 4 °C. The supernatant was collected and added with 200 μl of chloroform in fresh tubes. The tubes were vortexed rigorously and incubated at ice for 10 min followed by centrifugation at 21,000 × g. The upper aqueous phase was collected for RNA extraction and lower organic phase was collected for protein extraction as per instructions provided in the manual. In brief, ethanol was added, and DNA pallet was removed. Isopropanol was added to the collected supernatant and incubated at ice for 10 min. The samples were then centrifuged and protein pellets were washed three times with guanidinium chloride (0.3 m in 95% ethanol). The pellets were then washed in 100% ethanol, air dried, and dissolved in sample buffer. Equal amount of proteins as estimated by Pierce 660 nm protein assay reagent (Thermo Fisher Scientific cat. no. 22660) were separated by SDS-PAGE and transferred onto PVDF membrane (GE Healthcare cat. no. 10600021). Membranes were blocked with 5% milk in TBS with 0.1% Tween 20 (TBST) and incubated overnight with primary antibodies as indicated in Table S2. The blots were washed three times with TBST for 10 min each and incubated further with anti-rabbit HRP conjugated antibody (Cell Signaling Technology cat. no. 7074) diluted 1:2000 in blocking solution and incubated for 1 h at RT. Blots were then washed three times with TBST, and the signals were developed with SuperSignal West Femto maximum sensitivity substrate (Thermo Fisher Scientific cat. no. 34095) and exposed to X-ray film for further quantification. The bands were analyzed by ImageJ software (National Institutes of Health). GAPDH was used as reference protein for normalization.
Statistical analysis
Statistical analyses were performed using Prism 5 software (GraphPad, SPSS IBM Corporation) and Microsoft Excel. Results are presented as mean ± S.E. of the mean. Student's t test, one-way ANOVA or two-way ANOVA were performed to test statistical significance according the requirements of the experiment. Statistical significance: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
Author contributions
R. K. S., H. S., and C. F. I. conceptualization; R. K. S. and C. F. I. data curation; R. K. S., A. M., H. S., and C. F. I. formal analysis; R. K. S. and C. F. I. supervision; R. K. S., H. S., and C. F. I. funding acquisition; R. K. S., A. M., E.-S. L., and H. S. investigation; R. K. S., A. M., E. N., E. S., C. L., and H. S. methodology; R. K. S. and C. F. I. writing-original draft; R. K. S. and C. F. I. project administration; R. K. S. and C. F. I. writing-review and editing.
Supplementary Material
Acknowledgment
We thank New Chih Sheng for experiment help and assistance.
This work was supported by Singapore National Medical Research Council Grants NMRC/CBRG/0107/2016 (to C. F. I.) and NMRC/BnB/018b/2015 (to H. S.), National University of Singapore Aspiration Fund Partner (to C. F. I.), and Swedish Research Council (Vetenskapsrådet) Grant 2016-01538 (to C. F. I.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1 and S2.
- WAT
- white adipose tissue
- SVF
- stromal vascular fraction
- iWAT
- inguinal WAT
- TNF
- tumor necrosis factor
- Q-PCR
- quantitative PCR
- DAPI
- 4′,6-diamidino-2-phenylindole
- CL
- EdU
- ethynyl-deoxyuridine
- ANOVA
- analysis of variance
- RT
- room temperature.
References
- 1. Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., and Nedergaard J. (2010) Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shabalina I. G., Petrovic N., de Jong J. M. A., Kalinovich A. V., Cannon B., and Nedergaard J. (2013) UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 5, 1196–1203 10.1016/j.celrep.2013.10.044 [DOI] [PubMed] [Google Scholar]
- 3. Harms M., and Seale P. (2013) Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 19, 1252–1263 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- 4. Sanchez-Gurmaches J., Hung C.-M., and Guertin D. A. (2016) Emerging complexities in adipocyte origins and identity. Trends Cell Biol. 26, 313–326 10.1016/j.tcb.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang W., and Seale P. (2016) Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 10.1038/nrm.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A.-H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W. D., Hoeks J., Enerbäck S., Schrauwen P., and Spiegelman B. M. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Q. A., Tao C., Gupta R. K., and Scherer P. E. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenwald M., Perdikari A., Rülicke T., and Wolfrum C. (2013) Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 10.1038/ncb2740 [DOI] [PubMed] [Google Scholar]
- 9. Dharmadhikari B., Wu M., Abdullah N. S., Rajendran S., Ishak N. D., Nickles E., Harfuddin Z., and Schwarz H. (2016) CD137 and CD137L signals are main drivers of type 1, cell-mediated immune responses. Oncoimmunology 5, e1113367 10.1080/2162402X.2015.1113367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thum E., Shao Z., and Schwarz H. (2009) CD137, implications in immunity and potential for therapy. Front. Biosci. 14, 4173–4188 10.2741/3521 [DOI] [PubMed] [Google Scholar]
- 11. Ussar S., Lee K. Y., Dankel S. N., Boucher J., Haering M.-F., Kleinridders A., Thomou T., Xue R., Macotela Y., Cypess A. M., Tseng Y.-H., Mellgren G., and Kahn C. R. (2014) ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 6, 247ra103–247ra103 10.1126/scitranslmed.3008490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jong J. M. A., Larsson O., Cannon B., and Nedergaard J. (2015) A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 308, E1085–E1105 10.1152/ajpendo.00023.2015 [DOI] [PubMed] [Google Scholar]
- 13. Garcia R. A., Roemmich J. N., and Claycombe K. J. (2016) Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr. Metab. (Lond). 13, 24 10.1186/s12986-016-0081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee M.-W., Odegaard J. I., Mukundan L., Qiu Y., Molofsky A. B., Nussbaum J. C., Yun K., Locksley R. M., and Chawla A. (2015) Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 10.1016/j.cell.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Igarashi Y., Nawaz A., Kado T., Bilal M., Kuwano T., Yamamoto S., Sasahara M., Jiuxiang X., Inujima A., Koizumi K., Imura J., Shibahara N., Usui I., Fujisaka S., and Tobe K. (2018) Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci. Rep. 8, 14567 10.1038/s41598-018-32803-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim C.-S., Tu T. H., Kawada T., Kim B.-S., and Yu R. (2010) The immune signaling molecule 4–1BB stimulation reduces adiposity, insulin resistance, and hepatosteatosis in obese mice. Endocrinology 151, 4725–4735 10.1210/en.2010-0346 [DOI] [PubMed] [Google Scholar]
- 17. Kim C.-S., Kim J. G., Lee B.-J., Choi M.-S., Choi H.-S., Kawada T., Lee K.-U., and Yu R. (2011) Deficiency for costimulatory receptor 4–1BB protects against obesity-induced inflammation and metabolic disorders. Diabetes 60, 3159–3168 10.2337/db10-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo T., Marmol P., Moliner A., Björnholm M., Zhang C., Shokat K. M., and Ibáñez C. F. (2014) Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. Elife 3, e03245 10.7554/eLife.03245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishibashi J., and Seale P. (2010) Medicine. Beige can be slimming. Science 328, 1113–1114 10.1126/science.1190816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwarz H., Valbracht J., Tuckwell J., Kempis von, J., and Lotz M. (1995) ILA, the human 4–1BB homologue, is inducible in lymphoid and other cell lineages. Blood 85, 1043–1052 [PubMed] [Google Scholar]
- 21. Zhu Y., Zhu G., Luo L., Flies A. S., and Chen L. (2007) CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood 109, 4882–4889 10.1182/blood-2006-10-043463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.