Abstract
BACKGROUND:
Optical coherence tomography (OCT) is a non-invasive diagnosing tool used in clinics. Due to its high resolution (<10um), it is appropriate for the early detection of tiny infections. It has been widely used in diagnosis and treatment of diseases, evaluation of therapeutic efficacy, and monitoring of various physiological and pathological processes.
OBJECTIVE:
To systemically review literature to summarize the clinic application of OCT in recent years.
METHODS:
For clinic applications that OCT has been applied, we selected studies that describe the most relevant works. The discussion included: 1) which tissue could be used in the OCT detection, 2) which character of different tissue could be used as diagnosing criteria, 3) which diseases and pathological process have been diagnosed or monitored using OCT imaging, and 4) the recent development of clinic OCT diagnosing.
RESULTS:
The literature showed that the OCT had been listed as a routine test choice for ophthalmic diseases, while the first commercial product for cardiovascular OCT detection had gotten clearance. Meanwhile, as the development of commercial benchtop OCT equipment and tiny fiber probe, the commercial application of OCT in dermatology, dentistry, gastroenterology and urology also had great potential in the near future.
CONCLUSIONS:
The analysis and discussions showed that OCT, as an optical diagnosing method, has been used successfully in many clinical fields, and has the potential to be a standard inspection method in several clinic fields, such as dermatology, dentistry and cardiovascular.
Keywords: Optical coherence tomography (OCT), clinical application of OCT
1. Introduction
Optical coherence tomography (OCT) was proposed for the first time in the 1990 s by Fujimoto [1]. Analogous to B-scan ultrasound imaging, OCT acquires cross-sectional images by measuring the intensity of back-scattered light from biological tissue. As the echo delay time is too short to measure due to the speed of light, OCT uses a low coherence light source and an interfering setup with the biological tissue in its sample arm.
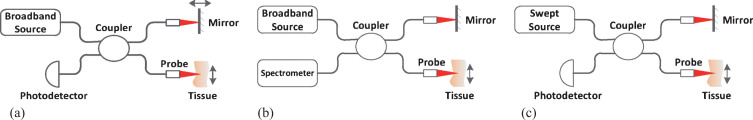
OCT was introduced firstly in a time-domain modality, called as time-domain OCT (TD-OCT) [2], as shown in Fig. 1(a). It uses a broadband source and a Michelson interferometer. The light backscattered from biological tissue interferes with light reflected from the reference mirror when the optical distance difference between the two light beams is less than the coherence length decided by the broadband source. The reference mirror moves periodically along the direction of light propagation (called as axial direction), forming axial scan and acquiring the information of biological tissue within the depth of scan. The interfering light is received by a photodetector which generates A-lines signals. The 3D OCT image is acquired through the cruising of sample probe across the tissue surface perpendicular to the axial direction. TD-OCT is able to achieve an axial resolution of 10μm or less. The drawback of this modality is that the moving reference mirror limits the imaging speed.
Fig.1.
Demonstration of several OCT modalities: (a) Time-domain OCT (b) Spectral-domain OCT (c) Swept source OCT.
Fourier-domain OCT (FD-OCT) is another modality which has two branches, spectral-domain OCT (SD-OCT) [3] and swept source OCT (SS-OCT) [4], both need no reference mirror movement. The SD-OCT, as shown in Fig. 1(b), uses a spectrometer instead of photodetector to acquire the interfering spectrum, and gets the A-lines through inverse Fourier-transform (IFFT). The SS-OCT, as shown in Fig. 1(c), uses a swept light source which sweeps the bandwidth in a sweeping period and outputs a sequence of narrow-band (several hundredth of a nanometer) light. A photodetector acquires the interfering light sequence and arranges them together according to time received as the interfering spectrum. As the imaging speed of FD-OCT is decided by the responding time of the spectrometer or the sweeping period of the swept light source, the development of technology and equipment makes it possible for FD-OCT to image in real time [5].
Both TD-OCT and FD-OCT have been widely used in clinic. And there are a serial of extensions of OCT which enrich the information of OCT images and have been put into use in clinic, such as polarization sensitive OCT (PS-OCT) [6], Doppler OCT [7], OCT angiography (OCTA) [8], dynamic OCT [9] and so on.
In this paper, the clinic applications of OCT in recent years are reviewed and discussed, revealing the progress and prospect of OCT in medical imaging.
2. Clinic application
2.1. OCT in ophthalmology
Human eye is a multi-layer chamber-like structure filled with semitransparent media, which makes it possible for the near-infrared light used in the OCT system to go deep into the fundus oculi to acquire the whole tomography of eyes. Since the semitransparency of eye, OCT achieves the biggest success in the ophthalmology and the OCT equipment for eyes is the only commercial OCT product that has been used as a standard inspection method in clinic.
The vision-affecting-diseases and therapeutic progress change the characters of eye layers [10–14]. The most commonly used markers in ophthalmological OCT diagnosing are retinal thickness, retinal layer structure and choroidal thickness. Kim [11] measured subfoveal choroidal and central foveal thicknesses of patients with acute anterior uveitis using a SD-OCT, and found a significant increase in patients during the active period of acute anterior uveitis. Schönfeldt-Lecuona [15] measured the total retinal volume and thickness as well as the volumes and thickness for 10 retinal layers of major depression patients and healthy controls with a SD-OCT, and found a 0.03mm3 retinal total volume difference between the right and left eye in the major depression patients, which was the only difference between the patients and the healthy controls. Martinez-Lapiscina [16] studied retinal nerve fibre layer thickness and macular volume in multiple sclerosis patients, proved a higher disability worsening rate in patients with retinal nerve fibre layer thickness of less than or equal to 87μm. El-Shazly [17] performed retinal nerve fibre layer and ganglion cell layer thickness measurement using SD-OCT on active smokers and passive smokers, and proved that there was retinal nerve fibre layer thinning which was related to the number of cigarettes/day in active smokers.
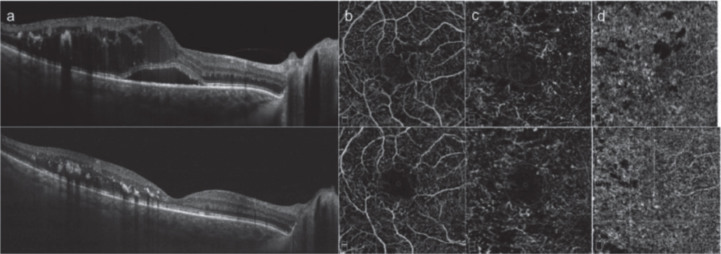
Another commonly used predictor of pathological changes in ophthalmology is the distribution of vessels which is acquired by OCTA. Toto [18] compared the superficial and deep capillary plexus density, choricapillary density and optic disc vessel density in the eyes of type 2 diabetic retinopathy patients before and after the dexamethasone implant. The results showed reduced retinal vascular density and choriocapillar density, and an increase tendency in the choroid vessel density after the dexamethasone implan. Shen [14] compared the superficial vessel distribution of diabetic eyes using a spectral-domain OCTA, and found a lower vessel density while the common OCT showed no obvious difference between the patients and healthy controls.
In most of the clinic studies, commercial OCT equipments were used. Since the first commercial ophthalmologic OCT equipment was produced by Carl Zeis Meditec Inc. in 1996, more and more ophthalmologic OCT products were pushed out by manufacturers like Canon and Optos. The majority of the commercial OCT products have a high resolution of 5μm-10μm, which makes them essential tools for ophthalmologic examination.
2.2. OCT in Cardiovascular
Another successful application of OCT is cardiovasology. In the cardiovascular inspection, a tiny OCT probe is sent into the cardiovascular through the peripheral artery to scan and acquire the morphology and blood flow of the cardiovascular. Among the recent applications, the drug-eluting stents and thin cap fibroatheroma are the top widely studied topics.
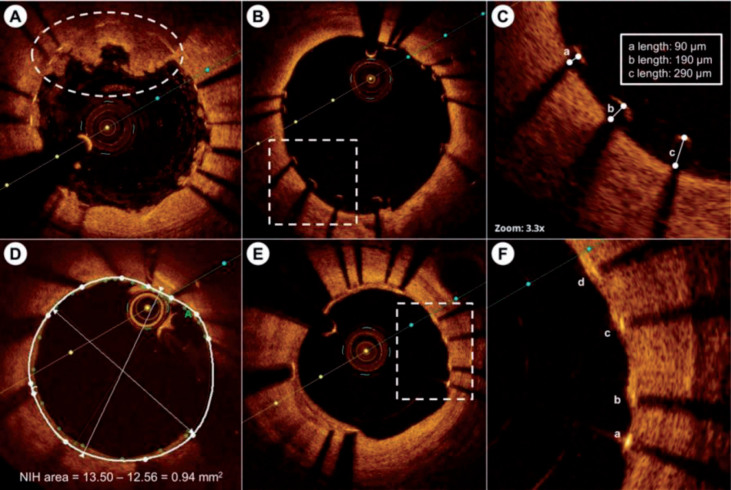
In patients with drug-eluting stents, the intimal coverage and apposition are critical indicator for the success of implantation. Since its high resolution, OCT makes the visualization of the stent strut morphology and residual stenosis possible. Iliescu [19] measured the luminal cross-sectional area, stent cross-sectional area, thickness of coverage and distance from the intimal wall for the inadequately apposed strut in OCT images of cancer patients with recently placed drug-eluting stents, and validated the ability of OCT in evaluating the risk of stent thrombosis caused by the dual antiplatelet therapy discontinuation for cancer-related procedures. Sato [20] measured the diameter of stent lumen and vessels and the thickness of plaque, and classified the plaques into calcific, fibro and mix types according to their morphology in the OCT images. The results from 39 patients with everolimus-eluting bioresorbable scaffold showed that the plaque eccentricity and plaque components were potential predictors for the acute scaffold recoil. Barber-Chamoux [21] attempted to conform and guide the treatment of iatrogenic coronary dissection using OCT, and succeed in 4 suspicions. Kim [22] compared the neointimal response between high- and moderate-intensity dosing of Vytorin after biodegradable polymer Biolimus-eluting stent implantation in patients with acute myocardial infarction using a FD-OCT. The images gotten 12 mothes after implantation demonstrated that both dosing led to lower percentages of uncovered struts and malapposed struts and thin neointimal thickness.
Besides, as its high resolution and easy-to-use fiber probe, OCT is the best choice to quantify the thickness of plaque fiber cap which is a critical predictor for plaque vulnerability. Wang [23] measured the fibrous cap thickness in the FD-OCT images of patients with acute coronary syndrome, and found thinner fibrous cap and higher occurrence frequency of thin-cap fibroatheroma on patients with higher platelet to lymphocyte ratio.
Nowadays, OCT is one of the three most important methods in cardiovascular study (the other two are intravascular ultrasound and coronary angiography). In clinic, it is a favorite tool for guiding coronary interventions [19, 28], evaluating effect of therapies and drugs [23], suggesting risk predictors [25], and analyzing and monitoring symtoms [21, 26, 27]. Since C7XR(TM) FD-OCT Imaging System and Companion C7 Dragonfly(TM) Imaging Catheter (St. Jude Medical, USA) got FDA clearance and CE mark in 2009, cardiovascular has been another clinic field that OCT has the potential to be in the list of the routine test.
2.3. OCT in Dermatology
Skin is a multi-layer structure whose thickness ranges from 0.5-4 mm. In OCT images, a thin hyporeflective line marks the top of the skin surface [28]. Below that are epidermis, dermal-epidermal junction and dermis in depth order. The main criteria of OCT that used in dermatological pathology are skin layer thickness, vascular network and the hypo-structure. Wessels [29] acquired 2D and 3D images of patients with suspicious penile lesion using a SS-OCT, and the results showed that the (pre) malignant tissue have higher average epidermal layer thickness and attenuation coefficient. Manfredini [30] tested acne lesions with DOCT. Compared with uninvolved skin, the closed comedos, open comedos, papules and pustules showed variably increased infundibular diameter, epidermal thickness and vascular network. Besides, the follow-up OCT scanning indicated an early normalization with the systemic antibiotic treatment, proving the potential ability of DOCT in guiding the acne therapy. Niculescu [31] imaged patients with basal cell carcinoma. With the criteria as thickening of epidermis and oval signal-poor structures surrounded by white stroma, OCT showed its advantage compared with dermatoscopy in identifying residual basal cell carcinoma lesions after the photodynamic therapy and distinguishing the completely cured lesions with the residual ones. Pires [32] measured the inferior labial mucosa thickness of systemic sclerosis patients, and found the thickening of labial mucosa epithelium a potential predictor of systemic sclerosis.
The success of OCT in dermatology lies on the small layer thickness of skin and the touchable probe used in the dermatological OCT. The former makes the limited imaging depth of OCT not a problem and the latter promises a stable imaging which is not affected by the movement of patients. Although there’s no commercial dermatological OCT equipment, many OCT products with a hand-held probe, such as Telesto serial of thorlabs, can be used directly for skin imaging without modification.
2.4. OCT in Urology
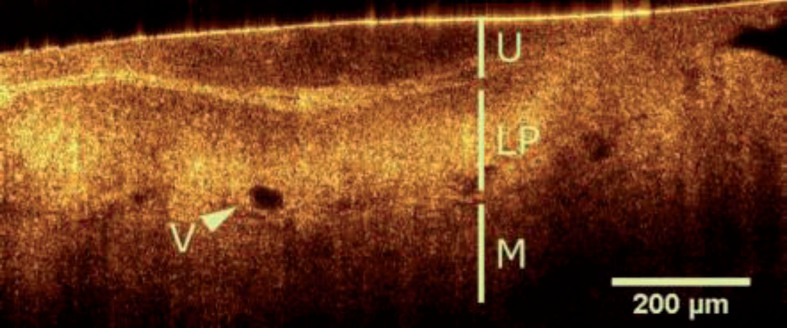
The combination of OCT and endoscope/cystoscope and the development of tiny fiber probes make the application of OCT in cavity and tubulous organs like bladder and urinary tracts possible. Using a special-designed OCT probe cystoscopy, Daniltchenko [33] got images of normal tissue, inflammation and transitional cell carcinoma, and found that the thicker epithelial layer and the absence of the ordered arrangement of internal structure were key indicators for urinary bladder lesion. Gladkova [34] acquired the cross-polarization OCT images of patients with flat local lesions. Using the criteria that the benign lesion has uniform signal and horizontal oriented structure and malignant lesion has dark or vertically oriented structure, the detection of flat malignant bladder lesions achieved a sensitivity of 93.7%, a specificity of 84% and an accuracy of 85.3%.
Other than layer structure, OCT was proved to have the ability of evaluating the optical parameters of urothelium, lamina propria, andmuscle layer which cancer always alters [35]. Bus [36] conducted ureterorenoscopic OCT on patients with upper urinary tract urothelial carcinoma to stage and grade the disease. The appearance of anatomical layers was used as criteria for staging, achieving a sensitivity of 100% and a specificity of 92%. Meanwhile, the decrease in light intensity was quantified for grading and reached a sensitivity of 87%, a specificity of 90% with 2.4 mm-1 as the boundary of low and high grade.
2.5. OCT in Gastroenterology
Digestive tract is the first human tissue which endoscopic OCT is applied to [37]. Similar with the urinary tract, the OCT is applied in the digestive tract in vivo with the assistance of endoscope or during operation. The capsule endomicroscopy is also used in OCT detection recently, whose coverage of upper GI wall, esophagus and duodenum is 85.4%, 90.3% and 84.8% [38].
The main application of OCT in gastroenterology is disease diagnosing and classification. Masci [39] obtained OCT images from patients with celiac disease during esophago-gastro-duodenoscopy. Using the histology results as baseline, the classification of villous atrophy had a sensitivity of 82% and a specificity of 100%, and the concordance between OCT and histology in determining patchy lesions was 75%. Hatta [40] proposed three criteria, including the thickness of esophageal wall layer, the involvement of the tumor signal into layer and destruction of layers, to classify the stage of tumor infiltration in superficial esophageal squamous cell carcinoma. The classifying results of 46 patients got an accuracy of 92.7%. Later, Hatta [41] studied patients with Barrett’s adenocarcinoma using an endoscopic OCT, with the subepithelial glandular structure and the loss of distinct layer structure under a distinct homogeneous superficial layer as criteria, the predictive accuracy, sensitivity and specificity of the Barrett’s mucosa buried underneath esophageal squamous epithelium were 85.7%, 77.8% and 100%. Testoni [42] introduced the OCT probe into the standard endoscopic retrograde cholangio-pancreatography catheter and imaged 5 controls with sphincter of Oddi dysfunction and 5 patients with pancreatic adenocarcinoma not involving the papillary region. Compared with the controls, the patients had a marked increase in the thickness of the intermediate fibro-uscular layer and increased infrared light back-scattering.
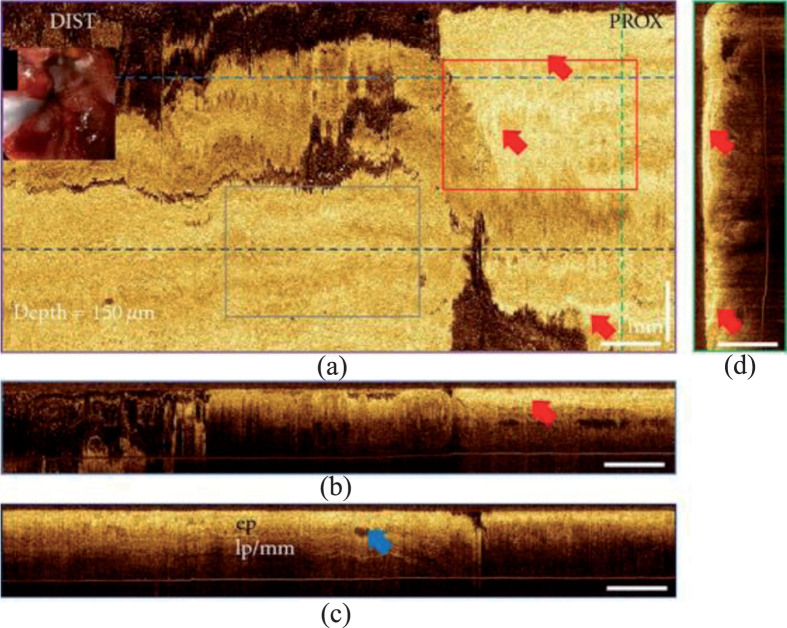
Besides, OCT is able to provide guidance for surgery. Desai [43] conducted endoscopic OCT on patients with achalasia. By directing the peroral endoscopic myotomy tunnel toward thicker muscle or away from submucosal vessels, the bleeding was significantly less than the patients without the OCT guidance. Tsai [44] inspected patients with Barrett’s esophagus using 3D OCT, evalutating the tissue architectural changes after radiofrequency ablation and cryospray ablation which is hard for white light and NBI endoscope. The images showed that the patients receiving radiofrequency ablation had burned tissues with hyperscattering desquamation and debris, while the patients receiving cryospray ablation had edema-like changes.
2.6. OCT in Dentistry
OCT was used in the dentistry for the first time in 1998 [45]. Since then, countless experiments have been completed on animal and human teeth ex vivo and in vivo, including cervical lesion [46, 47], demineralization and remineralization [48–50], caries [51], adhesive and sealants character [52, 53], and so on [54, 55].
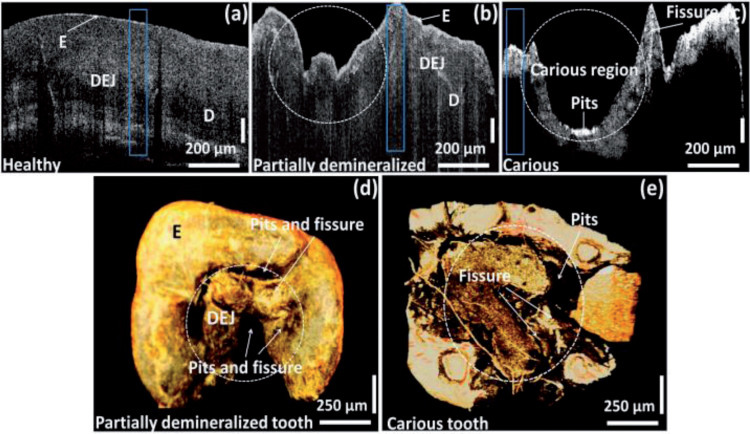
Nowadays, the main clinic OCT applications focused on the dental demineralization/remineralization and the materials used in restorative dentistry and esthetic dentistry. Nee [48] monitored premolars from 20 patients with orthodontic brackets using a cross-polarization OCT in a period of 12 months, and found increasing demineralization indicated by a small but significant increase in the lesion depth and reflectivity over that depth. Sugiura [49] studied the effect of chewing gums on the white spot lesion remineralization using OCT. The OCT images from 20 person chewing POs-Ca and POs-Ca+F gums showed a decrease of the enamel boundary depth which indicates the remineralization, while the OCT images of 9 person chewing no-POs-Ca gums expressed no remineralization. Wijesinghe [51] studied the progression of dental caries by imaging healthy, partially demineralized and completely demineralized tooth samples of 4 orthodontic patients using a SD-OCT. The results proved that the enamel thickness, depth direction total intensity fluctuations and enamel residual volume were promising parameters to evaluate the progression of caries. Ishibashi [52] investigated 52 patients with resin-based composite restorations and validated the potential of SS-OCT to provide restoration defects as marginal crevice, porosity and gap formation, which is unavailable with other imaging methods. Haak [53] studied the interfacial adhesive defect of adhesive Scotchbond UniversalTM applied with different etching modes using adhesive OptiBondTM as control. The high resolution of SD-OCT images made it possible to show subtle adhesive defect difference among these four adhesive application in different interfaces and time, which is hard for clinic assessment.
As teeth and gum can be easily accessed, both OCT products designed specifically for dentistry [52] and commercial OCT products with hand-held probe as Telesto II (Thorlabs) [48] and IVS-3000-CP (Santec) [53] are used in the researches above. The easy usage of commercial OCT products in clinic studies makes the future application of OCT in dentistry convenient, and dentistry has great potential to be another clinic domain that takes OCT as a standard inspection choice.
2.7. Advantage and Limitation
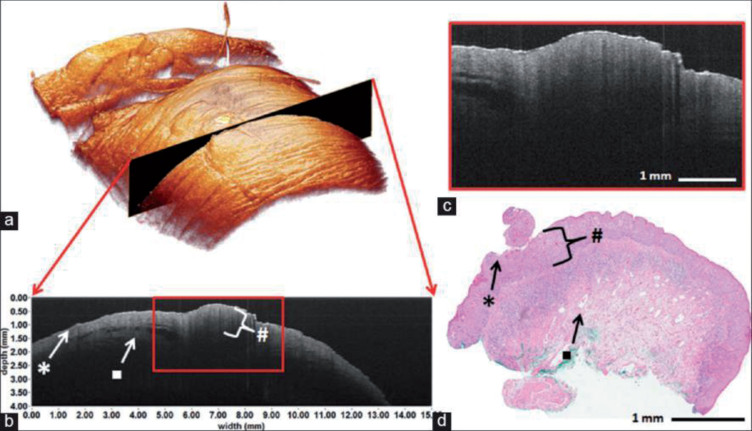
Figures 2 to 7 show several OCT images acquired from different tissues discussed above and Table 1 displays the commonly used criteria in these OCT applications. It can be seen that the main criteria in the OCT detection are layer thickness and tiny structure of mucosa. The high resolution details have been proved to be consistent to those in the anatomic and histological images [29, 35, 57, 58], as an example shown in Figure 4, which makes OCT to be called “optical biopsy”. Actually, compared with widely used clinic tomography modalities, the key advantage of OCT is its micrometer resolution which enables the detection of tiny pathological changes and validated the comparable performance of OCT in clinic with some gold diagnosing standards [14, 59, 60], making OCT a potential clinic alternative in condition of contraindication, tissue removal impossible, avoiding tissue destruction and so on. Take ultrasonic tomography which has similar theory and ability to be used in endoscopy with OCT as example, Mueller-Lisse [61] compared the endoluminal ultrasonography and OCT images of porcine upper urinary tract, and demonstrated the better performance of OCT in distinguishing between urothelium, lamina propria, and muscle layer. Horeman [60] displayed OCT and IVUS images of cartilage with defects, and found that the OCT has better performance in indicating defects smaller than 1 mm.
Fig.2.
SD-OCT (a) and OCTA (b-d) images of a patient with diabetic macular edema before (top) and after (bottom) intravitreal dexamethasone implant [18]. (Used under the terms of the Creative Commons Attribution 4.0 license)
Fig.7.
The OCT images of healthy, partially demineralized, and completely demineralized (carious) molar tooth samples [51]. (Used under the terms of the Creative Commons Attribution 4.0 license)
Table 1.
Commonly used criteria for clinical OCT applications
| Applications | Key Criteria |
| Ophthalmology | Retinal thickness (retinal nerve fiber layer thickness, ganglion cell-inner plexiform layer thickness, nuclear layer thickness, photoreceptor layer thickness), retinal volume, retinal vessel density, choroidal thicknesses, choroidal reflectivity, macular thickness, morphology (foveal contour, choroidal surface irregularity, submacular retinal pigment epithelium disorganization, macular optical empty spaces) |
| Cardiovascular | Fibrous cap thickness, lipid arc, vessel diameter, blood-flow velocity, neointimal thickness, plaque eccentricity, plaque composition |
| Dermatology | Epidermal thickness, surface-to-first-vessel distance, vascular network, morphology (epidermal attenuation coefficient, hyporefletive flaccid structure, hypoechogenic structure) |
| Urology | Epithelial attenuation coefficient, urothelium thickness, lamina propria structure |
| Gastroenterology | Muscular layer thickness, mucosal and submucosal blood-flow velocity, organization and existence of esophageal wall tissue (squamous epithelium, lamina propria mucosa, muscularis mucosa, submucosa, muscularis propria, glands, villous) |
| Dentistry | Enamel and dentin attenuation coefficient, ceramics prosthese reflectivity, gingival tissue organization (sulcus, epithelium) |
Fig.4.
3D and 2D OCT images of glans penis carcinoma, showing horny layer (*), thickened epidermal layer (#) and a blood vessel (•) [29]. (Used under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License)
Fig.3.
The postimplantation OCT image [22]. (Used under the terms of the Creative Commons Attribution 4.0 license)
Fig.5.
OCT image of a healthy bladder wall indicating urothelium (U), intact lamina propria (LP) and muscularis (M) [56]. (Used under the terms of the Creative Commons Attribution 4.0 license)
Fig.6.
The 3D OCT images of Barrett’s esophagus after radiofrequency ablation [44]. (Used under the terms of the Creative Commons Attribution license)
On the other hand, accompanied by its high resolution, OCT has low imaging depth. For turbid tissue such as mucosa, vessel, skin and teeth, OCT has a limited imaging depth of less than 3 mm [45, 60], which prevents the detection of pathological changes in the deep tissue. It makes OCT a good imaging tool for just superficial tissue. To take its advantage, OCT could be used in the follow-up or synchronous steps of imaging modalities with large depth and low resolution, such as ultrasound [62] and CT [63].
3. Discussion
Since its application in medical imaging in 1991, OCT has been used in various clinical fields, including pathology detecting, pathological mechanism analyzing, pathological process monitoring, and therapy assessment, as shown in Fig. 1. Although OCT has only been listed as a routine test choice in the ophthalmology, commercial OCT equipments are widely used in clinical researches. A lot of producers have developed commercial products and modules that can be integrated and used in different clinical studies. Besides, the development of tiny fiber probe promotes the combination of OCT and various medical introscopes, which enhances the imaging depth of OCT and enables its application in inner body cavity and organs.
As commercial benchtop OCT products can be used in dermatology and dentistry conveniently with only a little modification, either a custom-made probe or a self-made image-processing software, it is believed that OCT has the potential to be applied in the daily diagnosing of dermal and dental pathology in the near future.
Other than these mature applications, there are still some OCT applications that need further studies to be used in clinic, like in orthopedics and neurology. The main difficulty of these OCT applications is the limited penetration ability of the near infrared light, either in the tissue outside the joints or the thick skull covering the brain. Although using OCT in open surgery or the combination of OCT and the fiber probe [64–67] maybe potential solutions to the problem, further researches and improved components are essential for the sake of safety.
Acknowledgments
This work is sponsored by the National Science and Technology Support Program 2018YFC0116202, 2017YFC0109702 and 2017YFC0109901.
References
- [1]. Huang D., Swanson E.A., Lin C.P., et al. , Optical coherence tomography, Science 22 254 (1991), 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Pan Y., Birngruber R., Rosperich J., et al. , Low-coherence optical tomography in turbid tissue: Theoretical analysis, Applied Optics 34 (1995), 6564. [DOI] [PubMed] [Google Scholar]
- [3]. Leitgeb R., Wojtkowski M., Kowalczyk A., et al. , Spectral measurement of absorption by spectroscopic frequency-domain optical coherence tomography, Optics Letters 25 (2000), 820–822. [DOI] [PubMed] [Google Scholar]
- [4]. Yun S.H., Tearney G.J., de Boer J.F., et al. , High-speed optical frequency-domain imaging, Optics Express 11 (2003), 2953–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Potsaid B., Gorczynska I., Srinivasan V.J., et al. , Ultrahigh speed Spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second, Optics Express 16 (2008), 15149–15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. de Boer J.F., Milner T.E. and van Gemert M.J.C., et al. , Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography, Optics Letters 22 (1997), 934–936. [DOI] [PubMed] [Google Scholar]
- [7]. Wong R.C.K., Izatt J.A., Sivak M.V. Jr., et al. , The visualization of submucosal blood vessels by color doppler flow imaging using optical coherence tomography: Potential use in peptic ulcer hemorrhage, Gastrointestinal Endoscopy 45 (1997), AB43. [Google Scholar]
- [8]. Yu Lingfeng and Chen Zhongping, Doppler variance imaging for three-dimensional retina and choroid angiography, Journal of Biomedical Optics 15 (2010), 016029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Tuchin V.V., Xu X., Wang R.K. Dynamic optical coherence tomography in studies of optical clearing, sedimentation, and aggregation of immersed blood, Applied Optics 41 (2002), 258–271. [DOI] [PubMed] [Google Scholar]
- [10]. Mano N., Mitsutsuji T., Yoshikawa Y., et al. , Optical Coherence Tomography in an Infant with Walker-Warburg Syndrome, Case Reports in Ophthalmology 6 (2015), 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Kim M., Choi S.Y., Park Y. Analysis of choroidal and central foveal thicknesses in acute anterior uveitis by enhanced-depth imaging optical coherence tomography, BMC Ophthalmology 17 (2017), 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Qian C.X., Charran D., Strong C.R., et al. , Optical Coherence Tomography Examination of the Retinal Pigment Epithelium in Best Vitelliform Macular Dystrophy, Ophthalmology 124 (2017), 456–463. [DOI] [PubMed] [Google Scholar]
- [13]. Dastiridou A.I., Bousquet E., Kuehlewein L., et al. , Choroidal Imaging with Swept-Source Optical Coherence Tomography in Patients with Birdshot Chorioretinopathy, Ophthalmology 124 (2017), 8, 1186–1195. [DOI] [PubMed] [Google Scholar]
- [14]. Shen C., Yan S., Du M., et al. , Assessment of capillary dropout in the superficial retinal capillary plexus by optical coherence tomography angiography in the early stage of diabetic retinopathy, BMC Ophthalmology 18 (2018), 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Schönfeldt-Lecuona C., Schmidt A., Kregel T., et al. , Retinal changes in patients with major depressive disorder – A controlled optical coherence tomography study, Journal of Affective Disorders 227 (2018), 665–671. [DOI] [PubMed] [Google Scholar]
- [16]. Martinez-Lapiscina E.H., Arnow S., Wilson J.A., et al. , Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study, Lancet Neurology 15 (2016), 574–584. [DOI] [PubMed] [Google Scholar]
- [17]. El-Shazly A.A.E., Farweez Y.A.T., Elewa L.S., et al. , Effect of Active and Passive Smoking on Retinal Nerve Fibre Layer and Ganglion Cell Complex, Journal of Ophthalmology 2017 (2017), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Toto L., D’Aloisio R., Nicola M.D., et al. , Qualitative and Quantitative Assessment of Vascular Changes in Diabetic Macular Edema after Dexamethasone Implant Using Optical Coherence Tomography Angiography, International Journal of Molecular Sciences 18 (2017), 1181, doi: 10.3390/ijms18061181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Iliescu C.A., Cilingiroglu M., Giza D. E., et al. , “Bringing on the light” in a complex clinical scenario: Optical coherence tomography–guided discontinuation of antiplatelet therapy in cancer patients with coronary artery disease (PROTECT-OCT registry), American Heart Journal 194 (2017), 83–91. [DOI] [PubMed] [Google Scholar]
- [20]. Sato T., Jose J., El-Mawardy M., et al. , Predictors of acute scaffold recoil after implantation of the everolimus-eluting bioresorbable scaffold: an optical coherence tomography assessment in native coronary arteries, The International Journal of Cardiovascular Imaging 33 (2017), 145–152. [DOI] [PubMed] [Google Scholar]
- [21]. Barber-Chamoux N., Souteyrand G., Combaret N., et al. , Contribution of optical coherence tomography imaging in management of iatrogenic coronary dissection, Cardiovascular Revascularization Medicine 17 (2016), 138–142. [DOI] [PubMed] [Google Scholar]
- [22]. Kim Y., Hong Y.J., Kim S.W., et al. , Impact of Combination Therapy with Ezetimibe/Simvastatin Treatment on the Neointimal Response to Biodegradable Polymer Biolimus-Eluting Stent Implantation in Patients with Acute Myocardial Infarction: Serial Assessment with Optical Coherence Tomography, Applied Sciences 8 (2018), 1968, doi: 10.3390/app8101968 [DOI] [Google Scholar]
- [23]. Wang X., Xie Z., Liu X., et al. , Association of Platelet to lymphocyte ratio with non-culprit atherosclerotic plaque vulnerability in patients with acute coronary syndrome: An optical coherence tomography study, BMC Cardiovascular Disorders 17 (2017), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Jones D.A., Rathod K.S., Koganti S., et al. , Angiography Alone Versus Angiography Plus Optical Coherence Tomography to Guide Percutaneous Coronary Intervention, JACC: Cardiovascular Interventions 11 (2018), 1313–1321. [DOI] [PubMed] [Google Scholar]
- [25]. Fujino Y., Attizzani G.F., Tahara S., et al. , Association of skin autofluorescence with plaque vulnerability evaluated by optical coherence tomography in patients with cardiovascular disease, Atherosclerosis 274 (2018), 47–53. [DOI] [PubMed] [Google Scholar]
- [26]. Liu X., Xie Z., Sun M., et al. , Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography, International Journal of Cardiology 265 (2018), 18–23. [DOI] [PubMed] [Google Scholar]
- [27]. Clemmensen T.S., Holm N.R., Eiskjær H., et al. , “ST Elevation Infarction after Heart Transplantation Induced by Coronary Spasms and Mural Thrombus Detected by Optical Coherence Tomography”, Case Reports in Transplantation 2016 (2016), 1863869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Ulrich M., Themstrup L., de Carvalho N., et al. , Dynamic Optical Coherence Tomography in Dermatology, Dermatology 232 (2016), 298–311. [DOI] [PubMed] [Google Scholar]
- [29]. Wessels R., De Bruin D.M. and Faber D.J., et al. , Optical coherence tomography accurately identifies patients with penile (pre) malignant lesions: A single center prospective study, Urology Annals 7 (2015), 459–465, http://www.urologyannals.com/text.asp?2015/7/4/459/156147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Manfredini M., Greco M., Farnetani F., et al. , Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography, Journal of The European Academy of Dermatology and Venereology 31 (2017), 1541–1546. [DOI] [PubMed] [Google Scholar]
- [31]. Niculescu L., Bierhoff E., Hartmann D., et al. , Optical coherence tomography imaging of basal cell carcinoma undergoing photodynamic therapy: A pilot study, Photodiagnosis and Photodynamic Therapy 18 (2017), 133–137. [DOI] [PubMed] [Google Scholar]
- [32]. Pires N.S.M., Dantas A.T., Duarte A.L.B.P., et al. , Labial mucosa evaluation in systemic sclerosis using optical coherence tomography, Conference on Lasers and Electro-Optics, OSA Technical Digest (online) (2017), JW2A.51. [Google Scholar]
- [33]. Daniltchenko D., Sachs M., Lankenau E., et al. , Optical Coherence Tomography of the Urinary Bladder: The Potential of a High-Resolution Visual Investigation Technique for Endoscopic Diagnostics, Optics and Spectroscopy 101 (2006), 40–45. [Google Scholar]
- [34]. Gladkova N., Streltsova O., Zagaynova E., et al. , Cross-polarization optical coherence tomography for early bladder-cancer detection: Statistical study, Journal of Biophotonics 4 (2011), 519–532. [DOI] [PubMed] [Google Scholar]
- [35]. Ikeda M., Matsumoto K., Choi D., et al. , The impact of real-time 3d imaging by ultra-high speed optical coherence tomography in urothelial carcinoma, BMC Urology 13 (2013), 65, doi: 10.1186/1471-2490-13-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Bus M.T.J., de Bruin D.M. and Faber D.J., et al. , Optical Coherence Tomography as a Tool for In Vivo Staging and Grading of Upper Urinary Tract Urothelial Carcinoma: A Study of Diagnostic Accuracy, The Journal of urology 196 (2016), 1749–1755. [DOI] [PubMed] [Google Scholar]
- [37]. Rollins A.M., Ung-arunyawee R., Chak A., et al. , Real-time in vivo imaging of human gastrointestinal ultrastructure by use of endoscopic optical coherence tomography with a novel efficient interferometer design, Optics Letters 24 (1999), 1358–1360. [DOI] [PubMed] [Google Scholar]
- [38]. Gora M.J., Quénéhervé L., Carruth R.W., et al. , Tethered capsule endomicroscopy for microscopic imaging of the esophagus, stomach, and duodenum without sedation in humans (with video), Gastrointestinal Endoscopy 88 (2018), 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Masci E., Mangiavillano B., Barera G., et al. , Optical coherence tomography in pediatric patients: A feasible technique for diagnosing celiac disease in children with villous atrophy, Digestive and Liver Disease 41 (2009), 639–643. [DOI] [PubMed] [Google Scholar]
- [40]. Hatta W., Uno K., Koike T., et al. , Optical coherence tomography for the staging of tumor infiltration in superficial esophageal squamous cell carcinoma, Gastrointestinal Endoscopy 71 (2010), 899–906. [DOI] [PubMed] [Google Scholar]
- [41]. Hatta W., Uno K., Koike T., et al. , Feasibility of optical coherence tomography for the evaluation of Barrett’s mucosa buried underneath esophageal squamous epithelium, Digestive Endoscopy 28 (2016), 427–433. [DOI] [PubMed] [Google Scholar]
- [42]. Testoni P.A., Mangiavillano B., Mariani A., et al. , Investigation of Oddi sphincter structure by optical coherence tomography in patients with biliary-type 1 dysfunction: A pilot in vivo study, Digestive and Liver Disease 41 (2009), 907–912. [DOI] [PubMed] [Google Scholar]
- [43]. Desai A.P., Tyberg A., Kedia P., et al. , Optical coherence tomography (OCT) prior to peroral endoscopic myotomy (POEM) reduces procedural time and bleeding: A multicenter international collaborative study, Surgical Endoscopy 30 (2016), 5126–5133. [DOI] [PubMed] [Google Scholar]
- [44]. Tsai T., Zhou C., Lee H., et al. , Comparison of Tissue Architectural Changes between Radiofrequency Ablation and Cryospray Ablation in Barrett’s Esophagus Using Endoscopic Three-Dimensional Optical Coherence Tomography, Gastroenterology Research and Practice 2012 (2012), 684832, doi: 10.1155/2012/684832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Colston B.W. Jr., Sathyam U.S. and DaSilva L.B., Dental OCT, Optics Express 3 (1998), 230–238. [DOI] [PubMed] [Google Scholar]
- [46]. Wada I., Shimada Y., Ikeda M., et al. , Clinical assessment of non carious cervical lesion using swept-source optical coherence tomography, Journal of Biophotonics 8 (2015), 846–854. [DOI] [PubMed] [Google Scholar]
- [47]. Sugita I., Nakashima S., Ikeda A., et al. , A pilot study to assess the morphology and progression of non-carious cervical lesions, Journal of Dentistry 57 (2017), 51–56. [DOI] [PubMed] [Google Scholar]
- [48]. Nee A., Chan K., Kang H., et al. , Longitudinal monitoring of demineralization peripheral to orthodontic brackets using cross polarization optical coherence tomography, Journal of Dentistry 42 (2014), 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Sugiura M., Kitasako Y., Sadr A. White spot lesion remineralization by sugar-free chewing gum containing bio-available calcium and fluoride: A double-blind randomized controlled trial, Journal of Dentistry 54 (2016), 86–91. [DOI] [PubMed] [Google Scholar]
- [50]. Zhou Y., Shimada Y., Matin K., et al. , Assessment of bacterial demineralization around composite restorations using swept-source optical coherence tomography (SS-OCT), Dental Materials 32 (2016), 1177–1188. [DOI] [PubMed] [Google Scholar]
- [51]. Wijesinghe R.E., Cho N.H., Park K., et al. , Bio-Photonic Detection and Quantitative Evaluation Method for the Progression of Dental Caries Using Optical Frequency-Domain Imaging Method, Sensors 16 (2016), 2076. doi: 10.3390/s16122076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Ishibashi K., Ozawa N., Tagami J., et al. , Swept-source optical coherence tomography as a new tool to evaluate defects of resin-based composite restorations, Journal of Dentistry 39 (2011), 543–548. [DOI] [PubMed] [Google Scholar]
- [53]. Haak R., Schmidt P., Park K., et al. , OCT for early quality evaluation of tooth-composite bond in clinical trials, Journal of Dentistry 76 (2018), 46–51. [DOI] [PubMed] [Google Scholar]
- [54]. Duma V.-F., Sinescu C., Bradu A., et al. , A Optical Coherence Tomography Investigations and Modeling of the Sintering of Ceramic Crowns, Materials 12 (2019), 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Oguro R., Nakajima M., Seki N., et al. , The role of enamel thickness and refractive index on human tooth colour, Journal of Dentistry 51 (2016), 36–44. [DOI] [PubMed] [Google Scholar]
- [56]. Bovenkamp D., Sentosa R., Rank E., et al. , Combination of High-Resolution Optical Coherence Tomography and Raman Spectroscopy for Improved Staging and Grading in Bladder Cancer, Applied Science 8 (2018), 2371. doi: 10.3390/app8122371 [DOI] [Google Scholar]
- [57]. Niemelä T., Virén T., Liukkonen J., et al. , Application of optical coherence tomography enhances reproducibility of arthroscopic evaluation of equine joints, Acta Veterinaria Scandinavica 56 (2014), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Cernohorsky P., Jansen S.M., de Bruin D.M., et al. , Quantitative Assessment of Optical Properties in Healthy Cartilage and Repair Tissue by Optical Coherence Tomography and Histology, IEEE Journal of Selected Topics in Quantum Electronics 22 (2016), 6801407. [Google Scholar]
- [59]. Pilge H., der Velden K.H. and Herten M., et al. , Comparison of hip joint cartilage degeneration assessed by histology and ex vivo optical coherence tomography, Orthopedic Reviews 6 (2014), 5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Horeman T., Buiter E.C., Pouran B., et al. , In-Vitro Detection of Small Isolated Cartilage Defects: Intravascular Ultrasound Vs. Optical Coherence Tomography, Annals of Biomedical Engineering 45 (2018), 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Mueller-Lisse U.L., Bader M., Bauer M., et al. , Optical coherence tomography of the upper urinary tract: Review of initial experience ex vivo and in vivo , Medical Laser Application 25 (2010), 44–52. [Google Scholar]
- [62]. Zhou C., Wang J., Jiao S. Dual channel dual focus optical coherence tomography for imaging accommodation of the eye, Optics Express 17 (2009), 8947–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Kim Y., Jeong M.H., Kim M.C., et al. , Optimal drug-eluting stent implantation with the aid of optical coherence tomography in the stenotic lesion of ectatic coronary artery, Cardiology Journal 25 (2018), 534–535. [DOI] [PubMed] [Google Scholar]
- [64]. Chu C.R., Lin D., Geisler J.L., et al. , Arthroscopic Microscopy of Articular Cartilage Using Optical Coherence Tomography, The American Journal of Sports Medicine 32 (2004), 699–709. [DOI] [PubMed] [Google Scholar]
- [65]. Chu C.R., Williams A., Tolliver D. Clinical Optical Coherence Tomography of Early Articular Cartilage Degeneration in Patients With Degenerative Meniscal Tears, Arthritis & Rheumatism 62 (2010), 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Xie Y., Bonin T., Loffler S., et al. , Coronal in vivo forward-imaging of rat brain morphology with an ultra-small optical coherence tomography fiber probe, Physics in Medicine & Biology 58 (2013), 555–568. [DOI] [PubMed] [Google Scholar]
- [67]. Li Y., Choi W.J. and Wei W., et al. , Aging-associated changes in cerebral vasculature and blood flow as determined by quantitative optical coherence tomography angiography, Neurobiology of Aging 70 (2018), 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]