Abstract
Iron is an essential nutrient for all living organisms. To acquire iron, many pathogens have developed elaborate systems to steal it from their hosts. The iron acquisition system in the opportunistic pathogen Staphylococcus aureus comprises nine proteins, called iron-regulated surface determinants (Isds). The Isd components enable S. aureus to extract heme from hemoglobin (Hb), transport it into the bacterial cytoplasm, and ultimately release iron from the porphyrin ring. IsdB and IsdH act as hemoglobin receptors and are known to actively extract heme from extracellular Hb. To limit microbial pathogenicity during infection, host organisms attempt to restrict the availability of nutrient metals at the host–pathogen interface. The human acute phase protein haptoglobin (Hp) protects the host from oxidative damage by clearing hemoglobin that has leaked from red blood cells and also restricts the availability of extracellular Hb-bound iron to invading pathogens. To investigate whether Hp serves an additional role in nutritional immunity through a direct inhibition of IsdH-mediated iron acquisition, here we measured heme extraction from the Hp–Hb complex by UV-visible spectroscopy and determined the crystal structure of the Hp–Hb–IsdH complex at 2.9 Å resolution. We found that Hp strongly inhibits IsdH-mediated heme extraction and that Hp binding prevents local unfolding of the Hb heme pocket, leaving IsdH unable to wrest the heme from Hb. Furthermore, we noted that the Hp–Hb binding appears to trap IsdH in an initial state before heme transfer. Our findings provide insights into Hp-mediated IsdH inhibition and the dynamics of IsdH-mediated heme extraction.
Keywords: Staphylococcus aureus (S. aureus), hemoglobin, inhibitor, infection, iron, Haptoglobin, Hemoglobin, iron, IsdH, Staphylococcus aureus
Introduction
To establish a successful infection, pathogens must be able to obtain nutritional iron from their host because it is essential for a variety of metabolic processes (1). Consequently, pathogens have developed a number of different strategies to take up iron from their surroundings. The Gram-positive bacteria Staphylococcus aureus acquires iron via two different strategies. One strategy is to secrete various siderophores that chelate free iron and are subsequently reimported by the bacteria (2). Another strategy is to obtain iron via the extraction and uptake of heme from host hemoproteins, e.g. Hb.2 The S. aureus heme acquisition system is an elaborate system of nine proteins called the iron-regulated surface determinants (IsdA–IsdI) (3). In cooperation, the Isd proteins extract heme from Hb on the surface of the bacterium, transport it into the cytoplasm, and liberate the iron. As indicated by the name, expression of the components of the Isd system is regulated by the level of available iron via the ferric uptake regulator (4).
Initially, serum Hb is captured by either IsdB or IsdH, which are both covalently attached to the peptidoglycan layer of the bacterium (5–7). IsdB and IsdH use a similar mechanism to actively extract heme from Hb (8, 9) and transfer it to the likewise peptidoglycan-anchored IsdA and IsdC (10–12). From there, heme is delivered to IsdE and transported across the plasma membrane by a ABC transporter constituted by IsdD, IsdE, and IsdF (13, 14). In the cytoplasm, iron is liberated from heme by the actions of the two heme oxygenases IsdG and IsdI (15).
The four peptidoglycan-anchored proteins IsdA, IsdB, IsdC, and IsdH are structurally related and contain a variable number of conserved near iron transporter (NEAT) domains (16). NEAT domains are found in a range of bacterial proteins, where they primarily function in iron acquisition. NEAT domains adopt a β-sandwich fold that consists of two five-stranded antiparallel β-sheets (17). Although sharing the same overall fold, NEAT domains are functionally distinct, because some are directly involved in Hb binding, whereas others bind heme (17). The two functionally distinct groups are distinguished by a YXXXY motif only found in the heme-binding NEAT domains (18). The first tyrosine residue in the motif is directly involved in heme binding by coordinating the heme iron.
The number and types of NEAT domains vary between the different Isd proteins. IsdA and IsdC both contain a single heme-binding NEAT domain, whereas IsdB and IsdH contain two and three NEAT domains, respectively (6, 7, 16). NEAT domains N1 and N2 of IsdB and NEAT domains N2 and N3 of IsdH are structurally and functionally homologous because both have an Hb-binding NEAT domain (IsdBN1 and IsdHN2) and a heme-binding NEAT domain (IsdBN2 and IsdHN3) separated by a helical linker region, which is essential for heme extraction (19). The additional N-terminal NEAT domain of IsdH (IsdHN1) is also capable of binding Hb (6), but its significance in heme extraction is not yet fully understood.
To defend themselves against pathogenic invaders, host organisms have developed various methods to limit the availability of the essential iron (20, 21). Specifically, free iron is removed from the circulation by the actions of transferrin and the transferrin-receptor (22), effectively restricting siderophore access to noncomplexed iron. Free heme is captured by hemopexin and subsequently cleared from circulation by LRP1(CD91)-mediated endocytosis (23). However, iron complexed by globin-bound heme by far constitutes the greatest pool of iron in the human body and is therefore an attractive target for pathogen iron-acquisition mechanisms. Hb-bound heme is cleared from circulation by the combined action of the acute phase protein haptoglobin (Hp) and the scavenger receptor CD163 (24, 25). In solution, Hb exists in equilibrium between tetrameric Hb (α2β2Hb) and dimeric Hb (αβHb). Hp forms extensive interactions with both α- and β-subunits of αβHb(26). Because the Hp-binding site overlaps with the interface between αβHb dimers in tetrameric α2β2Hb, dissociation of α2β2Hb is a prerequisite for Hp–Hb complexation. After formation of the Hp–Hb complex, it is removed from circulation by macrophages via CD163-mediated endocytosis. Hp is composed of a proteolytically inactive serine protease (HpSP) domain and one (Hp1 allele) or two (Hp2 allele) CCP domains (27, 28). The HpSP domain binds Hb, whereas the CCP domains are involved in Hp dimerization in Hp1–Hp1 genotypes and dimerization/multimerization in Hp1–Hp2 and Hp2–Hp2 genotypes. From here on Hp–Hb denotes the complex between dimeric Hp (Hp1-1) and two αβHb dimers.
A well-described function of Hp is to protect the vasculature from oxidative damage by capturing Hb and neutralizing the oxidative properties of the Hb-bound heme groups (29). In addition, by forming large molecular weight complexes, Hp prevents Hb from being filtered in the kidneys, which are highly sensitive to oxidative damage (30). In this work we investigate whether Hp, in addition to protecting the human body against oxidative damage, also plays a role in nutritional immunity by preventing IsdH from extracting heme from Hb. Using spectroscopy, we show that Hp significantly inhibits IsdH-mediated Hb-heme extraction. The crystal structure of the HpSP–αβHb–IsdHN2-N3 complex shows that Hp prevents the unfolding of the αHb F-helix and FG corner, which could explain the inhibition. Furthermore, the HpSP–αβHb–IsdHN2-N3 structure may represent an initial state of the Hb–IsdH complex prior to heme transfer and therefore provides new insights into the mechanism of IsdH-mediated Hb heme extraction.
- Direct transfer
- Hp-Hb dissociation
- Spontaneous release
Results
Haptoglobin inhibits IsdH-mediated extraction of hemoglobin heme
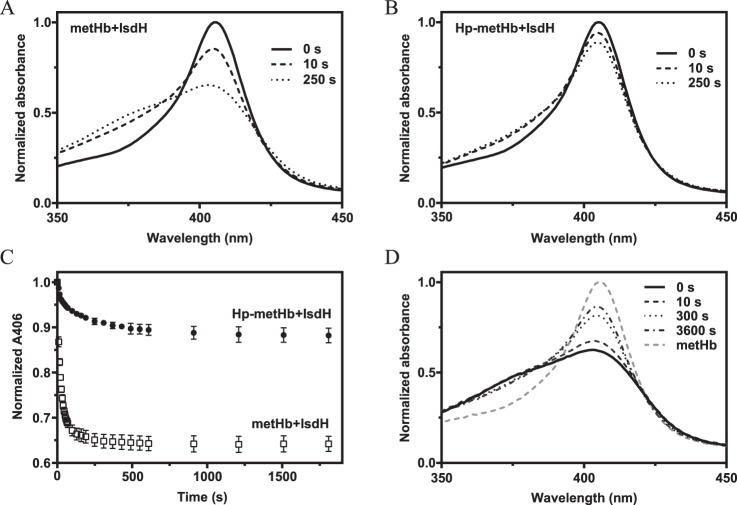
To investigate the effect of Hp binding on extraction of Hb-heme by IsdH, we recorded UV-visible spectral changes after mixing either metHb or Hp-bound metHb (Hp–metHb) with an N-terminal truncation variant of IsdH(IsdHN2-N3) (Fig. 1, A and B). As previously shown, heme transfer to IsdH can be determined by measuring decrease in absorbance at a wavelength of 406 nm (19), which is accompanied by a concomitant increase of ∼370 nm. Whereas considerable heme transfer occurs within seconds after mixing metHb with IsdHN2-N3, a much slower extraction of heme is observed with Hp–metHb as a substrate for IsdHN2-N3 (Fig. 1C). This shows that Hp is a potent but not complete inhibitor of IsdH-mediated heme extraction. Interestingly, prolonged incubation of Hp–metHb with IsdHN2-N3 does not result in complete extraction of heme (Fig. 1C), showing that Hp does not simply slow down the rate of heme extraction but rather seems to alter the equilibrium of heme transfer between metHb and IsdHN2-N3. This implies that the transfer of heme from metHb or Hp–metHb to IsdHN2-N3 is not irreversible but an equilibrium governed by binding kinetics. To test this, we allowed metHb and IsdH to incubate until all heme appeared to have been transferred to IsdH. Subsequent addition of Hp immediately reversed the flow of heme, reaching equilibrium within minutes (Fig. 1D). These results confirm that the transfer of heme from Hb to IsdH is reversible.
Figure 1.
Heme transfer from metHb and Hp–metHb to IsdHN2-N3. A and B, spectral changes over time for metHb+IsdHN2-N3 (A) and Hp–metHb+IsdHN2-N3 (B). C, absorbances at 406 nm for metHb + IsdHN2-N3 and Hp–metHb + IsdhN2-N3 followed for 30 min. Normalized absorbances at 406 nm are represented as mean values ± S.D. (n = 3). D, spectral changes over time for metHb preincubated with IsdHN2-N3. Hp was added at time point 0. After 1 h, no further spectral change was observed indicating that the system reached equilibrium.
Because Hp does not completely inhibit the IsdH-mediated Hb-heme extraction, we speculated that the observed transfer of heme from Hp–metHb could arise from other reactions than the direct transfer of heme from Hp–Hb to IsdH (reaction 1). One possibility is that the observed transfer arises from dissociation of the Hp–Hb complex and subsequent extraction from the liberated Hb (reaction 2). Another possibility is that heme is spontaneously released from Hp–Hb and then captured by IsdH (reaction 3).
Kinetics of the complex between Hb and Hp
To investigate whether the dissociation of the Hp–Hb complex (reaction 2) could account for the measured heme transfer in Fig. 1C, we determined the binding kinetics of the Hp–Hb complex. If free Hb is the only substrate for IsdH, we expect the heme-extraction rate to be limited by the Hp-dissociation rate. Heme-extraction rates from metHb have previously been determined by fitting of a two-phase decay model to experimental data, yielding kfast and kslow values (31). Being well-suited for modeling of a unidirectional transfer process, this model cannot be used for the equilibrium transfer of heme back and forth between IsdH and Hb–Hp. However, by plotting the early time points from Fig. 1C and fitting a one-phase decay model to it, we can estimate the initial rate of heme extraction as ∼0.004 s−1.
In the literature, the affinity of the complex between Hb and Hp is often stated as being one of the strongest known noncovalent interactions in serum, reporting affinities ranging from immeasurable to 10−15 m (32–34), which is not compatible with free metHb as the only substrate for IsdH. Since these reports, numerous technical advances have improved the ability to perform precise kinetics studies. Using surface plasmon resonance, we determined the kinetic parameters for metHb binding to both full-length Hp (Hp1-1 genotype) and a recombinant fragment of Hp encompassing the SP domain (HpSP) (Fig. 2A and Table 1). The estimated dissociation rate constants (kd) are ∼50 times (Hp1-1) and 30 times (HpSP) slower than the estimated heme extraction rate of 0.004 s−1. Therefore, we conclude that dissociation of Hp–Hb only accounts for a marginal fraction of the IsdH-mediated Hp–Hb heme extraction.
Figure 2.
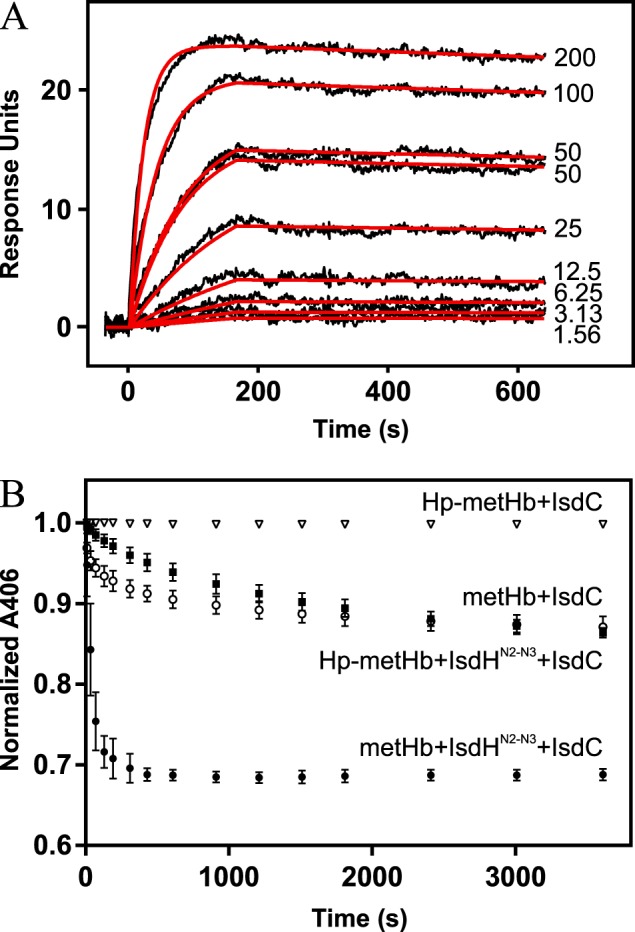
Hp–Hb binding kinetics and spontaneous release of heme from Hp–metHb and metHb. A, surface plasmon resonance determination of Hp1-1 affinity for Hb. Sensogram data are shown as black curves. Concentrations (in nm) of the injected Hb are shown next to the curves. A 1:1 Langmuir model was fitted to the sensogram (red curves). B, absorbances at 406 nm followed for 60 min. Normalized absorbances at 406 nm are represented as mean values ± S.D. (n = 3).
Table 1.
Kinetic parameters of metHb binding to Hp
| Ka | kd | KD | S.E. |
||
|---|---|---|---|---|---|
| (ka) × 10−3 | (kd) × 10−6 | ||||
| μm−1 s−1 | s−1 × 10−5 | nm | |||
| Hp1-1 | 0.22 | 7.85 | 0.36 | 1.07 | 1.23 |
| HpSP | 0.30 | 12.90 | 0.43 | 1.30 | 1.09 |
Spontaneous release of heme from Hp–Hb
Next, we investigated whether spontaneous release of heme from the Hp–Hb complex could account for the measured transfer (reaction 3). For measuring spontaneous dissociation of heme from Hb or Hp–Hb we used IsdC as a scavenger of free heme. Holo-IsdC and holo-IsdH show similar UV-Vis spectra, allowing spectral changes to be interpreted as heme dissociation from Hb and subsequent scavenging by IsdC. Compared with metHb, Hp–metHb shows an extremely low spontaneous heme dissociation (Fig. 2B), which agrees with previous reports showing that Hp-binding increases the affinity of Hb for heme (35). The very low rate of spontaneous heme dissociation from Hp–metHb can only account for a small fraction of the IsdH-mediated heme extraction from Hp–metHb. Furthermore, addition of IsdHN2-N3 significantly increases the rate of heme transfer from both metHb and Hp–metHb, confirming that IsdH actively promotes heme extraction from both Hb and Hp–Hb. Therefore, we conclude that direct transfer of heme from Hp–Hb to IsdH (reaction 1) is the primary source of the measured heme transfer in Fig. 1C.
Structure of the HpSP–αβHb–IsdHN2-N3 complex
To investigate the structural basis of Hp inhibition of IsdH-mediated heme extraction, we determined the structure of the HpSP–αβHb–IsdHN2-N3 complex. For structure determination we used the truncated IsdHN2-N3 carrying a Y642A mutation previously shown to disrupt heme transfer (9, 19). Accordingly, we were unable to measure any heme extraction from Hp–Hb in the presence of IsdHN2-N3(Y642A) (Fig. S1). Initial crystallization experiments using Hp–Hb were not successful. Hence, we switched to recombinant HpSP (residues 148–406) with mutations (N184S, N207S, N211S, and N241S) that disrupt N-linked glycosylation sites. The HpSP(N184S, N207S, N211S, N241S)-αβHb–IsdHN2-N3(Y642) complex crystallized in the P212121 space group, and data extending to 2.9 Å resolution were collected (Table 2). The structure was determined using molecular replacement with human Hp–Hb (PDB accession code 4WJG) (36) and S. aureus IsdHN2-N3(Y642A) (PDB accession code 4XS0) (37) as search models. Manual rebuilding and refinement yielded a final structure with Rwork of 23.46% and Rfree of 26.95%. The asymmetric unit contains a single HpSP–αβHb complex with IsdHN2-N3 bound to both αHb and βHb (Fig. 3A). The electron density for the entire complex is generally well-defined, although the B-factors are higher than expected at the current resolution, which probably reflects an inherent disorder of the crystals.
Table 2.
X-ray data collection and refinement statistics for HpSP–αβHb–IsdHN2-N3
| Data collection | |
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (Å) | 83.54, 86.74, 218.15 |
| α, β, γ (°) | 90, 90, 90 |
| Wavelength | 1.03 |
| Resolution (Å) | 50–2.9 (3.0–2.9)a |
| No. of total reflections | 463,827 |
| No. of unique reflections | 35,967 |
| Redundancy | 12.90 (13.95) |
| Completeness (%) | 100.0 (100.0) |
| I/σI | 15.12 (1.11) |
| CC½ | 99.8 (42.0) |
| Rmeas | 8.4 (296.5) |
| Refinement | |
| Resolution (Å) | 50–2.9 |
| Rwork/Rfreeb | 23.46/26.95 |
| Root-mean-square deviation | |
| Bond lengths | 0.006 |
| Bond angles | 1.120 |
| No. of atoms | |
| Proteins | 9,621 |
| Ligands | 86 |
| Waters | 0 |
| Average B-factors | |
| Hb | 114.96 |
| Hp | 111.96 |
| IsdHN2-N3 | 151.31 |
| Ligands | 126.19 |
| Geometry (%) | |
| Favored region | 86.7 |
| Allowed region | 12.9 |
| Outliers | 0.4 |
a The numbers in parentheses are values for the highest resolution shell.
b Rwork = Σ||Fobs| − |Fcalc||/Σ|Fobs|. Rfree = Σ||Fobs| − |Fcalc||/Σ|Fobs|, where all reflections belong to a test set of randomly selected data.
Figure 3.
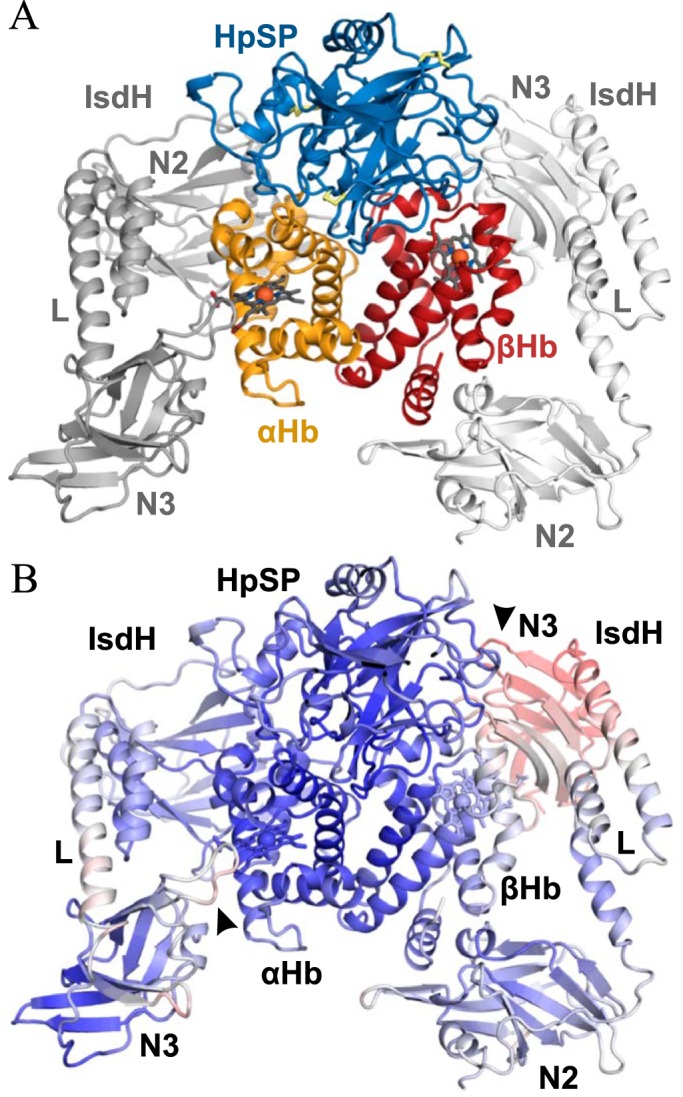
Structure of the HpSP–αβHb–IsdHN2-N3 complex. A, Cartoon representation of the 2.9 Å resolution crystal structure of HpSP–αβHb–IsdHN2-N3 complex. Hp is shown in blue, αHb is in orange, βHb is in red, and IsdHN2-N3 is light and dark gray. N2 and N3 denotes IsdHN2 and IsdHN3, respectively. L denotes the IsdHlinker domain. Heme groups are represented by dark gray sticks, and iron atoms are represented by red spheres. Disulfides are shown as yellow sticks. B, B-factors of the determined structure of Hp–Hb–IsdHN2-N3 are plotted on a cartoon representation of the structure with blue representing low B-factor, white representing intermediate, and red representing high. The interfaces αHb–IsdHN3 and βHb-IsdhN3 are marked with arrows.
Except for minor variations in loop regions of Hp, the structure of HpSP–αβHb bound to IsdHN2-N3 is highly similar to the previously determined structure of Hp–Hb (root-mean-square deviation of 0.489 over 3291 atoms) (Fig. S2A). It has previously been suggested that IsdH interacts with Hp (7, 38). However, no interaction is observed between IsdHN2-N3 and HpSP, supporting previous surface plasmon resonance analysis of Hp and IsdH (39). IsdHN2-N3 binds both αHb and βHb as observed in the previous structures of Hb–IsdHN2-N3 (9, 37) with IsdHN2 binding to the A-helix and the EF corner of each Hb subunit and with IsdHN3 positioned outside the heme-binding pocket (Fig. 3A). The conformations of IsdHN2-N3 molecules bound to the respective αHb and βHb subunits are essentially the same (Fig. S2B).
Electron density is observed for the entire IsdHN2-N3, and loop regions connecting the linker domain (IsdHlinker) with IsdHN2 and IsdHN3 can be modeled. IsdHN3 positioned outside the βHb heme-binding pocket has significantly higher B-factors than the other domains of IsdH and is hardly involved in any crystal contacts, suggesting a weak interaction between IsdHN3 and βHb (Fig. 3B). The IsdHN3 bound to αHb on the other hand is involved in multiple crystal contacts and has lower B-factors compared with the corresponding domain at βHb. However, the B-factors of the IsdHN3 regions contacting αHb are higher than the rest of the domain (Fig. 3B). Overall, the structure suggests a weak coordination of IsdHN3 by both αHb and βHb and that IsdHN3 is merely held in place outside the heme-binding pocket by its connection to IsdHN2 via the IsdHlinker.
Hp prevents IsdH-mediated unfolding of the αHb F-helix
When comparing the structure of HpSP–αβHb–IsdHN2-N3 with Hb–IsdHN2-N3 (PDB entry 4XS0) (9), we observe identical interfaces between αHb and IsdHN2. However, the positions of the IsdHlinker and IsdHN3 are markedly different in the two structures. In the Hp-inhibited structure, the IsdHlinker and IsdHN3 are positioned further away from the αHb heme-binding pocket. This novel conformation of IsdHN2-N3 appears to be a result of a conformational change internally in IsdHN2 and not within the presumed flexible loop regions connecting the individual domains of IsdHN2-N3 (Fig. 4A). This altered conformation of IsdHN2-N3 is most likely a result of Hp binding to αβHb.
Figure 4.
Interactions between the IsdHlinker and Hb in Hb–IsdHN2-N3versus HpSP–αβHb–IsdHN2-N3. A, structural comparison of IsdHN2-N3 bound to αHb in Hb–IsdHN2-N3 (light color scheme) (PDB entry 4XS0) (9) and HpSP–αβHb–IsdHN2-N3 (dark color scheme). The structures are aligned on IsdHN2. DynDom analysis reveal that the two structures are related by a 5° rotation of the IsdHlinker and IsdHN3. The axis of rotation is marked by a black rod. Residues that are rotated as a rigid body are shown in light blue, residues in a fixed position are shown in red, and flexible residues are shown in green. B, interface between the αHb F-helix and IsdHlinker in the structure of Hb–IsdHN2-N3 (PDB entry 4XS0) (9). C, interface between the αHb F-helix and IsdHlinker in the structure of HpSP–αβHb–IsdHN2-N3. D, structural comparison of αHb–IsdHN2-N3 and βHb–IsdHN2-N3 in the structure of HpSP–αβHb–IsdHN2-N3. The structures are aligned on IsdHN2. The globin chains of αHb and βHb are structurally distinct, which is emphasized by differences in the position of the F-helix. In βHb, the position of the F-helix is closer to the IsdHlinker. E, interactions between βHb and IsdHlinker. In contrast to αHb, we observe direct interactions between IsdHlinker and the βHb F-helix. Hydrogen bond and salt bridges are shown as dashed black lines.
The previous structure of IsdHN2-N3 in complex with αHb indicates that IsdH promotes the release of heme from αHb by partially unfolding the F-helix (Fig. 4B) (37). This unfolding is suspected to be induced by interactions between the IsdHlinker and the αHb F-helix, which presumably lowers the αHb affinity for heme and allows it to be transferred from αHb to IsdHN3. A similar unfolding of the αHb F-helix was recently reported in the complex between IsdB and αHb, suggesting that the two Hb receptors extract heme using similar mechanisms (8). In the structure of HpSP–αβHb–IsdHN2-N3, we do not observe a partially unfolded αHb F-helix, and the IsdHlinker does not engage in interactions with αHb because of its outwards rotated conformation (Fig. 4C). This suggests that Hp via its interaction with αHb prevents interactions between the IsdHlinker and αHb and concomitant unfolding of the αHb F-helix, which again explains the inhibitory effect of Hp binding.
Although structurally similar, the globin chains of αHb and βHb are distinct. This is emphasized in Fig. 4D, showing the two globin chains aligned on the IsdHN2-binding region. Here, the βHb F-helix is naturally positioned closer to the IsdHlinker compared with the αHb F-helix. This allows the Tyr-495 from the IsdHlinker to form a hydrogen bond with βHb Glu-90, even though IsdH is in an outwards rotated conformation. A similar interaction is suspected to cause unfolding of the αHb F-helix in the uninhibited IsdH–Hb complex (Fig. 4B). However, despite the interaction between the IsdHlinker and βHb, no unfolding of the βHb F-helix has occurred in the Hp-inhibited complex (Fig. 4E). Unbiased omit maps of the αHb and βHb F-helices are shown in Fig. S3, confirming the structural observations described above.
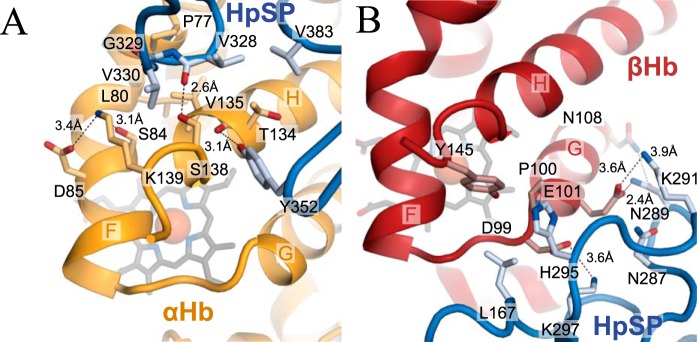
HpSP forms extensive interactions with both subunits of αβHb, in particular around the heme-binding pockets (26, 36, 40). At αHb, HpSP interacts directly with the F-helix through contacts between HpSP residues Gly-329 and Val-330 and αHb residues Pro-77 and Leu-80 (Fig. 5A). In addition, HpSP engages in multiple interactions with the αHb H-helix, which in turn is tightly linked to the F-helix. These interactions most likely prevent the F-helix from unfolding and interacting with the IsdHlinker whereby the heme transfer to IsdHN3 is inhibited. HpSP forms multiple interactions with the βHb FG-corner and G-helix (Fig. 5B), which probably leads to a general stabilization of the heme-binding pocket and inhibition of heme transfer to IsdHN3.
Figure 5.
HpSP interactions with the αHb and βHb heme-binding pockets. A and B, selected interactions between HpSP and the regions surrounding the heme-binding pockets of αHb (A) and βHb (B) in the structure of HpSP–αβHb-IsdhN2-N3. Hydrogen bond and salt bridges are shown as dashed black lines.
The βHb heme iron is partially coordinated by the distal histidine
Well-defined electron density for the heme group is observed at the αHb heme-binding pocket, with the heme iron coordinated by the proximal histidine residue (Fig. 6A). At βHb, the electron density for the heme group is less well-defined (Fig. 6B). Electron density is observed close to both the proximal and distal histidine residues, indicating two alternative positions of the heme group. One is the canonical position where the heme iron is coordinated by the proximal histidine. In the second position the heme group has moved ∼6 Å out of the heme-binding pocket and is now coordinated by the distal histidine residue. This novel heme binding could represent an intermediate step in the transfer of heme from the proximal histidine of Hb to Tyr-642 of IsdHN3.
Figure 6.
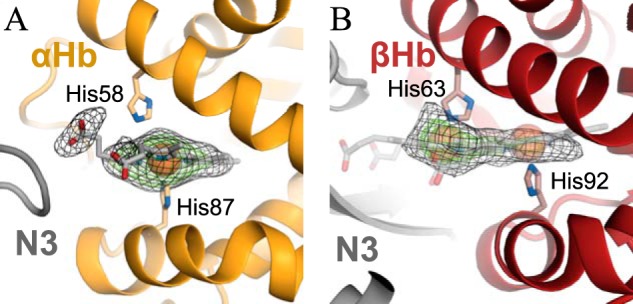
Heme-binding sites in HpSP–αβHb–IsdHN2-N3. A and B, Electron density maps (mFo − DFc, heme omit maps contoured at 2.5 σ (black mesh) and 5.0 σ (green mesh)) of the heme-groups bound to αHb (A) and βHb (B). Well-defined electron density for the heme-group is observed at αHb at the canonical site with the heme iron coordinated by the proximal histidine (His-87). At βHb, the electron density is not well-defined and indicates that the heme group can be in two alternative positions, either at the canonical site or in a novel position with the heme iron coordinated by the distal histidine (His-63).
Discussion
Hp is well-known for its important function in clearing of toxic Hb escaped from red blood cells. Here, we show that Hp likely serves an additional role in nutritional immunity, by limiting access of iron to bacterial pathogens. This is achieved by tightly binding Hb and preventing the S. aureus Hb receptors IsdH and IsdB from extracting the iron-containing heme. Hp is present in human serum at high concentrations (41), and under normal conditions the vast majority of serum Hb will be complexed by Hp, making Hp–Hb complexes the largest source of iron for infecting microorganisms. Whether S. aureus is able to grow on Hp–Hb as the only source of iron appears to be controversial, and studies with conflicting results have been published (8, 38, 42). In the present paper we show that although Hp is a potent inhibitor of IsdH-mediated heme extraction, relatively low levels of extraction from Hp–Hb complexes is observed, which could explain the discrepancies in the literature. Hence, at high Hp–Hb concentrations, the less efficient iron acquisition from Hp–Hb could be sufficient for S. aureus growth, whereas at low Hp–Hb concentrations, the iron acquisition will not meet the demands of the bacteria. Consequently, the protective effect of Hp against invading S. aureus is probably largest during the early stages of infection where serum concentrations of Hp–Hb are low. As the infection progresses into the hemolytic stage, the concentration of Hp–Hb in serum will rise because of the activity of α-hemolysin, and the pool of globin-bound iron available to the bacteria increases. In addition, Hp may become saturated with Hb, granting IsdH and IsdB easy access to heme from free Hb.
IsdH and IsdB are structurally and functionally homologous but differ in the number of NEAT domains. The additional NEAT domain IsdHN1 binds αHb at the same site as IsdHN2 but does not bind βHb (43). IsdHN1 and IsdHN2 are separated by a linker region constituted by 93 residues with a high percentage of hydrophilic residues (76%). Secondary structure prediction indicates that the linker is devoid of any secondary structure elements and likely has a random coil structure (Fig. S4) (53). In principle, this can permit a spatial separation of IsdHN1 and IsdHN2 by up to 300 Å and potentially allow IsdHN1 to extend above the peptidoglycan layer on the surface of the bacteria. Hence, a proposed function of IsdHN1 is to engage in the initial binding of Hb (or Hp–Hb) and deliver it to IsdHN2-N3 for subsequent heme extraction (19). Another possible function of IsdHN1 is to prevent the bacteria from being engulfed by host macrophages. The binding of the multimeric Hp–Hb complexes on the surface of the bacteria is a potential risk because Hp–Hb also is a substrate for the macrophage-specific receptor CD163. We recently showed that IsdHN1 inhibits CD163-mediated uptake of Hp–Hb by sterically blocking the CD163-binding site on Hp–Hb (39). Considering the length of the IsdHN1–IsdHN2 linker, it is possible for IsdHN2-N3 to extract heme from one site of multimeric Hp–Hb, whereas IsdHN1 blocks CD163 from binding to the other sites (Fig. 7). The inhibition of IsdH-mediated Hb heme extraction by Hp and the blocking of CD163-mediated clearance of Hp–Hb by IsdH are both examples of evolutionary adaptations in the battle for iron between bacterial pathogens and their vertebrate hosts.
Figure 7.
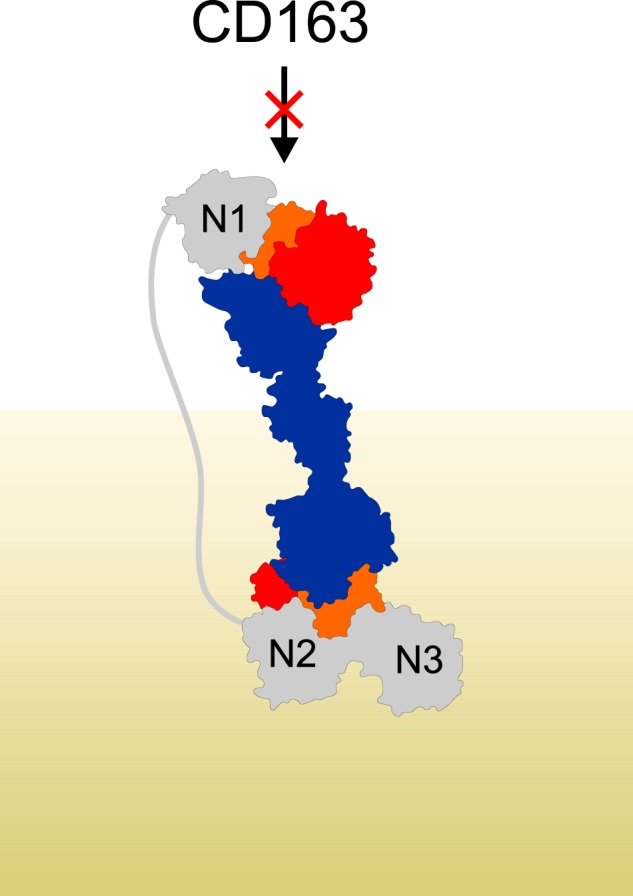
IsdHN1 blocks interaction between Hp–Hb and CD163. Shown is a model of IsdHN1-N3 binding to Hp–Hb. The model was generated based on the structures of HpSP–αβHb–IsdHN2-N3 and Hp–Hb–IsdHN1 (PDB entry 4WJG) (36). The distance between IsdHN1 C terminus and IsdHN2 N terminus is ∼180 Å. IsdH is shown in gray, Hp is in blue, αHb is in orange, and βHb is in red. The peptidoglycan layer on the surface of the bacterium is indicated in khaki.
Because the globin chains of αHb and βHb are structurally different, so are the environments of their respective heme groups (44). This nonequivalence leads to differences in both redox properties and heme affinities (45), with βHb being more prone to oxidation and with lower heme affinity compared with αHb. Correspondingly, the mechanisms of heme extraction by IsdH and IsdB could also be different between the two Hb subunits. Previous structural analysis has not provided any insight into the mechanism of heme extraction from βHb. Hence, it is not known whether a partial unfolding of the βHb F-helix is required for heme transfer as suggested for αHb. The facts that βHb has lower heme affinity compared with αHb and that we observe a partial transfer of heme from βHb without unfolding of the F-helix suggest that the unfolding may not be required for heme transfer from βHb. Also, the F-helix of βHb is naturally positioned closer to the IsdHlinker, and interaction between the two is observed without distortion of the βHb structure. Therefore, it is possible that IsdH actively promotes the transfer of heme from αHb by inducing structural changes in the globin chain that lowers its heme affinity, whereas the transfer from βHb to IsdH may have a more or less passive character.
The inhibitory effect of Hp on IsdH-mediated heme extraction from αHb most likely stems from Hp engaging in interactions with the αHb F-helix and H-helix and thereby preventing the partial unfolding of the αHb F-helix and interactions with the IsdHlinker. The inhibitory effect of Hp on βHb is less clear from the structural data, although Hp interacts with regions around the heme-binding pocket. Because we observe a partial dissociation of heme from the proximal histidine, the extraction of heme from βHb appears not to be as strongly inhibited as αHb. Hence, the low level of extraction of heme by IsdH from Hp–Hb probably stems from βHb.
In the structure of HpSP–αβHb–IsdHN2-N3, we observe a novel intermediate state in heme transfer, where the βHb heme group has moved ∼6 Å out of the heme-binding pocket and is coordinated by the distal histidine (His-63). A different intermediate state is observed in the structure of IsdB–Hb (8). Here, the αHb heme is coordinated by both the distal histidine (His-58) and His-89 from the unfolded F-helix. A similar bis-His state does not seem possible in βHb, because the residue corresponding to His-89 is an aspartate in βHb (Asp-94). Thus, the extraction of heme from the respective αHb and βHb subunits likely occurs via different intermediate states.
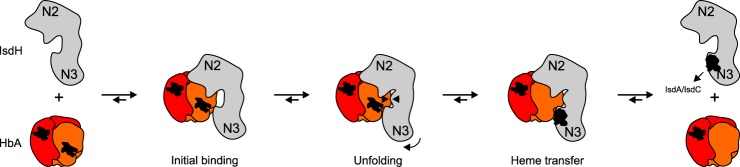
In complex with HpSP–αβHb, IsdHN2-N3 adopts a novel and outwards rotated conformation, which could represent a state of IsdHN2-N3 after Hb binding but prior to heme transfer. Consequently, the structure may provide new information on the mechanism of IsdH-mediated Hb heme extraction. Based on this novel structural information and previous structures of Hb–IsdHN2-N3, we propose a preliminary model of the mechanism, in which IsdHN2-N3 functions as a “magnet on a spring” to promote unfolding of the Hb F-helix (Fig. 8). After binding to Hb, there is a potential electrostatic attraction between the IsdHlinker and Hb F-helix. Because of tension within IsdHN2 and possibly also repulsion between IsdHN3 and Hb, the IsdHlinker is likely prevented from moving all the way to the Hb F-helix. Instead, the IsdHlinker and F-helix meet halfway, which causes unfolding of the F-helix and allows the heme to be transferred to IsdHN3. After heme transfer, IsdHN2-N3 is released from Hb and can transfer the heme to either IsdC or IsdA.
Figure 8.
Suggested mechanism of IsdH mediated heme extraction. The structure of HpSP–αβHb–IsdHN2-N3 possibly represents a novel conformational state of IsdHN2-N3 after Hb binding (initial binding) but prior to Hb unfolding and heme transfer. The proposed mechanism is obtained by combining our results with the previous structural analysis of Hb–IsdHN2-N3 (37).
Experimental procedures
Plasmids
We used a plasmid construct with IsdHN2-N3 (residues 321–655) cloned into the XhoI and BamHI sites of the pET15b vector (Novagen) (39). IsdHN2-N3 mutants were generated using the QuikChange site-directed mutagenesis kit (Agilent Technologies). IsdC (residues 29–192) was cloned into the NdeI/XhoI sites of pET15b. The serine protease domain of Hp (HpSP, residues 148–406) was cloned into the BamHI and XhoI sites of the mammalian expression vector pcDNA5/FRT (Thermo Fisher Scientific).
Cell lines
Flp-In CHO cells (Invitrogen) were maintained in HyClone serum-free medium for CHO cells (ThermoFisher Scientific). Stably transfected Flp-In CHO cells expressing Hp (residues 148–406) were established using the FuGENE 6 transfection reagent (Promega) and subsequent selection with 750 μg/ml hygromycin B (Invitrogen).
Production and purification of proteins
IsdHN2-N3 and IsdC constructs were transformed into the BL21 Escherichia coli strain. The cells were grown in LB growth medium with 50 μg/ml ampicillin, and protein expression was induced by the addition of 1 mm isopropyl β-d-thiogalactopyranoside when cultures had reached mid-log growth phase. After induction, the cultures were incubated overnight at 20 °C with continuous shaking at 200 rpm and harvested by centrifugation at 4,000 × g for 20 min. The cells were resuspended in lysis buffer (20 mm Tris-HCl, pH 7.5, 500 mm NaCl, and 20 mm imidazole) and disrupted by sonication (Branson Sonifier 250) followed by centrifugation at 25,000 × g for 30 min. The supernatant was applied to a HisTrap FF Crude column (GE Healthcare), and bound protein was eluted with a linear gradient from 20 to 500 mm imidazole in 20 mm Tris-HCl, pH 7.5, and 500 mm NaCl. As a final step of purification, IsdHN2-N3 and IsdC were purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 GL column (GE Healthcare) equilibrated in 50 mm Hepes, pH 7.5, and 100 mm NaCl.
Hb was obtained from red blood cells from a healthy donor as previously described (36). Briefly, blood cells harvested by centrifugation for 20 min at 4,000 g were lysed by the addition of water in a 1:1 water:red blood cells ratio. Cellular debris was removed by centrifugation at 8,000 × g for 15 min. Before storage at −20 C, the essentially pure Hb samples were dialyzed against 20 mm Hepes, pH 7.5, and 60 mm KCl.
Large-scale cultivation of Flp-In CHO cells expressing HpSP(N184S, N207S, N211S, N241S) was performed using a WAVE Bioreactor 2/10 system (GE Healthcare). For purification of secreted HpSP(N184S, N207S, N211S, N241S), the conditioned cell medium was adjusted to pH 7.5 with Tris-HCl and supplemented with 500 mm NaCl, before being applied to a cOmpleteTM His tag purification column (Roche). Bound protein was eluted with a linear gradient from 0 to 250 mm imidazole in 50 mm Hepes, pH 7.6, and 500 mm NaCl.
UV-visible analysis
Extraction or spontaneous dissociation of heme from metHb and from Hp–metHb was measured at 25 °C in the 350–450-nm range on a Cary 60 (Agilent Technologies, Glostrup, Denmark) UV-visible spectrophotometer. MetHb was produced by oxidation of oxygenated Hb using ferricyanide. Briefly, oxygenated Hb at 1 mm concentration was incubated in the dark at room temperature for 1 h with a 2-fold molar excess of potassium ferricyanide. To remove excess ferricyanide, metHb was exchanged into 50 mm Hepes, pH 7.5, and 100 mm NaCl on a PD-10 column (GE Healthcare).
For UV-visible analysis, IsdHN2-N3 and IsdC proteins were prepared in their apo form by acid-acetone extraction, based on the original protocol for production of apoHb (46). Briefly, IsdHN2-N3 and IsdC were dialyzed against Milli-Q water and added dropwise to 3 mm HCl in acetone at −20 °C with vigorous stirring. This was kept at −20 °C for 15 min before centrifugation at 500× g for 5 min. The supernatant was removed, and the colorless pellet was redissolved in Milli-Q water. Finally, apo IsdHN2-N3 and apo IsdC were purified using size-exclusion chromatography on a Superdex 200 Increase column (GE Healthcare) equilibrated in 50 mm Hepes, pH 7.5, and 100 mm NaCl.
Surface plasmon resonance
Surface plasmon resonance experiments were carried out using a protein G sensor chip on a Biacore 3000 instrument (GE Healthcare). A double capture experiment was developed as follows. Polyclonal antibody (15 μg/ml) against Hp (A0030, DAKO) was captured in flow cells 1 and 2 (FC1 and 2) to ∼4,000 response units. The system was allowed to stabilize before either Hp1-1 (Sigma–Aldrich) or recombinant HpSP (see above) was injected in FC2 only to a capture level of ∼50 response units. Next, a 2-fold serial dilution (200 nm to 1.56 nm) of metHb was injected in both flow cells. The association phase was 180 s, followed by a 900-s dissociation phase. At the end of each binding cycle, both surfaces were regenerated by a 60 s injection of 10 mm glycine, pH 1.5. All proteins were diluted in running buffer (10 mm HEPES, pH 7.5, 150 mm NaCl, and 0.05% Tween 20). A flow rate of 30 μl/min was used in all steps of the experiments. Binding analysis was performed at 25 °C, and the data were collected at a rate of 1 Hz. Recorded signals from FC2 were double referenced; the signal from the in-line reference flow cell (FC1) was subtracted, as was the signal from a blank run (0 nm analyte). A 1:1 Langmuir model was fitted to the data, using BIAevaluation 4.1.1 software (GE Healthcare). Although not usually recommended, Rmax values were fitted locally. However, this may be justified by the heterogeneous nature of the Hb analyte. In the range of concentrations examined here, Hb exists in concentration-dependent equilibria between tetrameric, dimeric, and monomeric species (47), of which only dimeric αβHb will bind.
Crystallization and data collection
Purified HpSP(N184S, N207S, N211S, N241S) was mixed with 2-fold excess of Hb and dialyzed overnight at 4 °C against buffer A (20 mm MES, 20 mm MOPS, 70 mm KCl, pH 6.1). The sample was centrifuged at 20,000 × g for 10 min, and the supernatant was applied to a 1-ml Source 15S column (GE Healthcare). Bound protein was eluted using a linear pH gradient between buffers A and B (20 mm MES, 20 mm MOPS, 60 mm KCl, pH 8.0). Fractions containing the HpSP–αβHb complex were pooled and incubated with a 2-fold molar excess of IsdHN2-N3(Y642A). The sample was concentrated using an Amicon Ultra centrifugal filter (10-kDa molecular mass cutoff; Millipore) and subjected to size-exclusion chromatography using a Superdex 200 Increase 10/300 GL column (GE Healthcare), equilibrated in 20 mm Tris, pH 7.6, and 75 mm KCl. Fractions containing the HpSP–αβHb–IsdHN2-N3(Y642A) complex were pooled and concentrated to 8 mg/ml using an Amicon Ultra centrifugal filter (10-kDa molecular mass cutoff; Millipore). The crystals were obtained in 0.2 m sodium citrate tribasic dihydrate, 20% (w/v) PEG 3350 using the sitting-drop vapor-diffusion method by mixing 2 μl of protein sample with 2 μl of reservoir solution. Prior to data collection, the crystals were transferred into cryoprotection buffer (0.2 m sodium citrate tribasic dihydrate, 25% PEG 3350, 15% glycerol) and flash-frozen in liquid nitrogen.
Structure determination and analysis
X-ray diffraction data extending to 2.9 Å resolution were collected at the P13 Beamline (DESY, Hamburg, Germany) using a wavelength of 1.0 Å at a temperature of 100 K. Diffraction data were processed and scaled using the XDS package (48). Initial phases were calculated by molecular replacement using PHASER (49) with the structures of human Hp–Hb (PDB accession 4WJG) (36) and S. aureus IsdHN2-N3 (PDB accession 4XS0) (9) as search models. The model was refined using iterative cycles of refinement in PHENIX (49) followed by manual model building in COOT (50). The figures were prepared with PyMOL (51), and IsdHN2-N3 domain movement analysis was done with the DynDom server (52).
Author contributions
J. H. M., K. R., and C. B. F. A. formal analysis; J. H. M., K. R., and C. B. F. A. investigation; J. H. M., K. R., and C. B. F. A. methodology; J. H. M. writing-original draft; C. B. F. A. supervision; C. B. F. A. funding acquisition; C. B. F. A. validation; C. B. F. A. project administration; C. B. F. A. writing-review and editing.
Supplementary Material
Acknowledgments
We thank Gitte Petersen Ratz for technical assistance and the staff at Deutsches Elektronen Synchrotron for help with data collection.
The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
The atomic coordinates and structure factors (code 6TB2) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- Hb
- hemoglobin
- Hp
- haptoglobin
- NEAT
- near iron transporter
- PDB
- Protein Data Bank
- Isd
- iron-regulated surface determinant.
References
- 1. Nairz M., Schroll A., Sonnweber T., and Weiss G. (2010) The struggle for iron: a metal at the host–pathogen interface. Cell. Microbiol. 12, 1691–1702 10.1111/j.1462-5822.2010.01529.x [DOI] [PubMed] [Google Scholar]
- 2. Conroy B. S., Grigg J. C., Kolesnikov M., Morales L. D., and Murphy M. E. P. (2019) Staphylococcus aureus heme and siderophore-iron acquisition pathways. Biometals 32, 409–424 10.1007/s10534-019-00188-2 [DOI] [PubMed] [Google Scholar]
- 3. Maresso A. W., and Schneewind O. (2006) Iron acquisition and transport in Staphylococcus aureus. Biometals 19, 193–203 10.1007/s10534-005-4863-7 [DOI] [PubMed] [Google Scholar]
- 4. Torres V. J., Attia A. S., Mason W. J., Hood M. I., Corbin B. D., Beasley F. C., Anderson K. L., Stauff D. L., McDonald W. H., Zimmerman L. J., Friedman D. B., Heinrichs D. E., Dunman P. M., and Skaar E. P. (2010) Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 78, 1618–1628 10.1128/IAI.01423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torres V. J., Pishchany G., Humayun M., Schneewind O., and Skaar E. P. (2006) Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 188, 8421–8429 10.1128/JB.01335-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dryla A., Hoffmann B., Gelbmann D., Giefing C., Hanner M., Meinke A., Anderson A. S., Koppensteiner W., Konrat R., von Gabain A., and Nagy E. (2007) High-affinity binding of the staphylococcal HarA protein to haptoglobin and hemoglobin involves a domain with an antiparallel eight-stranded β-barrel fold. J. Bacteriol. 189, 254–264 10.1128/JB.01366-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilpa R. M., Robson S. A., Villareal V. A., Wong M. L., Phillips M., and Clubb R. T. (2009) Functionally distinct NEAT (NEAr Transporter) domains within the Staphylococcus aureus IsdH/HarA protein extract heme from methemoglobin. J. Biol. Chem. 284, 1166–1176 10.1074/jbc.M806007200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowden C. F. M., Chan A. C. K., Li E. J. W., Arrieta A. L., Eltis L. D., and Murphy M. E. P. (2018) Structure-function analyses reveal key features in Staphylococcus aureus IsdB-associated unfolding of the heme-binding pocket of human hemoglobin. J. Biol. Chem. 293, 177–190 10.1074/jbc.M117.806562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickson C. F., Kumar K. K., Jacques D. A., Malmirchegini G. R., Spirig T., Mackay J. P., Clubb R. T., Guss J. M., and Gell D. A. (2014) Structure of the hemoglobin–IsdH complex reveals the molecular basis of iron capture by Staphylococcus aureus. J. Biol. Chem. 289, 6728–6738 10.1074/jbc.M113.545566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazmanian S. K., Ton-That H., Su K., and Schneewind O. (2002) An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 2293–2298 10.1073/pnas.032523999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu H., Xie G., Liu M., Olson J. S., Fabian M., Dooley D. M., and Lei B. (2008) Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J. Biol. Chem. 283, 18450–18460 10.1074/jbc.M801466200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muryoi N., Tiedemann M. T., Pluym M., Cheung J., Heinrichs D. E., and Stillman M. J. (2008) Demonstration of the iron-regulated surface determinant (Isd) heme transfer pathway in Staphylococcus aureus. J. Biol. Chem. 283, 28125–28136 10.1074/jbc.M802171200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pluym M., Vermeiren C. L., Mack J., Heinrichs D. E., and Stillman M. J. (2007) Heme binding properties of Staphylococcus aureus IsdE. Biochemistry 46, 12777–12787 10.1021/bi7009585 [DOI] [PubMed] [Google Scholar]
- 14. Mazmanian S. K., Skaar E. P., Gaspar A. H., Humayun M., Gornicki P., Jelenska J., Joachmiak A., Missiakas D. M., and Schneewind O. (2003) Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299, 906–909 10.1126/science.1081147 [DOI] [PubMed] [Google Scholar]
- 15. Wu R., Skaar E. P., Zhang R., Joachimiak G., Gornicki P., Schneewind O., and Joachimiak A. (2005) Staphylococcus aureus IsdG and IsdI, heme-degrading enzymes with structural similarity to monooxygenases. J. Biol. Chem. 280, 2840–2846 10.1074/jbc.M409526200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrade M. A., Ciccarelli F. D., Perez-Iratxeta C., and Bork P. (2002) NEAT: a domain duplicated in genes near the components of a putative Fe3+ siderophore transporter from Gram-positive pathogenic bacteria. Genome Biol. 3, 47 10.1186/gb-2002-3-9-research0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilpa R. M., Fadeev E. A., Villareal V. A., Wong M. L., Phillips M., and Clubb R. T. (2006) Solution structure of the NEAT (NEAr Transporter) domain from IsdH/HarA: the human hemoglobin receptor in Staphylococcus aureus. J. Mol. Biol. 360, 435–447 10.1016/j.jmb.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 18. Watanabe M., Tanaka Y., Suenaga A., Kuroda M., Yao M., Watanabe N., Arisaka F., Ohta T., Tanaka I., and Tsumoto K. (2008) Structural basis for multimeric heme complexation through a specific protein-heme interaction: the case of the third neat domain of IsdH from Staphylococcus aureus. J. Biol. Chem. 283, 28649–28659 10.1074/jbc.M803383200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spirig T., Malmirchegini G. R., Zhang J., Robson S. A., Sjodt M., Liu M., Krishna Kumar K., Dickson C. F., Gell D. A., Lei B., Loo J. A., and Clubb R. T. (2013) Staphylococcus aureus uses a novel multidomain receptor to break apart human hemoglobin and steal its heme. J. Biol. Chem. 288, 1065–1078 10.1074/jbc.M112.419119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinberg E. D. (1975) Nutritional immunity: host's attempt to withold iron from microbial invaders. JAMA 231, 39–41 10.1001/jama.231.1.39 [DOI] [PubMed] [Google Scholar]
- 21. Hood M. I., and Skaar E. P. (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Silva D. M., Askwith C. C., and Kaplan J. (1996) Molecular mechanisms of iron uptake in eukaryotes. Physiol. Rev. 76, 31–47 10.1152/physrev.1996.76.1.31 [DOI] [PubMed] [Google Scholar]
- 23. Hvidberg V., Maniecki M. B., Jacobsen C., Højrup P., Møller H. J., and Moestrup S. K. (2005) Identification of the receptor scavenging hemopexin-heme complexes. Blood 106, 2572–2579 10.1182/blood-2005-03-1185 [DOI] [PubMed] [Google Scholar]
- 24. Shim B. S., Lee T. H., and Kang Y. S. (1965) Immunological and biochemical investigations of human serum haptoglobin: composition of haptoglobin–haemoglobin intermediate, haemoglobin-binding sites and presence of additional alleles for β-chain. Nature 207, 1264–1267 10.1038/2071264a0 [DOI] [PubMed] [Google Scholar]
- 25. Kristiansen M., Graversen J. H., Jacobsen C., Sonne O., Hoffman H. J., Law S. K., and Moestrup S. K. (2001) Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 10.1038/35051594 [DOI] [PubMed] [Google Scholar]
- 26. Andersen C. B., Torvund-Jensen M., Nielsen M. J., de Oliveira C. L., Hersleth H.-P., Andersen N. H., Pedersen J. S., Andersen G. R., and Moestrup S. K. (2012) Structure of the haptoglobin–haemoglobin complex. Nature. 489, 456–459 10.1038/nature11369 [DOI] [PubMed] [Google Scholar]
- 27. Smithies O., and Walker N. F. (1956) Notation for serum-protein groups and the genes controlling their inheritance. Nature 178, 694–695 10.1038/178694a0 [DOI] [PubMed] [Google Scholar]
- 28. Maeda N., Yang F., Barnett D. R., Bowman B. H., and Smithies O. (1984) Duplication within the haptoglobin Hp2 gene. Nature 309, 131–135 10.1038/309131a0 [DOI] [PubMed] [Google Scholar]
- 29. Lim S. K., Kim H., Lim S. K., bin Ali A., Lim Y. K., Wang Y., Chong S. M., Costantini F., and Baumman H. (1998) Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92, 1870–1877 10.1182/blood.V92.6.1870.418k38_1870_1877 [DOI] [PubMed] [Google Scholar]
- 30. Fagoonee S., Gburek J., Hirsch E., Marro S., Moestrup S. K., Laurberg J. M., Christensen E. I., Silengo L., Altruda F., and Tolosano E. (2005) Plasma protein haptoglobin modulates renal iron loading. Am. J. Pathol. 166, 973–983 10.1016/S0002-9440(10)62319-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sjodt M., Macdonald R., Marshall J. D., Clayton J., Olson J. S., Phillips M., Gell D. A., Wereszczynski J., and Clubb R. T. (2018) Energetics underlying hemin extraction from human hemoglobin by Staphylococcus aureus. J. Biol. Chem. 293, 6942–6957 10.1074/jbc.RA117.000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiancone E., Alfsen A., Ioppolo C., Vecchini P., Agrò A. F., Wyman J., Antonini E. (1968) Studies on the reaction of haptoglobin with haemoglobin and haemoglobin chains: I. Stoichiometry and affinity. J. Mol. Biol. 34, 347–356 10.1016/0022-2836(68)90258-1 [DOI] [PubMed] [Google Scholar]
- 33. Hwang P. K., and Greer J. (1980) Interaction between hemoglobin subunits in the hemoglobin·haptoglobin complex. J. Biol. Chem. 255, 3038–3041 [PubMed] [Google Scholar]
- 34. Adams E. C., and Weiss M. R. (1969) Calorimetric studies of the haemoglobin–haptoglobin reaction. Biochem. J. 115, 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bunn H. F., and Jandl J. H. (1968) Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 243, 465–475 [PubMed] [Google Scholar]
- 36. Stødkilde K., Torvund-Jensen M., Moestrup S. K., and Andersen C. B. (2014) Structural basis for trypanosomal haem acquisition and susceptibility to the host innate immune system. Nat Commun. 5, 5487 10.1038/ncomms6487 [DOI] [PubMed] [Google Scholar]
- 37. Dickson C. F., Jacques D. A., Clubb R. T., Guss J. M., and Gell D. A. (2015) The structure of haemoglobin bound to the haemoglobin receptor IsdH from Staphylococcus aureus shows disruption of the native α-globin haem pocket. Acta Crystallogr. D Biol. Crystallogr. 71, 1295–1306 10.1107/S1399004715005817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dryla A., Gelbmann D., von Gabain A, Nagy E. (2003) Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 49, 37–53 10.1046/j.1365-2958.2003.03542.x [DOI] [PubMed] [Google Scholar]
- 39. Sæderup K. L., Stødkilde K., Graversen J. H., Dickson C. F., Etzerodt A., Hansen S. W., Fago A., Gell D., Andersen C. B., and Moestrup S. K. (2016) The Staphylococcus aureus protein IsdH inhibits host hemoglobin scavenging to promote heme acquisition by the pathogen. J. Biol. Chem. 291, 23989–23998 10.1074/jbc.M116.755934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lane-Serff H., MacGregor P., Lowe E. D., Carrington M., and Higgins M. K. (2014) Structural basis for ligand and innate immunity factor uptake by the trypanosome haptoglobin-haemoglobin receptor. Elife 3, e05553 10.7554/eLife.05553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Katnik I., and Jadach J. (1996) Haptoglobin concentration in serum and other body fluids measured by comparison of its reactivity with hemoglobin and concanavalin A. Arch. Immunol. Ther. Exp. (Warsz.) 44, 45–50 [PubMed] [Google Scholar]
- 42. Francis R. T. Jr., Booth J. W., and Becker R. R. (1985) Uptake of iron from hemoglobin and the haptoglobin-hemoglobin complex by hemolytic bacteria. Int. J. Biochem. 17, 767–773 10.1016/0020-711X(85)90262-9 [DOI] [PubMed] [Google Scholar]
- 43. Krishna Kumar K., Jacques D. A., Pishchany G., Caradoc-Davies T., Spirig T., Malmirchegini G. R., Langley D. B., Dickson C. F., Mackay J. P., Clubb R. T., Skaar E. P., Guss J. M., and Gell D. A. (2011) Structural basis for hemoglobin capture by Staphylococcus aureus cell-surface protein, IsdH. J. Biol. Chem. 286, 38439–38447 10.1074/jbc.M111.287300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bolton W., and Perutz M. F. (1970) Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature 228, 551–552 10.1038/228551a0 [DOI] [PubMed] [Google Scholar]
- 45. Banerjee R., and Lhoste J. M. (1976) Non-equivalence of human hemoglobin chains in the oxidation-reduction and heme-transfer reactions: a 13C nuclear-Magnetic-resonance study. Eur. J. Biochem. 67, 349–356 10.1111/j.1432-1033.1976.tb10698.x [DOI] [PubMed] [Google Scholar]
- 46. Fanelli A. R., Antonini E., and Caputo A. (1958) Studies on the structure of hemoglobin: I. Physicochemical properties of human globin. Biochim. Biophys. Acta 30, 608–615 10.1016/0006-3002(58)90108-2 [DOI] [PubMed] [Google Scholar]
- 47. Chiancone E. (1968) Dissociation of hemoglobin into subunits: II. Human oxyhemoglobin: gel filtration studies. J. Biol. Chem. 243, 1212–1219 [PubMed] [Google Scholar]
- 48. Kabsch W. (2010) XDS. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 51. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schroedinger, LLC, New York [Google Scholar]
- 52. Poornam G. P., Matsumoto A., Ishida H., and Hayward S. (2009) A method for the analysis of domain movements in large biomolecular complexes. Proteins 76, 201–212 10.1002/prot.22339 [DOI] [PubMed] [Google Scholar]
- 53. Drozdetskiy A., Cole C., Procter J., and Barton G. J. (2015) JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 43, W389–W394 10.1093/nar/gkv332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.