Summary
Background
Antidepressants, opioids for non-cancer pain, gabapentinoids (gabapentin and pregabalin), benzodiazepines, and Z-drugs (zopiclone, zaleplon, and zolpidem) are commonly prescribed medicine classes associated with a risk of dependence or withdrawal. We aimed to review the evidence for these harms and estimate the prevalence of dispensed prescriptions, their geographical distribution, and duration of continuous receipt using all patient-linked prescription data in England.
Methods
This was a mixed-methods public health review, comprising a rapid evidence assessment of articles (Jan 1, 2008, to Oct 3, 2018; with searches of MEDLINE, Embase, and PsycINFO, and the Cochrane and King's Fund libraries), an open call-for-evidence on patient experience and service evaluations, and a retrospective, patient-linked analysis of the National Health Service (NHS) Business Services Authority prescription database (April 1, 2015, to March 30, 2018) for all adults aged 18 years and over. Indirectly (sex and age) standardised rates (ISRs) were computed for all 195 NHS Clinical Commissioning Groups in England, containing 7821 general practices for the geographical analysis. We used publicly available mid-year (June 30) data on the resident adult population and investigated deprivation using the English Indices of Multiple Deprivation (IMD) quintiles (quintile 1 least deprived, quintile 5 most deprived), with each patient assigned to the IMD quintile score of their general practitioner's practice for each year. Statistical modelling (adjusted incident rate ratios [IRRs]) of the number of patients who had a prescription dispensed for each medicine class, and the number of patients in receipt of a prescription for at least 12 months, was done by sex, age group, and IMD quintile.
Findings
77 articles on the five medicine classes were identified from the literature search and call-for-evidence. 17 randomised placebo-controlled trials (6729 participants) reported antidepressant-associated withdrawal symptoms. Almost all studies were rated of very low, low, or moderate quality. The focus of qualitative and other reports was on patients' experiences of long-term antidepressant use, and typically sudden onset, severe, and protracted withdrawal symptoms when medication was stopped. Between April 1, 2017, and March 31, 2018, 11·53 million individuals (26·3% of residents in England) had a prescription dispensed for at least one medicine class: antidepressants (7·26 million [16·6%]), opioids (5·61 million [12·8%]), gabapentinoids (1·46 million [3·3%]), benzodiazepines (1·35 million [3·1%]), and Z-drugs (0·99 million [2·3%]). For three of these medicine classes, more people had a prescription dispensed in areas of higher deprivation, with adjusted IRRs (referenced to quintile 1) ranging from 1·10 to 1·24 for antidepressants, 1·20 to 1·85 for opioids, and 1·21 to 1·85 for gabapentinoids across quintiles, and higher ISRs generally concentrated in the north and east of England. In contrast, the highest ISRs for benzodiazepines and Z-drugs were generally in the southwest, southeast, and east of England, with low ISRs in the north. Z-drugs were associated with increased deprivation, but only at the highest quintile (adjusted IRR 1·11 [95% CI 1·01–1·22]). For benzodiazepines, prescribing was reduced for people in quintiles 4 (0·90 [0·85–0·96]) and 5 (0·89 [0·82–0·97]). In March, 2018, for each of medicine class, about 50% of patients who had a prescription dispensed had done so continuously for at least 12 months, with the highest ISRs in the north and east. Long-term prescribing was associated with a gradient of increased deprivation.
Interpretation
In 1 year over a quarter of the adult population in England had a prescription dispensed for antidepressants, opioids (for non-cancer pain), gabapentinoids, benzodiazepines, or Z-drugs. Long-term (>12 months) prescribing is common, despite being either not recommended by clinical guidelines or of doubtful efficacy in many cases. Enhanced national and local monitoring, better guidance for personalised care, and better doctor–patient decision making are needed.
Funding
Public Health England.
Research in context.
Evidence before this study
Antidepressants, opioids (for chronic non-cancer pain), gabapentinoids, benzodiazepines, and Z-drugs are associated with dependence or withdrawal, but, to date, there have been no national estimates of prescribing prevalence and practices, and no public health review of patients' experiences of taking these medicines. Unpleasant physical and psychological withdrawal symptoms can occur if the medicine is stopped without tapering, and fear of withdrawal alongside these cognitive and behaviour symptoms can induce chronic dependence. As part of our rapid evidence assessment for the current study, we searched for articles published in English between Jan 1, 2008, and Oct 3, 2018, on risk factors for drug use disorder and withdrawal symptoms, the harms experienced, and prevention and treatment (excluding end-of-life and palliative care for cancer, and treatment of epilepsy). We searched for systematic reviews, randomised and non-randomised trials and qualitative studies in the Cochrane Library, Epistemonikos, the Database of Promoting Health Effectiveness Reviews, Health Evidence, Medline, Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Daily, Embase, PsycINFO, Health Technology Appraisals, the Trials Register of Promoting Health Interventions and the Applied Social Sciences Index and Abstracts. The King's Fund Library and the National Institute for Health Research Journals Library were searched for reports on patients' experience and service models.
Added value of this study
We did a mixed-methods public health review and study in England, comprising a rapid evidence assessment, an open call-for-evidence on patient experience and service evaluations, and a retrospective, patient-linked analysis of the UK National Health Service Business Services Authority (NHSBSA) prescription database. NHSBSA data showed that 11·53 million adults (26·3% of adults residing in England) had a prescription dispensed for one or more of the five medicine classes (antidepressants, opioids, gabapentinoids, benzodiazepines, and Z-drugs) between April, 2017, and March, 2018. Greater local area deprivation was associated with higher standardised rates of dispensed antidepressants, opioids, and gabapentinoids (particularly in urban centres in the north and east of England), and slightly lower rates for benzodiazepines and Z-drugs (with the highest rates in the southwest, southeast, and east, and markedly lower rates in the north). With some exceptions, the proportion of patients with a dispensed prescription for at least a 12-month period was higher in the north and east than south and west, but the geographical pattern was less clear for benzodiazepines and Z-drugs. An association between prescribing frequency and deprivation was also found for most of the medicine classes (although not for benzodiazepines, and for Z-drugs only at the highest quintile of deprivation). For each class, about 50% of patients who had received a prescription in March, 2018, had done so continuously for at least 12 months. Longer-term prescribing was not associated with sex for any of the medicine classes, but increased with age (except in the oldest age group for opioids) and deprivation.
Implications of all the available evidence
In England, around half of patients who were dispensed a prescription for opioids (for chronic non-cancer pain), gabapentinoids, benzodiazepines, or Z-drugs had been treated continuously for at least 12 months, a practice either not recommended by clinical guidelines or of doubtful efficacy in many cases. Given evidence from clinical studies and patients' experiences, sudden cessation of these medicines can lead to physical and psychological withdrawal symptoms. If there is a decision to stop, patients treated long-term with any of these medicine classes studied should be offered careful tapering and support. These findings highlight the need for improved guidance, monitoring, and effective patient care.
Introduction
A fundamental principle in evidence-based clinical practice is to balance the likelihood of benefit for the patient against the risk of harms.1 Some medicines need to be titrated carefully (the classic example being strong opioid analgesics) and the risk of side effects, as well as any non-prescribed medication use and dependence (ie, drug use disorder) monitored and managed.2 In the short-term, opioids are effective for neuropathic (nerve damage), musculoskeletal, and cancer pain, but have little effectiveness for chronic non-cancer pain (pain lasting or recurring for more than 3 months).3, 4 Since the mid-1990s, the incidence of fatal opioid overdoses has risen substantially in many countries.5, 6
Gabapentinoids (the γ-aminobutyric-acid [GABA] analogue medications pregabalin and gabapentin) were introduced in the late 1970s in the UK for seizure control and later for neuropathic pain, with pregabalin also introduced to treat anxiety. Early on, these medicines were known to cause reinforcing euphoria, sedation, and dissociation, but were initially believed to carry a relatively low risk of dependence and withdrawal symptoms.7, 8 Evidence now shows that when gabapentinoids are taken with opioids a dangerous respiratory depression can occur.9
Benzodiazepines, which are effective for short-term treatment of severe anxiety and insomnia,10 also carry a risk of dependence. Prescribing in the UK (and globally) was widespread in the 1970s and 1980s, but evidence accumulated that longer-term treatment led to risks of adverse neurological effects, dependence, and withdrawal symptoms.11, 12 Although benzodiazepines have a relatively low toxicity profile, taking them alongside opioids increases the risk of overdose due to cumulative and synergistic effects on respiratory depression.13
In the 1990s, three non-benzodiazepine hypnotic agents—zopiclone, zaleplon, and zolpidem—were licensed in the UK for insomnia. Known as Z-drugs, these medicines were developed with the aim of preventing the over-sedation and daytime sleepiness associated with benzodiazepines, and were initially believed to carry a low risk of dependence.14
Antidepressants are not associated with a risk of dependence, are used long-term for severe depression, and can bring transformative clinical benefit.15, 16, 17 However, antidepressants have become controversial, with claims that they have minimal efficacy and can be harmful.18, 19 Since the late 1950s, it has been recognised that abrupt discontinuation of an antidepressant can lead to (sometimes protracted) withdrawal symptoms.20 Withdrawal symptoms appear to be more likely and can be more severe with some SSRI and SNRI antidepressants, especially paroxetine and venlafaxine, than with other types of antidepressant.21
In England, between 2008 and 2018, dispensing of antidepressants from community pharmacies increased from 36·0 million to 70·9 million items, while opioids increased from 33·1 million to 40·5 million dispensed items.22 In the same period, gabapentinoids were less commonly dispensed but still increased substantially, from 2·7 million to 14·4 million. By contrast, dispensed prescriptions of benzodiazepines fell from 10·5 million to 7·7 million, and dispensed prescriptions of Z-drugs increased from 5·4 million to 6·5 million until 2014, before falling to 6·0 million.21 In addition to dispensing from community retail pharmacies, hospitals have also been a source of medicines in all these classes. A national audit conducted by IQVIA, with data purchased by Public Health England (PHE), indicated that between November, 2016, and October, 2018, hospital pharmacies dispensed around 1·4 million antidepressant packs and 6·2 million opioid packs per year.23 Hospital dispensing was lower for gabapentinoids and benzodiazepines (each around 0·5 million prescriptions per year) and Z-drugs (210 000 per year).
There have been repeated calls in the UK for a review of prescribed medicines associated with dependence or withdrawal.24, 25, 26 In October, 2017, the UK minister for public health and primary care commissioned PHE to identify the scale, distribution, and causes of prescription drug dependence, and what might be done to address it. The full report of this review is available online. In this Article, we present our evaluation.
Methods
Design and scope
This was a mixed-methods public health review and study of the prevalence of dispensed prescriptions for antidepressants, opioids, gabapentinoids, benzodiazepines, and Z-drugs in England, and the number of patients in continuous receipt of such a prescription for at least 12 months. The study comprised a rapid evidence assessment, an open call-for-evidence on patient experiences and service evaluations, and a retrospective, patient-linked analysis of the national prescription database. The study population was all adults aged 18 years and older who were registered with a GP practice in England.
We tabulated and mapped data using the boundary locations of all 195 National Health Service (NHS) Clinical Commissioning Groups (CCGs) operational in April, 2018. CCGs are statutory bodies that plan and commission community and hospital services for their local population. Within the boundaries of these CCGs were 7821 general practices (including practices that closed during the study).
In preparation, we asked the National Institute for Health and Care Excellence (NICE) to tabulate all potentially in-scope medicines by their British National Formulary (BNF) category code and class, as well as their legal category, indications, usual dose, index events (informing the need for clinical review and change in medication or dose), and recommended limits on prescribing duration (taken from the manufacturer's statement of product characteristics, the BNF, guidance from NICE, and guidance from the Faculty of Pain Medicine; appendix pp 2–62).27
After consultation with the study's expert reference group, we selected 30 antidepressants, 16 opioids, the two gabapentinoids, nine benzodiazepines, and the three Z-drugs for study, as the classes of medicine most commonly associated with dependence and withdrawal (appendix pp 63–64). The opioids included medicines containing codeine and dihydrocodeine that can be purchased without a prescription in pharmacies, because of the risk of harms and opioid use disorder associated with self-directed treatment.28
Literature search and rating procedure
The rapid evidence assessment was done in accordance with PRISMA guidelines.29 All searches were completed between Sept 19, 2018, and Oct 3, 2018. We searched for systematic reviews, randomised and non-randomised trials and qualitative studies in the Cochrane Library, Epistemonikos, the Database of Promoting Health Effectiveness Reviews, Health Evidence, MEDLINE (Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily), Embase, PsycINFO, Health Technology Appraisals, the Trials Register of Promoting Health Interventions and the Applied Social Sciences Index and Abstracts (see appendix [pp 65–81] for full search strategy). The King's Fund Library and the National Institute for Health Research Journals Library were searched for reports on patients' experience and service models.
All titles and abstracts retrieved were screened by two independent reviewers, with discrepancies resolved by a third party. For potentially relevant publications, full texts were obtained and checked. Risk of bias was rated with use of a checklist appropriate to the study design (ie, Cochrane RoB30 and ROBINS-I31 for randomised and non-randomised studies; Quality in Prognosis Studies32 for prognostic studies; the Institute of Health Economics Quality Appraisal of Case Series Studies;33 and the Critical Appraisal Skills Programme34 checklist for qualitative studies). Studies were assessed for risk of bias; data were extracted into evidence tables and the quality of the evidence rated. Where possible, a meta-analysis was done using the Cochrane Review Manager (RevMan software, version 5.3) and GRADEpro was used to rate evidence quality.35 When a full data analysis was not possible, a narrative summary of the results was presented along with risk of bias rating. Findings from qualitative studies were tabulated thematically, and GRADE-CERQual was used to rate confidence.
All protocols were pre-registered at PROSPERO (CRD42018111310, CRD42018111319, CRD42018111349, CRD42018111356, CRD42018111357).
National prescriptions data
Data on dispensed prescriptions were obtained from the NHS Business Services Authority (NHSBSA) database, which records all medications submitted to NHSBSA for reimbursement after being prescribed by a medical professional and dispensed by a retail pharmacy in England, excluding items identified as not dispensed, disallowed, or returned. Apart from in specific circumstances related to some long-term conditions, NHS guidance is that all prescribed medicines are dispensed with no more than 28 days' supply. Prescriptions should specify the total quantity to be supplied or the number of days, along with the strength, dose, and frequency. Additional requirements apply to prescriptions for medicines controlled under schedules 2 and 3 of the Misuse of Drugs Act.
There is no comprehensive national database that records diagnostic information on indications, dependence, or withdrawal management. However, since April 1, 2015, the NHSBSA database has included each patient's NHS number (a unique identifier for each person registered with the NHS), enabling the duration of dispensed medicines to be estimated by linking two or more dispensed items of the same medicine class. Because the exact date a medication is dispensed is not always available, we used the month of submission to NHSBSA as the base time unit.
Data extraction and case selection
We first extracted data on all antidepressants, opioids, gabapentinoids, benzodiazepines, and Z-drugs reported to the NHSBSA from April 1, 2015, to June 30, 2018, that had been prescribed by general practitioners' (GP) practices in England and dispensed to patients by retail pharmacies. In addition to NHS number (with pseudonymisation to protect confidentiality),36 the following variables from the NHSBSA database were used: patient's sex and age; type of prescription form used; medicine class prescribed (using codes from the BNF); and the GP practice code. We assumed that methadone and buprenorphine prescriptions that used the BNF pain code on the FP10-MDA forms, used for controlled drug instalment dispensing, were for the treatment of opioid use disorder (ie, that the pain code was used in error), and these entries were removed. Using these forms, we removed less common instalment prescribing of other medicine classes (mainly benzodiazepines). We also removed dispensed opioids that had been prescribed by dentists using the FP10-D form.
After data extraction, the National Cancer Registration and Analysis Service matched the data to the National Cancer Registration Dataset (using a pseudonymised identifier) to allow removal of dispensed prescriptions for opioid use in cancer. This method led to the exclusion of all opioids issued 6 months before and 5 years after a cancer diagnosis, or up to 1 year before death from cancer. Exclusion of opioids dispensed during terminal care to patients who were not otherwise recorded in the cancer registration dataset was not possible, nor was exclusion of gabapentinoids dispensed for epilepsy.
Using all prescriptions dispensed between April 1, 2015, and June 30, 2018, we created an assignment rule to identify periods of continuous receipt of a prescription of one or more drugs from the same medicine class. We judged it reasonable to assume that prescriptions had been issued and dispensed around the same time as the pharmacist's report to NHSBSA. Consecutive monthly dispensed prescriptions for one or more medicines from the same class indicated continuous receipt of a prescription. A single month's missing data might indicate a prescription for longer than 28 days or a delay in submission to NHSBSA, and treatment was regarded as ongoing. However, if there were two adjacent months in which no prescriptions were dispensed, we took this to indicate that the prescription of that medicine class had ended (at the time when the previous prescription was dispensed). The dispensing of a medicine from the same class prescribed after a delay marked the start of a new period of prescribing. Any change in GP practice did not affect this assignment.
Additional data
Before doing the analysis, we added publicly available data on the English population, boundary data on all local treatment systems, and social deprivation. Published mid-year (June 30) resident adult population estimates were obtained from the Office for National Statistics (ONS) website37 and registered practice populations at April 1 for each year from the NHS Digital website.38 The national registered adult population (46·3 million) exceeds the ONS-estimated resident adult population (43·8 million) by about 6% because of differences in definitions, delays in removing decedents from registers, and some overcounting.
Deprivation was measured by English Indices of Multiple Deprivation (IMD).39 The IMD is a weighted combination of 37 indicators of income, employment, education, skills and training, health and disability, crime, barriers to housing and services, and living environment. We grouped IMD scores into quintiles (quintile 1 being least deprived and quintile 5 most deprived). For each year, each patient was assigned the IMD quintile score of their GP practice. In England, with some exceptions, local areas with the highest level of deprivation are large urban conurbations (particularly in the North and parts of London) and coastal towns, although there are local areas of deprivation surrounded by less deprived areas in every region (see appendix p 88 for deprivation quintiles for each CCG-registered population for April, 2017).
Statistical analysis
Data extraction was done in Structured Query Language. Statistical analysis was done in RStudio (version 1.1.463), R (version 3.5.2), and STATA (version 15.1). To display differences in prevalence and longer-term prescribing, we used the ONS Open Geography Portal to construct shapefiles using ultra-generalised clipped boundaries for April, 2018.40 Choropleth maps at the CCG level were drawn in ArcGIS Desktop (version 10.5) with ArcMap (version 10.7).
For a very small proportion of patients, information on sex, age, and IMD quintile was invalid or missing. Overall totals include these patients. Proportions at CCG level and national analyses of deprivation were calculated using the local registered adult population at each GP practice (at April 1 each year) before aggregation. We expected that a national analysis by CCG level and deprivation (which both use the registered rather than resident population as the denominator) would yield slightly lower prevalence estimates and would not be directly comparable to the remaining national-level analysis.
Monthly national numbers of patients who received a dispensed prescription were aggregated from CCG totals, apart from analyses by IMD quintile, in which GP practice totals were used. As some patients changed their practice during the month, the summed counts by IMD quintile could exceed other totals. For each CCG area for each year, we computed indirectly standardised rates (ISRs) using national sex-specific and age-specific prescribing rates for the year as a benchmark. An ISR greater than 1 indicates that prescribing in the CCG is greater than would be expected given the local population, based on national rates, whereas values less than 1 indicate lower-than-expected prescribing.
For the analysis of duration, we aggregated data retrospectively to estimate the number of patients who had a prescription dispensed each month, broken down by the duration of continuous receipt of a prescription up to that month. We then tabulated the following periods for those identified in March, 2018: 1 month, 2–5 months, 6–11 months, 12–34 months, and 35–36 months (the latter taken to be continuous at least from April, 2015, to March, 2018). This method does not yield a likelihood estimate of long-term prescribing, and there is no consensus on what constitutes long-term for each medicine class. As an indicator, we used a threshold of at least 12 months' consecutive duration for our estimates and models.
We planned to use Poisson regression to model the count of patients prescribed each medicine class and the count of patients treated continuously for at least 12 months, with adjustment by sex, age, and deprivation, computing these associations as unadjusted and covariate-adjusted incident rate ratios (IRRs) with 95% CIs. However, the conditional variance exceeded the conditional mean, so multivariable negative binomial regression was used (STATA command nbreg). The total number of registered patients and the total number estimated to have had a prescription dispensed at each level in the data were used as a frequency weight and in each exposure model. As multiple observations were recorded per CCG, standard errors were adjusted to allow for intra-group correlation at the CCG level.
As a sensitivity analysis, given the few covariates available in the NHSBSA database, we calculated the E-value.41 On the risk ratio scale, the E-value (and the lower bound of the CI as its uncertainty estimate) indicated the minimum strength-of-association that an unmeasured confounder would need to have to account for the effect of an observed confounder on a dependent variable, conditional upon model covariates. If the E-value and its uncertainty estimate are close in value to an adjusted estimate, then the association is threatened by unmeasured confounding. E-values were computed with Excel software (version 16.0).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
In the rapid evidence assessment, 77 articles were identified (PRISMA flowchart in appendix [p 87]). Evidence from 17 randomised placebo-controlled trials (6729 participants) pointed to a risk of withdrawal symptoms for patients taking antidepressants at discontinuation, as well as more adverse events on tapering or post-study compared with patients receiving placebo. The evidence suggested that, compared with the placebo group, the antidepressant group had more cases of withdrawal syndrome (one study; evidence rated high quality), vertigo (one study), dizziness (seven studies), and suicidal ideation (four studies; all evidence rated low quality), and nausea (six studies; evidence rated very low quality) after discontinuation of treatment, and more adverse events emergent on tapering or after the study (nine studies; evidence rated low quality). For suicidal ideation, there was evidence of greater occurrence in people taking antidepressants, but the event rates were very low (one or two occurrences of suicidal ideation per study). No clear differences were found with regard to rebound insomnia, depression, upper respiratory tract infection, vomiting, headache, or diarrhoea (evidence rated low or very low quality). Little information regarding withdrawal was found for the other medicine classes studied (appendix pp 84–85, 95–113). 17 studies on risk factors were identified: 12 for longer-term use of opioids, or opioid use disorder, and five for benzodiazepine use disorder (all evidence rated low or very-low quality). Meta-analysis was not feasible given wide variation in outcome definition, risk factor measurement, and confounder adjustment (appendix pp 91–92, 100–207). 26 randomised and two non-randomised studies reported on prevention and treatment efforts: three for antidepressants, 12 for opioids, eight for benzodiazepines, one for Z-drugs, and four targeting several medicine classes. Interventions varied considerably and meta-analysis was not feasible (appendix pp 89–90, 100–207). Four reports on seven services were identified (appendix pp 92–93). Three reported on opioids, the other on several prescription drugs, including antidepressants, benzodiazepines, and Z-drugs (all studies rated with high risk of bias). 12 articles were identified on patients' experiences (three qualitative thematic analysis, three other reports, one HTA, and five analyses of online material). 11 reported on longer-term use of antidepressants, one reported on antidepressants and benzodiazepines, and one reported on several medicine classes (appendix p 92, 100–207). Patients' reports of antidepressants included side-effects, adverse reactions, and withdrawal symptoms following dose reduction and cessation. There were some positive but also negative beliefs about treatment, with calls for discussion of the risk of side-effects, for better support, and better monitoring of medication response.
For the most recent year in the study (April 1, 2017, to March 31, 2018), among the total population of adult residents in England (43·75 million), 26·3% (11·53 million adults) received at least one dispensed prescription for antidepressants, opioids, gabapentinoids, benzodiazepines, or Z-drugs (table 1). Antidepressants were the most commonly prescribed medicine class, followed by opioids, gabapentinoids, benzodiazepines, and Z-drugs.
Table 1.
Estimates of adults in England receiving at least one dispensed prescription by medicine class (April, 2017 to March, 2018)
| Antidepressants | Opioids | Gabapentinoids | Benzodiazepines | Z-drugs | Any | ||
|---|---|---|---|---|---|---|---|
| All | 7 260 707 (16·6%) | 5 607 684 (12·8%) | 1 460 049 (3·3%) | 1 350 001 (3·1%) | 992 384 (2·3%) | 11 528 376 (26·3%) | |
| Sex* | |||||||
| Male | 2 478 368 (11·6%) | 2 164 396 (10·1%) | 558 380 (2·6%) | 483 782 (2·3%) | 379 292 (1·8%) | 4 322 760 (20·2%) | |
| Female | 4 760 640 (21·3%) | 3 418 223 (15·3%) | 899 948 (4·0%) | 860 125 (3·8%) | 609 566 (2·7%) | 7 151 166 (32·0%) | |
| Age, years† | |||||||
| 18–24 | 511 758 (10·6%) | 162 631 (3·4%) | 25 072 (0·5%) | 50 110 (1·0%) | 40 589 (0·8%) | 646 953 (13·4%) | |
| 25–44 | 2 075 669 (14·1%) | 1 116 657 (7·6%) | 278 529 (1·9%) | 365 056 (2·5%) | 238 908 (1·6%) | 2 909 368 (19·8%) | |
| 45–64 | 2 728 549 (19·2%) | 2 040 143 (14·3%) | 613 062 (4·3%) | 467 255 (3·3%) | 352 176 (2·5%) | 4 106 498 (28·9%) | |
| 65–74 | 982 746 (17·9%) | 1 086 503 (19·8%) | 274 858 (5·0%) | 210 345 (3·8%) | 161 832 (2·9%) | 1 857 853 (33·8%) | |
| ≥75 | 939 918 (20·7%) | 1 174 263 (25·9%) | 266 977 (5·9%) | 250 557 (5·5%) | 195 167 (4·3%) | 1 949 329 (43·0%) | |
| IMD quintile‡ | |||||||
| 1 (least deprived) | 1 554 433 (14·5%) | 1 043 295 (9·7%) | 264 339 (2·5%) | 333 723 (3·1%) | 241 703 (2·3%) | 2 445 311 (22·8%) | |
| 2 | 1 597 629 (15·7%) | 1 152 337 (11·3%) | 296 208 (2·9%) | 318 404 (3·1%) | 223 561 (2·2%) | 2 508 627 (24·6%) | |
| 3 | 1 514 100 (15·7%) | 1 171 137 (12·2%) | 310 907 (3·2%) | 280 303 (2·9%) | 204 732 (2·1%) | 2 398 334 (24·9%) | |
| 4 | 1 435 295 (16·1%) | 1 185 409 (13·3%) | 313 251 (3·5%) | 238 311 (2·7%) | 176 448 (2·0%) | 2 285 631 (25·6%) | |
| 5 (most deprived) | 1 154 028 (16·8%) | 1 047 405 (15·2%) | 274 360 (4·0%) | 177 678 (2·6%) | 145 280 (2·1%) | 1 878 391 (27·3%) | |
Data are n (%); percentages are of the adult population residing in England (restricted by sex or age as applicable), except for IMD quintiles, for which the adult population registered with English general practices was used and grouped by the quintile of the IMD score of the practice. IMD=Indicators of Multiple Deprivation.
Analysis excludes cases (≤0·5%) for whom sex was not available.
Analysis excludes cases (≤0·5%) for whom no valid age record was available.
Analysis excludes cases (≤0·1%) for whom no IMD score was available.
Two years previously (April 1, 2015, to March 31, 2016), 11·34 million adults received at least one dispensed prescription. This increase of 1·7% from 2015–16 to 2017–18 is essentially static in the context of population growth. However, changes were observed among the different medicine classes. From 2015–16 to 2017–18, the number of people with a dispensed prescription for gabapentinoids increased by 18·5% (from 1·23 million) and antidepressants increased by 6·7% (from 6·80 million). By contrast, the number of people with a dispensed prescription for opioids decreased by 3·9% (from 5·84 million), benzodiazepines decreased by 5·1% (from 1·42 million), and Z-drugs by 5·0% (from 1·04 million).
The crude proportion of women who received at least one of the medicine classes was higher than that of men (table 1). This difference ranged from 1·5 times higher for opioids to 1·8 times higher for antidepressants. The prevalence of prescribing in all the medicine classes increased with age in both women and men. Prescribing increased by IMD quintile for antidepressants, opioids, and gabapentinoids, and decreased slightly for benzodiazepines and Z-drugs.
In the latest month of the study (March, 2018), for each of the medicine classes, around 50% of patients were estimated to have been prescribed to continuously for at least 12 months (ie, 1·17 million patients treated with opioids [50·0%]; 212 000 with benzodiazepines [50·4%]; 456 000 with gabapentinoids [53·3%]; 2·34 million with antidepressants [52·1%]; and 194 000 with Z-drugs [53·3%]; table 2, appendix pp 82–86). Compared with the equivalent values from April, 2016, these proportions increased across all five classes by March, 2018, with differences ranging from 3·4% for benzodiazepines to 7·3% for gabapentinoids. The proportion who had been prescribed to continuously from April, 2015, to March, 2018, ranged from 22·5% (for gabapentinoids) to 31·5% (for benzodiazepines).
Table 2.
Duration of prescribing by medicine class using latest estimated retrospective duration in months at March, 2018, and those in receipt of a prescription for 12 months or more, overall and by sex, age group, and deprivation
| Antidepressants | Opioids | Gabapentinoids | Benzodiazepines | Z-drugs | ||
|---|---|---|---|---|---|---|
| People estimated to have a prescription in March, 2018* | ||||||
| All | 4 480 468 | 2 344 177 | 854 165 | 419 564 | 364 258 | |
| Sex† | ||||||
| Male | 1 489 501 | 873 463 | 329 201 | 148 923 | 136 947 | |
| Female | 2 987 201 | 1 468 290 | 524 719 | 270 091 | 226 947 | |
| Age, years‡ | ||||||
| 18–24 | 212 161 | 27 466 | 9653 | 7545 | 5969 | |
| 25–44 | 1 108 877 | 336 188 | 143 273 | 80 239 | 60 940 | |
| 45–64 | 1 807 506 | 896 241 | 373 084 | 141 001 | 131 373 | |
| 65–74 | 687 213 | 517 011 | 163 880 | 78 783 | 70 279 | |
| ≥75 | 659 978 | 564 629 | 164 012 | 111 421 | 95 322 | |
| IMD quintile§ | ||||||
| 1 (least deprived) | 959 309 | 399 703 | 148 980 | 94 604 | 86 062 | |
| 2 | 992 394 | 471 107 | 170 902 | 96 463 | 81 646 | |
| 3 | 931 730 | 494 277 | 181 227 | 87 809 | 76 019 | |
| 4 | 887 519 | 520 861 | 187 442 | 79 492 | 66 515 | |
| 5 (most deprived) | 716 610 | 462 495 | 167 392 | 62 111 | 54 749 | |
| People estimated to have been in receipt of a prescription for more than 12 months | ||||||
| All | 2 335 779 (52·1%) | 1 171 313 (50·0%) | 455 502 (53·3%) | 211 546 (50·4%) | 194 203 (53·3%) | |
| Sex† | ||||||
| Male | 771 857 (51·8%) | 425 522 (48·7%) | 178 663 (54·3%) | 73 628 (49·4%) | 70 626 (51·6%) | |
| Female | 1 563 667 (52·3%) | 745 691 (50·8%) | 276 793 (52·8%) | 137 898 (51·1%) | 123 559 (54·4%) | |
| Age, years‡ | ||||||
| 18–24 | 50 925 (24·0%) | 4915 (17·9%) | 3143 (32·6%) | 1043 (13·8%) | 663 (11·1%) | |
| 25–44 | 448 292 (40·4%) | 140 571 (41·8%) | 68 758 (48·0%) | 28 163 (35·1%) | 23 050 (37·8%) | |
| 45–64 | 981 733 (54·3%) | 469 309 (52·4%) | 203 062 (54·4%) | 72 229 (51·2%) | 71 794 (54·6%) | |
| 65–74 | 425 769 (62·0%) | 273 010 (52·8%) | 88 830 (54·2%) | 45 335 (57·5%) | 41 454 (59·0%) | |
| ≥75 | 428 853 (65·0%) | 283 417 (50·2%) | 91 666 (55·9%) | 64 760 (58·1%) | 57 230 (60·0%) | |
| IMD quintile§ | ||||||
| 1 (least deprived) | 467 779 (49·0%) | 183 621 (46·2%) | 73 590 (49·7%) | 41 683 (44·3%) | 42 362 (49·4%) | |
| 2 | 507 084 (51·3%) | 227 350 (48·5%) | 88 336 (51·9%) | 46 400 (48·4%) | 42 187 (52·0%) | |
| 3 | 483 289 (51·5%) | 247 805 (49·8%) | 95 994 (52·6%) | 44 969 (50·7%) | 41 417 (54·0%) | |
| 4 | 477 994 (53·7%) | 271 009 (51·9%) | 103 345 (55·0%) | 43 407 (54·5%) | 37 487 (56·2%) | |
| 5 (most deprived) | 404 173 (56·4%) | 244 549 (52·9%) | 95 347 (57·0%) | 35 708 (57·5%) | 31 258 (57·1%) | |
Data are n (%); percentages are of the total (restricted by age, sex, or IMD quintile, as applicable) estimated to have a prescription in March, 2018, by medicine class. IMD=Indicators of Multiple Deprivation.
Includes individuals who did not have a dispensed prescription reported in March, 2018, but had one reported in the months either side.
Analysis excludes cases (≤0·1%) in which sex was not available.
Analysis excludes cases (≤0·1%) in which no valid age record was available.
Analysis from general practice data and excludes cases in which no IMD score was available.
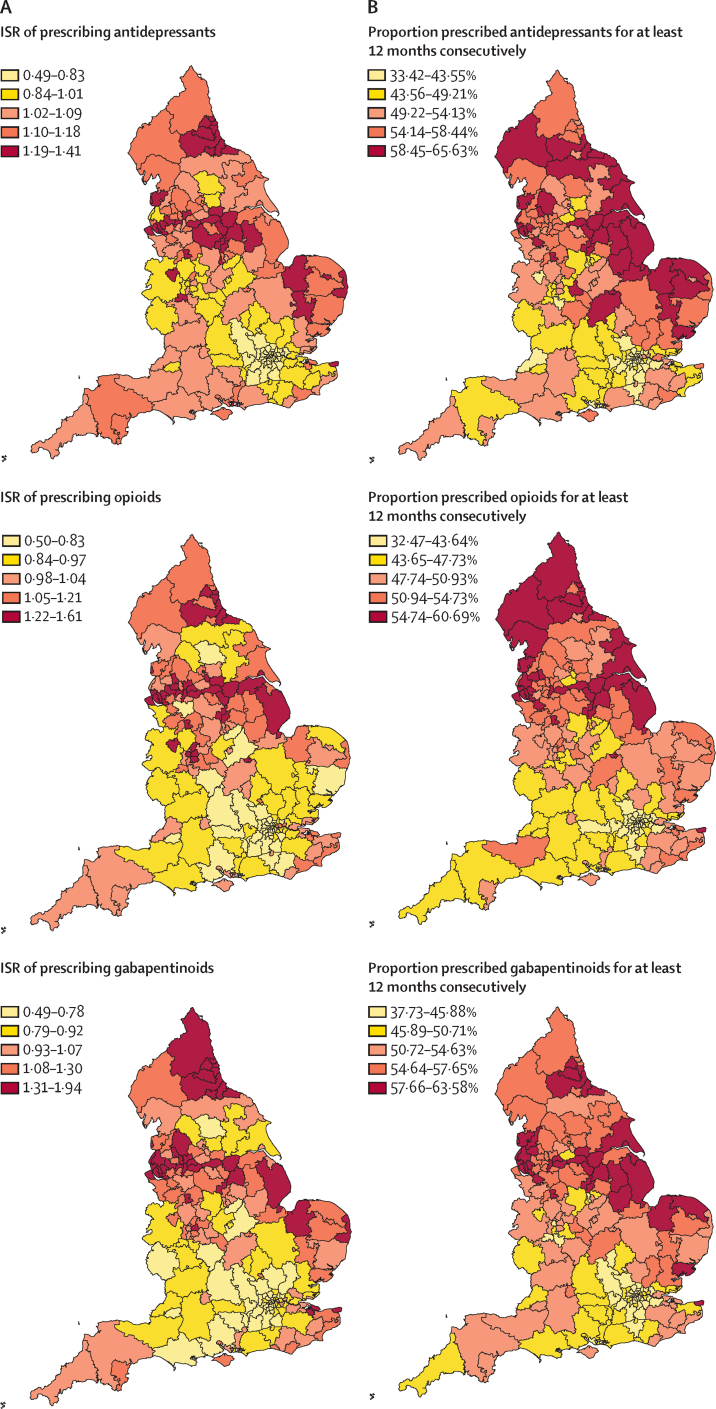
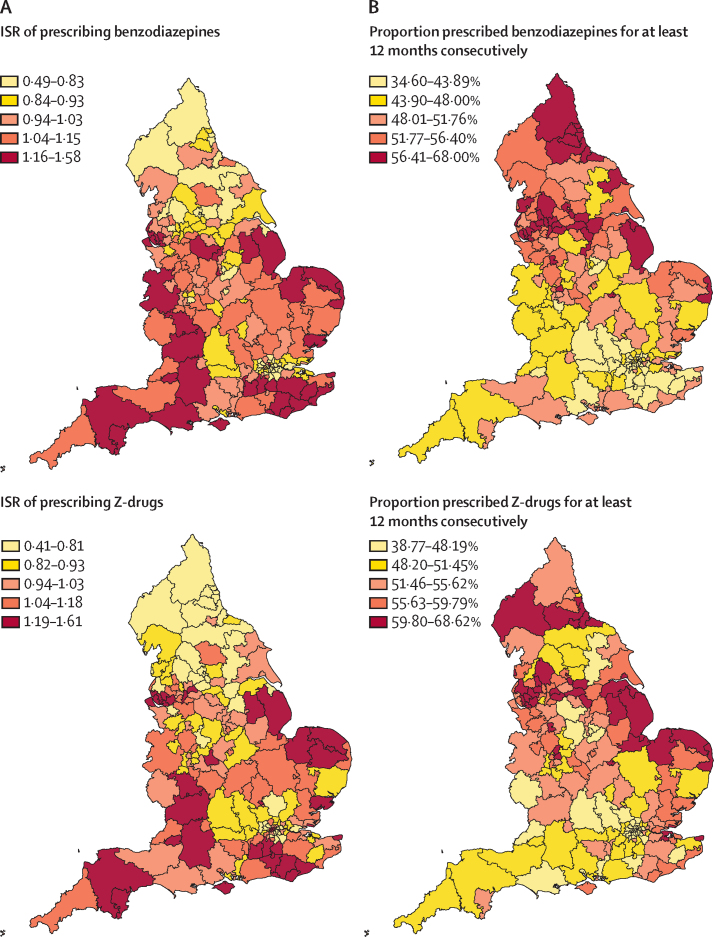
For the period from April 1, 2017, to March 31, 2018, for antidepressants, gabapentinoids and opioids higher ISRs were associated with CCGs concentrated in urban centres in the north of England and, for antidepressants in particular, the east (figure). For most CCGs in London and some surrounding areas, the ISRs indicated markedly lower-than-expected prescribing rates for these three medicine classes. By contrast, CCGs with the highest ISRs for benzodiazepines and Z-drugs were in the southwest, southeast, and east, with markedly lower prescribing rates in many areas in the north.
Figure.
Prescribing of five medicine classes in England, 2017–18
(A) ISRs (standardised by age and sex) of prescribing from April 1, 2017, to March 31, 2018. An ISR of 1 is the expected rate based on national observations. (B) Proportions of individuals with a prescription in March, 2018, who were estimated to have been prescribed to continuously for at least 12 months. The colour gradient represents the quintile boundaries for ISR and proportions. ISR=indirectly standardised rate.
The univariate and multivariable negative binomial models (table 3) showed that, across all five medicine classes, women were more likely than men to have a dispensed prescription. IRRs generally increased with age for both women and men for each medicine class, with the effect most pronounced for patients aged 75 years and older. For antidepressants, opioids, and gabapentinoids, an increasing gradient of association with deprivation quintile was observed. For Z-drugs, only the adjusted IRR for the most deprived quintile (1·11 [95% CI 1·01–1·22]) was significantly greater than that of the least deprived quintile. For benzodiazepines, significantly reduced prescribing with increased deprivation was seen for quintiles 4 and 5 only.
Table 3.
Negative binomial regression of patients receiving prescriptions for antidepressants, opioids, gabapentinoids, benzodiazepines, or Z-drugs in 2017–18, adjusted by sex, age, and deprivation category, for the national registered adult population (N=46 324 965)
| Unadjusted IRR | Adjusted IRR | E-value* | ||
|---|---|---|---|---|
| Antidepressants | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·89 (1·87–1·90) | 1·89 (1·88–1·91) | 3·19 (3·16) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 1·27 (1·24–1·30) | 1·29 (1·25–1·32) | 1·89 (1·81) | |
| 45–64 | 1·82 (1·76–1·89) | 1·85 (1·79–1·92) | 3·11 (2·98) | |
| 65–74 | 1·77 (1·70–1·83) | 1·81 (1·74–1·88) | 3·02 (2·88) | |
| ≥75 | 2·07 (2·00–2·14) | 2·07 (2·00–2·14) | 3·56 (3·42) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·08 (1·05–1·12) | 1·10 (1·06–1·13) | 1·42 (1·32) | |
| 3 | 1·08 (1·03–1·14) | 1·12 (1·06–1·17) | 1·48 (1·32) | |
| 4 | 1·11 (1·03–1·19) | 1·16 (1·09–1·24) | 1·59 (1·40) | |
| 5 (most deprived) | 1·15 (1·08–1·24) | 1·24 (1·16–1·33) | 1·78 (1·58) | |
| Model constant | .. | 0·06 (0·06–0·06) | .. | |
| Opioids | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·55 (1·54–1·56) | 1·61 (1·60–1·63) | 2·61 (2·58) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 2·16 (2·09–2·23) | 2·21 (2·13–2·29) | 3·84 (3·68) | |
| 45–64 | 4·31 (4·10–4·53) | 4·59 (4·36–4·83) | 8·65 (8·20) | |
| 65–74 | 6·18 (5·87–6·50) | 6·77 (6·41–7·15) | 13·01 (12·29) | |
| ≥75 | 8·18 (7·81–8·56) | 8·79 (8·38–9·22) | 17·07 (16·25) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·16 (1·12–1·21) | 1·20 (1·15–1·24) | 1·68 (1·57) | |
| 3 | 1·25 (1·18–1·32) | 1·34 (1·27–1·40) | 2·01 (1·86) | |
| 4 | 1·36 (1·28–1·45) | 1·52 (1·44–1·61) | 2·41 (2·23) | |
| 5 (most deprived) | 1·56 (1·48–1·66) | 1·85 (1·76–1·95) | 3·11 (2·91) | |
| Model constant | .. | 0·02 (0·02–0·02) | .. | |
| Gabapentinoids | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·59 (1·57–1·60) | 1·66 (1·64–1·68) | 2·70 (2·67) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 3·52 (3·34–3·71) | 3·51 (3·32–3·71) | 6·47 (6·09) | |
| 45–64 | 8·50 (7·93–9·11) | 8·67 (8·06–9·33) | 16·83 (15·60) | |
| 65–74 | 10·25 (9·54–11·01) | 10·98 (10·18–11·84) | 21·45 (19·86) | |
| ≥75 | 12·25 (11·45–13·11) | 13·04 (12·16–13·97) | 25·56 (23·82) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·18 (1·13–1·23) | 1·21 (1·17–1·27) | 1·73 (1·60) | |
| 3 | 1·31 (1·23–1·39) | 1·39 (1·31–1·48) | 2·13 (1·95) | |
| 4 | 1·42 (1·32–1·54) | 1·56 (1·44–1·68) | 2·48 (2·24) | |
| 5 (most deprived) | 1·62 (1·48–1·76) | 1·85 (1·69–2·01) | 3·09 (2·78) | |
| Model constant | .. | 0·00 (0·00–0·00) | .. | |
| Benzodiazepines | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·76 (1·74–1·77) | 1·71 (1·70–1·73) | 2·82 (2·79) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 2·29 (2·21–2·37) | 2·32 (2·25–2·41) | 4·08 (3·92) | |
| 45–64 | 3·21 (3·07–3·37) | 3·27 (3·11–3·43) | 5·99 (5·68) | |
| 65–74 | 3·90 (3·68–4·12) | 3·89 (3·67–4·11) | 7·24 (6·81) | |
| ≥75 | 5·65 (5·35–5·96) | 5·44 (5·16–5·73) | 10·35 (9·79) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·00 (0·97–1·04) | 1·03 (0·99–1·06) | 1·19 (1·00) | |
| 3 | 0·93 (0·88–0·98) | 0·97 (0·93–1·02) | 1·19 (1·00) | |
| 4 | 0·85 (0·79–0·91) | 0·90 (0·85–0·96) | 1·46 (1·26) | |
| 5 (most deprived) | 0·82 (0·75–0·89) | 0·89 (0·82–0·97) | 1·50 (1·23) | |
| Model constant | .. | 0·01 (0·01–0·01) | .. | |
| Z-drugs | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·60 (1·58–1·61) | 1·57 (1·55–1·59) | 2·51 (2·47) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 1·84 (1·78–1·90) | 1·84 (1·78–1·91) | 3·09 (2·96) | |
| 45–64 | 2·97 (2·84–3·11) | 2·98 (2·84–3·13) | 5·41 (5·12) | |
| 65–74 | 3·68 (3·49–3·87) | 3·70 (3·51–3·89) | 6·85 (6·47) | |
| ≥75 | 5·40 (5·12–5·69) | 5·35 (5·08–5·64) | 10·18 (9·63) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 0·97 (0·93–1·01) | 1·02 (0·98–1·07) | 1·18 (1·00) | |
| 3 | 0·94 (0·88–1·00) | 1·03 (0·97–1·09) | 1·21 (1·00) | |
| 4 | 0·87 (0·80–0·93) | 0·99 (0·92–1·06) | 1·13 (1·00) | |
| 5 (most deprived) | 0·93 (0·85–1·02) | 1·11 (1·01–1·22) | 1·45 (1·11) | |
| Model constant | .. | 0·01 (0·01–0·01) | .. | |
IRR=incident rate ratio.
Values in parentheses are the lower bound of the 95% CI (towards the null).
With regard to the sex and age group adjustment, the E-values (ranging from 1·89 to 25·56) and uncertainty estimates (lower bound of CI towards the null ranging from 1·81 to 23·82) were greater than the adjusted IRRs (table 3). The associations with sex and age could be accounted for by an unmeasured confounder, but this would need to have an adjusted IRR with a value above the observed covariates, and weaker confounding could not have done so.
There was no strong evidence that unmeasured confounding could account for the associations of antidepressants, opioids, or gabapentinoids with deprivation, with all E-values and CIs greater than the adjusted IRRs. By contrast, the associations of benzodiazepines and Z-drugs with lower levels of deprivation were threatened by unmeasured confounding: E-values were all greater than 1 and the adjusted IRRs, but the uncertainty estimates ranged from 1·00 to 1·26 for benzodiazepines, and 1·00 to 1·11 for Z-drugs.
With some exceptions, the estimated proportion of patients in each CCG who had a dispensed prescription in March, 2018, and who had been prescribed to continuously for at least 12 months was highest in CCGs in the north and east (figure), while most CCGs in London and some surrounding areas had lower proportions of patients who had been prescribed to continuously for at least 12 months. For antidepressants, ISRs were markedly higher in the north and east than in the south and west. Areas in the north and east generally had higher proportions of patients prescribed to continuously for at least 12 months for opioids, gabapentinoids, and benzodiazepines compared with areas in the south and west. ISRs for Z-drugs were highest in CCGs in the north and east and lowest in the southwest (figure).
In the unadjusted and adjusted models, continuous receipt of a dispensed prescription for at least 12 months showed no association with sex (table 4). However, IRR increased with age group for each medicine class except opioids, where the association was slightly attenuated among patients 75 years and older compared with those aged 65–74 years. In each model, continuous receipt of a prescription for at least 12 months was associated with increasing levels of deprivation. E-values and their uncertainty estimates were all greater than the IRR values, indicating no marked threat of unmeasured confounding in the associations with age or deprivation.
Table 4.
Negative binomial regression of patients estimated to have been receiving an antidepressant, opioid, gabapentinoid, benzodiazepine, or Z-drug for at least 12 months, adjusted by sex, age, and deprivation category
| Unadjusted IRR | Adjusted IRR | E-value* | ||
|---|---|---|---|---|
| Antidepressants (N=4 482 793) | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·01 (1·00–1·01) | 1·00 (1·00–1·01) | 1·07 (1·02) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 1·71 (1·68–1·75) | 1·70 (1·67–1·73) | 2·79 (2·72) | |
| 45–64 | 2·28 (2·23–2·34) | 2·27 (2·21–2·32) | 3·96 (3·85) | |
| 65–74 | 2·60 (2·54–2·67) | 2·61 (2·54–2·67) | 4·65 (4·52) | |
| ≥75 | 2·72 (2·64–2·79) | 2·74 (2·66–2·82) | 4·93 (4·77) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·05 (1·02–1·08) | 1·06 (1·03–1·10) | 1·32 (1·20) | |
| 3 | 1·05 (1·02–1·09) | 1·07 (1·03–1·11) | 1·35 (1·22) | |
| 4 | 1·10 (1·06–1·14) | 1·13 (1·08–1·17) | 1·50 (1·39) | |
| 5 (most deprived) | 1·15 (1·11–1·20) | 1·19 (1·14–1·25) | 1·68 (1·55) | |
| Model constant | .. | 0·22 (0·21–0·23) | .. | |
| Opioids (N=2 345 796) | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·03 (1·03–1·04) | 1·05 (1·04–1·05) | 1·27 (1·25) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 2·45 (2·36–2·53) | 2·45 (2·37–2·54) | 4·34 (4·17) | |
| 45–64 | 2·99 (2·87–3·11) | 3·02 (2·90–3·15) | 5·49 (5·25) | |
| 65–74 | 3·01 (2·89–3·14) | 3·07 (2·94–3·21) | 5·60 (5·33) | |
| ≥75 | 2·88 (2·75–3·00) | 2·95 (2·83–3·08) | 5·35 (5·10) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·06 (1·04–1·07) | 1·06 (1·04–1·07) | 1·31 (1·25) | |
| 3 | 1·09 (1·06–1·12) | 1·09 (1·07–1·12) | 1·41 (1·34) | |
| 4 | 1·14 (1·10–1·17) | 1·14 (1·11–1·18) | 1·55 (1·47) | |
| 5 (most deprived) | 1·15 (1·11–1·19) | 1·17 (1·13–1·21) | 1·61 (1·50) | |
| Model constant | .. | 0·15 (0·15–0·16) | .. | |
| Gabapentinoids (N=855 680) | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 0·97 (0·96–0·97) | 0·97 (0·97–0·98) | 1·19 (1·18) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 1·54 (1·49–1·60) | 1·50 (1·44–1·56) | 2·37 (2·25) | |
| 45–64 | 1·72 (1·66–1·79) | 1·68 (1·62–1·74) | 2·75 (2·62) | |
| 65–74 | 1·70 (1·64–1·76) | 1·68 (1·63–1·74) | 2·76 (2·64) | |
| ≥75 | 1·71 (1·66–1·77) | 1·72 (1·66–1·79) | 2·84 (2·71) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·07 (1·04–1·10) | 1·08 (1·05–1·10) | 1·36 (1·27) | |
| 3 | 1·09 (1·05–1·12) | 1·09 (1·06–1·13) | 1·41 (1·31) | |
| 4 | 1·14 (1·11–1·18) | 1·15 (1·11–1·18) | 1·56 (1·47) | |
| 5 (most deprived) | 1·19 (1·14–1·24) | 1·20 (1·15–1·25) | 1·68 (1·56) | |
| Model constant | .. | 0·30 (0·29–0·31) | .. | |
| Benzodiazepines (N=419 903) | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·03 (1·02–1·04) | 1·00 (0·98–1·01) | 1·05 (1·00) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 2·85 (2·62–3·10) | 2·80 (2·58–3·04) | 5·05 (4·60) | |
| 45–64 | 3·98 (3·63–4·38) | 3·90 (3·55–4·27) | 7·26 (6·57) | |
| 65–74 | 4·46 (4·05–4·92) | 4·47 (4·05–4·92) | 8·40 (7·57) | |
| ≥75 | 4·54 (4·12–5·01) | 4·65 (4·21–5·13) | 8·76 (7·89) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·10 (1·08–1·13) | 1·12 (1·09–1·15) | 1·49 (1·42) | |
| 3 | 1·15 (1·11–1·19) | 1·18 (1·13–1·22) | 1·63 (1·52) | |
| 4 | 1·24 (1·20–1·28) | 1·28 (1·24–1·32) | 1·89 (1·79) | |
| 5 (most deprived) | 1·30 (1·24–1·36) | 1·37 (1·31–1·43) | 2·08 (1·95) | |
| Model constant | .. | 0·11 (0·10–0·12) | .. | |
| Z-drugs (N=364 617) | ||||
| Sex | ||||
| Male | 1·00 (ref) | 1·00 (ref) | .. | |
| Female | 1·05 (1·04–1·06) | 1·05 (1·04–1·06) | 1·27 (1·23) | |
| Age, years | ||||
| 18–24 | 1·00 (ref) | 1·00 (ref) | .. | |
| 25–44 | 4·10 (3·60–4·66) | 4·03 (3·55–4·58) | 7·52 (6·55) | |
| 45–64 | 5·62 (4·90–6·44) | 5·61 (4·90–6·44) | 10·71 (9·26) | |
| 65–74 | 6·01 (5·24–6·90) | 6·20 (5·39–7·13) | 11·88 (10·26) | |
| ≥75 | 6·08 (5·29–6·98) | 6·37 (5·53–7·34) | 12·22 (10·53) | |
| IMD quintile | ||||
| 1 (least deprived) | 1·00 (ref) | 1·00 (ref) | .. | |
| 2 | 1·05 (1·02–1·09) | 1·07 (1·04–1·11) | 1·35 (1·25) | |
| 3 | 1·11 (1·06–1·16) | 1·15 (1·09–1·21) | 1·56 (1·40) | |
| 4 | 1·15 (1·10–1·20) | 1·22 (1·17–1·27) | 1·74 (1·62) | |
| 5 (most deprived) | 1·16 (1·11–1·21) | 1·27 (1·21–1·32) | 1·85 (1·72) | |
| Model constant | .. | 0·08 (0·07–0·10) | .. | |
IRR=incident rate ratio.
Lower bound of the CI towards the null.
Discussion
We report the first mixed-methods public health study of antidepressants, opioids for non-cancer pain, gabapentinoids, benzodiazepines, and Z-drugs, and their associations with dependence and withdrawal. In March, 2018, 11·53 million people (26·3% of residents) in England had a prescription dispensed for antidepressants, opioids for chronic non-cancer pain, gabapentinoids, benzodiazepines, or Z-drugs. Around 50% of these patients had been dispensed a prescription for one or more such medicines continuously for at least 12 months, and between 23% (gabapentinoids) and 32% (benzodiazepines) had done so continuously since April, 2015.
These findings highlight the variance of current practice from clinical guidelines. Given the risk of adverse drug reactions and dependence, doctors are advised to prescribe benzodiazepines and Z-drugs for no more 4 weeks.42, 43 For opioids, no national clinical guideline on prescribing duration for non-cancer pain has been established, but clinical consensus is that these drugs are rarely effective for more than 12 weeks.27 National guidance for prescribers on gabapentinoids focuses on avoiding risks of misuse and dependence, without stipulating any limit on duration of treatment.44
We cannot say how many of the 1·17 million people in England estimated to be continuously prescribed an opioid for at least a 12-month period had opioid use disorder, but many will need careful clinical management if they wish to stop taking opioids. Of the 854 165 people estimated to have had a prescription for gabapentinoids in March, 2018, 53% were estimated to have been treated for at least 12 months. Although such prescribing practice might be necessary for those being treated for epilepsy, some individuals being treated for other conditions might be dependent and need support to stop. The situation is different with antidepressants: although we did not collect data on clinical response in this study, long-term prescribing (6 months to 2 years or more) is indicated for many people with severe depression.45 Our study highlights the size of the population of individuals who might require careful assessment and help from their doctor if a decision to stop taking antidepressants is made.
Prescribing and long-term prescribing estimates showed differences by region. GP practices in the north and the east of England were more likely to prescribe antidepressants, opioids, and gabapentinoids, whereas benzodiazepines and Z-drugs were prescribed more frequently in the southwest, southeast, and east. The lower rate of prescribing of benzodiazepines and Z-drugs in much of the north might be related to several local non-governmental support services for patients experiencing long-term harms from benzodiazepines in these regions, the action and legacy of which has raised awareness in these CCGs.
Antidepressants, gabapentinoids, and opioids showed a strong and increasing association with deprivation (in contrast to Z-drugs, which showed a weak association with the highest level of deprivation, with a risk of unmeasured confounding). For opioid prescribing, our findings align with previous studies in which a similar pattern of regional variation was observed in England for all opioid prescribing,46, 47 and the association with increasing deprivation is reflected in the association between chronic pain and unemployment.48
The placebo-controlled trial evidence points to a risk of some types of withdrawal symptoms for patients taking antidepressants at discontinuation, as well as more adverse events emerging on tapering or post-study than in the placebo group, but the evidence quality is generally low. To add to this evidence, we summarised a wide range of patient experience reports from qualitative and other studies. These views are important because they are rarely captured in conventional randomised placebo-controlled trials of medication efficacy. Generally, patient reports were focused on the experience of taking antidepressants long-term. Patients reported adverse physical, affective, social, and sexual reactions which they attributed to taking these medicines. It is not possible to determine from these reports whether the cause of perceived negative effects was the medicine, withdrawal from it, or the primary condition itself, although some clinical indicators have been suggested.49
With regard to stopping long-term antidepressants, we found patient reports of a range of severe withdrawal symptoms, including severe anxiety, agitation, low mood, and somatic and other symptoms. These symptoms had sudden onset and were protracted and disabling. Although antidepressants are not associated with dependence, anxiety about withdrawal symptoms could dissuade some patients from stopping.50, 51
Notably, patients frequently felt that their experiences of withdrawal symptoms were either discounted by clinicians or attributed only as an indication of relapse to depression. The qualitative evidence gathered in this study suggested some positive views of support services, but also a call for better information and discussion of treatment options, better monitoring of progress during treatment, and more community support. Determining optimal tapering procedures to minimise withdrawal symptoms for patients who wish to discontinue antidepressants is a current topic of debate among clinicians.49, 52
Our geographical analysis showed that many areas with the highest prescribing prevalence have high levels of deprivation. These areas are likely to have constrained resources for treatment and support. Many factors influence the demand for medicine and prescribing decisions. Poverty and deprivation, and the social environments they create, can be drivers for seeking medical treatment if they cause anxiety, depression, or insomnia, or make pain less bearable.53 The physical health effects of poor diets, smoking, and lack of exercise can also contribute to a demand for more treatments. Furthermore, the choice of treatment can be affected: hard-pressed clinicians in poor areas, with little recourse to alternative treatments, might be more likely to prescribe medicines.54
Our findings should be considered in the context of several limitations. First, the primary function of the NHSBSA database is to reimburse retail pharmacies for their medication purchasing and dispensing costs, rather than for population epidemiology of prescribing for particular conditions; our estimates include, for example, opioids given appropriately in terminal care to patients (other than those recorded in the cancer registration dataset) and gabapentinoids necessarily used in the long term for epilepsy. Although we were able to link dispensed prescriptions over time for patients, we were reliant on the date the dispensed prescription was submitted to NHSBSA for reimbursement rather than the actual date of dispensing. We sought advice from the study expert reference group (which included pharmacists and pharmacy experts in PHE), and believe that this time difference is usually small. However, several scenarios exist in which our assessment of duration could yield invalid estimates, including the possibility of identifying false breaks in prescribing where, for example, the patient takes the medicine more slowly than prescribed, or there is a delay in the pharmacy submitting prescriptions to NHSBSA for payment. Given the lack of specific date information, it is also possible that our use of the month of reporting could link dispensed prescriptions and classify these as continuous where there was, in fact, a short break in treatment.
Second, the retrospective method used to estimate duration necessitates careful interpretation. The method reflects a snapshot of the population with a dispensed prescription for these medicine classes at any time, but the resulting proportions cannot be generalised to assess the individual likelihood of how long a prescribed course of treatment will last.
Third, the short time span of usable NHSBSA data that includes linkable NHS numbers poses challenges for the estimate of duration. In our study, duration was truncated to a maximum of 36 months by March, 2018. Therefore, a person could have had 35 months of prescriptions recorded retrospectively to May, 2015, with a submitted prescription for April, 2015, missing, for example, but we did not have such information. We took a pragmatic view that a person who was identified continuously from May, 2015, to March, 2018, probably had a dispensed prescription covering April, 2015, so had been prescribed to for the entire reporting period.
This study indicates the need for increased awareness among the public and clinicians of the risks and benefits of medicines associated with dependence and withdrawal, and of non-medication or supplementary treatment options. Patients reported that withdrawal symptoms might not be well understood by prescribers, so improved training and guidance for clinicians are required. Our findings on long-term prescribing must be interpreted carefully. Prescribing should not be restricted if a patient with an acute or legitimate long-term need can benefit, and any actions to limit long-term prescribing must not be allowed to generate unintended negative consequences, including stigmatising or limiting the appropriate and safe use of potentially helpful medicines. Such actions could prevent people from gaining clinical benefit and could lead to an increase in harms, including suicide and the seeking of medicines from illicit or unregulated sources (such as online), or even resorting to taking illicit drugs to treat symptoms or withdrawal symptoms. Concerns about online prescribing and supply are receiving attention from the Medicines and Healthcare products Regulatory Agency, Care Quality Commission, and General Pharmaceutical Council. Recent guidelines from the General Pharmaceutical Council are an important step towards better regulation of online prescribing and online pharmacies.55
Our recommendations (panel) encompass a new approach to national and local monitoring of the prescribing of these medicine classes to support improvements in clinical practice. Patients must be safeguarded against developing dependence or experiencing withdrawal. Shared decision making with patients, informed choice, and regular clinical reviews of benefit and harm are the tenets of effective personalised care. Although the evidence identified was insufficient to draw firm conclusions on best practice, it is clear that many patients need better help with dependence or withdrawal management, as well as ongoing treatment for underlying conditions. Treatment and support pathways might include social prescribing options and support groups, self-guided and high-intensity psychological therapies from Improving Access to Psychological Therapies clinics, and referral to mental health teams, specialist pain clinics, and medical services. It will be crucial that clinicians have the time and resources to explore these options with their patients.
Panel. Recommendations.
Monitoring and using data on prescribing
-
•
Care Quality Commission to use relevant prescribing data when inspecting primary care
-
•
Regional Medicines Optimisation Committees to use prescribing data to support improvements in clinical practice
-
•
Commissioners, primary care staff, and clinicians, including clinical pharmacists, to use available data on prescribing patterns to identify need
-
•
Department of Health and Social Care (DHSC), NHS England & NHS Improvement (NHSE&I), and NHSBSA to review the data collected nationally on prescribing to establish whether it can be enhanced
Guidance
-
•
National Institute for Health and Care Excellence (NICE) to consider dependence and withdrawal when making prescribing recommendations
-
•
NICE and NHSE&I to support the implementation of NICE's planned guideline and quality standard on withdrawal management
-
•
NICE to develop clinical decision aids to help prescribers better manage dependence and withdrawal
-
•
NHSE&I to enhance medication safety monitoring within the Quality Outcomes Framework
-
•
As part of its opioids review, the Commission on Human Medicines (CHM) to examine the evidence and related guidance on over-the-counter opioid medicines (principally those containing low-dose codeine, usually in combination with another, non-opioid, painkiller), and consider options for reducing easy access to them
-
•
Health Education England to review the availability of relevant training
-
•
The Medicines and Healthcare products Regulatory Agency (MHRA) and CHM to consider how marketing authorisation applications and pharmacovigilance could further assess risks of dependence and withdrawal.
Information, shared decision making and support
-
•
CHM to consider whether to label medicines that do not already warn about the risks of dependence and withdrawal
-
•
NHSE&I, CCGs, and primary care services to ensure that patients can access information on risk of dependence on and withdrawal from medications and Care Quality Commission assesses primary care provision
-
•
Government, through its relevant organisations, to investigate behaviour change marketing and other ways of supporting the public to make informed decisions about treatment options
-
•
DHSC to consider supporting the development of a time-limited national helpline and website to provide expert advice and support to patients
-
•
Primary care services, pharmacists, and general practitioners to enhance competence in assessing and treating dependence and withdrawal
-
•
Local health and social care commissioners to ensure treatment pathways are available for dependence and withdrawal and for ongoing care of underlying or related conditions
-
•
Medication reviews by clinical pharmacists in primary care to have sufficient focus on medicines that can cause dependence or withdrawal
-
•
As part of its commitment to universal personalised care, NHSE&I to ensure there is a significant focus on medicines that might cause dependence and withdrawal
Research questions
-
•
Commission qualitative studies of patients' experience of medications and services
-
•
Identify patient, prescriber, and system-level risk factors for harms of medicines associated with dependence or withdrawal
-
•
Ascertain the duration, nature, and severity of withdrawal from antidepressants, including enduring symptoms, and isolate these from the clinical condition
-
•
In context of individualised care, determine optimal medication treatment regimens
-
•
Develop and evaluate novel prevention and treatment interventions to manage harms of medicines associated with dependence or withdrawal
Acknowledgments
Acknowledgments
We kindly acknowledge Agnès Cuyàs, Nicole Downes, Lina Gulhane, Clare Jones, Sharangini Rajesh, Eleanor Samarasekera, and Maria Smyth (all at the National Guideline Centre) for undertaking the searches, screening, and analysis for the systematic review; Kate Ashmore (National Guideline Centre), Paul Cosford (PHE; expert reference group meeting chair), Rachel Clark (PHE; from February, 2019), Lauren Oyinloye (PHE; from October, 2018), Clare Perkins (PHE), and Garry Stillwell (April, 2018, to December, 2018) for management and administrative support; Georgina Anderson, Olivia Box Power, and Sacha Wyke (all at PHE's Health Intelligence Division) for data quality assurance support; Gareth Franklin (National Institute for Health and Care Excellence [NICE]), Nikki Dodds and Phil Godfrey (NHS Business Services Authority), Thomas Bacon, Gabrielle Emanuel, Katherine Henson, Alan MacDonald, Jem Rashbass, and Brian Shand (all at the PHE National Cancer Registration and Analysis Service), Caryl Beynon (PHE Research, Translation and Innovation Health Improvement Directorate), Sally McManus, Rukmen Sehmi, and Neil Smith (all at the National Centre for Social Research for the Public Health Research Consortium) for technical advice and support. We also acknowledge the following individuals who formed the study's expert reference group: Yasir Abbasi (Mersey Care NHS Foundation Trust), Navjot Alhuwalia (Rotherham, Doncaster and South Humber NHS Foundation Trust), Louis Appleby (Centre for Mental Health and Safety), Tim Ballard (Care Quality Commission), Ruth Bastable (Royal College of General Practitioners), Paul Chrisp (NICE), James Davies (All-Party Parliamentary Group on Prescribed Drug Dependence), Sarah Dennison (Care Quality Commission), Colin Drummond (Royal College of Psychiatrists), David Geddes (NHS England); Natalie Gold (PHE Behavioural Insights Team), Andrew Green (British Medical Association), Graeme Henderson (University of Bristol), Gregor Henderson (PHE), Tracey Hogan (The Bridge Project), John Hughes (Faculty of Pain Medicine, Royal College of Anaesthetists), Martyn Hull (Turning Point, Primary Care), Cathryn Kemp (expert by experience), Jim McManus (Association of Directors of Public Health), Luke Montagu (expert by experience), Peter Pratt (NHS England), Sarah Rann (Royal College of General Practitioners), John Read (British Psychological Society), Gul Root (Department of Health and Social Care), Graham Sanderson (Specialist GP in Drugs and Alcohol, Bradford), Cathy Stannard (Consultant in Pain Medicine, NHS Gloucestershire, CCG), Arabella Tresillian (expert by experience), and Lucy Williams (Royal College of Anaesthetists).
Contributors
The review and study design were developed by PB, FG, MK, JK, JM, AT, ST, and MW. SC led the National Guideline Centre team for literature review. FA managed the implementation process with PB and ST, with input from FG, MK, JK, JM, AT, and MW. MW was the lead on the National Health Service Business Services Authority analysis with JK and JM. PW oversaw all mapping. BE, JM, and MW led on the statistical modelling, with input from PB, FG, JK, and ST. JM drafted the first version of the manuscript with input from all authors. All authors contributed to the interpretation of the data analysis and the further drafting of the manuscript and approved the final version.
Declaration of interests
In the past 3 years, JM has received research grants from the National Institute for Health Research (NIHR) for a randomised controlled trial of depot naltrexone for opioid use disorder, and a randomised controlled trial of acamprosate for alcohol use disorder; and research grants from the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley National Health Service (NHS) Mental Health Foundation Trust (SLaM) for a randomised controlled trial of novel cognitive therapy for cocaine use disorder. JM has worked part-time as the Senior Academic Advisor for the Alcohol, Drugs, Tobacco and Justice Division, of the Public Health England (PHE) Health and Wellbeing Directorate, and is a clinical academic consultant for the US National Institute on Drug Abuse, Centre for Clinical Trials Network. JM also declares an unrestricted research grant at the King's College London Institute of Psychiatry, Psychology & Neuroscience (IoPPN) and SLaM from Indivior via Action on Addiction for a completed randomised controlled trial of personalised psychosocial intervention in opioid agonist medication for opioid use disorder; unrestricted research grant funding at IoPPN and SLaM from Indivior for a 3-year, multicentre, randomised controlled trial of injectable depot buprenorphine for opioid use disorder (2019–21); and honoraria and travel support from Reckitt-Benckiser (2016; treatment of opioid use disorder) and PCM Scientific and Martindale for the Improving Outcomes in Treatment of Opioid Dependence conference (2018; contribution and chairing). MK has received a research grant from NIHR (for a randomised controlled trial of depot naltrexone); is the site principal investigator for a randomised controlled trial of extended-release buprenorphine for the treatment of opioid use disorder funded by Camurus and Braeburn Pharmaceuticals; is a research collaborator for a study of treatment for hepatitis C virus (HCV) for people who inject drugs, funded by Merck; has received support from Cepheid for a finger prick testing unit for HCV and HIV; has received an unrestricted research grant at IoPPN and SLaM from Indivior via Action on Addiction for a completed randomised controlled trial of personalised psychosocial intervention in opioid agonist medication for opioid use disorder and unrestricted research grant funding at IoPPN and SLaM from Indivior for a 3-year, multicentre, randomised controlled trial of injectable depot buprenorphine for opioid use disorder (2019–21). MK also declares honoraria and travel support from Mundipharma (2016; expert panel discussion on novel pharmacotherapies for opioid use disorder) and Abbvie and Gilead (2017 and 2018; discussion with hepatology specialists on HCV treatment for people who inject drugs). FG is a clinical senior lecturer at Imperial College London and has received funding from the National Institute for Health Research (NIHR), in particular the Collaboration for Leadership in Applied Health Research and Care programme and the Applied Research Collaboration for Northwest London. All other authors declare no competing interests. No author received support from the US National Institutes for Health for their contribution to this study.
Supplementary Material
References
- 1.National Institute for Health and Care Excellence Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Clinical guideline [CG76] January, 2009. https://www.nice.org.uk/guidance/cg76
- 2.Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth. 2018;120:1335–1344. doi: 10.1016/j.bja.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Turner JA, Devine EB. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 5.Berterame S, Erthal J, Thomas J. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet. 2016;387:1644–1656. doi: 10.1016/S0140-6736(16)00161-6. [DOI] [PubMed] [Google Scholar]
- 6.Organisation for Economic Co-operation and Development . OECD Publishing; Paris: 2019. OECD health policy studies—addressing problematic opioid use in OECD countries. [Google Scholar]
- 7.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111:1160–1174. doi: 10.1111/add.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77:403–426. doi: 10.1007/s40265-017-0700-x. [DOI] [PubMed] [Google Scholar]
- 9.Lyndon A, Audrey S, Wells C. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112:1580–1589. doi: 10.1111/add.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldwin DS, Aitchison K, Bateson A. Benzodiazepines: risks and benefits. A reconsideration. J Psychopharmacol. 2013;27:967–971. doi: 10.1177/0269881113503509. [DOI] [PubMed] [Google Scholar]
- 11.Tyrer P. Dependence on benzodiazepines. Br J Psychiatry. 1980;137:576–577. doi: 10.1192/bjp.137.6.576. [DOI] [PubMed] [Google Scholar]
- 12.Ashton H. Risks of dependence on benzodiazepine drugs: a major problem of long term treatment. BMJ. 1989;298:103–104. doi: 10.1136/bmj.298.6666.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. doi: 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt J, Leong C. Benzodiazepines and Z-Drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17:493–507. doi: 10.1007/s40268-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller I, Ashby D, Chambers M. Medicines and Healthcare products Regulatory Agency; London: 2005. Report of the CSM Expert Working Group on the safety of selective serotonin reuptake inhibitor antidepressants. [Google Scholar]
- 16.Geddes JR, Carney SM, Davies C. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 17.Cipriani A, Furukawa TA, Salanti G. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timimi S, Moncrieff J, Gøtzche P. Network meta-analysis of antidepressants. Lancet. 2018;392:1011–1012. doi: 10.1016/S0140-6736(18)31784-7. [DOI] [PubMed] [Google Scholar]
- 19.Hengartner MP, Plöderl M. Statistically significant antidepressant-placebo differences on subjective symptom-rating scales do not prove that the drugs work: effect size and method bias matter! Front Psychiatry. 2018;9:517. doi: 10.3389/fpsyt.2018.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen M, Hansen EH, Gøtzsche PC. What is the difference between dependence and withdrawal reactions? A comparison of benzodiazepines and selective serotonin re-uptake inhibitors. Addiction. 2012;107:900–908. doi: 10.1111/j.1360-0443.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 21.Committee on Safety of Medicines . Medicines and Healthcare products Regulatory Agency; London: 2004. Report of the CSM Expert Working Group on the safety of selective serotonin reuptake inhibitor antidepressants. [Google Scholar]
- 22.NHS Digital Prescription cost analysis—England, 2018 [PAS]. Prescription cost analysis 2018—Trends—Items. March 28, 2019. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018
- 23.Taylor S, Annand F, Burkinshaw P. Public Health England; London: 2019. Dependence and withdrawal associated with some prescribed medicines: an evidence review; pp. 89–92.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/829777/PHE_PMR_report.pdf [Google Scholar]
- 24.Behan M. Alternative report on an inquiry carried out by the All-Party Parliamentary Drug Misuse Group. Jan 22, 2009. https://www.benzo.org.uk/appg3d.htm
- 25.All-Party Parliamentary Group for Prescribed Drug Dependence Antidepressant withdrawal: a survey of patients' experience by the All-Party Parliamentary Group for Prescribed Drug Dependence. September, 2018. https://www.prescribeddrug.org/wp-content/uploads/2018/09/APPG-PDD-Antidepressant-Withdrawal-Patient-Survey.pdf
- 26.British Medical Association Prescribed drugs associated with dependence and withdrawal—building a consensus for action. Analysis report. October, 2015. http://bmaopac.hosted.exlibrisgroup.com/exlibris/aleph/a23_1/apache_media/H6IB5G1BL8SX1KJ7VY4MCRCXVG6EV7.pdf
- 27.Faculty of Pain Medicine. Public Health England Opioids Aware: a resource for patients and healthcare professionals to support prescribing of opioid medicines for pain. https://www.rcoa.ac.uk/faculty-of-pain-medicine/opioids-aware/
- 28.Fingleton N, Duncan E, Watson M, Matheson C. Specialist clinicians' management of dependence on non-prescription medicines and barriers to treatment provision: an exploratory mixed methods study using behavioural therapy. Pharmacy (Basel) 2019;7:E25. doi: 10.3390/pharmacy7010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gøtzsche PC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JAC, Hernán MA, Reeves BC. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Health Economics IHE quality appraisal checklist for case series studies. March 3, 2016. https://www.ihe.ca/publications/ihe-quality-appraisal-checklist-for-case-series-studies
- 34.Critical Appraisal Skills Programme CASP qualitative checklist. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf
- 35.Lewin S, Booth A, Glenton C. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci. 2018;13(suppl 1):2. doi: 10.1186/s13012-017-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henson KE, Brock R, Shand B. Cohort profile: prescriptions dispensed in the community linked to the national cancer registry in England. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Office for National Statistics Population esimates. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates
- 38.NHS Digital Patients registered at a GP practice. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/general-practice-data-hub/patients-registered-at-a-gp-practice
- 39.Ministry of Housing. Communities & Local Government English indices of deprivation 2015. Sept 30, 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015
- 40.Office for National Statistics Clinical Commissioning Groups (April 2018) ultra generalised clipped boundaries in England. https://geoportal.statistics.gov.uk/datasets/5252644ec26e4bffadf9d3661eef4826_4?geometry=-43.958%2C48.023%2C-2.605%2C57.305
- 41.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 42.National Institute for Health and Care Excellence British National Formulary. Hypnotics and Anxiolytics. https://bnf.nice.org.uk/treatment-summary/hypnotics-and-anxiolytics.html
- 43.National Institute for Health and Care Excellence Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of insomnia. Technology appraisal guidance [TA77] April 28, 2004. https://www.nice.org.uk/guidance/ta77
- 44.Public Health England. NHS England Advice for prescribers on the risk of the misuse of pregabalin and gabapentin. December, 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/385791/PHE-NHS_England_pregabalin_and_gabapentin_advice_Dec_2014.pdf
- 45.National Institute for Health and Care Excellence Depression in adults: recognition and management. Clinical guideline [CG90] October, 2009. https://www.nice.org.uk/guidance/cg90 [PubMed]
- 46.Mordecai L, Reynolds C, Donaldson LJ, de C Williams AC. Patterns of regional variation of opioid prescribing in primary care in England: a retrospective observational study. Br J Gen Pract. 2018;68:e225–e233. doi: 10.3399/bjgp18X695057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis HJ, Croker R, Walker AJ, Richards GC, Quinlan J, Goldacre B. Opioid prescribing trends and geographical variation in England, 1998-2018: a retrospective database study. Lancet Psychiatry. 2019;6:140–150. doi: 10.1016/S2215-0366(18)30471-1. [DOI] [PubMed] [Google Scholar]
- 48.Grotle M, Foster NE, Dunn KM, Croft P. Are prognostic indicators for poor outcome different for acute and chronic low back pain consulters in primary care? Pain. 2010;151:790–797. doi: 10.1016/j.pain.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry. 2019;6:538–546. doi: 10.1016/S2215-0366(19)30032-X. [DOI] [PubMed] [Google Scholar]
- 50.Leydon GM, Rodgers L, Kendrick T. A qualitative study of patient views on discontinuing long-term selective serotonin reuptake inhibitors. Fam Pract. 2007;24:570–575. doi: 10.1093/fampra/cmm069. [DOI] [PubMed] [Google Scholar]
- 51.Gibson K, Cartwright C, Read J. Patient-centered perspectives on antidepressant use: A narrative review. Int J Ment Health. 2014;43:81–99. [Google Scholar]
- 52.Selvaraj S, Jauhar S, Baldwin DS. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry. 2019;6:560–561. doi: 10.1016/S2215-0366(19)30183-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsimtsiou Z, Ashworth M, Jones R. Variations in anxiolytic and hypnotic prescribing by GPs: a cross-sectional analysis using data from the UK Quality and Outcomes Framework. Br J Gen Pract. 2009;59:e191–e198. doi: 10.3399/bjgp09X420923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen TC, Chen LC, Kerry M, Knaggs RD. Prescription opioids: Regional variation and socioeconomic status—evidence from primary care in England. Int J Drug Policy. 2019;64:87–94. doi: 10.1016/j.drugpo.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 55.General Pharmaceutical Council New safeguards for people seeking medicines online. April 16, 2019. https://www.pharmacyregulation.org/news/new-safeguards-people-seeking-medicines-online
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.