Abstract
Background
Although oral food challenge (OFC) is an important clinical procedure for diagnosing food allergy, there is a paucity of literature on the outcome of the procedure and specifically the patients on whom the procedure is performed from the aspects of their age, sex, race/ethnicity, health insurance status, and serum specific IgE to the food tested.
Objective
We aimed to review results of OFC and determine the impact of patient age, sex, race/ethnicity, insurance status, private or public, and food specific serum IgE on the outcome of OFC.
Methods
A retrospective chart review was performed of patients undergoing OFCs at a children's hospital outpatient allergy clinic over a two-year period. The outcome of OFC was allergic or non-allergic based on determination and documentation by the treating physician. A logistic regression model was built to determine the association between the OFC outcomes, age, and symptoms at the time of OFC. A Chi-square analysis was performed to check for any significant relationship between the OFC outcome and age when stratified by insurance status.
Results
Five hundred and eight children underwent 641 OFCs. Twenty nine percent of OFCs had an allergic outcome with the most commonly challenged foods being peanuts, eggs, and milk. Patient age and gender, when stratified by insurance status, did not have a significant effect on OFC outcomes. Serum IgE to peanuts and egg was significantly different between allergic OFC and non-allergic outcome. Vomiting and urticaria/angioedema correlated with an allergic OFC outcome.
Conclusion
OFCs confirm the food allergy diagnosis in about one-third of patients tested, and they should continue to be used when possible for an accurate diagnosis. Age, sex, and insurance status do not have a significant association with the outcome of OFC and cannot be added as predictive factors.
Background
The prevalence of childhood food allergy within the United States is approximately 8%, and among these individuals, 38.7% have a history of severe reactions.1 Immunoglobulin E- (IgE) mediated food reactions typically occur within 2 hours of consumption and may involve the skin, gastrointestinal, respiratory, and cardiovascular systems.2 The clinical history is a cornerstone of the diagnosis of food allergy and can be supported to some extent with skin prick testing and food-specific serum IgE testing. Oral food challenge (OFC) is a clinical procedure that is performed, when possible, to confirm or refute the diagnosis of food allergy. Because OFC involves feeding the patient the food they may be allergic to, it carries the risk of an allergic reaction. OFC is thus a procedure that patients and health care providers might be hesitant to undertake.3, 4, 5 This may result in a delay in performing an OFC, reasoning that the older the patient, the more likely they are to be able to express symptoms of a start of a reaction. There may also be differences in the racial and socioeconomic class attitudes towards risk taking, especially when there is an alternative of continuing to avoid the food.
Scant data exist regarding the influence of patient factors on food allergy and its diagnosis.6,7 Regarding factors associated with the development of food allergy or allergic diseases, low socioeconomic class seemed to be protective in a Canadian study.8 A United States based study found higher odds of food allergy in African American and Asian children compared to Caucasian children, but lower odds of having the allergy diagnosed. The study found that those with food allergies from a lower socioeconomic class are at a disadvantage when it comes to provision of care.1,8,9 Racial differences were reported among Caucasians, African Americans, and Hispanics in the prevalence of food allergy, the food allergens, and the co-morbidities of food allergy, with the latter ethnic groups showing shorter periods of follow up by their allergists.10 A recent study examined the epidemiology of multicenter clinic-based OFCs and found that anaphylaxis occurrence was higher in the Northeast, Midwest, and North Midwest regions of the United States, and that males were more likely than females to have anaphylaxis.11 In the United States, insurance type, Medicaid vs non-Medicaid, distinguishes low vs high socioeconomic class. Medicaid is an insurance that is provided by state governments to adults and children with low incomes and limited resources. The term non-Medicaid lumps commercial insurances that are carried by parents who are employed or pay for the insurance by other means. Thus, children insured by Medicaid would be of low socioeconomic class, while children who are insured by a non-Medicaid insurance would be of a higher socioeconomic class. Given the previously listed factors that may impact the outcome of OFC, we examined the outcome of OFC conducted in our outpatient clinics in the population as stratified by race/ethnicity, insurance type, age, and gender and the effect of food specific serum IgE.
Methods
Data collection
We conducted a retrospective chart review on patients who had OFCs at our allergy and immunology outpatient clinics from December 2013 through November 2015. Institutional Review Board (IRB) approval was obtained prior to chart review. OFCs that were part of a desensitization study and those that did not have the outcome clearly determined were excluded; all other OFCs were included. The following data were extracted: patient race/ethnicity, sex, age at OFC, insurance payor whether medicaid or non-medicaid, zip code, foods tested in the OFCs, outcomes of the OFCs as documented by a physician, symptoms documented during OFCs, and serum food specific IgE results dating back up to a year prior to the date of OFC. Age at OFC was stratified into 4 groups: 0–5, 6–10, 11–15, and 16 years and above.
Statistical analysis
Statistical analysis was performed using SAS 9.3 (SAS Institute, Cary, North Carolina). Descriptive statistics were performed to analyze the outcome of OFCs by patient age, sex, race/ethnicity, and type of food challenged. A logistic regression model was used to determine if age at OFC, insurance type, symptoms at OFC, and food specific serum IgE results were associated with OFC outcome. A Chi-Square test in SAS was performed to determine any associations. Food specific serum IgE results lower than 0.35 kUA/L were replaced by the level of detection/√2 and results ≥100 kUA/L were converted to 101.12,13 Food specific serum IgE levels were not normally distributed and were therefore compared using Mann-Whitney U test.
Results
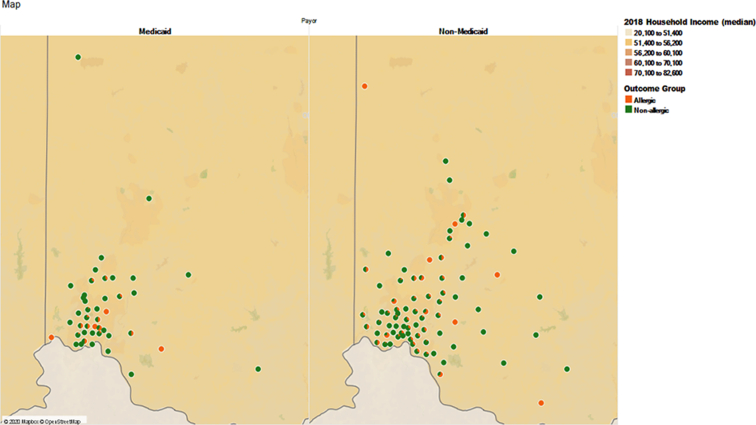
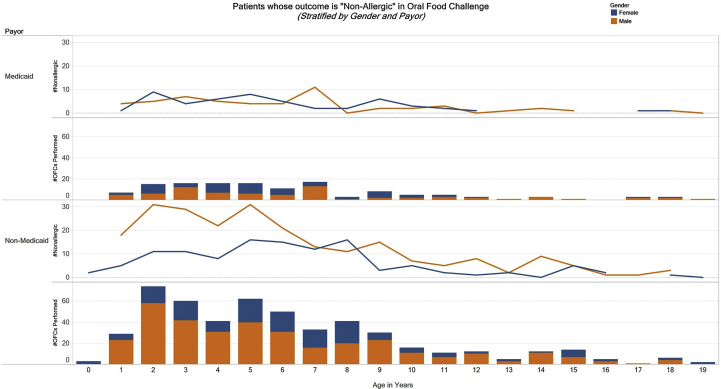
A total of 641 OFCs performed during the period of study were included. They were performed on 508 children with ages ranging from 0 to 19 years old. All OFCs were performed as outpatient procedures and were open challenges. Zip code distribution showed that Medicaid patients were concentrated around the medical center, while non-medicaid patients came from a wider area including the suburbs (Fig. 1). Of note, our academic center provides health care to all children regardless of insurance status and their ability to pay for medical care, so there is no population of children that does not have access to medical care. When the data was stratified by age group, sex and insurance status, the majority of the OFCs (30.3%) were performed on non-medicaid males in the age group 0–5 years (Table 1a). Out of the 641 challenges, 188 (29%) were designated as patient is allergic to the challenge food, and the rest were non-allergic (Fig. 2). A majority of the patients tested (76%) were Non-Hispanic and Caucasian (Table 1b). The most common food allergens tested by OFC were peanut (32%), egg (29%), and milk (13%) followed by soy and mixed nuts (4% each). These foods accounted for 82% of the OFCs performed. The remainder were OFCs to individual tree nuts, fruits, wheat, fish, shellfish, sunflower, sesame, oats, poultry, coconut, beef, pork, peas, orange, corn, and chocolate (Table 2). Analysis of median specific IgE level (most recent value collected up to 1 year prior to OFC) of peanut, egg, and milk showed that median specific IgE associated with allergic OFC outcomes in all 3 food allergens was higher than that associated with non-allergic outcomes, though only the data for peanut and egg were statistically significant (Table 3).
Fig. 1.
Map of zip codes of patients who had OFCs divided by insurance type: Medicaid and non-Medicaid
Table 1a.
Number of oral food challenges stratified by age, sex and insurance type
| Age Group | Gender | Insurance Type | Number of OFCs | % OFCs | Median age in years | Inter-Quartile range |
|---|---|---|---|---|---|---|
| 0–5yrs | Female | Medicaid | 34 | 5.3% | 4 | 2–5 |
| Non-Medicaid | 75 | 11.7% | 3 | 2–5 | ||
| Male | Medicaid | 36 | 5.6% | 3 | 2–4 | |
| Non-Medicaid | 194 | 30.3% | 3 | 2–4 | ||
| 6–10 yrs | Female | Medicaid | 21 | 3.3% | 8 | 6–9 |
| Non-Medicaid | 69 | 10.8% | 7 | 6–8 | ||
| Male | Medicaid | 23 | 3.6% | 7 | 7–7 | |
| Non-Medicaid | 101 | 15.8% | 8 | 6–9 | ||
| 11–15 yrs | Female | Medicaid | 3 | 0.5% | 11 | 11–12 |
| Non-Medicaid | 16 | 2.5% | 13.5 | 11.5–15 | ||
| Male | Medicaid | 10 | 1.6% | 12.5 | 11–14 | |
| Non-Medicaid | 38 | 5.9% | 13 | 12–14 | ||
| 16 & Above | Female | Medicaid | 2 | 0.3% | 17.5 | 17–18 |
| Non-Medicaid | 6 | 0.9% | 18 | 16–18 | ||
| Male | Medicaid | 5 | 0.8% | 18 | 17–18 | |
| Non-Medicaid | 8 | 1.2% | 17.5 | 16–18 |
Fig. 2.
Outcome of food challenges as non-allergic by age, sex and insurance type as Medicaid or non-Medicaid
Table 1b.
Number of oral food challenges stratified by ethnicity and race
| Ethnicity | Race | Number of OFCs (n) | Percent of all OFCs |
|---|---|---|---|
| Hispanic | White or Caucasian | 5 | 0.8% |
| Other | 22 | 3.4% | |
| Asian | 1 | 2.0% | |
| Non-Hispanic | White or Caucasian | 487 | 76.1% |
| Black or African American | 82 | 12.8% | |
| Other | 12 | 1.9% | |
| Asian | 20 | 3.1% | |
| Unknown | 4 | 0.6% | |
| Unknown | White or Caucasian | 3 | 0.5% |
| Other | 2 | 0.3% | |
| Unknown | 2 | 0.3% |
OFC = oral food challenge
Table 2.
Food allergens tested by an oral food challenge and outcome
| Type of Food | Performed OFC (n) | Percent Allergic |
|---|---|---|
| Peanut | 200 | 35% |
| Egg | 181 | 33% |
| Milk | 81 | 32% |
| Mixed nuts | 23 | 39% |
| Soy | 22 | 14% |
| Almond | 18 | 6% |
| Fruit | 12 | 8% |
| Wheat | 11 | 27% |
| Fish | 10 | 10% |
| Walnut | 9 | 56% |
| Hazelnut | 6 | 0% |
| Cashew | 6 | 50% |
| Shellfish | 6 | 0% |
| Pistachio | 6 | 17% |
| Pecan | 3 | 33% |
| Sesame | 3 | 0% |
| Chicken | 3 | 0% |
| Sunflower | 3 | 0% |
| Oat | 3 | 0% |
| Turkey | 2 | 50% |
| Coconut | 2 | 0% |
| Beef | 2 | 0% |
| Pork | 1 | 0% |
| Chocolate | 1 | 0% |
| Brazil Nut | 1 | 0% |
| Corn | 1 | 0% |
| Orange | 1 | 0% |
| Pea | 1 | 0% |
| Other | 23 | 17% |
Table 3.
OFC outcome and serum specific IgE levels for peanut, egg, and milk
| % Non-allergic OFC | Median IgE Level in KAU/L, Non-allergic OFCs | Interquartile Range, Non-allergic OFCs | Median IgE level in KAU/L, Allergic OFCs | Interquartile Range, Allergic OFCs | P-Value | |
|---|---|---|---|---|---|---|
| Peanut (n = 102) | 71% | 0.29 | 0.07–0.96 | 1.03 | 0.48–2.85 | <0.0001 |
| Egg (n = 141) | 67% | 0.71 | 0.27–2.02 | 2.73 | 1.2–6.23 | <0.0001 |
| Milk (n = 61) | 70% | 0.83 | 0.18–4.09 | 1.46 | 0.26–5.13 | 0.092 |
A logistic regression model was built to determine if age at OFC, insurance type, food specific serum IgE values, and symptoms occurring at the OFC, had a significant effect on OFC outcome. A backward elimination selection method was deployed to remove factors that were not significant. The following symptoms were found to have a significant effect on OFC outcome: erythematous rash (P < 0.0001), pruritis (P = 0.0003), urticaria/angioedema (P < 0.0001), rhinorrhea (P = 0.003), cough (P < 0.0049), nausea (P < 0.0049), vomiting (P < 0.0006), or other complaints (P < 0.0001). A frequency distribution of the symptoms that are associated with OFC outcomes are listed in Table 4.
Table 4.
Frequency distribution of significant symptoms associated with OFC outcome
| Symptom | Allergic (n = 188) | Non-Allergic (n = 453) |
|---|---|---|
| Vomiting | 11% (n = 20) | 0.2% (n = 1) |
| Urticaria/Angioedema | 51% (n = 96) | 8% (n = 36) |
| Nausea | 10% (n = 18) | 1% (n = 4) |
| Cough | 8% (n = 15) | 1% (n = 6) |
| Erythematous rash | 38% (n = 71) | 11% (n = 48) |
| Rhinorrhea | 16% (n = 30) | 2% (n = 9) |
| Pruritis | 30% (n = 56) | 6% (n = 27) |
| Other Complaints | 30% (n = 57) | 7% (n = 31) |
Age at the time of OFC and food specific serum IgE values were non-significant and consequently removed from the final model.
The Chi-square results showed no significant relationship between OFCs outcomes i.e. allergic (p = 0.40) and non-allergic (p = 0.74) and age, sex, and insurance status.
Discussion
In this study, conducted to examine patient factors that may impact the outcome of OFCs, 508 children underwent 641 OFCs. Twenty nine percent of OFCs had an allergic outcome with the most commonly challenged foods being peanuts, eggs, and milk.
Patient age and sex, when stratified by insurance status, did not have a significant effect on OFC outcomes. Serum IgE to peanuts and egg was significantly different between allergic OFC and non-allergic outcome. Vomiting and urticaria/angioedema correlated with an allergic OFC outcome.
This study was conducted in an academic center that serves a mix of Medicaid and non-Medicaid insured pediatric populations. The center is unique in the community in its ability to service Medicaid insured populations. By the same token this study is unique in examining the effect of insurance type on the result of OFC. Medicaid insurance is more common in the inner city population, which was demonstrated in the results where the challenges for the Medicaid population were from the area surrounding the medical center, where people of low socioeconomic status reside, while the non-Medicaid were spread in the suburbs, where people of high socioeconomic status reside. Despite this insurance mix, the majority of the population was non-Hispanic Caucasian, so a difference by race/ethnicity was not noted in the analysis. The race/ethnicity, however, mirrors the population of the county which is 65.2% Caucasian alone, 25.3% African American alone, and 3.27% Hispanic or Latino.14 The reason for conducting the challenges were clinical indications, which were either to confirm the allergy or to determine whether the food allergy has resolved. In the 641 OFCs on 508 children, ages 0–19 years-old, 29% of OFCs had an allergic outcome with the foods most commonly challenged being peanuts (32% of OFCs performed), eggs (29%), and milk (13%). Our data is similar to prior studies which found egg, milk, and peanut to be among the most commonly challenged foods.15,16 Spergel et al. did a retrospective chart review of 998 oral food challenges with egg, milk, and peanut accounting for 30%, 27%, and 14% of OFCs performed, respectively.15 Lieberman et al. performed a retrospective chart review of 701 OFCs and reported peanut, tree nuts, egg, and milk accounting for 18%, 17%, 16%, and 7.8% of OFCs, respectively.16
Serum IgE to food allergens has previously been studied for its predictive value in determining outcomes of OFCs. Our data found that the median sIgEs for peanut, egg, and milk are higher in patients with allergic OFC outcomes to the respective foods, compared to patients with non-allergic OFC outcomes, but this was only statistically significant for peanut and eggs. Comparing to other studies, food-specific serum IgE have been shown to predict allergic OFC outcomes, with age of patients acting as a confounding variable in the predicted probabilities between these studies.17, 18, 19 Rolinck-Werninghaus et al. found food specific serum IgE level, young age, and history of eczema were predictors of allergic outcome of OFC to milk and egg.20 Cortot et al. found skin prick tests, food-specific serum IgE levels, and age were not good predictors of OFC results to baked egg.21 Cianferoni et al. found that age was not, but food-specific serum IgE and skin prick test wheal size were associated with allergic outcome of OFC.22 Ahrens et al. found that younger age was a predictor of an allergic outcome of OFC.23 Horimukai et al. found that age did not affect OFC outcome.24
Looking at adverse symptoms occurring in relation to OFC outcome in our study, patients with vomiting had much greater odds ratio of being diagnosed as allergic to the OFC food (OR = 41.2, 95%CI = 7.0–786.7, P < 0.0006), followed by urticaria/angioedema (OR = 27.4, 95%CI = 12.7–65.0, P < 0.0001). Previous studies have reported cutaneous system complaints being the most common symptom during OFCs.15,16,25, 26, 27 Spergel et al. found cutaneous reactions to be most common during OFCs, followed by multi-organ reactions.15 Similarly, Ahrens et al. and Mankad et al. reported that the majority of clinical reactions were cutaneous, followed by gastrointestinal symptoms.25,26 All of these studies found that positive OFCs were mostly to egg and cow's milk. Likewise, Lieberman et al. reported that cutaneous reactions were the most common during OFCs, and cow's milk was the most common food to be associated with an allergic OFC result.16 Yanagida et al. and Gupta et al. retrospectively evaluated 393 and 2304 OFCs, respectively.28,29 Similar to our results, they found that gastrointestinal symptoms were the most common OFC-provoked reaction. This may be a reflection of peanut and egg being the most commonly challenged foods in our study, which differs from other OFC studies where cow's milk and egg are challenged more frequently. Recent studies have shown the development of gastrointestinal symptoms more likely in OFC with egg and peanut compared to OFC with cow's milk, soy and wheat.25,29
Age did not have a significant effect on OFC outcome in our data, similar to previous studies.21,22,24 Moreover, stratifying OFC outcomes by age, sex, and insurance type did not result in any significant association with outcome of OFC.
This study examined the outcome of OFC as allergic or non-allergic in children based on their serum IgE to the food, but in the context of the patients’ age at the time of the OFC, gender, race/ethnicity, and the socio-economic statuses, determined by the patient insurance with Medicaid and non-Medicaid insurances representing low and high socio-economic statuses, respectively. There is a potential limitation in the study. While the various races/ethnicities and socio-economic statuses are all represented in the study population, the patient population who attend our clinics are skewed towards a majority Caucasian, non-Hispanic, and high socio-economic status. This may have impacted the analysis showing no association of these factors with the outcome of OFC. The serum IgE continued to be associated with the outcome of OFC, specifically for peanut and egg. Future studies should recruit minority populations with low socio-economic status.
In conclusion, our study found that: 1) out of 641 OFCs, 29% of OFCs had an allergic outcome; 2) the most commonly challenged foods were peanuts, eggs, and milk; 3) higher median sIgE for peanut and egg was associated with allergic OFC outcome; 4) vomiting and urticaria/angioedema were highly associated with allergic OFC outcome; and 5) Age, sex, and insurance type were not associated with OFC outcome.
Funding
Andrew T. Dang was supported by National Research Service Award for Fellowship in Immunology and Allergy, National Institute of Allergy and Infectious Diseases T32 AI60515. Amanda Beyer was supported by funds from the 2017 University Of Cincinnati College Of Medicine Medical Student Summer Research Fellowship. Somboon Chansakulporn was supported by funds for a visiting fellowship from the Department of Pediatrics, HRH Princess Maha Chakri Siridhorn Medical Center, Srinakharinwirot University, Nakhon Nayok, Thailand. Tesfaye B. Mersha was supported by the National Institutes of Health (NIH) grant R01HL132344.
Declaration of Competing Interest
I, Andrew Dang, do not have any conflicts of interest to disclose.
I, Pavan Chundi, do not have any conflicts of interest to disclose.
I, Nadeem Mousa, do not have any conflict of interest to disclose.
I, Amanda Beyer, do not have any conflicts of interest to disclose.
I, Somboon Chansakulporn, do not have any conflicts of interest to disclose.
I, Carina Venter, have received honorariums from Danone, Mead Johnson and Abbott. None constitute a conflict of interest.
I, Tesfaye B. Mersha, do not have any conflicts of interest to disclose.
I, Amal Assa'ad, have received research grants to my institution associated with Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi and ABBVIE and the National Institute of Health, USA. I have a patent. I have no other financial affiliations. None constitute a conflict of interest.
Acknowledgments
Great thanks to the faculty, fellows, and staff at our Allergy and Immunology Division for their involvement and care of the patients and oral food challenges assessed in this study.
Footnotes
Full list of author information is available at the end of the article.
Institutional review board: The study received approval from the Institutional Review Board of Cincinnati Children's Hospital Medical Center. The approval number and title are: 2015–4861: Factors affecting oral food challenge outcomes in children. Due to the retrospective chart review nature of the study, the approval did not require that subjects sign consent or assent forms.
References
- 1.Gupta R.S., Springston E.E., Warrier M.R. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Burks A.W., Tang M., Sicherer S. ICON: food allergy. J Allergy Clin Immunol. 2012;129(4):906–920. doi: 10.1016/j.jaci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bird J.A., Groetch M., Allen K.J. Conducting an oral food challenge to peanut in an infant. J Allergy Clin Immunol Pract. 2017;5(2):301–311. doi: 10.1016/j.jaip.2016.07.019. e301. [DOI] [PubMed] [Google Scholar]
- 4.Nowak-Wegrzyn A., Assa'ad A.H., Bahna S.L. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123(6 Suppl):S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Wilson B.G., Cruz N.V., Fiocchi A. American college of allergy A, immunology adverse reactions to food C. survey of physicians' approach to food allergy, part 2: allergens, diagnosis, treatment, and prevention. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2008;100(3):250–255. doi: 10.1016/S1081-1206(10)60450-4. [DOI] [PubMed] [Google Scholar]
- 6.Sindher S., Long A.J., Purington N. Analysis of a large standardized food challenge data set to determine predictors of positive outcome across multiple allergens. Front Immunol. 2018;9:2689. doi: 10.3389/fimmu.2018.02689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purington N., Chinthrajah R.S., Long A. Eliciting dose and safety outcomes from a large dataset of standardized multiple food challenges. Front Immunol. 2018;9:2057. doi: 10.3389/fimmu.2018.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Shoshan M., Harrington D.W., Soller L. Demographic predictors of peanut, tree nut, fish, shellfish, and sesame allergy in Canada. J Allergy. 2012;2012 doi: 10.1155/2012/858306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilaver L.A., Kester K.M., Smith B.M. Socioeconomic disparities in the economic impact of childhood food allergy. Pediatrics. 2016;137(5) doi: 10.1542/peds.2015-3678. [DOI] [PubMed] [Google Scholar]
- 10.Mahdavinia M., Fox S.R., Smith B.M. Racial differences in food allergy phenotype and health care utilization among US children. J Allergy Clin Immunol Pract. 2017;5(2):352–357. doi: 10.1016/j.jaip.2016.10.006. e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akuete K., Guffey D., Israelsen R.B. Multicenter prevalence of anaphylaxis in clinic-based oral food challenges. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2017;119(4):339–348. doi: 10.1016/j.anai.2017.07.028. e331. [DOI] [PubMed] [Google Scholar]
- 12.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 13.Amin M.R., Khoury J.C., Assa'ad A.H. Food-specific serum immunoglobulin E measurements in children presenting with food allergy. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2014;112(2):121–125. doi: 10.1016/j.anai.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 14.https://datausa.io/profile/geo/hamilton-county-oh/ Accessed.
- 15.Spergel J.M., Beausoleil J.L., Fiedler J.M. Correlation of initial food reactions to observed reactions on challenges. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2004;92(2):217–224. doi: 10.1016/S1081-1206(10)61550-5. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman J.A., Cox A.L., Vitale M. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128(5):1120–1122. doi: 10.1016/j.jaci.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampson H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 18.Celik-Bilgili S., Mehl A., Verstege A. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2005;35(3):268–273. doi: 10.1111/j.1365-2222.2005.02150.x. [DOI] [PubMed] [Google Scholar]
- 19.Benhamou A.H., Zamora S.A., Eigenmann P.A. Correlation between specific immunoglobulin E levels and the severity of reactions in egg allergic patients. Pediatr Allergy Immunol: Off Publ Eur Soc Pediatr Allergy Immunol. 2008;19(2):173–179. doi: 10.1111/j.1399-3038.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- 20.Rolinck-Werninghaus C., Niggemann B., Grabenhenrich L. Outcome of oral food challenges in children in relation to symptom-eliciting allergen dose and allergen-specific IgE. Allergy. 2012;67(7):951–957. doi: 10.1111/j.1398-9995.2012.02838.x. [DOI] [PubMed] [Google Scholar]
- 21.Cortot C.F., Sheehan W.J., Permaul P. Role of specific IgE and skin-prick testing in predicting food challenge results to baked egg. Allergy Asthma Proc. 2012;33(3):275–281. doi: 10.2500/aap.2012.33.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cianferoni A., Garrett J.P., Naimi D.R. Predictive values for food challenge-induced severe reactions: development of a simple food challenge score. Isr Med Assoc J: Isr Med Assoc J. 2012;14(1):24–28. [PubMed] [Google Scholar]
- 23.Ahrens B., Niggemann B., Wahn U. Positive reactions to placebo in children undergoing double-blind, placebo-controlled food challenge. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2014;44(4):572–578. doi: 10.1111/cea.12284. [DOI] [PubMed] [Google Scholar]
- 24.Horimukai K., Hayashi K., Tsumura Y. Total serum IgE level influences oral food challenge tests for IgE-mediated food allergies. Allergy. 2015;70(3):334–337. doi: 10.1111/all.12562. [DOI] [PubMed] [Google Scholar]
- 25.Ahrens B., Niggemann B., Wahn U. Organ-specific symptoms during oral food challenge in children with food allergy. J Allergy Clin Immunol. 2012;130(2):549–551. doi: 10.1016/j.jaci.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 26.Mankad V.S., Williams L.W., Lee L.A. Safety of open food challenges in the office setting. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. 2008;100(5):469–474. doi: 10.1016/S1081-1206(10)60473-5. [DOI] [PubMed] [Google Scholar]
- 27.Calvani M., Berti I., Fiocchi A. Oral food challenge: safety, adherence to guidelines and predictive value of skin prick testing. Pediatr Allergy Immunol: Off Publ Eur Soc Pediatr Allergy Immunol. 2012;23(8):755–761. doi: 10.1111/pai.12016. [DOI] [PubMed] [Google Scholar]
- 28.Yanagida N., Sato S., Asaumi T. Risk factors for severe reactions during double-blind placebo-controlled food challenges. Int Arch Allergy Immunol. 2017;172(3):173–182. doi: 10.1159/000458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta M., Grossmann L.D., Spergel J.M. Egg food challenges are associated with more gastrointestinal reactions. Children. 2015;2(3):371–381. doi: 10.3390/children2030371. [DOI] [PMC free article] [PubMed] [Google Scholar]