Abstract
Background
An occlusion or stenosis of intracranial large arteries can be detected in the acute phase of ischaemic stroke in about 42% of patients. The approved therapies for acute ischaemic stroke are thrombolysis with intravenous recombinant tissue plasminogen activator (rt‐PA), and mechanical thrombectomy; both aim to recanalise an occluded intracranial artery. The reference standard for the diagnosis of intracranial stenosis and occlusion is intra‐arterial angiography (IA) and, recently, computed tomography angiography (CTA) and magnetic resonance angiography (MRA), or contrast‐enhanced MRA. Transcranial Doppler (TCD) and transcranial colour Doppler (TCCD) are useful, rapid, noninvasive tools for the assessment of intracranial large arteries pathology. Due to the current lack of consensus regarding the use of TCD and TCCD in clinical practice, we systematically reviewed the literature for studies assessing the diagnostic accuracy of these techniques compared with intra‐arterial IA, CTA, and MRA for the detection of intracranial stenosis and occlusion in people presenting with symptoms of ischaemic stroke.
Objectives
To assess the diagnostic accuracy of TCD and TCCD for detecting stenosis and occlusion of intracranial large arteries in people with acute ischaemic stroke.
Search methods
We limited our searches from January 1982 onwards as the transcranial Doppler technique was only introduced into clinical practice in the 1980s. We searched MEDLINE (Ovid) (from 1982 to 2018); Embase (Ovid) (from 1982 to 2018); Database of Abstracts of Reviews of Effects (DARE); and Health Technology Assessment Database (HTA) (from 1982 to 2018). Moreover, we perused the reference lists of all retrieved articles and of previously published relevant review articles, handsearched relevant conference proceedings, searched relevant websites, and contacted experts in the field.
Selection criteria
We included all studies comparing TCD or TCCD (index tests) with IA, CTA, MRA, or contrast‐enhanced MRA (reference standards) in people with acute ischaemic stroke, where all participants underwent both the index test and the reference standard within 24 hours of symptom onset. We included prospective cohort studies and randomised studies of test comparisons. We also considered retrospective studies eligible for inclusion where the original population sample was recruited prospectively but the results were analysed retrospectively.
Data collection and analysis
At least two review authors independently screened the titles and abstracts identified by the search strategies, applied the inclusion criteria, extracted data, assessed methodological quality (using QUADAS‐2), and investigated heterogeneity. We contacted study authors for missing data.
Main results
A comprehensive search of major relevant electronic databases (MEDLINE and Embase) from 1982 to 13 March 2018 yielded 13,534 articles, of which nine were deemed eligible for inclusion. The studies included a total of 493 participants. The mean age of included participants was 64.2 years (range 55.8 to 69.9 years). The proportion of men and women was similar across studies. Six studies recruited participants in Europe, one in south America, one in China, and one in Egypt. Risk of bias was high for participant selection but low for flow, timing, index and reference standard. The summary sensitivity and specificity estimates for TCD and TCCD were 95% (95% CI = 0.83 to 0.99) and 95% (95% CI = 0.90 to 0.98), respectively. Considering a prevalence of stenosis or occlusion of 42% (as reported in the literature), for every 1000 people who receive a TCD or TCCD test, stenosis or occlusion will be missed in 21 people (95% CI = 4 to 71) and 29 (95% CI = 12 to 58) will be wrongly diagnosed as harbouring an intracranial occlusion. However, there was substantial heterogeneity between studies, which was no longer evident when only occlusion of the MCA was considered, or when the analysis was limited to participants investigated within six hours. The performance of either TCD or TCCD in ruling in and ruling out a MCA occlusion was good. Limitations of this review were the small number of identified studies and the lack of data on the use of ultrasound contrast medium.
Authors' conclusions
This review provides evidence that TCD or TCCD, administered by professionals with adequate experience and skills, can provide useful diagnostic information for detecting stenosis or occlusion of intracranial vessels in people with acute ischaemic stroke, or guide the request for more invasive vascular neuroimaging, especially where CT or MR‐based vascular imaging are not immediately available. More studies are needed to confirm or refute the results of this review in a larger sample of stroke patients, to verify the role of contrast medium and to evaluate the clinical advantage of the use of ultrasound.
Keywords: Humans; Brain Ischemia; Brain Ischemia/diagnostic imaging; Cerebral Arteries; Cerebral Arteries/diagnostic imaging; Constriction, Pathologic; Constriction, Pathologic/diagnostic imaging; Infarction, Middle Cerebral Artery; Randomized Controlled Trials as Topic; Stroke; Stroke/diagnostic imaging; Ultrasonography, Doppler, Transcranial; Ultrasonography, Doppler, Transcranial/methods
Plain language summary
Transcranial colour Doppler (TCD) and transcranial colour‐coded duplex (TCCD), in patients with acute ischaemic stroke for detecting intracranial vessel occlusion or stenosis
What is the aim of this review?
The aim of this review was to find out how accurate two imaging techniques ‐ transcranial colour Doppler (TCD) and transcranial colour‐coded duplex (TCCD) ‐ are for detecting a blockage of the arteries in the brain in the first hours after a stroke and whether they can be used to select patients who may need to receive more invasive and expensive imaging methods such as intra‐arterial angiography (IA), computed tomography angiography (CTA), and magnetic resonance angiography (MRA). There is currently no agreement on the use of TCD and TCCD in the management of people with acute stroke, and the use of TCD and TCCD varies between and within countries.
Key messages
TCD and TCCD may provide clinically helpful information for detecting a blockage of arteries in the brain when compared with IA, CTA, and MRA.
What was studied in the review?
Ischaemic stroke is the third leading cause of death and the most common cause of long‐term disability. It is usually caused by a blockage of the blood supply to one part of the brain. When stroke is caused by a blockage of a large artery due to a blood clot, the prognosis, without treatment, is often poor and can lead to severe disability. Curently, there are two effective treatment options that can be used to dissolve the blood clot: to administer a thrombolytic drug, or to physically extract the blood clot from the artery (mechanical thrombectomy). Both treatments work best within the first few hours of stroke onset. Ultrasound scans (TCD and TCCD) are a quick and simple way to detect the blockage of blood vessels in the brain. We reviewed the current literature for clinical studies assessing the accuracy of these diagnostic techniques compared with IA, CTA, and MRA for the detection of blocked blood vessels in the brain in people with symptoms of ischaemic stroke.
What are the main results of the review
A comprehensive search of major relevant electronic databases from 1982 to 13 March 2018 identified 13,534 articles but only nine studies met the prespecified inclusion criteria. The nine identified studies included a total of 493 stroke patients with similar proportions of men and women. The average age of included participants was 64.2 years (range 55.8 to 69.9 years). Six studies recruited participants in Europe, one in South America, one in China, and one in Egypt. The results of this review indicate that if TCD or TCCD were to be used in a group of 1000 people with symptoms of acute stroke, which in 420 (42%) of them is caused by a blockage of large arteries in the brain, then 428 would have a positive test result but 29 of these (29/428, 7%) would be wrongly identified as positives even though they would not have a blockage of large arteries. Similarly, an estimated 572 would have a negative test result indicating that their symptoms are not caused by a blockage of large arteries in the brain but 21 (21/572, 4%) of these negative cases would actually have a blockage of the large arteries, which TCD or TCCD have missed. In brief, for people with acute ischaemic stroke, TCD or TCCD can provide clinically helpful information for detecting blockage of large arteries in the brain compared with IA, CTA and MRA. Both tests studied in the review (TCD and TCCD) have shown similar accuracy.
How reliable are the results of the studies in the review?
The main limitation of this review is the small number of people assessed by TCD and TCCD in the individual studies. Not enough people have been studied to be really confident about these results. Further larger studies are needed to confirm or refute these results.
Summary of findings
Summary of findings'. '.
| What is the diagnostic accuracy of TCD and TCCD for detecting stenosis and occlusion of intracranial large arteries in people with acute ischaemic stroke? | ||||||
| Patient population | Adults with acute ischaemic stroke | |||||
| Settings | Emergency departments or specialist units (i.e. stroke units, neurological departments) in 8 studies, not specified in 1 study (Martinez‐Sanchez 2015) | |||||
| Expertice of sonographer | Experienced sonographer in 4 studies (Kenton 1997; Brunser 2009; Bar 2010; Guan 2013), not specified in 5 studies (Zanette 1989; Camerlingo 1993; Kamel 2010; Martinez‐Sanchez 2015; Panebianco 2016) | |||||
| Index test | TCD in 5 studies (Zanette 1989; Camerlingo 1993; Brunser 2009; Kamel 2010; Guan 2013); TCCD in 4 studies (Kenton 1997; Bar 2010; Martinez‐Sanchez 2015; Panebianco 2016) | |||||
| Reference standard | IA in 2 studies (Zanette 1989; Camerlingo 1993); CTA in 5 studies (Brunser 2009; Bar 2010; Guan 2013; Martinez‐Sanchez 2015; Panebianco 2016); MRA in 2 studies (Kenton 1997; Kamel 2010) | |||||
| Target condition | Stenosis and occlusion of intracranial large arteries. Occlusion is defined as a complete obstruction of a vessel (i.e. no signal on IA, CTA or MRA) whereas stenosis as a narrowing of a vessel of any degree (high velocity signal on doppler) | |||||
| Timing of performance of index test and reference standard from the ischaemic stroke onset | < 3 hours in 1 study (Bar 2010); < 6 hours in 3 studies (Zanette 1989; Camerlingo 1993; Panebianco 2016); < 8 hours in 1 study (Martinez‐Sanchez 2015); < 24 hours in 4 studies (Kenton 1997; Brunser 2009; Kamel 2010; Guan 2013) | |||||
| Included studies | 8 prospective studies and 1 retrospective study. 7 studies published in full and 2 presented as proceedings (total number of participants = 493) | |||||
| Limitations | High risk of selection bias in three studies (Camerlingo 1993; Bar 2010; Kamel 2010); relatively small number of patients; subgroup analyses not prespecified; lack of data on the use of ultrasound contrast medium | |||||
|
Pooled estimates (95% CI) |
Consequences in a cohort of 1000 with a 42% disease prevalence (prevalence taken fromWardlaw 2014) |
|||||
| Target disease |
Included Studies N |
Included individuals N |
Sensitivity | Specificity | Missed cases | False positives |
| Any artery | 9 | 493 | 0.95 (0.83 to 0.99) |
0.95 (0.90 to 0.98) |
21 (95% CI 4 to 71) | 29 (95% CI 12 to 58) |
| MCA | 8 | 393 | 0.95 (0.82 to 0.99) | 0.96 (0.90 to 0.99) | 21(95% CI 4 to 76) | 23 (95% CI 6 to 58) |
| Investigation of heterogeneity and sensitivity analysis | Statistical heterogeneity was substantial according to the results of the estimated variance in the logit sensitivity and specificity values. Heterogeneity was also evident by visually inspecting the prediction regions for summary sensitivity and specificity provided by the bivariate analyses. The performed sensitivity analyses led to summary sensitivity and specificity close to the values observed in the primary analysis, though a substantial reduction of statistical heterogeneity. | |||||
| Conclusions | With summary sensitivity of 95% (CI 95% 0.83 to 0.99) and a summary specificity of 95% (95% CI 0.95 to 0.98), TCD and TCCD provide reasonably accurate diagnostic information to rule in or rule out occlusion or stenosis of intracranial arteries in patients with acute ischaemic stroke and could be particularly useful in centres that do not have access to more sophisticated neuroimaging techniques. | |||||
CTA: computed tomography angiography IA: intra‐arterial angiography MCA: middle cerebral artery TCCD: transcranial colour doppler TCD: transcranial doppler
Background
Ischaemic stroke is the third leading cause of death and the most common cause of permanent disability (Warlow 2003). An occlusion (complete obstruction of a vessel) or stenosis (a narrowing of a vessel) of intracranial large arteries can be detected in the acute phase of stroke in about 42% of patients (Wardlaw 2014). The frequency of occlusion and stenosis could change with time after stroke onset because of spontaneous recanalisation. Other different studies have shown different prevalence: an occlusion in around 66% (range 34% to 80%) of people and a stenosis in about 14% (range 11% to 17%) (Alexandrov 1999; Gerriets 2000; Kassem‐Moussa 2002; Arnold 2004; Brunser 2009), and it is commonly associated with a worse prognosis (Smith 2006). In a continuous series of acute ischaemic stroke patients, the prevalence of intracranial pathology was 8.9% (Baracchini 2016).
The approved therapy for acute ischaemic stroke is recombinant tissue plasminogen activator (rt‐PA), a thrombolytic agent given intravenously, whose therapeutic benefits are greater, the earlier the therapy is administered (Wardlaw 2012; Wardlaw 2015), and mechanical thrombectomy, but this procedure can be offered in few centres and should be concluded within six hours (Yarbrough 2015;Rodrigues 2016),
Recent large studies suggest the benefit of thrombolytic therapy may be greater among people with intracranial artery stenosis or occlusion compared to those without stenosis or occlusion (Fiebach 2012, Wardlaw 2015).
A recent systematic review (Ricci 2012), and the NOR‐SASS RCT (Nacu 2017), have shown that sonothrombolysis might increase recanalisation in people with acute ischaemic stroke and intracranial occlusion of the middle cerebral artery (MCA) although the clinical effects of sonothrombolysis in reducing death or dependency have still to be definitely proven.
In addition to a person's clinical status and the time passed since stroke symptoms onset, neuroimaging findings play an important role in the decision process about new acute stroke therapies. Whilst the technology to identify an ischaemic penumbra (i.e. ischaemic tissue that is not yet irreversibly injured and hence has the potential to be saved by acute reperfusion therapy) is complex and not widely available, the detection of vessel occlusion or stenosis by ultrasound is a more practical strategy for selecting people who might benefit from acute treatments. A correct diagnosis of intracranial stenosis or occlusion has the potential to influence the management in the early phase of stroke and thus to improve outcome.
Transcranial colour Doppler (TCD) and transcranial colour‐coded duplex (TCCD) are imaging techniques that can be used in the acute phase of stroke to detect the presence of intracranial pathology (stenosis or occlusion, or both). Furthermore, ultrasound contrast agents (UCAs) can be safely administered to acute ischaemic stroke patients with inadequate temporal bone window (Baracchini 2017).
Target condition being diagnosed
The target conditions are stenosis and occlusion of intracranial large arteries. For the purpose of this review, we defined stenosis of an intracranial vessel as any reduction of the lumen of the vessel, while occlusion is any complete interruption of the blood flow. We have considered these two conditions separately, but we have analysed them together as intracranial pathology in our main analysis. The traditional reference standard for the diagnosis of intracranial stenosis and occlusion is intra‐arterial angiography (IA), an invasive method that carries a 1% to 2% risk of stroke or death (Hankey 1990). Intra‐arterial angiography involves the injection of iodine contrast agent after selective catheterisation of the vessels to the brain. X‐ray images are taken before ('the mask') and during contrast injection. The mask is then subtracted from the post‐contrast images. All that remains should be the blood vessels that were filled with contrast material. Since the early 1990s, new less‐invasive imaging methods, such as computed tomography angiography (CTA) and magnetic resonance angiography (MRA), or contrast‐enhanced MRA, have been increasingly used in clinical practice to identify people with intracranial stenosis and occlusion. Computed tomogrpahy (CT) combines the use of x‐rays with computerised analysis of the images. In CTA, the injection of intravenous radio‐opaque contrast agent accentuates blood vessels during the arterial phase of contrast perfusion. Magnetic resonance (MR) uses the electromagnetic properties of protons to produce high‐quality cross‐sectional images of the brain. MRA is a group of techniques based on MR imaging. They use intravenous contrast to highlight vessels (contrast‐enhanced MRA) or take advantage of blood flow to distinguish the vessels from other static tissue (e.g. time‐of‐flight techniques: TOF). In clinical practice, IA has gradually been replaced by CTA and MRA, which have shown satisfactory accuracy in the detection of intracranial stenosis and occlusion (Latchaw 2009). As of today, most centres use angio CT, which is widely available 24 hours per day, whereas angio MR (mostly TOF) is done in a few centres.
Index test(s)
TCD and TCCD are becoming increasingly popular in clinical practice. TCD uses frequencies of 1 to 2.5 MHz to send ultrasounds (insonate) to cerebral vessels through several windows in the skull (transtemporal, transforaminal and transorbital). This technique can detect intracranial flow velocities and the direction of flow measured by a pulsed Doppler. TCCD uses frequencies of 1.5 to 4 MHz to obtain the same information, and add 'B‐mode' and 'colour Doppler' imaging that displays a two‐dimensional image of cerebral vessels and parenchyma as seen by the ultrasound probe. Ultrasound contrast agent is used if the temporal acoustic bone window has insufficient signal intensity, or proximal branches of the circle of Willis are not visible. Intracranial stenosis is detected as a colour and a pulsed‐wave pattern of increased blood flow velocity. Occlusion is detected as the absence of a colour Doppler signal and pulsed‐wave spectrum on the index artery whilst any other artery is detectable from the same acoustic window. Some parameters can be used to quantify the stenosis grade: peak systolic velocity, which is defined as the highest velocity of the systolic wave within the Doppler frequency spectrum, and mean flow velocity, which consists of an averaging of all instantaneous velocity values within a cardiac cycle. TCD and TCCD are useful, rapid, non‐invasive tools for the assessment of acute stroke. The equipment is portable and can, therefore, be used for bedside investigations, especially for those people who cannot tolerate conventional neuroimaging. They provide clinical results in a short period of time (minutes) and can be repeated over time, hence might have potential advantages in the evaluation and monitoring of people in the early phase of stroke. Furthermore, they are relatively inexpensive, and may be more accessible than neuroimaging and IA in many city hospitals in many countries.
Clinical pathway
The rapid and accurate identification of occlusion or stenosis of large intracranial arteries is crucial for establishing proper management of stroke patients and improving clinical outcomes. In particular, the detection of a vascular occlusion allows the identification of people with acute ischaemic stroke who are more likely to benefit from endovascular thrombectomy and intra‐arterial thrombolysis. At present in clinical practice, invasive and expensive neuroimaging methods (i.e. IA, CTA, MRA, and contrast‐enhanced MRA) are used to detect intracranial stenosis or occlusion of intracranial arteries. However, in many centres these methods are not available or, in some cases (i.e. IA), they cannot be performed quickly with the negative consequence of delaying treatment. TCD and TCCD, which are more widely available, can be done quickly and with little discomfort for the patient, have the potential to improve the identification of people with intracranial stenosis and/or occlusion and can be used to select patients who may require more invasive and complex imaging methods. Usually, when TCD or TCCD shows an occlusion, further neuroimaging techniques (CTA, MRA, or even IA) are considered in order to decide whether the patient should undergo thrombectomy. However, if TCD or TCCD does not show an occlusion, and both the clinical symptoms (i.e. National Institutes of Health Stroke Scale (NIHSS) score criteria) and the initial plain CT, which all stroke patients receive, exclude the presence of an occlusion of a large artery, no further investigation is requested and, if indicated, the stroke patient is treated with thrombolysis. The clinical pathway diagram is presented in Figure 1.
1.
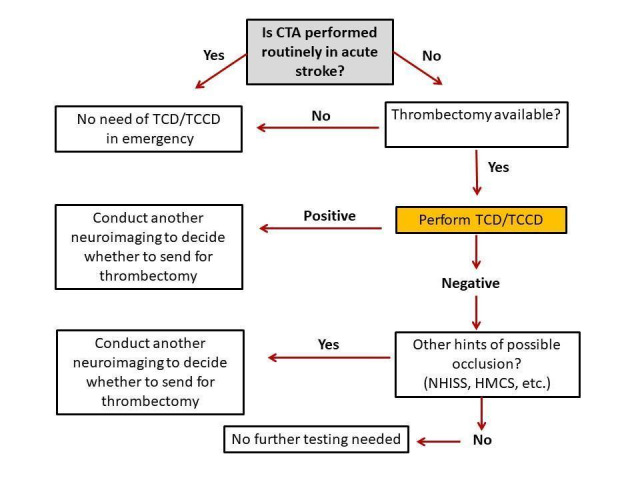
Possible role of TCD/TCCD in the current stroke care pathway
Rationale
In people who present to the emergency department with symptoms of ischaemic stroke, the rapid and accurate identification of occlusion or stenosis of large intracranial arteries is important to establish proper management and improve clinical outcomes. The detection of a vascular occlusion, for example, allows selection of people with acute ischaemic stroke who might benefit from treatment with intravenous fibrinolytic therapy (even beyond current time limits), endovascular thrombectomy, and intra‐arterial thrombolysis. Even though IA, CTA, MRA, and contrast‐enhanced MRA are considered the best available methods for the diagnosis of intracranial occlusion and stenosis, they are invasive, time‐consuming, expensive, and not available 24 hours per day in many centres. Moreover, they are not tolerated by some people. TCD and TCCD could be used prior to, or instead of, IA, CTA, MRA, or contrast‐enhanced MRA in people with ischaemic acute stroke. The Neurosonology in Acute Ischaemic Stroke (NAIS) study suggested that ultrasound techniques could be used to predict clinical outcomes at a very early stage, thereby allowing therapeutic interventions to be tailored to suit individuals' needs (Allendoerfer 2006). However, there is currently no formal consensus on the use of neurosonology in the management of the acute phase of stroke. The use of TCD and TCCD varies between countries. In the USA, TCD is considered useful in assessing people within eight hours of stroke onset, monitoring the effects of thrombolytic therapy over time, and establishing prognosis (Adams 2007). According to the ESO Guidelines 2008, TCD can contribute to the selection of people with stroke who might receive intra‐arterial treatments in specialised centres. The Italian stroke guidelines indicate that, in the acute phase of stroke, neurosonology of intracranial arteries may be a valid alternative to more invasive techniques (SPREAD 2016). Furthermore, a recent study using the SITS‐ISTR database showed a possible beneficial outcome associated with the use of TCD in the hyperacute phase. Studies used for patients' selection for endovascular therapy are warranted, in particular in settings with limited round‐the‐clock availability of CTA and MRA (Mazya 2018). Due to the current lack of consensus regarding the use of TCD and TCCD in clinical practice, we systematically reviewed the literature for studies assessing the diagnostic accuracy of these techniques compared with IA, CTA, and MRA for the detection of intracranial stenosis and occlusion in people with ischaemic stroke.
Objectives
To assess the diagnostic accuracy of TCD and TCCD for detecting stenosis and occlusion of intracranial large arteries in people with acute ischaemic stroke.
Secondary objectives
We planned to assess the accuracy of different thresholds of mean flow velocity or peak systolic velocity, or both, in the grading of intracranial artery stenosis. However, due to the lack of data on different thresholds we were not able to do so.
Methods
Criteria for considering studies for this review
Methods
Criteria for considering studies for this review
Types of studies
We have included all studies comparing TCD or TCCD with IA, CTA, MRA, or contrast‐enhanced MRA in people with acute ischaemic stroke, where all participants underwent both the index test and the reference standard within 24 hours of symptom onset. We have included prospective cohort studies and randomised clinical studies of test comparisons in which participants were randomised to TCD or TCCD and verified by the reference standards. We have also considered retrospective studies as eligible for inclusion where the original population sample was recruited prospectively but the results were analysed retrospectively.
Participants
Adults (as defined by the studies' investigators) presenting in emergency departments or specialist units (i.e. stroke units, neurological departments) with acute ischaemic stroke confirmed by imaging (CT or MR). We included people irrespective of the severity of their disease as long as they were stable enough to undergo the tests under investigation.
Index tests
We assessed the following index tests.
TCD: with or without echo contrast agents ‐ performed with a low frequency probe device (1.0 to 2.5 MHz).
TCCD: with or without echo contrast agents ‐ performed with a low frequency probe device (1.5 to 4.0 MHz).
We accepted the definition of stenosis as reported by the studies' investigators (i.e. peak systolic velocity criteria or mean flow velocity criteria, or both).
Target conditions
Stenosis or occlusion of intracranial large arteries. Occlusion is defined as a complete obstruction of a vessel (i.e. no signal on reference standard techniques: IA, CTA, MRA) whereas a stenosis is a narrowing of a vessel of any degree (high velocity signal on Doppler).
Reference standards
We considered the following reference standards as suitable for inclusion.
IA
CTA
MRA (time‐of‐flight technique or contrast‐enhanced MRA)
We considered the above imaging tests interchangeable as their technical characteristics and accuracy are considered similar in clinical practice.
Search methods for identification of studies
We performed a comprehensive literature search with no language restrictions to identify relevant studies in the literature. We limited our searches from January 1982 onwards as the transcranial Doppler technique was only introduced into clinical practice in the 1980s.
Electronic searches
We searched the following electronic bibliographic databases:
MEDLINE (Ovid) (from 1982 to 13 March 2018) (Appendix 1);
Embase (Ovid) (from 1982 to 13 March 2018) (Appendix 2);
Database of Abstracts of Reviews of Effects (DARE) at www.crd.york.ac.uk/crdweb/ (from 1982 to 13 March 2018);
Health Technology Assessment Database (HTA) at www.hta.ac.uk (from 1982 to 13 March 2018).
We used a combination of controlled vocabulary and relevant free‐text terms for each database. Although stenosis of the intracranial internal carotid artery is relevant for this review, we did not include controlled vocabulary and free‐text terms to describe the general concept of 'carotid stenosis' in the search strategies. This is justified by the fact that the focus of this systematic review is stenosis or occlusion of intracranial arteries and not on carotid stenosis of the neck arteries. Inclusion of terms relating to 'carotid stenosis' and 'carotid artery disease' would have retrieved an unmanageable number of irrelevant citations. We developed the MEDLINE and Embase search strategies in collaboration with the Cochrane Stroke Group Information Specialist following the recommendations of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (De Vet 2008). Both search strategies were constructed in a very complex way using a number of combinations of concepts. Both strategies also included a methodological search filter for studies of diagnostic accuracy. The use of a search filter, even though it may reduce the overall sensitivity of the literature searches, is justified by the fact that a literature search combining MeSH terms and text words for the target condition (stroke) with those for the imaging tests under evaluation (as for the current recommendation of the Screening and Diagnostic Tests Methods Group) would retrieve a number of citations that would be unmanageable within the timeline for the completion of this review. Furthermore, the methodological filter is based on the diagnostic component of the search strategy developed and validated by Astin and colleagues to identify diagnostic accuracy studies on imaging (Astin 2008). We adapted the MEDLINE search strategy to search the other relevant electronic databases.
Searching other resources
In order to identify further published, unpublished and ongoing studies we have also:
perused the reference lists of all relevant articles identified by the electronic searches as well as previously published review articles on this clinical area;
handsearched relevant conference proceedings;
contacted experts in the field;
-
searched the following relevant websites using the terms 'intracranial stenosis'; 'intracranial occlusion'; 'stroke'; 'transcranial doppler':
National Institute of Neurological Diseases and Stroke NINDS (www.ninds.nih.gov; searched 13 March 2018);
American Heart Association AHA (www.heart.org/HEARTORG/; searched 13 March 2018);
American Stroke Association ‐ ASA (www.strokeassociation.org/STROKEORG/; searched 13 March 2018);
National Stroke Association ‐ NSA (www.stroke.org; searched 13 March 2018);
Scottish Intercollegiate Guidelines Network ‐ SIGN (www.sign.ac.uk; searched 13 March 2018);
US National Institutes of Health Ongoing Trials register ClinicalTrials.gov (www.clinicaltrials.gov; searched 13 March 2018);
Google Scholar (scholar.google.co.uk/; searched 13 March 2018).
We used Science Citation Index Cited Reference Search for forward tracking of important articles.
We imported the citations identified by the search strategies into the Reference Manager bibliographic database (Reference Manager 10).
Data collection and analysis
Selection of studies
Three review authors (MM, VS and TM for TCD studies; MDS, CG and CF for TCCD studies) independently screened the titles and abstracts of all records generated by the electronic searches for relevance. We excluded duplicates and studies that did not meet the inclusion criteria. We retrieved copies of all remaining potentially relevant reports in full. Three review authors (same as above) independently assessed the full‐text reports for inclusion using a study eligibility screening form based on the prespecified inclusion criteria. We referred any disagreement that could not be resolved by discussion to a third review author (AM, SC, SR). For studies presented as conference proceedings, we attempted to identify an existing full‐text publication or obtained detailed information from the study author. We considered studies suitable for inclusion if the absolute numbers of true positives, false positives, false negatives, and true negatives observations were available or could be derived from the data reported in the primary studies.
Data extraction and management
We designed a data extraction form to record details from included studies. Two review authors (AM, SC) independently extracted the following information from each individual study (without concealing the study authorship or other publication details).
Bibliographic details of included studies: author, title, year of publication, journal.
Study design: method of recruitment, sampling procedures, geographical location.
Clinical and demographic characteristics of participants: number of participants, age, gender, setting, severity of stroke, stroke clinical syndrome (i.e. anterior, posterior), concomitant diseases, inclusion and exclusion criteria.
Details of the index tests: technical characteristics of TCD and TCCD, criteria used for defining stenosis and occlusion, timing of test, expertise of the clinician or technician performing the procedure, location of stenosis or occlusion, adverse events and participant acceptability.
Details of the reference standards: type of reference standards (IA, CTA, MRA, or contrast‐ enhanced MRA), timing, location of stenosis or occlusion.
Numerical results: data for 2 x 2 tables (true positives, true negatives, false positives, and false negatives).
Any disagreement between review authors was resolved by discussion or arbitration (MB, SR). We entered 'consensus' data in a separate electronic form. We contacted study authors for missing data.
Assessment of methodological quality
Two review authors (AM, SC) independently assessed the methodological quality of each included study using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) developed by the NHS Centre for Reviews and Dissemination at the University of York, UK (Whiting 2011). The QUADAS‐2 comprises four domains: 1) participant selection, 2) index test, 3) reference standard, and 4) flow of participants through the study and timing of the index tests and reference standard (flow and timing). We assessed each domain for risk of bias, and we also assessed the first three domains for concerns regarding applicability. We judged risk of bias and concerns about applicability as 'low', 'high' or 'unclear'. We added signalling questions, specific to this review topic, separately.
We considered an appropriate spectrum of participants presenting with acute ischaemic stroke symptoms evaluated within 24 hours of symptoms onset. With regard to the time interval between index test and reference standard we accepted any time interval reported by the study investigators, providing that both tests (index and reference standard) were performed within 24 hours of symptoms onset (i.e. on day 1).
The modified QUADAS‐2 checklist is shown in Appendix 3. We resolved any disagreements between review authors by discussion or arbitration (MB, SR). For each individual study we tabulated the agreed results of the quality assessment. We also presented the results of the methodological quality assessment graphically.
Statistical analysis and data synthesis
We carried out the statistical analyses following the recommendations reported in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Chapter 10) (Macaskill 2010). We used Review Manager 5 software for analyses and plots (RevMan 2014). We extracted or derived indices of diagnostic performance from data presented in each primary study for each test, and generated 2 x 2 contingency tables of true positive cases, false positive cases, false negative cases, and true negative cases. We calculated sensitivity and specificity with 95% confidence intervals (CI) for each test in each study. We used forest plots to display the sensitivity and specificity estimates measured in each study and to illustrate the variation in estimates between studies. We plotted the results of studies for individual diagnostic tests in a receiver operator characteristic (ROC) space. We meta‐analysed the pairs of sensitivities and specificities. The primary analysis aimed at comparing TCD or TCCD versus CTA, MRA, contrast‐enhanced MRA or IA. We investigated the performance of each index test versus each reference standard. We used STATA 13 to fit the bivariate model (Reitsma 2005). We transferred parameter estimates (logit and variances) to Review Manager to produce the summary operating point (i.e. summary values for sensitivity and specificity) and 95% confidence region. We fitted the bivariate model as it is recommended for purely binary tests or when different studies report similar thresholds (Leeflang 2014). We have also fitted univariate random‐effects models in case of non‐convergence of the bivariate model estimation due to the small number of available studies (Takwoingi 2017). We accepted the definitions of stenosis and occlusion given in any individual study. Initially, we analysed stenosis and occlusion of any intracranial artery together as one condition. We decided to perform 'post hoc' analyses because of the clear clinical interest related to them: 1) patient seen within six hours (possible treatment based on the result of the test); and 2) occlusion of the MCA (the most assessed artery). We summarised findings with absolute values on 1000 tested participants with estimated numbers of false positives (undue treatment) and false negatives (missing appropriate treatment).
Investigations of heterogeneity
In addition to the proposed subgroup analyses, we planned to add covariates related to clinical criteria for occlusion/stenosis (i.e. mean flow velocity and peak systolic velocity), operator expertise (as defined in the various studies), and stroke severity (defined by the National Institutes of Health Stroke Scale ‐ NIHSS or any other scale used in the study).
However, due to the small sample size and missing data, we were only able to add covariates related to type of reference standard (CTA, MRA, contrast‐enhanced MRA, or IA) and index test (TCC, TCCD).
Sensitivity analyses
We did not perform several sensitivity analyses planned in the original protocol due to the lack of suitable data. It was possible to perform a sensitivity analysis based on data from studies at low risk of bias. We also decided to perform two post hoc sensitivity analyses; one excluding studies published exclusively as conference proceedings and not in full (Martinez‐Sanchez 2015; Panebianco 2016), and one restricted to studies that assessed the MCA only.
Assessment of reporting bias
Considering that the Deek's funnel plot asymmetry test is likely to be underpowered when only a small number of studies are available, we have presented it only for the main analysis.
Results
Results of the search
The results of the literature searches are outlined below in Figure 2. MEDLINE and Embase searches from 1982 to 13 March 2018 yielded 13,534 citations. After removal of duplicates, the total number of citations was 12,736.
2.

Study flow diagram.
After an initial screening based on titles and abstracts, we excluded 12,651 reports because they did not meet the prespecified inclusion/exclusion criteria and we retrieved in full 85 articles. After full‐text assessment, we excluded a further 78 articles. The main reasons for exclusion were: inclusion of patients with transient ischaemic attack (TIA), inappropriate timing of imaging, and insufficient numerical data to create a 2 x 2 table (even though we contacted study authors for missing data).
We also reviewed the conference proceedings of major stroke meetings in the last three years and identified two additional papers published as abstracts, which we included in the review (Martinez‐Sanchez 2015; Panebianco 2016). After contacting the corresponding authors, we were able to obtain complete data from one of the two abstracts (Panebianco 2016).
We included nine independent studies, which met our prespecified inclusion criteria.
Zanette 1989 compared transcranial Doppler with angiography in an acute (within six hours from onset) ischaemic population. The target condition was occlusion of the MCA (main stem, branches and siphon). It was the oldest study identified in the literature.
Camerlingo 1993 assessed a patient population in a stroke unit within six hours from the onset. The study aimed to compare transcranial Doppler with angiography in the diagnosis of occlusion or stenosis of the MCA territories.
Kenton 1997 assessed 30 participants with ischaemic stroke within 24 hours from onset and compared TCCD with MRA time of flight (TOF) as the reference standard for the diagnosis of occlusion or stenosis of the MCA.
Brunser 2009 assessed a sample of 100 stroke patients within 24 hours and compared TCD and CTA for the diagnosis of occlusion of anterior and posterior arteries.
Kamel 2010 compared TCD with MRA time of flight as a reference standard for the diagnosis of occlusion or stenosis of the MCA.
Bar 2010 compared TCCD with CTA for the diagnosis of occlusion of the MCA and intracranial internal carotid artery (IC‐ICA) It was the study with the shortest timing (within three hours from onset).
Guan 2013 assessed a sample of 128 stroke participants (the largest stroke patient population) who underwent CTA and TCD within 24 hours for the diagnosis of occlusion of the anterior and posterior arteries.
Martinez‐Sanchez 2015 assessed 65 stroke patients who underwent CTA and TCCD within 8 hours for the diagnosis of occlusion of the MCA.
Panebianco 2016 is a report of a recent study that compared TCCD with CTA within 12 hours from symptoms onset for the diagnosis of occlusion or stenosis of the MCA.
We have presented further details about the nine included studies in the Characteristics of included studies table.
Description of included studies
There were seven studies published in full and two studies presented as proceedings from 1989 to 2018 that we selected for inclusion (Zanette 1989; Camerlingo 1993; Kenton 1997; Brunser 2009; Kamel 2010; Bar 2010; Guan 2013; Martinez‐Sanchez 2015; Panebianco 2016). The characteristics of these studies are summarised in the Characteristics of included studies section. Additional features of these studies are also presented in the Table 1.
The identified studies included a total of 493 participants. The mean age of participants was 64.2 years (range 55.8 to 69.9 years) across studies.
Studies that provided participants' demographic details reported a similar proportion of men (53.8%) and women (46.2%). Six studies recruited participants in Europe, one in South America, one in China, and one in Egypt.
Eight studies used a prospective method for participant recruitment, and one a retrospective method (Martinez‐Sanchez 2015). Five studies recruited participants consecutively and in four studies, the data were unclear (Zanette 1989; Kamel 2010; Martinez‐Sanchez 2015; Panebianco 2016).
In four studies, the index test was TCCD (Kenton 1997; Bar 2010; Martinez‐Sanchez 2015; Panebianco 2016), while in five studies, the index test was TCD (Zanette 1989; Camerlingo 1993; Brunser 2009; Kamel 2010; Guan 2013). In five studies, the reference standard was CTA (Brunser 2009; Bar 2010; Guan 2013; Martinez‐Sanchez 2015; Panebianco 2016), in two studies, MRA (Kenton 1997; Kamel 2010), and in other two studies, angiography (Zanette 1989; Camerlingo 1993). None of the studies reported any complication related to the use of the diagnostic tests under investigation.
In one study, the index test and reference standard were performed within three hours from symptom onset (Bar 2010), in three studies, within six hours (Zanette 1989; Camerlingo 1993; Panebianco 2016), in one study, within eight hours (Martinez‐Sanchez 2015), and in four studies, within 24 hours (Kenton 1997; Brunser 2009; Kamel 2010; Guan 2013).
The target condition was stenosis or occlusion in seven studies (Camerlingo 1993; Kenton 1997; Bar 2010; Kamel 2010; Guan 2013; Martinez‐Sanchez 2015; Panebianco 2016), and occlusion in two studies (Zanette 1989, Brunser 2009).
All studies ‐ except for Guan 2013 and Martinez‐Sanchez 2015 ‐ reported that participants were recruited from a stroke unit or an emergency department. Two studies (Camerlingo 1993; Bar 2010) excluded individuals with mild or severe stroke, and only included participants who were alert or had a NIHSS score that ranged from 5 to 20.
The baseline prevalence of occlusion or stenosis in the overall study samples was 51.5% and varied from 27.3% (Guan 2013), to 90.8% (Martinez‐Sanchez 2015). In studies that assessed only the MCA, the prevalence of occlusion or stenosis was 53.4%.
Methodological quality of included studies
The results of QUADAS‐2 assessment for each of the nine included studies are presented in Figure 3. We judged three studies as being at low risk of bias in the participant selection, flow and timing, index test, and reference standard (Zanette 1989; Brunser 2009; Guan 2013).
3.
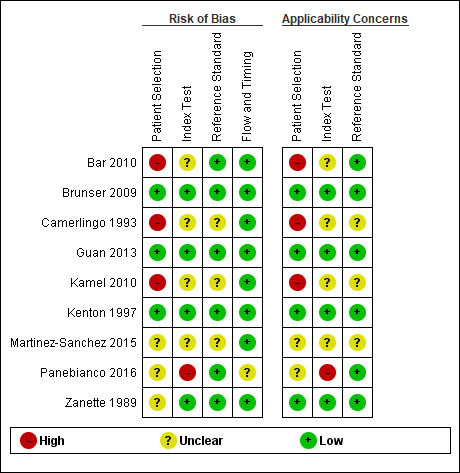
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
It was unclear in four studies whether the index test results were interpreted without knowledge of the results of the reference standard (Camerlingo 1993; Bar 2010; Kamel 2010; Martinez‐Sanchez 2015). In six studies, the reference standard test was administered and interpreted without knowledge of the index test results. In three studies, this information was not clearly reported (Camerlingo 1993; Kamel 2010; Martinez‐Sanchez 2015).
For Zanette 1989, Kamel 2010, Martinez‐Sanchez 2015, and Panebianco 2016, the method of recruitment and the sampling procedures were unclear.
Camerlingo 1993, Bar 2010 and Kamel 2010 did not avoid inappropriate exclusions for a variety of reasons (age 40 to 80 years, first‐ever stroke, alert patients, absence of severe concomitant disease, presence of ischaemic area visible at CT control for Camerlingo 1993; restless patients, NIHSS score 5 to 20, age 18 to 80 years for Bar 2010; and concurrent severe systemic disease for Kamel 2010).
One study reported that the sonographer was an experienced sonographer certified by the American Society of Neuroimaging (Brunser 2009), another study specified that the sonographer had at least five years of experience (Bar 2010), three studies described the sonographer as "experienced sonographer" (Kenton 1997; Guan 2013; Panebianco 2016), and four studies did not report information on the expertise of the sonographer (Zanette 1989; Camerlingo 1993; Kamel 2010; Martinez‐Sanchez 2015).
We provide a summary of the 'Risk of bias' assessment in Figure 4.
4.
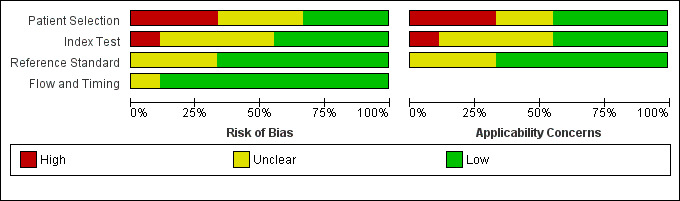
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
Findings
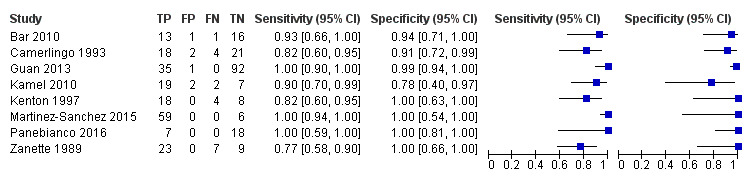
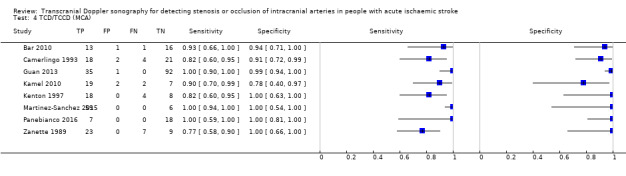
For the detection of occlusion or stenosis of any intracranial artery, the sensitivity estimates of TCD or TCCD ranged from 0.77 to 1.00, while the specificity estimates ranged from 0.78 to 1.00 (Figure 5) across studies. Using the bivariate model, the summary estimate of sensitivity was 0.95 (95% CI 0.83 to 0.99) and the summary estimate of specificity was 0.95 (95% CI 0.90 to 0.98) (Figure 6). Deek's funnel plot asymmetry test was significant (P = 0.036). A meta‐regression analysis showed that the type of index test did not impact on the estimates of accuracy (sensitivity and specificity) (TCD versus TCCD: P = 0.421) while the type of reference standard did (CTA versus others: P = 0.005). In fact, according to the results of the meta‐regression analysis, CTA as the reference standard showed a summary estimate for sensitivity of 0.98 (95% CI 0.94 to 0.99) and a summary estimate for specificity of 0.96 (95% CI 0.90 to 0.99) while in IA/MRA studies, the summary estimate for sensitivity was 0.82 (95% CI 0.73 to 0.88) and the summary estimate for specificity was 0.92 (95% CI 0.76 to 0.98).
5.
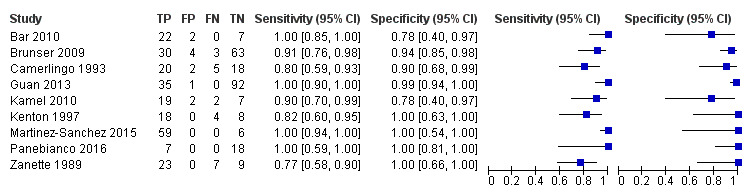
Forest plot of paired sensitivity and specificity estimated for studies assessing stenosis or occlusion in any intracranial artery with either TCD or TCCD as index test.
6.
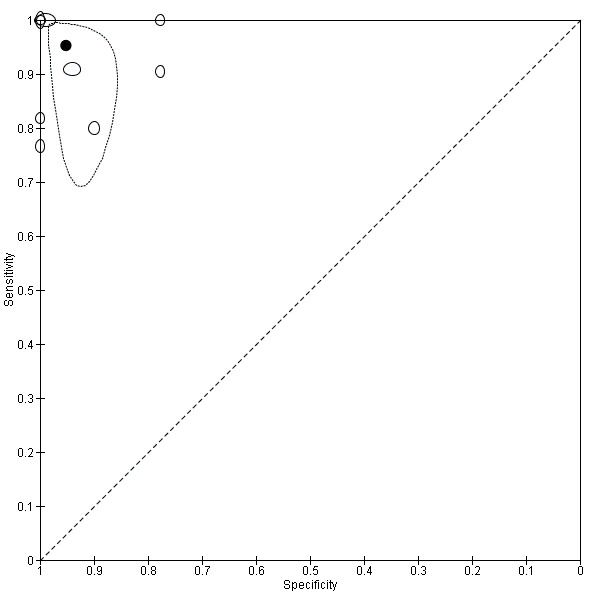
Summary ROC plot for studies assessing stenosis or occlusion in any intracranial artery with either TCD or TCCD as index test.
Post hoc subgroup analyses
All subgroup analyses are presented in additional Table 2. Using a univariate random‐effects model, a subgroup analysis of five studies that used TCD as the index test for detection of occlusion or stenosis in any intracranial artery gave a summary estimate for sensitivity of 0.90 (95% CI 0.78 to 0.96) and a summary estimate for specificity of 0.95 (95% CI 0.88 to 0.98). For TCCD, the summary estimates for sensitivity and specificity (four studies) were 1.00 (95% CI 0.32 to 1.00) and 0.98 (95% CI 0.40 to 1.00), respectively. A subgroup analysis looking at studies that examined participants within six hours from stroke onset (which is the relevant time window for treatment decision) showed a summary estimate for sensitivity of 0.89 (95% CI 0.62 to 0.98) and for specificity of 0.93 (95% CI 0.78 to 0.98).
1. Subgroup analyses.
| Target artery | Analysis |
Included Studies N |
Included Individuals N |
Sensitivity | Specificity |
Consequences in a cohort of 1000 with a 42% disease prevalence (prevalence taken fromWardlaw 2014) |
Model | |
| Missed cases | False positives | |||||||
| Any artery | Reference: CTA | 5 | 349 | 1.00 (0.57 to 1.00) |
0.97 (0.91 to 0.99) |
0 (95% CI 0 to 181) | 17 (95% CI 6 to 52) | BREM |
| Reference: MRA/IA | 4 | 144 | 0.82 (0.72 to 0.88) |
0.91 (0.76 to 0.97) |
76 ((95% CI 50 to 118) | 62 (95% CI 17 to 139) | BREM | |
| Index: TCD | 5 | 342 | 0.90 (0.78 to 0.96) |
0.95 (0.88 to 0.98) |
42 (95% CI 17 to 92) | 29 (95% CI 12 to 70) | BREM | |
| Index: TCCD | 4 | 151 | 1.00 (0.32 to 1.00) |
0.98 (0.40 to 1.00) |
0 (95% CI 0 to 286) | 12 (95% CI 0 to 348) | BREM | |
| Timing < 6 hours | 4 | 140 | 0.89 (0.62 to 0.98) |
0.93 (0.78 to 0.98) |
46 (95% CI 8 to 160) | 41 (95% CI 12 to 128) | BREM | |
| Timing > 6 hours | 5 | 353 | 0.97 (0.82 to 0.99) |
0.96 (0.88 to 0.99) |
13 (95% CI 4 to 76) | 23 (95% CI 4 to 70) | BREM | |
| MCA | Reference: CTA | 4 | 249 | 0.99 (0.84 to 1.00) |
0.99 (0.94 to 1.00) |
4 (95% CI 0 to 67) | 6 (95% CI 0 to 35) | UREM |
| Reference:MRA/IA | 4 | 144 | 0.82 (0.73 to 0.99) |
0.92 (0.77 to 0.98) |
76 (95% CI 4 to 113) | 46 (95% CI 12 to 133) | BREM | |
| Index: TCD | 4 | 242 | 0.90 (0.74 to 0.97) |
0.96 (0.84 to 0.99) |
42 (95% CI 13 to 109) | 23 (95% CI 6 to 93) | UREM | |
| Index: TCCD | 4 | 151 | 0.97 (0.74 to 1.00) |
0.98 (0.84 to 1.00) |
13 (95% CI 0 to 109) | 12 (95% CI 0 to 93) | BREM | |
| Timing < 6 hours | 4 | 140 | 0.84 (0.73 to 0.90) |
0.96 (0.87 to 0.99) |
67 (95% CI 42 to 113) | 23 (95% CI 6 to 71) | UREM | |
| Timing > 6 hours | 4 | 253 | 0.98 (0.72 to 1.00) |
0.97 (0.82 to 1.0) |
8 (95% CI 0 to 118) | 17 (95% CI 0 to 104) | BREM | |
BREM: bivariate random effects meta‐analysis CTA: computed tomography angiography MCA: middle cerebral artery TCCD: transcranial colour doppler TCD: transcranial doppler UREM: univariate random effects meta‐analysis
In studies assessing occlusion or stenosis of the MCA (the most investigated artery) using TCD, the summary estimate for sensitivity (using a univariate random‐effects model) was 0.90 (95% CI 0.74 to 0.97) and the summary estimate for specificity was 0.96 (95% CI 0.84 to 0.99). We observed similar estimates for studies evaluating MCA using TCCD (sensitivity 0.97, 95% CI 0.74 to 1.00; specificity 0.98, 95% CI 0.84 to 1.00).
For studies assessing occlusion or stenosis of the MCA using CTA as the reference standard (the most commonly used reference standard for occlusion of intracranial arteries in acute stroke), the summary estimate for sensitivity (using a univariate random‐effects model) was 0.99 (95% CI 0.84 to 1.00) and the summary estimate for specificity was 0.99 (95% CI 0.94 to 1.00). For studies assessing the MCA using MRA/IA as the reference standard, the summary estimate for sensitivity was 0.82 (0.73 to 0.99) and that of specificity 0.92 (95% CI 0.77 to 0.98).
When we considered specifically the evaluation of the MCA within six hours of stroke onset, the summary estimate for sensitivity (univariate random‐effects model) was 0.84 (95% CI 0.73 to 0.90) and that for specificity was 0.96 (95% CI 0.87 to 0.99).
Sensitivity analyses
The exclusion of Panebianco 2016 and Martinez‐Sanchez 2015, which were published as conference proceedings, had no impact on the results: the summary estimate for sensitivity was 0.91 (95% CI 0.80 to 0.96) and that for specificity was 0.94 (95% CI 0.86 to 0.98).
For the three studies judged to be at low risk of bias, the summary sensitivity and specificity estimates (univariate random‐effects model) were 0.94 (95% CI 0.77 to 0.98) and 0.97 (95% CI 0.91 to 0.99), respectively. For the four studies judged to be at low risk of bias on Domain 2 of the QUADAS‐2 assessment, the summary estimates for sensitivity and specificity (univariate random‐effects model) were 0.90 (95% CI 0.75 to 0.97) and 0.97 (95% CI 0.92 to 0.99), respectively. See also additional Table 3.
2. Sensitivity analyses.
| Target artery | Analysis |
Included studies N |
Included individuals N |
Sensitivity | Specificity |
Consequences in a cohort of 1000 with a 42% disease prevalence (prevalence taken fromWardlaw 2014) |
Model | |
| Missed cases | False positives | |||||||
| Any artery | Exclusion of conference proceedings | 7 | 403 | 0.91 (0.80 to 0.96) |
0.94 (0.86 to 0.98) |
38 (95% CI 8 to 59) | 35 (95% CI 12 to 81) | BREM |
| Low risk of bias | 3 | 258 | 0.94 (0.77 to 0.98) |
0.97 (0.91 to 0.99) |
25 (95% CI 8 to 97) | 17 (95% CI 6 to 52) | UREM | |
| Low risk of bias on Domain 2 of the QUADAS‐2 |
4 | 297 | 0.90 (0.75 to 0.97) |
0.97 (0.92 to 0.99) |
42 (95% CI 13 to 105) | 17 (95% CI 6 to 46) | UREM | |
| MCA | Exclusion of conference proceedings | 6 | 303 | 0.89 (0.78 to 0.95) |
0.95 (0.88 to 0.98) |
46 (95% CI 21 to 92) | 29 (95% CI 12 to 70) | BREM |
| Low risk of bias | 2 | 158 | 0.97 (0.41 to 1.00) |
0.99 (0.93 to 1.00) |
13 (95% CI 0 to 248) | 6 (95% CI 0 to 41) | UREM | |
| Low risk of bias on Domain 2 of the QUADAS‐2 |
3 | 197 | 0.91 (0.62 to 0.98) |
0.99 (0.94 to 1.00) |
38 (95% CI 8 to 160) | 6 (95% CI 0 to 35) | UREM | |
BREM: bivariate random effects meta‐analysis MCA: middle cerebral artery UREM: univariate random‐effects meta‐analysis
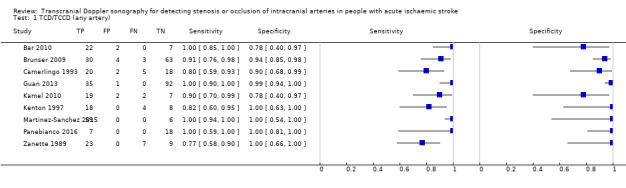
A sensitivity analysis restricted to studies that evaluated the MCA only (Figure 7) gave similar results to those observed among studies assessing any intracranial artery (summary sensitivity 0.95, 95% CI 0.82 to 0.99; summary specificity of 0.96, 95% CI 0.90 to 0.99) (Figure 8).
7.
Forest plot of paired sensitivity and specificity estimates for studies assessing stenosis or occlusion in the middle cerebral artery with either TCD or TCCD as index test.
8.
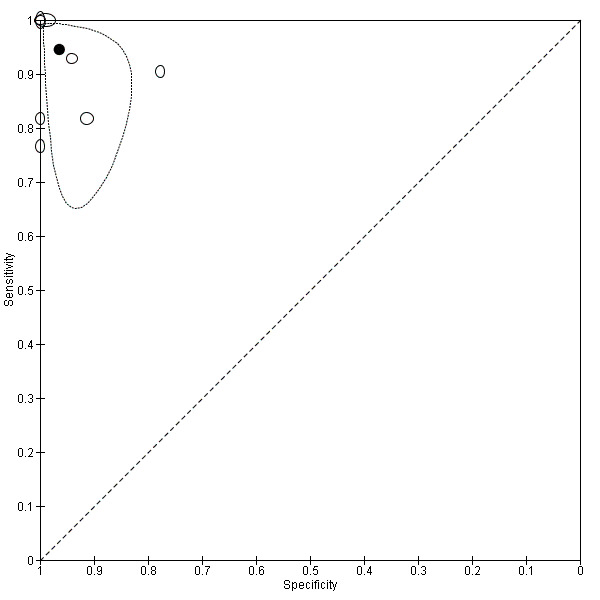
Summary ROC plot for studies assessing stenosis or occlusion in the middle cerebral artery with either TCD or TCCD as index test.
Discussion
Summary of main results
The results suggested that among patients with acute ischaemic stroke, the tests provide reasonably accurate diagnostic information to rule in or rule out occlusion or stenosis of intracranial arteries with summary sensitivity of 95% and specificity of 95% respectively (Table 1). In a patient group with a prevalence of stenosis or occlusion of 42% (prevalence taken from Wardlaw 2014 in the literature), for every 1000 people who receive a TCD or TCCD evaluation, stenosis or occlusion will be missed in 21 people and 29 people will be wrongly diagnosed as harbouring an intracranial occlusion. Although the number of included participants (493) is small compared with other reviews, we decided to perform some post hoc subgroup and sensitivity analyses, guided by clinical interest and relevance. We analysed the subgroup of occlusion of the MCA diagnosed within six hours (M1 or M2 tract), because that is the most common reason for sending a patient to the neuroangiographic suite. At present, the most commonly used reference test is CTA; therefore, we decided to perform a comparison between ultrasound‐based diagnosis and CTA. Finally, we looked at TCD separately, because it was used in five of the nine included studies. However, in our assessment, the two index tests did not show relevant differences in terms of diagnostic ability. Although the observed summary estimates showed a good accuracy of these imaging techniques, confidence intervals were wide, particularly for sensitivity estimates. This could be explained by the small number of included studies as well as by the heterogeneity observed across studies. Deek's funnel plot test showed evidence of reporting bias.
Strengths and weaknesses of the review
TCD and TCCD have shown high sensitivity and specificity estimates for detection of intracranial arterial pathology (stenosis and/or occlusion) when compared with reference standards commonly used in clinical practice. However, the estimates of sensitivity and specificity varied between studies. In particular, statistical heterogeneity was substantial according to the estimated variance in the logit sensitivity and specificity and the visual inspection of the prediction regions from the bivariate analyses. We found a high risk of selection bias in three studies and this could have implications in terms of the applicability and generalisability of the observed results (Camerlingo 1993; Bar 2010; Kamel 2010). The main limitation of this review is the relatively small number of participants assessed by the included studies and the high risk of selection bias observed in some studies. Moreover, due to lack of data, we were not able to assess whether the use of ultrasound contrast medium increased accuracy (Baracchini 2016). The use of contrast medium is thought to increase the number of patients who can be correctly detected in the emergency setting; in fact, when a signal from the MCA cannot be detected, the use of contrast medium could reduce the false positive results by showing the artery flow. New studies comparing TCCD with contrast medium against CTA are definitely warranted.
Applicability of findings to the review question
Participants and index tests of the included studies were sufficiently similar to those of our research question. Our findings are encouraging, although more data are needed to confirm them.
Authors' conclusions
Implications for practice.
The results of this review indicates that TCD and TCCD can be considered for guiding imaging decisions in acute stroke. Both techniques have provided reasonably accurate diagnostic information to rule in or rule out occlusion or stenosis of intracranial arteries in people with acute ischaemic stroke and could be of particular use in centres that do not have access to more sophisticated vascular imaging techniques when a decision on whether to transfer a stroke patient to another hospital needs to be taken. It is worth stressing the point that TCD and TCCD are operator‐dependent and, in order to get reliable findings, they should be administered by an operator with well‐established experience and technical skills. TCD and TCCD could also have a potential role in the monitoring of the status of intracranial arteries during treatment.
Implications for research.
Further research is needed to confirm or refute the findings of this review in a larger sample of patients. For instance, a study in which the accuracy of ultrasound with use of contrast medium is evaluated using CTA as the reference standard could be of great value. If such a study will confirm the high accuracy of TCD and TCCD, then the clinical advantage of these techniques could be formally evaluated.
Acknowledgements
We are grateful to Brenda Thomas and Joshua Cheyne, previous and current Information Specialists of the Cochrane Stroke Group, for their assistance with the literature searches, and to Hazel Fraser, Managing Editor of the Cochrane Stroke Group, for her support, patience, and guidance.
We are grateful to Kathy Mahan for her help in preparing the plain language summary.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy – January 1982 onwards
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or brain infarction/ or exp brain stem infarctions/ or cerebral infarction/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or hypoxia‐ischemia, brain/ or intracranial arterial diseases/ or cerebral arterial diseases/ or Intracranial Arteriosclerosis/ or exp "intracranial embolism and thrombosis"/ or stroke/ 2. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or hypoxi$)).tw. 3. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw. 4. ((intracranial or intra‐cranial or brain arter$ or cerebral arter$ or basilar arter$ or vertebral art$ or intracerebral or intra‐cerebral) adj5 (stenosis or stenoses or steno‐occlu$ or occlusion or occluded or occlusive or narrow$ or arteriosclero$ or atherosclero$ or plaque or plaques)).tw. 5. basilar artery/ or exp cerebral arteries/ or vertebral artery/ 6. Constriction, Pathologic/ or arterial occlusive diseases/ or arteriosclerosis/ or (stenosis or stenoses or steno‐occlu$ or occlusion or occluded or occlusive or narrow$ or arteriosclero$ or atherosclero$ or plaque or plaques).tw. 7. 5 and 6 8. 1 or 2 or 3 or 4 or 7 9. ultrasonography, doppler, transcranial/ or ultrasonography, doppler/ or ultrasonography, doppler, duplex/ or ultrasonography, doppler, color/ or ultrasonography, doppler, pulsed/ or echoencephalography/ 10. ultrasonography/ and blood flow velocity/ 11. ((transcranial or duplex or color or colour) adj5 doppler).tw. 12. (TCD or TCCD).tw. 13. ((doppler or duplex) adj5 (sonogra$ or ultrasonogra$)).tw. 14. Transcranial Color‐Coded Duplex.tw. 15. 9 or 10 or 11 or 12 or 13 or 14 16. Magnetic Resonance Angiography/ 17. angiography/ or cerebral angiography/ 18. Magnetic Resonance Imaging/ 19. 17 and 18 20. ((magnetic resonance or MR or MRI or NMR) adj5 (angiogra$ or arteriogra$)).tw. 21. MRA.tw. 22. exp Tomography, X‐Ray Computed/ 23. angiography/ or cerebral angiography/ 24. 22 and 23 25. ((Compute$ tomograph$ or CT or CAT) adj5 (angiogra$ or arteriogra$)).tw. 26. CTA.tw. 27. angiography, digital subtraction/ 28. angiography/ and (subtraction technique/ or subtraction.tw.) 29. ((digital subtract$ or catheter or cerebral or brain or intra‐arterial) adj5 (angiogra$ or arteriogra$)).tw. 30. (DSA or IADSA).tw. 31. 16 or 19 or 20 or 21 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. *cerebrovascular disorders/di, pa, ra, us or *basal ganglia cerebrovascular disease/di, pa, ra, us or *brain ischemia/di, pa, ra, us or *brain infarction/di, pa, ra, us or exp *brain stem infarctions/di, pa, ra, us or *cerebral infarction/di, pa, ra, us or *infarction, anterior cerebral artery/di, pa, ra, us or *infarction, middle cerebral artery/di, pa, ra, us or *infarction, posterior cerebral artery/di, pa, ra, us or *hypoxia‐ischemia, brain/di, pa, ra, us or *intracranial arterial diseases/di, pa, ra, us or *cerebral arterial diseases/di, pa, ra, us or *Intracranial Arteriosclerosis/di, pa, ra, us or exp *"intracranial embolism and thrombosis"/di, pa, ra, us or *stroke/di, pa, ra, us 33. *basilar artery/us or exp *cerebral arteries/us or *vertebral artery/us 34. 32 or 33 35. exp "sensitivity and specificity"/ 36. (sensitiv$ or specificity).tw. 37. (predictive adj5 value$).tw. 38. exp diagnostic errors/ 39. ((false adj positive$) or (false adj negative$)).tw. 40. (observer adj variation$).tw. 41. (roc adj curve).tw. 42. (likelihood adj3 ratio$).tw. 43. likelihood function/ 44. 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 45. cerebrovascular disorders/us or basal ganglia cerebrovascular disease/us or brain ischemia/us or brain infarction/us or exp brain stem infarctions/us or cerebral infarction/us or infarction, anterior cerebral artery/us or infarction, middle cerebral artery/us or infarction, posterior cerebral artery/us or hypoxia‐ischemia, brain/us or intracranial arterial diseases/us or cerebral arterial diseases/us or Intracranial Arteriosclerosis/us or exp "intracranial embolism and thrombosis"/us or stroke/us 46. 33 or 45 47. 8 and 15 and 31 48. 15 and 34 49. 8 and 15 and 44 50. 44 and 46 51. 47 or 48 or 49 or 50 52. (1982$ or 1983$ or 1984$ or 1985$ or 1986$ or 1987$ or 1988$ or 1989$ or 199$ or 20$).ed. 53. 51 and 52
Appendix 2. Embase (Ovid) search strategy – January 1982 onwards
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ or brain atherosclerosis/ 2. stroke patient/ or stroke unit/ 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or hypoxi$)).tw. 4. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw. 5. ((intracranial or intra‐cranial or brain arter$ or cerebral arter$ or basilar arter$ or vertebral art$ or intracerebral or intra‐cerebral) adj5 (stenosis or stenoses or steno‐occlu$ or occlusion or occluded or occlusive or narrow$ or arteriosclero$ or atherosclero$ or plaque or plaques)).tw. 6. exp brain artery/ or vertebral artery/ 7. "stenosis, occlusion and obstruction"/ or stenosis/ or occlusion/ or blood vessel occlusion/ or artery occlusion/ or obstruction/ or arteriosclerosis/ or artery thrombosis/ or atherosclerotic plaque/ or atherosclerosis/ or artery intima proliferation/ 8. (stenosis or stenoses or steno‐occlu$ or occlusion or occluded or occlusive or narrow$ or arteriosclero$ or atherosclero$ or plaque or plaques).tw. 9. 7 or 8 10. 6 and 9 11. 1 or 2 or 3 or 4 or 5 or 10 12. echography/ or doppler echography/ or real time echography/ or color ultrasound flowmetry/ 13. echography/ and blood flow velocity/ 14. ((transcranial or duplex or color or colour) adj5 doppler).tw. 15. (TCD or TCCD).tw. 16. ((doppler or duplex) adj5 (sonogra$ or ultrasonogra$)).tw. 17. Transcranial Color‐Coded Duplex.tw. 18. 12 or 13 or 14 or 15 or 16 or 17 19. magnetic resonance angiography/ 20. angiography/ or arteriography/ or exp brain angiography/ 21. nuclear magnetic resonance imaging/ 22. 20 and 21 23. ((magnetic resonance or MR or MRI or NMR) adj5 (angiogra$ or arteriogra$)).tw. 24. MRA.tw. 25. computed tomographic angiography/ 26. angiography/ or arteriography/ or exp brain angiography/ 27. computer assisted tomography/ or spiral computer assisted tomography/ 28. 26 and 27 29. ((compute$ tomograph$ or CT or CAT) adj5 (angiogra$ or arteriogra$)).tw. 30. CTA.tw. 31. conventional angiography/ or digital subtraction angiography/ 32. image subtraction/ or subtraction.tw. 33. angiography/ or arteriography/ or exp brain angiography/ 34. 32 and 33 35. ((digital subtract$ or catheter or cerebral or brain) adj5 (angiogra$ or arteriogra$)).tw. 36. (DSA or IADSA).tw. 37. 19 or 22 or 23 or 24 or 25 or 28 or 29 or 30 or 31 or 34 or 35 or 36 38. *cerebrovascular disease/di or *cerebral artery disease/di or *cerebrovascular accident/di or *stroke/di or *vertebrobasilar insufficiency/di or exp *brain infarction/di or exp *brain ischemia/di or exp *occlusive cerebrovascular disease/di or *brain atherosclerosis/di 39. "sensitivity and specificity"/ 40. receiver operating characteristic/ 41. diagnostic accuracy/ 42. exp diagnostic error/ 43. observer variation/ 44. "limit of detection"/ 45. "diagnostic test accuracy study".sh. 46. (sensitivity or specificity).tw. 47. (predictive adj3 value$).tw. 48. ((false adj positive$) or (false adj negative$)).tw. 49. observer variation$.tw. 50. (roc adj curve$).tw. 51. (likelihood adj3 ratio$).tw. 52. or/39‐51 53. 11 and 18 and 37 54. 18 and 38 55. 11 and 18 and 52 56. 53 or 54 or 55 57. (1982$ or 1983$ or 1984$ or 1985$ or 1986$ or 1987$ or 1988$ or 1989$ or 199$ or 20$).em. 58. 56 and 57 59. (animal/ or nonhuman/ or animal experiment/) and human/ 60. animal/ or nonhuman/ or animal experiment/ 61. 60 not 59 62. 58 not 61
Appendix 3. QUADAS tool
Domain 1 ‐ Patient selection
1.1 Was a consecutive or random sample of patients enrolled?
Yes is scored when either a consecutive or random sample of patients with acute ischaemic stroke was enrolled.
No is scored when a consecutive or random sample of patients with acute ischaemic stroke was not enrolled.
Unclear is scored when there is insufficient information to make a decision.
1.2 Was a case‐control design avoided?
Yes is scored when a case‐control design is avoided.
No is scored when a case‐control design is not avoided.
Unclear is scored when there is insufficient information to make a decision.
1.3 Did the study avoid inappropriate exclusions?
Yes is scored when inappropriate exclusions were avoided (i.e. all patients assessed with TCD/TCCD against either CTA, MRA, contrast‐enhanced MRA or IA angiography are considered).
No is scored when inappropriate exclusions were not avoided (e.g. patients with test findings ‘difficult’ to interpret or patients with severe stroke symptoms are not considered).
Unclear is scored when there is insufficient information to make a decision.
Domain 2 – Index test
2.1 Were the index test results interpreted without knowledge of the results of the reference standard?
Yes is scored when results of the TCD/TCCD are interpreted without knowledge of the results of either CTA, MRA, contrast‐enhanced MRA or IA angiography.
No is scored when results of the TCD/TCCD are interpreted knowing already the results of either CTA, MRA, contrast‐enhanced MRA or IA angiography.
Unclear is scored when there is insufficient information on the way TCD/TCCD and the reference tests were interpreted.
2.2 If a threshold was used, was it prespecified?
Yes is scored when a TCD/TCCD threshold was prespecified (e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s).
No is scored when a TCD/TCCD threshold was not prespecified.
Unclear is scored when there is insufficient information to make a decision.
Domain 3 – Reference standard
3.1 Is the reference standard likely to correctly classify the target condition?
Yes is scored when the reference standard used is either CTA, MRA or contrast‐enhanced MRA, or IA angiography.
No is scored when another test, different from IA angiography, CTA, MRA or contrast‐enhanced MRA, is used as reference standard.
Unclear is scored when there is insufficient information on the reference standard used.
3.2 Were the reference standard results interpreted without knowledge of the results of the index test?
Yes is scored when results of the reference tests are interpreted without knowledge of the TCD/TCCD findings in cases when reference tests are used before the index standard.
No is scored when results of the reference tests are interpreted knowing already the findings of the TCD/TCCD in cases when index tests are used before the reference tests.
Unclear is scored when there is insufficient information on the way TCD/TCCD and reference tests were interpreted.
Domain 4 – Flow and timing
4.1 Was there an appropriate interval between index tests and reference standard?
Yes is scored when intracranial arteries are examined by both the reference standard and the index tests within 24 hours.
No is scored when the time period between index tests and reference standard is more than 24 hours.
Unclear is scored when there is insufficient information on the time interval between tests.
4.2 Did all patients receive a reference standard?
Yes is scored when the whole sample of acute stroke patients or a random selection of the sample or a selection of the sample with consecutive series receive verification using either CTA, MRA, contrast‐enhanced MRA or IA angiography.
No is scored when a part of the sample of acute stroke patients that is non‐randomly or non‐consecutively selected receives verification with either CTA, MRA, contrast‐enhanced MRA or IA angiography.
Unclear is scored when there is insufficient information to ascertain if the whole sample or a random selection of the sample received verification with the reference standard.
4.3 Did all patients receive the same reference standard?
Yes is scored when study participants are tested with the same reference standard (either CTA, MRA, contrast‐enhanced MRA or IA angiography) regardless of TCD/TCCD results.
No is scored when choice of the reference standard test is based on the TCD/TCCD results.
Unclear is scored when there is insufficient information on the different reference standard tests used.
4.4 Were all patients included in the analysis?
Yes is scored when all acute stroke patients were included in the analysis.
No is scored when all acute stroke patients were not included in the analysis.
Unclear is scored when there is insufficient information to make a decision.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 TCD/TCCD (any artery) | 9 | 493 |
| 4 TCD/TCCD (MCA) | 8 | 393 |
1. Test.
TCD/TCCD (any artery).
4. Test.
TCD/TCCD (MCA).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bar 2010.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 31 participants (mean age 64.5) Department of Neurology. Geographical location: Czech Republic |
||
| Index tests | TCCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries: MCA (M1 and MCA branches), IC, ICA. Reference standard: CTA | ||
| Flow and timing | Timing < 3 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Brunser 2009.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 100 participants (mean age 69.9) Emergency Department. Geographical location: Chile |
||
| Index tests | TCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries: MCA, ACA, terminal ICA, VA, BA, PCA. Reference standard: CTA | ||
| Flow and timing | Timing < 24 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Camerlingo 1993.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 45 participants (mean age 68.1) Stroke unit. Geographical location: Italy |
||
| Index tests | TCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries/stenosis: IC‐ICA, MCA (mainstem, distal and arterial branches). Reference standard: IA | ||
| Flow and timing | Timing < 6 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Guan 2013.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 128 participants (mean age 61.4) Setting unclear. Geographical location: China |
||
| Index tests | TCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries/stenosis: MCA, VA, BA, PCA, syphon, terminal ICA, ACA. Reference standard: CTA | ||
| Flow and timing | Timing < 3 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kamel 2010.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 30 participants (mean age 60) ICU neurology department. Geographical location: Egypt |
||
| Index tests | TCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries/stenosis: MCA. Reference standard: MRA time of flight | ||
| Flow and timing | Timing < 24 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kenton 1997.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 30 participants (mean age 74.8) Acute stroke setting. Geographical location: UK |
||
| Index tests | TCCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries/stenosis: MCA. Reference standard: MRA time of flight | ||
| Flow and timing | Timing < 24 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Martinez‐Sanchez 2015.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 65 participants (mean age 63.8) Geographical location: Spain |
||
| Index tests | TCCD | ||
| Target condition and reference standard(s) | Occlusion of MCA Reference standard: CTA |
||
| Flow and timing | Timing < 8 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Panebianco 2016.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 30 participants (mean age 60.7) Stroke unit. Geographical location: Italy |
||
| Index tests | TCCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries/stenosis: MCA. Reference standard: CTA | ||
| Flow and timing | Timing < 12 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Zanette 1989.
| Study characteristics | |||
| Patient sampling | |||
| Patient characteristics and setting | 39 participants (mean age 66.2) Stroke unit. Geographical location: Italy |
||
| Index tests | TCD | ||
| Target condition and reference standard(s) | Occlusion of intracerebral arteries: MCA (mainstem and branches), syphon. Reference standard: IA | ||
| Flow and timing | Timing < 6 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Index test | |||
| If a TCD/TCCD threshold was used, was it pre‐specified e.g. MFV thresholds ranging from ≥ 80 cm/s to ≥ 120 cm/s and ≥ 120 cm/s to ≥ 230 cm/s? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
ACA: anterior cerebral artery BA: basilar artery CTA: computed tomography angiography IA: intraarterial angiography IC‐ICA: intracranial‐internal carotid artery ICA: internal carotid artery MCA: middle cerebral artery MFV: maximum flow velocity MRA: magnetic resonance angiography PCA: posterior cerebral artery TCCD: transcranial colour‐coded duplex TCD: transcranial colour doppler VA: vertebral artery
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bang 2003a | Timing > 24 hours |
| Bang 2003b | Timing > 24 hours |
| Barlinn 2013 | Timing > 24 hours |
| Chen 2014 | Nonconsecutive patient sample. Timing > 24 hours |
| Felberg 2002 | Timing > 24 hours |
| Gerriets 2002 | Lack of data for 2 x 2 tables |
| Khan 2009 | Inclusion of asymptomatic patients |
| Kim 2014 | Occlusions causing significant haemodynamic disorders in a different artery from the target arteries |
| Lange 2015 | Timing > 24 hours |
| Ley‐Pozo 1990 | Inclusion of TIA patients |
| Nasr 2013 | Inclusion of TIA patients |
| Postert 1998 | Lack of data for 2 x 2 tables |
| Postert 1999 | Lack of data for 2 x 2 tables |
| Rorick 1994 | Timing > 24 hours |
| Suwanwela 2002 | Timing > 24 hours |
| Tsivgoulis 2007 | Inclusion of TIA patients |
| Zhou 2013 | Lack of data for 2 x 2 tables |
TIA: transient ischaemic attack
Characteristics of ongoing studies [ordered by study ID]
Palmieri 2017.
| Trial name or title | Accuracy of transcranial colour‐coded duplex sonography in the diagnosis of acute intracranial large vessel occlusion |
| Target condition and reference standard(s) | Target condition: occlusion or major stenosis of a large intracranial artery Reference standards: CTA and IA |
| Index and comparator tests | Index test: TCCD |
| Starting date | 2016 |
| Contact information | Baracchini C |
| Notes |
CTA: computed tomography angiography IA: intraarterial angiography TCCD: transcranial colour‐coded duplex
Differences between protocol and review
Due to the small number of included studies, we were not able to perform all the sensitivity analyses and meta‐regressions we originally planned and detailed in the research protocol. Instead we decided to perform a sensitivity analysis excluding the studies which were not published in full. We introduced also two posthoc analyses in order to investigate the diagnostic accuracy according to the recent changes in the clinical indication for endovascular treatment.
We summarised sensitivity and specificity estimates using the bivariate model instead of the hierarchical summary ROC model (HSROC). The latter is recommended for continuous tests when the included studies report different thresholds for test positivity (Leeflang 2014).
We planned to assess the accuracy of different thresholds of mean flow velocity or peak systolic velocity, or both, in the grading of intracranial artery stenosis. However, due to the lack of data on different thresholds, we were not able to do so.
Contributions of authors
AM, SR and SC conceived the research question and contributed to the design of the review. AM drafted the initial version of the protocol with additional input from MB and SR. AM and MB developed the search strategies with additional input from Brenda Thomas and Joshua Cheyne, former and current Information Specialists of the Cochrane Stroke Group, and designed the data extraction form. MDS, CG, CF reviewed the literature on TCCD while MM and VS reviewed the literature on TCD. AM undertook the review and drafted its initial version with assistance from TM, SR and MB. SC and TM contributed to data extraction and tabulation of results. PE double checked all the extracted data and performed statistical analyses. All authors critically reviewed and approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
European Federation Neurological Society grant, Italy.
Declarations of interest
Alessia Mattioni: none Silvia Cenciarelli: none Paolo Eusebi: none Miriam Brazzelli: none Tatiana Mazzoli: none Massimo Del Sette: none Carlo Gandolfo: none Marinella Marinoni: none Cinzia Finocchi: none Valentina Saia: none Stefano Ricci: none
New
References
References to studies included in this review
Bar 2010 {published data only}
- Bar M, Skoloudik D, Roubec M, Hradiek P, Chmelovà J, Czerny D, et al. Transcranial Duplex sonography and CT angiography in acute stroke patients. Journal of Neuroimaging 2010;20:240‐5. [DOI] [PubMed] [Google Scholar]
Brunser 2009 {published data only}
- Brunser AM, Lavados PM, Hoppe A, Lopez J, Valenzuela M, Rivas R. Accuracy of transcranial Doppler compared with CT angiography in diagnosing arterial obstructions in acute ischemic strokes. Stroke 2009;40:2037‐41. [DOI] [PubMed] [Google Scholar]
Camerlingo 1993 {published data only}
- Camerlingo M, Casto L, Censori B, Ferraro B, Gazzaniga GC, Mamoli A. Transcranial doppler in acute ischemic stroke of the middle cerebral artery territories. Acta Neurologica Scandinavica 1993;88:108‐11. [DOI] [PubMed] [Google Scholar]
Guan 2013 {published data only}
- Guan J, Zhou Q, Ouyang H, Zhang S, Lu Z. The diagnostic accuracy of TCD for intracranial arterial stenosis/occlusion in patients with acute ischemic stroke: the importance of time interval between detection of TCD and CTA. Neurological Research 2013;35(9):930‐6. [DOI] [PubMed] [Google Scholar]
Kamel 2010 {published data only}
- Kamel A, Aziz SA, El‐Ebyary MM, El‐Fattah DA, Shawky KM. Ischemic stroke of anterior circulation: sensitivity and prognostic value of early ultrasound and imaging studies. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2010;47(2):303‐10. [Google Scholar]
Kenton 1997 {published data only}
- Kenton AR, Martin PJ, Abbott RJ, Moody AR. Comparison of transcranial color‐coded sonography and magnetic resonance angiography in acute stroke. Stroke 1997;28:1601‐6. [DOI] [PubMed] [Google Scholar]
Martinez‐Sanchez 2015 {published data only}
- Martinez‐Sanchez P, Garcia‐Pastor A, Arenillas‐Lara JF, Ayo‐Martin O, Ruiz‐Ares G, Diaz‐Otero F, et al. Accuracy of transcranial duplex sonography, compared with CT angiography, for detection of intracranial arterial occlusions in acute stroke. International Journal of Stroke 2015;10 Suppl 2:77‐438. [Google Scholar]
Panebianco 2016 {published data only}
- Panebianco M, Zavanone C, Perrone P, Callone MV. Transcranial color doppler ultrasonography: the "stethoscope" of vascular neurologist for assessment of medium cerebral artery in acute ischemic stroke patients. Conference proceeding ‐ Congresso Nazionale SNO 2016, Catania, Italy.
Zanette 1989 {published data only}
- Zanette EM, Fieschi C, Bozzao L, Roberti C, Toni D, Argentino C, et al. Comparison of cerebral angiography and transcranial Doppler sonography in acute stroke. Stroke 1989;20:899‐903. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bang 2003a {published data only}
- Bang OY, Joo IS, Huh K, Kim SY. The role of transcranial Doppler in symptomatic striatocapsular small deep infarction. Journal of Neuroimaging 2003;13(1):48‐52. [PubMed] [Google Scholar]
Bang 2003b {published data only}
- Bang OY, Cho JH, Han BI, Joo IS, Kim DI, Huh K. Transcranial Doppler findings in middle cerebral arterial occlusive disease in relation to degree of stenosis and presence of concomitant stenoses. Journal of Clinical Ultrasound 2003;31(3):142‐51. [DOI] [PubMed] [Google Scholar]
Barlinn 2013 {published data only}
- Barlinn K, Zivanovic Z, Zhao l, Kesani M, Balucani C, Tsivgoulis G, et al. Intracranial vessel localization with power motion Doppler (PMD‐TCD) compared with CT angiography in patients with acute ischaemic stroke. International Journal of Stroke 2013;8(6):398‐402. [DOI] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen J, Wang L, Bai J, Lun Z, Zhang J, Xing Y. The optimal velocity criterion in the diagnosis of unilateral middle cerebral artery stenosis by transcranial Doppler. Cell Biochemisty and Biophysics 2014;69(1):81‐7. [DOI] [PubMed] [Google Scholar]
Felberg 2002 {published data only}
- Felberg RA, Christou I, Demchuk AM, Malkoff M, Alexandrov AV. Screening for intracranial stenosis with transcranial Doppler: the accuracy of mean flow velocity thresholds. Journal of Neuroimaging 2002;12(1):9‐14. [DOI] [PubMed] [Google Scholar]
Gerriets 2002 {published data only}
- Gerriets T, Goertler M, Stolz E, Postert T, Sliwka U, Schlachetzki F, et al. Feasibility and validity of transcranial duplex sonography in patients with acute stroke. Journal of Neurology, Neurosurgery and Psychiatry 2002;73(1):17‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2009 {published data only}
- Khan S, Rich P, Clifton A, Markus HS. Noninvasive detection of vertebral artery stenosis: a comparison of contrast‐enhanced MR angiography, CT angiography, and ultrasound. Stroke 2009;40(11):3499‐503. [DOI] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim JS, Lee SW, Eun MY, Seo WK. Power motion‐mode Doppler signature: a useful tool for assessing middle cerebral artery stenosis. Journal of Clinical Ultrasound 2014;42(6):348‐54. [DOI] [PubMed] [Google Scholar]
Lange 2015 {published data only}
- Lange MC, Bruch TP, Pedrozo JC, Maranha L, Sakae TM, Pacheco R, et al. The use of neurovascular ultrasound versus digital subtraction angiography in acute ischemic stroke. Arquivos de Neuro‐Psiquiatria 2015;73(3):218‐22. [DOI] [PubMed] [Google Scholar]
Ley‐Pozo 1990 {published data only}
- Ley‐Pozo J, Ringelstein EB. Noninvasive detection of occlusive disease of the carotid siphon and middle cerebral artery. Annals of Neurology 1990;28(5):640‐7. [DOI] [PubMed] [Google Scholar]
Nasr 2013 {published data only}
- Nasr N, Ssi‐Yan‐Kai G, Guidolin B, Bonneville F, Larrue V. Transcranial color‐coded sonography to predict recurrent transient ischaemic attack/stroke. European Journal of Neurology 2013;20(8):1212‐7. [DOI] [PubMed] [Google Scholar]
Postert 1998 {published data only}
- Postert T, Braun B, Federlein J, Przuntek H, Köster O, Büttner T. Diagnosis and monitoring of middle cerebral artery occlusion with contrast‐enhanced transcranial color‐coded real‐time sonography in patients with inadequate acoustic bone windows. Ultrasound in Medicine and Biology 1998;24(3):333‐40. [DOI] [PubMed] [Google Scholar]
Postert 1999 {published data only}
- Postert T, Braun B, Meves S, Köster O, Przuntek H, Weber S, et al. Contrast‐enhanced transcranial color‐coded sonography in acute hemispheric brain infarction. Stroke 1999;30(9):1819‐26. [DOI] [PubMed] [Google Scholar]
Rorick 1994 {published data only}
- Rorick MB, Nichols FT, Adams RJ. Transcranial Doppler correlation with angiography in detection of intracranial stenosis. Stroke 1994;25(10):1931‐4. [DOI] [PubMed] [Google Scholar]
Suwanwela 2002 {published data only}
- Suwanwela NC, Phanthumchinda K, Suwanwela N. Transcranial doppler sonography and CT angiography in patients with atherothrombotic middle cerebral artery stroke. American Journal of Neuroradiology 2002;23(8):1352‐5. [PMC free article] [PubMed] [Google Scholar]
Tsivgoulis 2007 {published data only}
- Tsivgoulis G, Sharma VK, Lao AY, Malkoff MD, Alexandrov AV. Validation of transcranial Doppler with computed tomography angiography in acute cerebral ischemia. Stroke 2007;38(4):1245‐9. [DOI] [PubMed] [Google Scholar]
Zhou 2013 {published data only}
- Zhou C, Zhang Pl. Comparative study of transcranial Doppler ultrasonography and magnetic resonance angiography on recanalization of intravenous thrombolysis with alteplase in acute ischemic stroke patients. Chinese Journal of Contemporary Neurology and Neurosurgery 2013;13(12):1027‐32. [Google Scholar]
References to ongoing studies
Palmieri 2017 {published data only}
- Palmieri A, Farina FM, Kulyk C, Viaro F, Baracchini C, Vodret FR, et al. Accuracy of transcranial colorcoded duplex sonography in the diagnosis of acute intracranial large vessel occlusion. International Journal of Stroke 2017;12(1 Supplement 1):18. [Google Scholar]
Additional references
Adams 2007
- Adams HP, Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke. A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Circulation 2007;115:e478‐e534. [DOI] [PubMed] [Google Scholar]
Alexandrov 1999
- Alexandrov AV, Demchuk AM, Wein TH, Grotta JC. Yield of transcranial doppler in acute cerebral ischemia. Stroke 1999;30:1604‐9. [DOI] [PubMed] [Google Scholar]
Allendoerfer 2006
- Allendoerfer J, Goertler M, Reutern GM, Neurosonology in Acute Ischemic Stroke Study Group. Prognostic relevance of ultra‐early doppler sonography in acute ischaemic stroke: a prospective multicentre study. Lancet Neurology 2006;5:835‐40. [DOI] [PubMed] [Google Scholar]
Arnold 2004
- Arnold M, Nedeltchev K, Brekenfeld C, Fischer U, Remonda L, Schroth G, et al. Outcome of acute stroke patients without visible occlusion on early arteriography. Stroke 2004;35:1135‐8. [DOI] [PubMed] [Google Scholar]
Astin 2008
- Astin M, Brazzelli M, Fraser C, Counsell C, Needham G, Grimshaw J. Developing a sensitive search strategy in MEDLINE to retrieve studies on assessment of the diagnostic performance of imaging techniques. Radiology 2008;247:365‐73. [DOI] [PubMed] [Google Scholar]
Baracchini 2016
- Baracchini C, Anzola GP, Cenciarelli S, Diomedi M, Bella R, Tono A, et al. Italian symptomatic intracranial atherosclerosis study (ISIDE). Neurological Sciences 2016;37(10):1645‐51. [DOI] [PubMed] [Google Scholar]
Baracchini 2017
De Vet 2008
- Vet HCW, Eisinga A, Riphagen II, Aertgeerts B, Pewsner D. Chapter 7: Searching for studies. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.4 [updated September 2008]. The Cochrane Collaboration, 2008. Available from srdta.cochrane.org.
ESO Guidelines 2008
- The European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascular Diseases 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
Fiebach 2012
- Fiebach JB, Al‐Rawi Y, Wintermark M, Furlan AJ, Rowley HA, Lindsten A, et al. Vascular occlusion enables selecting acute ischemic stroke patients for treatment with desmoteplase. Stroke 2012;43:1561‐6. [DOI] [PubMed] [Google Scholar]
Gerriets 2000
- Gerriets T, Postert T, Goertler M, Stolz E, Schlachetzki F, Sliwka U, the DIAS (Duplex Sonography in Acute Stroke) Study Group. DIAS I: Duplex‐sonographic assessment of the cerebrovascular status in acute stroke. A useful tool for future stroke trials. Stroke 2000;31:2342‐5. [DOI] [PubMed] [Google Scholar]
Hankey 1990
- Hankey GJ, Warlow CP, Molyneux AJ. Complications of cerebral angiography for patients with mild carotid territory ischaemia being considered for carotid endarterectomy. Journal of Neurology, Neurosurgery and Psychiatry 1990;53:542‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassem‐Moussa 2002
- Kassen‐Moussa H, Graffagnino C. Nonocclusion and spontaneous recanalization rates in acute ischemic stroke. Archives of Neurology 2002;59:1870‐3. [DOI] [PubMed] [Google Scholar]
Latchaw 2009
- Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 2009;40:3646‐78. [DOI] [PubMed] [Google Scholar]
Leeflang 2014
- Leeflang MMG. Systematic reviews and meta‐analyses of diagnostic test accuracy. Clinical Microbiology and Infection 2014;20(2):105‐13. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Chapter 10 Analysing and presenting results. In: Deeks JJ, Harbord RM, Takwoingi Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0. The Cochrane Collaboration, 2010. Available from http://srdt.cochrane.org/.
Mazya 2018
- Mazya MV, Ahmed N, Azevedo E, Davalos A, Dorado L, Karlinski M, the SITS Investigators. Impact of transcranial doppler ultrasound on logistics and outcomes in stroke thrombolysis. Results from the SITS‐ISTR. Stroke 2018;49(7):1695‐700. [DOI] [PubMed] [Google Scholar]
Nacu 2017
- Nacu A, Kvistad CE, Naess H, Øygarden H, Logallo N, Assmus J, et al. NOR‐SASS (Norwegian Sonothrombolysis in Acute Stroke Study). Randomized controlled contrast‐enhanced sonothrombolysis in an unselected acute ischemic stroke population. Stroke 2017;48(2):335‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference Manager 10 [Computer program]
- ISI ResearchSoft. Reference Manager. Version Professional, 10. ISI ResearchSoft, 1984‐2001.
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, Cochrane Collaboration. Review Manager 5 (RevMan5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration, 2014.
Ricci 2012
- Ricci S, Dinia L, Sette M, Anzola P, Mazzoli T, Cenciarelli S, et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008348.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodrigues 2016
- Rodrigues FB, Neves JB, Caldeira D, Ferron JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta‐analysis. BMJ 2016;353:i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2006
- Smith WS, Tsao JW, Billings ME, Johnston C, Hemphill JC, Bonovich DC, et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocritical Care 2006;4:14‐7. [DOI] [PubMed] [Google Scholar]
SPREAD 2016
- Gruppo SPREAD. Stroke PRevention and Educational Awareness Diffusion (SPREAD). VIII Edizione. Ictus cerebrale: linee guida italiane di prevenzione e trattamento. Linee Guida ISO SPREAD 21 luglio 2016 http://www.iso‐spread.it/.
Takwoingi 2017
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2017;26(4):1896‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wardlaw 2012
- Wardlaw JM, Murray V, Berge E, Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta‐analysis. Lancet 2012;379:2364‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wardlaw 2014
- Warldlaw JM, Carpenter T, Sakka E, Mair G, Cohen G, Shuler K, et al. Imaging perfusion deficits, arterial patency and thrombolysis safety and efficacy in acute ischaemic stroke. An observational study of the effect of advanced imaging methods in the Third International Stroke Trial (IST‐3), a randomised controlled trial. Efficacy and Mechanism Evaluation, No. 1.1. Vol. 1, Southampton, UK: NIHR Journals Library, 2014. [DOI: 10.3310/eme01010] [DOI] [PubMed] [Google Scholar]
Wardlaw 2015
- Wardlaw JM, Dennis MS. Thrombectomy for acute ischemic stroke. JAMA 2015;314(17):1803‐5. [DOI] [PubMed] [Google Scholar]
Warlow 2003
- Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet 2003;362:1211‐24. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, the QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155:529‐36. [DOI] [PubMed] [Google Scholar]
Yarbrough 2015
- Yarbrough CK, Ong CJ, Beyer AB, Lipsey K, Derdeyn CP. Endovascular thrombectomy for anterior circulation stroke. Systematic review and meta‐analysis. Stroke 2015;46:3177‐83. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mattioni 2013
- Mattioni A, Brazzelli M, Cenciarelli S, Mazzoli T, Sette M, Gandolfo C, et al. Transcranial Doppler sonography for detecting stenosis or occlusion of intracranial arteries in people with acute ischaemic stroke. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010722] [DOI] [PMC free article] [PubMed] [Google Scholar]


