Abstract
Osteoarthritis (OA) is a prevalent whole joint disease characterised by cartilage degradation, subchondral bone sclerosis and bone remodelling, and synovium inflammation, leading to pain, deformity, and cartilage dysfunction. Currently, there is no appropriate therapy for OA, and available treatments simply aim to reduce pain and swelling. Exosomes are membrane-bound extracellular vesicles secreted by almost all cells, receiving increasing interest because of their effect in cell-to-cell communication. Increasing evidence suggests that exosomes play an important role in cartilage physiological and pathological effects. This article reviews the potential role of exosomes in OA regenerative medicine. Special attention is given to mesenchymal stem cells-derived exosomes due to the extensive research on their cartilage repair property and their function as miRNA cargo. More investigations are needed for the effects of exosomes from synovial fluid and chondrocytes in joints. A better understanding of the mechanisms will contribute to a novel and promising therapy for OA patients.
The translational potential of this article
A better understanding of the role of extracellular vesicles in regenerative medicine will contribute to a novel and promising therapy for OA patients.
Keywords: Chondrocytes, Exosomes, Mesenchymal stem cells, MicroRNA, Osteoarthritis, Synovial inflammation
Introduction
Osteoarthritis (OA) is a leading cause of pain and disability in the elderly, affecting more than 250 million people worldwide. OA is mainly characterised by inflammation in the synovium, progressive loss of articular cartilage, degradation and degeneration of menisci and ligaments, thickening of the subchondral bone, and formation of osteophytes [1]. Multiple pathophysiological processes are involved in OA progression, including the activation of the innate and immune systems and imbalance between the anabolic and catabolic factors, eventually leading to the degradation of cartilage and bone [2]. Numerous risk factors may be associated with OA, such as aging, obesity, and trauma. To date, drugs for OA patients can only relieve pain without actually curing the disease. The treatment for OA has always been a problem because of the lack of blood supply to the articular cartilage, the poor proliferation, and the migration potential of chondrocytes [3]. The most widely used treatment in end-stage patients is surgical therapy, which is not only expensive, but also needs a second surgery in some cases [4]. Therefore, an appropriate disease-modifying therapy that targets the onset and progression of OA is urgently needed. In recent years, exosomes have been proposed to play important roles in the pathogenesis and progression of OA, thus providing exciting therapeutic prospects.
Exosomes are membrane-bound extracellular vesicles (EVs) surrounded by a phospholipid bilayer that can be released from normal and pathological cells existing in the blood, urine, saliva, breast milk, bronchoalveolar lavage, pleural effusions, malignant ascites, and amniotic and synovial fluids (Figure 1) [5,6]. EVs are divided into three categories: exosomes, microparticles, and apoptotic bodies (Table 1). Exosomes, the diameters of which vary from 30 to 100 nm, originate from the cell membrane during endocytic internalisation [7]. Previously, the size was considered to be a main determining factor in distinguishing these three particles [8], but recent research points to the fact that protein composition may be a major mechanism in identifying them as well [9]. In this review, EVs refer to exosomes. Exosomes contain RNAs, DNAs, mRNAs, microRNA (miRNAs), proteins, and lipids [10], and their cargo reflects the biological state of the parent cell. They mostly function during cell-to-cell communication via paracrine, juxtacrine, and endocrine signalling [11]. The mRNAs contained in exosomes can be translated within the recipient cells via exosome–cell interaction for silencing gene expression and posttranscriptional regulation [10]. Besides, the bilayer phospholipid membranes of exosomes offer a controlled internal microenvironment, as well as protection, to its contents that guarantee degradation-free travel over cell distances [12]. In OA pathogenesis, exosomes can be pathogenic or protective [2,7]. Since exosomes are secreted by almost all cell types, in this review, we demonstrate the role of exosomes derived from certain typical cell types that are involved in OA, such as mesenchymal stem cells (MSCs), fibroblast-like synoviocytes (FLS), and articular chondrocytes.
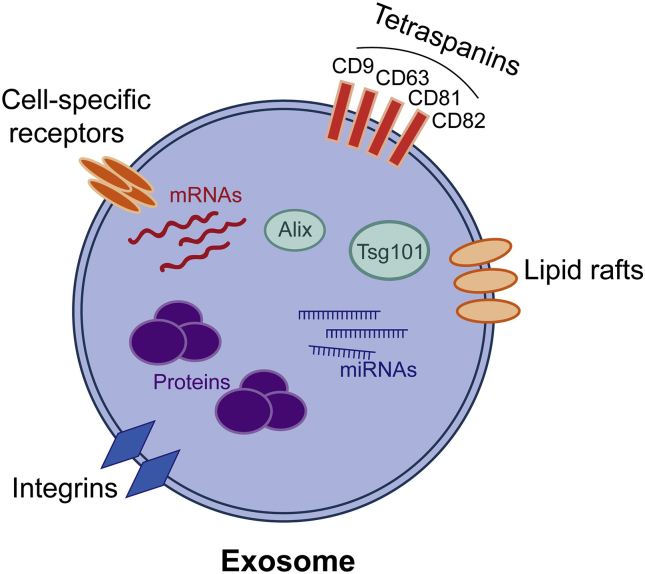
Figure 1.
Diagram of the exosome. Exosomes are membrane-bound extracellular vesicles and have an endosome origin, with a size ranging from 30 nm to 100 nm. Cell-specific receptors, integrins, and lipid rafts can be found in the phospholipid bilayer of the membrane, participating in cell-to-cell communication. Almost all exosomes contain tetraspanins (CD9, CD63, CD81, CD82), multivesicular body biogenesis-related proteins (Alix, Tsg101), heat shock proteins, and some phospholipases. Besides proteins, exosomes also contain mRNAs as well as miRNAs. The composition of exosomes not only reflects the biological state of their parent cells but also affects their functions.
Table 1.
Different types of extracellular vesicles.
| Characteristics | Exosomes | Microvesicles | Apoptotic bodies |
|---|---|---|---|
| Size | 30–100 nm [7] | 50–1000 nm [13] | 1–5000 nm [13] |
| Morphology | Cup-shaped [14] | Heterogeneous [14] | Heterogeneous [14] |
| Origin | Luminal budding into multivesicular bodies (MVBs); release by fusion of MVBs with the cell membrane [13] | Budding off the plasma membrane [15] | Outward blebbing of the cell membrane [13] |
| Composition | Coding RNA, noncoding RNA, proteins, antigen presentation molecules, DNA [10,14] | miRNA, mRNA, proteins, lipids, cytosol [15] | Proteins, lipids, DNA, rRNA, cytosol [15] |
Role of mesenchymal stem cell-derived exosomes in OA
Characteristics of mesenchymal stem cells
MSCs can be divided into three types: embryonic stem cells, pluripotent stem cells, and adult stem cells [16]. Multipotent MSCs have been isolated from various tissues, such as bone marrow, adipose tissue, dental pulp, umbilical cord, and others [17]. MSCs display remarkable antiinflammatory functions and immunosuppressive properties in terms of decreasing inflammation and activating monocytes. They can also modulate immune responses by interacting with cells existing in both the innate and adaptive immune systems. Furthermore, they inhibit macrophage polarisation toward proinflammatory macrophages (M1), and instead favor the antiinflammatory macrophage (M2) phenotype. Additionally, MSCs have the potential to differentiate into chondrocytes, osteoblasts, adipocytes, endothelial cells, neurogenic cells, and cardiovascular cells [18,19]. They can also secrete other bioactive molecules such as insulin-like growth factor 1 (IGF-1), interleukin 6 (IL-6), prostaglandin E2 (PGE2), matrix metalloproteinase-2 (MMP-2), MMP-9 and platelet-derived growth factor (PDGF), which culminate in anti-apoptotic, immunomodulatory, hematopoietic stem cell support, and anti-fibrotic functions [20]. As MSCs are able to mediate inflammation, modulate immune responses, differentiate into chondrocytes, and accelerate tissue repair, they are considered to be a suitable form of therapy for cartilage repair. Previously, Murphy et al. [21] reported the regeneration of the medial meniscus and a reduction in articular cartilage degradation after intraarticular injection of bone marrow MSCs in goats with surgery-induced knee joint OA. At one point, their ability to repair cartilage damage was attributed to chondrocyte differentiation; however, emerging evidence suggests the MSC secretome (mainly referring to exosomes) as an important component in modulating abnormal injury tissue microenvironments and regenerative processes, such as cell migration, proliferation, and matrix synthesis [22]. Although MSC-derived exosomes have been proven to be therapeutically efficient in different studies spanning cardiovascular disease, graft-versus-host disease, and bone fracture, their potential mechanisms in OA still remain unclear. Here, we attempt to summarise the role of MSC-derived exosomes in OA.
Potential role of MSC-Derived exosomes in OA
MSC-derived exosomes in cartilage repair and OA therapy
The articular cartilage, which is mostly responsible for distributing loads evenly and reducing friction, is a unique hypocellular and avascular tissue. In this case, the common regenerative processes upon damage (with respect to stem cell recruitment through the blood) do not occur easily [23], which means that it has very limited capability of self-repair. Therefore, a chronic articular cartilage defect would ultimately cause OA. One of the current surgical treatment options involves autologous chondrocyte implantation, which is still confronted with several problems, including donor site morbidity, shortage of cell source, tissue hypertrophy, and high surgery costs [24]. Another option is joint arthroplasty, which could possibly cause severe complications and injuries at a later stage. Hence, a safe and promising cell-based therapy is urgently needed in terms of regenerative medicine. Although MSC therapy is safer than other types of stem cells, the direct application of stem cells has many limitations, such as tumor growth stimulation, bio-distribution, or ectopic grafting, and immune rejection [25]. Notably, research has increasingly indicated that the therapeutic effects of MSCs may not be limited merely in terms of cell differentiation, but also with respect to their paracrine secretion of bioactive molecules and trophic factors.
Several studies conducted on animals suggested that MSC exosomes possibly facilitated cartilage repair and contributed to promoting articular conditions. Zhang et al. first reported that femurs treated with human embryonic MSC-derived exosomes showed thorough rehabilitation of the subchondral bone and cartilage along with a smooth, regular surface on the hyaline cartilage and almost normal extracellular matrix (ECM) deposition in a surgical rat model. Similar results showed that EVs released by human bone marrow-derived MSCs (hBMSC-EVs) were able to accelerate OA cartilage repair not only by promoting ECM production via OA chondrocytes, but also ameliorating inflammatory responses [26]. Despite their ability to regenerate, MSC exosomes exhibit antiapoptotic capabilities, which may also relate to cartilage repair. Since chondrocytes are the only resident cells in the cartilage, their apoptosis is of great pathogenic importance in OA. Zhang et al. further observed an increase in proliferating nuclear antigen + cells and a different change in cyclic citrullinated peptide 3 + apoptotic cells in exosome-treated groups, which implied that MSC-derived exosomes possibly induced cell proliferation and weakened apoptosis [27]. Similarly, Cosenza et al. also reported that MSC exosomes influenced apoptotic chondrocyte levels in a murine apoptosis model [28].
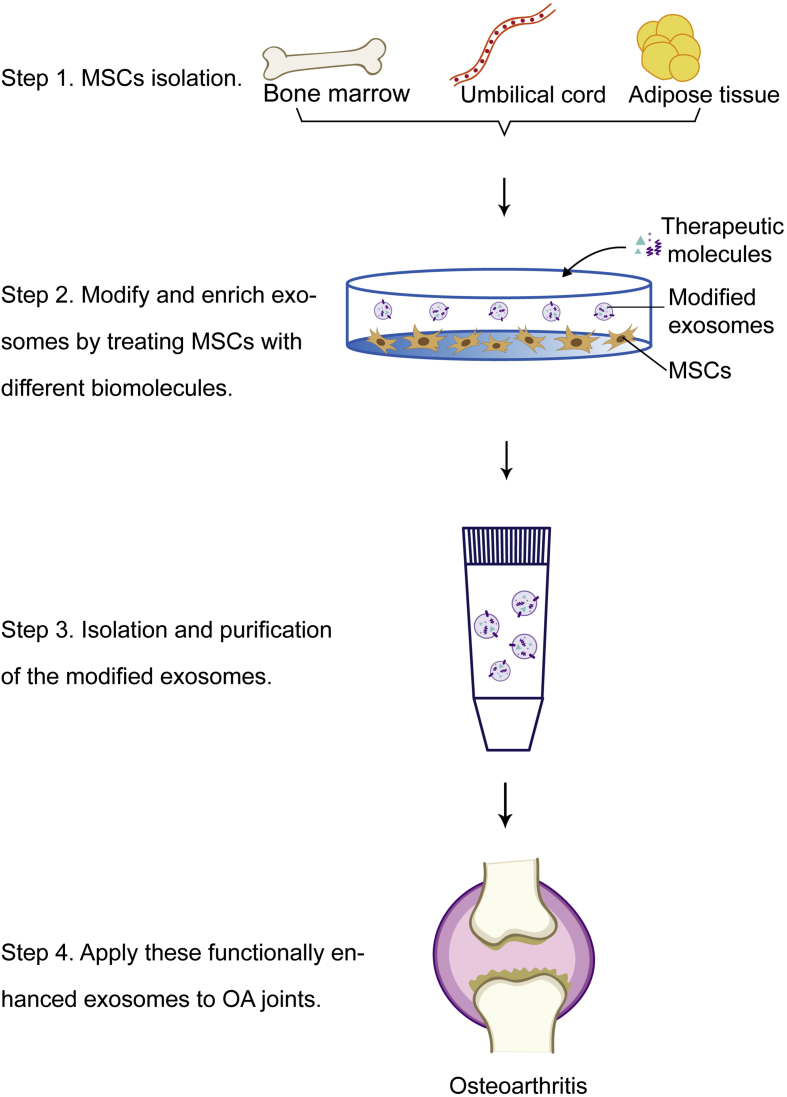
For many years, stem cell therapy has been considered to be an effective and promising strategy in treating many diseases. Exosome-based therapy is obviously superior when compared to MSC therapy not only because exosomes are relatively more sustainable and reproducible, but also because of their probable safety levels, which is enforced by their reduced toxicity and immunogenicity issues [29,30]. In addition, MSC-derived exosomes could communicate with their target cells directly, which significantly reduces the risks and limits of carrying toxicity and rapid clearance [31]. They can also maintain the immune-privileged characteristics of their parental cells and prevent immunogenicity because of the absence of cell surface major histocompatibility complex I/II proteins [14]. Previously, a study showed that MSC-derived EVs induced antiinflammatory cytokine expression, while simultaneously triggering cell death in activated T cells [32]. Exosomes can also be engineered by changing their contents (proteins, miRNAs, nucleic acids, etc.) so that they can be used in various treatments [33]. To date, exosome-based therapies have been reportedly applied in different disease investigations, such as graft-versus-host disease [34] and cardiovascular disease [30]. In terms of treating OA, exosomes from different kinds of MSCs have shown different effects. For instance, Zhu et al. reported that injecting exosomes secreted by both human-induced pluripotent stem cell-derived MSCs (iMSCs-Exos) and synovial membrane-derived MSCs suppressed OA in a mouse model; however, iMSCs-Exos demonstrated superior therapeutic abilities as they were able to better stimulate chondrocyte migration and proliferation [35]. According to this study, exosome therapy for OA is possible because of the non-invasive collection process, inexhaustible cell source, and immunosuppressive properties of iMSCs. Another study by Wang et al. reported that embryonic stem cell-induced MSC-derived exosomes could alleviate OA by balancing the formation and degradation of chondrocyte ECM [36]. Recently, MSCs derived from adipose tissue have shown promising therapeutic prospects in treating OA. In recent research, Wu et al. [37] reported that exosomes secreted by infrapatellar fat pad MSCs (IPFP-MSCs) had the potential to protect the cartilage and prevent its destruction and meanwhile attenuate gait abnormality in OA mice models. In addition, they also found a significant enhancement of the chondrocyte autophagy level via inhibiting the mammalian target of the rapamycin signalling pathway, which was closely related to exosomes derived from IPFP-MSCs. Taken together, these findings above point out that exosome-based therapy may have the great potential to open new possibilities for cell-free treatments in the future. In addition to the direct application of exosomes, modified exosomes have also attracted remarkable scientific interest (Figure 2). Parental cells are well-known to impact the properties and functions of their exosomes by affecting their bioactive cargos. Besides, their specific cell-targeting potential is also noteworthy. Increasing investigations regarding exosomal enrichment with biomolecules for therapeutic purposes suggested another novel strategy for OA treatment based on gene targeting (also known as genetically engineered exosome therapy). Such functionally enhanced exosomes have already been applied successfully in other fields to treat cancer, mediate immune responses, and ameliorate neural deficits. In a recent study, modified exosomes were used to deliver siRNA to human epidermal growth factor receptor 2-positive breast cancer cells [38].
Figure 2.
Schematic diagram of modified MSC-derived exosomes production and their application in treating OA.
Despite the advantages and potential of MSC-derived exosome therapy, certain limitations and risks, in terms of uncontrolled transfer of gene information [10] and problems associated with biodistribution, still exist. Moreover, many challenges with respect to the intrinsic properties of exosomes, such as the lack of targeting property, and the difficulty to load specific biomolecules [39], should be given due attention. In addition, this application requires high-quality standardisation of isolation and professional techniques to harvest sustainable and reproducible exosomes Future investigations may focus on the method to purify exosomes, simultaneously lowering the cost and time of exosome production.
Exosomal miRNA derived from MSCs
As demonstrated above, exosomes can contain RNAs, DNAs, mRNAs, miRNAs, and other biomolecules, through which miRNAs have constantly received increased research interest. Among the different small RNAs, the proportion of exosomal miRNAs has been reported to be higher than miRNAs in their respective parent cells [40].
Notably, particular miRNAs are closely associated with cartilage formation and degeneration. For example, a study by Miyaki suggested that miRNA-140, known to be the most cartilage-specific miRNA, was significantly downregulated in OA articular cartilage and was involved in both chondrocyte differentiation and the imbalance of anabolic and catabolic processes in OA [41]. To be more specific, some other studies implied that exosomal miR-140-5p was of great importance in cartilage homeostasis and slowing down OA progression. Furthermore, Tao et al. found that exosomes derived from miR-140-5p-overexpressed synovial mesenchymal cells (SMSC-140-Exos) enhanced chondrocyte migration and proliferation in a rat OA model [42]. Another study showed that miR-320c enhanced human MSC chondrogenesis and downregulated MMP-13 expression [43]. Sun et al. demonstrated that exosomes derived from hBMSCs overexpressing miR-320c increased SOX9 [SRY (Sex determining region Y)-box 9] and decreased MMP-13 expression in OA chondrocytes, thereby indicating its potent effects in promoting chondrogenesis [44]. Exosomal miR-92a-3p derived from MSCs was also found to mediate cartilage development and homeostasis via WNT5A. Moreover, cartilage proliferation and matrix gene expression were both found to be promoted after MSC-miR-92a-3p-Exosome treatment in OA primary human chondrocytes [45]. The main role of miRNAs in OA pathogenesis is summarised in Table 2. It is important to note that the amount and composition of the miRNAs packaged into exosomes follow diverse disciplines and that exosomal miRNAs are expressed at different levels under various physiological conditions [46]. Unfortunately, present research regarding these topics is very limited. Therefore, the role of MSC-derived exosomal miRNAs in OA needs further investigation.
Table 2.
Role of microRNAs in the pathogenesis of OA.
| miRNAs | Roles | Reference |
|---|---|---|
| miR-93 | Inhibit chondrocyte apoptosis and inflammation in OA | [47] |
| miR-31 | Promote chondrocyte proliferation by targeting C-X-C motif chemokine ligand 12 | [48] |
| miR-485-5p | Increase the release of inflammatory factors and inhibit the cartilage differentiation | [49] |
| miR-486-5p | Inhibit chondrocyte proliferation and migration by suppressing Mothers against decapentaplegic homolog 2 (SMAD2) | [50] |
| miR-10a-5p | Promote chondrocyte apoptosis in OA by targeting Homeobox A1 (HOXA1) | [51] |
| miR-92a | Involved in chondrogenesis and positively regulate co19a2 and aggrecan expression | [52] |
| miR-320 | Inhibit MMP-13 expression in chondrogenesis | [43] |
| miR-181b | Negatively regulate the cartilage development and chondrocyte differentiation | [53] |
| miR-125b | Suppress the expression of ADAMTS-4 mRNA in osteoarthritic chondrocytes | [54] |
| miR-455 | Exacerbate cartilage destruction through diminishing TGFβsignalling | [55] |
| miR-27b | Regulate MMP-13 expression in human osteoarthritis chondrocytes | [56] |
| miR-146a | Intensely expressed in low-grade OA cartilage and decrease in parallel with the level of MMP-13 expression | [57] |
| miR-9 | Inhibit secretion of the collagen type Ⅱ-targeting metalloproteinase MMP-13 in OA chondrocytes | [58] |
Role of exosomes in OA synovial inflammation
Synovial inflammation in OA
Synovial inflammation is a potential cause of OA progression. The synovial membrane is a membranous structure lining the cavities of joints, and its main functions are to secrete synovial fluid and provide nutrients to the cartilage [59]. Evidence-based medicine shows that OA patients experience acute or chronic synovitis in varying degrees [59]. Although OA is always considered to be a degenerative disease with significant debilitating cartilage disorder, research increasingly indicates that chronic, low-grade inflammatory processes leading to synovial inflammation also contribute to OA pathogenesis and disease progression. The underlying mechanisms probably involve the activation of macrophages by some cartilage matrix catabolic molecules and stimulation of other immune cells that release inflammatory cytokines, gradually resulting in chondrocyte dysfunction [60].
Exosomes in synovial inflammation
During the synovial inflammation process, macrophages play a significant role in disease progression [61]. Macrophage accumulation in the synovial lining is a significant characteristic of synovitis. Once stimulated by certain environmental stimuli and inflammatory mediators, these highly plastic cells can change their phenotype, going from M1 macrophages to M2 macrophages [62]. M1 macrophages are capable of presenting antigen and activating Th1 responses, thus displaying proinflammatory functions. M2 macrophages can be divided into three different subtypes: M2a, which are stimulated by IL-4 or IL-13 and are considered to ameliorate proinflammatory stimuli [63]; M2b, which are induced by immune complexes and Toll-like receptors or IL-1 receptor agonists [64]; and M2c, which are stimulated by IL-10 or transforming growth factor beta (TGF-β) and glucocorticoids and suppress inflammation by downregulating proinflammatory cytokines while promoting neovascularisation and tissue remodelling [64,65]. Generally, M2 macrophages contribute to antiinflammation and the repair of injured tissue [66]. Lo Sicco et al. reported for the first time that exosomes secreted by adipose tissue-derived MSCs induced the polarisation of macrophages toward the M2 phenotype, which exhibited antiinflammatory ability [67]. Similarly, Cosenza et al. showed that BMSC-derived exosomes inhibited macrophage activation and likely induced a shift to the M2 phenotype [68]. Interestingly, M2 macrophages have been widely reported to show an antiinflammatory effect during the immune response process, which could promote cartilage repair and ideal joint conditions by suppressing inflammation in arthrosis. Therefore, the immunological property of exosomes may also lay a foundation for clinical therapy in OA patients by manipulating macrophage polarisation in synovial tissues (Figure 3).
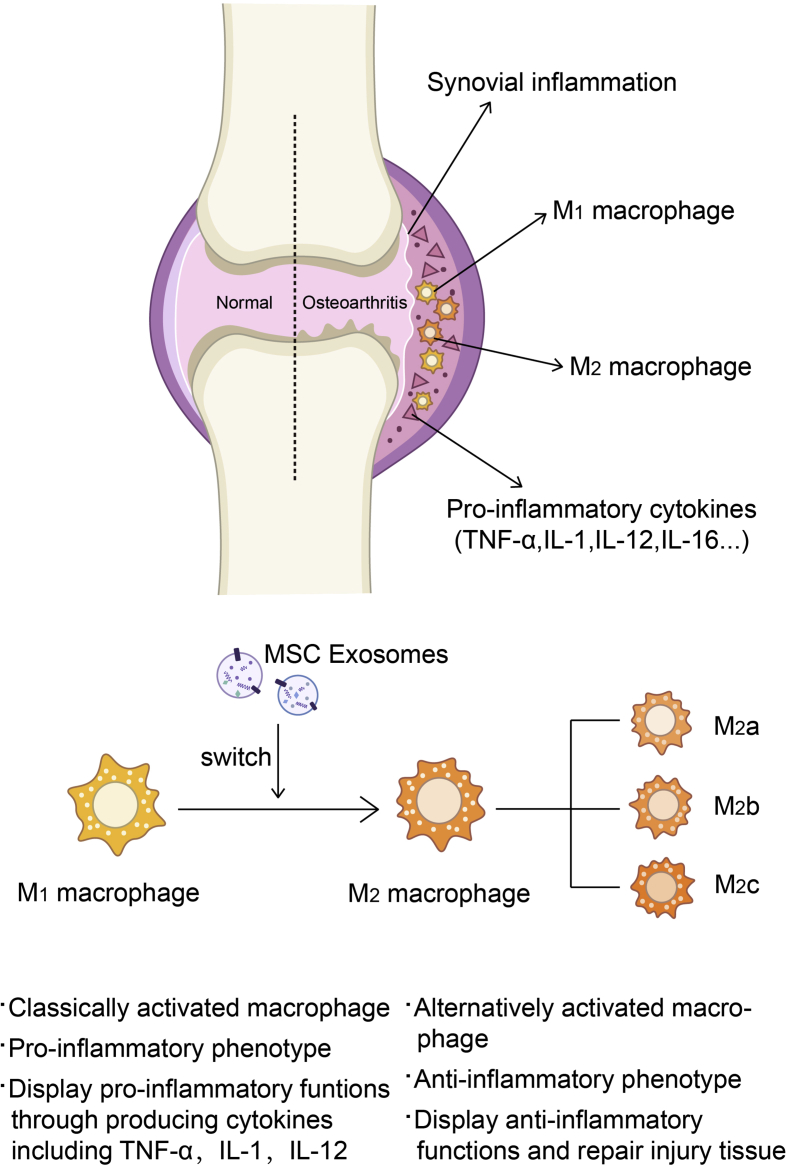
Figure 3.
Macrophage cell accumulation in the synovial lining is one of the characteristics of synovial inflammation. Synovial inflammation promotes the polarisation of macrophages into the M1 phenotype, and they could produce pro-inflammatory cytokines. TNF-α, IL-1, IL-12, and IL-16 abound in inflamed synovium. Previous studies showed that MSCs could change the phenotype of macrophage. Consistent with this, exosomes derived from MSCs may also be macrophage polarisation switchers. Polarised from the M1 phenotype into the M2 phenotype (subdivided into M2a, M2b, and M2c), macrophages could display anti-inflammatory functions and repair the injured tissue.
Fibroblast-like synoviocyte-derived exosomes in OA
Unfortunately, the role of FLS-derived exosomes in OA has not been investigated as much as MSC-derived exosomes (Figure 4). FLS, also known as synovial fibroblasts, are important constituents of the intimal lining layer of the synovial membrane. They are mesenchymal-derived cells, and their main function is to maintain the normal structure and integrity of the joints by stabilising ECM and synovial fluid homeostasis [69]. FLS are capable of releasing exosomes, and a recent study reported that IL-1β-stimulated FLS could increase exosomes secretion [70]. FLS-derived exosomes in OA might be pathogenic because they contribute to OA pathogenesis mainly by inducing OA-related gene expression and promoting angiogenic activities. Kato et al. showed that FLS-derived exosomes induced OA-like changes in articular chondrocytes by upregulating MMP-13 and A disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5) and downregulating Aggrecan (ACAN) and Collagen type II alpha 1 (COL2A1), which suggested that FLS-derived exosomes induced OA-related gene expression patterns, especially in articular chondrocytes. Another study by Kato et al. demonstrated that exosomes from IL-1β-stimulated FLS also induced angiogenesis [70]. The formation of new capillary blood vessels is commonly found in the OA synovium, which is closely associated with chronic inflammation. Exosomes are also involved in chondrocytes-FLS communication. Investigations showed that chondrocyte-derived exosomes treated with inflammatory cytokines could increase COX-2, IL-1β, tumor necrosis factor alpha (TNF-α), and MMP-13 production via FLS, which contributed to ECM degradation, thereby promoting OA progression.
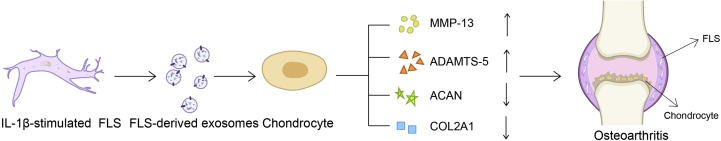
Figure 4.
FLS-derived exosomes induce the gene expression patterns related to OA. Exosomes secreted by IL-1β-stimulated FLS promote MMP-13 and TNF-α expression while inhibiting ACAN and COL2A1 expression. MMP-13 is the most important collagenase that causes type Ⅱ collagen degradation. The upregulation or aberrant activation of MMP-13 contributes to the progression of different stages of OA. TNF-α is also involved in the cartilage degradation process. Simultaneously, the expression of anabolic genes (ACAN and COL2A1) is decreased.
Besides inflammatory cytokines and chemokines, miRNAs have also attracted great interest because miRNAs expressed in exosomes play different roles in chondrocytes. Notably, a selective process to miRNA release is likely to exist. Kato et al. reported the differences in miRNA expression in exosomes derived from IL-1β-stimulated FLS. This study observed that while 340 miRNAs were upregulated in FLS, only 11 miRNAs were upregulated in exosomes and that, while 24 miRNAs were downregulated in FLS, 39 were downregulated in exosomes. 5 miRNAs were upregulated in both FLS and exosomes, namely miR-720, miR-199b-5p, miR-500b, miR-4454, and miR-3154 [70]. Research showed that miR-720 promoted cell migration in cancer tissues [71,72], while miR-199b-5p was associated with the osteogenesis process in hBMSCs by promoting osteoblast differentiation [73] and that it was also upregulated in chondrogenesis [74]. Unfortunately, research related to miR-500b, miR-4454, and miR-3154 in the musculoskeletal system remains poorly understood. Moreover, miR-200c in EVs derived from the synovial fluid of OA patients was shown to increase by 2.5-fold when compared to non-OA subjects. It was also reported to promote the expression of type Ⅱ collagen while ameliorating IL-6-dependent inflammation [68]. Interestingly, Kolhe et al. found that miRNAs detected in the EVs of FLS-secreted synovial fluids in OA patients differed with gender, i.e., a greater amount of differentially-regulated miRNAs were observed in the female OA group when compared to the male OA group. They also revealed that OA-specific miRNAs in women were estrogen responsive and were involved in Toll-like receptor signalling pathways, thereby indicating that these gender-specific miRNAs were likely related to the higher incidence and severity of OA in females [75].
Role of chondrocyte-derived exosomes in OA
Chondrocytes are extremely important in maintaining normal cartilage function as they are the only cells in the cartilage that produce ECM. Chondrogenesis is essential in forming cartilage and involves several processes, such as mesenchymal cell recruitment and migration, condensation of progenitors, and chondrocyte differentiation/maturation [76]. Various underlying mechanisms contribute to the pathogenesis of OA, and aside from the potential risk factors mentioned previously, increasing evidence suggests that epigenetic factors are also involved. Histone modifications, DNA methylation, and non-coding RNAs form the basic regulatory mechanisms in epigenetics [77]. These epigenetic regulatory processes in chondrocytes are related to the catabolic and anabolic factor instability, which results in articular cartilage injury that gradually leads to OA [78]. In a recent investigation, Mao et al. showed the potential role of miRNAs secreted by exosomes derived from chondrocytes in both cartilage development and OA pathogenesis. Twenty-two miRNAs were found to be upregulated, while 29 miRNAs were downregulated in OA chondrocyte-derived exosomes when compared to the control, among which miR-95-5p was the most studied. They found that miR-95-5p was reduced 2.54-fold in OA chondrocyte exosomes. Furthermore, exosomal miR-95-5p could not only delay the development of OA and maintain cartilage homoeostasis but could also directly inhibit histone deacetylases 2/8, thereby possibly regulating chondrocyte development and participating in hypertrophic responses [79]. Therefore, exosomes derived from miR-95-5p-overexpressing chondrocytes exhibit the potential to be novel therapeutic drugs for OA patients [45]. Other findings also demonstrate the potential role of extracellular vesicles secreted by chondrocytes in OA. Kirsch et al. reported that matrix vesicles (MVs) derived from OA chondrocytes contained annexins Ⅱ, Ⅴ, and Ⅵ, which were closely associated with the pathological mineralisation of the cartilage. These MVs also contained alkaline phosphatase, which participates in mineral formation [80]. Since excessive and pathological mineralisation may contribute to OA, the components of those MVs released from chondrocytes can provide a therapeutic target for OA. To the best of our knowledge, investigations concerning the potential role of chondrocyte-derived exosomes in OA are very limited, and the mechanisms are poorly understood. Therefore, the role of chondrocyte-derived exosomes in OA needs to be further elucidated.
Conclusions
OA is a very common and complex joint disease, which unfortunately still lacks appropriate clinical therapy options. In this review, we illustrated the potential role of exosomes in OA regenerative medicine and paid special attention to exosomes derived from MSCs. MSC-derived exosomes have been widely investigated. Their efficacy in terms of cartilage repair, miRNA transport, and several other functions showcase their great potential and enable them to be promising cell-free therapeutic agents with respect to OA treatments. However, their clinical application in patients requires abundant clinical trials. Unfortunately, studies concerning exosomes from the synovial fluid and chondrocytes are limited; thus, their role in OA is not yet fully understood.
Significantly, much research is ongoing on the role of other biomolecules in OA, which would help in promoting the development of OA diagnosis and treatments. For instance, long noncoding RNA (lncRNA) is a type of RNA consisting of more than 200 nucleotides, and the regulated expression of lncRNAs plays an important role in many diseases. It is reported that over 24 kinds of lncRNAs were upregulated in different vivo or vitro OA models, but only a few now focus on those derived from extracellular vesicles. A recent study reported that the expression of lncRNA Prostate-specific transcript 1 increased gradually in normal, early, and late-stage OA, which indicated that some lncRNA could be used as an indicator for distinguishing the different stages of OA [81]. In another study, it was reported that MSC-derived exosomal lncRNA Kruppel Like Factor 3 Antisense RNA 1 (KLF3-AS1) promoted cartilage repair and chondrocyte proliferation by inhibit apoptosis of chondrocytes via KLF3-AS1/miR206/GTPase-activating Protein axis in OA [82,83]. More future work needs to emphasise the underlying mechanisms and the role of other extracellular vesicles derived from different categories of cells apart from what has been discussed above, such as subchondral bone, which is believed to change earlier than cartilage [84,85]. Besides, we are still confronted with various obstacles, such as the time-consuming and expensive isolation and purification processes in exosome production. Therefore, continued investigation in this field is needed so that these different exosomes may benefit OA patients in the future.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
Authors wish to acknowledge funding support from Arthritis Australia, The Prince Charles hospital foundation research fellowship to Dr. Indira Prasadam (RF-01), The National Natural Science Foundation of China for Prof. Xinzhan Mao (81371997) and The Project of Training Programs of Innovation for Research Students from Central South University (502211809). We also acknowledge Fuyiting He from the South China University of Technology and Mengyuan Zhao from Central South University for figures assistance.
Contributor Information
Xinzhan Mao, Email: xinzhan.mao@csu.edu.cn.
Indira Prasadam, Email: i.prasadam@qut.edu.au.
References
- 1.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domenis R., Zanutel R., Caponnetto F., Toffoletto B., Cifu A., Pistis C. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediat Inflamm. 2017;2017:4814987. doi: 10.1155/2017/4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinert A.F., Ghivizzani S.C., Rethwilm A., Tuan R.S., Evans C.H., Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9(3):213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okoro T., Morrison V., Maddison P., Lemmey A.B., Andrew J.G. An assessment of the impact of behavioural cognitions on function in patients partaking in a trial of early home-based progressive resistance training after total hip replacement surgery. Disabil Rehabil. 2013;35(23):2000–2007. doi: 10.3109/09638288.2013.770082. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M., Raposo G., Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 6.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 7.Li Z., Wang Y., Xiao K., Xiang S., Li Z., Weng X. Emerging role of exosomes in the joint diseases. Cell Physiol Biochem. 2018;47(5):2008–2017. doi: 10.1159/000491469. [DOI] [PubMed] [Google Scholar]
- 8.Witwer K.W., Buzas E.I., Bemis L.T., Bora A., Lasser C., Lotvall J. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotvall J., Hill A.F., Hochberg F., Buzas E.I., Di Vizio D., Gardiner C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 11.Lo Cicero A., Stahl P.D., Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Eldh M., Ekstrom K., Valadi H., Sjostrand M., Olsson B., Jernas M. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5(12):e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal Stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y., Sun R., Wu C., Wang L., Zhang C. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpman D., Stahl A.L., Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13(9):545–562. doi: 10.1038/nrneph.2017.98. [DOI] [PubMed] [Google Scholar]
- 16.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pers Y.M., Ruiz M., Noel D., Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027–2035. doi: 10.1016/j.joca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Crapnell K., Blaesius R., Hastings A., Lennon D.P., Caplan A.I., Bruder S.P. Growth, differentiation capacity, and function of mesenchymal stem cells expanded in serum-free medium developed via combinatorial screening. Exp Cell Res. 2013;319(10):1409–1418. doi: 10.1016/j.yexcr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Maumus M., Jorgensen C., Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95(12):2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Murphy J.M., Fink D.J., Hunziker E.B., Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Meirelles L., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5):419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y.Z., Zhang S.F., Qi Y.Y., Wang L.L., Ouyang H.W. Cell transplantation for articular cartilage defects: principles of past, present, and future practice. Cell Transplant. 2011;20(5):593–607. doi: 10.3727/096368910X532738. [DOI] [PubMed] [Google Scholar]
- 25.Herberts C.A., Kwa M.S., Hermsen H.P. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9(1):29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonk L.A., van Dooremalen S.F.J., Liv N., Klumperman J., Coffer P.J., Saris D.B.F. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8(4):906–920. doi: 10.7150/thno.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q., Zhang Y., Yang G., Chen X., Zhang Y., Cao G. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008;36(8):2690–2699. doi: 10.1093/nar/gkn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleury A., Martinez M.C., Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:370. doi: 10.3389/fimmu.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellavia D., Raimondi L., Costa V., De Luca A., Carina V., Maglio M. Engineered exosomes: a new promise for the management of musculoskeletal diseases. Biochim Biophys Acta Gen Subj. 2018;1862(9):1893–1901. doi: 10.1016/j.bbagen.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Mokarizadeh A., Delirezh N., Morshedi A., Mosayebi G., Farshid A.A., Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Akyurekli C., Le Y., Richardson R.B., Fergusson D., Tay J., Allan D.S. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015;11(1):150–160. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 34.Kordelas L., Rebmann V., Ludwig A.K., Radtke S., Ruesing J., Doeppner T.R. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Yu D., Liu Z., Zhou F., Dai J., Wu B. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J., Kuang L., Chen C., Yang J., Zeng W.-N., Li T. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Limoni S.K., Moghadam M.F., Moazzeni S.M., Gomari H., Salimi F. Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl Biochem Biotechnol. 2019;187(1):352–364. doi: 10.1007/s12010-018-2813-4. [DOI] [PubMed] [Google Scholar]
- 39.Jafari D., Malih S., Eslami S.S., Jafari R., Darzi L., Tarighi P. The relationship between molecular content of mesenchymal stem cells derived exosomes and their potentials: opening the way for exosomes based therapeutics. Biochimie. 2019;165:76–89. doi: 10.1016/j.biochi.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Goldie B.J., Dun M.D., Lin M., Smith N.D., Verrills N.M., Dayas C.V. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42(14):9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyaki S., Nakasa T., Otsuki S., Grogan S.P., Higashiyama R., Inoue A. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60(9):2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao S.C., Yuan T., Zhang Y.L., Yin W.J., Guo S.C., Zhang C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng F., Zhang Z., Chen W., Huang G., He A., Hou C. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1beta-induced chondrocyte responses. Osteoarthritis Cartilage. 2016;24(5):932–941. doi: 10.1016/j.joca.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Sun H., Hu S., Zhang Z., Lun J., Liao W., Zhang Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 2019;120(1):171–181. doi: 10.1002/jcb.27289. [DOI] [PubMed] [Google Scholar]
- 45.Mao G., Kang Y., Zhang Z., Zhang Z., Liao W. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and prevent the development of osteoarthritis. Osteoarthritis Cartilage. 2018;26:S103. [Google Scholar]
- 46.Zhang J., Li S., Li L., Li M., Guo C., Yao J. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinform. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Y., Wang L., Zhao Q., Wu Z., Kong L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFkappaB signaling pathway. Int J Mol Med. 2019;43(2):779–790. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai Y., Liu S., Xie X., Ding M., Zhou Q., Zhou X. MicroRNA31 promotes chondrocyte proliferation by targeting CXC motif chemokine ligand 12. Mol Med Rep. 2019;19(3):2231–2237. doi: 10.3892/mmr.2019.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H.O., Zhang L., Tang Z.Y., Gong Z.M. MiR-485-5p promotes the development of osteoarthritis by inhibiting cartilage differentiation in BMSCs. Eur Rev Med Pharmacol Sci. 2018;22(11):3294–3302. doi: 10.26355/eurrev_201806_15148. [DOI] [PubMed] [Google Scholar]
- 50.Shi J., Guo K., Su S., Li J., Li C. miR4865p is upregulated in osteoarthritis and inhibits chondrocyte proliferation and migration by suppressing SMAD2. Mol Med Rep. 2018;18(1):502–508. doi: 10.3892/mmr.2018.8931. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y., Wu Y., Chen J., Huang K., Ji B., Chen Z. miR-10a-5p promotes chondrocyte apoptosis in osteoarthritis by targeting HOXA1. Mol Ther Nucleic Acids. 2018;14:398–409. doi: 10.1016/j.omtn.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou C., Zhang Z., Zhang Z., Wu P., Zhao X., Fu M. Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Mol Med Rep. 2015;12(4):4877–4886. doi: 10.3892/mmr.2015.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song J., Lee M., Kim D., Han J., Chun C.H., Jin E.J. MicroRNA-181b regulates articular chondrocytes differentiation and cartilage integrity. Biochem Biophys Res Commun. 2013;431(2):210–214. doi: 10.1016/j.bbrc.2012.12.133. [DOI] [PubMed] [Google Scholar]
- 54.Matsukawa T., Sakai T., Yonezawa T., Hiraiwa H., Hamada T., Nakashima M. MicroRNA-125b regulates the expression of aggrecanase-1 (ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res Ther. 2013;15(1):R28. doi: 10.1186/ar4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swingler T.E., Wheeler G., Carmont V., Elliott H.R., Barter M.J., Abu-Elmagd M. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012;64(6):1909–1919. doi: 10.1002/art.34314. [DOI] [PubMed] [Google Scholar]
- 56.Akhtar N., Rasheed Z., Ramamurthy S., Anbazhagan A.N., Voss F.R., Haqqi T.M. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62(5):1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamasaki K., Nakasa T., Miyaki S., Ishikawa M., Deie M., Adachi N. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum. 2009;60(4):1035–1041. doi: 10.1002/art.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones S.W., Watkins G., Le Good N., Roberts S., Murphy C.L., Brockbank S.M. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17(4):464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Sun A.R., Friis T., Sekar S., Crawford R., Xiao Y., Prasadam I. Is synovial macrophage activation the inflammatory link between obesity and osteoarthritis? Curr Rheumatol Rep. 2016;18(9):57. doi: 10.1007/s11926-016-0605-9. [DOI] [PubMed] [Google Scholar]
- 60.van Lent P.L., Grevers L., Blom A.B., Sloetjes A., Mort J.S., Vogl T. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann Rheum Dis. 2008;67(12):1750–1758. doi: 10.1136/ard.2007.077800. [DOI] [PubMed] [Google Scholar]
- 61.Goichberg P. Current understanding of the pathways involved in adult stem and progenitor cell migration for tissue homeostasis and repair. Stem Cell Rev. 2016;12(4):421–437. doi: 10.1007/s12015-016-9663-7. [DOI] [PubMed] [Google Scholar]
- 62.Spiller K.L., Anfang R.R., Spiller K.J., Ng J., Nakazawa K.R., Daulton J.W. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 64.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Lech M., Grobmayr R., Weidenbusch M., Anders H.J. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments. Mediators Inflamm. 2012;2012:951390. doi: 10.1155/2012/951390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C.K. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185(10):2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lo Sicco C., Reverberi D., Balbi C., Ulivi V., Principi E., Pascucci L. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6(3):1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cosenza S., Ruiz M., Maumus M., Jorgensen C., Noel D. Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bottini N., Firestein G.S. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Y., Lin Y., Li C., Hu X., Liu Y., He M. MicroRNA-720 promotes in vitro cell migration by targeting Rab35 expression in cervical cancer cells. Cell Biosci. 2015;5:56. doi: 10.1186/s13578-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das S.G., Romagnoli M., Mineva N.D., Barille-Nion S., Jezequel P., Campone M. miR-720 is a downstream target of an ADAM8-induced ERK signaling cascade that promotes the migratory and invasive phenotype of triple-negative breast cancer cells. Breast Cancer Res. 2016;18(1):40. doi: 10.1186/s13058-016-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao R., Li Y., Lin Z., Wan J., Xu C., Zeng Y. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3beta/beta-catenin signaling pathway. Biochem Biophys Res Commun. 2016;477(4):749–754. doi: 10.1016/j.bbrc.2016.06.130. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z., Kang Y., Zhang Z., Zhang H., Duan X., Liu J. Expression of microRNAs during chondrogenesis of human adipose-derived stem cells. Osteoarthritis Cartilage. 2012;20(12):1638–1646. doi: 10.1016/j.joca.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Kolhe R., Hunter M., Liu S., Jadeja R.N., Pundkar C., Mondal A.K. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldring M.B., Tsuchimochi K., Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 77.Barter M.J., Bui C., Young D.A. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. 2012;20(5):339–349. doi: 10.1016/j.joca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Kim H., Kang D., Cho Y., Kim J.-H. Epigenetic regulation of chondrocyte catabolism and anabolism in osteoarthritis. Mol Cells. 2015;38(8):677–684. doi: 10.14348/molcells.2015.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao G., Hu S., Zhang Z., Wu P., Zhao X., Lin R. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med 22(11) 2018:5354–5366. doi: 10.1111/jcmm.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirsch T. Annexins - their role in cartilage mineralization. Front Biosci. 2005;10:576–581. doi: 10.2741/1553. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Y., Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42(12):2865–2872. doi: 10.1007/s00264-018-4093-6. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y., Zou R., Wang Z., Wen C., Zhang F., Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475(22):3629–3638. doi: 10.1042/BCJ20180675. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17(21–22):2411–2422. doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prasadam I., Crawford R., Xiao Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes – possible pathogenic role in osteoarthritis. J Rheumatol. 2012;39(3):621–634. doi: 10.3899/jrheum.110777. [DOI] [PubMed] [Google Scholar]
- 85.Prasadam I., van Gennip S., Friis T., Shi W., Crawford R., Xiao Y. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010;62(5):1349–1360. doi: 10.1002/art.27397. [DOI] [PubMed] [Google Scholar]