Abstract
In addition to needing acute emergency management, blast-mediated traumatic brain injury (TBI) is also a chronic disorder with delayed-onset symptoms that manifest and progress over time. While the immediate consequences of acute blast injuries are readily apparent, chronic sequelae are harder to recognize. Indeed, the identification of individuals with mild-TBI or TBI-induced symptoms is greatly impaired in large part due to the lack of objective and robust biomarkers. The purpose of this study was to address these need by identifying candidates for serum-based biomarkers of blast TBI, and also to identify unique or differentially regulated protein expression in the thalamus in C57BL/6J mice exposed to blast using high throughput qualitative screens of protein expression. To identify thalamic proteins differentially or uniquely associated with blast exposure, we utilized an antibody-based affinity-capture strategy (referred to as “proteomics-based analysis of depletomes”; PAD) to deplete thalamic lysates from blast-treated mice of endogenous thalamic proteins also found in control mice. Analysis of this “depletome” detected 75 unique proteins, many with associations to the myelin sheath. To identify blast-associated proteins eliciting production of circulating autoantibodies, serum antibodies of blast-treated mice were immobilized, and their immunogens subsequently identified by proteomic analysis of proteins specifically captured following incubation with thalamic lysates (a variant of a strategy referred to as “proteomics-based expression library screening”; PELS). This analysis identified 46 blast-associated immunogenic proteins, including 6 shared in common with the PAD analysis (ALDOA, PHKB, HBA-A1, DPYSL2, SYN1, and CKB). These proteins and their autoantibodies are appropriate for further consideration as biomarkers of blast-mediated TBI.
Keywords: Biochemistry, Neuroscience, Proteins, Retinal ganglion cell, Biomarker, Blast injury, Traumatic brain injury
Biochemistry; Neuroscience; Proteins; Retinal ganglion cell; Biomarker; Blast injury; Traumatic brain injury.
1. Introduction
Blast-mediated traumatic brain injury (TBI) is a common condition among active and recently-active military personnel, and also affects civilian populations [1]. Blast-mediated TBI is a traumatic event that needs both acute and chronic management, and symptoms typically manifest and progress chronically [2]. Identification of individuals with mild TBI or TBI-induced symptoms is difficult for multiple reasons, including self-reporting of blast-exposure. In addition, improvements in protective armor have improved survivability in recent conflicts, which has resulted in an increased incidence of TBI [3]. Even if TBI is suspected based on the reported history, a confounding factor for symptom-based diagnosis is that individuals with TBI can present with a wide constellation of symptoms which include cognitive, behavioral, neuropsychological, motor and visual impairment [4, 5]. Many of these symptoms may not be immediately apparent and may only manifest months to years after the initial injury, or are diagnosed post-mortem [6, 7]. Thus, there is a significant unmet need for objective blood-based biomarkers for mild injuries that can be used to help confirm diagnosis.
A significant challenge to developing tools for diagnosis and therapeutic treatments for blast-mediated mild TBI is that the timing of neuronal loss and the pathways leading to neuronal impairment and degeneration are not well understood. The pathophysiology of blast-mediated TBI is precipitated by the interaction of a blast wave with neuronal tissue. Following this interaction, multiple mechanisms that lead to the death and dysfunction of neurons after exposure to TBI have been reported from several models [8, 9, 10]. These mechanisms include immediate [11, 12, 13] and delayed [14, 15] neuronal changes. In many models, classic markers of apoptotic cell death are not present [16]. Cellular mechanisms that are believed to lead to neuronal death and dysfunction after mild TBI or repetitive TBI include diffuse axonal injury and myelin damage, excitotoxicity, dysregulation of the neurovascular unit, inflammation, and oxidative stress [17, 18, 19, 20, 21]. Each of these processes has been individually implicated in contributing to neuronal death; the possibility of their complex combinatorial interactions seems likely, but remains largely untested.
In part because of the difficulties in symptom-based diagnosis and an incomplete knowledge of damage-inducing mechanisms, developing protein-based biomarkers in the body that can serve as a proxy for poorly-accessible organs such as the brain has been a focus of considerable research effort [22, 23, 24]. Many proteins that are expressed differentially or uniquely during pathogenesis of TBI have been identified. Among the most promising candidates for biomarkers of blast-mediated TBI are several which have proven useful for other forms of acquired brain injury, including: S-100β, neuron specific enolase (NSE), glial fibrillary acid protein (GFAP), myelin basic protein (MBP), and BDNF [25]. While these proteins have been implicated with varying levels of TBI, their utility in detecting patients with mild blast-mediated TBI has not been completely validated.
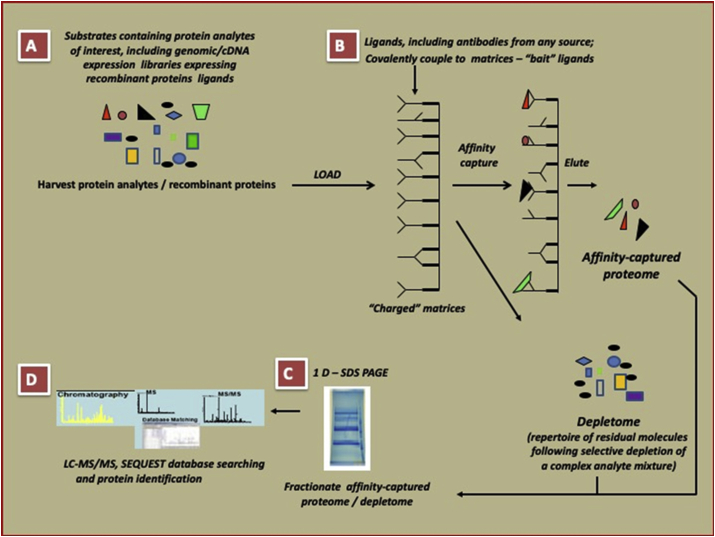
Here, we report the discovery of novel candidates for serum-based biomarkers of blast TBI in addition to discovering unique and upregulated proteins in the thalamus in C57BL/6J mice exposed to blast using novel, robust qualitative screens for rapid identification of proteins and analytes of interest. To achieve this, we first identified thalamic proteins differentially or uniquely associated with blast exposure. Next, we identified blast-associated proteins eliciting production of circulating autoantibodies. Finally, results of both approaches were compared to identify the small subset shared in common, i.e. proteins induced by blast that also solicit sustained autoantibody production. We performed these analyses using variations of proteomics-based approaches: proteomics-based analysis of depletomes (PAD) and proteomics-based expression library screening (PELS), which each utilize antibody-based affinity capture of proteins and their characterization using tandem mass spectrometry in different iterations (Figure 1). The results identify six proteins with properties appropriate for further consideration as biomarkers of blast-mediated TBI, as well as several others previously not known to be associated with blast-injury but potentially relevant to mechanisms of damage caused by blast-exposure. These proteins are: fructose-biphosphate aldolase A, phosphorylase b kinase regulatory subunit beta, alpha globin 1, dihydropyrimidinase-related protein 2, isoform 1b of synapsin 1, and creatine kinase B-type.
Figure 1.
The PELS principle for generation of affinity-captured proteome/depletome used in this study.
2. Methods
2.1. Animals
All animal studies were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Iowa City Veterans Affairs Institutional Animal Care and Use Committee. The study utilized two experimental groups of mice, which both consisted entirely of male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), and were randomly assigned to one of the following treatment groups at 8 weeks of age: 1) mice were exposed to one single blast injury (referred to throughout as “TBI-mice”), or 2) mice were subjected to a sham-injury (“sham-mice”). A total of 92 mice were used in this study.
2.2. Blast injury induction
An enclosed blast chamber was used for the purpose of these studies to investigate the effect of primary blast exposure. One half of this tank was pressurized, with a 13-cm opening between the sides of the chamber, as described previously [11, 12]. A Mylar membrane (Mylar A, 0.00142 gauge; Country Plastics, Ames, IA, USA) was placed over the opening on the pressurized side of the chamber. The unpressurized side of the tank contained a padded polyvinyl chloride (PVC) protective restraint for positioning of an anesthetized mouse 30 cm from the Mylar membrane. Compressed air was pumped into the pressurized side of the tank to 20 psi, at which point the membrane ruptured and created a blast wave. Mice were anesthetized with a combination of ketamine (0.03 mg/g, intraperitoneal [IP]) and xylazine (0.005 mg/g, IP) and positioned within the blast chamber with the left side of the head and eye oriented toward the source of the blast wave. To ensure that the primary effect of the blast wave was at the level of the head, only the head of TBI-mice was exposed to the blast wave, with the rest of the body shielded. The head was allowed to move freely and was not fixed in position, although care was taken to ensure that the head did not impact hard surfaces, and was supported from major movement with thick foam placed behind the head. Immediately following exposure to the blast wave, TBI-mice were placed on a heating pad to facilitate recovery from general anesthesia and to prevent hypothermia. Xylazine anesthesia was reversed with yohimbine chloride (0.001 mg/g, IP) to speed the recovery from anesthesia. Control sham-mice used in this study were anesthetized and placed in the blast chamber, but did not receive a blast exposure. TBI-mice and sham-mice equally received analgesic via subcutaneous injection (0.1 mL/20 g body weight) of buprenorphine (0.003 mg/mL) immediately after the blast or sham-blast, respectively.
2.3. Sample collection
For studies of thalamic proteins, TBI-mice (n = 12; 4 weeks post blast) and sham-mice (n = 12; 4 weeks post sham injury) were humanely euthanized and thalamic regions micro-dissected. Homogenates from each subject group were pooled together and stored at -80 °C prior to analysis. For studies of circulating auto-antibodies, serum specimens were collected from cardiac punctures of TBI-mice (n = 12) and sham-mice (n = 12) 8 weeks following blast exposure. Serum specimens were pooled and polyclonal antibodies purified via Protein A affinity chromatography using HiTrap Protein A HP (1 ml) columns (GE Healthcare) per manufacturer guidelines.
2.4. Proteomics-based analysis of depletomes (PAD)
The term “depletome” refers to the complement of interesting molecules resident in a complex mixture, following selective depletion of irrelevant components. To derive the depletome of the thalamus from blast-exposed mice, bait polyclonal antibodies were generated in chickens (IgY) against proteins from pooled thalami of sham-mice (C57BL/6J Male mice, 8 weeks of age at the beginning of the study) using the services of a commercial vendor (Aves Labs, OR), and affinity purified using anti-chicken IgY polyclonal generated in goats. The bait IgY-polyclonal antibodies (titer assessed to be >1:10,000 in dot immunoblotting against 2 μg of the immunogen mixture) were then covalently coupled to Dynabeads M-280 Tosylactivated (Invitrogen/Life Technologies, CA) and HiTrap NHS-activated columns (1 ml; GE Healthcare Life Sciences) per manufacturer guidelines. The thalamus protein extracts from TBI-mice (complex mixture; 5 mg total protein in 5 mls of PBS [pH 7.4]) were reacted first with charged Dynabeads M-280 Tosylactivated and then passed through charged HiTrap NHS-activated columns per manufacturer guidelines. This process of selective depletion of confounding proteins from the complex mixture and the simultaneous enrichment for relevant proteins, resulted in a depletome constituted by proteins that were either differentially (i.e., produced in larger amounts in thalami of TBI-mice than in those of untreated mice, defined as an increase of 1 or more identified peptides compared to untreated mice) or uniquely expressed in thalami of TBI-mice 4 weeks post injury. Increases in protein peptides are commonly used for analysis of high-throughput, qualitative assays of protein expression [26]. The proteins comprising the depletome were processed and subjected to tandem mass spectrometry for identification. Protein identifications were linked to gene symbols for 75 proteins in the depletome; 2 peptides were excluded (IPI00987580, IPI00224605) because they linked to predicted pseudogenes.
2.5. Proteomics-based expression library screening (PELS)
The overall strategy followed a published PELS protocol [27], with variations [28] to identify host thalamus proteins shed in body fluids following blast-mediated injury. First, “bait” polyclonal antibodies (bait PAbs) were generated from the pooled sera of TBI-mice (8 weeks post blast) and were covalently coupled to HiTrap NHS-activated columns (1 ml; GE Healthcare Life Sciences) creating “charged columns”. Next, pooled thalamic protein extracts from TBI-mice (4 weeks post blast) containing the analytes of interest were subjected to immunoaffinity capture by passage through the charged columns. The captured proteins were then eluted and subjected to tandem mass spectrometry for identification. Elutions of the same extracts loaded on NHS columns charged with bait PAbs affinity purified from sera collected from untreated mice and on NHS columns without covalently coupled polyclonal antibodies, but quenched active groups (“uncharged”) served as controls for assessing both specificity of bait PAbs and nonspecific adsorption to the column matrix. Protein identifications were linked to gene symbols for 46 proteins identified by PELS; 3 peptides were excluded because they linked to predicted pseudogenes (IPI00987580, IPI00265107) or could not be linked to a gene (IPI00462809).
2.6. Tandem mass spectrometry
Tandem mass spectra were extracted by ABI Analyst version 2.0. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.2.2). Mascot was set up to search the IPI-Mouse FASTA database assuming digestion enzyme trypsin. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified unique peptides. Proteins with single peptide hits were included if they exhibited high confidence based on low false discovery rates [29]. Relative protein abundance was estimated using the normalized total spectral counts [30]. Protein probabilities were assigned using the Protein Prophet algorithm [31]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
2.7. Functional annotation and pathway analysis
DAVID [32, 33] and WebGestalt [34, 35] were used to compare protein lists against a C57BL/6J mouse brain proteome [36]. Settings for functional annotation using DAVID utilized the gene symbols for the 75 proteins in the depletome for “gene list” the brain proteome list as “background”, and “Mus musculus” as species. Outputs, and their abbreviations used herein, for UP_KEYWORDS (keywords), GOTERM_CC_DIRECT (cellular compartment), GOTERM_MF_DIRECT (molecular function), GOTERM_BP_DIRECT (biological process), KEGG_PATHWAY (KEGG pathway), INTERPRO (protein domains) were compiled into spreadsheets with only terms surpassing statistical significance after multiple hypothesis testing performed by DAVID using the Benjamini-Hochberg method. Settings for WebGestalt utilized the same gene lists described above, with “mmusculus” for species and default settings for all other parameters. One of the 75 genes in the depletome (Atp5f1b, IPI00468481) was not recognized by DAVID or WebGestalt because of ortholog ambiguity.
3. Results
To identify thalamic proteins differentially or uniquely associated with blast exposure, we utilized the PAD strategy to identify a depletome of proteins over-represented in the thalamus of TBI-mice at 4 weeks after blast, compared to the thalamus of age-, sex-, and strain-matched sham-mice. This analysis identified 75 proteins (Table 1). To identify blast-associated proteins eliciting production of circulating autoantibodies, we utilized a variant of the PELS strategy to identify serum antibodies of TBI-mice at 8 weeks post blast that recognize thalamic proteins of TBI-mice at 4 weeks post blast. This analysis identified 46 blast-associated immunogenic proteins (Table 2). A comparison of the results in common to both approaches, i.e. proteins differentially/uniquely associated with blast exposure and those that elicit a sustained production of autoantibodies, identified six proteins (Table 3).
Table 1.
Depletome proteins identified using PAD.
| Identified proteins | Accession number | Molecular weight | Number of unique peptides in thalamus of untreated mouse | Number of unique peptides in depletome | UniProtKB | Gene Symbol |
|---|---|---|---|---|---|---|
| Gamma-enolase | IPI00331704 | 47 kDa | 4 | 12 | P17183 | Eno2 |
| Serum albumin | IPI00131695 | 69 kDa | 2 | 9 | P07724 | Alb |
| Creatine kinase B-type | IPI00136703 | 43 kDa | 4 | 8 | Q04447 | Ckb |
| Malate dehydrogenase, mitochondrial | IPI00323592 | 36 kDa | 1 | 7 | P08249 | Mdh2 |
| Heat shock cognate 71 kDa protein | IPI00323357 | 71 kDa | 0 | 6 | P63017 | Hspa8 |
| Dihydropyrimidinase-related protein 2 | IPI00114375 | 62 kDa | 2 | 6 | O08553 | Dpysl2 |
| Isoform Ib of Synapsin-1 | IPI00136372 (+1) | 70 kDa | 3 | 6 | O88935 | Syn1 |
| 14-3-3 protein gamma | IPI00230707 | 28 kDa | 2 | 5 | P61982 | Ywhag |
| Isoform M2 of Pyruvate kinase isozymes M1/M2 | IPI00407130 (+1) | 58 kDa | 0 | 5 | P52480 | Pkm |
| Aspartate aminotransferase, cytoplasmic | IPI00230204 | 46 kDa | 0 | 5 | P05201 | Got1 |
| Actin, cytoplasmic 1 | IPI00110850 (+4) | 42 kDa | 2 | 4 | P60710 | Actb |
| Superoxide dismutase [Cu–Zn] | IPI00130589 | 16 kDa | 2 | 4 | P08228 | Sod1 |
| Alpha-enolase | IPI00462072 (+2) | 47 kDa | 0 | 4 | P17182 | Eno1 |
| Tubulin beta-4 chain | IPI00109073 (+1) | 50 kDa | 0 | 4 | Q9D6F9 | Tubb4a |
| Isoform 1 of Alpha-synuclein | IPI00115157 (+1) | 14 kDa | 0 | 4 | O55042 | Snca |
| Tubulin alpha-1A chain | IPI00110753 (+3) | 50 kDa | 0 | 3 | P68369 | Tuba1a |
| Stathmin | IPI00551236 | 17 kDa | 0 | 3 | P54227 | Stmn1 |
| Malate dehydrogenase, cytoplasmic | IPI00336324 | 37 kDa | 0 | 3 | P14152 | Mdh1 |
| Isoform 1 of Microtubule-associated protein 1A | IPI00408909 (+1) | 300 kDa | 1 | 2 | Q9QYR6 | Map1a |
| Triosephosphate isomerase | IPI00467833 (+1) | 32 kDa | 1 | 2 | P17751 | Tpi1 |
| SH3 domain-binding glutamic acid-rich-like protein 3 | IPI00127358 | 10 kDa | 1 | 2 | Q91VW3 | Sh3bgrl3 |
| Isoform HuC-L of ELAV-like protein 3 | IPI00122451 | 40 kDa | 0 | 2 | Q60900 | Elavl3 |
| Fructose-bisphosphate aldolase C | IPI00119458 | 39 kDa | 0 | 2 | P05063 | Aldoc |
| 2-iminobutanoate/2-iminopropanoate deaminase | IPI00130640 | 14 kDa | 0 | 2 | P52760 | Rida |
| Peroxiredoxin-2 | IPI00117910 (+1) | 22 kDa | 0 | 2 | Q61171 | Prdx2 |
| Isoform 1 of Serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform | IPI00121545 (+2) | 59 kDa | 0 | 2 | P63328 | Ppp3ca |
| Annexin A5 | IPI00317309 | 36 kDa | 0 | 2 | P48036 | Anxa5 |
| Heat shock protein HSP 90-alpha | IPI00330804 | 85 kDa | 0 | 2 | P07901 | Hsp90aa1 |
| Elongation factor 1-alpha 2 | IPI00119667 | 50 kDa | 0 | 2 | P62631 | Eef1a2 |
| Isoform Mitochondrial of Peroxiredoxin-5, mitochondrial | IPI00129517 (+3) | 22 kDa | 0 | 2 | P99029 | Prdx5 |
| L-lactate dehydrogenase B chain | IPI00229510 | 37 kDa | 0 | 2 | P16125 | Ldhb |
| Ras-related protein Rab-3A | IPI00122965 | 25 kDa | 0 | 2 | P63011 | Rab3a |
| Ubiquitin carboxyl-terminal hydrolase isozyme L1 | IPI00313962 (+1) | 25 kDa | 0 | 2 | Q9R0P9 | Uchl1 |
| Fructose-bisphosphate aldolase A | IPI00221402 | 39 kDa | 0 | 2 | P05064 | ALDOA |
| Alpha globin 1 | IPI00845802 | 15 kDa | 1 | 2 | Q91VB8 | Hba-a1 |
| Cytochrome P450, family 2, subfamily c, polypeptide 68 | IPI00405136 (+1) | 56 kDa | 0 | 1 | Q8VCP4 | Cyp2c68 |
| Translationally-controlled tumor protein | IPI00129685 | 19 kDa | 0 | 1 | P63028 | Tpt1 |
| Proteasomal ubiquitin receptor ADRM1 | IPI00331155 | 42 kDa | 0 | 1 | Q9JKV1 | Adrm1 |
| Isoform 1 of Serine/threonine-protein kinase SMG1 | IPI00403352 | 410 kDa | 0 | 1 | Q8BKX6 | Smg1 |
| Isoform 2 of Neurogenic locus notch homolog protein 2 | IPI00621767 | 243 kDa | 0 | 1 | O35516 | Notch2 |
| Isoform 4 of Myocyte-specific enhancer factor 2C | IPI00318314 (+1) | 47 kDa | 0 | 1 | Q8CFN5 | Mef2c |
| Parvalbumin alpha | IPI00230766 | 12 kDa | 0 | 1 | P32848 | Pvalb |
| Neurogranin | IPI00380227 | 7 kDa | 0 | 1 | P60761 | Nrgn |
| Polyadenylate-binding protein 1 | IPI00124287 (+2) | 71 kDa | 0 | 1 | P29341 | Pabpc1 |
| Histidine triad nucleotide-binding protein 1 | IPI00108189 (+1) | 14 kDa | 0 | 1 | P70349 | Hint1 |
| Isoform 1 of 60 kDa heat shock protein, mitochondrial | IPI00308885 (+1) | 61 kDa | 0 | 1 | P63038 | Hspd1 |
| Isoform 1 of Microtubule-associated protein 6 | IPI00115833 | 96 kDa | 0 | 1 | Q7TSJ2 | Map6 |
| Tubulin alpha-4A chain | IPI00117350 | 50 kDa | 0 | 1 | P68368 | Tuba4a |
| Ras-related protein Rab-1A | IPI00114560 (+3) | 23 kDa | 0 | 1 | P62821 | Rab1a |
| V-type proton ATPase subunit B, brain isoform | IPI00119113 | 57 kDa | 0 | 1 | P62814 | Atp6v1b2 |
| Protein kinase C and casein kinase substrate in neurons protein 1 | IPI00123613 | 51 kDa | 0 | 1 | Q61644 | Pacsin1 |
| Isoform IIb of Synapsin-2 | IPI00134492 (+2) | 52 kDa | 0 | 1 | Q64332 | Syn2 |
| Isoform 1 of Alpha-adducin | IPI00136000 (+4) | 81 kDa | 0 | 1 | Q9QYC0 | Add1 |
| Profilin-1 | IPI00224740 (+1) | 15 kDa | 0 | 1 | P62962 | Pfn1 |
| Phosphoglycerate mutase 2 | IPI00230706 (+1) | 29 kDa | 0 | 1 | O70250 | Pgam2 |
| Rho GDP-dissociation inhibitor 1 | IPI00322312 | 23 kDa | 0 | 1 | Q99PT1 | Arhgdia |
| Rab GDP dissociation inhibitor alpha | IPI00323179 | 51 kDa | 0 | 1 | P50396 | Gdi1 |
| Isoform 1 of Structural maintenance of chromosomes protein 5 | IPI00380203 (+1) | 129 kDa | 0 | 1 | Q8CG46 | Smc5 |
| Amphiphysin | IPI00400180 | 75 kDa | 0 | 1 | Q7TQF7 | Amph |
| ATP synthase subunit beta, mitochondrial | IPI00468481 | 56 kDa | 0 | 1 | P56480 | Atp5f1b |
| Choline transporter-like protein 3 | IPI00122287 | 73 kDa | 0 | 1 | Q921V7 | Slc44a3 |
| Ras-related protein Rab-11B | IPI00135869 (+5) | 24 kDa | 0 | 1 | P46638 | Rab11b |
| Ubiquitin-60S ribosomal protein L40 | IPI00138892 (+11) | 15 kDa | 0 | 1 | P62984 | Uba52 |
| Isoform 3 of NSFL1 cofactor p47 | IPI00387232 (+2) | 41 kDa | 0 | 1 | Q9CZ44 | Nsfl1c |
| Mitogen-activated protein kinase 1 | IPI00119663 (+1) | 41 kDa | 0 | 1 | P63085 | Mapk1 |
| Neurocan core protein | IPI00135563 (+1) | 137 kDa | 0 | 1 | P55066 | Ncan |
| Tyrosine-protein phosphatase non-receptor type substrate 1 | IPI00756790 (+3) | 17 kDa | 0 | 1 | P97797 | Sirpa |
| Vomeronasal 2, receptor 112 | IPI00660717 | 98 kDa | 0 | 1 | L7N221 | Vmn2r112 |
| Adaptin ear-binding coat-associated protein 1 | IPI00225533 | 30 kDa | 0 | 1 | Q9CR95 | Necap1 |
| Transcriptional activator protein Pur-beta | IPI00128867 | 34 kDa | 0 | 1 | O35295 | Purb |
| Isoform 1 of D-aminoacyl-tRNA deacylase 1 | IPI00133713 | 23 kDa | 0 | 1 | Q9DD18 | Dtd1 |
| Endophilin-A1 | IPI00331110 (+3) | 40 kDa | 0 | 1 | Q62420 | Sh3gl2 |
| Macrophage migration inhibitory factor | IPI00230427 | 13 kDa | 0 | 1 | P34884 | Mif |
| 14-3-3 protein epsilon | IPI00118384 (+1) | 29 kDa | 0 | 1 | P62259 | Ywhae |
| Phosphorylase b kinase regulatory subunit beta | IPI00380735 | 124 kDa | 0 | 1 | Q7TSH2 | Phkb |
Table 2.
Identification of proteins shed into circulation after TBI that elicited the formation of autoantibodies in the serum of TBI-mice and identified with PELS.
| Identified proteins | Accession number | Molecular weight | Number of unique peptides | UniProtKB | Gene symbol |
|---|---|---|---|---|---|
| Fructose-bisphosphate aldolase A | IPI00221402 | 39 kDa | 11 | P05064 | ALDOA |
| 40S ribosomal protein S19 | IPI00113241 (+5) | 16 kDa | 7 | Q9CZX8 | Rps19 |
| Alpha globin 1 | IPI00845802 | 15 kDa | 7 | Q91VB8 | Hba-a1 |
| 40S ribosomal protein S13 | IPI00125901 (+2) | 17 kDa | 6 | P62301 | Rps13 |
| 60S ribosomal protein L27 | IPI00122421 (+1) | 16 kDa | 6 | P61358 | Rpl27 |
| Histone H2B type 1-F/J/L | IPI00114642 (+10) | 14 kDa | 5 | P10853 | Hist1h2bf |
| Hemoglobin subunit beta-1 | IPI00762198 (+3) | 16 kDa | 5 | P02088 | Hbb-b1 |
| 40S ribosomal protein S15 | IPI00319231 (+1) | 17 kDa | 4 | P62843 | Rps15 |
| Isoform 2 of 40S ribosomal protein S24 | IPI00402981 (+4) | 15 kDa | 4 | P62849 | Rps24 |
| 60S ribosomal protein L29 | IPI00222548 (+9) | 18 kDa | 3 | P47915 | Rpl29 |
| Histone H1.4 | IPI00223714 | 22 kDa | 3 | P43274 | Hist1h1e |
| 60S ribosomal protein L13 | IPI00224505 (+1) | 24 kDa | 3 | P47963 | Rpl13 |
| 60S ribosomal protein L6 | IPI00313222 (+2) | 34 kDa | 3 | P47911 | Rpl6 |
| 40S ribosomal protein S10 | IPI00112448 | 19 kDa | 3 | P63325 | Rps10 |
| 40S ribosomal protein S18 | IPI00317590 (+2) | 18 kDa | 3 | P62270 | Rps18 |
| 60S ribosomal protein L39 | IPI00273974 (+4) | 6 kDa | 2 | P62892 | Rpl39 |
| Isoform 1 of GRB10-interacting GYF protein 2 | IPI00473912 (+1) | 149 kDa | 2 | Q6Y7W8 | Gigyf2 |
| 40S ribosomal protein S7 | IPI00136984 (+1) | 22 kDa | 2 | P62082 | Rps7 |
| 40S ribosomal protein S29 | IPI00222553 | 7 kDa | 2 | P62274 | Rps29 |
| 60S ribosomal protein L35a | IPI00115902 (+1) | 13 kDa | 2 | O55142 | Rpl35a |
| Histone H2A.J | IPI00153400 (+13) | 14 kDa | 2 | Q8R1M2 | H2afj |
| Isoform Ib of Synapsin-1 | IPI00136372 (+1) | 70 kDa | 2 | O88935 | Syn1 |
| Isoform 2 of Heterogeneous nuclear ribonucleoprotein Q | IPI00406118 (+3) | 63 kDa | 1 | Q7TMK9 | Syncrip |
| Isoform 2 of Calcium-activated potassium channel subunit alpha-1 | IPI00120643 (+12) | 135 kDa | 1 | Q08460 | Kcnma1 |
| 60S ribosomal protein L18 | IPI00555113 (+1) | 22 kDa | 1 | P35980 | Rpl18 |
| CCAAT/enhancer-binding protein beta | IPI00116613 (+3) | 31 kDa | 1 | P28033 | Cebpb |
| 60S ribosomal protein L8 | IPI00137787 | 28 kDa | 1 | P62918 | Rpl8 |
| 60S ribosomal protein L35 | IPI00263879 (+1) | 15 kDa | 1 | Q6ZWV7 | Rpl35 |
| Histone H1.3 | IPI00331597 | 22 kDa | 1 | P43277 | Hist1h1d |
| Anti-colorectal carcinoma light chain | IPI00462809 (+7) | 26 kDa | 1 | Q7TS98 | N/A |
| Isoform 1 of Protein FAM126B | IPI00226426 (+1) | 59 kDa | 1 | Q8C729 | Fam126b |
| V-type proton ATPase subunit G 2 | IPI00123817 (+1) | 14 kDa | 1 | Q9WTT4 | Atp6v1g2 |
| Plexin-A2 | IPI00137313 | 212 kDa | 1 | P70207 | Plxna2 |
| Nuclear receptor subfamily 1 group D member 2 | IPI00119178 | 64 kDa | 1 | Q60674 | Nr1d2 |
| Serine/arginine-rich splicing factor 2 | IPI00121135 (+2) | 25 kDa | 1 | Q62093 | Sfrs2 |
| Keratin, type II cytoskeletal 6B | IPI00131366 (+7) | 60 kDa | 1 | Q9Z331 | Krt6b |
| Isoform 1 of Dynamin-1 | IPI00272878 (+5) | 98 kDa | 1 | P39053 | Dnm1 |
| 60S ribosomal protein L24 | IPI00323806 (+3) | 18 kDa | 1 | Q8BP67 | Rpl24 |
| Isoform 1 of Syntaxin-binding protein 1 | IPI00415402 (+2) | 68 kDa | 1 | O08599 | Stxbp1 |
| Peptidyl-prolyl cis-trans isomerase A | IPI00554989 | 18 kDa | 1 | P17742 | Ppia |
| Probable G-protein coupled receptor 158 | IPI00465871 | 134 kDa | 1 | Q8C419 | Gpr158 |
| Succinyl-CoA ligase [GDP-forming] subunit alpha, mitochondrial | IPI00406442 (+1) | 36 kDa | 1 | Q9WUM5 | Suclg1 |
| LYR motif-containing protein 4 | IPI00169804 | 11 kDa | 1 | Q8K215 | Lyrm4 |
| Phosphorylase b kinase regulatory subunit beta | IPI00380735 | 124 kDa | 1 | Q7TSH2 | Phkb |
| Dihydropyrimidinase-related protein 2 | IPI00114375 | 62 kDa | 1 | O08553 | Dpysl2 |
| Creatine kinase B-type | IPI00136703 | 43 kDa | 1 | Q04447 | Ckb |
Table 3.
Potential TBI biomarkers simultaneously identified with PELS and PAD.
| Identified proteins | Accession number | Molecular weight | Number of unique peptides in thalamus of untreated mouse | Number of unique peptides in depletome | Number of unique peptides identified as immunogenic with PELS | UniProtKB | Gene symbol |
|---|---|---|---|---|---|---|---|
| Fructose-bisphosphate aldolase A | IPI00221402 | 39 kDa | 0 | 2 | 11 | P05064 | ALDOA |
| Phosphorylase b kinase regulatory subunit beta | IPI00380735 | 124 kDa | 0 | 1 | 1 | Q7TSH2 | Phkb |
| Alpha globin 1 | IPI00845802 | 15 kDa | 1 | 2 | 7 | Q91VB8 | Hba-a1 |
| Dihydropyrimidinase-related protein 2 | IPI00114375 | 62 kDa | 2 | 6 | 1 | O08553 | Dpysl2 |
| Isoform Ib of Synapsin-1 | IPI00136372 (+1) | 70 kDa | 3 | 6 | 2 | O88935 | Syn1 |
| Creatine kinase B-type | IPI00136703 | 43 kDa | 4 | 8 | 1 | Q04447 | Ckb |
To test whether these protein lists included an over-representation of any gene ontology terms, the DAVID (Table 4) and WebGestalt (Table 5) databases were utilized to compare our results to a previously published C57BL/6J mouse brain proteome [36]. Analysis of the PAD-identified depletome indicated a wide-range of over-represented ontology terms. From analysis with DAVID, the most statistically significant over-represented terms of the depletome were both in the “Cellular Component” category, “Myelin sheath” (27 members, P = 3.7E-24) and “Extracellular exosome” (41 members, P = 1.7E-10). From analysis with WebGestalt, the statistical significance levels were less pronounced and more closely clustered, with several of the top terms in the “Cellular Component” category involving synapse- or axon-related ontology terms (including, “Neuron projection, 17 members, P = 2.09E-5; and “Axon”, 9 members, P = 6.0E-4).
Table 4.
Gene ontology term and pathway analysis using the DAVID database.
| Depleteome (74) | |
|---|---|
| Keywords: | Acetylation (37) 1.3E-4 |
| Phosphoprotein (52) 1.9E-4 | |
| Glycolysis (6) 1.0E-4 | |
| Cytoplasm (35) 1.0E-3 | |
| Nitration (5) 7.8E-3 | |
| Methylation (14) 3.3E-2 | |
| Biological Process: | Glycolytic process (6) 6.8E-4 |
| Cellular Component: | Myelin sheath (27) 3.7E-24 |
| Extracellular exosome (41) 1.7E-10 | |
| Cytosol (28) 5.6E-6 | |
| Cytoplasm (46) 4.9E-4 | |
| Extracellular space (16) 9.0E-5 | |
| Axon (11) 6.3E-4 | |
| Terminal bouton (9) 9.5E-6 | |
| Synaptic vesicle (8) 5.7E-4 | |
| Neuron projection (10) 1.6E-2 | |
| Neuronal cell body (11) 1.5E-2 | |
| Axon terminus (5) 1.6E-2 | |
| Protein complex (12) 3.3E-3 | |
| Blood microparticle (5) 1.7E-2 | |
| Molecular Function: | MHC Class II protein complex binding (4) 2.1E-2 |
| Catalytic activity (12) 1.5E-2 | |
| KEGG Pathway: | Carbon metabolism (9) 2.3E-3 |
| Glycolysis/Gluconeogenesis (7) 2.3E-3 | |
| Biosynthesis of antibiotics (10) 6.1E-3 | |
| Biosynthesis of amino acids (7) 4.6E-3 |
Table 5.
Gene ontology term and pathway analysis using the WebGestalt database.
| Depleteome (74) | |
|---|---|
| Biological Process: | Multicellular organismal process (32) 1.0E-4 |
| Catabolic process (22) 4.0E-4 | |
| Single-multicellular organism process (32) 1.0E-4 | |
| Organonitrogen combound metabolic process (20) 5.0E-4 | |
| Nucleobase-containing small molecular metabolic process (16) 3.0E-4 | |
| Monosaccharide catabolic process (6) 3.0E-4 | |
| Hexose catabolic process (6) 3.0E-4 | |
| Generation of precursor metabolites and energy (10) 4.0E-1 | |
| Glucose catabolic process (6) 3.0E-4 | |
| Glycolysis (6) 1.0E-4 | |
| Cellular Component: | Synapse (13) 1.0E-4 |
| Synapse part (10) 6.0E-4 | |
| Cell projection (21) 1.8E-5 | |
| Neuron projection (17) 2.09E-5 | |
| Axon (9) 6.0E-4 | |
| Cytoplasm (51) 2.45E-5 | |
| Cytosol (22) 9.56E-8 | |
| Coated vesicle (9) 3.32E-5 | |
| Clathrin-coated vesicle (7) 6.0E-4 | |
| Synaptic vesicle (6) 7.0E-4 | |
| Molecular Function: | Binding (50) 4.9E-3 |
| Catalytic activity (36) 2.6E-3 | |
| Cell surface binding (4) 8.0E-4 | |
| Protein binding (40) 1.1E-3 | |
| Enzyme binding (15) 2.6E-3 | |
| Phosphoprotein binding (4) 5.8E-3 | |
| Protein phosphorylated amino acid binding (3) 4.9E-3 | |
| Malate dehydrogenase activity (2) 9.1E-0.03 | |
| Phosphopyruvate hydratase activity (2) 4.9 E-3 | |
| L-malate dehydrogenase activity (2) 2.6E-3 | |
| KEGG Pathway: | Glycolysis/Gluconeogenesis (6) 4.0E-4 |
| Pyruvate metabolism (4) 1.5E-2 | |
| Neurotrophin signaling pathway (4) 4.9E-2 | |
| Phagosome (4) 4.9E-2 | |
| Oocyte meiosis (4) 4.9E-2 | |
| Phenylalanine metabolism (2) 4.9E-2 |
The cognitive function of mice was tested with the Morris water maze, a measure of hippocampal dependent learning and memory, in order to demonstrate that bTBI was inducing damage in the brain. Our results demonstrate that blast exposure did not affect learning during the training period of the task (Supplemental Figure 1A), but did result in a significant decrease in memory retention in mice with TBI compared to sham-blast mice (Supplemental Figure 1B, P = 0.0021). The average speed of each mouse calculated during the probe-test was not significantly different between sham and TBI-mice (Supplemental Figure 1C). The range of distributions for each parameter demonstrated that TBI induction results in consistent phenotypes without significant outliers. Anti-GFAP staining was performed in the brain to evaluate the astrocytic response to blast exposure (Supplemental Figure 2). These results demonstrate that there is not significant GFAP reactivity in the brains of mice 4 weeks following blast exposure when compared to sham-mice. These results also demonstrate that the intra-animal response to blast is uniform.
4. Discussion
We have utilized novel proteomics-based qualitative approaches to identify candidates for serum-based biomarkers of TBI. We focused our study on the thalamus, as it is a major sensory relay station in the brain, and previous findings of both auditory and visual difficulties have been observed in humans following blast-mediated TBI [37, 38, 39, 40, 41, 42, 43, 44]. Thus, the thalamus may be a site particularly prone to damage and appropriate for developing biomarkers. Using a previously described mouse model for studying blast-induced mild TBI, our current analysis identified six proteins with properties appropriate for further consideration as biomarkers of blast-mediated TBI, as well as several others previously not known to be associated with blast-injury and which may be relevant to ongoing mechanistic studies of damage caused by blast-exposure.
If developed, biomarkers could be of particular use with patients in which overt traumatic blast injuries were not sustained, but mild or chronic TBI is suspected. The identification and routine use of biomarkers could help to particularly improve the quality of life of Warfighters and Veterans, for whom mild to moderate cases of TBI might otherwise go undiagnosed. Many Veterans who have been exposed to blast complain of sensory impairment chronically post injury, but lack clearly identifiable diagnoses. For example, many blast-exposed Veterans complain of vision problems, have no measurable deficits in the visual pathway, but none-the-less progress to develop chronic visual impairments. Biomarkers could lead to the earlier identification of such patients and also promote their better monitoring and possible treatment.
A history of exposure to a blast from an explosion is common among Veterans of recent military conflicts. Blast exposures account for nearly 75% of combat-related injuries – 50% of which result in a diagnosis of mild TBI [45, 46]. Blast exposure results in axonal damage in the brain [47], and individuals exposed to blast often report chronic dysfunction of sensory organs [48]. Sensory system dysfunction in humans increases with repetitive blast exposure [49], and is reported in greater numbers further from the time of injury [50]. Damage to the visual system is found in both humans and preclinical models exposed to blast [51], although the mechanism of injury has not been elucidated [37]. Damage to the visual system has been reported in a variety of rodent bTBI models [11, 52, 53], which replicates visual dysfunction observed in Veterans exposed to blast [54, 55]. Visual dysfunction can be observed immediately following injury [56], with deficits persisting months after the injury [57]. The visual system damage observed after blast exposure is similar to damage observed in weight drop models of TBI [52, 58, 59], suggesting similar mechanisms. These mechanisms are varied and include axonal damage, activation of microglia, tissue swelling, infiltration of immune cells, upregulation of chemoattractants, and neuroinflammation [8, 60, 61, 62, 63, 64, 65, 66, 67].
The current study was designed based on using damage to the eye as a surrogate for sensory impairment in general, as sensory impairment, particularly visual function, is diminished by blast and non-blast TBI exposure [41, 68]. Thus, we sought to identify protein changes at a time point after blast exposure (4 weeks) when visual function has a transient recovery from deficits observed 1 h to one week following bTBI [11], but subtle physiologic abnormalities to visual stimuli can none-the-less be detected [12]. The visual function in this model declines significantly below baseline by two months post injury, and remains suppressed until at least 10 months post bTBI [11]. Although these time frames might all be considered “acute” for humans, in the context of the comparatively shortened life-span of a mouse, it is reasonable to surmise that they may roughly correlate to the pertinent time frame for a human who has been exposed to blast, sustained a mild TBI injury, and is manifesting an early stage of chronic sensory pathology when additional diagnosis tools could be useful.
Among the autoantibodies that were detected, there were identifiable links with other diseases and damage to CNS tissues. Notably, a recent study of autoantibodies that increase at the subacute phase of human spinal cord injury [69] discovered elevated levels of autoantibodies of three proteins also identified by our analyses: alpha globin alpha (HBA-A1), alpha globin beta (HBA-B1), and peptidyl-prolyl cis-trans isomerase A (PPIA). Amongst these, HBA-A1 stands out because it was identified by both our depletome and PELS analyses. As well as functioning in red blood cells, HBA and HBB have also been detected in brain neurons and hypothesized to have a role in response to injury [70]. PPIA, also referred to as cyclophilin A, is the intracellular receptor for the immunosuppressant cyclosporin A, and functions physiologically as a chaperone or foldase [71]. Though speculative, Arevalo-Martin et al. have previously hypothesized that autoantibodies to PPIA may promote immunosenescence after spinal cord injury [69]. Our results also included a large signature for antibodies directed against ribosomal proteins. Although autoantibodies to three ribosomal proteins (commonly referred to as P0, P1, and P2; encoded by RPLP0, RPLP1, and RPLP2, respectively) have previously been associated with systemic lupus erythematosus [72], autoantibodies to these specific ribosomal proteins were not detected in our analysis. Also of relevance, our analysis did not detect autoantibodies to myelin-associated proteins such as myelin oligodendrocyte glycoprotein (MOG) or myelin basic protein (MBP) which are hallmarks of multiple sclerosis [73].
Our findings also have implications with respect to mechanisms of blast-mediated injury. In our analysis of the thalamic depletome, the most pronounced pathway perturbation was clearly to proteins with links to the myelin sheath. Perturbations involving myelin have previously been observed in differing mouse models of blast-induced TBI [74, 75, 76]. In this regard, the common identification of this pathway hints that although each of these models evokes blast damage in differing ways, that there are none-the-less similarities in some of the basic mechanisms of injury. As reviewed by Fehily and Fitzgerald [10], demyelination or dysmyelination can involve multiple mechanisms, including sensitivity of oligodendrocytes, oligodendrocyte progenitor cells, glutamate excitotoxicity, calcium overload, oxidative stress, and/or altered metabolism, among others.
A second pathway with potential mechanistic relevance, which was specifically identified by WebGestalt, was “L-malate dehydrogenase activity” (2 members, P = 2.6E-3). The malate-aspartate shuttle is a biochemical reaction pathway that is important in regenerating NADH from NAD(+) within the mitochondrial matrix [77]. It has previously been shown that disruption of the NAD(+) pathway results in mitochondrial damage and neuronal death. Analysis of models of moderate brain injury has shown presence of chronic mitochondrial dysfunction and reduced antioxidant activity [78], and that lack of efficient re-synthesis of NAD(+) controlled by NAMPT leads to tissue damage in ischemic tissue [78, 79]. We, and others, have previously shown that treatment with a neuroprotective compound that increases NAD salvage via NAMPT [80] reduces the severity of neuronal damage in blast-exposed mice [12, 13] and mice with fluid-percussion injury [81, 82].
Some proteins identified in our study were previously identified as upregulated in a proteomic analysis of optic nerves from mice subjected to repetitive blunt impact TBI [59]. These proteins included ALB, MAP1A, and TUBA4A. A down-regulation of DPYSL2 was observed following repetitive impact TBI in the optic nerve. While we did not observe a change in DPYSL2 in the thalamus, we did detect auto-antibodies to DPYSL2 in the serum. These models also shared increases in similar classes of proteins, including peroxiredoxins, heat shock proteins and ATPases. Taken together these data suggest that similar molecular mechanisms are responsible for cell death and dysfunction in blast and blunt-trauma mediated TBI.
Our current study had caveats that are important to note. Regarding the injury, it is relevant that brain injury comes in many forms and intensities. Our study focused on mild blast-mediated TBI, which will have a very different presentation and protein profile than moderate to severe TBIs, or penetrating and concussive-mediated TBIs. The mild nature of our injury may explain why classic markers of TBI such as GFAP, UCH-L1 and S-100β were not detected. We have characterized many of the physical features of our blast protocol (including a pressure/time profile, overpressure, positive phase and duration) [83], which will help the comparison of these studies to other models of blast-mediated and non-blast mediated TBI. It should be noted that the visual and cognitive dysfunction we have previously reported using our model [11, 12, 13, 84] is bilateral, suggesting a lack of effect from blunt impacts, and these same dysfunctions are frequently observed in Soldiers and Veterans exposed to blast injury [37, 38, 42, 43, 85, 86]. This study also only focused on a single brain region, whereas past studies have focused globally on the brain or brain-connected fluids [23]. While this does not limit the impact of our study in beginning to define the molecular events that lead to sensory damage after blast-mediated TBI, we do not know if other regions of the brain, or other CNS neurons, respond similarly to blast injury. Our study, performed at a single time point following injury, is also only a “snapshot” of the changes in brain proteins and it does not address how protein expression changes over time. Completion of additional replicates, whether by PELS or by other technologies, will be an important component of future work, in addition to analysis of expression change over time.
Regarding the modeling with mice, our study was conducted in a single strain and sex of mice. We selected the strain (C57BL/6J) because it represents the most widely utilized genetic background of mice. However, our choice of species, genetic background, and sex could all bias the results, and it will be important to extend future studies to females and also different strains and species. Also of relevance, the brain tissue and sera were pooled to analyze proteins that change after blast injury. While it is important to examine the expression profiles of these proteins and auto-antibodies in individual mice, it was beyond the current scope to perform replication studies of candidates within individual mice to assess individual variability of the findings. It should be noted that the method of inducing blast-mediated TBI that was used in this study results in uniform damage among mice exposed to blast. Previously published transcriptomic studies using this model demonstrates very little intra-animal variability in gene expression changes [87].
Finally, regarding our proteomic methodology, it is important to re-iterate that we only sought to identify proteins with increased abundance in response to blast. We did not attempt to identify proteins that might have decreased abundance in response to blast. As with all protein studies, there is the possibility that protein-protein interactions may exist, although we believe that the likelihood of this happening in this study is low, given the high confidence of the identification methods used, the fact that proteins identified in certain fractions were expected, and the fact that our fractionation and differentiation approaches are widely used and designed to reduce this possibility.
In summary, a combined PAD and PELS analysis identified six proteins with properties appropriate for further consideration as biomarkers of blast-mediated TBI (ALDOA, PHKB, HBA-A1, DPYSL2, SYN1, and CKB), as well as several others potentially relevant to the incompletely understood mechanisms of damage caused by blast-exposure. In our opinion, HBA-A1 stands out as a leading candidate for further testing as a biomarker, and cellular responses involving myelin seem likely to be of mechanistic importance, both of which we intend to test in our ongoing work.
Declarations
Author contribution statement
Matthew M. Harper, Manohar John: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Danielle Rudd, Kacie J. Meyer, Edwin Vázquez-Rosa, Min-Kyoo Shin, Kalyani Chaubey, Yeojung Koh, Lucy P. Evans, Michael G. Anderson, Indira T. Kudva: Analyzed and interpreted the data; Wrote the paper.
Anumantha G Kanthasamy, Vellareddy Anantharam: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Andrew A. Pieper, Alexander G. Bassuk: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Laura Dutca: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Department of Veterans Affairs (RX000952), Department of Defense (W81XWH-14-1-0583), and the Iowa City VA Center for the Prevention and Treatment of Visual Loss. AAP is supported by the Brockman Medical Research Foundation, Hamilton, Bermuda, the Elizabeth Ring Mather & William Gwinn Mather Fund, Cleveland, OH, USA, the S. Livingston Samuel Mather Trust, Cleveland, OH, USA, and Department of Veterans Affairs Merit Review 1IO1BX002444 to AAP. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs, Department of Defense, or the U.S. Government.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Brundage J.F., Taubman S.B., Hunt D.J., Clark L.L. Whither the "signature wounds of the war" after the war: estimates of incidence rates and proportions of TBI and PTSD diagnoses attributable to background risk, enhanced ascertainment, and active war zone service, active component, U.S. Armed Forces, 2003-2014. MSMR. 2015;22(2):2–11. [PubMed] [Google Scholar]
- 2.Mac Donald C.L., Barber J., Jordan M., Johnson A.M., Dikmen S., Fann J.R. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol. 2017;74(7):821–829. doi: 10.1001/jamaneurol.2017.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg M.S. Casualty rates of US military personnel during the wars in Iraq and Afghanistan. Defence Peace Econ. 2018;29(1):44–61. [Google Scholar]
- 4.Greer N., Sayer N., Kramer M., Koeller E., Velasquez T. 2016. Prevalence and Epidemiology of Combat Blast Injuries from the Military Cohort 2001-2014. VA Evidence-Based Synthesis Program Reports. Washington (DC) [PubMed] [Google Scholar]
- 5.Wang E.W., Huang J.H. Understanding and treating blast traumatic brain injury in the combat theater. Neurol. Res. 2013;35(3):285–289. doi: 10.1179/1743132812Y.0000000138. [DOI] [PubMed] [Google Scholar]
- 6.Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6(2):244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4(134):134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H.J., Sajja V.S., Vandevord P.J., Lee Y.W. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience. 2013;253:9–20. doi: 10.1016/j.neuroscience.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 9.Evans L.P., Newell E.A., Mahajan M., Tsang S.H., Ferguson P.J., Mahoney J. Acute vitreoretinal trauma and inflammation after traumatic brain injury in mice. Ann. Clin. Transl. Neurol. 2018;5(3):240–251. doi: 10.1002/acn3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehily B., Fitzgerald M. Repeated mild traumatic brain injury: potential mechanisms of damage. Cell Transplant. 2017;26(7):1131–1155. doi: 10.1177/0963689717714092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan K., Kecova H., Hernandez-Merino E., Kardon R.H., Harper M.M. Retinal ganglion cell damage in an experimental rodent model of blast-mediated traumatic brain injury. Invest. Ophthalmol. Vis. Sci. 2013;54(5):3440–3450. doi: 10.1167/iovs.12-11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutca L.M., Stasheff S.F., Hedberg-Buenz A., Rudd D.S., Batra N., Blodi F.R. Early detection of subclinical visual damage after blast-mediated TBI enables prevention of chronic visual deficit by treatment with P7C3-S243. Invest. Ophthalmol. Vis. Sci. 2014;55(12):8330–8341. doi: 10.1167/iovs.14-15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin T.C., Britt J.K., De Jesus-Cortes H., Lu Y., Genova R.M., Khan M.Z. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 2014;8(6):1731–1740. doi: 10.1016/j.celrep.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouzon B., Bachmeier C., Ojo J., Acker C., Ferguson S., Crynen G. Chronic white matter degeneration, but No tau pathology at one-year post-repetitive mild traumatic brain injury in a tau transgenic model. J. Neurotrauma. 2018 doi: 10.1089/neu.2018.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold E.M., Vasilevko V., Hasselmann J., Tiefenthaler C., Hoa D., Ranawaka K. Repeated mild closed head injuries induce long-term white matter pathology and neuronal loss that are correlated with behavioral deficits. ASN Neuro. 2018;10 doi: 10.1177/1759091418781921. 1759091418781921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawyer T.W., Wang Y., Ritzel D.V., Josey T., Villanueva M., Shei Y. High-fidelity simulation of primary blast: direct effects on the head. J. Neurotrauma. 2016;33(13):1181–1193. doi: 10.1089/neu.2015.3914. [DOI] [PubMed] [Google Scholar]
- 17.Gyoneva S., Kim D., Katsumoto A., Kokiko-Cochran O.N., Lamb B.T., Ransohoff R.M. Ccr2 deletion dissociates cavity size and tau pathology after mild traumatic brain injury. J. Neuroinflammation. 2015;12:228. doi: 10.1186/s12974-015-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin N., Kim H.G., Shin H.J., Kim S., Kwon H.H., Baek H. Uncoupled endothelial nitric oxide synthase enhances p-tau in chronic traumatic encephalopathy mouse model. Antioxidants Redox Signal. 2018 doi: 10.1089/ars.2017.7280. [DOI] [PubMed] [Google Scholar]
- 19.Wu L., Chung J.Y., Saith S., Tozzi L., Buckley E.M., Sanders B. Repetitive head injury in adolescent mice: a role for vascular inflammation. J. Cerebr. Blood Flow Metabol. 2018 doi: 10.1177/0271678X18786633. 271678X18786633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouzon B., Saltiel N., Ferguson S., Ojo J., Lungmus C., Lynch C. Impact of age on acute post-TBI neuropathology in mice expressing humanized tau: a Chronic Effects of Neurotrauma Consortium Study. Brain Inj. 2018;32(10):1285–1294. doi: 10.1080/02699052.2018.1486457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marion C.M., Radomski K.L., Cramer N.P., Galdzicki Z., Armstrong R.C. Experimental traumatic brain injury identifies distinct early and late phase Axonal conduction deficits of white matter pathophysiology, and reveals intervening recovery. J. Neurosci. 2018;38(41):8723–8736. doi: 10.1523/JNEUROSCI.0819-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch R.D., Ayaz S.I., Lewis L.M., Unden J., Chen J.Y., Mika V.H. Ability of serum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J. Neurotrauma. 2016;33(2):203–214. doi: 10.1089/neu.2015.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X.J., Glushakova O., Mondello S., Van K., Hayes R.L., Lyeth B.G. Acute temporal profiles of serum levels of UCH-L1 and GFAP and relationships to neuronal and astroglial pathology following traumatic brain injury in rats. J. Neurotrauma. 2015;32(16):1179–1189. doi: 10.1089/neu.2015.3873. [DOI] [PubMed] [Google Scholar]
- 24.Mondello S., Schmid K., Berger R.P., Kobeissy F., Italiano D., Jeromin A. The challenge of mild traumatic brain injury: role of biochemical markers in diagnosis of brain damage. Med. Res. Rev. 2014;34(3):503–531. doi: 10.1002/med.21295. [DOI] [PubMed] [Google Scholar]
- 25.Chmielewska N., Szyndler J., Makowska K., Wojtyna D., Maciejak P., Plaznik A. Looking for novel, brain-derived, peripheral biomarkers of neurological disorders. Neurol. Neurochir. Pol. 2018;52(3):318–325. doi: 10.1016/j.pjnns.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Koutroukides T.A., Guest P.C., Leweke F.M., Bailey D.M., Rahmoune H., Bahn S. Characterization of the human serum depletome by label-free shotgun proteomics. J. Separ. Sci. 2011;34(13):1621–1626. doi: 10.1002/jssc.201100060. [DOI] [PubMed] [Google Scholar]
- 27.Kudva I.T., Krastins B., Sheng H., Griffin R.W., Sarracino D.A., Tarr P.I. Proteomics-based expression library screening (PELS): a novel method for rapidly defining microbial immunoproteomes. Mol. Cell. Proteomics. 2006;5(8):1514–1519. doi: 10.1074/mcp.T600013-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudva I., Krastins B., Torres A., Griffin R., Sheng H., Sarracino D. The Escherichia coli O157:H7 cattle immune-proteome includes outer membrane protein A (OmpA), a modulator of adherence to bovine recto-anal junction squamous epithelial (RSE) cells. Proteomics. 2015;15:1829–1842. doi: 10.1002/pmic.201400432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y.F., Radivojac P. Computational approaches to protein inference in shotgun proteomics. BMC Bioinf. 2012;13(Suppl 16):S4. doi: 10.1186/1471-2105-13-S16-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlwain S., Mathews M., Bereman M.S., Rubel E.W., MacCoss M.J., Noble W.S. Estimating relative abundances of proteins from shotgun proteomics data. BMC Bioinf. 2012;13:308. doi: 10.1186/1471-2105-13-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nesvizhskii A.I., Keller A., Kolker E., Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75(17):4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 32.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Kirov S., Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Duncan D., Shi Z., Zhang B. WEB-based GEne SeT AnaLysis toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–83. doi: 10.1093/nar/gkt439. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Qian W.J., Chin M.H., Petyuk V.A., Barry R.C., Liu T. Characterization of the mouse brain proteome using global proteomic analysis complemented with cysteinyl-peptide enrichment. J. Proteome Res. 2006;5(2):361–369. doi: 10.1021/pr0503681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cockerham G.C., Goodrich G.L., Weichel E.D., Orcutt J.C., Rizzo J.F., Bower K.S. Eye and visual function in traumatic brain injury. J. Rehabil. Res. Dev. 2009;46(6):811–818. doi: 10.1682/jrrd.2008.08.0109. [DOI] [PubMed] [Google Scholar]
- 38.Cockerham G.C., Lemke S., Glynn-Milley C., Zumhagen L., Cockerham K.P. Visual performance and the ocular surface in traumatic brain injury. Ocul. Surf. 2013;11(1):25–34. doi: 10.1016/j.jtos.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Gallun F.J., Diedesch A.C., Kubli L.R., Walden T.C., Folmer R.L., Lewis M.S. Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. J. Rehabil. Res. Dev. 2012;49(7):1005–1025. doi: 10.1682/jrrd.2012.03.0038. [DOI] [PubMed] [Google Scholar]
- 40.Gallun F.J., Lewis M.S., Folmer R.L., Diedesch A.C., Kubli L.R., McDermott D.J. Implications of blast exposure for central auditory function: a review. J. Rehabil. Res. Dev. 2012;49(7):1059–1074. doi: 10.1682/jrrd.2010.09.0166. [DOI] [PubMed] [Google Scholar]
- 41.Goodrich G.L., Flyg H.M., Kirby J.E., Chang C.Y., Martinsen G.L. Mechanisms of TBI and visual consequences in military and veteran populations. Optom. Vis. Sci. 2013;90(2):105–112. doi: 10.1097/OPX.0b013e31827f15a1. [DOI] [PubMed] [Google Scholar]
- 42.Lemke S., Cockerham G.C., Glynn-Milley C., Cockerham K.P. Visual quality of life in veterans with blast-induced traumatic brain injury. JAMA Ophthalmol. 2013;131(12):1602–1609. doi: 10.1001/jamaophthalmol.2013.5028. [DOI] [PubMed] [Google Scholar]
- 43.Lemke S., Cockerham G.C., Glynn-Milley C., Lin R., Cockerham K.P. Automated perimetry and visual dysfunction in blast-related traumatic brain injury. Ophthalmology. 2016;123(2):415–424. doi: 10.1016/j.ophtha.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Saunders G.H., Frederick M.T., Arnold M., Silverman S., Chisolm T.H., Myers P. Auditory difficulties in blast-exposed Veterans with clinically normal hearing. J. Rehabil. Res. Dev. 2015;52(3):343–360. doi: 10.1682/JRRD.2014.11.0275. [DOI] [PubMed] [Google Scholar]
- 45.Owens B.D., Kragh J.F., Jr., Wenke J.C., Macaitis J., Wade C.E., Holcomb J.B. Combat wounds in operation Iraqi freedom and operation enduring freedom. J. Trauma. 2008;64(2):295–299. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 46.Murray C.K., Reynolds J.C., Schroeder J.M., Harrison M.B., Evans O.M., Hospenthal D.R. Spectrum of care provided at an echelon II medical unit during operation Iraqi freedom. Mil. Med. 2005;170(6):516–520. doi: 10.7205/milmed.170.6.516. [DOI] [PubMed] [Google Scholar]
- 47.Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swan A.A., Nelson J.T., Pogoda T.K., Amuan M.E., Akin F.W., Pugh M.J. Sensory dysfunction and traumatic brain injury severity among deployed post-9/11 veterans: a chronic effects of neurotrauma consortium study. Brain Inj. 2018;32(10):1197–1207. doi: 10.1080/02699052.2018.1495340. [DOI] [PubMed] [Google Scholar]
- 49.Reid M.W., Miller K.J., Lange R.T., Cooper D.B., Tate D.F., Bailie J. A multisite study of the relationships between blast exposures and symptom reporting in a post-deployment active duty military population with mild traumatic brain injury. J. Neurotrauma. 2014;31(23):1899–1906. doi: 10.1089/neu.2014.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belanger H.G., Proctor-Weber Z., Kretzmer T., Kim M., French L.M., Vanderploeg R.D. Symptom complaints following reports of blast versus non-blast mild TBI: does mechanism of injury matter? Clin. Neuropsychol. 2011;25(5):702–715. doi: 10.1080/13854046.2011.566892. [DOI] [PubMed] [Google Scholar]
- 51.DeWalt G.J., Eldred W.D. Visual system pathology in humans and animal models of blast injury. J. Comp. Neurol. 2017;525(13):2955–2967. doi: 10.1002/cne.24252. [DOI] [PubMed] [Google Scholar]
- 52.Choi J.H., Greene W.A., Johnson A.J., Chavko M., Cleland J.M., McCarron R.M. Pathophysiology of blast-induced ocular trauma in rats after repeated exposure to low-level blast overpressure. Clin. Exp. Ophthalmol. 2015;43(3):239–246. doi: 10.1111/ceo.12407. [DOI] [PubMed] [Google Scholar]
- 53.Hines-Beard J., Marchetta J., Gordon S., Chaum E., Geisert E.E., Rex T.S. A mouse model of ocular blast injury that induces closed globe anterior and posterior pole damage. Exp. Eye Res. 2012;99:63–70. doi: 10.1016/j.exer.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capo-Aponte J.E., Urosevich T.G., Temme L.A., Tarbett A.K., Sanghera N.K. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil. Med. 2012;177(7):804–813. doi: 10.7205/milmed-d-12-00061. [DOI] [PubMed] [Google Scholar]
- 55.Magone M.T., Kwon E., Shin S.Y. Chronic visual dysfunction after blast-induced mild traumatic brain injury. J. Rehabil. Res. Dev. 2014;51(1):71–80. doi: 10.1682/JRRD.2013.01.0008. [DOI] [PubMed] [Google Scholar]
- 56.Shedd D.F., Benko N.A., Jones J., Zaugg B.E., Peiffer R.L., Coats B. Long term temporal changes in structure and function of rat visual system After blast exposure. Invest. Ophthalmol. Vis. Sci. 2018;59(1):349–361. doi: 10.1167/iovs.17-21530. [DOI] [PubMed] [Google Scholar]
- 57.Allen R.S., Motz C.T., Feola A., Chesler K.C., Haider R., Ramachandra Rao S. Long-term functional and structural consequences of primary blast overpressure to the eye. J. Neurotrauma. 2018;35(17):2104–2116. doi: 10.1089/neu.2017.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vest V., Bernardo-Colon A., Watkins D., Kim B., Rex T.S. Rapid repeat exposure to subthreshold trauma causes synergistic axonal damage and functional deficits in the visual pathway in a mouse model. J. Neurotrauma. 2019;36(10):1646–1654. doi: 10.1089/neu.2018.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzekov R., Dawson C., Orlando M., Mouzon B., Reed J., Evans J. Sub-chronic neuropathological and biochemical changes in mouse visual system after repetitive mild traumatic brain injury. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon D.W., McGeachy M.J., Bayir H., Clark R.S.B., Loane D.J., Kochanek P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017;13(9):572. doi: 10.1038/nrneurol.2017.116. [DOI] [PubMed] [Google Scholar]
- 61.Puntambekar S.S., Saber M., Lamb B.T., Kokiko-Cochran O.N. Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury. Brain Behav. Immun. 2018;71:9–17. doi: 10.1016/j.bbi.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 62.Di Battista A.P., Rhind S.G., Hutchison M.G., Hassan S., Shiu M.Y., Inaba K. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J. Neuroinflammation. 2016;13:40. doi: 10.1186/s12974-016-0500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Javidi E., Magnus T. Autoimmunity after ischemic stroke and brain injury. Front. Immunol. 2019;10:686. doi: 10.3389/fimmu.2019.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Struebing F.L., King R., Li Y., Chrenek M.A., Lyuboslavsky P.N., Sidhu C.S. Transcriptional changes in the mouse retina after ocular blast injury: a role for the immune system. J. Neurotrauma. 2018;35(1):118–129. doi: 10.1089/neu.2017.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y., Yang Z., Liu B., Valdez C., Chavko M., Cancio L.C. Low-level primary blast induces neuroinflammation and neurodegeneration in rats. Mil. Med. 2019;184(Supplement_1):265–272. doi: 10.1093/milmed/usy330. [DOI] [PubMed] [Google Scholar]
- 67.Newell E.A., Todd B.P., Mahoney J., Pieper A.A., Ferguson P.J., Bassuk A.G. Combined blockade of interleukin-1alpha and -1beta signaling protects mice from cognitive dysfunction after traumatic brain injury. eNeuro. 2018;5(2) doi: 10.1523/ENEURO.0385-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capo-Aponte J.E., Jorgensen-Wagers K.L., Sosa J.A., Walsh D.V., Goodrich G.L., Temme L.A. Visual dysfunctions at different stages after blast and non-blast mild traumatic brain injury. Optom. Vis. Sci. 2017;94(1):7–15. doi: 10.1097/OPX.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 69.Arevalo-Martin A., Grassner L., Garcia-Ovejero D., Paniagua-Torija B., Barroso-Garcia G., Arandilla A. Elevated autoantibodies in subacute human spinal cord injury are naturally occurring antibodies. Front. Immunol. 2018;9:2365. doi: 10.3389/fimmu.2018.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richter F., Meurers B.H., Zhu C., Medvedeva V.P., Chesselet M.F. Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J. Comp. Neurol. 2009;515(5):538–547. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis T.L., Walker J.R., Campagna-Slater V., Finerty P.J., Paramanathan R., Bernstein G. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010;8(7) doi: 10.1371/journal.pbio.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toubi E., Shoenfeld Y. Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmun. Rev. 2007;6(3):119–125. doi: 10.1016/j.autrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Egg R., Reindl M., Deisenhammer F., Linington C., Berger T. Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult. Scler. 2001;7(5):285–289. doi: 10.1177/135245850100700503. [DOI] [PubMed] [Google Scholar]
- 74.Song H., Konan L.M., Cui J., Johnson C.E., Langenderfer M., Grant D. Ultrastructural brain abnormalities and associated behavioral changes in mice after low-intensity blast exposure. Behav. Brain Res. 2018;347:148–157. doi: 10.1016/j.bbr.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Rubovitch V., Zilberstein Y., Chapman J., Schreiber S., Pick C.G. Restoring GM1 ganglioside expression ameliorates axonal outgrowth inhibition and cognitive impairments induced by blast traumatic brain injury. Sci. Rep. 2017;7:41269. doi: 10.1038/srep41269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubovitch V., Ten-Bosch M., Zohar O., Harrison C.R., Tempel-Brami C., Stein E. A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 2011;232(2):280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minarik P., Tomaskova N., Kollarova M., Antalik M. Malate dehydrogenases--structure and function. Gen. Physiol. Biophys. 2002;21(3):257–265. [PubMed] [Google Scholar]
- 78.Chen H., Chan Y.L., Nguyen L.T., Mao Y., de Rosa A., Beh I.T. Moderate traumatic brain injury is linked to acute behaviour deficits and long term mitochondrial alterations. Clin. Exp. Pharmacol. Physiol. 2016;43(11):1107–1114. doi: 10.1111/1440-1681.12650. [DOI] [PubMed] [Google Scholar]
- 79.Jing Z., Xing J., Chen X., Stetler R.A., Weng Z., Gan Y. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J. Cerebr. Blood Flow Metabol. 2014;34(10):1613–1621. doi: 10.1038/jcbfm.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang G., Han T., Nijhawan D., Theodoropoulos P., Naidoo J., Yadavalli S. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158(6):1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blaya M.O., Bramlett H.M., Naidoo J., Pieper A.A., Dietrich W.D. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J. Neurotrauma. 2014;31(5):476–486. doi: 10.1089/neu.2013.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blaya M.O., Wasserman J.M., Pieper A.A., Sick T.J., Bramlett H.M., Dietrich W.D. Neurotherapeutic capacity of P7C3 agents for the treatment of traumatic brain injury. Neuropharmacology. 2019;145(Pt B):268–282. doi: 10.1016/j.neuropharm.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harper M.M. Author response: pressure wave dosimetry for "Retinal ganglion cell damage in an experimental rodent model of blast-mediated traumatic brain injury. Invest. Ophthalmol. Vis. Sci. 2014;55(3):1350–1351. doi: 10.1167/iovs.13-13692. [DOI] [PubMed] [Google Scholar]
- 84.Yin T.C., Voorhees J.R., Genova R.M., Davis K.C., Madison A.M., Britt J.K. Acute axonal degeneration drives development of cognitive, motor, and visual deficits after blast-mediated traumatic brain injury in mice. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0220-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barker F., Cockerham G., Goodrich G., Hartwick A., Kardon R., Mick A.B. Brain injury impact on the eye and vision. Optom. Vis. Sci. 2017;94(1):4–6. doi: 10.1097/OPX.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 86.Cockerham G.C., Rice T.A., Hewes E.H., Cockerham K.P., Lemke S., Wang G. Closed-eye ocular injuries in the Iraq and Afghanistan wars. N. Engl. J. Med. 2011;364(22):2172–2173. doi: 10.1056/NEJMc1010683. [DOI] [PubMed] [Google Scholar]
- 87.Harper M.M., Woll A.W., Evans L.P., Delcau M., Akurathi A., Hedberg-Buenz A. Blast preconditioning protects retinal ganglion cells and reveals targets for prevention of neurodegeneration following blast-mediated traumatic brian injury. Invest. Ophthalmol. Vis. Sci. 2019;60(13):4159–4170. doi: 10.1167/iovs.19-27565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.