Abstract
The current obesity pandemic results from a physiological imbalance in which energy intake chronically exceeds energy expenditure (EE), and prevention and treatment strategies remain generally ineffective. Approaches designed to increase EE have been informed by decades of experiments in rodent models designed to stimulate adaptive thermogenesis, a long-term increase in metabolism, primarily induced by chronic cold exposure. At the cellular level, thermogenesis is achieved through increased rates of futile cycling, which are observed in several systems, most notably the regulated uncoupling of oxidative phosphorylation from ATP generation by uncoupling protein 1, a tissue-specific protein present in mitochondria of brown adipose tissue (BAT). Physiological activation of BAT and other organ thermogenesis occurs through β-adrenergic receptors (AR), and considerable effort over the past 5 decades has been directed toward developing AR agonists capable of safely achieving a net negative energy balance while avoiding unwanted cardiovascular side effects. Recent discoveries of other BAT futile cycles based on creatine and succinate have provided additional targets. Complicating the current and developing pharmacological-, cold-, and exercise-based methods to increase EE is the emerging evidence for strong physiological drives toward restoring lost weight over the long term. Future studies will need to address technical challenges such as how to accurately measure individual tissue thermogenesis in humans; how to safely activate BAT and other organ thermogenesis; and how to sustain a negative energy balance over many years of treatment.
Keywords: adipocyte, adrenergic receptor, energy metabolism, imaging, pharmacology, obesity, energy expenditure, metabolic disorder, sympathomimetic, thermogenesis
Introduction
An alarming rise in the rates of obesity and the numerous associated health complications, from diabetes to heart disease and cancers, has occurred over the last 50 years. Prevention of the multiple interrelated and even unknown causes of the obesity pandemic has proven difficult to implement. The billions of dollars spent on reversing obesity have not solved and perhaps even worsened the pandemic. This bleak picture has encouraged creative responses, one of which is based on utilizing physiological thermogenesis to achieve the necessary net negative energy balance. Exercise is a key component, but is rarely effective enough. Although effective, bariatric surgery (1) comes with a high initial cost, risk of long-term morbidities, and recidivism (2). Therefore, the field is turning to environmental, pharmacological, and other novel interventions to boost thermogenesis and treat obesity and its related health complications.
To appreciate the regulation of mammalian thermogenesis, it is appropriate to start with fundamental principles. The second law of thermodynamics—entropy always increases—stipulates that for biological reactions in an organism to occur, there must be a release and dilution of thermal energy. Thus, thermogenesis is an essential component of animal physiology. All mammals are endothermic, meaning they generate their own heat rather than being at the mercy of the environment. Most mammals are also homeothermic and maintain a tightly-controlled, stable body temperature, which is advantageous for consistent chemical reaction rates, blood pH, and enzymatic functions, and it may help minimize parasitic fungal growth (3, 4). Thus such “warm-blooded” animals, or homeotherms, can live and function similarly in widely varying thermal environments. In this review, we illustrate and promote the process of thermogenesis, from the molecular interactions that generate heat to its current heightened relevance at the clinical level. We do this by first clarifying the lexicon of the field studying whole-body thermogenesis and then detailing the sources of thermogenesis at the molecular level. With that background, we describe the challenges of measuring human thermogenesis. Understanding the mechanisms and methodologies enables one to appreciate why activating thermogenesis is of such interest and holds promise as a treatment for obesity-related diseases. The background also helps the reader appreciate why targeting thermogenesis has been fraught with disappointments. In the final section of this review, we provide our perspective on what the future may hold for the field.
Concepts
Whole-body thermogenesis
Thermogenesis is generally defined as any metabolic process that releases heat, so whole-body thermogenesis is equivalent to total energy expenditure (EE).3 Total EE includes three principal components: basal metabolic rate (BMR); thermic effect of food (TEF); and activity EE (Fig. 1). The BMR is regarded as the minimum energy required to perform all of the basic chemical reactions of the body and is the primary contributor (60–75%) to total EE in a typical human adult. The BMR remains relatively constant, is mainly determined by lean body mass, and can be regulated by hormones. TEF is the heat generated during digestion of macronutrients. Activity-related EE is necessary for movement and is the most variable contributor to total EE. These components of EE are not for the specific purpose of heat generation, so thermogenesis from them is an obligatory by-product. For the context of this paper, we define the term thermogenesis more functionally as any additional metabolism used for heat production, either for defending body temperature or to burn off calories determined to be in excess of the body's needs, a process known as diet-induced thermogenesis.
Figure 1.
Sources of human thermogenesis and pharmacological approaches to increase it. There are three principal sources of thermogenesis in adult humans. Most derive from the BMR, which is the minimum energy required to perform all the basic chemical reactions of the body and is the primary contributor (60–75%) to total energy expenditure in an adult human. Movement-related thermogenesis includes exercise and nonexercise activity thermogenesis (10–30%). Adaptive thermogenesis is a physiological feedback system in which energy demands are assessed over a longer period of time, and the body responds by increasing energy expenditure. A related but different process is facultative thermogenesis, which is a temporary increase in energy expenditure only when extra heat is required acutely, such as for defending body temperature. There are three principal pharmacological approaches to stimulating human thermogenesis that use some combination of appetite suppression, impaired absorption of ingested food, and increased energy expenditure. Adapted from Ref. 67.
Cannon and Nedergaard (5) categorized thermogenesis as having both facultative and adaptive components. Facultative thermogenesis is activated when extra heat is required acutely, such as for maintaining body temperature during short-term cold exposure, whereas adaptive thermogenesis refers to metabolic adaptation in response to additional heat needs over a longer duration, such as during chronic or repeated cold exposure. The detailed relationship between EE and environmental temperature is largely based on classical animal studies by Kleiber (6) and Scholander et al. (7), and more recently by Cannon, Nedergaard, and co-workers (5, 8) and Abreu-Vieira et al. (9). The thermoneutral zone (TNZ) is the environmental temperature range over which resting EE is minimum and equal to the BMR. The BMR helps to maintain the normal and optimal human core temperature, 37 °C, which is also called the “defended” body temperature. The lower and upper critical temperatures are the environmental temperature limits of the TNZ. Although resting metabolism is constant throughout the TNZ, to maintain constant core temperature as the environmental temperature drops from the upper to lower critical temperatures, autonomic (e.g. vasoconstriction and piloerection) and behavioral (e.g. huddling and environment selection) responses occur to mitigate heat-loss without increasing heat production. Thus, measures such as skin surface temperature and heart rate will be different when assessed at the upper versus lower critical temperatures, reflecting these thermoregulatory responses. Below the lower critical temperature, heat conservation responses are insufficient to defend body temperature, and EE must increase linearly as environmental temperature drops. This increase above the BMR to maintain core temperature is defined as cold-induced thermogenesis (CIT). According to Fourier's law of heat conduction, there is no heat transfer at the x-intercept after the CIT line is extrapolated, demonstrating that the environmental temperature is equal to the defended body temperature (6). For greater detail, a comparison of the EE responses to different environmental temperatures in and around the TNZ in lean and obese young men have been published recently (10).
The neuronal pathways involved in mammalian thermogenesis for thermoregulation are described by Morrison et al. (11). These thermoregulated neuronal pathways can activate BAT and skeletal muscle through thermoreceptors in the case of CIT or through nonthermal means, such as stress. Thermal sensations on the skin are transmitted as signals through the dorsal root ganglia and to the hypothalamic preoptic area where warm-sensitive (W-S) neurons regulating BAT and skeletal muscle are inhibited by cool-activated neurons in the lateral part of the parabrachial nucleus. The W-S neurons function to inhibit BAT sympathoexcitatory neurons, so when these neurons are disinhibited in the cold, BAT and skeletal muscle sympathoexcitatory neurons are stimulated (11).
Homeostatic regulation of heat conservation/dissipation and heat production mechanisms results in constant body temperature. Loss of body temperature control can cause death from either hypothermia or hyperthermia. A rise of 1 °C in body temperature due to fever is associated with a 13% increase of heat production (12), albeit with large individual variability.
Cellular thermogenesis
The principal organs involved in adaptive thermogenesis are skeletal muscle, BAT, and likely WAT (Fig. 2). The majority of reactions regulating biological energy production occurs in the mitochondria (Fig. 3). Energy stored in dietary nutrients is released through the tricarboxylic acid (TCA) cycle and the electron transport chain (ETC). These processes are normally facilitated through interconnected enzyme-facilitated reactions, which release energy stored in chemical bonds to fuel ATP synthesis (13). ATP can then be shuttled throughout the cell to provide the energy to drive essential processes, notably macromolecular synthesis, intermembrane ion pump function, protein folding, and metabolite store mobilization (14).
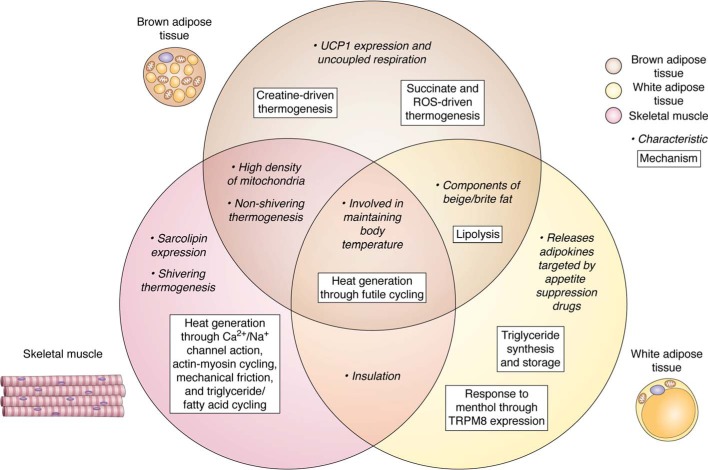
Figure 2.
Summary of brown adipose tissue, white adipose tissue, and skeletal muscle characteristics and mechanisms related to thermogenesis. Molecular, cellular, and physiological characteristics are shown in lightface text, and the molecular mechanisms are shown in bold text. Brown adipose tissue, white adipose tissue, and skeletal muscle are represented by brown, yellow, and red circles, respectively. Characteristics and mechanisms relevant to two or three tissues are shown in regions where the circles overlap.
Figure 3.
Molecular mechanisms underlying adaptive cellular thermogenesis. Mechanisms relevant to coupled respiration, which occurs in most tissues, are illustrated within the box. In the mitochondrial inner membrane, succinate dehydrogenase (SDH) forms complex II of the ETC and is one of the enzymes of the TCA cycle. Brown adipocytes expressing uncoupling protein 1 (UCP1) in response to β-adrenergic signaling can uncouple the tricarboxylic acid cycle (TCA) and oxidative phosphorylation by the electron transport chain, composed of coenzyme Q (CoQ), cytochrome c (Cyt C), and complexes I–IV, increasing the rates of the associated exergonic reactions and generating heat instead of harnessing it to make ATP. In beige/brite fat, thermogenic hydrolysis of ETC-generated ATP occurs when the mitochondrial ADP/ATP carrier transports ATP from the mitochondrial matrix into the intermembrane space where mitochondrial CK (Mi-CK) catalyzes the conversion of creatine to phosphocreatine, which is then hydrolyzed by enzymes to generate heat.
This typical mitochondrial energy conversion does not break down all energy intake efficiently despite being considered “coupled.” Nevertheless, there are other cellular processes that are considered entirely “uncoupled” or futile, as energy is only expended and released as excess heat. Cation leakage across concentration gradients is a common example of “uncoupled” reactions which occurs in all cells, and this is the fate of up to 20% of all mitochondrial energy (15). During cation leakage, the ETC and TCA cycle work faster to pump protons out of the mitochondrion, which requires more ATP hydrolysis and associated thermogenesis. Heat is released from collisional interactions as protons move out of the mitochondrion or potentially from the amount of energy required to maintain the appropriate concentration of enzymes to drive futile cycling.
Muscle tissue can also expend energy through nonmitochondrial futile cycling (Fig. 4). These include Ca2+ and Na+ channel action; actin–myosin cycling during muscle contraction; the heat produced due to mechanical friction; and triglyceride/fatty acid cycling (16). All of these processes involve futile cycling, which generates heat from the same basic principle: ATP hydrolysis. The futile cycle results from an uncoupling process that causes the ETC and TCA cycle to speed up, thus generating heat as a result. Energy lost from triglyceride/fatty-acid cycling comes from hydrolysis of ester bonds in triglycerides (17), and it has been advanced as the source of thermogenesis in acute burns (18), cancer cachexia (19), and following exercise (20).
Figure 4.
Muscle-specific mechanisms of thermogenesis and therapies that can increase thermogenesis. Repeated excitation–contraction cycling with actin and myosin is stimulated by the release of sarcoplasmic calcium through the ryanodine receptor and is used by skeletal muscle for shivering thermogenesis. Muscular nonshivering thermogenesis is mediated by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and sarcolipin. Triglyceride/fatty acid synthesis and lipolysis, which occur in skeletal muscle and other tissues, are parts of a thermogenic futile cycle. Certain therapies, such as β3-adrenergic receptor agonists, up-regulate cellular signaling pathways that lead to increased lipolysis and thus thermogenesis. Much like UCP1, DNP, and controlled-release mitochondrial protonophore (CRMP) uncouple the ETC from ATP synthesis, increasing thermogenesis.
Thermogenesis from shivering, the involuntary contraction of muscles caused by cold exposure is the main contributor to heat production in moderate to extreme cold (21). Nonshivering thermogenesis (NST), however, is any heat production independent of shivering, and can be both facultative and adaptive (5). There are thought to be two contributors to NST: brown adipose tissue (BAT) via uncoupling protein 1 (UCP1) and skeletal muscle via sarcolipin. Both of these processes cause futile cycling with ATP hydrolysis and therefore generate heat through an exergonic reaction. UCP1 releases energy as heat during the dissipation of the proton-motive force across the mitochondrial membrane. Sarcolipin controls thermogenesis in skeletal muscle by mediating heat production from the sarco/endoplasmic reticulum Ca2+-ATPase pump. Despite a wealth of recent articles about NST and BAT, the capacity of, physiological mechanism behind, and primary contributor to NST in humans all remain to be determined (22).
The thermodynamic inefficiency of mammalian energy metabolism may offer flexibility for various cellular processes (16), but the proton leak regulated by UCP1 in human BAT serves a distinct purpose. UCP1 is found in brown and beige/brite fat, made up of “brown-able” adipocytes that develop in white fat, and it permits proton flow from the intermembrane space to the mitochondrial matrix (23). This results in the uncoupling of ATP production from the proton gradient established by the ETC. The UCP1-mediated proton leak is thereby specific to BAT thermogenesis: a diminished proton gradient permits an increased rate in the series of exothermic reactions generating the protein gradient, from the TCA cycle through the ETC, and increases the rate of collisions between protons and other molecules, generating substantial heat (24). Mitochondrial dysfunction and age-related hormonal changes that regulate UCP1 expression may also explain why BAT activation correlates negatively with age (25).
Recent advancements have identified alternative mechanisms for facultative and adaptive thermogenesis in BAT (26). One of these is a mitochondrial substrate futile cycle based on creatine. Creatine, an amino acid found predominantly in skeletal muscle, supports substrate futile cycling by increasing ADP-dependent respiration in mitochondria of beige fat (27), which shares many functional similarities with brown fat. Such a pathway requires creatine and creatine kinase–mediated hydrolysis of ATP to drive a catalytic mechanism that stimulates cycling of ATP production and consumption (28). Both in vivo and genetic evidence support the role of creatine in thermogenesis (28, 29). Additionally, it has been recently shown that the TCA cycle and ETC intermediate succinate is present in higher levels in rodent BAT after cold exposure. This is specific to succinate and not other related metabolites, and this process occurs through transport from the extracellular milieu. Succinate therefore can specifically drive mitochondrial respiration in a dose-dependent fashion and stimulates BAT thermogenesis via succinate dehydrogenase, which oxidizes succinate to drive this process (30). Of note, studies of these mechanisms have been completed in rodent models, and it still needs to be determined what contribution they have toward thermogenesis in humans as methods for measuring tissue and organ thermogenesis are being developed.
Methods to measure human thermogenesis
It is clear from the previous sections that the past decades have witnessed advances in the mechanistic understanding of cellular thermogenesis, and its measurement has become more precise. Where a key challenge arises is in integrating the processes occurring in each cell to accurately describe what is happening at the level of individual organs and the entire body. This section describes the methods currently used to measure both whole-body energy expenditure and tissue/organ metabolism. The goal is 2-fold: the first is to explicate how the quantification is done, and second is to justify why measuring human thermogenesis needs to be considered differently from measuring other cellular processes at the whole-body level. As will be demonstrated, measuring whole-body thermogenesis requires rigorous control of external factors in study design and accuracy and precision of calorimetry methods. Although human thermogenesis has been investigated for more than two centuries, technological advancements have improved the measurement precision that is critical for quantifying small, but significant, components of total energy expenditure (31). A better understanding of potential mechanisms of human thermogenesis can be gained by combining measurement of whole-body energy expenditure with in vivo quantification of organ and tissue metabolic processes and fuel utilization via minimally-invasive imaging methodologies. In the summary, we will revisit these methods when assessing the state of the field and identify areas that require the most attention.
Body size is known to be the major determinant of whole-body EE. Thus, allometric scaling is necessary when comparing thermogenesis between species and between individuals of the same species. The simplest method to normalize metabolism to body size is to divide by body mass, as proposed by Brody and Procter (32) and by Kleiber (33), or body surface area, as proposed by Benedict (34) and Rubner (35). Normalizing to body weight is a common approach when comparing the metabolism of animals of different weights (36), whereas the surface area is often used to scale parameters of human heat loss and production (e.g. W/m2) (37, 38). Although body mass and surface area are easy to obtain—the latter is typically derived from predictive equations from height, body mass, and other characteristics—these allometric scaling approaches are known to reflect between subject differences less accurately, especially those attributed to age, sex, and race. Alternatively, Scholander et al. (7) suggested expressing CIT as a percentage of BMR, which is useful for cross-species comparisons because CIT magnitude varies drastically on account of the differences in surface–to–volume ratio and thermal physiology. A more physiologically-based approach is to use detailed measures of body composition to scale metabolic rate. Multiple regression analysis shows that fat-free mass (FFM), which consists of all nonadipose tissue such as muscle, bone, and viscera, is the main determinant of BMR, followed by sex, fat mass, age, and race (39). However, expressing metabolic rate as a simple quotient, e.g. BMR/FFM, creates artifacts because such models contain nonzero intercepts as constants. Thus, it is recommended to scale metabolism, particularly BMR, by using FFM and other factors as covariates. Going one step further, investigators can use organ and tissue masses measured with computed tomography (CT) or magnetic resonance imaging (MRI) to explain more of the variability in BMR. For example, Heymsfield et al. (40) showed that using masses of organs and tissues such as brain, liver, skeletal muscles, and adipose tissue can predict individual BMR with 18% less error than using FFM alone, and the regression intercept is close to zero, suggesting this is a more biologically accurate scaling model. Thus, to minimize the influence of body composition on metabolism, it is essential to use the most accurate measurements of body composition available, which currently are performed via dual-energy X-ray absorptiometry, CT, MRI, or other validated methodologies. The goal is to scale metabolism when comparing human subjects of different body size or composition or when assessing change in metabolism resulting from an intervention that may alter body composition. In summary, understanding of obesity requires measurement of EE, which itself depends on the accurate measure of body composition.
Measurement accuracy and precision of thermogenesis also depend on the specific methodology used to quantify energy expenditure. Direct and indirect calorimetry have been used to measure heat loss and EE, respectively, as reviewed in detail elsewhere (31). Direct calorimetry quantifies heat exchange with the environment using well-controlled environmental chambers. Limitations of this method include the high cost of equipment and complex engineering. Thus, most studies of human thermogenesis are done via indirect calorimetry, whereby thermogenesis is quantified by measuring the amount of oxygen consumed and carbon dioxide produced, with the ratio being the respiratory quotient that provides information about substrate utilization, i.e. carbohydrate and fat oxidation.
Lavoisier (41) was the first to note that oxygen consumption in resting men increased in cold temperatures. Classic studies performed in the 1930s to the 1950s extended our understanding of the effects of environmental temperature on human metabolism by using whole-room calorimeters capable of measuring direct heat loss and indirect gas exchange simultaneously (42–46). Because of the technical limitations of these early calorimeters, EE measurements were labor-intensive. Thus, studies were limited; subjects were few; and documented CIT was small to moderate in magnitude (0–36%). More recent studies of CIT have involved EE measurement via indirect calorimetry either using portable carts with canopies/hoods for short exposure periods or calorimetry rooms for exposures up to 24 h. Despite the improved precision of newer measurement devices, reported CIT has varied considerably, from 0 to 280% and peaking at 500% of the BMR at “peak shivering” (47), which is likely dependent on experimental conditions and subject characteristics. Greater detail of the variations in CIT measurement is provided elsewhere (31). These variations demonstrate the need for standardized protocols to measure CIT and other changes in metabolism. Current best practices include ensuring careful measurement of the BMR in thermoneutral temperatures with standardized clothing while minimizing energetic contributions from activity and diet. In addition, it is essential to employ systematic intervention protocols, e.g. uniform dosing of cold exposure or pharmacological agents, optimized timing for measuring changes in energy expenditure, and appropriate study power and sample size to detect clinically meaningful changes. When designing such protocols, researchers should also recognize the limited temporal resolution of room calorimeters and the greater time–to–steady-state for metabolic cart measurements—both requiring ∼30 min of steady-state energy-expenditure measurement at each intervention stage to accurately quantify changes in metabolism.
Organ-level measurement of thermogenesis is necessary to better understand the mechanisms underlying CIT and other metabolic changes and to design more targeted drugs capable of selectively elevating metabolism from these organs. However, as with BMR, organ-level contributions to CIT are currently difficult to quantify. As discussed previously, skeletal muscle, through shivering thermogenesis, and BAT, through NST, are thought to be the main contributors to human CIT (21). Given BAT's prominence in rodent energy expenditure and its potential for treating obesity and the associated metabolic diseases, substantial focus over the past decade has been devoted to measuring its volume and metabolic activity. The challenges, pitfalls, and successes have been discussed in several extensive reviews and consensus papers (48–51) and therefore will not be addressed in detail here. In brief, the principal challenges to accurately assess human BAT's contributions to energy expenditure are anatomical and technical. First, although human BAT is a solid organ, the individual thermogenic adipocytes are mixed with different combinations of metabolically quiet white adipocytes and dormant brown and beige/brite adipocytes that can be activated with cold exposure or chronic treatment with adrenergic agonists (27, 52–54). Second, because the thermogenic adipocytes are distributed throughout the body, any imaging whose goal is to characterize whole-body BAT activity needs to sample from the base of the skull to the umbilicus via PET computed tomography (PET/CT) (55). Among the radiotracers available that reflect oxygen consumption, the most commonly used is 2-deoxy-2-[18F]fluoroglucose ([18F]fluorodeoxyglucose (18F-FDG)), which behaves similarly to blood glucose yet gets trapped into most metabolically-active cells; after entry and phosphorylation to become fluorodeoxyglucose 6-phosphate, it can proceed no further through glycolysis and cannot be dephosphorylated in most cells because they lack glucose-6-phosphatase. Because glucose uptake is a real-time marker of tissue oxygen consumption (56), 18F-FDG is a useful biomarker of tissue energy expenditure in the fasted state. The principal drawback of 18F-FDG is that it does not provide a quantitative measure of cellular EE. Other tracers that are more reflective include [11C]acetate (57), which measures oxidative phosphorylation, and 15O2, which provides a direct measure of oxygen consumption (58, 59). Because the latter two molecules have much shorter half-lives (seconds to a few minutes), are not routinely produced for clinical use, and can be visualized only in a small region of the body during a single scan, studies using them are more limited in number but quite promising in terms of learning about the therapeutic potential of human BAT (60). MRI-based methods are also gaining in interest and application toward measuring thermogenesis. In addition to assessing changes in tissue fat fraction to detect changes in BAT fuel consumption (61, 62), MRI and functional MRI have the ability to directly measure thermogenesis (63). Given the absence of ionizing radiation and the wide range of MRI pulse-sequences that can be developed, it is likely that in the future substantial advances in understanding human thermogenesis will rely on MRI-based techniques.
Shivering thermogenesis, which can consume more calories that the BMR itself (64), is also difficult to measure due to inherent technical limitations and highly-variable muscle recruitment patterns during shivering. Traditional methods to quantify shivering include using indwelling and surface-based sensors (21) to measure muscle electrical activity, or electromyography (EMG), rather than thermogenesis directly. EMG sensors primarily record from superficial, but not deeper, muscle. Alternatively, the same PET/CT-based imaging techniques used to quantify BAT metabolic activity can also be used to quantify the metabolic activity of shivering skeletal muscle. Concurrent measures of surface EMGs and PET/CT imaging demonstrate that the two methods are correlated (64). However, PET/CT has also demonstrated that surface and deep muscles are involved in shivering-based heat production (65).
In summary, the methods used over the past decades to measure thermogenesis have enabled multiple advances in understanding human physiology, the development of obesity, and a characterization of how the body adapts to weight loss. What is lacking is the ability to actively manipulate and then accurately measure processes happening at the individual tissue and cellular level. One critical area in this regard relates to human BAT. Estimates of energy expenditure in response to BAT activation span over 2 orders of magnitude (60), which makes it currently impossible to determine BAT's roles in human physiology. Beyond BAT, the field of metabolism is in great need of noninvasive measures to study the intercommunication among the organs' regulation energy storage and utilization. The breakthroughs will likely come through new radiotracers and stable isotopes (66). The needs—and the potential discoveries—are vast.
Pharmacological interventions
Having discussed the origins and function of mammalian thermogenesis, we now turn to pharmacological regulation of thermogenesis. The various classes of drugs that attempt to alter the caloric balance by suppressing appetite, inhibiting fat absorption, or increasing energy consumption (Fig. 1) have been summarized (67, 68). The activation of BAT is of particular interest because it is an organ whose principal physiological role is thermogenesis. An extensive treatment of the translational pharmacology involved in human BAT activation has been recently published (69). What follows here is a more focused discussion of the cellular physiology underlying pharmacological activation of thermogenesis and includes human BAT.
Adrenergic receptors
The sympathetic nervous system (SNS) is the principal network that regulates thermogenesis. The receptors of the SNS can be divided into two main types based on their function, the α- and the β-adrenoreceptors (ARs). There are at least two types of α-ARs, α1-AR and α2-AR, and there are three types of β-ARs, β1-, β2-, and β3-ARs (70, 71). The α-AR and β-AR differ in their specificity for coupling to the heterotrimeric G-proteins, which results in complex and multidimensional stimulatory and inhibitory signaling pathways. α1-ARs couple mainly to Gαq/11 proteins that activate phospholipase C to generate inositol trisphosphate and diacylglycerol. These two second messengers increase intracellular Ca2+ and protein kinase C, respectively, which stimulate a myriad of intracellular processes specific to each distinct cell type (70, 71). In contrast, α2-AR couples primarily to Gαi/o to inhibit adenylyl cyclase. β-ARs predominantly couple to Gαs and activate adenylyl cyclase to increase intracellular cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA). The endogenous ligands of ARs are catecholamines, principally norepinephrine (NE), a neurotransmitter released from the sympathetic nerve endings as well as directly into the blood by the adrenal medulla, and/or epinephrine, a hormone synthesized only in the adrenal medulla and released into the systemic circulation. A detailed description of the molecular interactions underlying AR ligand binding can be seen in Refs. 72, 73 and additional references for downstream signaling (74–76).
In adipose tissue, β-ARs stimulate physiologically-relevant thermogenesis and bind NE to acutely increase cellular catabolism by stimulating lipolysis in white adipocytes and coupled lipolysis and thermogenesis in brown adipocytes. Activation of the mitochondrial protein UCP1 in BAT generates heat by allowing protons in the mitochondrial intermembrane space to re-enter the matrix without uncoupling the respiratory chain, accelerating substrate oxidation and ATP hydrolysis, and allowing energy to be released as heat rather than ATP (77). PKA phosphorylates hormone-sensitive lipase, a key enzyme in lipolysis, and induces UCP1 activation in two ways: 1) fatty acids released by lipolysis, which, besides being fuel for oxidation within the mitochondria, also bind to UCP1 to activate and increase its function (23, 71, 78–80); and 2) PKA phosphorylates and activates cAMP-response element-binding protein and the p38 MAPK pathway. p38 MAPK activates PGC1α that, together with PPARα and PPARγ, stimulates transcription of UCP1 and other thermogenic genes (71, 81).
In rodents, all three β-ARs are expressed in brown adipocytes, with β1/β2/β3-AR mRNA transcripts at a ratio of 3:1:150 (82), suggesting that the β3-AR plays a dominant role in BAT metabolism. Although β3-ARs are thought to be the main contributors to BAT activation and NST, each member of β-ARs contributes to BAT thermogenesis at a different magnitude (83), and all are physiologically relevant in mediating β-AR signal transduction in BAT. Gene knockout of the individual β-ARs results in increased obesity, possibly due to the incomplete functional redundancy of the β-ARs in adipocytes (84–88). Disruption of all three β-ARs produces “β-less mice,” which increases their susceptibility to cold-induced hypothermia and diet-induced obesity (89, 90). However, knocking out β3-AR (β3AR-KO) increases β1-AR mRNA in adipocytes, brown more than white, suggesting cross-talk between β3-AR and β1-AR. These mice displayed only a modest increase in fat stores, suggesting compensatory mechanisms in response to β3-AR deficiency to maintain BAT function (84). In fact, deletion of β1-AR has also caused cold-induced hypothermia, decreased catecholamine-stimulated BAT response, and susceptibility to diet-induced obesity, suggesting that the β1-AR plays an important role in SNS stimulation of adaptive thermogenesis (91). Moreover, following cold exposure, compared with WT, β3AR-KO display the same thermogenic genes expression (Ucp1, Pgc1a, Dio2, and Cidea) in BAT and WAT, indicating that the β3-AR is dispensable for browning of the adipose tissue (92). These animal data suggest that increased EE through activation of NST is not exclusively mediated via β3-AR. Translation and comparison of studies on rodent β-ARs to humans is progressing as well, as several epidemiological and mechanistic studies have linked mutations in the β3-AR to obesity and insulin resistance, although the precise mechanisms remain to be determined (93–96).
Sympathomimetics
Ephedrine was one of the earliest sympathomimetic drugs used to increase EE in rodents and humans (97–104). It mediates its thermogenic effects by directly activating β-ARs (105) and indirectly enhancing the release of NE and epinephrine from sympathetic nerve terminals (106). The substantial increase in EE does not come from activation of human BAT (107, 108), but more likely is from activation of skeletal muscle (98). While chronic administration of ephedrine does lead to a net increase in EE, there is a reduction in BAT activity in response to the greater muscle thermogenesis (109). Most trials utilizing ephedrine for weight loss rely on its central suppression of appetite in addition to its sympathomimetic properties and have been short-term (less than 6 months), so the long-term effects are not well-known. A meta-analysis of 20 clinical trials using ephedrine and ephedra (with and without caffeine, discussed below) for weight loss found they were associated with a 2 to 3 times increased risk of heart palpitations as well as psychiatric, autonomic, and upper gastrointestinal symptoms (110). As will be seen in the upcoming discussions, the experience with ephedrine for obesity treatment has a profile similar to several other classic drugs described here in other places: short-term increases in energy expenditure; early reductions in weight without evidence of long-term efficacy; cardiovascular side effects; and several types of long-term risks.
Caffeine is an extremely popular drug that is thought to increase thermogenesis by inhibiting the phosphodiesterase-induced degradation of intracellular cAMP (111), and its effects on human energy expenditure are well-documented (101, 112, 113). In nonhuman animal models, ephedrine and caffeine combined are stimulatory thermogenic agents that cause NST via β-AR–cAMP-mediated BAT activation (100, 101, 114, 115). Aside from the increase in energy expenditure, ephedrine and caffeine have also been reported to decrease fat accumulation (106). Conflicting human data have been reported on the effects on body weight with some studies reporting significant decreases (116, 117), whereas others report negligible effects (118). An elegant study using innervated interscapular BAT in control and chemically-sympathectomized rats showed that both ephedrine and caffeine have a dose-dependent synergistic action (119). At low concentrations, the thermogenic effects of both are due to presynaptic NE release from nerve terminals, whereas in higher doses (1 μm ephedrine and 2 mm caffeine), BAT stimulation is postsynaptic (119). The thermogenic effects of ephedrine and caffeine are regulated by two negative feedback mechanisms: 1) extracellular via adenosine and prostaglandin binding to Gi-coupled heptahelical receptors (119, 120),and 2) intracellular by phosphodiesterase degradation of cAMP (120). Therefore, the effect of ephedrine and caffeine is further exaggerated when combined with aspirin, 8-phenyltheophylline (a potent adenosine receptor antagonist), and 3-propylxanthine (an inhibitor of phosphodiesterase) (120). Interestingly, the effect of ephedrine and caffeine is attenuated in obesity, consistent with the growing body of literature demonstrating catecholamine resistance in obese rodent models (121). This resistance was eliminated by adding aspirin, through a mechanism that is not fully understood (122). This could be explained by obesity-mediated PDE3B up-regulation (121) that causes a reduction in cAMP and reduced BAT activity in an obese animal after ephedrine stimulation (108, 109, 121, 122).
Like caffeine and ephedrine, the sympathomimetic amine phentermine has been available for decades for weight loss. Phentermine targets the CNS by modulating catecholamines in the satiety centers of the hypothalamus to act as an anorectic agent that negatively regulates energy balance (123). It has been used as a short-term management of obesity (123) and is approved for short-term (up to 12 weeks) weight management. Like other sympathomimetic agents, phentermine is associated with elevations of blood pressure and heart rate and is not recommended in individuals with preexisting cardiac issues (124). A retrospective cohort study (125) found that cardiovascular disease was rare in individuals taking phentermine for 12 and 24 months, although a slight increase in heart rate was observed. Current recommendations do not support long-term administration of this drug.
For several decades, it has been observed that animals treated with nicotine gain less weight and at a slower rate compared with untreated animals. This is achieved by increasing their energy expenditure without decreasing food intake (126). Similarly, smokers have been reported to have a higher resting metabolic rate compared with nonsmokers, and when they stop smoking, their metabolic rate appears to decrease (127). Mechanistically, nicotine increases EE by stimulating the SNS, increasing NE turnover and blood concentrations of NE and epinephrine, and by increasing the binding of the purine nucleotide GDP to BAT mitochondria (128) promoting thermogenesis and consequently weight loss. In mice, nicotine was reported to increase BAT weight and, when combined with caffeine, increase BAT thermogenesis (129, 130). In a clinical study evaluating the effect of smoking on energy expenditure, nicotine exposure caused a 10% increase in total 24-h energy expenditure with a significantly higher mean diurnal urinary excretion of NE in cigarette smokers. This suggests that nicotine induces its thermogenic effects via increased NE secretion (131) that could likely be due to activation of the sympathetic central outflow, as reported in rodents (128). A second mechanism proposed for nicotine-induced weight loss and increased thermogenesis in rodents and humans is by inactivation of the hypothalamic AMP-activated protein kinase (AMPK)/BAT axis. This is a pathway in which reduced AMPK levels in the ventromedial nucleus of the hypothalamus activate the SNS to stimulate BAT thermogenesis (132). Nicotine also induces an increase in anorexic signaling in the hypothalamus, decreasing hunger and feeding, and an increase in physical activity and substrate oxidation, leading to increased NST (132–134). Nicotine and liraglutide, a glucagon-like peptide 1 (GLP-1) receptor agonist, promote body weight reduction via increasing satiety and mediating BAT thermogenesis through the hypothalamic AMPK axis (131, 134–136). The principal limitations of nicotine are the stimulation of the cardiovascular system and its ability to cause physical and psychological addiction (137). Smoking is also specifically associated with an increased risk for developing various cancers (127).
Topiramate (TPM) was initially approved in 1996 for—and primarily used to treat—CNS disorders, including epilepsy, migraines, bipolar disorder, and neuropathic pain (138). Patients receiving TPM displayed significant weight loss, leading to the drug's subsequent approval by the FDA as an anti-obesity drug. TPM exerts multiple mechanisms of action: it increases the opening of GABA-mediated chloride channels (139, 140); decreases the action of glutamate at kainite/AMPA receptors (141); decreases L-type voltage-sensitive calcium currents (142); inhibits carbonic anhydrase (143); and increases potassium conductance (144). The negative effect of TPM on body weight is thought to be mainly achieved by the modulation of GABA and antagonizing AMPA/PKA receptors. Studies in lean and obese rodent models show that TPM reduces fat accumulation independent of either diet or food intake (145, 146) by reducing appetite and decreasing lipoprotein lipase (LPL) activity in WAT depots (145). Although no thermogenic action of TPM has been demonstrated yet, a possible mechanism could be the increased LPL activity in BAT, skeletal muscle, and cardiac muscle, thus potentially increasing substrate oxidation and thermogenesis (147, 148). Because both phentermine and TPM reduce appetite, an extended-release combination therapy (Qsymia® by Vivus) was approved by the FDA in 2012 for the treatment of obesity. This combination has been shown to be efficacious in inducing and maintaining negative energy balance alongside improved lipid profile and blood pressure (149). These long-term effects suggest an increase in metabolic rate, yet no effects on BAT thermogenesis have been reported.
The class of β3-AR agonists has been a focus of the pharmaceutical industry for over 3 decades as a drug target for stimulating fatty acid oxidation and thermogenesis (150–152). To date, no drug has been approved for that indication, yet the high expression of the β3-AR on urinary bladder smooth muscle facilitated its approval to treat overactive bladder. β3-AR agonists such as mirabegron cause relaxation of the bladder's smooth muscle, thereby allowing greater urine capacity and mitigating the effects of overactive bladder. The commercial availability of mirabegron led to the testing of the ability of this highly-selective β3-AR agonist to activate human BAT thermogenesis and WAT lipolysis. A single oral dose of 200 mg in young healthy men increased EE by 203 kcal/day (+13%) and stimulated BAT thermogenesis (153). While encouraging, there was an increase in heart rate and blood pressure that were not seen at the maximum approved dosage of 50 mg daily, which itself did not affect EE (154). It remains to be determined whether other doses of mirabegron will be clinically effective and safe, both acutely (155) and after chronic treatment (156).
In summary, sympathomimetics are logical choices for stimulating thermogenic EE, suppressing appetite, and ultimately treating obesity. Although these drug options have achieved desired metabolic effects, such as weight loss, increased energy expenditure, increased BAT activity, or decreased food intake, the sympathetic innervation–activation of β1- and β2-AR leads to an increase in heart rate and blood pressure that have negative health effects. Although the prolonged use of such agents leads to chronic stimulation of the SNS, the long-term pathological effects to humans are not easily predicted nor are they restricted to elevated BP. Fisher et al. (157) summarized the consequences to include the following: vascular effects (hypertension and atherosclerosis), metabolic effects (insulin resistance and dyslipidemia), cardiac effects (arrhythmia and tachycardia), and/or renal effects (renal vasoconstriction and glomerulosclerosis). Similar consequences are also described in middle- to older-age adults when chronic SNS activation occurs as an intended physiological response to stimulate thermogenesis (158). Therefore, despite the conceptual appeal, anti-obesity drugs have been developed and mostly withdrawn due to adverse effects affecting their safety and tolerability (68).
Uncoupling agents
NST is achieved by mitochondrial uncoupling mediated by UCP1 in BAT. Uncoupling can be also induced by ionophores such as 2,4-dinitrophenol (DNP), which generates heat by allowing protons to shuttle more freely across the inner mitochondrial membrane into the matrix. As with UCP1, when uncouplers reduce the backpressure from the protons in the intermembrane space, the TCA cycle and electron transport chain run faster, generating heat through the increased rate of exothermic processes and intermolecular friction (23). In contrast to UCP1, DNP's actions occur in every cell and are unregulated, which leads to uncontrolled hyperthermia (159). Despite its success in reducing body weight (up to 3 lbs per week) in high doses, DNP caused substantial side effects, including rashes, cataracts, and even death due to hyperthermia (160). Nevertheless, DNP was used as an off-label weight-loss medication in the 1930s (160) and has the distinction of being one of the first drugs banned by the FDA (161). Besides direct uncoupling of oxidative phosphorylation, DNP increases intracellular cAMP, likely through the activation of adenylyl cyclase and/or inhibition of phosphodiesterase activity (160). Elevated cAMP could then activate PKA and then downstream lipolysis and thermogenesis. The door appears to be shut on the possibility of using ionophores to treat obesity due to the numerous studies demonstrating adverse reactions indicative of toxicity in the muscle, liver, and heart when people took DNP (161). However, recent studies by the Shulman and co-workers (162) have focused on using a controlled-release oral formulation of DNP, called CRMP (controlled-release mitochondrial protonophore). In a proof–of–concept rat model, CRMP produced mild hepatic mitochondrial uncoupling and ameliorated hypertriglyceridemia, insulin resistance, hepatic steatosis, and diabetes (162).
TRP superfamily
Transient receptor potential channels are a family of intermembrane ion channels that play a role in sensory reception and can be thermally or chemically activated. The chemoreceptive properties of the channels provide a target for pharmacological interventions affecting sensory reception of temperature. Capsaicin is the compound responsible for the burning sensation associated with hot peppers. It belongs to a family of chemicals known as capsaicinoids present in all species of Capsicum peppers (163). Capsaicin binds transient receptor potential vanilloid receptor 1 (TRPVR1), which detects painful heat and harmful chemicals (164), and is currently approved by the FDA as a topical agent for the treatment of neuropathic pain associated with post-herpetic neuralgia. Another class of TRPV1 receptor agonists found in Capsicum peppers are known as capsinoids and lack the pungency of capsaicin (165). Both capsaicinoids and capsinoids are thought to increase energy expenditure through TRPV1 receptor-mediated sympathetic nervous system response (166) and release of adrenal catecholamines (167). The effect of capsaicin and capsinoids on human metabolism is not entirely clear. Many studies are conducted using extracts of red peppers and other natural products, which makes it difficult to determine which chemical agent induced the observed effects. Pure capsaicin was evaluated in a study of weight maintenance after weight loss in 140 overweight men and women. The group that consumed 22.5 mg of capsaicin had a significantly higher increase in resting energy expenditure (REE) (167 ± 120 kcal/day) than controls (48 ± 120 kcal/day) (168). Overall results have been mixed, however. Another study of 78 healthy men who ingested 3 mg of dihydrocapsiate for 28 days had a nonsignificant increase in REE compared with controls, whereas another study of 13 men found no changes in metabolic rate with the consumption of 1, 3, 6, and 12 mg of capsinoid capsules over a 5-day inpatient stay (169). Capsaicin has also been associated with BAT activation (170), although there has been no demonstration that capsaicin activates human BAT, either directly through cell-surface receptors or indirectly via the SNS. Thus, despite the large interest in this natural compound, it is unclear whether it has any potential utility in treating obesity-related energy imbalance.
Menthol, also known as mint camphor, is a compound isolated from the plant genus Mentha that is responsible for the cooling sensation associated with mint extracts (171). Menthol binds the transient receptor potential melatstatin-8 (TRPM8) on peripheral nerve fibers, which detect environmental cold (172). These nerves transverse the dorsal horn of the spinal cord to the lateral parabrachial nucleus in the dorsolateral pons and then on to the preoptic area of the hypothalamus (173). Efferent signals leave the brain descending the spinal cord to sympathetic nerves that release norepinephrine causing vasoconstriction in blood vessels and BAT activity. TRPM8 receptors have been found to be expressed on the surface of both mouse brown and white adipocytes as well (174, 175). Treatment of mouse white adipocytes with menthol increased UCP1 expression, thought to be due to TRPM8 activation. Mice fed a high-fat diet with 1% menthol for 18 weeks had a significantly-reduced weight gain compared with high-fat diet mice (174). TRPM8 expression has been detected in human white adipocytes as well, and when treated with menthol the adipocytes showed an increase in UCP1 expression, mitochondrial membrane potential, and heat production, displaying a similarity to brown adipose tissue during cold exposure (176). Although there have yet to be any in vivo human studies on the effect of menthol and energy expenditure, these results show potential for menthol-induced increases in REE.
In summary, it is useful to conceptualize the pharmacological treatment of energy imbalanced-induced obesity as one would approach other chronic conditions such as essential hypertension and insulin resistance. The undesirable retention of food calories is similarly multigenic and produced by multiple interactions between genetic background and environment. Success routinely requires several medications from distinct classes that address the different contributors to the chronic illnesses. Just as hypertension is often treated with combinations of sympatholytics, ion channel blockers, and enzyme inhibitors, the pharmacological approach to obesity will likely require a combination of drugs that suppress appetite, impair absorption, and increase energy expenditure (Fig. 1). Given the challenges associated with combating the strong evolutionary drive to preserve caloric stores, focus should be toward treating obesity-related metabolic disease. Success is more likely in this arena where substantial advances have already been made in reducing insulin resistance, hypertension, and dyslipidemia.
Impacts of pharmacological regulation of energy balance, overweight, and obesity
Metabolic adaptations
As the previous section shows, there is great interest in developing pharmacological approaches to achieving a net negative energy balance and treating obesity. However, the small number of approved anti-obesity drugs reflect many conceptual and practical inadequacies. The limitations are not just safety or even short-term efficacy. A larger challenge is the need over the long term to combat homeostatic drives within humans to return to higher weights. Despite a seemingly simple equation, “energy in less than energy out,” there are complex adaptations to energy regulation. Although body weight can be modulated acutely, it remains difficult to achieve long-term weight loss as the body tends to adapt or resist by modulating energy expenditure, hormonal, and psychological drive.
Even in short-duration studies of energy deficit, energy balance tends to reestablish. For example, in a 1-day diet-induced or exercise-induced net-negative energy balance, subjects the following day compensated calorically to the energy lost on the previous day and returned to energy balance after 48 h (177). In cases where energy expenditure increases due to exercise training, there is evidence to suggest that energy intake will also compensatively increase (178, 179), and activity outside volitional exercise tends to decrease (180). Similarly, in mice, cold-induced increases in energy expenditure lead to increased food intake in a dose-dependent manner (181). Although the relationship between energy expenditure and energy intake may be unclear, energy maintenance is still tightly regulated (182). It is true that with longer-term energy deficits, there is weight loss. However, decreases in weight are accompanied by reductions in circulating leptin, peptide YY, cholecystokinin, and amylin and increases in ghrelin—a coordinated hormonal response that promotes weight gain. Even after the initial dynamic phase of weight loss, these changes are maintained, likely playing a part in the body's continual resistance to weight loss (183). In fact, hormonal regulations that alter intake and satiety can persist for greater than a year after a 10-week very low energy diet (183).
To the frustration of most dieters, there is a lowering of REE with weight loss. It is a widely-held but false belief that a daily energy deficit of 500 kcal leads to a loss of a pound of fat per week and over 50 lbs per year (184). Rather, weight loss is more asymptotic, and a larger energy deficit is required to continue losing weight at the same rate (https://www.niddk.nih.gov/health-information/weight-management/body-weight-planner) (185). The extent of weight change has both hormonal and genetic components (186–188). At first, the body can adjust energy expenditure, up or down, according to a 10% weight gain or loss, respectively (189). However, this adaptation is only able to adjust to a certain level: in subjects who lost 20% of their initial weight, REE did not decrease any further, suggesting a maximal adaptation to the maintenance of reduced body weight (189). In subjects who lost over one-third of their initial body weight and maintained adequate fat-free mass during the “Biggest Loser” competition, the measured BMR decreased by 504 kcal/day more than that predicted based on fat-free mass, fat mass, age, and sex (190). The degree of metabolic adaptation was inversely correlated with changes in thyroid-stimulating hormone levels but was not correlated to changes in triiodothyronine levels, which declined with weight loss. This pattern suggests that metabolic adaptation was centrally mediated with the hypothalamic–pituitary–thyroid axis potentially playing a large role (190). Other studies have shown that leptin, an anorexigenic adipokine, may play a role in metabolic adaptation (191–193). Metabolic slowing not only adds to the difficulty of losing weight but may also contribute to weight regain. For instance, in a subset of the “Biggest Loser” contestants measured 6 years after their initial weight loss, the percentage of original weight regained was directly related to the amount of metabolic adaptation, i.e. the difference between the measured and predicted BMR at 6 years (194). Interestingly, the amount of weight regained by the participants after 6 years was not correlated with a change from pre-weight loss energy intake but was inversely correlated with changes from pre-weight loss physical activity levels. These observations suggest that physical activity, although not necessarily effective as the primary approach for weight loss, is important for successfully preventing the regaining of lost weight (195).
Approaches to maximize thermogenesis
Given that humans and related mammals are physiologically designed to resist weight loss, it raises the following question: does the method of altered energy intake or output change metabolic responses? This was addressed in rats whose weight loss from diet was compared with exercise. Both groups had similar reductions in adiposity, but the exercised rats displayed improvements in insulin resistance, greater insulin sensitivity, and lower low-density lipoprotein (196), and they showed increased mitochondrial functioning in BAT (196). Other reports on exercise suggest that endurance exercise promotes high rates of oxidative metabolism in skeletal muscle. With endurance exercise, there is a shift to type I myofibers with high mitochondrial content and thus fatty-acid oxidation profile (197). This switch is similar to the one that occurs in skeletal muscle from cold exposure, where cold exposure increases expression of PGC-1α in skeletal muscle promoting mitochondrial biogenesis and a switch to oxidative fibers (198).
Besides reduced oral caloric consumption and increased exercise, cold exposure is another intervention that can lead to a net negative energy balance. Mice exposed intermittently to cold for 0, 1, 4, or 8 h a day three times a week for 10 weeks saw an increase in total energy expenditure (181). Joy (199) tested human subjects repeatedly exposed to cold temperatures (5 °C) for 8 h over the course of 5 weeks. The cold-adapted subjects could further increase their metabolic rates in response to an infusion of NE, demonstrating an increase in the body's ability to perform nonshivering thermogenesis. These early findings were corroborated by studies of subjects who were exposed to 17 °C for 2 h daily over 6 weeks, which resulted in increased BAT activity and an ∼167% increase in the CIT response to acute exposure to 19 °C (170). Further study by Lee et al. (200) demonstrated that sleeping in a 19 °C room at night for 1 month increased BAT volume and activity compared with 1 month of sleeping in 24 °C. Physiological responses in these subjects also included increased expression of adiponectin and GLUT4 in subcutaneous WAT. White adipocyte insulin resistance decreased, and the Matsuda index for insulin sensitivity increased (200). However, as with increased exercise and reduced caloric intake, the increased metabolism in response to cold may only be temporary “adjustments” seen in those who normally live in a thermoneutral climate. Over the longer term, the body undergoes “adaptations” that lead to blunted metabolic rate increases (201). Additionally, people with obesity, for whom therapeutic BAT activation would be desirable, have less activated BAT than lean men after cold exposure, despite a greater amount of fat in BAT-containing depots (55).
Thus, depending on the type and amount of exposure to cold, caloric changes, and exercise regimen, there are multiple ways the body will manipulate cellular energy expenditure, hormones, and physiological responses to re-achieve a perceived homeostasis. The body, over chronic periods of energy abundance or deprivation, will adapt. The decreasing return on investment, whether it be to lose weight, improve the lipoprotein profile, etc., posits a question for how often and frequently we can manipulate our actions and environments to receive the desired results.
The future and conclusions
In summary, the prospect of using pharmacological and other nonsurgical means to increase thermogenesis and reduce obesity is in a state of equipoise. Current methodologies to measure whole-body energy expenditure are quite precise, yet advances are urgently needed in the area of measuring thermogenesis at the level of the individual tissue. Available approaches to stimulate thermogenesis are effective acutely, but long-term efficacy and safety are still not assured. There remains promise in new approaches to increasing EE, particularly human BAT as it was only recently determined to be functional in adult humans. Much remains to be learned about its roles in human physiology, both in terms of thermogenesis and other contributions. Finally, as with any intervention that perturbs homeostasis, solutions are needed for the physiological compensation that occurs when a negative energy balance is achieved. It may be that small, sustained increases in energy expenditure or reducing metabolic adaptation will be the most effective over the years to achieve and maintain lower weights.
These sober perspectives will remain the fate of the field unless—or until—the ultimate breakthrough in mammalian energy balance is discovered: how the body knows and maintains its own weight. As described above, numerous studies point to hypothalamic nuclei as regions where the brain receives input from the periphery regarding energy stores. It has also been demonstrated that the brain contains the sites where metabolic adaptation slows metabolism after weight loss. If new research determines how to directly adjust the body's “baristat,” then the therapeutic activation of human energy expenditure and thermogenesis to treat obesity would be attainable. Such systems have already been described and manipulated beneficially to treat other endocrine disorders, from the thyroid to the reproductive axes. The same may be true for the regulation of energy balance, yet the big discoveries await. The obesity pandemic means the demand for treatments is high, and so must be the ingenuity and resourcefulness among those in the field.
This work was supported by National Institutes of Health Intramural Research Program of the NIDDK Grants DK075112, DK075115, DK075116, DK071013, and DK071014. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- EE
- energy expenditure
- BMR
- basal metabolic rate
- ETC
- electron transport chain
- AR
- adrenoreceptor
- DNP
- 2,4-dinitrophenol
- CRMP
- controlled-release mitochondrial protonophore
- TRP
- transient receptor potential
- BAT
- brown adipose tissue
- CIT
- cold-induced thermogenesis
- W-S
- warm-sensitive
- TEF
- thermic effect of food
- TNZ
- thermoneutral zone
- NST
- nonshivering thermogenesis
- WAT
- white adipose tissue
- NE
- norepinephrine
- PKA
- protein kinase A
- TPM
- topiramate
- FDA
- Food and Drug Administration
- GABA
- γ-aminobutyric acid
- CT
- computed tomography
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- TCA
- tricarboxylic acid cycle
- FFM
- fat-free mass
- MRI
- magnetic resonance imaging
- 18F-FDG
- [18F]fluorodeoxyglucose
- EMG
- electromyography
- SNS
- sympathetic nervous system
- PPAR
- peroxisome proliferator-activated receptor
- LPL
- lipoprotein lipase
- PET
- positron emission tomography
- CNS
- central nervous system
- REE
- resting energy expenditure.
References
- 1. Schauer P. R., Bhatt D. L., Kirwan J. P., Wolski K., Brethauer S. A., Navaneethan S. D., Aminian A., Pothier C. E., Kim E. S., Nissen S. E., Kashyap S. R., and STAMPEDE Investigators (2014) Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N. Engl. J. Med. 370, 2002–2013 10.1056/NEJMoa1401329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gadde K. M., Martin C. K., Berthoud H. R., and Heymsfield S. B. (2018) Obesity: pathophysiology and management. J. Am. Coll. Cardiol. 71, 69–84 10.1016/j.jacc.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geiser F., Stawski C., Wacker C. B., and Nowack J. (2017) Phoenix from the ashes: fire, torpor, and the evolution of mammalian endothermy. Front. Physiol. 8, 842 10.3389/fphys.2017.00842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert V. A., and Casadevall A. (2009) Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 200, 1623–1626 10.1086/644642 [DOI] [PubMed] [Google Scholar]
- 5. Cannon B., and Nedergaard J. (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 10.1242/jeb.050989 [DOI] [PubMed] [Google Scholar]
- 6. Kleiber M. (1961) The Fire of Life: An Introduction to Animal Energetics, pp. 105–174, John Wiley & Sons, Inc., New York [Google Scholar]
- 7. Scholander P. F., Hock R., Walters V., Johnson F., and Irving L. (1950) Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 99, 237–258 10.2307/1538741 [DOI] [PubMed] [Google Scholar]
- 8. Fischer A. W., Csikasz R. I., von Essen G., Cannon B., and Nedergaard J. (2016) No insulating effect of obesity. Am. J. Physiol. Endocrinol. Metab. 311, E202–E213 10.1152/ajpendo.00093.2016 [DOI] [PubMed] [Google Scholar]
- 9. Abreu-Vieira G., Xiao C., Gavrilova O., and Reitman M. L. (2015) Integration of body temperature into the analysis of energy expenditure in the mouse. Mol. Metab. 4, 461–470 10.1016/j.molmet.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brychta R. J., Huang S., Wang J., Leitner B. P., Hattenbach J. D., Bell S. L., Fletcher L. A., Perron Wood R., Idelson C. R., Duckworth C. J., McGehee S., Courville A. B., Bernstein S. B., Reitman M. L., Cypess A. M., and Chen K. Y. (2019) Quantification of the capacity for cold-induced thermogenesis in young men with and without obesity. J. Clin. Endocrinol. Metab. 104, 4865–4878 10.1210/jc.2019-00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison S. F., Madden C. J., and Tupone D. (2014) Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756 10.1016/j.cmet.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roth J. V. (2009) Some unanswered questions about temperature management. Anesth. Analg. 109, 1695–1699 10.1213/ANE.0b013e3181b763ae [DOI] [PubMed] [Google Scholar]
- 13. Green D. E., and Zande H. D. (1981) Universal energy principle of biological systems and the unity of bioenergetics. Proc. Natl. Acad. Sci. U.S.A. 78, 5344–5347 10.1073/pnas.78.9.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clapham J. C., and Arch J. R. (2007) Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes. Metab. 9, 259–275 10.1111/j.1463-1326.2006.00608.x [DOI] [PubMed] [Google Scholar]
- 15. Jastroch M., Divakaruni A. S., Mookerjee S., Treberg J. R., and Brand M. D. (2010) Mitochondrial proton and electron leaks. Essays Biochem. 47, 53–67 10.1042/bse0470053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rolfe D. F., and Brown G. C. (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 10.1152/physrev.1997.77.3.731 [DOI] [PubMed] [Google Scholar]
- 17. Randle P. J., Garland P. B., Hales C. N., and Newsholme E. A. (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 10.1016/s0140-6736(63)91500-9 [DOI] [PubMed] [Google Scholar]
- 18. Wolfe R. R., Herndon D. N., Jahoor F., Miyoshi H., and Wolfe M. (1987) Effect of severe burn injury on substrate cycling by glucose and fatty acids. N. Engl. J. Med. 317, 403–408 10.1056/NEJM198708133170702 [DOI] [PubMed] [Google Scholar]
- 19. Klein S., and Wolfe R. R. (1990) Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J. Clin. Invest. 86, 1403–1408 10.1172/JCI114854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolfe R. R., Klein S., Carraro F., and Weber J. M. (1990) Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. J. Physiol. 258, E382–E389 10.1152/ajpendo.1990.258.2.E382 [DOI] [PubMed] [Google Scholar]
- 21. Haman F., and Blondin D. P. (2017) Shivering thermogenesis in humans: origin, contribution and metabolic requirement. Temperature 4, 217–226 10.1080/23328940.2017.1328999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bal N. C., Maurya S. K., Sopariwala D. H., Sahoo S. K., Gupta S. C., Shaikh S. A., Pant M., Rowland L. A., Bombardier E., Goonasekera S. A., Tupling A. R., Molkentin J. D., and Periasamy M. (2012) Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 10.1038/nm.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertholet A. M., and Kirichok Y. (2017) UCP1: a transporter for H(+) and fatty acid anions. Biochimie 134, 28–34 10.1016/j.biochi.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golozoubova V., Cannon B., and Nedergaard J. (2006) UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am. J. Physiol. Endocrinol. Metab. 291, E350–E357 10.1152/ajpendo.00387.2005 [DOI] [PubMed] [Google Scholar]
- 25. Zoico E., Rubele S., De Caro A., Nori N., Mazzali G., Fantin F., Rossi A., and Zamboni M. (2019) Brown and beige adipose tissue and aging. Front. Endocrinol. 10, 368 10.3389/fendo.2019.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chouchani E. T., and Kajimura S. (2019) Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189–200 10.1038/s42255-018-0021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W. D., Hoeks J., Enerbäck S., Schrauwen P., and Spiegelman B. M. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kazak L., Chouchani E. T., Jedrychowski M. P., Erickson B. K., Shinoda K., Cohen P., Vetrivelan R., Lu G. Z., Laznik-Bogoslavski D., Hasenfuss S. C., Kajimura S., Gygi S. P., and Spiegelman B. M. (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 10.1016/j.cell.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perna M. K., Kokenge A. N., Miles K. N., Udobi K. C., Clark J. F., Pyne-Geithman G. J., Khuchua Z., and Skelton M. R. (2016) Creatine transporter deficiency leads to increased whole body and cellular metabolism. Amino Acids 48, 2057–2065 10.1007/s00726-016-2291-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mills E. L., Pierce K. A., Jedrychowski M. P., Garrity R., Winther S., Vidoni S., Yoneshiro T., Spinelli J. B., Lu G. Z., Kazak L., Banks A. S., Haigis M. C., Kajimura S., Murphy M. P., Gygi S. P., et al. (2018) Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 10.1038/s41586-018-0353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brychta R. J., and Chen K. Y. (2017) Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 71, 345–352 10.1038/ejcn.2016.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brody S., and Procter R. C. (1932) Relation between basal metabolism and mature body-weight in different species of mammals and birds. pp. 89–101, University of Missouri, Agricultural Experiment Station, Research Bulletin, Columbia, MO [Google Scholar]
- 33. Kleiber M. (1947) Body size and metabolic rate. Physiol. Rev. 27, 511–541 10.1152/physrev.1947.27.4.511 [DOI] [PubMed] [Google Scholar]
- 34. Benedict F. G. (1938) Vital Energetics. A Study in Comparative Basal Metabolism, Carnegie Institute, Washington Publication [Google Scholar]
- 35. Rubner M. (1883) Ueber den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Zeitschrift für Biologie 19, 535–562 [Google Scholar]
- 36. Tschöp M. H., Speakman J. R., Arch J. R., Auwerx J., Brüning J. C., Chan L., Eckel R. H., Farese R. V. Jr., Galgani J. E., Hambly C., Herman M. A., Horvath T. L., Kahn B. B., Kozma S. C., Maratos-Flier E., et al. (2011) A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 10.1038/nmeth.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buskirk E. R., Thompson R. H., Moore R., and Whedon G. D. (1960) Human energy expenditure studies in the National Institute of Arthritis and Metabolic Diseases metabolic chamber. 1. Interaction of cold environment and specific dynamic effect. 2. Sleep. Am. J. Clin. Nutr. 8, 602–613 10.1093/ajcn/8.5.602 [DOI] [Google Scholar]
- 38. Rennie D. W., Covino B. G., Blair M. R., and Rodahl K. (1962) Physical regulation of temperature in Eskimos. J. Appl. Physiol. 17, 326–332 10.1152/jappl.1962.17.2.326 [DOI] [PubMed] [Google Scholar]
- 39. Ravussin E., and Bogardus C. (1989) Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am. J. Clin. Nutr. 49, 968–975 10.1093/ajcn/49.5.968 [DOI] [PubMed] [Google Scholar]
- 40. Heymsfield S. B., Thomas D., Bosy-Westphal A., Shen W., Peterson C. M., and Müller M. J. (2012) Evolving concepts on adjusting human resting energy expenditure measurements for body size. Obes. Rev. 13, 1001–1014 10.1111/j.1467-789X.2012.01019.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. West J. B. (2013) The collaboration of Antoine and Marie-Anne Lavoisier and the first measurements of human oxygen consumption. Am. J. Physiol. Lung Cell Mol. Physiol. 305, L775–L785 10.1152/ajplung.00228.2013 [DOI] [PubMed] [Google Scholar]
- 42. Dubois E. F., Ebaugh F. G. Jr., and Hardy J. D. (1952) Basal heat production and elimination of 13 normal women at temperatures from 22 °C to 35 °C. J. Nutr. 48, 257–293 10.1093/jn/48.2.257 [DOI] [PubMed] [Google Scholar]
- 43. Hardy J. D., and Dubois E. F. (1937) Regulation of heat loss from the human body. Proc. Natl. Acad. Sci. U.S.A. 23, 624–631 10.1073/pnas.23.12.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swift R. W. (1932) The effects of low environmental temperature upon metabolism I. Technic and respiratory quotient. J. Nutr. 5, 213–225 10.1093/jn/5.3.213 [DOI] [Google Scholar]
- 45. Swift R. W. (1932) The effects of low environmental temperature upon metabolism II. The influence of shivering, subcutaneous fat, and skin temperature on heat production. J. Nutr. 5, 227–249 10.1093/jn/5.3.227 [DOI] [Google Scholar]
- 46. Winslow C. E. A., Herrington L. P., and Gagge A. P. (1937) Physiological reactions of the human body to varying environmental temperatures. Am. J. Physiol. 120, 1–22 10.1152/ajplegacy.1937.120.1.1 [DOI] [Google Scholar]
- 47. Eyolfson D. A., Tikuisis P., Xu X., Weseen G., and Giesbrecht G. G. (2001) Measurement and prediction of peak shivering intensity in humans. Eur. J. Appl. Physiol. 84, 100–106 10.1007/s004210000329 [DOI] [PubMed] [Google Scholar]
- 48. van der Lans A. A., Wierts R., Vosselman M. J., Schrauwen P., Brans B., and van Marken Lichtenbelt W. D. (2014) Cold-activated brown adipose tissue in human adults: methodological issues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R103–R113 10.1152/ajpregu.00021.2014 [DOI] [PubMed] [Google Scholar]
- 49. Cypess A. M., Haft C. R., Laughlin M. R., and Hu H. H. (2014) Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 20, 408–415 10.1016/j.cmet.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen K. Y., Cypess A. M., Laughlin M. R., Haft C. R., Hu H. H., Bredella M. A., Enerbäck S., Kinahan P. E., Lichtenbelt Wv., Lin F. I., Sunderland J. J., Virtanen K. A., and Wahl R. L. (2016) Brown adipose reporting criteria in imaging studies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 24, 210–222 10.1016/j.cmet.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ong F. J., Ahmed B. A., Oreskovich S. M., Blondin D. P., Haq T., Konyer N. B., Noseworthy M. D., Haman F., Carpentier A. C., Morrison K. M., and Steinberg G. R. (2018) Recent advances in the detection of brown adipose tissue in adult humans: a review. Clin. Sci. 132, 1039–1054 10.1042/CS20170276 [DOI] [PubMed] [Google Scholar]
- 52. Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., and Nuutila P. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- 53. Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., and Nedergaard J. (2010) Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jespersen N. Z., Feizi A., Andersen E. S., Heywood S., Hattel H. B., Daugaard S., Peijs L., Bagi P., Feldt-Rasmussen B., Schultz H. S., Hansen N. S., Krogh-Madsen R., Pedersen B. K., Petrovic N., Nielsen S., and Scheele C. (2019) Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol. Metab. 24, 30–43 10.1016/j.molmet.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leitner B. P., Huang S., Brychta R. J., Duckworth C. J., Baskin A. S., McGehee S., Tal I., Dieckmann W., Gupta G., Kolodny G. M., Pacak K., Herscovitch P., Cypess A. M., and Chen K. Y. (2017) Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. U.S.A. 114, 8649–8654 10.1073/pnas.1705287114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marette A., and Bukowiecki L. J. (1989) Stimulation of glucose transport by insulin and norepinephrine in isolated rat brown adipocytes. Am. J. Physiol. 257, C714–C721 10.1152/ajpcell.1989.257.4.C714 [DOI] [PubMed] [Google Scholar]
- 57. Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., Turcotte E. E., Richard D., and Carpentier A. C. (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 10.1172/JCI60433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muzik O., Mangner T. J., Leonard W. R., Kumar A., Janisse J., and Granneman J. G. (2013) 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J. Nucl. Med. 54, 523–531 10.2967/jnumed.112.111336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. U Din M., Raiko J., Saari T., Kudomi N., Tolvanen T., Oikonen V., Teuho J., Sipilä H. T., Savisto N., Parkkola R., Nuutila P., and Virtanen K. A. (2016) Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus. Eur. J. Nucl. Med. Mol. Imaging 43, 1878–1886 10.1007/s00259-016-3364-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marlatt K. L., Chen K. Y., and Ravussin E. (2018) Is activation of human brown adipose tissue a viable target for weight management? Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R479–R483 10.1152/ajpregu.00443.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gifford A., Towse T. F., Walker R. C., Avison M. J., and Welch E. B. (2016) Characterizing active and inactive brown adipose tissue in adult humans using PET-CT and MR imaging. Am. J. Physiol. Endocrinol. Metab. 311, E95–E104 10.1152/ajpendo.00482.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fraum T. J., Crandall J. P., Ludwig D. R., Chen S., Fowler K. J., Laforest R. A., Salter A., Dehdashti F., An H., and Wahl R. L. (2019) Repeatability of quantitative brown adipose tissue imaging metrics on positron emission tomography with 18F-fluorodeoxyglucose in humans. Cell Metab. 30, 212–224.e4 10.1016/j.cmet.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 63. Zhang L., Burant A., McCallister A., Zhao V., Koshlap K. M., Degan S., Antonacci M., and Branca R. T. (2017) Accurate MR thermometry by hyperpolarized 129Xe. Magn. Reson. Med. 78, 1070–1079 10.1002/mrm.26506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blondin D. P., and Haman F. (2018) Shivering and nonshivering thermogenesis in skeletal muscles. Handb. Clin. Neurol. 156, 153–173 10.1016/B978-0-444-63912-7.00010-2 [DOI] [PubMed] [Google Scholar]
- 65. Blondin D. P., Labbé S. M., Phoenix S., Guérin B., Turcotte É. E., Richard D., Carpentier A. C., and Haman F. (2015) Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J. Physiol. 593, 701–714 10.1113/jphysiol.2014.283598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weir G., Ramage L. E., Akyol M., Rhodes J. K., Kyle C. J., Fletcher A. M., Craven T. H., Wakelin S. J., Drake A. J., Gregoriades M. L., Ashton C., Weir N., van Beek E. J. R., Karpe F., Walker B. R., and Stimson R. H. (2018) Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab. 27, 1348–1355.e4 10.1016/j.cmet.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tseng Y. H., Cypess A. M., and Kahn C. R. (2010) Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 9, 465–482 10.1038/nrd3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cheung B. M., Cheung T. T., and Samaranayake N. R. (2013) Safety of antiobesity drugs. Ther. Adv. Drug. Saf. 4, 171–181 10.1177/2042098613489721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Larson C. J. (2019) Translational pharmacology and physiology of brown adipose tissue in human disease and treatment. Handb. Exp. Pharmacol. 251, 381–424 10.1007/164_2018_184 [DOI] [PubMed] [Google Scholar]
- 70. Evans B. A., Merlin J., Bengtsson T., and Hutchinson D. S. (2019) Adrenoceptors in white, brown, and brite adipocytes. Br. J. Pharmacol. 176, 2416–2432 10.1111/bph.14631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Collins S., Cao W., and Robidoux J. (2004) Learning new tricks from old dogs: β-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol. Endocrinol. 18, 2123–2131 10.1210/me.2004-0193 [DOI] [PubMed] [Google Scholar]
- 72. Oldham W. M., and Hamm H. E. (2008) Heterotrimeric G protein activation by G-protein–coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 10.1038/nrm2299 [DOI] [PubMed] [Google Scholar]
- 73. Weis W. I., and Kobilka B. K. (2018) The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87, 897–919 10.1146/annurev-biochem-060614-033910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vanderbeld B., and Kelly G. M. (2000) New thoughts on the role of the β-γ subunit in G-protein signal transduction. Biochem. Cell Biol. 78, 537–550 10.1139/o00-075 [DOI] [PubMed] [Google Scholar]
- 75. Neves S. R., Ram P. T., and Iyengar R. (2002) G protein pathways. Science 296, 1636–1639 10.1126/science.1071550 [DOI] [PubMed] [Google Scholar]
- 76. Weinstein L. S. (2014) Role of Gsα in central regulation of energy and glucose metabolism. Horm. Metab. Res. 46, 841–844 10.1055/s-0034-1387798 [DOI] [PubMed] [Google Scholar]
- 77. Lowell B. B., and Spiegelman B. M. (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 10.1038/35007527 [DOI] [PubMed] [Google Scholar]
- 78. MacPherson R. E., Castellani L., Beaudoin M. S., and Wright D. C. (2014) Evidence for fatty acids mediating CL 316,243-induced reductions in blood glucose in mice. Am. J. Physiol. Endocrinol. Metab. 307, E563–E570 10.1152/ajpendo.00287.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fedorenko A., Lishko P. V., and Kirichok Y. (2012) Mechanism of fatty-acid–dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 10.1016/j.cell.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Divakaruni A. S., Humphrey D. M., and Brand M. D. (2012) Fatty acids change the conformation of uncoupling protein 1 (UCP1). J. Biol. Chem. 287, 36845–36853 10.1074/jbc.M112.381780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cao W., Daniel K. W., Robidoux J., Puigserver P., Medvedev A. V., Bai X., Floering L. M., Spiegelman B. M., and Collins S. (2004) p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 10.1128/MCB.24.7.3057-3067.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Collins S., Daniel K. W., Rohlfs E. M., Ramkumar V., Taylor I. L., and Gettys T. W. (1994) Impaired expression and functional activity of the β3- and β1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol. Endocrinol. 8, 518–527 10.1210/mend.8.4.7914350 [DOI] [PubMed] [Google Scholar]
- 83. Bengtsson T., Cannon B., and Nedergaard J. (2000) Differential adrenergic regulation of the gene expression of the β-adrenoceptor subtypes β1, β2, and β3 in brown adipocytes. Biochem. J. 347, 643–651 [PMC free article] [PubMed] [Google Scholar]
- 84. Susulic V. S., Frederich R. C., Lawitts J., Tozzo E., Kahn B. B., Harper M. E., Himms-Hagen J., Flier J. S., and Lowell B. B. (1995) Targeted disruption of the β3-adrenergic receptor gene. J. Biol. Chem. 270, 29483–29492 10.1074/jbc.270.49.29483 [DOI] [PubMed] [Google Scholar]
- 85. Revelli J. P., Preitner F., Samec S., Muniesa P., Kuehne F., Boss O., Vassalli J. D., Dulloo A., Seydoux J., Giacobino J. P., Huarte J., and Ody C. (1997) Targeted gene disruption reveals a leptin-independent role for the mouse β3-adrenoceptor in the regulation of body composition. J. Clin. Invest. 100, 1098–1106 10.1172/JCI119620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang J., Hanada K., Staus D. P., Makara M. A., Dahal G. R., Chen Q., Ahles A., Engelhardt S., and Rockman H. A. (2017) Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat. Commun. 8, 1706 10.1038/s41467-017-01855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sharma A. M., Pischon T., Hardt S., Kunz I., and Luft F. C. (2001) Hypothesis: β-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension 37, 250–254 10.1161/01.HYP.37.2.250 [DOI] [PubMed] [Google Scholar]
- 88. Lee P., Kengne A. P., Greenfield J. R., Day R. O., Chalmers J., and Ho K. K. (2011) Metabolic sequelae of β-blocker therapy: weighing in on the obesity epidemic? Int. J. Obes. 35, 1395–1403 10.1038/ijo.2010.284 [DOI] [PubMed] [Google Scholar]
- 89. Bachman E. S., Dhillon H., Zhang C. Y., Cinti S., Bianco A. C., Kobilka B. K., and Lowell B. B. (2002) βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 10.1126/science.1073160 [DOI] [PubMed] [Google Scholar]
- 90. Jimenez M., Léger B., Canola K., Lehr L., Arboit P., Seydoux J., Russell A. P., Giacobino J. P., Muzzin P., and Preitner F. (2002) β(1)/β(2)/β(3)-adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett. 530, 37–40 10.1016/S0014-5793(02)03387-2 [DOI] [PubMed] [Google Scholar]
- 91. Ueta C. B., Fernandes G. W., Capelo L. P., Fonseca T. L., Maculan F. D., Gouveia C. H., Brum P. C., Christoffolete M. A., Aoki M. S., Lancellotti C. L., Kim B., Bianco A. C., and Ribeiro M. O. (2012) β(1)-Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J. Endocrinol. 214, 359–365 10.1530/JOE-12-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. de Jong J. M. A., Wouters R. T. F., Boulet N., Cannon B., Nedergaard J., and Petrovic N. (2017) The β3-adrenergic receptor is dispensable for browning of adipose tissues. Am. J. Physiol. Endocrinol. Metab. 312, E508–E518 10.1152/ajpendo.00437.2016 [DOI] [PubMed] [Google Scholar]
- 93. Widén E., Lehto M., Kanninen T., Walston J., Shuldiner A. R., and Groop L. C. (1995) Association of a polymorphism in the β3-adrenergic-receptor gene with features of the insulin resistance syndrome in Finns. N. Engl. J. Med. 333, 348–351 10.1056/NEJM199508103330604 [DOI] [PubMed] [Google Scholar]
- 94. Clément K., Vaisse C., Manning B. S., Basdevant A., Guy-Grand B., Ruiz J., Silver K. D., Shuldiner A. R., Froguel P., and Strosberg A. D. (1995) Genetic variation in the β3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N. Engl. J. Med. 333, 352–354 10.1056/NEJM199508103330605 [DOI] [PubMed] [Google Scholar]
- 95. Shuldiner A. R., Silver K., Roth J., and Walston J. (1996) β3-Adrenoceptor gene variant in obesity and insulin resistance. Lancet 348, 1584–1585 10.1016/S0140-6736(05)66197-1 [DOI] [PubMed] [Google Scholar]
- 96. Li L. S., Lönnqvist F., Luthman H., and Arner P. (1996) Phenotypic characterization of the Trp64Arg polymorphism in the β3-adrenergic receptor gene in normal weight and obese subjects. Diabetologia 39, 857–860 10.1007/s001250050521 [DOI] [PubMed] [Google Scholar]
- 97. Astrup A., Bülow J., Christensen N. J., and Madsen J. (1984) Ephedrine-induced thermogenesis in man: no role for interscapular brown adipose tissue. Clin. Sci. 66, 179–186 10.1042/cs0660179 [DOI] [PubMed] [Google Scholar]
- 98. Astrup A., Bülow J., Madsen J., and Christensen N. J. (1985) Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am. J. Physiol. 248, E507–E515 10.1152/ajpendo.1985.248.5.E507 [DOI] [PubMed] [Google Scholar]
- 99. Astrup A., Lundsgaard C., Madsen J., and Christensen N. J. (1985) Enhanced thermogenic responsiveness during chronic ephedrine treatment in man. Am. J. Clin. Nutr. 42, 83–94 10.1093/ajcn/42.1.83 [DOI] [PubMed] [Google Scholar]
- 100. Astrup A., and Toubro S. (1993) Thermogenic, metabolic, and cardiovascular responses to ephedrine and caffeine in man. Int. J. Obes. Relat. Metab. Disord. 17, Suppl. 1, S41–S43 [PubMed] [Google Scholar]
- 101. Dulloo A. G., and Miller D. S. (1989) Ephedrine, caffeine and aspirin: “over-the-counter” drugs that interact to stimulate thermogenesis in the obese. Nutrition 5, 7–9 [PubMed] [Google Scholar]
- 102. Pasquali R., Baraldi G., Cesari M. P., Melchionda N., Zamboni M., Stefanini C., and Raitano A. (1985) A controlled trial using ephedrine in the treatment of obesity. Int. J. Obes. 9, 93–98 [PubMed] [Google Scholar]
- 103. Shekelle P., and Hardy M. (2002) Safety and efficacy of ephedra and ephedrine for enhancement of athletic performance, thermogenesis and the treatment of obesity. Phytomedicine 9, 78 [PubMed] [Google Scholar]
- 104. Shannon J. R., Gottesdiener K., Jordan J., Chen K., Flattery S., Larson P. J., Candelore M. R., Gertz B., Robertson D., and Sun M. (1999) Acute effect of ephedrine on 24-h energy balance. Clin. Sci. 96, 483–491 [PubMed] [Google Scholar]
- 105. Vansal S. S., and Feller D. R. (1999) Direct effects of ephedrine isomers on human β-adrenergic receptor subtypes. Biochem. Pharmacol. 58, 807–810 10.1016/S0006-2952(99)00152-5 [DOI] [PubMed] [Google Scholar]
- 106. Dulloo A. G., and Miller D. S. (1986) The thermogenic properties of ephedrine/methylxanthine mixtures: human studies. Int. J. Obes. 10, 467–481 [PubMed] [Google Scholar]
- 107. Cypess A. M., Chen Y. C., Sze C., Wang K., English J., Chan O., Holman A. R., Tal I., Palmer M. R., Kolodny G. M., and Kahn C. R. (2012) Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. U.S.A. 109, 10001–10005 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Carey A. L., Formosa M. F., Van Every B., Bertovic D., Eikelis N., Lambert G. W., Kalff V., Duffy S. J., Cherk M. H., and Kingwell B. A. (2013) Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 56, 147–155 10.1007/s00125-012-2748-1 [DOI] [PubMed] [Google Scholar]
- 109. Carey A. L., Pajtak R., Formosa M. F., Van Every B., Bertovic D. A., Anderson M. J., Eikelis N., Lambert G. W., Kalff V., Duffy S. J., Cherk M. H., and Kingwell B. A. (2015) Chronic ephedrine administration decreases brown adipose tissue activity in a randomised controlled human trial: implications for obesity. Diabetologia 58, 1045–1054 10.1007/s00125-015-3543-6 [DOI] [PubMed] [Google Scholar]
- 110. Shekelle P. G., Hardy M. L., Morton S. C., Maglione M., Mojica W. A., Suttorp M. J., Rhodes S. L., Jungvig L., and Gagné J. (2003) Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 289, 1537–1545 10.1001/jama.289.12.1537 [DOI] [PubMed] [Google Scholar]
- 111. Daly P. A., Krieger D. R., Dulloo A. G., Young J. B., and Landsberg L. (1993) Ephedrine, caffeine and aspirin: safety and efficacy for treatment of human obesity. Int. J. Obes. Relat. Metab. Disord. 17, Suppl 1, S73–78 [PubMed] [Google Scholar]
- 112. Bracco D., Ferrarra J. M., Arnaud M. J., Jéquier E., and Schutz Y. (1995) Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am. J. Physiol. 269, E671–E678 10.1152/ajpendo.1995.269.4.E671 [DOI] [PubMed] [Google Scholar]
- 113. Napolitano G., Amente S., Castiglia V., Gargano B., Ruda V., Darzacq X., Bensaude O., Majello B., and Lania L. (2010) Caffeine prevents transcription inhibition and P-TEFb/7SK dissociation following UV-induced DNA damage. PLoS ONE 5, e11245 10.1371/journal.pone.0011245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dulloo A. G. (2002) Herbal simulation of ephedrine and caffeine in treatment of obesity. Int. J. Obes. Relat. Metab. Disord. 26, 590–592 10.1038/sj.ijo.0802036 [DOI] [PubMed] [Google Scholar]
- 115. Tulp O. L., and Buck C. L. (1986) Caffeine and ephedrine stimulated thermogenesis in LA-corpulent rats. Comp. Biochem. Physiol. C 85, 17–19 10.1016/0742-8413(86)90045-9 [DOI] [PubMed] [Google Scholar]
- 116. Molnár D., Török K., Erhardt E., and Jeges S. (2000) Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents. Int. J. Obes. Relat. Metab. Disord. 24, 1573–1578 10.1038/sj.ijo.0801433 [DOI] [PubMed] [Google Scholar]
- 117. Boozer C. N., Daly P. A., Homel P., Solomon J. L., Blanchard D., Nasser J. A., Strauss R., and Meredith T. (2002) Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int. J. Obes. Relat. Metab. Disord. 26, 593–604 10.1038/sj.ijo.0802023 [DOI] [PubMed] [Google Scholar]
- 118. Vukovich M. D., Schoorman R., Heilman C., Jacob P. 3rd., Benowitz N. L. (2005) Caffeine-herbal ephedra combination increases resting energy expenditure, heart rate and blood pressure. Clin. Exp. Pharmacol. Physiol. 32, 47–53 10.1111/j.1440-1681.2005.04152.x [DOI] [PubMed] [Google Scholar]
- 119. Dulloo A. G., Seydoux J., and Girardier L. (1991) Peripheral mechanisms of thermogenesis induced by ephedrine and caffeine in brown adipose tissue. Int. J. Obes. 15, 317–326 [PubMed] [Google Scholar]
- 120. Dulloo A. G. (1993) Ephedrine, xanthines and prostaglandin-inhibitors: actions and interactions in the stimulation of thermogenesis. Int. J. Obes. Relat. Metab. Disord. 17, Suppl. 1, S35–S40 [PubMed] [Google Scholar]
- 121. Mowers J., Uhm M., Reilly S. M., Simon J., Leto D., Chiang S. H., Chang L., and Saltiel A. R. (2013) Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKϵ and TBK1. Elife 2, e01119 10.7554/eLife.01119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Horton T. J., and Geissler C. A. (1991) Aspirin potentiates the effect of ephedrine on the thermogenic response to a meal in obese but not lean women. Int. J. Obes. 15, 359–366 [PubMed] [Google Scholar]
- 123. Fleming J. W., McClendon K. S., and Riche D. M. (2013) New obesity agents: lorcaserin and phentermine/topiramate. Ann. Pharmacother. 47, 1007–1016 10.1345/aph.1R779 [DOI] [PubMed] [Google Scholar]
- 124. Lotfi K., Palmer K., and Apovian C. M. (2015) Case study: weight loss in a patient with type 2 diabetes: challenges of diabetes management. Obesity 23, Suppl. 1, S11–S12 10.1002/oby.21095 [DOI] [PubMed] [Google Scholar]
- 125. Lewis K. H., Fischer H., Ard J., Barton L., Bessesen D. H., Daley M. F., Desai J., Fitzpatrick S. L., Horberg M., Koebnick C., Oshiro C., Yamamoto A., Young D. R., and Arterburn D. E. (2019) Safety and effectiveness of longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity 27, 591–602 10.1002/oby.22430 [DOI] [PubMed] [Google Scholar]
- 126. McNair E., and Bryson R. (1983) Effects of nicotine on weight change and food consumption in rats. Pharmacol. Biochem. Behav. 18, 341–344 10.1016/0091-3057(83)90451-3 [DOI] [PubMed] [Google Scholar]
- 127. Glauser S. C., Glauser E. M., Reidenberg M. M., Rusy B. F., and Tallarida R. J. (1970) Metabolic changes associated with the cessation of cigarette smoking. Arch. Environ. Health 20, 377–381 10.1080/00039896.1970.10665607 [DOI] [PubMed] [Google Scholar]
- 128. Lupien J. R., and Bray G. A. (1988) Nicotine increases thermogenesis in brown adipose tissue in rats. Pharmacol. Biochem. Behav. 29, 33–37 10.1016/0091-3057(88)90269-9 [DOI] [PubMed] [Google Scholar]
- 129. Wager-Srdar S. A., Levine A. S., Morley J. E., Hoidal J. R., and Niewoehner D. E. (1984) Effects of cigarette smoke and nicotine on feeding and energy. Physiol. Behav. 32, 389–395 10.1016/0031-9384(84)90252-X [DOI] [PubMed] [Google Scholar]
- 130. Wellman P. J., Marmon M. M., Reich S., and Ruddle J. (1986) Effects of nicotine on body weight, food intake and brown adipose tissue thermogenesis. Pharmacol. Biochem. Behav. 24, 1605–1609 10.1016/0091-3057(86)90493-4 [DOI] [PubMed] [Google Scholar]
- 131. Hofstetter A., Schutz Y., Jéquier E., and Wahren J. (1986) Increased 24-hour energy expenditure in cigarette smokers. N. Engl. J. Med. 314, 79–82 10.1056/NEJM198601093140204 [DOI] [PubMed] [Google Scholar]
- 132. López M. (2018) Hypothalamic AMPK and energy balance. Eur. J. Clin. Invest. 48, e12996 10.1111/eci.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. López M., and Tena-Sempere M. (2017) Estradiol effects on hypothalamic AMPK and BAT thermogenesis: a gateway for obesity treatment? Pharmacol. Ther. 178, 109–122 10.1016/j.pharmthera.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 134. Martínez de Morentin P. B., Whittle A. J., Fernø J., Nogueiras R., Diéguez C., Vidal-Puig A., and López M. (2012) Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes 61, 807–817 10.2337/db11-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Seoane-Collazo P., Martínez de Morentin P. B., Fernø J., Diéguez C., Nogueiras R., and López M. (2014) Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology 155, 1679–1689 10.1210/en.2013-1839 [DOI] [PubMed] [Google Scholar]
- 136. Lynch L., Hogan A. E., Duquette D., Lester C., Banks A., LeClair K., Cohen D. E., Ghosh A., Lu B., Corrigan M., Stevanovic D., Maratos-Flier E., Drucker D. J., O'Shea D., and Brenner M. (2016) iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 24, 510–519 10.1016/j.cmet.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Benowitz N. L. (2010) Nicotine addiction. N. Engl. J. Med. 362, 2295–2303 10.1056/NEJMra0809890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kudin A. P., Debska-Vielhaber G., Vielhaber S., Elger C. E., and Kunz W. S. (2004) The mechanism of neuroprotection by topiramate in an animal model of epilepsy. Epilepsia 45, 1478–1487 10.1111/j.0013-9580.2004.13504.x [DOI] [PubMed] [Google Scholar]
- 139. White H. S. (1997) Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia 38, Suppl. 1, S9–S17 10.1111/j.1528-1157.1997.tb04523.x [DOI] [PubMed] [Google Scholar]
- 140. White H. S., Brown S. D., Woodhead J. H., Skeen G. A., and Wolf H. H. (2000) Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia 41, Suppl. 1, S17–S20 [PubMed] [Google Scholar]
- 141. Gryder D. S., and Rogawski M. A. (2003) Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J. Neurosci. 23, 7069–7074 10.1523/JNEUROSCI.23-18-07069.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang X., Velumian A. A., Jones O. T., and Carlen P. L. (2000) Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia 41, Suppl. 1, S52–S60 10.1111/j.1528-1157.2000.tb02173.x [DOI] [PubMed] [Google Scholar]
- 143. Dodgson S. J., Shank R. P., and Maryanoff B. E. (2000) Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia 41, Suppl. 1, S35–S39 10.1111/j.1528-1157.2000.tb06047.x [DOI] [PubMed] [Google Scholar]
- 144. Herrero A. I., Del Olmo N., Gonzáalez-Escalada J. R., and Solís J. M. (2002) Two new actions of topiramate: inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology 42, 210–220 10.1016/S0028-3908(01)00171-X [DOI] [PubMed] [Google Scholar]
- 145. Richard D., Ferland J., Lalonde J., Samson P., and Deshaies Y. (2000) Influence of topiramate in the regulation of energy balance. Nutrition 16, 961–966 10.1016/S0899-9007(00)00452-4 [DOI] [PubMed] [Google Scholar]
- 146. Biton V. (2003) Effect of antiepileptic drugs on bodyweight: overview and clinical implications for the treatment of epilepsy. CNS Drugs 17, 781–791 10.2165/00023210-200317110-00002 [DOI] [PubMed] [Google Scholar]
- 147. Richard D., Picard F., Lemieux C., Lalonde J., Samson P., and Deshaies Y. (2002) The effects of topiramate and sex hormones on energy balance of male and female rats. Int. J. Obes. Relat. Metab. Disord. 26, 344–353 10.1038/sj.ijo.0801873 [DOI] [PubMed] [Google Scholar]
- 148. Astrup A., and Toubro S. (2004) Topiramate: a new potential pharmacological treatment for obesity. Obes. Res. 12, Suppl. 167S–173S 10.1038/oby.2004.284 [DOI] [PubMed] [Google Scholar]
- 149. Jordan J., Astrup A., Engeli S., Narkiewicz K., Day W. W., and Finer N. (2014) Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity. J. Hypertens. 32, 1178–1188 10.1097/HJH.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Arch J. R. (2011) Challenges in β(3)-adrenoceptor agonist drug development. Ther. Adv. Endocrinol. Metab. 2, 59–64 10.1177/2042018811398517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sacco E., and Bientinesi R. (2012) Mirabegron: a review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 4, 315–324 10.1177/1756287212457114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schena G., and Caplan M. J. (2019) Everything you always wanted to know about β3-AR* (* But Were Afraid to Ask). Cells 8, E357 10.3390/cells8040357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cypess A. M., Weiner L. S., Roberts-Toler C., Franquet Elía E., Kessler S. H., Kahn P. A., English J., Chatman K., Trauger S. A., Doria A., and Kolodny G. M. (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Baskin A. S., Linderman J. D., Brychta R. J., McGehee S., Anflick-Chames E., Cero C., Johnson J. W., O'Mara A. E., Fletcher L. A., Leitner B. P., Duckworth C. J., Huang S., Cai H., Garraffo H. M., Millo C. M., et al. (2018) Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3-adrenergic receptor agonist. Diabetes 67, 2113–2125 10.2337/db18-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Loh R. K. C., Formosa M. F., La Gerche A., Reutens A. T., Kingwell B. A., and Carey A. L. (2019) Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes Obes. Metab. 21, 276–284 10.1111/dom.13516 [DOI] [PubMed] [Google Scholar]
- 156. Finlin B. S., Memetimin H., Confides A. L., Kasza I., Zhu B., Vekaria H. J., Harfmann B., Jones K. A., Johnson Z. R., Westgate P. M., Alexander C. M., Sullivan P. G., Dupont-Versteegden E. E., and Kern P. A. (2018) Human adipose beiging in response to cold and mirabegron. JCI Insight 3, 121510 10.1172/jci.insight.121510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fisher J. P., Young C. N., and Fadel P. J. (2009) Central sympathetic overactivity: maladies and mechanisms. Auton. Neurosci. 148, 5–15 10.1016/j.autneu.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Seals D. R., and Bell C. (2004) Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes 53, 276–284 10.2337/diabetes.53.2.276 [DOI] [PubMed] [Google Scholar]
- 159. Hoch F. L., and Hogan F. P. (1973) Hyperthermia, muscle rigidity, and uncoupling in skeletal muscle mitochondria in rats treated with halothane and 2,4-dinitrophenol. Anesthesiology 38, 237–243 10.1097/00000542-197303000-00007 [DOI] [PubMed] [Google Scholar]
- 160. Geisler J. G. (2019) 2,4-Dinitrophenol as medicine. Cells 8, 280 10.3390/cells8030280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Colman E. (2007) Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul. Toxicol. Pharmacol. 48, 115–117 10.1016/j.yrtph.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 162. Perry R. J., Zhang D., Zhang X. M., Boyer J. L., and Shulman G. I. (2015) Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256 10.1126/science.aaa0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ludy M. J., Moore G. E., and Mattes R. D. (2012) The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem. Senses 37, 103–121 10.1093/chemse/bjr100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Caterina M. J., and Julius D. (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517 10.1146/annurev.neuro.24.1.487 [DOI] [PubMed] [Google Scholar]
- 165. Lee T. A., Li Z., Zerlin A., and Heber D. (2010) Effects of dihydrocapsiate on adaptive and diet-induced thermogenesis with a high protein very low calorie diet: a randomized control trial. Nutr. Metab. 7, 78 10.1186/1743-7075-7-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ohnuki K., Haramizu S., Oki K., Watanabe T., Yazawa S., and Fushiki T. (2001) Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci. Biotechnol. Biochem. 65, 2735–2740 10.1271/bbb.65.2735 [DOI] [PubMed] [Google Scholar]
- 167. Watanabe T., Kawada T., Kato T., Harada T., and Iwai K. (1994) Effects of capsaicin analogs on adrenal catecholamine secretion in rats. Life Sci. 54, 369–374 10.1016/0024-3205(94)00793-4 [DOI] [PubMed] [Google Scholar]
- 168. Lejeune M. P., Kovacs E. M., and Westerterp-Plantenga M. S. (2003) Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br. J. Nutr. 90, 651–659 10.1079/BJN2003938 [DOI] [PubMed] [Google Scholar]
- 169. Galgani J. E., Ryan D. H., and Ravussin E. (2010) Effect of capsinoids on energy metabolism in human subjects. Br. J. Nutr. 103, 38–42 10.1017/S0007114509991358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., Iwanaga T., and Saito M. (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 10.1172/JCI67803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pergolizzi J. V. Jr., Taylor R. Jr, LeQuang J. A., Raffa R. B., and NEMA Research Group. (2018) The role and mechanism of action of menthol in topical analgesic products. J. Clin. Pharm. Ther. 43, 313–319 10.1111/jcpt.12679 [DOI] [PubMed] [Google Scholar]
- 172. McKemy D. D. (2007) in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades (Liedtke W. B., and Heller S., eds) CRC Press/Taylor & Francis, Boca Raton, FL: [PubMed] [Google Scholar]
- 173. Johnson C. D., Melanaphy D., Purse A., Stokesberry S. A., Dickson P., and Zholos A. V. (2009) Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 296, H1868–H1877 10.1152/ajpheart.01112.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jiang C., Zhai M., Yan D., Li D., Li C., Zhang Y., Xiao L., Xiong D., Deng Q., and Sun W. (2017) Dietary menthol-induced TRPM8 activation enhances WAT “browning” and ameliorates diet-induced obesity. Oncotarget 8, 75114–75126 10.18632/oncotarget.20540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ma S., Yu H., Zhao Z., Luo Z., Chen J., Ni Y., Jin R., Ma L., Wang P., Zhu Z., Li L., Zhong J., Liu D., Nilius B., and Zhu Z. (2012) Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell. Biol. 4, 88–96 10.1093/jmcb/mjs001 [DOI] [PubMed] [Google Scholar]
- 176. Rossato M., Granzotto M., Macchi V., Porzionato A., Petrelli L., Calcagno A., Vencato J., De Stefani D., Silvestrin V., Rizzuto R., Bassetto F., De Caro R., and Vettor R. (2014) Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell. Endocrinol. 383, 137–146 10.1016/j.mce.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 177. Goldberg G. R., Murgatroyd P. R., McKenna A. P., Heavey P. M., and Prentice A. M. (1998) Dietary compensation in response to covert imposition of negative energy balance by removal of fat or carbohydrate. Br. J. Nutr. 80, 141–147 10.1017/S0007114598001044 [DOI] [PubMed] [Google Scholar]
- 178. Doucet E., McInis K., and Mahmoodianfard S. (2018) Compensation in response to energy deficits induced by exercise or diet. Obes. Rev. 19, Suppl. 1, 36–46 10.1111/obr.12783 [DOI] [PubMed] [Google Scholar]
- 179. Thomas D. M., Bouchard C., Church T., Slentz C., Kraus W. E., Redman L. M., Martin C. K., Silva A. M., Vossen M., Westerterp K., and Heymsfield S. B. (2012) Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes. Rev. 13, 835–847 10.1111/j.1467-789X.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Melanson E. L., Keadle S. K., Donnelly J. E., Braun B., and King N. A. (2013) Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med. Sci. Sports Exerc. 45, 1600–1609 10.1249/MSS.0b013e31828ba942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ravussin Y., Xiao C., Gavrilova O., and Reitman M. L. (2014) Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PLoS ONE 9, e85876 10.1371/journal.pone.0085876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Blundell J. E., Caudwell P., Gibbons C., Hopkins M., Naslund E., King N., and Finlayson G. (2012) Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis. Model. Mech. 5, 608–613 10.1242/dmm.009837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Sumithran P., Prendergast L. A., Delbridge E., Purcell K., Shulkes A., Kriketos A., and Proietto J. (2011) Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 10.1056/NEJMoa1105816 [DOI] [PubMed] [Google Scholar]
- 184. Guth E. (2014) JAMA patient page. Healthy weight loss. JAMA 312, 974 10.1001/jama.2014.10929 [DOI] [PubMed] [Google Scholar]
- 185. Hall K. D., Sacks G., Chandramohan D., Chow C. C., Wang Y. C., Gortmaker S. L., and Swinburn B. A. (2011) Quantification of the effect of energy imbalance on bodyweight. Lancet 378, 826–837 10.1016/S0140-6736(11)60812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Presby D. M., Jackman M. R., Rudolph M. C., Sherk V. D., Foright R. M., Houck J. A., Johnson G. C., Orlicky D. J., Melanson E. L., Higgins J. A., and MacLean P. S. (2019) Compensation for cold-induced thermogenesis during weight loss maintenance and regain. Am. J. Physiol. Endocrinol. Metab. 316, E977–E986 10.1152/ajpendo.00543.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hollstein T., Ando T., Basolo A., Krakoff J., Votruba S. B., and Piaggi P. (2019) Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Am. J. Clin. Nutr 110, 593–604 10.1093/ajcn/nqz062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Piaggi P. (2019) Metabolic determinants of weight gain in humans. Obesity 27, 691–699 10.1002/oby.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Leibel R. L., Rosenbaum M., and Hirsch J. (1995) Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 10.1056/NEJM199503093321001 [DOI] [PubMed] [Google Scholar]
- 190. Johannsen D. L., Knuth N. D., Huizenga R., Rood J. C., Ravussin E., and Hall K. D. (2012) Metabolic slowing with massive weight loss despite preservation of fat-free mass. J. Clin. Endocrinol. Metab. 97, 2489–2496 10.1210/jc.2012-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Knuth N. D., Johannsen D. L., Tamboli R. A., Marks-Shulman P. A., Huizenga R., Chen K. Y., Abumrad N. N., Ravussin E., and Hall K. D. (2014) Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity 22, 2563–2569 10.1002/oby.20900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., Gallagher D., Mayer L., Murphy E., and Leibel R. L. (2005) Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115, 3579–3586 10.1172/JCI25977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rosenbaum M., Nicolson M., Hirsch J., Murphy E., Chu F., and Leibel R. L. (1997) Effects of weight change on plasma leptin concentrations and energy expenditure. J. Clin. Endocrinol. Metab. 82, 3647–3654 10.1210/jcem.82.11.4390 [DOI] [PubMed] [Google Scholar]
- 194. Fothergill E., Guo J., Howard L., Kerns J. C., Knuth N. D., Brychta R., Chen K. Y., Skarulis M. C., Walter M., Walter P. J., and Hall K. D. (2016) Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 24, 1612–1619 10.1002/oby.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kerns J. C., Guo J., Fothergill E., Howard L., Knuth N. D., Brychta R., Chen K. Y., Skarulis M. C., Walter P. J., and Hall K. D. (2017) Increased physical activity associated with less weight regain 6 years after “The Biggest Loser” competition. Obesity 25, 1838–1843 10.1002/oby.21986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Welly R. J., Liu T. W., Zidon T. M., Rowles J. L. 3rd., Park Y. M., Smith T. N., Swanson K. S., Padilla J., and Vieira-Potter V. J. (2016) Comparison of diet versus exercise on metabolic function and gut microbiota in obese rats. Med. Sci. Sports Exerc. 48, 1688–1698 10.1249/MSS.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., and Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 10.1016/S0092-8674(00)81410-5 [DOI] [PubMed] [Google Scholar]
- 198. Zhang L., Zhou Y., Wu W., Hou L., Chen H., Zuo B., Xiong Y., and Yang J. (2017) Skeletal muscle-specific overexpression of PGC-1α induces fiber-type conversion through enhanced mitochondrial respiration and fatty acid oxidation in mice and pigs. Int. J. Biol. Sci. 13, 1152–1162 10.7150/ijbs.20132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Joy R. J. (1963) Responses of cold-acclimatized men to infused norepinephrine. J. Appl. Physiol 18, 1209–1212 10.1152/jappl.1963.18.6.1209 [DOI] [PubMed] [Google Scholar]
- 200. Lee P., Smith S., Linderman J., Courville A. B., Brychta R. J., Dieckmann W., Werner C. D., Chen K. Y., and Celi F. S. (2014) Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698 10.2337/db14-0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Andersen K. L., Loyning Y., Nelms J. D., Wilson O., Fox R. H., and Bolstad A. (1960) Metabolic and thermal response to a moderate cold exposure in nomadic Lapps. J. Appl. Physiol. 15, 649–653 10.1152/jappl.1960.15.4.649 [DOI] [PubMed] [Google Scholar]