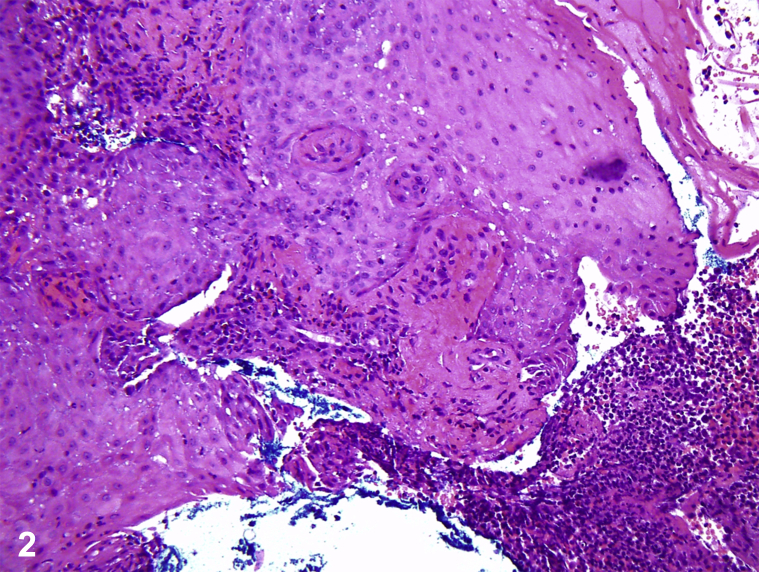
A teenage boy with Crohn's disease, treated with 6-mercaptopurine (6-MP) and mesalamine, had tender, hemorrhagic, vesiculobullous plaques on the hands, metacarpophalangeal joints, tongue, and face, which began as small red bumps (Fig 1). He was febrile to 38.7°C, pancytopenic, and neutropenic, with elevated erythrocyte sedimentation rate and C-reactive protein. Lesional pathology findings showed a neutrophilic infiltrate with occasional histiocytes but negative acid-fast bacilli and fungal stains (Fig 2). Lesional tissue culture revealed only methicillin-sensitive Staphylococcus aureus. Blood cultures were negative for bacteria and fungi. Positron emission tomography/computed tomography found axillary and mesenteric lymphadenopathy, deemed reactive. Bone marrow evaluation found hypocellular marrow, consistent with 6-MP toxicity, without malignancy.
Fig 1.
Fig 2.
Question 1: What is the most likely diagnosis?
-
A.
Acute febrile neutrophilic dermatosis
-
B.
Erythema multiforme
-
C.
Ecthyma gangrenosum
-
D.
Cutaneous anthrax
-
E.
Cutaneous small vessel vasculitis (CSSV)
Answers:
-
A.
Acute febrile neutrophilic dermatosis – Correct. The patient fulfilled both major criteria of Sweet syndrome (SS): (1) sudden onset of painful violaceous papules or nodules and (2) neutrophilic infiltrate without leukocytoclastic vasculitis. The patient fulfilled minor criteria of (1) accompaniment by inflammatory bowel disease and (2) fever greater than 38°C and constitutional symptoms.1 Rapid improvement with 60-mg prednisone confirmed the diagnosis of SS.
-
B.
Erythema multiforme – Incorrect. Although erythema multiforme favors acral surfaces, particularly the palms, the lesions characteristically display a targetoid morphology with 3 zones of coloration.
-
C.
Ecthyma gangrenosum – Incorrect. This diagnosis was initially suspected, given the patient's neutropenia, fever, and other systemic signs of infection (increased erythrocyte sedimentation rate and C-reactive protein). However, blood cultures did not show bacterial or fungal organisms, and lesional tissue did not culture Pseudomonas aeruginosa or another likely organism (only methicillin-sensitive Staphylococcus aureus was demonstrated).
-
D.
Cutaneous anthrax – Incorrect. Although the cutaneous manifestation of this rare disorder often begins as a nonpainful purpuric papule evolving into an ulcerating vesicle that forms a black eschar, the eschar is typically depressed and characteristically surprisingly painless, despite the necrosis. Lack of recent travel or exposures (such as to animal hides, wool, or contaminated soil) also makes this diagnosis less likely.
-
E.
CSSV – Incorrect. CSVV typically affects the lower extremities and usually consists of smaller purpuric macules and papules than the lesions seen in this patient, although vesicles and bullae within palpable purpuric lesions may be seen in the less common bullous variant.
Question 2: Which of the following is an uncommon cause of SS?
-
A.
Hematologic malignancies
-
B.
Solid-organ malignancies
-
C.
Granulocyte colony-stimulating factor (G-CSF)
-
D.
Azathioprine
-
E.
Hydroxychloroquine
Answers:
-
A.
Hematologic malignancies – Incorrect. Hematologic malignancies (most commonly acute myeloid leukemia) have been strongly associated with SS.2
-
B.
Solid-organ malignancies – Incorrect. Solid-organ malignancies are less commonly associated with SS than are hematologic malignancies, but associations of solid-organ ones with SS have been reported, most commonly with genitourinary tumors.
-
C.
G-CSF – Incorrect. Drug-induced SS have been reported, most commonly with G-CSF.3
-
D.
Azathioprine – Incorrect. Although drug-induced SS is more common with G-CSF than with azathioprine, azathioprine-associated SS has been reported.4 This patient had hypoplastic bone marrow consistent with azathioprine (6-MP) toxicity, which may have induced SS.
-
E.
Hydroxychloroquine – Correct. Hydroxychloroquine is not a common cause of SS; it has been reported as part of the treatment regimen for SS.5
Question 3: Which is a first-line treatment for SS?
-
A.
Systemic steroids
-
B.
Potassium iodide
-
C.
Colchicine
-
D.
Dapsone
-
E.
Ixekizumab
Answers:
-
A.
Systemic steroids – Correct. Systemic corticosteroids are the therapeutic standard for SS; for mild cases, intralesional or topical corticosteroids may be used as monotherapy or adjunctive therapy. Patients with extracutaneous manifestations, such as pulmonary, neurologic, gastrointestinal, renal, or cardiac SS, carry a higher mortality risk and warrant systemic treatment with corticosteroids. Our patient had acute kidney injury, which resolved with prednisone treatment. Any underlying malignancies, such as acute myeloid leukemia, should also be addressed appropriately.
-
B.
Potassium iodide – Incorrect. Steroid-sparing agents include potassium iodide, colchicine, and dapsone. These are typically used if systemic steroids are contraindicated or if a patient cannot tolerate systemic steroids.
-
C.
Colchicine – Incorrect. See explanation of choice B.
-
D.
Dapsone – Incorrect. See explanation of choice B.
-
E.
Ixekizumab – Incorrect. Although tumor necrosis factor-α inhibitors, such as adalimumab, etanercept, and infliximab, have been reported to treat SS in some cases, interleukin-17 inhibitors, such as ixekizumab, are not part of the therapeutic ladder for SS.
Footnotes
Drs Margaret Wat and Kord Honda contributed equally to this work.
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Nofal A., Abdelmaksoud A., Amer H. Sweet's syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15(11):1081–1088. doi: 10.1111/ddg.13350. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P.R. Sweet's syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draper B.K., Robbins J.B., Stricklin G.P. Bullous Sweet's syndrome in congenital neutropenia: association with pegfilgrastim. J Am Acad Dermatol. 2005;52(5):901–905. doi: 10.1016/j.jaad.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Aleissa M., Nicol P., Godeau M. Azathioprine hypersensitivity syndrome: two cases of febrile neutrophilic dermatosis induced by azathioprine. Case Rep Dermatol. 2017;9(1):6–11. doi: 10.1159/000454876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton J.L., Pincus L., Yazdany J. Association of Sweet's syndrome and systemic lupus erythematosus. Case Rep Rheumatol. 2011:242681. doi: 10.1155/2011/242681. [DOI] [PMC free article] [PubMed] [Google Scholar]