Abstract
Purpose
To assess the clinical efficacy of custom made PEEK patient-specific implants in treatment of orbital wall defects.
Methods
Forty-five patients with unilateral post-traumatic orbital wall defects were enrolled in the study. They underwent subsequent reconstructive procedures using PEEK patient-specific implants (PSI) or pre-bent titanium plates. All the patients were examined with the standardized algorithm, including local status examination, vision assessment and computer tomography (CT) with measurements of the orbital volume. A comparative analysis of the treatment outcomes in two groups of patients (pre-bent plates/PSI) was performed.
Results
The study findings show an absence of any postoperative infection, inflamation or decreased visual acuity in either group. In PSI group, diplopia after surgery was absent in 82.1% of patients versus 70.6% of controls. The mean duration of surgery was 54.25 ± 16.8 min with PSI application and 82.9 ± 10.8 min with pre-bent plates. The mean difference between the intact and damaged orbital volume was 1.9 ± 1.4 cm3 in the control group versus 0.74 ± 0.6 cm3 in PSI group (р<0.05).
Conclusion
PEEK PSI demonstrated higher clinical efficacy in comparison to pre-bent plates in orbital wall reconstruction especially in restoring the volume and shape of the damaged orbit.
Keywords: Orbital reconstruction, Patient-specific implants, PEEK
1. Introduction
Treatment of the orbital wall fractures is still a major challenge in maxillofacial surgery due to the high frequency and complexity of these injuries, which is associated with aesthetic and functional disorders.1, 2, 3 Traditional techniques commonly used for orbital reconstruction involve the application of standard titanium plates, meshes or polymeric implants.1,4 When using standard titanium plates, pre- or intraoperative bending and correction of their contours are needed. The proper installation and positioning of the implants inside the orbit are still difficult to accomplish. A lack of distal or medial support caused by the damage to the orbital ledge and/or intra-orbital buttress is a common problem associated with improper position of the pre-bent plates.3,5,6 The location of implants and their conformity to the individual anatomy of the damaged structures in size and shape are crucial for the integral success rate in orbital reconstruction.1,7,8, 9
Recent advances in CAD/CAM technology, which proved to be effective in management of facial bone defects, have attracted substantial interest among surgeons involved in orbital reconstruction.1,3,7,8,10 A number of authors have demonstrated the successful application of custom made patient-specific implants (PSI) for facial, including orbital reconstructions.1,3,10, 11, 12, 13, 14
Although the significant number of studies addresses the issues of cranioplasty and frontal bone reconstructions with PEEK implants, only few reports report the outcomes of the orbital wall reconstructions with PEEK PSI.15 This suggests the need for evidence-based improvement of PEEK PSI application in reconstructive surgery of the orbit.
The aim of this study was to assess the clinical efficacy of patient-specific PEEK implants in patients with post-traumatic orbital defects.
2. Material and methods
The medical records and CT data were collected for all patients operated for orbital defects at Kyiv Regional Centre for Maxillo-facial Surgery and Dentistry and at Kyiv Emergency Hospital, Kyiv, Ukraine during the period from 1/1/2013 to 31/12/2018. For the further analysis inclusion and exclusion criteria were applied to the sample.
Patients included to the study complied with the following criteria: unilateral fracture of the orbital floor and/or medial wall, orbital wall reconstruction with pre-bent titanium orbital plates or PEEK custom-made orbital implants, at least partial vision in both eyes before injury, written informed consent of the patient.
A total of 55 patients who had undergone orbital reconstruction procedures were selected. Of those, 45 patients (31 males and 14 females, aged from 17 to 54 years with mean age 35.3 ± 13.9 years) met inclusion and exclusion criteria and were enrolled in the study. All patients were divided in two groups according to the type of implants (PSI/pre-bent) used for orbital wall reconstruction. Group one (main) included 28 patients (7 females, 21 males), who had undergone orbital reconstruction procedures with PSI made of PEEK. Seventeen patients (5 females, 12 male), who had been treated with pre-bent orbital plates (KLS Martin orbital plate or Titamed BVBA orbital plate), composed group two (control). Both groups were equal regarding male/female ratio, mean age and trauma patterns.
Patients of both groups were examined preoperatively, then one week, one month and three months after surgery with the standardized algorithm, including the local status examination (enophthalm measuring), the evaluation of vision (visual acuity, diplopia and ocular mobility). Enophthalm evaluation was made with computer-assisted measurements and calculating tools by Zhang et al. (2010).16 Ocular motility and diplopia were assessed using the “follow my finger” test. All patients underwent computer tomography (CT) (Toshiba Activion 16 and Philips Diamond Select Brilliance CT 64, slice thickness - 0.5 mm) before and after surgery. Virtual orbital models of the damaged and intact sides were generated for all cases before and after surgery. Their volumes were measured in the software and compared for each individual case.16,17
Evaluation criteria for the clinical success rate were as follows: the comparison of residual enophthalmos degree in each group (values < 14 mm or a difference of >2 mm between two eyes were defined as enophthalmos),18 the accuracy of the orbital volume restoration compared to the volume of the intact orbit, the presence of diplopia, the duration of the surgical intervention, the occurrence of early and long-term postoperative complications.
PSI design was carried out in close collaboration between surgeons and biomedical engineers. Based on preoperative CT data (DICOM files without compression) biomedical engineers created the design of the implant and defined its optimal position inside the orbit with the participation and under control of surgeons. The implants were made by milling of radioopaque PEEK blocks (Merz Dental, Germany) on the machines with numerical control by (Imatek-Esco Ltd., Kyiv, Ukraine).
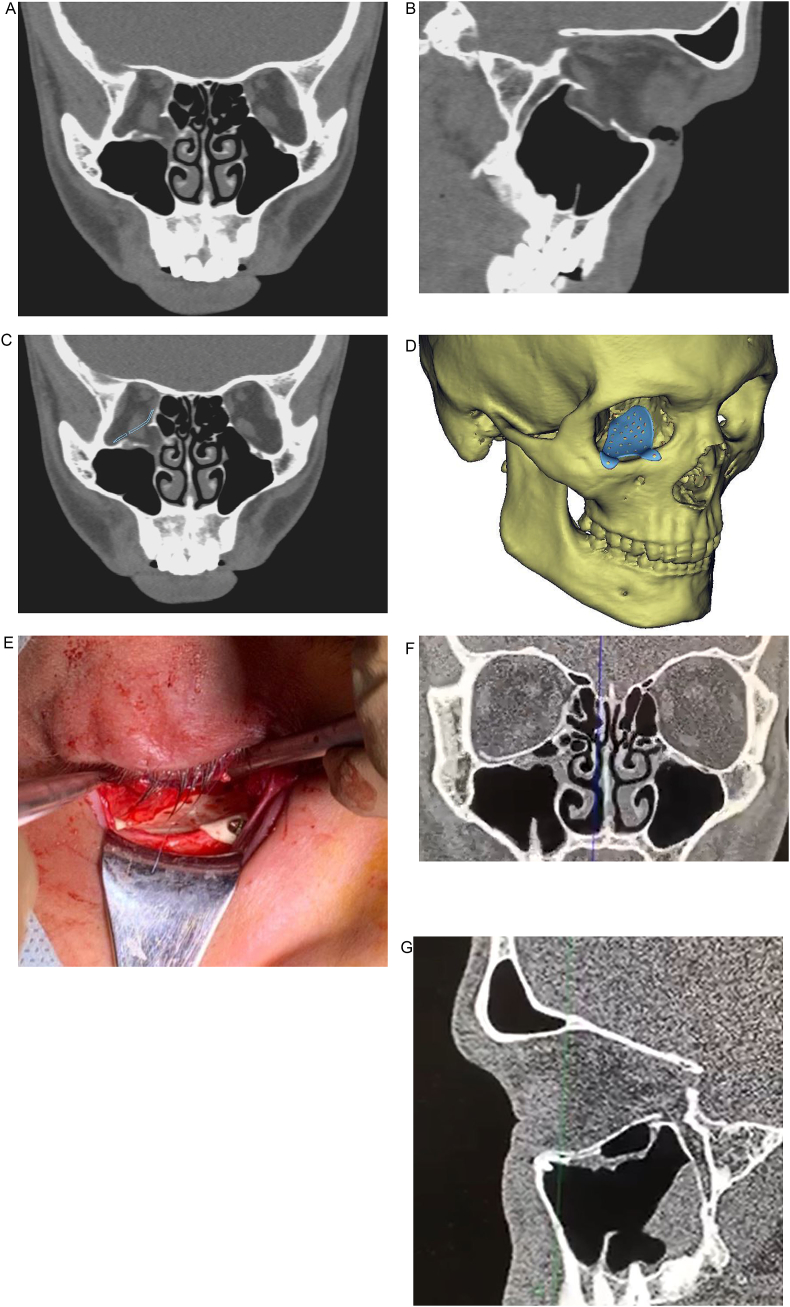
Titanium plates in control group were prefabricated manually before or during surgery using standard or individual plastic orbital models. All implants were sterilized the day before operation by autoclaving at 132 °C. Surgical procedures were equal in both groups (Fig. 1).
Fig. 1.
Patient A. with blowout fracture of the right orbit, three month after trauma: orbital reconstruction with PEEK PSI (a,b – CT-slices of the damaged orbital walls; с,d - virtual mode of the PSI positioned into the orbit; e − view of PSI in the wound after positioning in the orbit; f, g – CT-control with PSI reconstructed orbital floor).
Statistical analysis of the data included the calculation of mean values, and standard deviation for each parameter evaluated. Non-parametrical statistics was employed for analysis of the data. The Mann–Whitney U test and Pearson's chi-squared test were used to compare the differences between the parameters in the study groups. The level of significance was set at p < 0.05. The calculations were performed in SPSS Statistics software (IBM SPSS, USA).
3. Results
Post-traumatic defects arose from blowout orbital fractures. Clinical features and injury patterns of the patients are presented in Table 1. Preoperative examination revealed the presence of diplopia in 23 (82.1%) patients of the main group and in 15 (88.2%) controls. None of the patients of both groups developed inflammatory complications, decreased visual acuity or lost visual fields in postoperative period.
The measurements of volumes of 45 orbits on the intact side before and after surgery (actually, this volume did not change) showed the minor differences in all cases, which can be considered as measurement error. On average, they constituted 0.69 ± 0.7 cm3 (U = 987; Z = −0.206; p = 0.837). The average volume of the orbit on the intact side was 26.5 ± 2.9 cm3.
In the main group, diplopia was present in eight patients (28.6%) one month after surgery and in five patients (17.9%) three months after surgery. Ten patients of the control group had diplopia one month after surgery (58.8%) and only five (29.4%) - three months after the reconstruction. Motility disorders one month after surgery were totally absent in 26 cases (85.7%) in the main group versus 13 controls (76.5%) (χ2 = 2.458, р > 0.05). Three months after surgery, they were absent in 27 patients (92.8%) of the main group and in 15 controls (88.3%) (χ2 = 4.898, р > 0.05).
Statistical analysis of the functional disorders showed a higher rate of diplopia one month after surgery (χ2 = 4.03, р<0.05) in the control group. However, there were no statistically significant differences in presence of postsurgical diplopia three months after surgery (χ2 = 0.82, р > 0.05).
Mean duration of the surgery in the study groups was 54.25 ± 16.8 min with PSI application and 82.9 ± 10.8 min with pre-bent plates (U = 31.5; Z = −4.84; p < 0,001). Besides, there was a higher incidence of implant malpositioning in the control group (χ2 = 0,023, p < 0.05).
In the PSI group, the average volume of the intact orbits was 25.5 ± 2.4 cm3, whereas the average volume of the damaged orbits before reconstruction significantly increased and constituted 29.4 ± 4.0 cm3. The mean difference between the intact and injured sides was 3.9 ± 2.8 cm3.
After reconstructive surgery in this series, the average volume of the damaged orbits reduced to 25.6 ± 2.5 cm3. The mean difference with intact orbits after the surgical interventions was only 0.74 ± 0.6 cm3 (U = 365; Z = −0.44; p = 0.66).
In the control group, the average volume of the intact orbits was 27.4 ± 3.3 cm3, whereas the average volume of the damaged orbits before reconstruction significantly increased and constituted 32 ± 2.6 cm3. The mean difference between the intact and injured sides was 4.6 ± 2.4 cm3.
After reconstructive surgery in this series, the average volume of the damaged orbits reduced to 29.3 ± 2.7 cm3. The mean difference with intact orbits after the surgical interventions was 1.9 ± 1.4 (U = 89; Z = −1.91; p > 0.05).
Comparison of the findings in both groups showed that the mean orbital intact/damaged volume difference was significantly higher in the control group (U = 103; Z = −3.16; p < 0.01); controls also developed residual enophthalmos more frequently (χ2 = 0,023, p < 0.05).
4. Discussion
Orbital reconstruction is always a surgical challenge because of the complexity of its anatomy.2 The main goals of orbital reconstruction are elimination of the orbital wall defects with restoration of the orbital volume and correction of the globe position.3 This can be achieved by the use of different implants (titanium, PTFE, silicone, PE etc.). However, the major problem related to the their use is the complex and time-consuming adaptation to the shape of the injured orbit.3,4 The most difficult objective that determines functional recovery and a high aesthetic outcome of treatment is an accurate reconstruction of the orbital shape with restoration of its volume. This is especially important for the lower and medial walls, which form a ledge close to the orbital apex area and it is the main reason for unsatisfactory treatment results.3,5,7,8,17,19
Various studies demonstrate similar results regarding the efficacy of the pre-bent titanium plates in orbital volume restoration. The mean difference between the intact/damaged orbital volume is within the range of 1.6–2.4 cm3.16,20 Our study showed the same results: 1.9 ± 1.4 cm3 with 29.4% of residual enophthalmos. Whitehouse R. et al. reported that an increased orbital volume of >2 cm3 could result in significant functional and aesthetic sequelae such as diplopia and enophthalmos.19,21 The majority of studies devoted to pre-bent orbital plates, including this study, showed the results approaching this value. At the same time, according to Zieliński R. and co-authors,22 the use of standard plates was not associated with worse functional outcomes compared to PSI, but it required time-consuming intraoperative adaptation and demonstrated a higher level of bleeding during surgery.
Previous series proved high efficacy of PSI application in orbital volume and shape restoration as well as in correction of the residual enophthtalmos. Our study demonstrated similar results with 0.74 ± 0.6 cm3 of mean difference between damaged and intact orbit after surgery. Besides, the incidence of residual enophthalmos was low (3.7%).14,20,23,24
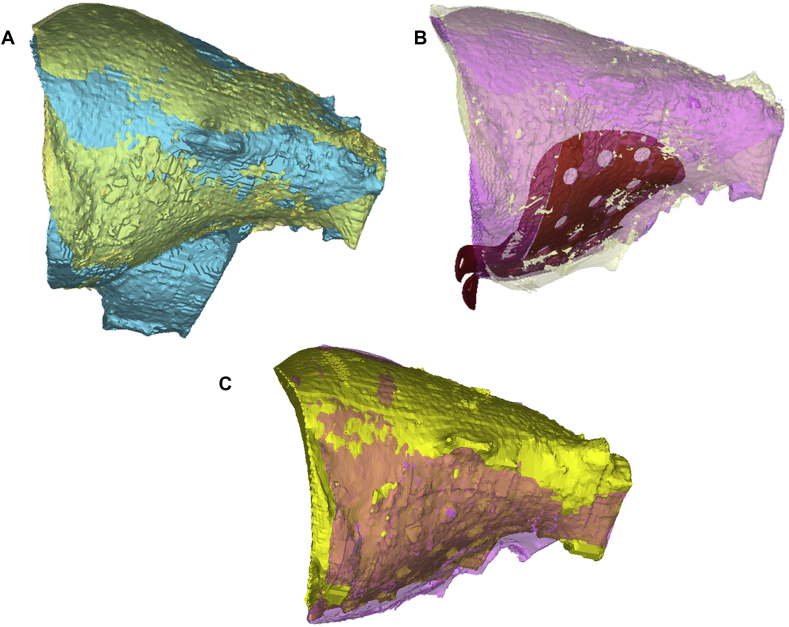
Virtual preoperative simulation was used for the estimation of the implant position inside the orbit, and its interrelationships with bony structures, nerves and oculomotor muscles. In the cases where collisions between the implant and anatomical structures were seen, the design was changed. (Fig. 2).
Fig. 2.
Comparison of presurgical planning and postsurgical outcome with PEEK PSI orbital reconstruction (a – superimposition of the damaged and intact virtual orbital models; b - superimposition of the pre-planned reconstructed (with designed PSI) and intact virtual orbital models; c - superimposition of the virtual orbital model: intact and damaged after surgical reconstruction).
Our results are indicative of higher clinical efficacy of PSIs when compared to pre-band titanium implants in orbital volume reconstruction. The incidence of residual enophthalmos in the PSI group was also significantly lower. There were no differences in motility or diplopia between both groups three months after surgery. However, the statistically significant differences in diplopia one month after surgery exhibited the presence of less favourable conditions for rehabilitation after the orbital trauma in patients of the control group.
The differences in duration of surgery, could be explained by lengthy procedures of the additional adaptation of the pre-bent standard implants inside the orbit. According to the literature, both techniques are quite time-consuming, due to pre- or intraoperative adaptation to the orbital anatomy (“pre-bending”) or CAD/CAM procedures. However, the time, spent to creation of the PSI design, is working time of the engineer, not surgeons, patient or anaesthesiologist without stress and with opportunity to verify virtually as much, as you can. Our study provides some evidence that the treatment of orbital wall fractures with PSI is clinically and technically quite simple and predictable.3,16,20,22,24
The results obtained correlate with those reported by other authors. Zieliński et al.22 showed the presence of motility disorders after CAD/CAM-assisted orbital reconstructions in 29% and 13% of cases one and six months after surgery, respectively. According to the multicentre study by R. M. Zimmerer et al.,20 when using PSIs, there was a statistically significant reduction in the time of surgical intervention. In the above study, the average time of surgery was about 60 min, which corresponds to the results obtained in the present study. Motility disorders after orbital reconstruction with PSI were seen in 15.8% of cases one month and in 3.3% of cases four months after surgery. Diplopia was found in 35.8% of cases one month after surgery. The value decreased to 24.6% four months after surgery. These results are almost similar to those obtained in our study.
The use PEEK for PSI manufacturing was conditioned by its favourable characteristics and the convenient manufacturing processes. PEEK is thermoplastic material with good imaging properties, stiffness, durability, light weight, fatigue, chemical resistance and modulus of elasticity close to the cortical bone. Allergic reactions to PEEK are extremely rare.11,13,25 As for our experience, the main advantages of PEEK PSI for orbital reconstruction are its mechanical properties, easy and fast manufacturing and high biocompatibility. We also took into consideration the manufacturer's recommendations which made it possible to produce the implants with the thickness of more than 0.4 mm by milling of PEEK. Well known, that it is difficult to achieve the same thickness by the selective laser sintering of titanium. Metal milling is more time-consuming technology, when timing in orbital reconstruction continues to be a very important factor, which sometimes limits PSI application for orbital reconstruction. In our series, neither clinical nor radiological manifestations of inflammatory complications, including sinusitis, caused by PEEK implants were observed at minimum follow-up of 1 year.
5. Conclusion
The obtained results are supportive PSI advantages such as reduced surgical time, precision and predictability in the treatment of orbital defects. PEEK PSI demonstrated higher clinical efficacy in comparison to pre-bent plates in orbital reconstruction especially in restoring the volume and shape of the damaged orbit. Possible advantages of PEEK PSI for orbital reconstruction are its mechanical properties, easy and fast manufacturing and high biocompatibility.
Funding
No funding received.
CRediT authorship contribution statement
Yurii Chepurnyi: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Writing - original draft. Denis Chernogorskyi: Formal analysis, Software, Visualization. Andrey Kopchak: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing. Oksana Petrenko: Formal analysis, Methodology, Supervision, Validation.
Declaration of competing interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. It was approved by the National Medical University Bioethics Committee (Protocol No 126). This article does not contain any studies with animals performed by any of the authors.
Informed consent: informed consent was obtained from all individual participants included in the study.
Acknowledgments
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript. The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2020.01.006.
Contributor Information
Yurii Chepurnyi, Email: 80667788837@ukr.net.
Denis Chernogorskyi, Email: cher103@meta.ua.
Andrey Kopchak, Email: kopchak@ua.fm.
Oksana Petrenko, Email: visionpetrenko@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baumann A., Sinko K., Dorner G. Late reconstruction of the orbit with patient-specific implants using computer-aided planning and navigation. J Oral Maxillofac Surg. 2015;73:101–106. doi: 10.1016/j.joms.2015.06.149. [DOI] [PubMed] [Google Scholar]
- 2.Patel N., Kim B., Zaid W. Use of virtual surgical planning for simultaneous maxillofacial osteotomies and custom polyetheretherketone implant in secondary orbito-frontal reconstruction: importance of restoring orbital volume. J Craniofac Surg. 2017;28:387–390. doi: 10.1097/SCS.0000000000003313. [DOI] [PubMed] [Google Scholar]
- 3.Mommaerts M., Büttner M., Vercruysse H., Jr., Wauters L., Beerens M. Orbital wall reconstruction with two-piece puzzle 3d printed implants: technical note Craniomaxillofac Trauma. Reconstr. 2016;9:55–61. doi: 10.1055/s-0035-1563392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Totir M., Ciuluvica R., Dinu I., Careba I., Gradinaru S. Biomaterials for orbital fractures repair. J Med Life. 2015;8(1):41–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois L., Jansen J., Schreurs R., Saeed Predictability in orbital reconstruction: a human cadaver study. Part I: endoscopic-assisted orbital reconsruction. J Cranio-Maxillo-Fac Surg. 2015;43(10):2034–2041. doi: 10.1016/j.jcms.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Kunz C., Audigé L., Cornelius C., Buitrago-Téllez C., Rudderman R., Prein J. The comprehensive AOCMF classification system: orbital fractures - level 3 tutorial. Craniomaxillofacial Trauma Reconstr. 2014;7(Suppl 1):S92–S102. doi: 10.1055/s-0034-1389562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois L., Jansen J., Schreurs R. Predictability in orbital reconstruction: a human cadaver study. Part II: navigation-assisted orbital reconsruction. J Cranio-Maxillo-Fac Surg. 2015;43(10):2042–2049. doi: 10.1016/j.jcms.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Dubois L., Jansen J., Schreurs R. Predictability in orbital reconstruction: a human cadaver study. Part III: implant – oriented navigation for optimized reconsruction. J Cranio-Maxillo-Fac Surg. 2015;43(10):2050–2056. doi: 10.1016/j.jcms.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Frodel J. Computer-designed implants for fronto-orbital defect reconstruction. Facial Plast Surg. 2008;24(1):22–34. doi: 10.1055/s-2007-1021459. [DOI] [PubMed] [Google Scholar]
- 10.Soleman J., Thieringer F., Beinemann J., Kunz C., Guzman R. Computer-assisted virtual planning and surgical template fabrication for frontoorbital advancement. Neurosurg Focus. 2015;38(5):E5. doi: 10.3171/2015.3.FOCUS14852. [DOI] [PubMed] [Google Scholar]
- 11.Goodson M., Farr D., Keith D., Banks R. Use of two-piece polyetheretherketone (PEEK) implants in orbitozygomatic reconstruction. Br J Oral Maxillofac Surg. 2012;50(3):268–269. doi: 10.1016/j.bjoms.2011.04.077. [DOI] [PubMed] [Google Scholar]
- 12.Williams V., Revington P. Novel use of an aerospace selective laser sintering machine for rapid prototyping of an orbital blowout fracture. Int J Oral Maxillofac Surg. 2010;39:182–184. doi: 10.1016/j.ijom.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Gerbino G., Zavattero E., Zenga F., Bianchi F., Garzino-Demo P., Berrone S. Primary and secondary reconstruction of complex craniofacial defects using polyetheretherketone custom-made implants. J Cranio-Maxillo-Fac Surg. 2015;43(8):1356–1363. doi: 10.1016/j.jcms.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Gander T., Essig H., Metzler P. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J Cranio-Maxillo-Fac Surg. 2015;3(1):126–130. doi: 10.1016/j.jcms.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Herford A., Miller M., Lauritano F., Cervino G., Signorino F., Maiorana C. The use of virtual surgical planning and navigation in the treatment of orbital trauma. Chin J Traumatol. 2017;20(1):9–13. doi: 10.1016/j.cjtee.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., He Y., Zhang Zh, An J. Evaluation of the application of computer-aided shape-adapted fabricated titanium mesh for mirroring-reconstructing orbital walls in cases of late post-traumatic enophthalmos. J Oral Maxillofac Surg. 2010;68:2070–2075. doi: 10.1016/j.joms.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Essig H., Dresse L., Rana Majeed. Precision of posttraumatic primary orbital reconstruction using individually bent titanium mesh with and without navigation: a retrospective study. Head Face Med. 2013;9:18. doi: 10.1186/1746-160X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cline R.A., Rootman J. Enophthalmos: a clinical review. Ophthalmology. 1984;91(3):229–237. doi: 10.1016/s0161-6420(84)34299-3. [DOI] [PubMed] [Google Scholar]
- 19.Jansen J., Dubois L., Schreurs R. Should virtual mirroring be used in the preoperative planning of an orbital reconstruction? J Oral Maxillofac Surg. 2018;76(2):380–387. doi: 10.1016/j.joms.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerer R., Ellis E., III, Aniceto G. Prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J Cranio-Maxillo-Fac Surg. 2016;44(9):1485–1497. doi: 10.1016/j.jcms.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Whitehouse R., Batterbury M., Jackson A., Noble J. Prediction of enophthalmos by computed tomography after ‘blow out’ orbital fracture. Br J Ophthalmol. 1994;78(8):618–620. doi: 10.1136/bjo.78.8.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zieliński R., Malińska M., Kozakiewicz M. Classical versus custom orbital wall reconstruction: selected factors regarding surgery and hospitalization. J Cranio-Maxillo-Fac Surg. 2017;45(5):710–715. doi: 10.1016/j.jcms.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Tarsitano A., Badiali G., Pizzigallo A., Marchetti C. Orbital reconstruction: patient-specific orbital floor reconstruction using a mirroring technique and a customized titanium mesh. J Craniofac Surg. 2016;27:1822–1825. doi: 10.1097/SCS.0000000000002907. [DOI] [PubMed] [Google Scholar]
- 24.Kärkkäinen M., Wilkman T., Mesimäki K., Snäll J. Primary reconstruction of orbital fractures using patient-specific titanium milled implants: the Helsinki protocol. Br J Oral Maxillofac Surg. 2018;56(9):791–796. doi: 10.1016/j.bjoms.2018.08.008. Epub 2018 Sep. 13. [DOI] [PubMed] [Google Scholar]
- 25.Jalbert F., Boetto S., Nadon F., Lauwers F., Schmidt E., Lopez R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J Cranio-Maxillo-Fac Surg. 2014;42(2):141–148. doi: 10.1016/j.jcms.2013.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.